Abstract
Temperate forests of eastern North America are subject to multiple invasions from non-native species that have the potential to drive long-term dynamics in biodiversity. Garlic mustard (Alliaria petiolata (M. Bieb.) Cavara and Grande) is an invasive plant in many deciduous forests, and management efforts often focus on removing this species to initiate native species restoration. Agrilus planipennis Fairmaire (emerald ash borer; Coleoptera: Buprestidae) is a non-native insect pest that has caused substantial loss of ash trees (Fraxinus spp. L.) in North America. Our goal was to understand how the herbaceous layer in an old-growth forest responded to the removal of a significant invasion of A. petiolata and the loss of Fraxinus spp. due to A. planipennis. Herbaceous diversity and environmental parameters were measured in 32 permanent plots (1 m2 each) from 2012 to 2020 in an old-growth forest remnant that had experienced A. petiolata invasion and subsequent removal as well as mortality of Fraxinus spp. due to A. planipennis. Near-total loss of Fraxinus spp. as a canopy tree was not associated with changes in the understory light environment, possibly due to rapid canopy closure by adjacent trees not susceptible to the insect. Alliaria petiolata removal was associated with changes in herbaceous species richness and possibly shifts in individual species importance. Vegetation–environment relationships remained stable throughout the sampling period, suggesting that resource-related factors that structure the herb layer prevailed throughout the changes associated with Fraxinus spp. mortality and A. petiolata management. From a natural area management perspective, our data offer support for the idea that A. petiolata removal influences herb-layer diversity and indicate that in stands with a diverse tree community, the loss of Fraxinus spp. may not directly influence understory biodiversity.
1. Introduction
The herbaceous layer of temperate deciduous forests is often species rich, plays an important role in ecosystem function, and is subject to effects from invasive species [1]. The nature and magnitude of the herbaceous layer’s contribution to ecosystem function depends upon its composition, which is regulated by a number of different biotic and abiotic drivers [1]. Herbaceous species distributions have been linked to abiotic gradients of topography [2,3], microtopographic features [4], soil fertility (e.g., nitrogen content and pH) [2,5], and soil moisture [5]. Overstory canopy cover is an important biotic regulator of resource availability (light, water, and nutrients) and microclimate in the understory environment [6], both of which can influence herbaceous species distributions at both local [2,7] and stand-level [8,9,10,11]. Tree canopy structure, both in terms of density and species composition, has been linked to understory temperature and light availability, both of which can influence herbaceous diversity [12] and growth [13].
A variety of non-native plant and insect species have been introduced to the forests in eastern North America with effects on native communities that may overlap spatially and temporally. One particularly impactful pest species introduction has been Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), a wood-boring beetle commonly known as emerald ash borer (EAB). Originating in Asia, EAB was discovered in North America in 2002 (Detroit, Michigan, and Windsor, Ontario), likely arriving via international cargo some years prior. It has since been extremely detrimental to both natural and horticultural ash tree (Fraxinus spp. L.) populations in the U.S. [14]. Larval consumption of the phloem and cambium causes high rates of tree mortality [14], and the long-term implications of EAB-related ash mortality may include significant alterations to forest composition and ecosystem function beyond natural gap dynamics [15,16,17,18]. Indirect changes to the understory community caused by EAB may be related to increased canopy gap formation, which could alter local environmental conditions and contribute to increased success of invasive plant species in areas with high overstory canopy loss [19,20]. In many forests, Fraxinus spp. is a relatively minor component of the overstory, so there are likely to be highly varied effects of EAB-related mortality, depending on forest structure and composition [16].
Many forests in which EAB is causing significant Fraxinus spp. mortality are also sites of invasion by other species, which could lead to synergistic negative effects on these ecosystems. A common co-occurring invasive species in such forests is Alliaria petiolata (M. Bieb.) Cavara and Grande (garlic mustard), a non-native herbaceous biennial [21]. Populations of A. petiolata are capable of rapid expansion [22], especially along forest edges and in moist areas with high light availability, although penetration into forest interiors also occurs [23]. Canopy gap formation due to the EAB-induced death of Fraxinus has the potential to create the disturbed, high-light conditions favorable for A. petiolata. Management of A. petiolata invasion has become an important goal for many stewards of natural areas [24]; however, the effects of removal on native plant communities have varied. For instance, although A. petiolata forms dense stands (Supplemental Figure S1) and may be allelopathic [25], some evidence suggests that removal efforts do not significantly impact the plant community in forests where it has invaded [26]. Other work has found modest positive effects of control [27] and suggested that, although species richness is not influenced by invasion, effects are discernable at the level of individual species [28].
Drew Woods State Nature Preserve (DWSNP) is an old-growth forest remnant in west central Ohio that provides a unique opportunity to explore long-term herb-layer dynamics in a setting with minimal anthropogenic disturbance. Due to its old-growth status, the herbaceous layer of this forest has not experienced the species loss or ecological homogenization that may be associated with large-scale disturbance [29,30,31]; therefore, it may be considered a reference site representing historical conditions of forests in this region. The long-term monitoring of forests using repeated sampling of permanent plots is an effective technique for assessing vegetation dynamics (e.g., CTFS, [32]; FIA, [33]) and is particularly useful in old-growth forests where experimental manipulations are not allowed [34]. Since the initiation of our research program there in 2010, DWSNP has experienced two ecological invasions (A. petiolata and EAB), and our long-term sampling provided an opportunity to assess the influences of these invaders on botanical diversity. Local environmental gradients also have a strong potential to control herb-layer composition despite these disturbances [2]; therefore, we also examined the underlying role of these background drivers of community composition. The objectives of this study were to (1) examine changes in herbaceous layer composition over an eight-year period (2012–2020), (2) assess how those changes relate to EAB-induced Fraxinus mortality and changes in the abundance of A. petiolata, and (3) assess whether herb-layer parameters (cover, diversity) maintained consistent relationships with environmental factors throughout the study period despite the aforementioned invasion-related perturbances.
2. Materials and Methods
2.1. Study Site
Drew Woods State Nature Preserve (DWSNP, 40°15′ N, 84°39′ W) consists of approximately 6 ha of old-growth oak-maple swamp forest located in Darke County, Ohio and is managed by Darke County Parks District (see [35] for site map). The topography is relatively flat with less than 5 m change in elevation across the site, and there are several vernal pools and semi-permanent ponds, indicating that the natural hydrology of the site is intact. Mean annual precipitation is 95.5 cm and mean annual temperature is 10.2 °C [36]. Boerner and Kooser [37] characterized the soil as Glynwood silt loam and Blount silt loam. DWSNP is in the Central Ohio Clayey Till Plain region [38], and the overstory is dominated by Acer saccharum Marsh., Carya spp. Nutt., Fraxinus spp., and Quercus spp. L. [35,36].
Goins et al. [35] found that Fraxinus spp. comprised 6.9% of the relative abundance in the overstory of DWSNP in 2011, and since that time EAB has invaded the region and caused significant mortality of ash trees throughout this forest stand. Emerald ash borer galleries and characteristic D-shaped exit holes are present on downed and dead standing Fraxinus spp. in our study site (pers. obs.) and the pattern of mortality matches the region-wide EAB-related loss [15,20].
Alliaria petiolata invasion in DWSNP was noticed in 2010 (it is not known when A. petiolata first arrived at this site), and by 2012 it was widespread, having the highest relative importance value (ca. 15%) of any herbaceous plant in the understory [39]. This prompted organized management activity to control the species via manual removal of plants early in the growing season [24]. In addition, whenever Alliaria petiolata was encountered in a study plot, its abundance was first recorded and then all plants were subsequently removed from the plot. Because this site is an old-growth forest reserve, it was inappropriate to set up experimental control plots where A. petiolata would be left in place; however, the effects of this adaptive management activity can be assessed using the long-term monitoring plots and our assessment began prior to any of the “pulls,” effectively providing before and after data.
2.2. Data Collection
Permanent plot centers (n = 32) were established in 2011 to sample the overstory, sapling, and seedling layers using a nested sampling design [35]. The initial survey of the herbaceous community was conducted in 2012 using the 1-m2 seedling layer plots [39]. Since then, the overstory, sapling, and seedling layers were resampled in 2017, and the herbaceous layer was resampled in early May 2013–2018 and late April 2020. Surveys were conducted in late April to early May to capture the phenological overlap of spring ephemeral and summer green species, which creates a seasonal peak in species richness during this time [39]. All species within a plot were identified to species-level when possible, and percentage cover was estimated using a modified Domin scale (<1%, 1%–5%, 6%–10%, 11%–25%, 26%–35%, 36%–50%, 51%–75%, 76%–90%, 91%–100%).
Soil moisture content (%) was measured in 2015 using a HydroSense volumetric water content probe (Campbell Scientific, Inc., Logan, UT, USA), taking four readings per plot and calculating a mean value. Plot center coordinates were collected using a Garmin eTrex Legend GPS Receiver (Garmin International, Inc., Olathe, KS, USA), then imported into ArcGIS 10.1 (ESRI, Redlands, CA, USA) to determine the distance in meters of each plot from the southern and western edges of DWSNP. In 2012 and 2017, canopy images were taken in the center of each plot using a CID-120 Canopy Imager (CID Bio-Science, Inc., Camas, WA, USA) and used to obtain leaf area index (LAI) values for each plot, representing the canopy cover. Botanical nomenclature follows Jones [40] and the USDA Plants Database [41] (https://plants.usda.gov; accessed on 25 September 2020).
2.3. Data Analysis
Relative importance values (RIV) were calculated for each species by averaging the relative cover and relative frequency within each year. Species richness, cover, Shannon diversity (presented as effective numbers of species [42]), and Pielou’s evenness were calculated for each plot in each sampling year. In order to assess the influence of changing A. petiolata abundance on herb-layer composition, A. petiolata abundance was treated as a separate independent variable and thus not included in the richness, cover, Shannon diversity, evenness, and beta diversity calculations. Plot-level changes in richness, cover, Shannon diversity, and evenness were calculated by subtracting 2012 plot values from the 2017 values (to compare to LAI and Fraxinus basal area change) and 2020 values (to compare with changes in A. petiolata cover). Herbaceous layer temporal turnover (beta diversity) values were calculated using the Bray-Curtis dissimilarity index, which ranges from 0 to 1 where values closer to 1 indicate greater change in species composition (greater dissimilarity). These values were calculated between consecutively sampled years (2012–2013, 2013–2014, 2014–2015, 2015–2016, 2016–2017, 2017–2018, and 2018–2020) to examine incremental changes, as well as between 2012 and 2017 and between 2012 and 2020 to examine long-term changes, Plot-level Fraxinus abundance values from the 2011 and 2017 overstory surveys (trees ≥ 2.5 cm diameter at breast height) were used to examine sitewide changes in living ash basal area. Changes in canopy cover were calculated by subtracting the 2012 LAI values from the 2017 values.
Repeated Measures ANOVA (one-way) was used to test for differences in herb-layer richness, cover, Shannon diversity, evenness, and A. petiolata cover among sampling years and beta diversity among sampling intervals. When violations of the assumption of sphericity occurred (Mauchly’s test of sphericity p < 0.05 [43]), the Greenhouse–Geisser correction [44] was applied to obtain adjusted p-values (PGG). Post hoc comparisons were made using pairwise t-tests with Bonferroni corrections.
Linear regression was used to test for significant relationships between environmental variables (soil moisture, canopy cover, and distances to southern and western forest edges) and herbaceous richness, cover, Shannon diversity, and evenness. Linear regression was used to test for significant relationships among 2012–2017 changes in canopy cover (LAI) and 2012–2017 changes in Fraxinus basal area and herb-layer richness, cover, Shannon diversity, and evenness over the same interval. We also examined whether significant relationships existed between changes in 2012–2020 A. petiolata cover and herb-layer richness, cover, Shannon diversity, and evenness. Beta diversity values were also compared to environmental variables, changes in A. petiolata cover, and changes in canopy cover (LAI) using linear regression. All analyses were performed using the R statistical programming language version 3.4.3 [45].
3. Results
3.1. Temporal Changes in Relative Importance Values
Numerous species exhibited noticeable shifts in relative importance over the sampling interval (Table 1). Alliaria petiolata generally decreased in importance over time, which was expected due to targeted removal efforts. Several native species experienced large increases in relative importance from 2012 to 2013: Floerkea proserpinacoides Willd., Trillium sessile L., Claytonia virginica L., Cardamine concatenata (Michx.) Sw., Allium tricoccum Aiton, and Erythronium spp. L. The relative importance of these species fluctuated in subsequent years but generally remained higher than the initial 2012 values. Sanicula odorata (Raf.) K.M. Pryer and L.R. Phillipe experienced a large increase in relative importance among later sampling years, dominating our sample of the herbaceous community from 2015 to 2018. Most other native herb species maintained fairly consistent levels of relative importance throughout the study period.

Table 1.
Relative Importance Values (RIV: mean of relative cover and relative frequency) of herbaceous species in Drew Woods State Nature Preserve, Darke County, Ohio, USA. Species are listed in descending order based on the 2012 values. Minor species are those with an RIV below 1.00 across all sampling years. Dashes indicate species’ absence in a particular year.
3.2. Temporal Changes in Herbaceous Diversity and Cover
All five of the herbaceous layer metrics (richness, cover, Shannon diversity, evenness, and beta diversity) varied significantly among sample years (Table 2). Species richness decreased significantly from 2012 to 2013, remained constant from 2013 to 2015, and then increased significantly in 2016 and again in 2018 (Figure 1A). Shannon diversity showed no significant differences among the years, spanning from 2012 to 2016, but there was a significant decrease from 2016 to 2017 followed by successive significant increases in 2018 and 2020 (Figure 1B). Evenness exhibited a similar pattern over time to that of Shannon diversity, where values were consistent from 2012 to 2016, followed by a decrease in 2017 that was significantly lower than 2014–2015, then two successive increases in 2018 and 2020 (Figure 1C). Herbaceous cover initially decreased significantly from 2012 to 2013, but later increased significantly in 2016 and 2020 compared to the preceding sample year (Figure 1D).

Table 2.
Repeated Measures ANOVA results testing for significant changes in herb-layer characteristics and Alliaria petiolata abundance from 2012 to 2020 (excluding 2019, which was not sampled) in DWSNP. Where Mauchly’s test of sphericity indicated significant (p < 0.05) departures from the assumption of sphericity, Greenhouse–Geisser corrections were applied to p-values. p-values less than 0.05 are significant. <<0.001 indicates that p-values were less than 1.0 × 10−7.
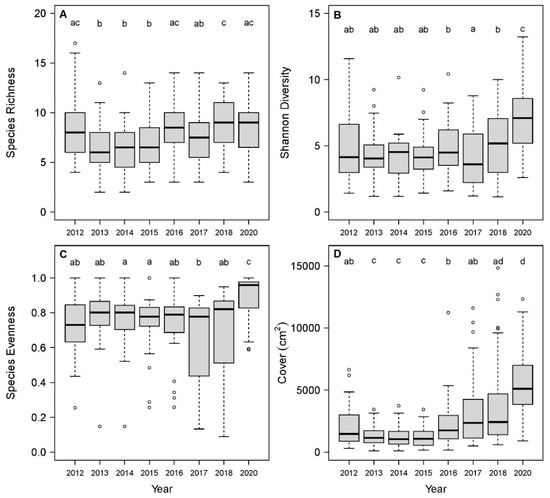
Figure 1.
Herbaceous layer species richness (A), Shannon diversity (B), evenness (C), and cover (D) from 2012 to 2018 and 2020 in Drew Woods State Nature Preserve (DWSNP) (plots were not sampled in 2019). Shannon diversity values are effective numbers of species. Letters indicate significant differences from post-hoc tests (pairwise paired t-tests) that followed repeated measures ANOVA.
Temporal turnover in community composition (beta diversity; Bray–Curtis dissimilarity) for the 2012–2013 interval was significantly higher than that of the 2013–2014, 2014–2015, 2015–2016, 2016–2017, and 2017–2018 intervals (Supplemental Figure S2). The lowest amounts of compositional change occurred in the 2013–2014 and 2014–2015 intervals, and these were significantly lower than all other sampling intervals, except 2017–2018. Turnover values in 2018–2020 were similar to 2012–2013, and both of these intervals were on par with temporal turnover measured over the entire study period from 2012 to 2020.
3.3. Responses to Removal of Alliaria petiolata
In 2012, Alliaria petiolata was the most important herb species in DWSNP (RIV = 15.4), but targeted removal efforts reduced its importance by almost half in the 2013 survey (RIV = 8.87; Table 1; Figure 2A). The relative importance of A. petiolata continued to decrease in subsequent years in response to continued management activities (Figure 2A). The reduction in A. petiolata abundance over time was significant based on the initial repeated measures ANOVA with sphericity assumed (p = 0.0014); however, the necessary application of the Greenhouse–Geisser correction resulted in a non-significant p-value of 0.067 (Table 2). Changes in species richness from 2012 to 2020 exhibited a significant relationship with changes in A. petiolata cover (R2 = 0.133, p = 0.023; Table 3), where greater increases in species richness were linked to decreases in A. petiolata cover. There were no significant relationships between the 2012–2020 change in A. petiolata cover and 2012–2020 changes in Shannon diversity, evenness, cover, or beta diversity (all p > 0.05; Table 3).
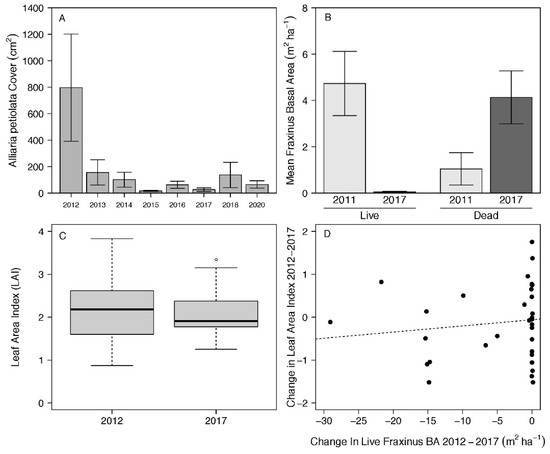
Figure 2.
Summary of emerald ash borer (Agrilus planipennis) and Alliaria petiolata perturbances in DWSNP. (A) Changes in mean cover of A. petiolata through time in response to removal efforts. (B) Mean basal area (m2 ha−1) of living and dead ash (Fraxinus spp.) in 2011 and 2017. (C) Leaf Area Index (LAI) measured in 2012 and 2017. (D) Comparison of change in leaf area index (LAI) and change in living Fraxinus spp. basal area (BA) from 2012 to 2017.

Table 3.
Regression results testing for significant relationships between changes in herb layer metrics (species richness, Shannon diversity, evenness, cover, and beta diversity) and changes in Alliaria petiolata cover (2012–2020), Leaf Area Index (LAI) (2012–2017), and living Fraxinus basal area (BA, m2 ha−1; 2012–2017).
3.4. Loss of Fraxinus spp. and Changes in Light Availability
The prevalence of Fraxinus spp. in DWSNP changed significantly over the sampling period as nearly all trees that were alive in 2011 had died by 2017 (Figure 2B). In 2011, mean basal area of Fraxinus spp. per plot was 4.73 m2 ha−1 among live trees, and dead Fraxinus basal area was 1.05 m2 ha−1 per plot (Figure 2B). By 2017, nearly all of the Fraxinus basal area consisted of dead trees (4.14 m2 ha−1), and the abundance of living ash trees was greatly reduced (0.05 m2 ha−1; Figure 2). Paired t-tests indicated that the reduction in living Fraxinus basal area was significant (t = 3.36, df = 31, p = 0.0021), as was the increase in dead basal area (t = −2.17, df = 31, p = 0.0038); however, changes in live Fraxinus basal area were not significantly associated with changes in herbaceous layer species richness, Shannon diversity, evenness, cover, or beta diversity from 2012 to 2017 (Table 3).
There was not a statistically discernable change in LAI over the course of the study (paired t-test; t = 0.889, df = 31, p = 0.408), indicating that Fraxinus spp. mortality did not alter the overall light level of the stand (Figure 2C). Of the 32 total plots in the site, 18 plots experienced a reduction in living Fraxinus spp. biomass from 2011 to 2017, but overall there was no statistically discernable relationship between change in LAI and loss of living ash basal area (R2 = −0.0155, p = 0.473; Figure 2D). The 2012–2017 changes in herb species richness and Shannon diversity were significantly related to changes in overstory LAI, though (Table 3). Both of these relationships were inverse—increases in LAI were associated with decreased herbaceous species richness and Shannon diversity.
3.5. Vegetation-Environment Relationships
Some significant underlying relationships were detected between environmental variables and herb layer metrics, a few of which were consistent through time (Table 4). Species richness exhibited a significant inverse relationship with distance to the southern edge of DWSNP across all sampling years (all p < 0.01), such that the sample plots with the highest species richness tend to occur closer to the southern edge of the stand. Species richness also exhibited a fairly consistent inverse relationship with soil moisture (significant in 2012 and 2014–2020; p < 0.05), where more waterlogged soils tended to support lower species richness. The level of species richness was unrelated to the distance to the western edge of DWSNP and was significantly related to LAI in 2012 (p = 0.016, inverse relationship), but not in 2017 (p = 0.920).

Table 4.
The relationships between herbaceous layer metrics and environmental gradients: distances to southern and western edges (m), soil moisture, and Leaf Area Index (LAI) in an old-growth temperate forest. LAI measurements were collected only in 2012 and 2017.
Shannon diversity exhibited a fairly consistent inverse relationship with soil moisture in 2012 (p = 0.036) and 2014–2017 (p < 0.05). Shannon diversity values were patterned along a north–south gradient in 2012, 2014, and 2020 (p < 0.02, inverse relationship), but this pattern was not consistent through time. Shannon diversity was unrelated to the distance from the western edge of DWSNP until 2017 and 2018 when there were weak positive relationships (p ≤ 0.043). Similar to species richness, Shannon diversity was significantly related to LAI only in 2012 (p = 0.016).
Evenness generally did not show any strong consistent patterning over the environmental gradients, although there were a few significant relationships with soil moisture in 2012, 2013, and 2017 (p < 0.05), and a significant north–south trend in 2012 (p = 0.024; Table 4). Herbaceous cover was also largely unrelated to environmental gradients except for weak significant relationships with distance to southern edge in 2016 (p = 0.044) and 2018 (p = 0.041) and distance to western edge in 2017 (p = 0.040) and 2018 (p = 0.044). Beta diversity was not patterned along these environmental gradients except for a single significant relationship with distance to the southern edge in 2016–2017 (p = 0.023) and distance to the western edge in 2018–2020 (p = 0.003; Table 4).
4. Discussion
Tree mortality is a baseline ecological process [46] that partly drives gap-dynamics in old-growth forests [47]. The relatively synchronized loss of overstory Fraxinus spp., due to EAB invasion, could create a widespread, stand-level alteration in the light regime at the forest floor with potentially significant effects on herbaceous species. Goins et al. [35] noted that Fraxinus spp. was the third largest contributor to overstory basal area in DWNSP, based on data collected in 2011. By 2017, live Fraxinus spp. trees were virtually absent from the stand with 18 (of 32) study plots experiencing a substantial reduction in live Fraxinus basal area over the study period. This loss of Fraxinus spp. was not statistically related to change in canopy openness at the plot level, suggesting that Fraxinus spp. mortality did not initiate a long-term change in light levels at the forest floor. There may have been short-term or localized changes in canopy cover and light availability; however, that influenced trends in species richness and Shannon diversity over the course of our study.
Host trees experience a relatively slow decline over several years after EAB infestation [48], and we posit that this extended period of Fraxinus spp. decline may initiate a gap-filling response among the surrounding canopy trees. As such, canopy gaps created by EAB-induced mortality may not form as abruptly as those generated by other disturbances (e.g., windthrow); thus, the environmental changes caused by Fraxinus-specific gap dynamics may be dampened. In DWNSP, temporal changes in herb-layer species richness and Shannon diversity were linked to temporal changes in LAI, but not to the loss of Fraxinus over the course of the study period (2012–2017). This suggests that the background gap dynamics of the stand have not been overwhelmed by the changes in overstory Fraxinus abundance. Future work across a range of forest stands varying in Fraxinus spp. dominance would be useful to discover whether a threshold may exist where EAB-related mortality initiates a unique disturbance regime and alters the understory light environment.
Alliaria petiolata invasion was ongoing and extensive in DWSNP at the initiation of our study (Supplemental Figure S1) and removal efforts to control this species had significant implications for herb-layer ecology. Mean A. petiolata cover per plot declined precipitously in the first few years of our control efforts, and we observed a variety of responses in the herbaceous layer. It is important to note that 2012 is our only pre-management data point and that the initial decline in A. petiolata observed in 2013 may in part be due to two-point cycling of different life stages of this biennial species [49]; however the lack of resurgence in later years suggests that our control efforts had an effect. At the individual species level, the removal of A. petiolata prompted increases in the relative importance of several native herb species. Sanicula odorata became the most important species in the herb-layer by 2017, followed by Geranium maculatum, Floerkea proserpinacoides, Cardamine concatenata, and Claytonia virginica. At the community level, species richness, cover, Shannon diversity, and evenness of the herb-layer varied significantly over the course of this study. The 2020 values of these four parameters generally matched or exceeded the 2012 values, and it is possible that some of these fluctuations were due to community recovery after A. petiolata removal. Our assessment of temporal beta diversity supports this notion, as we found that the removal of greater amounts of A. petiolata was associated with greater herb-layer compositional change through time.
Relationships between environmental gradients and herb layer composition were relatively stable over the sampling period despite the effects of EAB and A. petiolata removal. The north–south gradient of species richness identified by Chapman et al. [39] was persistent throughout the current study. This pattern is likely due to DWSNP’s orientation and location in the northern hemisphere [50], where greater yearly insolation along the southern edge creates a microclimatic gradient within the forest stand. Soil moisture was also a consistently important, underlying the environmental gradient driving herbaceous layer composition in DWSNP. These results are consistent with other studies, such as that of Murphy and McCarthy [51], where a north–south gradient of herbaceous species richness and Shannon diversity was observed in another old-growth Ohio forest and was partially attributed to lower soil moisture on the southern side. Such stability of vegetation–environment relationships through time indicates that these relationships supersede the anthropogenic disturbance processes associated with EAB, and A. petiolata invasion and removal, at least at the scale of our study site.
5. Conclusions
In summary, the loss of Fraxinus spp. due to EAB invasion in DWSNP created canopy gaps that were not obviously distinguishable from background overstory mortality in this system. Light is an important driver of herb-layer composition and dynamics, which we verified by comparing canopy openness to community parameters; however, it was not clear that tree mortality caused by EAB had a direct effect on herb-layer biodiversity. Removal of A. petiolata is a common practice in forests of eastern North America, and our data set indicated that the removal of A. petiolata initiated some changes in herbaceous community composition. Responses were most evident at the level of individual species where we saw some native herbs increase in dominance to occupy the niche space opened by A. petiolata removal. This was reflected in the higher temporal beta diversity values observed across 2012–2013 following the most drastic reduction in A. petiolata abundance. Vegetation–environment relationships remained stable over the sampling period, indicating that resource gradients structure the herbaceous community during perturbations of the scale represented in this study. As forests are increasingly subject to multiple interacting anthropogenic drivers of ecosystems dynamics, including co-occurring invasive species, long-term monitoring efforts that allow for distinguishing between background “natural” population dynamics and perturbation response are increasingly valuable.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/10/1069/s1, Figure S1: J. Chapman standing in a dense patch of Alliaria petiolata in DWNSP in 2012, Figure S2: Temporal turnover (Bray–Curtis dissimilarity) of the herbaceous layer in DWSNP sample plots.
Author Contributions
Conceptualization, J.I.C., R.W.M., and T.M.B.; methodology, J.I.C., R.W.M., and T.M.B.; validation, J.I.C., R.W.M., and T.M.B.; formal analysis, T.M.B. and J.I.C.; investigation, T.M.B. and J.I.C.; resources, R.W.M.; data collection and curation, J.I.C., T.M.B., and M.E.M.; writing—original draft preparation, T.M.B.; writing—review and editing, J.I.C., R.W.M., T.M.B., and M.E.M.; visualization, T.M.B. and J.I.C.; supervision, J.I.C. and R.W.M.; project administration, J.I.C.; funding acquisition, R.W.M. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this study was provided by the University of Dayton Department of Biology.
Acknowledgments
Thank you to Darke County Parks for allowing us to use DWSNP. Thanks to Eric Borth, Albert Burky, Keith Gilland, Sean Goins, Charlie Jackson, Mitchell Kukla, Corey Kuminecz, and Amy Myers for assisting with field work over the years.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- McEwan, R.W.; Muller, R.N. Dynamics, diversity, and resource gradient relationships in the herbaceous layer of an old-growth Appalachian forest. Plant Ecol. 2011, 212, 1179–1191. [Google Scholar] [CrossRef]
- Chapman, J.I.; McEwan, R.W. Spatiotemporal dynamics of a- and b-diversity across topographic gradients in the herbaceous layer of an old-growth deciduous forest. Oikos 2013, 122, 1–8. [Google Scholar] [CrossRef]
- Bratton, S.P. Resource division in an understory herb community: Responses to temporal and microtopographic gradients. Am. Nat. 1976, 110, 679–693. [Google Scholar] [CrossRef]
- Hutchinson, T.F.; Boerner, R.E.J.; Iverson, L.R.; Sutherland, S.; Sutherland, E.K. Landscape patterns of understory composition and richness across a moisture and nitrogen mineralization gradient in Ohio (U.S.A.) Quercus forests. Plant Ecol. 1999, 144, 177–189. [Google Scholar] [CrossRef]
- Dubbert, M.; Mosena, A.; Piayda, A.; Cuntz, M.; Correia, A.C.; Pereira, J.S.; Werner, C. Influence of tree cover on herbaceous layer development and carbon and water fluxes in a Portuguese cork-oak woodland. Acta Oecol. 2014, 59, 35–45. [Google Scholar] [CrossRef]
- Thompson, J.N. Treefalls and colonization patterns of temperate forest herbs. Am. Midl. Nat. 1980, 104, 176–184. [Google Scholar] [CrossRef]
- Brewer, R. A half-century of changes in the herb layer of a climax deciduous forest in Michigan. J. Ecol. 1980, 68, 823–832. [Google Scholar] [CrossRef]
- Whitney, G.G.; Foster, D.R. Overstorey composition and age as determinants of the understory flora of woods of central New England. J. Ecol. 1988, 76, 867–876. [Google Scholar] [CrossRef]
- Ford, M.F.; Odom, R.H.; Hale, P.E.; Chapman, B.R. Stand-age, stand characteristics, and landform effects on understory herbaceous communities in southern Appalachian cove-hardwoods. Biol. Conserv. 2000, 93, 237–246. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Roberts, M.R. Interactions between the herbaceous layer and overstory canopy of eastern forests. In The Herbaceous Layer in Forests of Eastern North America; Gilliam, F.S., Roberts, M.R., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 163–176. [Google Scholar]
- Zihan, J.; Ma, K.; Anand, M.; Zhang, Y. Interplay of temperature and woody cover shapes herb communities along an elevational gradient in a temperate forest in Beijing, China. Community Ecol. 2015, 16, 215–222. [Google Scholar]
- Wulf, M.; Naaf, T. Herb Layer Response to Broadleaf Tree Species with Different Leaf Litter Quality and Canopy Structure in Temperate Forests. J. Veg. Sci. 2009, 20, 517–526. [Google Scholar] [CrossRef]
- Poland, T.M.; McCullough, D.G. Emerald ash borer: Invasion of the urban forest and the threat to North America’s ash resource. J. For. 2006, 104, 118–124. [Google Scholar]
- Flower, C.E.; Knight, K.S.; Gonzalez-Meler, M.A. Impacts of the emerald ash borer (Agrilus planipennis Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol. Invasions 2013, 15, 931–944. [Google Scholar] [CrossRef]
- Kashian, D.M.; Witter, J.A. Assessing the potential for ash canopy tree replacement via current regeneration following emerald ash borer-caused mortality on southeastern Michigan landscapes. For. Ecol. Manag. 2011, 261, 480–488. [Google Scholar] [CrossRef]
- Knight, K.S.; Brown, J.P.; Long, R.P. Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biol. Invasions 2013, 15, 371–383. [Google Scholar] [CrossRef]
- Langenbruch, C.; Helfrich, M.; Flessa, H. Effects of beech (Fagus sylvatica), ash (Fraxinus excelsior) and lime (Tilia spec.) on soil chemical properties in a mixed deciduous forest. Plant Soil 2012, 352, 389–403. [Google Scholar] [CrossRef]
- Brothers, T.S.; Spingarn, A. Forest Fragmentation and alien plant invasion of central Indiana old-growth forests. Conserv. Biol. 1992, 6, 91–100. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef]
- Nuzzo, V. Invasion pattern of herb garlic mustard (Alliaria petiolata) in high quality forests. Biol. Invasions 1999, 1, 169–179. [Google Scholar] [CrossRef]
- Meekins, J.F.; McCarthy, B.C. Effect of population density on the demography of an invasive plant (Alliaria petiolata, Brassicaceae) population in a southeastern Ohio Forest. Am. Midl. Nat. 2002, 147, 256–278. [Google Scholar] [CrossRef]
- Meekins, J.F.; McCarthy, B.C. Effect of environmental variation on the invasive success of a non-indigenous forest herb. Ecol. Appl. 2001, 11, 1336–1348. [Google Scholar] [CrossRef]
- Chapman, J.I.; Cantino, P.D.; McCarthy, B.C. Seed production in garlic mustard (Alliaria petiolata) prevented by some methods of manual removal. Nat. Areas J. 2012, 32, 305–309. [Google Scholar] [CrossRef]
- Cipollini, D.; Cipollini, K. A review of garlic mustard (Alliaria petiolata, Brassicaceae) as an allelopathic plant. J. Torrey Bot. Soc. 2016, 143, 339–348. [Google Scholar] [CrossRef]
- Davis, M.A.; MacMillen, C.; LeFevre-Levy, M.; Dallavalle, C.; Kriegel, N.; Tyndel, S.; Martinez, Y.; Anderson, M.D.; Dosch, J.J. Population and plant community dynamics involving garlic mustard (Alliaria petiolata) in a Minnesota Oak Woodland: A four year study. J. Torrey Bot. Soc. 2014, 141, 205–216. [Google Scholar] [CrossRef]
- Carlson, A.M.; Gorchov, D.L. Effects of herbicide on the invasive biennial Alliaria petiolata (garlic mustard) and initial responses of native plants in a southwestern Ohio forest. Ecol. Restor. 2004, 12, 559–567. [Google Scholar] [CrossRef]
- Stinson, K.; Kaufman, S.; Durbin, L.; Lowenstein, F. Impacts of garlic mustard invasion on a forest understory community. Northeast. Nat. 2007, 14, 73–88. [Google Scholar] [CrossRef]
- Jules, E.S. Habitat fragmentation and demographic change for a common plant: Trillium in old-growth forest. Ecology 1998, 79, 1645–1656. [Google Scholar] [CrossRef]
- Vellend, M.; Verheyen, K.; Jacquemyn, H.; Kolb, A.; Calster, H.V.; Peterken, G.; Hermy, M. Extinction dept of forest plants persists for more than a century following habitat fragmentation. Ecology 2006, 87, 542–548. [Google Scholar] [CrossRef]
- Vellend, M.; Verheyen, K.; Flinn, K.M.; Jacquemyn, H.; Kolb, A.; Calster, H.V.; Peterken, G.; Graae, B.J.; Bellemare, J.; Honnay, O.; et al. Homogenization of forest plant communities and weakening of species-environment relationships via agricultural land-use. J. Ecol. 2007, 95, 565–573. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Bechtold, W.A.; Patterson, P.L. The Enhanced Forest Inventory and Analysis Program-National Sampling Design and Estimation Procedures; Gen. Tech. Rep. SRS-80; US Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2005; p. 80. [Google Scholar]
- McEwan, R.W.; Dyer, J.M.; Pederson, N. Multiple interacting ecosystem drivers: Toward an encompassing hypothesis of oak forest dynamics across eastern North America. Ecography 2011, 34, 244–256. [Google Scholar] [CrossRef]
- Goins, S.M.; Chapman, J.I.; McEwan, R.W. Composition shifts, disturbance, and canopy-accession strategy in an oldgrowth forest of Southwestern Ohio, USA. Nat. Areas J. 2013, 33, 384–394. [Google Scholar] [CrossRef]
- National Climate Data Center. Available online: http://www.ncdc.noaa.gov (accessed on 8 May 2012).
- Boerner, R.E.J.; Kooser, J.G. Vegetation of Drew Woods, an old-growth forest remnant in western Ohio, and issues of preservation. Nat. Areas J. 1991, 11, 48–54. [Google Scholar]
- Brockman, C.S. Physiographic Regions of Ohio; Ohio Department of Natural Resources, Ohio Division of Geological Survey: Columbus, OH, USA, 1998. [Google Scholar]
- Chapman, J.I.; Myers, A.L.; Burky, A.J.; McEwan, R.W. Edge Effects, invasion, and the spatial Pattern of herb-layer biodiversity in an old-growth deciduous forest fragment. Nat. Areas J. 2015, 35, 439–451. [Google Scholar] [CrossRef]
- Jones, R.L. Plant Life of Kentucky; University Press of Kentucky: Lexington, KY, USA, 2005. [Google Scholar]
- USDA. The PLANTS Database; National Plant Data Team: Greensboro, NC, USA. Available online: http://plants.usda.gov (accessed on 25 September 2020).
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Mauchly, J.W. Significance test for sphericity of a normal n-variate distribution. Ann. Math. Stat. 1940, 11, 204–209. [Google Scholar] [CrossRef]
- Greenhouse, S.W.; Geisser, S. On methods in the analysis of profile data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Franklin, J.F.; Shugart, H.H.; Harmon, M.E. Tree death as an ecological process: The causes, consequences, and variability of tree mortality. Bioscience 1987, 37, 550–556. [Google Scholar] [CrossRef]
- Runkle, J.R. Patterns of disturbance in some old-growth mesic forests of eastern North America. Ecology 1982, 63, 1533–1546. [Google Scholar] [CrossRef]
- Siegert, N.W.; McCullough, D.G.; Liebhold, A.M.; Telewski, F.W. Resurrected from the ashes: A historical reconstruction of emerald ash borer dynamics through dendrochronological analysis. In Proceedings of the Emerald ash borer and Asian Longhorned Beetle Research and Development Review Meeting, Cincinnati, OH, USA, 29 October–2 November 2006; FHTET 2007-04. U.S. Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2006; pp. 18–19. [Google Scholar]
- Pardini, E.A.; Drake, J.M.; Chase, J.M.; Knight, T.M. Complex population dynamics and control of the invasive biennial Alliaria petiolata (garlic mustard). Ecol. Appl. 2009, 19, 387–397. [Google Scholar] [CrossRef]
- Palik, B.J.; Murphy, P.G. Disturbance versus edge effects in sugar-maple/beech forest fragments. For. Ecol. Manag. 1990, 32, 187–202. [Google Scholar] [CrossRef]
- Murphy, S.J.; McCarthy, B.C. Temporal change in the herbaceous understory community of an old-growth forest: From seasons to decades. Plant Ecol. 2014, 215, 221–232. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).