Abstract
Yeast extract, which is environmentally friendly, nutritious, and convenient to use, has advantages over common plant growth regulators and soil conditioners. It is worth verifying the effect of the application of yeast extract to afforestation in a semiarid chestnut soil area. This study was conducted through a wild field afforestation experiment in Inner Mongolia, northern China. We designed an orthogonal experiment of 25 treatments with 30 repetitions on Pinus sylvestris (Pinus sylvestris var. mongolica Litv.) sand Armeniaca sibirica (Armeniaca sibirica (L.) Lam). Three factors with different levels were considered: application to rhizosphere soil with the amounts of 0 g, 10 g, 20 g, 30 g, and 40 g; foliar spraying with concentrations of 0%, 0.5%, 1%, 1.5%, and 2%; and spraying at three moments. The results showed that yeast extract could enhance the seedlings of Pinus sylvestris and Armeniaca sibirica. When applying 40 g of yeast extract, the survival rates and root parameters were significantly boosted. Foliar spraying plays an important role in promoting the growth of seedlings. It was most effective for the two species to be sprayed with a concentration of 2% after leafing of the seedlings. The data also revealed that the application of yeast extract improved the properties of the rhizosphere soil. The porosities and moisture contents were increased, and the bulk densities were reduced. Forty grams (40 g) was the best amount to apply, especially for the soil at 20–40 cm where the seedling roots are mainly located. The chemical properties were also improved, as there was a significant increase in the conductivities, organic matter, nitrogen, and phosphorus as well as a slight reduction in the calcium carbonate content and pH. Therefore, yeast extract has a beneficial effect on both seedling growth promotion and soil improvement. It is considered to be an environmentally efficient method for afforestation and ecological remediation in semiarid chestnut soil areas.
1. Introduction
As society has developed, the natural environment has been damaged by intensive human activities, attributed to excessive consumption and the destruction of natural resources. Thereby, the degradation of soil and vegetation and the decrease in diversity are becoming increasingly serious, which further enhances the erosion and massive loss of soil and water. Therefore, the restoration and improvement of the ecological environment has been the focus of much social and scientific research [1]. In order to prevent environmental deterioration, many ecological projects have been implemented, among which afforestation is one of the most commonly used [2].
Semiarid chestnut soil areas, where agriculture and animal husbandry intersect, are typically representative of a fragile ecological region. There is little precipitation; however, the evaporation is large. Chestnut soil is dominant in these areas, with the characteristics of seasonal leach and calcium accumulation [3]. Due to overgrazing, uncontrollable reclamation, the continuous degradation of vegetation, and the intensification of desertification are prevalent. To restore the ecotype, many afforestation projects have been carried out. To a certain extent, the vegetation coverage and species diversity have been restored, and soil and water have been meliorated [4]. However, on the one hand, the soil constituents, such as organic matter and soluble salt, are leached. Moreover, low rainfall and high evaporation lead to a lack of a water supply for seedlings to survive and grow [5]. On the other hand, as a result of artificial activities, such as grassland reclamation, the chemical properties of chestnut soil tend to deteriorate. Degraded soil further leads to a change in the humus state of soils, and the deterioration of soil fertility [6]. Thereby, the survival and preservation rates of afforested seedlings have remained low, and growth has been badly restricted.
On account of the unsolved afforestation issue in semiarid chestnut areas, it is necessary to apply a technical method during the afforestation process in order to improve the effectiveness of the afforestation and the restoration of semiarid chestnut soil areas. In the academic field, there have been some preliminary studies on afforestation in this area. Most of these are theoretical experience summaries from grassroots forestry workers that concentrate on the exploration of land preparation and the selection of species, the forestation season, and density [7]. These studies provide a reference for afforestation in a semiarid chestnut soil area from a macroscopic perspective. There are few scientific studies that have focused on improving seedling and chestnut soil by additives, such as plant growth regulators or soil conditioners, through large-scale wild afforestation trials.
Many experiments and applications have shown that growth regulators, such as naphthalene acetic acid and gibberellin, can not only promote high-diameter seedling growth, but also improve the restoration of injured roots of transplanted seedlings [8,9,10,11]. However, these regulators are all chemical agents that have slightly toxic side effects on humans and animals. Other studies have indicated that a rhizobia and organic–inorganic fertilizer combination could efficiently reform the rhizosphere, promote the development of roots, and enhance the acclimatization of seedlings [12,13,14,15,16,17]. Nevertheless, the produced organic and inorganic fertilizers also contain manufactured chemical agents, and the application of natural rhizobia fertilizers and organic fertilizers is inconvenient and has a high cost.
Lately, a great deal of attention has been paid to additives that are more natural, safe, and low-cost. Yeast extract has become a hot topic in academic fields as it is nontoxic, nutritious, and convenient to apply. It is a kind of soluble paste or powder made from brewer’s yeast, or fresh yeast with high biological activity. Yeast extract is rich in effective constituents, such as low-molecular-weight organic matter, amino acids, nucleotides, peptides, nitrogen, phosphorus, and trace elements. Moreover, yeast extract is free of chemically synthesized hormones and toxic ingredients. Studies have revealed that the extract yield obtained from beer yeast is higher than that of other yeasts [18,19].
Yeast extract has been studied in the field of agriculture [20], where it has been indicated that yeast extraction could improve the yield and quality of needle mushrooms (Flammulina velutiper (Fr.) Sing) [21]. The application of yeast significantly increased the vegetative growth, yield, and quality of vegetables such as cucumber (Cucumis sativus L.) [22], head lettuce (Lactuca sativa L.) [23], potato (Solanum tuberosum L.) [24], eggplant (Solanum melongena L.) [25], turnip (Raphanus sativus L.) [26], and soybean (Glycine max (L.) Merrill) [27], and also led to an increase in elemental content, such as N, P, K, Fe, and Zn, in vegetables. Some of these studies have proved that the enhancement effects of yeast extracts were more pronounced than other additives, such as Methanol [27].
In the field of forestry, the beneficial effect of yeast extract has been proven mainly in saline–alkali site conditions. Yeast extraction could promote the growth of red gum plants (Eucalyptus camaldulensis Dehn.) in the parameters of plant, stern, branch, and leaf. The percentage of volatile oil in leaves was the highest when sprayed with a concentration of active yeast extraction at 200 mL/L [28]. Leucaena leucocephala (Lam.) de Wit plants under salinity stress could grow better with the help of yeast extract. At the same time, the reduction of vegetative growth caused by salt could also be recovered to some extent [29]. A treatment of yeast extract could markedly improve the growth parameters of date palm (Phoenix dactylifera L.) at a few months after the end of the acclimatization stage [30], and enhance the tolerance of date palm plantlets to soil salinity [31]. The effect of yeast extract on forestry has been preliminarily proven. However, the application of yeast extract as both a plant growth regulator and soil conditioner has been infrequently reported. Furthermore, there have been few reports on the application of yeast extract in semiarid areas.
Overall, this study aimed to verify the effect of the application of yeast extract on seedling growth enhancement and soil improvement in afforestation in a semiarid chestnut soil area. Based on the characteristics of the yeast extract, the relative reference, and the practical situation, we designed a treatment from two routes of application. The first one was an application to rhizosphere with different amounts; the second one was foliar spraying with different concentrations at different times. According to the orthogonal treatment groups, a wild field afforestation trial was conducted with two local common species: Pinus sylvestris L. var. mongholica Litv. and Armeniaca sibirica (L.) Lam. Yeast extract was applied during afforestation. At the end of the growing season, we acquired two sets of parameters: the survival rate, increment, and root parameters for two species were studied to reveal the enhancement of seedling growth; and the physical and chemical properties of the rhizosphere soil were analyzed to compare the improvement to the rhizosphere soil. In general, this study could be a reference for the restoration of the ecological environment in a semiarid chestnut soil area.
2. Materials and Methods
2.1. Site Description
The research site was located in Xinghe County, Wulanchabu City in Inner Mongolia, which is a typical semiarid chestnut soil area. The altitude ranges from 1191 m to 1310 m. The annual average temperature of Xinghe is 4.2 °C, with an extreme minimum temperature of −33.8 °C, and an extreme maximum of 42 °C. The annual precipitation is 380 mm, while the evaporation is 2062 mm. Chestnut soil dominates this area, from which soluble salts are leached. The profile shows that calcium carbonate has been deposited, forming a grayish-white compact calcium layer in the range of 20–60 cm. The natural plant community is mainly temperate grassland with some forest of deciduous shrubs and aciculisilvae. Common plant species are Amorpha fruticosa Linn., Lespedeza daurica (Laxm.) Schindl, Artemisia gmelinii, and Thymus mongolicus Ronn. Locally, many afforestation projects have been implemented since the 1970s; however, the lack of technology and conservation management has led to many inefficiencies [32].
2.2. Materials
The yeast extract used in this study was produced by beer yeast from a biotechnological company (Tangshan Tuopo Biotechnology, Tangshan, China), and was a yellow dry powder. The nutritional contents are shown in the Table 1.

Table 1.
The nutritional contents of the yeast extract.
The seedlings were three-year Pinus sylvestris in a container, and catted two-year Armeniaca sibirica with a bare root. To ensure the uniformity of the utilized seedlings, seedlings with almost the same length and diameter were selected.
2.3. Treatment Groups
This study was conducted by an orthogonal experiment, and different levels of variables were considered, which are shown in Table 2. Five levels for the application amount to the rhizosphere soil, ranging from 0 g (control set) to 40 g per seedling, were set. At the same time, yeast extract was foliar sprayed with different concentrations ranging from 0% (control set) to 2% at three moments: after forestation (AF), after the leafing of the nursery stock (AL), and in the middle of the vigorous growth period (GP). After orthogonality, 25 treatment groups were finally obtained, and are shown in Table 3.

Table 2.
The factors and levels of the orthogonal experiment L25 (52 × 31). There were 2 factors with 5 levels and 1 factor with 3 levels.

Table 3.
The treatment groups L25 (52 × 31). There were 2 factors with 5 levels and 1 factor with 3 levels.
2.4. Preparation of the Experimental Site
The form of the site’s preparation was level trenches, which were completed in the fall of 2015 by an excavator with the specification of 250 cm × 80 cm × 80 cm. In April 2017, two 75 m × 40 m sites were selected for planting Pinus sylvestris and Armeniaca sibirica, respectively. The graphical representation of each site is shown in Figure 1. Corresponding to the 25 groups, each site had 25 planting holes along the trench. Fifteen rows were in the slope direction to be repetitions. Two seedlings were planted in each hole. Each treatment group had 30 repetitions. There were 750 seedlings for each plant in total. The experiment was located within a larger area also planted with seedlings, so edge effects are likely minimal.
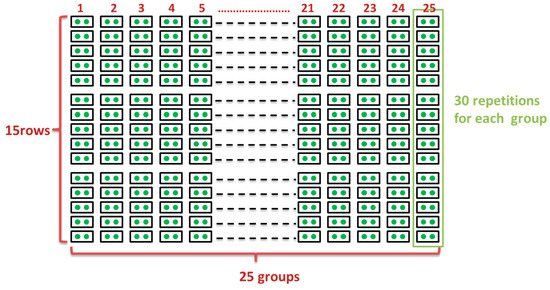
Figure 1.
A graphical representation of the experimental site. Corresponding to the 25 treatment groups, there were 25 planting holes along the level trench. Two seedlings were planted in each hole, and 15 rows were in the slope direction. Each treatment group had 30 repetitions.
The distribution of treatments in the study was non-random, and replicates within each treatment were not dispersed. This experimental arrangement could potentially allow environmental gradients in the soil or in the field to cause spurious treatment effects. However, due to the large number of repetitive treatments, and the fact that treatment effects and trends were consistent with hypothesized treatment effects, we suspect that environmental gradients had little discernible effect on our results.
2.5. Procedure of the Experiment
1. Afforestation preparation
The seedlings were brought to the field before dawn on the afforestation day. We selected the seedlings and dispersed them to the planting holes randomly.
2. Addition of yeast extract
The moderate soil of the planting holes was loosened with a shovel. According to the 25 treatments during the afforestation, the corresponding amounts of yeast extract were adequately mixed with loose soil. Then, the seedlings were planted while ensuring that the rhizosphere soil contained yeast extract.
3. Irrigation of water
In order to control the variable of water irrigation, the water control equipment [33] was used to irrigate the seedlings, ensuring 200 L of water for each planting hole.
4. Foliar Spraying
Different concentrations of diluted yeast extract were evenly and fully sprayed on the seedlings at the end of April, the end of May, and early July, respectively.
2.6. Recording, Sampling, and Measurement
After the end of the growing season in 2017, we recorded the survival and measured the growth of each seedling. To determine the growth of seedlings due to their growth characteristics, the increment of the main bough of Pinus sylvestris was measured, and the total length of the new branches of Armeniaca sibirica was detected. According to the growth results, the three seedlings closest to the mean vales were chosen to determine the root properties. The roots were dug out and cleaned cautiously, then the lengths, surface areas, and tips were determined and analyzed using the WinRHIZO-EC root analysis system (Regent Instrument Inc., Quebec, QC, Canada).
To determine the physicochemical properties of the soil, samples were collected from three layers of depth 0–20 cm, 20–40 cm, and 40–60 cm. The oven-drying method was used to measure the moisture of the soil at 105 °C. The bulk density and porosity were determined using the cutting-ring method [34]. The organic matter contents were measured by titration with potassium dichromate. Using the graphite digestion method, the contents of total nitrogen and total phosphorus were assayed by a Smartchem 200 Discrete Auto Analyzer (AMS Alliance, Rome, Italy) [35]. An Mp523-03 calcium concentration meter (Shanghai Sanxin, China) was used to assay the calcium carbonate contents. pH and conductivities were determined by MP522 precision pH and conductivity meters (Shanghai Sanxin, China), respectively [36].
2.7. Statistical Analysis
The data from the orthogonal experiment were subjected to an analysis of variance by SPSS 17.0. Multiple comparisons with the least significant difference (L.S.D.) at the 0.05 level were used for each investigated characteristic under different assigned treatments [37].
3. Results
3.1. Effects on Survival and Growth
The survival rates with different application amounts to the rhizosphere soil of the two species showed a trend of increment (Table 4). For Pinus sylvestris, there were significant differences between the application amounts of 20 g, 30 g, and 40 g and the control treatments at a level of 0.05. A significant effect on the survival rate of Armeniaca sibirica was also noticed under the three treatments. The treatment of 40 g of yeast extract gave the highest significant value of 95.13% and 88.19% for Pinus sylvestris and Armeniaca sibirica, respectively, 14.67% and 17.59% higher than the lowest mean value that occurred under the control treatment, respectively. Different levels of foliar spraying did not significantly affect the survival rates. Both species showed an increasing trend as the foliar concentration increased. The survival rate of Armeniaca sibirica was the highest when sprayed after the leafing of the seedlings. For Pinus sylvestris, spraying after forestation was the best treatment. It is obvious that the amount with a greater range influenced the survival of the two species the most, while foliar spraying had less of an effect on the survival rate. In general, the optimal combination to improve the survival rate of Pinus sylvestris and Armeniaca sibirica was 40 g, 2%, and AF and 40 g, 2%, and AL, respectively.

Table 4.
Effect of the application of yeast extract on the survival rates (%) and growth (m) of Pinus sylvestris and Armeniaca sibirica. AF, After Forestation; AL, After Leafing; GP, middle of the growth period. Averages with no common letters have a significant difference at the 0.05 level.
Progressive significant growth of Pinus sylvestris and Armeniaca sibirica was achieved with the application of yeast extract. When 40 g of yeast extract was applied to the rhizosphere soil, the growth of Pinus sylvestris reached 19.47 cm, and the total length of the new shoots of Armeniaca sibirica was 347.35 cm, which was significantly higher than that under the control treatment. Foliar spraying at concentrations of 1.5% and 2% had a remarkable effect on the growth of both species, with a significant difference at the 0.05 level. A 2% yeast extract treatment exhibited 21.22 cm in the growth of Pinus sylvestris, 9.04 cm higher than the yeast-free treatment. The length of the new branches of Armeniaca sibirica increased from 141.85 cm to 322.63 cm when sprayed with a 2% yeast extract. Spraying after leafing had the best effect on the growth of Pinus sylvestris, 5.51 cm higher than the growth obtained with spraying after forestation with a significant difference. The new branches of Armeniaca sibirica sprayed after leafing were also the longest, reaching 309.40 cm, which was significantly higher than the 184.73 cm of the AF treatment. According to the ranges, the amount applied to the rhizosphere soil and the concentration of the foliar spraying mainly affected the growth. To improve the growth of the two species, the optimal combination was 40 g, 2%, and AL.
3.2. Effects on Root Parameters
As expected, by augmenting the application amount of yeast extract, the root length of the two species gradually increased (Table 5). Accompanied by a significant difference, the 40 g treatment brought about the highest values of 14,109.7 cm and 7662.11 cm, respectively. There was no significant difference between the results under different foliar spraying treatments, although slight increases were induced for the two species. The 2% spraying concentration gave the longest root at 10,497.6 cm for Pinus sylvestris, which also resulted in the highest stimulation for the root length of Armeniaca sibirica. The mean value of the root length of Pinus sylvestris was the greatest when sprayed after leafing, while the length of Armeniaca sibirica sprayed in the middle of the vigorous growth period was the longest. On the basis of the ranges, the amount applied to the rhizosphere soil was the main influencing factor. The optimal combinations to improve the root length of Pinus sylvestris and Armeniaca sibirica were 40 g, 2%, and AL and 40 g, 2%, and GP, respectively.

Table 5.
Effect of the application of yeast extract on the root parameters of Pinus sylvestris and Armeniaca sibirica. AF, After Forestation; AL, After Leafing; and GP, middle of the growth period. Averages with no common letters have a significant difference at the 0.05 level.
The application of the yeast extract to the rhizosphere soil had a pronounced influence on the surface area of the root for Pinus sylvestris and Armeniaca sibirica (Table 5). When 40 g of yeast extract was applied, the surface areas of Pinus sylvestris and Armeniaca sibirica reached 5526.71 cm2 and 1583.14 cm2, respectively, which were more than doubled when compared to the control treatments with a significant difference. Spraying yeast did not significantly affect the root surface area of both species at the 0.05 level, and could be slightly enlarged with an increasing trend. The mean value of Pinus sylvestris sprayed with the 2% yeast extract increased by 732.46 cm2 over the mean value of Pinus sylvestris sprayed with the control treatment. The mean value of Armeniaca sibirica increased by 321.90 cm2. The amount applied to the rhizosphere soil with the largest ranges was the main influencing factor, and the effects of the foliar spraying concentration and time on the root surface were weak. For Pinus sylvestris and Armeniaca sibirica, the optimal combination to enlarge the surface area of the roots was 40 g, 2%, and AL and 40 g, 2%, and GP, respectively.
As shown in Table 5, the larger the application amount, the greater the number of root tips for the two species. Compared to the control treatment, the 40 g treatment gave the highest mean values of tips for Pinus sylvestris and Armeniaca sibirica of 127,636 and 92,786.6, respectively, with significant differences, while the foliar spraying of yeast extract aggrandized the root tips of the two species slightly with no significant difference. The values of the 2% treatment were increased by 35,695.4 and 18,007.2 for Pinus sylvestris and Armeniaca sibirica, respectively, when compared with the control treatment. The value of Armeniaca sibirica was greater when sprayed after leafing. For both species, the soil application amount was still the main influencing factor. The optimal combination to increase the number of root tips was 40 g, 2%, and AF and 40 g, 2%, and AL for Pinus sylvestris and Armeniaca sibirica, respectively.
3.3. Effects on Physical Properties of the Rhizosphere Soil
Since there were few direct effects of the foliar spray on the soil properties, only the amount applied to the rhizosphere soil was taken into account for the analysis of the soil properties.
As can be seen from Figure 2a, gradual increases in the capillary porosities of the rhizosphere soil occurred with an increasing amount of yeast extract. For soil at a depth of 0–20 cm, the capillary porosity was increased by 4.96% with the application amount of 40 g. There were significant increments in the mean values between the 20 g, 30 g, and 40 g treatments and the yeast-extract-free treatment for soil of the middle layer. The best effect on the soil at the deep layers also resulted under the 40 g treatment, which was increased by 5.12% with a significant difference at the 0.05 level.
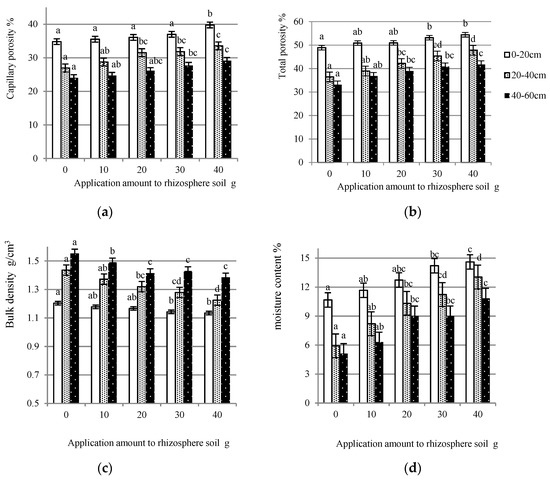
Figure 2.
The effect of the application of yeast extract on the physical properties of rhizosphere soil from the three layers. (a) Capillary porosities of rhizosphere soil to which has been applied different amounts of yeast extract; (b) Total porosities of rhizosphere soil to which has been applied different concentrations of yeast extract; (c) Bulk densities of rhizosphere soil to which has been applied different concentrations of yeast extract; and (d) Moisture contents of rhizosphere soil to which has been applied different concentrations of yeast extract. In each graph, the bars with the same color and no common letters have a significant difference at the 0.05 level.
Consistent with the capillary porosity, the application of yeast extract was recorded incrementally for the total porosities of the three layers soil (Figure 2b). Among the five levels, the amount of 40 g gave the highest mean value for the top layer at 54.40% with a significant difference. The total porosity of the 20–60 cm layer also increased observably at the amounts of 20 g, 30 g, and 40 g. The range between the 40-g treatment and the control treatment for the soil of the mid layer was 11.37%, which was significant at the 0.05 level.
As the application amount increased, the bulk densities of soil of the three layers decreased (Figure 2c). The values of the 0–20 cm layer decreased by 0.07 g/cm3 under the 40 g yeast extract treatment with a significant difference at the 0.05 level. The application amounts of 20 g, 30 g, and 40 g caused a conspicuous decrement in the rhizosphere soil at a depth of 20–60 cm, especially for the soil of the mid layer, which decreased from 1.44 g/cm3 to 1.22 g/cm3 by applying the 40 g yeast extract treatment.
As presented in Figure 2d, the application of yeast extract increased the moisture content of the rhizosphere soil. As the application amount reached 30 g and 40 g, a remarkable increase could be found for the soil of the top layer. For soil at a depth of 20–60 cm, there were significant differences between the mean moisture content values of the 20 g, 30 g, and 40 g treatments and the control treatment. The mean moisture content values could be increased to a maximum of 13.04% and 10.84%.
3.4. Effects on Chemical Properties of the Rhizosphere Soil
As shown in Figure 3a, the yeast extract slightly reduced the calcium carbonate content. The calcium carbonate of the 0–20 cm layer was reduced by 11.96 mg/g with a significant difference at the 0.05 level. However, the yeast extract did not have a significant effect on the decrease of the calcium carbonate content of the rhizosphere soil at the depth of 20–60 cm. The mean values were decreased by 20.35 mg/g and 17.08 mg/g for the 0–20 cm layer and the 20–60 cm layer, respectively.
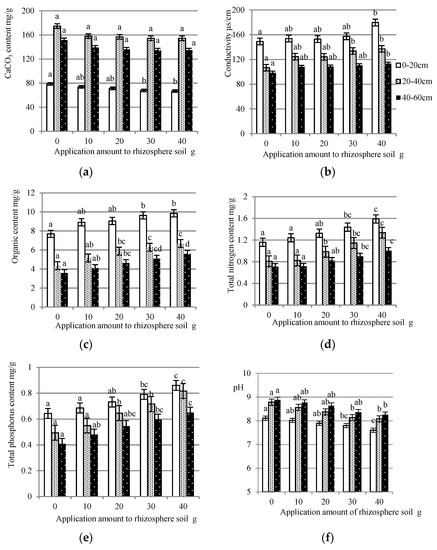
Figure 3.
The effect of the application of yeast extract on the chemical properties of rhizosphere soil from the three layers. (a) CaCO3 content of rhizosphere soil to which has been applied different amounts of yeast extract; (b) Conductivities of rhizosphere soil to which has been applied different concentrations of yeast extract; (c) Organic contents of rhizosphere soil to which has been applied different concentrations of yeast extract; (d) Total nitrogen contents of rhizosphere soil to which has been applied different concentrations of yeast extract; (e) Total phosphorus contents of rhizosphere soil to which has been applied different concentrations of yeast extract; and (f) pH of rhizosphere soil to which has been applied different concentrations of yeast extract. In each graph, the bars with the same color and no common letters have a significant difference at the 0.05 level.
It was noted that the application of yeast extract increased the conductivities of the rhizosphere soil (Figure 3b). Higher amounts of yeast extract were much more effective. When the amount reached 40 g, the conductivity of the top layer reached 179.72 μs/cm. The mean values of the middle and bottom layers were increased by 30.42 μs/cm and 14.64 μs/cm, respectively. The lowest result were found for the control treatment, which was significantly different from the result of the 30 g and 40 g treatments at the 0.05 level.
Progressive increments in the organic content of the soil are shown in Figure 3c. The greater the amount was, the higher the content. Thus, the 40 g yeast extract treatment had the most ameliorating effect on the organic content of the soil. The contents of the top, middle, and bottom layers were increased to a maximum of 2.16 mg/g, 2.33 mg/g, and 2.03 mg/g, respectively. For soil at a depth of 20–60 cm, there were significant differences between the control treatment and the 20 g, 30 g, and 40 g treatments at the 0.05 level. A much greater efficiency for the top layer was observed with the application amounts of 30 g and 40 g.
Figure 3d show that the application of the yeast extract raised the total nitrogen contents of the rhizosphere soil. For the soil of the top layer, the contents were significantly increased in response to the application of 30 g and 40 g of yeast extract, reaching 1.44 mg/g and 1.59 mg/g, respectively. The pronounced rise in the nitrogen content in the soil in the 20–40 cm layer occurred with the amounts of 20 g, 30 g, and 40 g, which had significant differences from the control treatment. Soil of the 40–60 cm layer had the most total nitrogen content of 1.00 mg/g under the 40 g treatment.
Figure 3e shows that the yeast extract had a remarkable positive effect on the total phosphorus content of the soil. Significant increments for the soil of the middle layer were noticed with the application of 20 g, 30 g, and 40 g of yeast extract, maximally reaching 0.86 mg/g and 0.82 mg/g. The lowest content of phosphorus resulted from the control treatment. For soil of the 0–20 cm and 40–60 cm layers, the application amounts of 30 g and 40 g were found to be effective for increasing the total phosphorus content, which increased by 0.22 mg/g and 0.24 mg/g, respectively.
As the application amount increased, the pH of the soil of each layer gradually decreased, as shown in Figure 3f. Significant differences at the 0.05 level were recorded at the application amounts of 30 g and 40 g for the 0–40 cm soil layer. The treatment of 40 g gave the best mean values of 7.61, 8.09, and 8.26 for the soil of the top, middle, and bottom layers, respectively.
4. Discussion
4.1. Survival and Growth
It was observed from the results on the survival rate and growth that the application of yeast extract to rhizosphere soil could improve the survival and growth of Pinus sylvestris and Armeniaca sibirica seedlings. The three levels of yeast extract showed a significant enhancement to the survival of Pinus sylvestris. Among them, the survival rate increased from 80.47% to 95.13% with the 40 g treatment. Amounts over 20 g were also effective for Armeniaca sibirica, where the survival rate was increased from 70.60% to 88.19%. Furthermore, the 30 g and 40 g treatments provided a significant improvement for the growth of Pinus sylvestris, while for Armeniaca sibirica, only the 40 g treatment significantly stimulated burgeon and growth. As a broad leaf seedling with a bare root, Armeniaca sibirica needed more yeast extract to improve its growth. These results correspond to those by Darwesh on date palm, who expounded that the growth parameters of date palm could be markedly increased by a combination of 50 cm/L yeast and 6 cm/L amino acids [31]. Sampedro et al., through studies on soybean, indicated that a 5% yeast extraction increased shoot initiation, shoot length, and fresh and dry weight [38]. These effects might be attributed to the nutrient supply from the yeast extract and its improvement on the rhizosphere soil’s properties. The organic fertilizer and elements from the yeast extract fulfilled the nutritional requirements of the seedlings. In addition, the components of yeast extract, which contain the precursor of indoleacetic acid, played a crucial role. The application of yeast extract to rhizosphere soil might significantly increase the indole content of seedlings. A high indole content contributes to building resilience in, and enhancing the suction of, plant cells.
The foliar spray did not have a significant influence on survival rates. However, it played a vital role in the growth of both Pinus sylvestris and Armeniaca sibirica. The concentrations of 1.5% and 2% significantly heightened the growth of both species, with the maximum ranges of 9.04 cm and 180.78 cm, respectively. By this token, compared with survival, the foliar spray of the yeast extract had a stronger direct impact on growth. With regard to spray time, new leaves sprouting and shooting is the best moment for the yeast extract to be fully absorbed and utilized. This is the most efficient time due to the yeast extract being rich in phytohormones, such as cytokinins, vitamins, enzymes, amino acids, and minerals, which have a stimulatory effect on cell division and enlargement. In addition to the synthesis of protein and nucleic acids, the yeast extract efficiently helped the seedlings to form chlorophyll [21]. Thus, the seedlings were well-germinated and supplied, especially at the time of initiation. Similar results were recorded by Nassar et al., who drew the conclusion that a foliar spray of yeast extraction could improve the growth characteristics of Leucaena plants to resist salinity stress [29]. Dawood reported that the growth, yield, and quality of soybean plants could be improved by a yeast extraction spray [26]. Mady, who foliar-sprayed yeast extraction at 50 mL/L and zinc at 75 ppm on faba bean, reported a positive effect on yield and seed quality [39].
4.2. Root Properties
The results on root properties indicate that the application of yeast extract to rhizosphere soil is beneficial for root development, with a rising trend. The application amounts of 30 g and 40 g significantly increased the root properties of Pinus sylvestris. The root length and tips of Armeniaca sibirica were affected by the 20 g, 30 g, and 40 g treatments. The surface area increased under the application amounts of 30 g and 40 g. In contrast to survival and growth, the bare root showed a brisker response to the increase in the amount, which may reflect that it is easier for yeast extract to be absorbed by bare root seedlings. The foliar spray improved the three root parameters of both species a little, but with no significant difference at the 0.05 level. These results were ascribed to the constituent of the yeast extract that acts on seedling roots. Firstly, the yeast extract had a nutrient addition effect on the growth of the root. Secondly, yeast treatments have been suggested to play a beneficial role through improving the content of auxins and cytokines [40]. Thirdly, beside the abovementioned indoleacetic acid, yeast extract could facilitate roots with an osmotic adjustment by improving and accumulating compatible solutes called ‘‘osmolytes’’ in the cells of seedling roots. “Osmolytes’’ are synthesized from the proteins, carbohydrates, and amino acids that were supplied by the yeast extract [30,41]. In this way, seedling roots could be maintained with yeast extract to grow better in a semiarid chestnut soil area. These results are in agreement with Darwesh on Phoenix dactylifera cv., who reported a positive effect of yeast on root length and numbers [30]. Shafeek on turnip indicated that the application of yeast extract had a stimulating effect on growth characteristics, such as root length [25].
4.3. Physical Properties
It can be seen from the results that the physical properties of chestnut soil were improved by the application of yeast extract. Higher treatment amounts resulted in better properties. The promotion effect on the physical properties was attributed to the agglutination of soil particles by the application of the yeast extract. The organic fertilizer from yeast extract may stimulate the microbial activity in soil, which brings about a more decomposed organic matter and aggregate structure in the soil. With the formation of a stable aggregate structure of the soil, more capillary puros appeared [16,42]. The 40 g treatment increased the total porosity of the top, middle, and bottom soil layers by 5.49%, 11.37%, and 8.61%, respectively. Greater porosity leads to a decrease in bulk densities and the increment space for water storage. Thus, the bulk densities of the chestnut soil of the top, middle, and bottom layers were lowered from 1.20 g/cm3, 1.44 g/cm3, and 1.55 g/cm3 to 1.13 g/cm3, 1.22 g/cm3, and 1.38 g/cm3, respectively. Moistures were augmented up to 14.61%, 13.04%, and 10.84%, respectively. These results are similar to those from a study on the improvement of the physical properties of chestnut soil by coal ash [43]. With the addition of coal ash, the blocky structure of the chestnut soil was improved. Porous soil led to an increment in the porosity and moisture content, and a decrement of the bulk density [43]. Wang’s study on the fertility of chestnut soil also confirmed a similar result [44]. It is worth mentioning that, compared with the soil a depth of 0–20 cm, the effect of a certain amount of yeast extract on deep soil was more remarkable, especially for soil at a depth of 20–40 cm. There is a possibility that, after irrigation, some of the yeast extract that was applied to the soil was leached to deeper layers with the permeation of water. Nevertheless, it is a good result for seedlings whose root is mainly distributed below 20 cm.
4.4. Chemical Properties
The results of this study showed that yeast extract had an effect on the chemical properties of chestnut soil. Due to the yeast extract being rich in nutrients, the fertility of the chestnut soil was improved as anticipated. The organic content of the top, middle, and bottom soil layers was improved up to 9.85 mg/g, 5.68 mg/g, and 5.60 mg/g, respectively. The content of total nitrogen and total phosphorus was also significantly increased with the application amounts of 30 g and 40 g. These results corresponded with those from the study on improving the chemical properties of chestnut soil by coal ash, which stated that coal ash could improve the chemical properties of chestnut soil [45]. Consistent with the physical properties, the effects on soil of the mid layer were more significant.
The conductivity of the soil was increased by the yeast extract, mainly because the ionic nutrients in the yeast extract supplemented the loss of leached ions in the chestnut soil. Thus, there were more soluble ions in the soil for absorption and utilization by the seedlings. Furthermore, the yeast extract, which is acidic, reduced the calcium pH with ranges of 0.51, 0.70, and 0.62.
However, it should be noted that with the increase in the application amount, the decrement of the calcium carbonate content in the soil below 20 cm was not significant. This might have resulted from the fact that the yeast extraction is rich in amino acids, which can be divided into two types according to their molecular structure: non-polar amino acids and polar amino acids. Non-polar amino acids, such as alanine (Ala) and tryptophan (Trp), are not electrolyzed in water, and have no effect on the mineralization of calcium carbonate. Polar amino acids, such as glycine (Gly) and glutamine (Gln), affect the mineralization of calcium carbonate. When the pH of the environment is higher than that of the amino acid, the polar amino acid releases protons, keeping a negative charge. At this time, the negatively charged amino acid will attract Ca2+ ions, causing an increase in the ion concentration of Ca2+, which would further lead to the oversaturation and accumulation of calcium carbonate. When the pH of the environment is lower than that of the polar amino acids, CO32− ions will be attracted, producing an oversaturation and accumulation of calcium carbonate [46]. Relevant studies have also shown that glycine, serine, cysteine, and lysine all promote the mineralization of calcium carbonate [47]. Changes in amino acid concentration affect the amount and size of the mineralized product. Calcium carbonate deposition was stabilized in the presence of alanine, lysine, and glutamic acid [48].
The economic cost of the yeast extract was about RMB 35 per kilogram, which means that RMB 1.4 for one seedling would bring about a remarkable improvement in afforestation. This was not inexpensive, but had a relatively high-performance cost ratio. So, it was considered that applying yeast extract is a method with some economic potential.
5. Conclusions
It was verified in this study that the application of yeast extract had a beneficial effect on promoting seedling growth and improving the soil in afforestation in a semiarid chestnut soil area. For Pinus sylvestris and Armeniaca sibirica, the amount applied to the rhizosphere soil was the key factor in increasing the survival rate, with the best treatment of 40 g, while foliar spraying played a crucial role in enhancing the growth of seedlings. The phytohormones from the yeast extract may be able to stimulate the growth of both species. The highest mean values were given by foliar spraying of a 2% yeast extract after the leafing of the seedlings. The application of the yeast extract to rhizosphere soil had a significant effect on root properties, possibly by increasing the content of auxin and cytokinin and facilitating an osmotic adjustment. The root length, surface area, and number of tips were increased by the 30 g and 40 g treatments.
The yeast extract had an effect on improving the physicochemical properties of chestnut soil at the optimized amount of 40 g, especially for the 20–40 cm layer, where the roots are mainly located. More agglutination and cementation led to an increase in the porosity and moisture content and a decrease in the bulk density. Due to the nutrients in the yeast extract, the fertility of chestnut soil could be improved due to the increased contents of organic matter, total nitrogen, total phosphorus, and conductivities. Furthermore, the pH and calcium carbonate content were slightly reduced, and accompanied by the mineralization of amino acids.
Overall, the application of yeast extract can be considered as a method for afforestation in a semiarid chestnut soil area as both a plant growth promoter and soil conditioner. The combination of applying 40 g to soil and foliar spraying of 2% after the leafing of the seedling is recommended. Notwithstanding, this is a tentative exploration of its application, and we offer a beneficial approach to solving the afforestation problem that can be applied in the ecological restoration of a semiarid chestnut soil area.
This study is an exploration of a yeast extract application in the afforestation of a semiarid chestnut soil area. The effects of greater levels of yeast extract on other species need to be studied. As a superior plant growth regulator and soil conditioner, yeast extract could probably be applied in combination with other materials to chestnut soil or even applied in other regions.
Author Contributions
Q.X. and T.Z. conceived and designed the study. Q.X., W.L., Y.C., and H.W. performed the experiments. Q.X., W.L., and Y.C. contributed to the sample measurement and data analysis. Q.X. and T.Z. wrote the paper.
Funding
This research was funded by the Study on Comprehensive Technology of Drought and Sand Resistance in Semiarid Chestnut Soil Area under grant number 2015HXFWSBXY014.
Acknowledgments
We would like to thank Japan Honda’s joint ventures in China for their financial support, and the Forestry Bureau of Xinghe County for their assistance with the labor and fieldwork. We are also grateful to the anonymous reviewers for their valuable comments that helped us to improve this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guan, Z.M.; Hao, C.Y. The fragile ecosystem types in arid and semi-arid region of China and their degradation causes. Ecol. Econ. 2013, 9, 158–162. [Google Scholar]
- Nunez-Mir, G.C.; Iannone, B.V.; Curtis, K.; Fei, S.L. Evaluating the evolution of forest restoration research in a changing world: A “big literature” review. New For. 2015, 46, 669–682. [Google Scholar] [CrossRef]
- Geng, Z.C.; Dai, W. Edaphology, 1st ed.; Science Press: Beijing, China, 2011; pp. 223–225. [Google Scholar]
- Bazykina, G.S. The ecological assessment of meadow-chestnut soils of the solonetzic complex ameliorated by means of agroforestry in nonirrigated conditions. Eurasian Soil Sci. 2000, 33, 1178–1185. [Google Scholar]
- Li, Z.H.; Zhao, L.B.; Dou, S. Edaphology, 1st ed.; Chemical Industry Press: Beijing, China, 2005; pp. 193–194. [Google Scholar]
- Merkusheva, M.G. The humus state and structure of microbial cenoses in deflated chestnut soils of barguzin depression (Western Transbaikal). Arid. Ecosyst. 2012, 2, 98–104. [Google Scholar] [CrossRef]
- Shi, X.F. The primary investigation of forestation effect of Pinus sylvestris var.mongolica Litv. on chestnut soil. J. Jilin For. Sci. Technol. 2014, 43, 14–15. [Google Scholar]
- Liu, X. Plant growth regulators on the control of forest. Mod. Agric. Res. 2009, 6, 49. [Google Scholar]
- Dai, F.; Dong, S.J.; Shan, S.T.; Ding, R.J. Effects of different soil substrates and growth regulators on the rooting of Armeniaca sibirica cutting. North. Hortic. 2012, 13, 32–34. [Google Scholar]
- Little, C.H.; Macdonald, J.E. Effects of exogenous gibberellin and auxin on shoot elongation and vegetative bud development in seedlings of Pinus sylvestris and Picea Glauca. Tree Physiol. 2003, 23, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Henrique, A.; Campinhos, E.N.; Ono, E.O.; de Pinho, S.Z. Effect of plant growth regulators in the rooting of Pinus cuttings. Braz. Arch. Boil. Technol. 2006, 49, 189–196. [Google Scholar] [CrossRef]
- Pena, R.; Simon, J.; Rennenberg, H.; Polle, A. Ectomycorrhiza affect architecture and nitrogen partitioning of beech (Fagus sylvatica L.) seedlings under shade and drought. Environ. Exp. Bot. 2013, 87, 207–217. [Google Scholar] [CrossRef]
- Qi, J.Y.; Song, R.Q. Effects of combined inoculation with Suillus luteus and Trichoderma virens on the roots of Pinus sylvestris var. mongolica in northwest Liaoning province. Sci. Silvae Sin. 2018, 54, 62–69. [Google Scholar]
- Wu, X.Q.; Zheng, L.; Ye, J.R. Root architecture differences and their relationships with the growth of Pinus thunbergii seedlings with three kinds of ectomycorrhizae. Acta Ecol. Sin. 2009, 29, 5493–5499. [Google Scholar]
- Khodjaeva, N.A.; Shustikova, E.P. Influence of various fertilizers on productivity grain-fallow crop rotation on chestnut soils. Dostizheniya Nauki Tekhniki APK 2012, 7, 23–26. [Google Scholar]
- Liang, L.B.; Xu, J.M.; Zhang, X.H. Effects of microbial fertilizer and organic-chemical fertilizer on physical properties of calcareous soil in north of China. J. Irrig. Drain. 2014, 33, 105–108. [Google Scholar]
- Huo, L.; Wang, C.B.; Pang, H.C.; Yang, S.C.; Li, Y.C.; Jiang, W.L. Effects of combined application of organic and inorganic fertilizers on physical and chemical properties and crop yields in alkali-saline soil. Agric. Res. Arid. Reg. 2015, 33, 105–111. [Google Scholar]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.; Ferreira, I. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Bi, H.J. Research on yeast extract. Chin. Condiment 2000, 2, 20–23. [Google Scholar]
- Li, G.J.; Wang, Y.S.; Pan, D.Z.; Zhang, S.R.; He, X. Physiological function of yeast extract and its application in poultry production. Feed. Ind. 2016, 37, 28–31. [Google Scholar]
- Zhu, Q. Study on the application of a yeast extract in the cultivation of needle mushroom. Edible Fungus 2009, 31, 32–34. [Google Scholar]
- Shehata, S.A.; Fawzy, Z.F.; El-Ramady, H.R. Response of Cucumber Plants to Foliar Application of Chitosan and Yeast under Greenhouse Conditions. Aust. J. Basic Appl. Sci. 2012, 6, 63–71. [Google Scholar]
- Fawzy Z., F. Increasing productivity of head lettuce by foliar spraying of some bio- and organic compounds. J. Appl. Sci. 2007, 22, 237–240. [Google Scholar]
- Ahmed, A.A.; El-Baky, M.M.H.A.; Zaki, M.F.; Abd El-Aal, F.S. Effect of foliar application of active yeast extract and zinc on growth, yield and quality of potato plant (Solanum tuberosum L.). J. Appl. Sci. Res. 2011, 7, 2479–2488. [Google Scholar]
- El-Tohamy, W.A.; El-Abagy, H.M.; El-Greadly, N.H.M. Studies on the effect of Putrescine, Yeast and Vitamin C on growth, yield and physiological responses of eggplant (Solanum melongena L.) under sandy soil conditions. Aust. J. Basic Appl. Sci. 2008, 2, 296–300. [Google Scholar]
- Shafeek, M.R.; Asmaa, R.M.; Aisha, H.A.; Magda, M.H.; Singer, S.M. Effect of different levels of potassium applied with foliar spraying of yeast on growth, yield and root quality of turnip under sandy soil conditions. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 868–877. [Google Scholar]
- Dawood, M.G.; El-Lethy, S.R.; Mervat, S. Role of methanol and yeast in improving growth, yield, nutritive value and antioxidants of soybean. World Appl. Sci. J. 2013, 26, 6–14. [Google Scholar]
- Reda, F.M.; Ismail, F.M. The influence of spraying with active yeast extract on vegetative growth and volatile oil of river red gum plant (Eucalyptus camaldulensis Dehn.). J. Agric. Sci. 2008, 33, 425–434. [Google Scholar]
- Nassar, R.M.A.; Shanan, N.T.; Reda, F.M. Active yeast extract counteracts the harmful effects of salinity stress on the growth of leucaena plant. Sci. Hortic. 2016, 201, 61–67. [Google Scholar] [CrossRef]
- Darwesh, R.S.S. Phoenix dactylifera cv. Medjol plantlets as affected by yeast extract and NPK fertilizers. Ann. Agric. Environ. Sci. 2016, 1, 7–14. [Google Scholar]
- Darwesh, R.S.S. Improving growth of date palm plantlets grown under salt stress with yeast and amino acids applications. Ann. Agric. Sci. 2013, 58, 247–256. [Google Scholar] [CrossRef]
- Li, G.F.; Zhang, Y.H.; Su, Z.C. Discussion on the causes of the current situation and transformation model of inefficient forests in Xinghe County. Inn. Mong. For. Investig. Des. 2016, 39, 58–59. [Google Scholar]
- Xi, Q.; Lai, W.H.; Cui, Y.Y. Irrigation Equipment and Systems. Utility model patent China ZL 201720580653.0, 30 January 2018. [Google Scholar]
- Cheng, D.J.; Zhang, Y.L. Soil Physics Experiment Instruction, 1st ed.; China Water Publish: Beijing, China, 2012; pp. 13–19, 43–44. [Google Scholar]
- Chen, L.X. Soil Experiment Tutorial, 1st ed.; Northeast Forestry University Press: Harbin, China, 2005; pp. 98–101. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2013; pp. 183–186. [Google Scholar]
- Shi, L.W. SPSS 19.0 Statistic Analysis, 1st ed.; Tsinghua University Press: Beijing, China, 2012; pp. 114–122. [Google Scholar]
- Sampedro, I.; Aranda, E.; Scervino, J.M.; Fracchia, S.; García-Romera, I.; Ocampo, J.A.; Godeas, A. Improvement by soil yeasts of arbuscular mycorrhizal symbiosis of soybean (Glycine max) colonized by Glomus mosseae. Mycorrhiza 2004, 14, 22–234. [Google Scholar] [CrossRef] [PubMed]
- Mady, M.A. Effect of foliar application with yeast extract and Zinc on fruit setting and yield of faba bean (Vicia faba L.). J. Biol. Chem. Environ. Sci. 2009, 4, 109–127. [Google Scholar]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. Yeasts Characteristics and Identification, 1st ed.; Cambridge University Press: London, UK, 1990; p. 999. [Google Scholar]
- Amal, A.M.; Bettina, E.L.; Ewald, S. Response of crops to salinity under Egyptian conditions: A review. Landbauforsch. Volkenrode 2007, 57, 119–125. [Google Scholar]
- Xu, Z.X. Effect of Combined Application of Organic and Inorganic Fertilizers on the Physical Characters of Soils. J. Irrig. Drain. 2010, 29, 11–13. [Google Scholar]
- Li, G.H.; Xu, H.; Shao, W.; Shen, J.F. Experimental study on improvement of physical properties of chestnut soil using fly ash in Baotou. J. Soil Water Conserv. 2002, 16, 113–115. [Google Scholar]
- Wang, D.W. Study on effect of organic and inorganic composition on chestnut soil in northwest plateau of Hebei province. Chin. J. Soil Sci. 1998, 3, 109–110. [Google Scholar]
- Li, G.H.; Sun, D.S.; Shen, J.F. Applied Research of Chemical Property on Ash-Improved Chestnut Soil in Baotou. Chin. J. Soil Sci. 2002, 33, 260–262. [Google Scholar]
- Chen, X.; Ren, D.N.; Feng, Q.L.; Li, S.R. Effect of several Amino Acids on the crystallization of CaCO3. Acta Minerlogica Sin. 2011, 31, 692–697. [Google Scholar]
- Chen, C.; Li, Q.F.; Zhang, Q.M.; Zhang, C.K.; Lv, Z.Z.; Yu, L.J. A simulation study on the effect of bacterial extracellular feature Amino Acids on calcium carbonate mineralization under low temperature. Geol. J. China Univ. 2017, 23, 606–614. [Google Scholar]
- Manoli, F.; Dalas, E. Calcium carbonates crystallization in the presence of glutamic acid. J. Cryst. Growth 2001, 222, 293–297. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).