Abstract
The aim of the study was to develop and evaluate the properties of MED610 material coated with a thin titanium (Ti) layer deposited by physical vapour deposition (PVD) for medical applications. The coating was designed to improve biocompatibility and selected functional characteristics, such as the material’s tribological resistance. Two groups of samples—unpolished and polished—were prepared to enable an assessment of the influence of surface topography on functional performance. A titanium layer was applied to both groups, followed by an analysis of the surface geometric structure, contact angle, and tribological properties in an artificial saliva environment with neutral pH, simulating oral cavity conditions. The results of these investigations allowed for a comprehensive assessment of the influence of surface topography and the presence of a Ti coating on the functional properties of MED610, a material approved for contact with soft tissues. The findings confirmed that the application of a titanium coating favourably affected the structure and durability of the material—the coating reduced surface roughness by 20–45% and exhibited good adhesion to the substrate. The polishing and coating processes significantly altered the tribological properties: they increased the coefficient of friction by approximately 31% while simultaneously reducing volumetric wear by up to 75% for uncoated samples and by 44% for samples with a Ti coating.
1. Introduction
The development of 3D printing technologies represents one of the key directions of modern manufacturing engineering, enabling the fabrication of components with complex geometries that are unattainable using conventional production methods. As knowledge of 3D printing technologies continues to expand, the number of their practical applications is also steadily increasing [1].
Additive manufacturing is currently used, among others, in the automotive and aerospace industries [2,3], as well as in medicine [4,5], where its design flexibility enables applications across numerous specialties, including surgery, dentistry, and orthopaedics. Such products include both ready-to-use components and highly personalised anatomical models, such as implants [6,7], surgical guides [8,9], preoperative simulations [7,10], biodegradable screws [11,12], functional prostheses and orthoses [1], as well as drug-delivery systems [13]. Moreover, MED610 can be used as an acrylic substitute in the production of replacement inserts for ocular plaque brachytherapy applicators [14], as well as for personalised nasoalveolar moulding devices designed for preoperative unilateral and bilateral cleft lip and palate treatment in infants [15]. It is also employed in the fabrication of customised mouthpieces for patients undergoing head and neck radiotherapy [16]. Consequently, intensive research is being conducted on new materials [4] that are characterised by biocompatibility [1,4] and good printability [1].
An example of a polymer used in medical applications is MED610. It is a biocompatible photopolymer employed in the PolyJet technology [17,18] used, among others, in dental applications. This material is approved for long-term skin contact—exceeding 30 days [18]—and for short-term contact with mucous membranes—up to 24 h [17,18]. MED610, obtained through the photopolymerisation of liquid polymer resins, was investigated by the authors of study [4]. The samples were manufactured in a high-accuracy mode with a layer thickness of 0.016 mm and three printing orientations in the X–Z plane: 0°, 45°, and 90°. The authors conducted tribological tests under technically dry friction conditions and with lubrication using a physiological saline solution (0.9% NaCl) at a load of 10 N. The counter samples were 6 mm diameter balls made of polyamide 6.6 and polyoxymethylene. The analysis of the results demonstrated that the printing orientation affects tribological wear, which is associated with the anisotropic nature of 3D printing technology. The lowest average coefficient of friction was obtained for samples printed in the 90° orientation.
In turn, the authors of study [18] conducted tribological tests on MED610 samples manufactured using PolyJet Modeling (PJM) technology. The tests were performed at three different loads (30, 45, and 60 N) and sliding speeds of 100, 150, and 200 m/s. Additionally, a metrological analysis was carried out to assess both the reliability of the printing technology and the material itself. The results showed that MED610 exhibits relatively good wear resistance. It was observed that the maximum wear depth and the wear track area decrease with increasing rotational speed. Nevertheless, this material is not suitable for use in friction nodes subjected to high loads. In turn, in the publication [19], the authors conducted tribological tests under rotary motion using samples made of M390 and M398 steels produced by powder metallurgy combined with HIP. These samples differed in surface roughness as well as carbide content—20% for M390 and 30% for M398. The counter sample was a 6.35 mm diameter Al2O3 ball. The tests were performed at a temperature of 200 °C under technically dry friction conditions. The authors demonstrated that M398 can replace M390, as it exhibits superior tribological properties. The M398 material showed more than a 400% reduction in wear compared with M390, along with a lower average friction coefficient by 0.05. However, in the publication [20], the authors investigated the effect of increasing irradiation doses of accelerated electrons on changes in the friction coefficient and surface roughness of the polymer materials PET, PTFE, and PE2000C. The materials were irradiated with three doses: 33 kGy, 129 kGy, and 300 kGy. Tribological tests were then carried out under reciprocating motion in technically dry friction conditions, with a G40 steel ball used as the counter sample. The applied load increased from 10 N to 100 N. Irradiation altered both the roughness and friction coefficient of the examined polymers: PET became smoother, whereas PE2000C became rougher. Higher doses—particularly 300 kGy—led to surface degradation and an increase in friction.
Materials used in medical applications, in addition to meeting specific mechanical and tribological requirements, must also exhibit non-toxicity. It is well known that uncured liquid photopolymer resins used in 3D printing processes display cytotoxic effects. They may cause severe eye irritation and allergic skin reactions upon prolonged contact. Only after the curing process do these materials become chemically stable and safe in their solid state. For this reason, all uncured residues must be removed during the cleaning stage of printed parts. However, depending on the printing orientation, uncured material may remain trapped within pores, channels, or internal cavities of the structure, which may lead to cytotoxicity during the final use of the product [1]. An effective way to reduce the toxic effects of uncured material residues is the application of thin, biocompatible protective coatings [21]. Such layers act as a barrier between the printed surface and the body’s tissues, ensuring the safe use of the medical device. An example of a material used for this purpose is titanium, which—due to its high strength-to-weight ratio and excellent biocompatibility [22]—is widely employed in implantology. This metal rarely induces allergic reactions, which is particularly important for components intended for long-term contact with the human body [23]. Moreover, titanium is generally regarded as non-toxic [23,24]—as it does not release harmful substances into the body, thereby ensuring patient safety over extended periods [23]. Consequently, titanium can be used in a wide range of medical applications, including surgical instruments, implants, and dental components [24]. According to Rodrigues et al. [25], a titanium (Ti) coating deposited on ultra-high molecular weight polyethylene (UHMWPE) exhibits superior mechanical properties—such as higher hardness and improved resistance to nanoscratching—as well as better tribological performance compared with tantalum (Ta) and zirconium (Zr) coatings. Among the analysed coatings, the Ti layer demonstrated the lowest coefficient of friction.
The authors of study [26], investigated metal coatings deposited on the polymer materials PEEK, UHMWPE, and PTFE, as well as the adhesion of osteoblasts to orthopaedic polymers coated with titanium or gold using the ion-plasma deposition process. As a result of this process, a nanostructured, surface-modified layer (with features below 100 nm) was obtained. It was reported that polymers coated with titanium or gold exhibited a significant increase in osteoblast adhesion compared with uncoated polymers. However, in study [27], the authors developed a new polymer coating process based on titanium-derived layers deposited using the PACVD method. Their findings indicated that thin and very smooth layers exhibit excellent adhesion to PET, PES, PTFE, and PVC polymers. For polyolefins such as PP and PE, the adhesion is lower, although it can be improved through preliminary plasma treatment. The researchers demonstrated that the coating enhances the biocompatibility and blood compatibility of the polymers. One possible factor contributing to improved biocompatibility may be the increased critical surface tension of the coating compared with uncoated polymers. Furthermore, the coating exhibited barrier properties, preventing the leaching of plasticisers—particularly from PVC—which is a widely discussed issue in the context of using PVC in medical applications.
In summary, despite the wide use of 3D printing technologies in medicine, photopolymer materials such as MED610 still require modification to improve their biocompatibility and wear resistance, as well as extremely precise and complex 3D printing procedures to eliminate toxicity associated with the printing process itself. Therefore, this study proposes an innovative solution involving the deposition of a thin titanium coating on MED610 prints, which also greatly facilitates the sterilisation of such models. The application of this modification aims to enhance the biological safety of the material, minimise the influence of uncured polymer residues, and improve its tribological properties under conditions simulating the oral environment.
2. Materials and Methods
2.1. Research Methodology
Figure 1 presents the research scheme and the equipment used, summarizing all relevant information on the performed tests and the devices on which they were conducted.
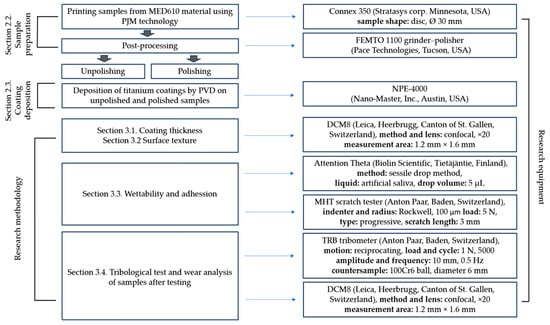
Figure 1.
Research scheme and equipment used.
Tribological tests were carried out in a classic ball-on-disc system with reciprocating motion at ambient temperature, with the artificial saliva solution being applied with a pipette to the sample surface and maintained at a constant volume throughout the test. The chemical composition of the lubricant is presented in Table 1 and a photograph of the friction node is presented in Figure 2.

Table 1.
Chemical composition of the lubricant—artificial saliva [28].
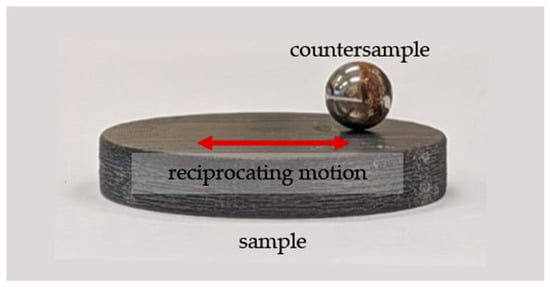
Figure 2.
View of the friction pair.
2.2. Sample Preparation
The test samples were manufactured using the PolyJet Matrix technology, which involves the photo-curing of liquid polymer resins. In this technology, fine droplets of liquid resin are jetted onto the surface of the currently formed layer of the model, corresponding to its cross-sectional geometry, as well as onto the regions requiring support material. The samples were fabricated using a Connex 350 printer. They were designed as discs with a diameter of 30 mm and a height of 6 mm to facilitate both the coating deposition process and subsequent wear analysis.
The 3D printing process was carried out using predefined technological parameters, including a layer thickness of 0.016 mm, the high-quality printing mode, and the matt printing type. The matt setting ensured complete coverage of every surface of the sample with support material, enabling the production of a uniform surface across the entire specimen. After printing, the samples were initially cleaned of support material using a water under heigh pressure. According to the manufacturer’s instructions, the PJM technology is characterised by a printing resolution of 600 dpi in the X-axis, 600 dpi in the Y-axis, and 1600 dpi in the Z-axis. Furthermore, as demonstrated in the authors’ previous studies, the manufacturing accuracy in the X–Y plane is typically on the order of 50 micrometres. In the Z-axis, this value is generally lower due to the very small layer thickness of 15 micrometres.
All samples were printed in a single orientation on the build platform—the specimen was positioned with its flat frontal surface placed directly on the platform. This approach allowed for highly precise reproduction of the cylindrical surface.
MED610 is a material with very good medical performance characteristics. According to the product specification, the manufacturer states that the material has been developed for medical and dental applications [29]. Moreover, this resin meets the normative requirements for:
- -
- irritation and type IV hypersensitivity [30];
- -
- cytotoxicity [31];
- -
- genotoxicity [32];
- -
- chemical characterisation [33].
According to the material manufacturer and the Material Safety Data Sheet [34] the key components of MED610 include:
- -
- Isobornyl acrylate, 15–30 wt %;
- -
- Acrylic monomer, 15–30 wt %;
- -
- Urethane acrylate, 10–30 wt %;
- -
- Acrylic monomer, 5–15 wt %;
- -
- Epoxy acrylate, 5–15 wt %;
- -
- Acrylate oligomer, 5–15 wt %;
- -
- Photoinitiator, 0.1–2 wt %.
Surface preparation constituted a crucial stage prior to coating deposition. A portion of the samples was subjected to grinding, while the remaining specimens were left in their as-printed state. Grinding was performed using silicon carbide abrasive papers with progressively increasing grit sizes—from 120 to 600. The coatings were applied both to the samples after initial cleaning following 3D printing (Figure 3a) and to the polished samples (Figure 3b). The surfaces prepared in this manner provided an appropriate substrate for the subsequent deposition of the titanium coating using the PVD method.

Figure 3.
Photographs of samples before coating deposition: unpolished (a), polished (b).
2.3. Coating Deposition
The titanium coating was deposited using the physical vapour deposition (PVD) method with a Nanomaster system. Prior to initiating the process, the samples were thoroughly cleaned and subsequently placed inside the deposition chamber. In the first stage, cleaning was carried out in an argon (Ar) atmosphere under vacuum conditions for 20 min. This was followed by the deposition stage, performed using a titanium target. The coating process lasted 2 h. Detailed parameters for both the cleaning stage and the coating deposition are summarised in Table 2. Photographs of the coated samples are presented in Figure 4. As shown in Figure 4a, certain imperfections of the process are clearly visible, including traces resulting from the spreading of material by individual printing heads, whereas in Figure 4b—after polishing—all such irregularities have been removed.

Table 2.
Coating deposition parameters.

Figure 4.
Photographs of samples with Ti coating: unpolished (a), polished (b).
3. Results
3.1. Coatings Thickness
The thickness of the Ti coating was measured using optical microscopy. To ensure accurate determination of the coating step height, the sample surface was mechanically polished prior to deposition, achieving a surface roughness of approximately Sa ≈ 100 nm. This preparation was necessary because measurements performed on unpolished surfaces are unreliable due to high topographical variability. After coating deposition, the thickness was determined by analysing the height difference (step height) between the coated and uncoated regions of the surface profile. The measurement was repeated five times at different locations to assess the uniformity of the coating. The results are presented in Figure 5. The mean thickness and standard deviation were calculated to evaluate the reproducibility of the deposition process.
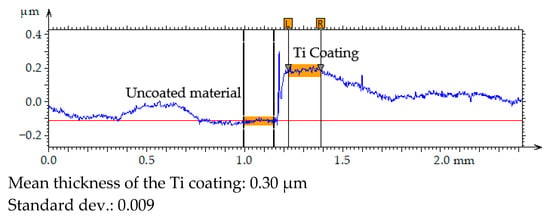
Figure 5.
Primary profile of the surface texture–coating/substrate transition area.
The results of the coating thickness measurements indicated that the 2 h PVD process produced a layer with a thickness of approximately 300 nm.
3.2. Surface Texture
Figure 6 presents the 3D axonometric images of the MED610 samples in both the unpolished and polished states, with and without the Ti coating. Figure 7 shows the primary surface profiles. The results of these analyses allow for an assessment of the influence of the polishing process and coating deposition on the microstructure and surface roughness in comparison with the base material. Table 3 summarises the amplitude parameters for all analysed surfaces, providing a quantitative assessment of topographical changes resulting from polishing and coating deposition.
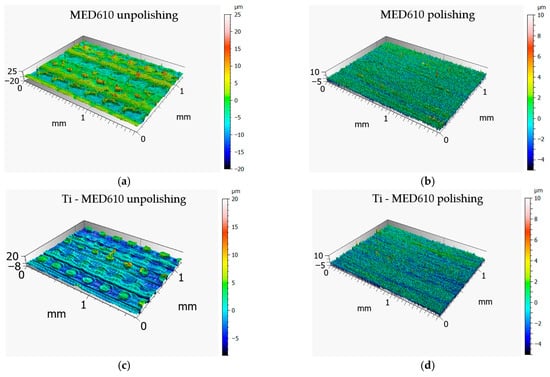
Figure 6.
Surface topography of MED610 (a,b) and Ti–MED610 (c,d) samples 3D printed using PJM technology: three-dimensional axonometric images.
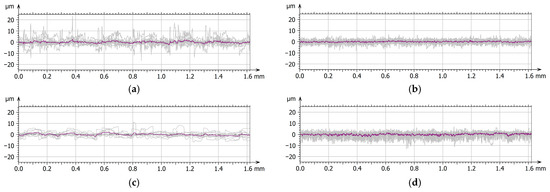
Figure 7.
Surface texture: primary profiles with the medium profile marked—MED610 unpolishing (a), MED610 polishing (b), Ti–MED610 unpolishing (c), Ti–MED610 polishing (d).

Table 3.
Amplitude parameters for representative samples.
On the surface of the unpolished, uncoated sample, characteristic droplets and irregularities typical of elements produced using PJM technology are visible, resulting from the deposition of successive polymer layers. The same droplet-like structures were also observed on the surface of the sample coated with the Ti layer; however, they were partially flattened—their height was approximately 20% lower compared with the uncoated surface. This phenomenon is likely due to the deposition of the coating, which fills micro-depressions and partially reduces height differences between individual layers of the polymer material. This observation is further supported by the amplitude parameters listed in Table 2. For the Sa (arithmetical mean height), Sq (root mean square height), and Sv (maximum pit depth) parameters, the differences between the unpolished coated and uncoated surfaces were approximately 20%. In contrast, for the Sp parameter (maximum peak height), a difference of up to 45% was observed. This result confirms that the deposited coating significantly smooths the highest points of the topography of the unpolished sample surface, reducing their height relative to uncoated areas. In the case of samples coated with the titanium layer, the effect of polishing was less pronounced, yet still noticeable. The values of the Sa and Sq parameters decreased by approximately 15% and 20%, respectively, whereas Sv and Sp increased by about 12% and 18%. The obtained results suggest that the presence of the Ti coating modifies the polishing process, limiting its effectiveness in reducing surface irregularities. The surface topography results further indicate that unpolished surfaces, due to their higher roughness and the presence of numerous micro-irregularities, may exhibit lower wear resistance under frictional conditions. These irregularities may promote local stress concentrations and intensify abrasive wear processes. From a biomedical standpoint, this is of particular importance—comparative studies of materials used in orthodontic appliances have shown that surfaces with lower roughness and higher smoothness display reduced tendencies for saliva, deposits, and bacterial accumulation, and therefore for biofilm formation. This is confirmed by the findings of Jeon et al. [35] as well as Ahn et al. [36], who demonstrated that surface roughness promotes strong bacterial adhesion.
3.3. Wettability and Adhesion
Figure 8 presents the mean contact angle values with error bars—standard deviation (SD) for the MED610 surface, calculated from ten measurements. Figure 9a shows images of artificial saliva droplets placed on the surface, while the corresponding 2D surface topography maps are shown in Figure 9b.
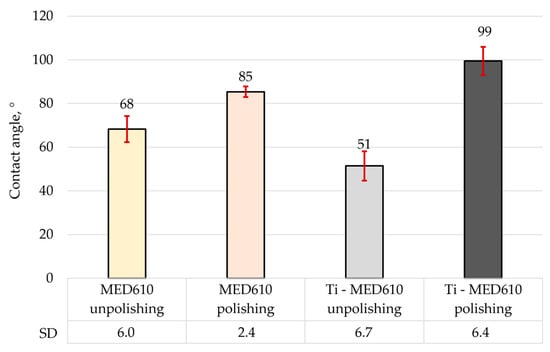
Figure 8.
Average contact angle of MED610 material and after PVD of Ti coating.
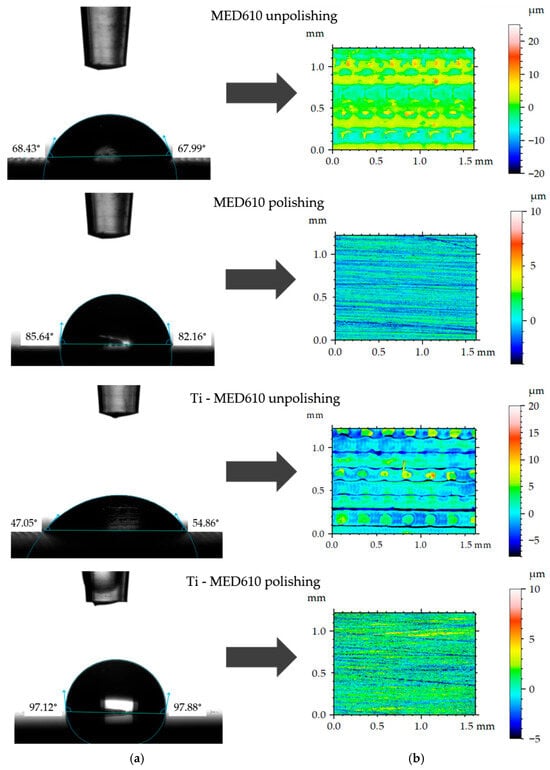
Figure 9.
Representative contact angle images (a) and corresponding 2-D topographical images (b).
The wettability analysis revealed a clear influence of both the polishing process and the presence of the titanium coating on the contact angle values. The unpolished samples exhibited relatively low contact angles, indicating a hydrophilic character of these surfaces. The lowest contact angle was recorded for the unpolished MED610 sample with the Ti coating, measuring 51°, whereas the unpolished uncoated MED610 sample showed a value of 60°. This indicates that the presence of the titanium coating imparts a hydrophilic character to unpolished samples. For the polished surfaces, the contact angle values increased significantly—by approximately 20% for the MED610 sample and by as much as 50% for the Ti-coated MED610 sample (Ti-MED610), compared with the unpolished specimens. This demonstrates a shift in surface character from hydrophilic to hydrophobic.
The literature indicates that surface roughness affects not only wettability but also bacterial adhesion. Jeon et al. [35] emphasised that rough surfaces are more wettable and promote bacterial colonisation, whereas smoother surfaces exhibit lower wettability and reduced bacterial adhesion. Similar observations were reported by Martínez Gil-Ortega et al. [37], who noted that hydrophilic surfaces with contact angles around 90° favour a high level of bacterial colonisation. On the other hand, superhydrophilic surfaces, which rapidly adsorb water, and superhydrophobic surfaces, which are surrounded by an air layer, limit bacterial contact with the material, as confirmed by Oliveira et al. [38]. In the context of our results, this suggests that polishing and titanium coating can modulate the surface character and potentially influence bacterial adhesion, which is important in designing biomedical materials with controlled microbial colonisation. Studies from other research centres [35,37,38] show that surfaces with moderate hydrophilicity, characterised by contact angles in the range of approximately 60–80°, exhibit the most favourable biological properties. Such surfaces hinder the permanent adsorption of proteins and bacteria—processes that occur more rapidly on highly hydrophilic surfaces—while simultaneously reducing excessive deposition, which is typical of strongly hydrophobic surfaces. Additionally, moderately hydrophilic surfaces facilitate uniform spreading of saliva, supporting natural surface cleaning and limiting biofilm formation in the oral environment.
The images of the measurement liquid droplets shown in Figure 9 indicate that, on the unpolished surfaces, the artificial saliva droplet spread more rapidly, which reflects stronger liquid–substrate interactions and a hydrophilic character of the surface. The micro-irregularities remaining after printing promoted liquid retention by increasing the number of contact points between the droplet and the material. In the case of the polished surfaces, the droplet maintained a more spherical shape, indicating reduced liquid adhesion to the substrate and limited spreading.
This is confirmed by Rupp et al. [39], who observed that hydrophilic surfaces can facilitate initial interactions between the surface and the wetting liquid, which is important for wound healing and osseointegration. Furthermore, surface roughness has a significant impact on wettability and also plays an important role in the osseointegration process. Similarly, Liber-Kneć et al. [40] concluded that a hydrophobic surface character may increase bacterial adhesion.
Figure 10 shows the results of the scratch test performed on Ti coatings deposited on MED610. The figure presents optical images of the scratches, along with plots of the applied loading force (Fn), acoustic emission (Ae), friction coefficient (µ), and the critical force values (LC1 and LC2).
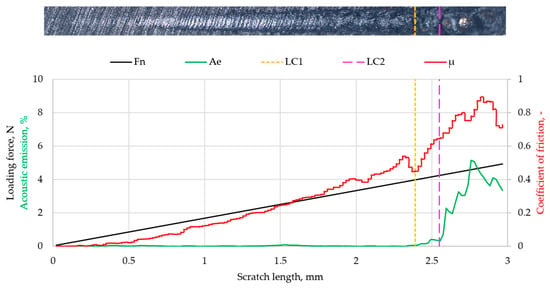
Figure 10.
Scratch test results for Ti coatings.
The results of the scratch test on the Ti coating deposited on MED610 indicated that the applied layer exhibited good adhesion to the substrate. A sudden increase in acoustic emission and friction coefficient was observed at a critical load of approximately 3.92 N (LC1). Since no spalling of the deposited layer was observed in the optical images, this jump is attributed to the surface roughness of the Ti–MED610 system. Increasing the load to 4.19 N (LC2) caused another jump in both parameters and led to delamination of the coating. For thin coatings (with thickness on the order of nanometres), the values of the critical forces LC1 and LC2 in this range indicate good adhesion of the coating to the substrate. This interpretation is consistent with the approach presented by Valli [41], who emphasised that LC1 typically corresponds to the initiation of cohesive damage within the coating, whereas LC2 is the critical adhesion parameter that marks the onset of coating detachment from the substrate. According to this framework, the LC1 and LC2 values obtained for the examined system—characteristic of thin coatings with nanometre-scale thickness—indicate good interfacial bonding between the Ti coating and the MED610 substrate.
3.4. Tribological Test and Wear Analysis of Samples After Testing
Figure 11 presents the mean friction coefficient values with error bars—standard deviation (SD) for the MED610 samples without the coating and those with the titanium (Ti) coating, while Figure 12 shows the three-dimensional axonometric views and primary profiles of the wear tracks, wherebuild-ups are indicated in green, whereas indentations are indicated in red. The use of a 10 mm amplitude and confocal microscopy enabled measurement of the entire wear area, allowing for a detailed analysis of the wear track and precise determination of the wear track volume (Figure 13). All values were calculated based on three independent measurement series.
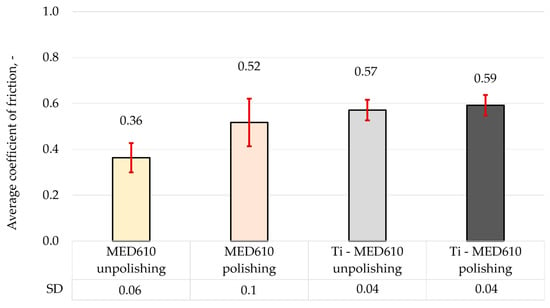
Figure 11.
Average coefficient of friction of MED610 material and after PVD of Ti coating.
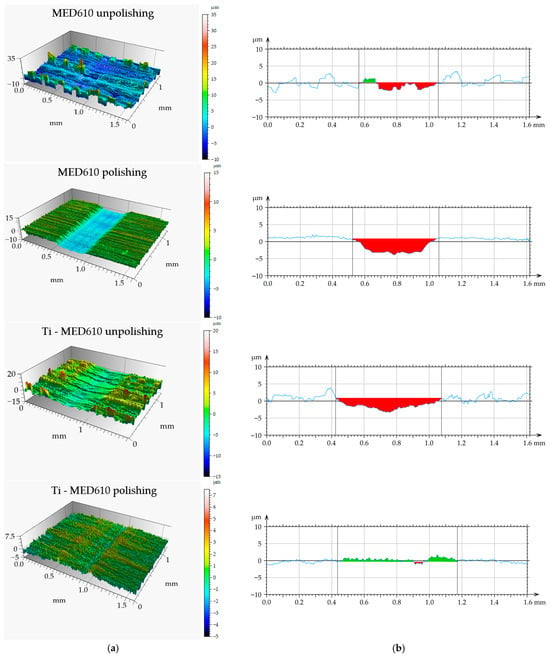
Figure 12.
Views of wear track (a) and primary profiles on the cross-section (b) of MED610 material and after PVD of Ti coating.
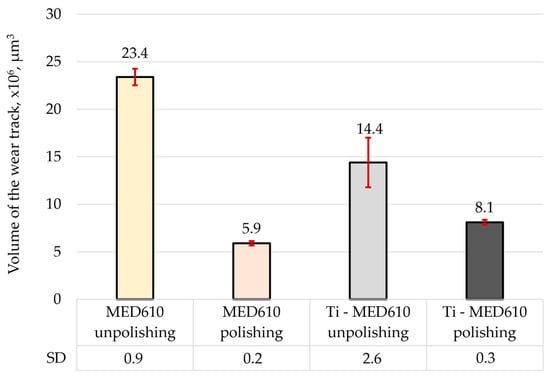
Figure 13.
Volume of the wear track for the tested samples.
The results of the friction coefficient measurements indicate that the polishing process contributes to an increase in its value by approximately 31% for the uncoated MED610 material. This phenomenon can be attributed to the smoothing of micro-irregularities, which reduces the surface’s ability to retain the lubricant (artificial saliva). Additionally, it was observed that the application of the titanium (Ti) coating to both unpolished and polished samples results in a further increase in the friction coefficient—by approximately 36% and 12%, respectively, compared with the base MED610 material. The friction coefficient values for the Ti-coated samples were similar (0.57 and 0.59), regardless of surface topography, suggesting that the presence of the coating stabilises the tribological behaviour of the material by reducing the influence of substrate roughness on the friction process. In the case of the polished samples, the increase in the friction coefficient after the coating deposition was associated with a rise in local surface roughness. This correlation between topography and friction value is also confirmed by Bartosova et al. [20], who demonstrated that surface bombardment increased the roughness of the samples, and consequently, their friction coefficient. According to Grzesik [42], in addition to the Sa and Sq parameters, Sku and Ssk also have a significant impact on friction and wear. A negative Ssk value contributes to an increase in the friction coefficient. This was confirmed for both polished and unpolished samples, as the deposition of the coating resulted in a negative Ssk value (Table 3), leading to higher average friction coefficient values.
The results of the volumetric wear analysis indicate that the fastest-wearing material in the friction pair with the steel ball was the unpolished and uncoated MED610 sample. It was observed that the polishing process significantly reduced the volumetric wear of the material—by approximately 75% for the uncoated samples and by 44% for the samples with the Ti coating. This effect can be attributed to the reduction in surface micro-irregularities (peaks), which limits local micro-impacts and decreases the intensity of abrasive wear. The application of the titanium (Ti) coating to the unpolished sample reduced the volumetric wear by approximately 40% compared with the uncoated surface. In contrast, for the polished sample, the coating resulted in a slight increase in wear—by around 27%. This indicates that the protective effectiveness of the Ti coating is strongly dependent on the substrate surface topography, and that its adhesion and ability to distribute loads uniformly may be limited on smoother surfaces. The obtained results demonstrate the crucial role of surface roughness in shaping the tribological properties of polymer materials. Moderate roughness promotes the retention of the lubricant (artificial saliva), which may reduce friction but simultaneously increase the intensity of abrasive wear. On the other hand, excessive smoothing of the surface reduces lubrication effectiveness while also decreasing the volumetric material loss. Furthermore, Al-Samarai et al. [43], Bayer et al. [44], and Barrett et al. [45] observed in their studies that the mass- and volume-specific wear rate decreases with a reduction in surface roughness, which was also confirmed in the present study.
Additionally, a statistical analysis was conducted to determine the probability of the influence of individual technological factors on the output parameters of the study.The effect of surface polishing and Ti coating on wettability, friction and wear of 3-D-printed samples is summarised in Table 4. In this table, a single asterisk indicates a lower probability of the outcome, while three asterisks represent the highest probability.

Table 4.
Effect of surface polishing and Ti coating on wettability, friction and wear of 3-D-printed samples (mean ± standard deviation).
A detailed analysis of the experimental results indicated that polishing leads to an increase in both the contact angle and the coefficient of friction, while it simultaneously reduces volumetric wear. Conversely, unpolished samples exhibited the opposite behaviour.
For samples coated with Ti, the most pronounced differences were observed in terms of wear. Unpolished Ti-coated samples demonstrated approximately 38% lower wear compared to their uncoated counterparts. In contrast, when these samples were polished, the wear increased by around 37%. These findings suggest that surface preparation significantly influences wear behaviour, with unpolished surfaces exhibiting reduced material loss.
4. Conclusions
Based on the experimental analyses performed, the following conclusions can be drawn regarding the effects of polishing and Ti-coating deposition on the surface and tribological properties of the MED610 material:
- The PVD process enabled the deposition of a uniform ~300 nm titanium coating with good adhesion to the MED610 substrate.
- Surface topography measurements showed that the Ti coating reduced the characteristic roughness of PJM-manufactured components, lowering amplitude parameters by 20–45%.
- Wettability tests demonstrated that unpolished samples exhibited lower contact angles, while polishing increased the contact angle and shifted the surface character from hydrophilic to hydrophobic.
- Tribological measurements revealed that polishing increased the friction coefficient of MED610 by approximately 31%, likely due to reduced lubricant retention, while Ti-coated samples exhibited similar friction values (~0.57–0.59), indicating stabilisation of frictional behaviour by the coating. Wear analyses showed that polishing significantly decreased volumetric wear (by ~75% for uncoated and ~44% for Ti-coated samples). Ti deposition reduced wear by ~40% on unpolished surfaces, whereas on polished surfaces it caused a slight wear increase of about 27%.
- Overall, the results confirm that both surface roughness and the titanium coating substantially affect the tribological behaviour and wettability of MED610. Improved wear resistance and favourable wettability in artificial saliva highlight the potential of this surface modification for medical devices intended for contact with mucous membranes, and provide a basis for further studies on enhancing the biocompatibility and durability of 3D-printed polymer materials.
Author Contributions
Conceptualization, K.P., J.K. and T.K.; investigation, K.P., J.K. and T.K.; writing—original draft preparation, K.P., J.K. and T.K.; writing—review and editing, K.P., J.K. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schneider, K.H.; Oberoi, G.; Unger, E.; Janjic, K.; Rohringer, S.; Heber, S.; Agis, H.; Schedle, A.; Kiss, H.; Podesser, B.K.; et al. Medical 3D Printing with Polyjet Technology: Effect of Material Type and Printing Orientation on Printability, Surface Structure and Cytotoxicity. 3D Print. Med. 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Bousnina, K.; Dridi, I.; Ben Yahia, N. Revolutionizing Automotive Design: The Impact of Additive Manufacturing. Vehicles 2025, 7, 24. [Google Scholar] [CrossRef]
- Khorasani, M.; Ghasemi, A.; Rolfe, B.; Gibson, I. Additive manufacturing a powerful tool for the aerospace industry. Rapid Prototyp. J. 2022, 28, 87–100. [Google Scholar] [CrossRef]
- Szczygieł, P.; Radoń-Kobus, K.; Madej, M.; Kozior, T. Tribological Properties of MED610 Medical Material Used in PolyJet Matrix 3D Printing Technology. Tribologia 2023, 4, 65–77. [Google Scholar] [CrossRef]
- Tanzli, E.; Kozior, T.; Hajnys, J.; Mesicek, J.; Brockhagen, B.; Grothe, T.; Ehrmann, A. Improved Cell Growth on Additively Manufactured Ti64 Substrates with Varying Porosity and Nanofibrous Coating. Heliyon 2024, 10, e25576. [Google Scholar] [CrossRef]
- Paxton, N.C. Navigating the Intersection of 3D Printing, Software Regulation and Quality Control for Point-of-Care Manufacturing of Personalized Anatomical Models. 3D Print. Med. 2023, 9, 9. [Google Scholar] [CrossRef]
- Zhao, C.X.; Yam, M. 3D Printing for Preoperative Planning and Intraoperative Surgical Jigs—A Prospective Study on Surgeon Perception. J. Orthop. Rep. 2024, 3, 100305. [Google Scholar] [CrossRef]
- l’Alzit, F.R.; Cade, R.; Naveau, A.; Babilotte, J.; Meglioli, M.; Catros, S. Accuracy of Commercial 3D Printers for the Fabrication of Surgical Guides in Dental Implantology. J. Dent. 2022, 117, 103909. [Google Scholar] [CrossRef]
- Cozzolino, E.; Risco, G.D.; von Windheim, N.; Gygi, C.; Astarita, A.; Ames, N. Scaling up the 3D Printing of Surgical Guides: Repeatability and Energy Efficiency. Rapid Prototyp. J. 2025, 31, 148–159. [Google Scholar] [CrossRef]
- Labakoum, B.; Farhan, A.; El Malali, H.; Mouhsen, A.; Lyazidi, A. Development of a Personalized and Low-Cost 3D-Printed Liver Model for Preoperative Planning of Hepatic Resections. Appl. Sci. 2025, 15, 9033. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U.; Weisman, J.A.; Ballard, D.H.; Wolford, D.D.; Pascual-Garrido, C.; Wolford, L.M.; Woodard, P.K.; Mills, D.K. 3D Printing Custom Bioactive and Absorbable Surgical Screws, Pins, and Bone Plates for Localized Drug Delivery. J. Funct. Biomater. 2019, 10, 17. [Google Scholar] [CrossRef]
- Hari Raj, K.; Gnanavel, S.; Ramalingam, S. Investigation of 3D Printed Biodegradable PLA Orthopedic Screw and Surface Modified with Nanocomposites (Ti–Zr) for Biocompatibility. Ceram. Int. 2023, 49, 7299–7307. [Google Scholar] [CrossRef]
- Saleh-Bey-Kinj, Z.; Heller, Y.; Socratous, G.; Christodoulou, P. 3D Printing in Oral Drug Delivery: Technologies, Clinical Applications and Future Perspectives in Precision Medicine. Pharmaceuticals 2025, 18, 973. [Google Scholar] [CrossRef]
- Sim, L. Novel application of 3D printing in brachytherapy using MED610 3D printed insert for I-125 ROPES eye plaque. Australas. Phys. Eng. Sci. Med. 2016, 39, 863–870. [Google Scholar] [CrossRef]
- Gong, X.; Dang, R.; Xu, T.; Yu, Q.; Zheng, J. Full Digital Workflow of Nasoalveolar Molding Treatment in Infants With Cleft Lip and Palate. J. Craniofacial Surg. 2020, 31, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Kitamori, H.; Sumida, I.; Tsujimoto, T.; Shimamoto, H.; Murakami, S.; Ohki, M. Evaluation of mouthpiece fixation devices for head and neck radiotherapy patients fabricated in PolyJet photopolymer by a 3D printer. Phys. Medica 2019, 58, 90–98. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Fang, H.-Y.; Shie, M.-Y.; Shen, Y.-F. The Mussel-Inspired Assisted Apatite Mineralized on PolyJet Material for Artificial Bone Scaffold. Int. J. Bioprint. 2019, 5, 197. [Google Scholar] [CrossRef]
- Rudnik, M.; Hanon, M.; Szot, W.; Beck, K.; Gogolewski, D.; Zmarzły, P.; Kozior, T. Tribological Properties of Medical Material (MED610) Used in 3D Printing PJM Technology. Teh. Vjesn. 2022, 29, 1100–1108. [Google Scholar] [CrossRef]
- Studeny, Z.; Krbata, M.; Dobrocky, D.; Eckert, M.; Ciger, R.; Kohutiar, M.; Mikus, P. Analysis of Tribological Properties of Powdered Tool Steels M390 and M398 in Contact with Al2O3. Materiały 2022, 15, 7562. [Google Scholar] [CrossRef]
- Bartosova, L.; Kohutiar, M.; Krbaťa, M.; Escherová, J.; Eckert, M.; Jus, M. The Influence of Accelerated Electron Irradiation on the Change of Tribological Behavior of Polymeric Materials PET, PTFE & PE2000C. Manuf. Technol. 2023, 23, 589–596. [Google Scholar] [CrossRef]
- Cyphert, E.L.; von Recum, H.A. Emerging Technologies for Long-Term Antimicrobial Device Coatings: Advantages and Limitations. Exp. Biol. Med. 2017, 242, 788–798. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K.H. Ion implantation of titanium based biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef]
- Marciniak, J. Biomaterials; Silesian University of Technology: Gliwice, Poland, 2002. [Google Scholar]
- Jung, H.-D. Titanium and Its Alloys for Biomedical Applications. Materials 2021, 11, 1945. [Google Scholar] [CrossRef]
- Rodrigues, M.M.; Fontoura, C.P.; Maddalozzo, A.E.D.; Leidens, L.M.; Quevedo, H.G.; dos Santos Souza, K.; da Silva Crespo, J.; Michels, A.F.; Figueroa, C.A.; Aguzzoli, C. Ti, Zr and Ta Coated UHMWPE Aiming Surface Improvement for Biomedical Purposes. Compos. Part B Eng. 2020, 189, 107909. [Google Scholar] [CrossRef]
- Yao, C.; Storey, D.; Webster, T.-J. Nanostructured metal coatings on polymers increase osteoblast attachment. Int. J. Nanomed. 2007, 2, 487–492. [Google Scholar] [PubMed Central]
- Breme, F.; Buttstaedt, J.; Emig, G. Coating of polymers with titanium-based layers by a novel plasma-assisted chemical vapor deposition process. Thin Solid Films 2000, 377, 755–759. [Google Scholar] [CrossRef]
- ISO 10993-15; Biological Evaluation of Medical Devices—Part 15: Identification and Quantification of Degradation Products of Metals and Alloys. International Organization for Standardization: Geneva, Switzerland, 2024.
- Stratasys. Biocompatible Clear MED610: 2018. Available online: https://support.stratasys.com/en/Materials/PolyJet/MED610 (accessed on 20 December 2025).
- EN ISO 10993-10; Biological Evaluation of Medical Devices—Part 10: Tests for Irritation and Skin Sensitization. International Organization for Standardization: Geneva, Switzerland, 2013.
- EN ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- EN ISO 10993-3; Biological Evaluation of Medical Devices—Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicit. International Organization for Standardization: Geneva, Switzerland, 2014.
- EN ISO 10993-18; Biological Evaluation of Medical Devices—Part 18: Chemical Characterization of Materials. International Organization for Standardization: Geneva, Switzerland, 2009.
- Stratasys MSDS Clear Bio-Compatible MED610. Available online: https://www.sys-uk.com/wp-content/uploads/2016/01/MSDS-Clear-Bio-Compatible-MED610-English-US-1.pdf (accessed on 20 December 2025).
- Jeon, D.-M.; An, J.-S.; Lim, B.-S.; Ahn, S.-J. Orthodontic Bonding Procedures Significantly Influence Biofilm Composition. Prog. Orthod. 2020, 21, 14. [Google Scholar] [CrossRef]
- Ahn, H.-B.; Ahn, S.-J.; Lee, S.-J.; Kim, T.-W.; Nahm, D.-S. Analysis of Surface Roughness and Surface Free Energy Characteristics of Various Orthodontic Materials. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 668–674. [Google Scholar] [CrossRef]
- Martínez Gil-Ortega, A.; Paz-Cortés, M.M.; Viñas, M.J.; Cintora-López, P.; Martín-Vacas, A.; Gil, J.; Aragoneses, J.M. Effect of Surface Wettability and Energy on Bacterial Adhesion to Dental Aligners: A Comparative In Vitro Study. Bioengineering 2025, 12, 898. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Kaizer, M.R.; Azevedo, M.S.; Ogliari, F.A.; Cenci, M.S.; Moraes, R.R. (Super)Hydrophobic Coating of Orthodontic Dental Devices and Reduction of Early Oral Biofilm Retention. Biomed. Mater. 2015, 10, 065004. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A Review on the Wettability of Dental Implant Surfaces I: Theoretical and Experimental Aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef]
- Valli, J. A review of adhesion test methods for thin hard coatings. J. Vac. Sci. Technol. A Vac. Surf. Film. 1986, 4, 3007–3014. [Google Scholar] [CrossRef]
- Grzesik, W. Effect of the machine parts surface topography features on the machine service. Mechanik 2015, 88, 587–593. [Google Scholar] [CrossRef]
- Al-Samarai, R.A.; Haftirman, K.R.A.; Al-Douri, Y. The Influence of Roughness on the Wear and Fric-Tion Coefficient under Dry and Lubricated Sliding. Int. J. Sci. Eng. Res. 2012, 3, 1–6. [Google Scholar]
- Bayer, R.G.; Sirico, J.L. The Influence of Surface Roughness on Wear. Wear 1975, 35, 251–260. [Google Scholar] [CrossRef]
- Barrett, T.S.; Stachowiak, G.W.; Batchelor, A.W. Effect of Roughness and Sliding Speed on the Wear and Friction of Ultra-High Molecular Weight Polyethylene. Wear 1992, 153, 331–350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.