Abstract
This paper investigates the effect of Ti3AlC2-MAX phase addition as a solid lubricant to Al2O3. The composites were prepared using powder metallurgy and consolidated by Spark Plasma Sintering (SPS). The influence of Ti3AlC2 addition on phase composition and mechanical and tribological properties was evaluated. The use of MAX phase allows for the fabrication of sintered materials characterized by high density, a hardness close to 2000 HV5, and a fracture toughness of 5.5 MPa*m0.5 for the sample containing 10 wt.% Ti3AlC2. Moreover, a significant reduction in wear of the composites was observed for friction tests conducted at 700 °C compared to the unreinforced sample. The research demonstrates that Ti3AlC2 can serve as a solid lubricant and exhibits the best performance at high temperatures.
1. Introduction
Alumina is a widely used ceramic due to its impressive properties, including high hardness, good thermal and chemical stability, good wear resistance, and affordability. These advantages make it suitable for various applications such as electronics, power generation, and cutting tools [1,2,3,4]. A major limitation of using Al2O3 in cutting tools is its low fracture toughness. To improve this, composites based on aluminum oxide are produced. However, particular attention should be paid to the wear of these composites during friction at elevated temperature, which is extremely important in the case of these specific applications. Solid lubricants offer significant advantages over conventional lubricants, particularly in high-temperature applications (above 350 °C) and oxidizing environments. These advantages include better lubricity, good thermal stability, and good chemical stability. This highlights the need for continued exploration and development of solid lubricants for demanding operating conditions [5]. The following types of compounds are used as solid lubricants: polymer composites, soft metals, layered materials (graphite, hBN, MoS2), oxides (B2O3, TiO2, V2O5 and MoO3), and MAX phases (Ti3SiC2, Ti2AlC) [6,7,8,9]. One of the most promising due to its properties are MAX phases. They combine the properties of ceramics and metals [10]. MAX phases are a large and diverse group of crystalline materials with a layered structure. They are typically denoted by the formula Mn + 1AXn, where M represents a light transition metal, A is a group 13 or 14 element, and X is carbon or nitrogen [11]. This layered structure contributes to MAX phases having properties of both ceramics and metals. Like ceramics, MAX phases exhibit high hardness, strength, and good thermal properties. Similar to metals, they show good electrical and thermal conductivity, high fracture toughness, and are relatively easy to machine [12]. These properties make MAX phases an ideal addition to ceramic matrix. Their high hardness will not significantly reduce the hardness of composites, while the flakelike structure will improve fracture toughness through mechanisms such as crack deflection and bridging. Moreover, due to their structure and properties, MAX phases can be used as an additive to ceramics to improve tribological properties at ambient and elevated temperatures.
The basic mechanism of MAX phase lubrication is very similar to that of graphite or MoS2. Due to the weak bonds between the M-X layers, they can slide over each other under relatively low shear stresses [13,14]. However, most layered materials lose their lubricating properties at elevated temperatures (above 350 °C) due to the degradation of their structure, especially in an oxidizing atmosphere. MAX phases have a much higher thermal stability. These materials retain their structure up to 600 °C in air [15] and over 1000 °C in a vacuum or inert gas [16]. MAX phases undergo oxidation during high-temperature friction. However, this process leads to the formation of a tribe-oxide film that significantly reduces material wear [17,18]. These properties have been confirmed in numerous scientific publications. Zhai et al. [19] investigated the tribological behavior of Ti3SiC2 compared to low-carbon steel. They employed a block-on-disc test method with a sliding speed of 20 m/s and applied loads corresponding to a stress range of 0.1 to 0.8 megapascals. The analysis revealed that the tribo-films formed on the surfaces were composed of TiO2, SiO2, and Fe2O3. El-Raghy et al. [20] investigated the grain size of Ti3SiC2 ceramic on tribological properties with the use of the pin-on-disk method. They showed that regardless of the grain size, the friction coefficient does not change significantly and is approximately 0.8. Sun et al. [21] investigated the tribological behaviour of a Ti3SiC2 ceramic containing 7 wt.% TiC against steel pins using a pin-on-disk tribometer. They showed no effect of normal load on COF and an increase in wear with increasing normal load. Moreover, they observed the formation of a tribofilm containing Fe. Hongxiang et al. [22] investigated the tribological properties of Ti3AlC2 ceramic. They employed a block-on-disc method and low-carbon steel as a counter sample. They obtained a very low COF of ∼0.1 and wear rate of ∼2.5 × 10−6 mm3/Nm, which they explained by the formation of a tribofilm containing Ti, O, Al and Fe.
Despite many publications on the tribological properties of MAX phase ceramics and composites based on them, there are no reports on the use of MAX phases as an additive to a ceramic matrix to improve tribological properties. The main objective of this article is to determine the influence of Ti3AlC2-MAX phase addition to Al2O3 on the improvement of tribological properties of composites both at room temperature and elevated temperature (700 °C) in air. The mechanisms responsible for the improvement of these properties will also be determined. Both the wear track of the sample and the counter-sample were subjected to detailed microscopic observations and chemical analysis. In addition, the basic mechanical properties of the composites were determined.
2. Materials and Methods
Table 1 provides a comprehensive overview of the fundamental characteristics of the powder substrates employed in the synthesis of the Ti3AlC2-MAX phase and the consolidation of Al2O3 matrix composites reinforced with MAX phase.

Table 1.
Parameters of powder substrates used for MAX phase synthesis and composite consolidation.
To prepare the MAX phases, titanium (GoodFellow, Cambridge, UK with a chemical purity of 99.6% and an average particle size below 20 µm), aluminum (Bend-Lutz Co. Skawina, Poland with a chemical purity of 99.7%), and carbon (Sigma-Aldrich, St. Louis, MO, USA with a chemical purity of 99.9% and the average particle size below 20 µm) powders were wet blended in isopropyl alcohol (no. 1759, Stanlab, Lublin, Poland) using a ball-type mill (Fritsch Pulverisette, Fritsch, Idar-Oberstein, Germany), then dried and sieved to #300 µm [23]. A molar ratio of Ti:Al:C = 3:1:1.9 was used. The reactive synthesis process was carried out using the SPS technique (FCT Systeme GMBH, Effelder Rauenstein, Germany) to synthesize the Ti3AlC2 MAX phases. The synthesis parameters were the following: a temperature of 1300 °C, a heating rate of 250 °C/min, and a vacuum atmosphere (p = 5 × 10−2 mbar). Following synthesis, the MAX phase was ground to a size below 45 µm using an automatic mortar grinder (Retsch KM100, Retsch GmbH, Haan, Germany), with a grinding bowl speed of 70 rpm and an applied force of 5–12.5 daN. In order to ensure the repeatability of the synthesis process, each batch of Ti3AlC2 was produced using powders from the same manufacturer with the same purity and particle size. Before each synthesis process, all powders were subjected to XRD analysis to verify the actual composition against the manufacturer’s declared specifications. Furthermore, great importance was attached to controlling the parameters of all manufacturing stages to minimize differences in the purity of the produced Ti3AlC2 powders. After drying and sieving, the powder mixture was synthesized using a Spark Plasma Sintering furnace (FCT HP D 10) under the following conditions: temperature: 1300 °C, heating rate: 250 °C/min, and vacuum atmosphere (p = 5 × 10−2 mbar); the powder was cooled immediately after reaching the maximum temperature. The synthesized Ti3AlC2-MAX phase was then ground with an automatic mortar grinder (Retsch KM100). The composites were fabricated using the powder metallurgy technique and consolidated via Spark Plasma Sintering (SPS). Initially, Al2O3-xTi3AlC2 powder mixtures, with varying weight percentages of Ti3AlC2 (x = 5, 10, 15, 20 wt.%), were wet-mixed using a horizontal mill for 24 h. Propan-2-ol and alumina grinding media (Nikkato, Osaka, Japan) were used during this step. Following drying, the powders were sieved (#300 µm). The composite mixtures were then sintered using Spark Plasma Sintering. The specific process parameters employed were the following: sintering temperature: 1400 °C, heating rate: 250 °C/min, dwell time: 4 min, applied pressure: 50 MPa, and vacuum atmosphere. As a reference, pure alumina samples were sintered under identical conditions. Fracture toughness (KIC) and hardness were evaluated using a Vickers Hardness Tester (FV-700e, Future Tech, Kanagawa, Japan) under a load of 49 N. Both measurements were performed on 3 samples, with at least 10 measurements taken on each. Friction tests were performed with the use of the high-temperature ‘pin-on-disc’ method (THT, CSM, Zurich, Switzerland). The friction radius was set at 4.5mm, linear velocity at 0.1 m/s, and load force at 1N. As a counter-sample, silicon nitride balls with a diameter of 6.35 mm were used. Two measurement temperatures were selected. For 20 °C, the test distances were 500 m (about 17,660 rotations) and 1000 m (about 35,320 rotations). For a high temperature of 700 °C, only a 500 m friction path was measured. The use of low linear velocity and low load force is associated with the potential use of these materials as cutting tools for materials that are difficult to process or require high precision, as well as for materials that are easily deformed. The selection of a temperature of 700 °C was associated with the higher thermal stability of the Ti3AlC2-MAX phase. The use of a lower temperature would not have caused the decomposition of the reinforcing phase during the friction test. The remaining friction test parameters such as linear velocity, load force, and friction distances were selected based on literature data for similar materials.
After the friction tests, the friction paths were measured with the use of optical profilometry (S-neox, Sensofar Metrology, Barcelona, Spain). In the case of the optical profiler studies, they were conducted in confocal mode. The objective magnification applied during the measurements was ×20. For each sample, eight different areas of the wear track were measured. Registered data, with dimensions of 850.08 × 709.32 µm2, were treated with the use of an academic license of OriginPro Version 2020 Software (OriginLab Corporation, Northampton, MA, USA). In the cases where the depth of the friction path was smaller than the surface roughness, the individual line profiles were analyzed to obtain proper worn volume estimation.
Integrated worn volumes in the friction paths were used to calculate the volumetric coefficients of wear from the following known formula:
where: Kw—coefficient of wear [mm3·N−1 · m−1], V—volume of wear [mm3], F—load force [N], S—test distance [m]. Based on the separate results, the average wear rate values were estimated for all composites.
Kw = V*F−1*S−1
Similarly, the wear coefficients for stationery ball counter-samples were appraised. The worn balls’ volume was determined by the diameter of the worn spherical bowl at a known radius of the balls used. Diameter measurements were made using SEM. Worn surfaces of the Al2O3 matrix composites and Si3N4 balls were examined using scanning electron microscopes (Axia ChemiSEM Thermo Scientific, Waltham, MA, USA and STEM Hitachi 5500, Tokyo, Japan). The degree of coverage of the wear track by the tribofilm was determined using image analysis. At least 10 images from different areas of the sample were used for the measurement. The images were analyzed using NIS Elements software 5. Phase analysis of the synthesized powders and consolidated composites was performed using a Rigaku, Tokyo, Japan MiniFlex II X-ray diffractometer with Cu Kα radiation (λ = 0.154059 nm). Additionally, the density of the Al2O3/MAX composites was measured using a helium pycnometer (Ultrapycnometer 1000 helium pycnometer, Quantachrome Instruments, Drive Boynton Beach, FL, USA).
3. Results
Figure 1a,b presents the morphology and phase composition of MAX powders used as a reinforcing phase for Al2O3-based composites. The morphology of the Ti3AlC2-MAX powders exhibits a layered structure with distinct flakes forming the powder particle. This indicates that the Ti3AlC2-MAX phase synthesis process was successful. Additionally, these observations are confirmed by the phase composition studies. The presence of the following four phases was observed: Ti3AlC2, TiC, Ti2AlC, and graphite. However, the most desired phase, Ti3AlC2, is the dominant phase, while the others are present in negligible amounts.
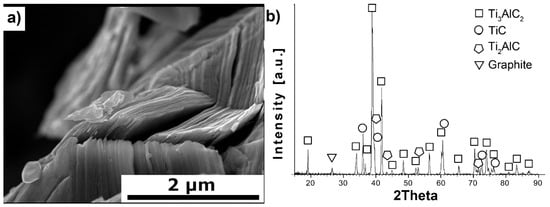
Figure 1.
Characterization of synthesized Ti3AlC2-MAX phase powders, (a) morphology and (b) XRD analysis.
The phase composition analysis was also performed for the Al2O3-based composites. The X-ray diffraction pattern for the Al2O3 composite with wt.% Ti3AlC2 is illustrated in Figure 2.
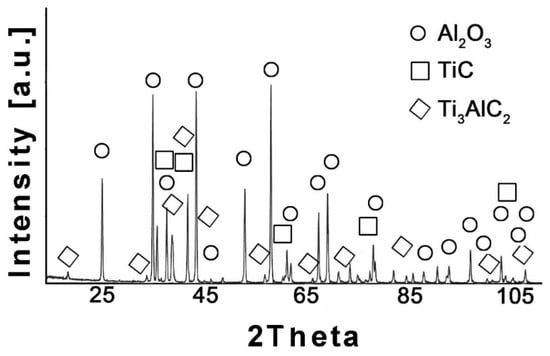
Figure 2.
XRD analysis of Al2O3 + 20 wt.% Ti3AlC2 addition showing the presence of three phases: Al2O3, TiC, and Ti3AlC2.
Figure 3 presents the results of density measurements for composites with varying Ti3AlC2-MAX phase content. An initial decrease in density is observed for the composite with the lowest Ti3AlC2-MAX phase content, followed by an increase in density with increasing Ti3AlC2 content. The maximum apparent density was achieved for composites containing 20 wt.% MAX phase. The increase in sinter density is caused by the addition of a Ti3AlC2-MAX phase with a higher density compared to Al2O3. Relative density was not determined due to the presence of TiC in the sintered structure and the associated difficulties in determining the correct theoretical density of the sintered samples. Only the theoretical density for composites containing pure Ti3AlC2 is presented. The measured values exhibit the same increasing trend as the theoretical values.
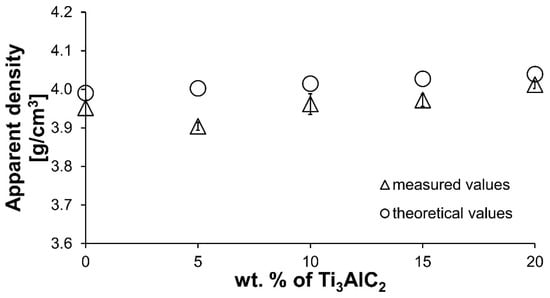
Figure 3.
The experimental apparent density of Al2O3-Ti3AlC2 composites and the associated theoretical density.
A decrease in hardness is observed for the composites in comparison to the reference sample (Figure 4). Moreover, the increase in the Ti3AlC2-MAX phase fraction does not significantly affect the hardness value. Only a slight decrease in hardness was observed with increasing Ti3AlC2 content. Much larger differences were observed in the case of fracture toughness (Figure 4). Even the smallest addition of MAX phase significantly increases KIC. However, no significant improvement in KIC is observed with increasing MAX phase fraction.
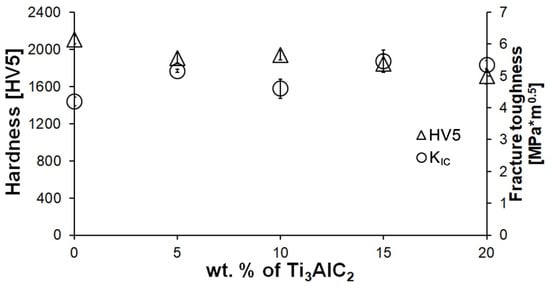
Figure 4.
Hardness (HV5) and fracture toughness (KIC) of Al2O3-Ti3AlC2 composites with varying Ti3AlC2 content.
Figure 5 shows the wear of composites as a function of MAX phase fraction for two temperatures and friction paths. In the case of samples tested at room temperature, no significant change in wear was observed with the change in the volume fraction of the reinforcing phase. Doubling the friction distance also did not significantly affect the wear value. Differences are visible in the case of the test conducted at 700 °C. There is a significant decrease in wear of Al2O3 − 5% Ti3AlC2-MAX phase compared to the reference sample. In the samples with 10 and 15% of Ti3AlC2-MAX phase after the high-temperature friction tests, the build-up formation was observed. The formation of the build-up prevented wear measurement, which explains the lack of points for the 10 and 15% of Ti3AlC2-MAX phase samples in Figure 5. The maximum volume fraction of the reinforcing phase allowed for an even lower wear value compared to the sample with 5% Ti3AlC2-MAX phase content.
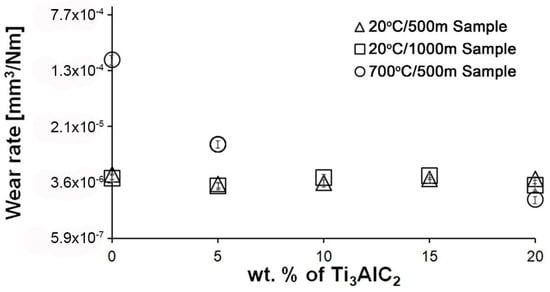
Figure 5.
Influence of Ti3AlC2-MAX phase content on wear rate of the Al2O3 matrix composites at different wear test conditions.
An almost identical wear profile was observed for the Si3N4 counterparts (Figure 6). For samples tested at room temperature, the wear value is almost identical regardless of the friction path and the MAX phase fraction in the composite. In the case of the test conducted at 700 °C, similar to the composites, the addition of Ti3AlC2-MAX phase reduces the wear of the counterpart. Lower wear values were observed for samples with 10% and 15% MAX, for which the formation of a build-up on the surface of the composites was observed. For the counterpart working with the composite containing 20% MAX, a slight increase in the wear value was observed.
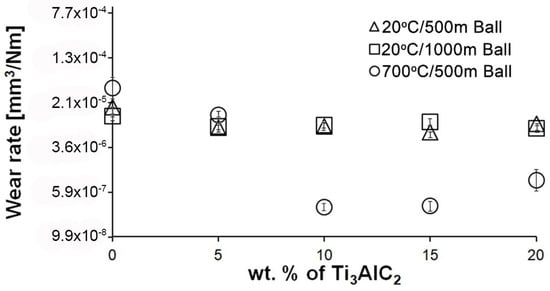
Figure 6.
Wear of Si3N4 counterparts working with composites with different Ti3AlC2 content at different wear test conditions.
Figure 7 shows the average friction coefficient values for all composites and tests conditions. Again, no significant changes were observed for the tests conducted at room temperature. Similar average friction coefficient values are observed for all samples. However, in the case of the test conducted at elevated temperature, a decrease and then an increase in the average friction coefficient value is observed for composites containing 10, 15, and 20% Ti3AlC2-MAX phase, respectively. This change is identical to that obtained for wear rate.
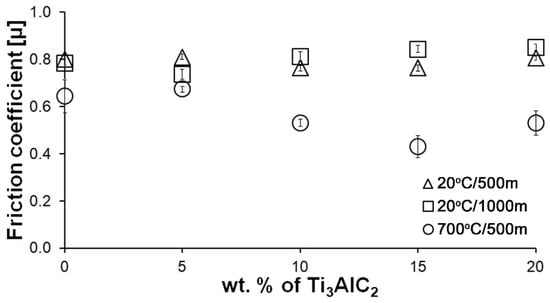
Figure 7.
Influence of Ti3AlC2 content on the friction coefficient of Al2O3-based composites.
Furthermore, the analysis of the friction coefficient changes as a function of the sliding distance reveals significant differences between the tested materials (Figure 8). The reference sample shows an increase in the friction coefficient to about 0.9, followed by a slow decrease and stabilization of its value after about 300 m. In contrast, no initial running stage is observed for the composites. The friction coefficient maintains a stable value throughout the entire test. For the samples tested at 700 °C, the friction coefficient is lower than the others and fluctuates significantly after reaching its maximum value.
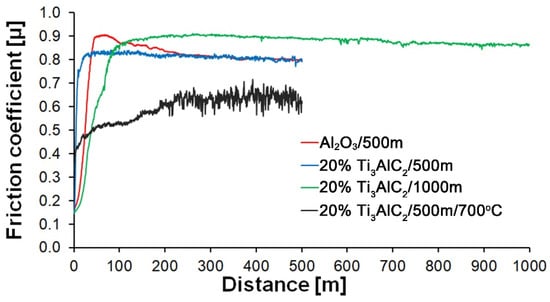
Figure 8.
Effect of sliding distance on friction coefficient of Al2O3 and Al2O3 + 20 wt.% Ti3AlC2 at different wear test conditions.
After the friction test, the surface of the samples was analysed using a scanning electron microscope. Exemplary images for composites containing 5% Ti3AlC2-MAX phase are presented in Figure 9a–f. For composites tested at room temperature (Figure 9a–d), a similar wear pattern was observed. The wear surface is relatively smooth with visible shallow grooves. Additionally, the presence of tribo-oxide films was observed. However, these areas are rather isolated and do not cover the entire wear track. The measured tribofilm coverage values for the wear surface are 5.5% and 7% for the samples Al2O3 + 5 wt.% Ti3AlC2 20 °C/500 m and Al2O3 + 5 wt.% Ti3AlC2 20 °C/1000 m, respectively. A completely different surface is observed for composites tested at elevated temperature (Figure 9e,f). For these samples, almost the entire wear surface (96%) is covered with a tribo-oxide film that cracks and spalling during friction.
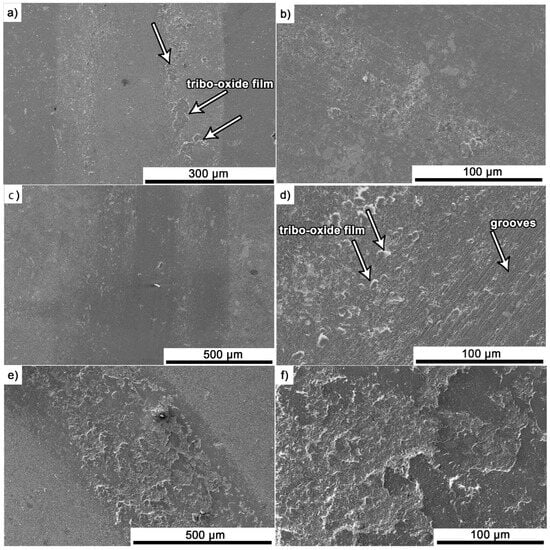
Figure 9.
Scanning electron microscopy (SEM) images of the worn surface of Al2O3 + 5 wt.% Ti3AlC2: (a,b) 20 °C/500m, (c,d) 20 °C/1000 m, (e,f) 700 °C/500 m.
Elemental analysis of the surfaces with visible build-up for the composite containing 10% MAX tested at 700 °C (Figure 10a,b) showed the presence of mainly Al and O, which confirms the formation of a tribo-oxide film. The presence of Ti originating from the MAX phase or TiC and Si from the counter sample was also observed.
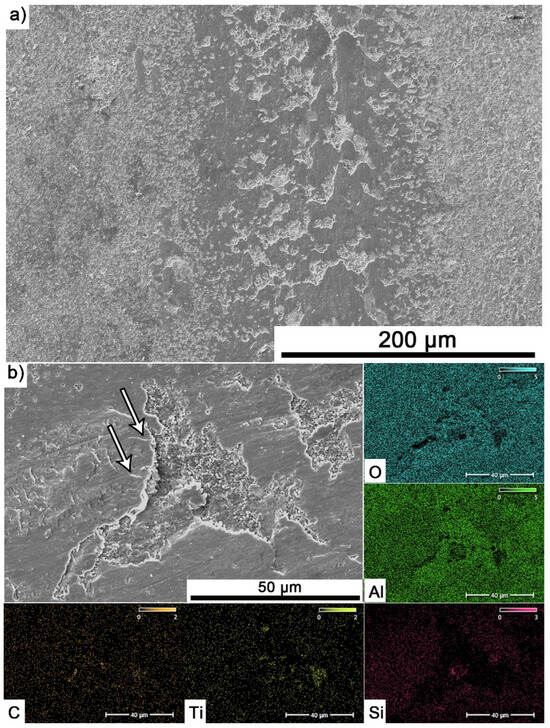
Figure 10.
SEM images with visible build-up for Al2O3 + 10 wt.% Ti3AlC2 specimen tested at 700 °C and 500 m sliding distance: (a) microstructure and (b) chemical composition.
The Si3N4 balls were also subjected to observation and EDS analysis. Figure 11a–c showed exemplary wear tracks for counter-samples that were a friction pair with reference sample and composites containing 20% Ti3AlC2-MAX phase. For Al2O3 (Figure 11a), the counter-samples exhibit uniform smooth wear with few grooves. Increasing the reinforcement content (Figure 11b) resulted in the appearance of a tribo-oxide film on the counter-sample surface, containing mainly Al2O3. In the case of the counter-sample tested at 700 °C (Figure 11c), a tribo-oxide film is also present, but its thickness is much larger and its elemental composition includes titanium, which is a product of the decomposition of Ti3AlC2-MAX phase.
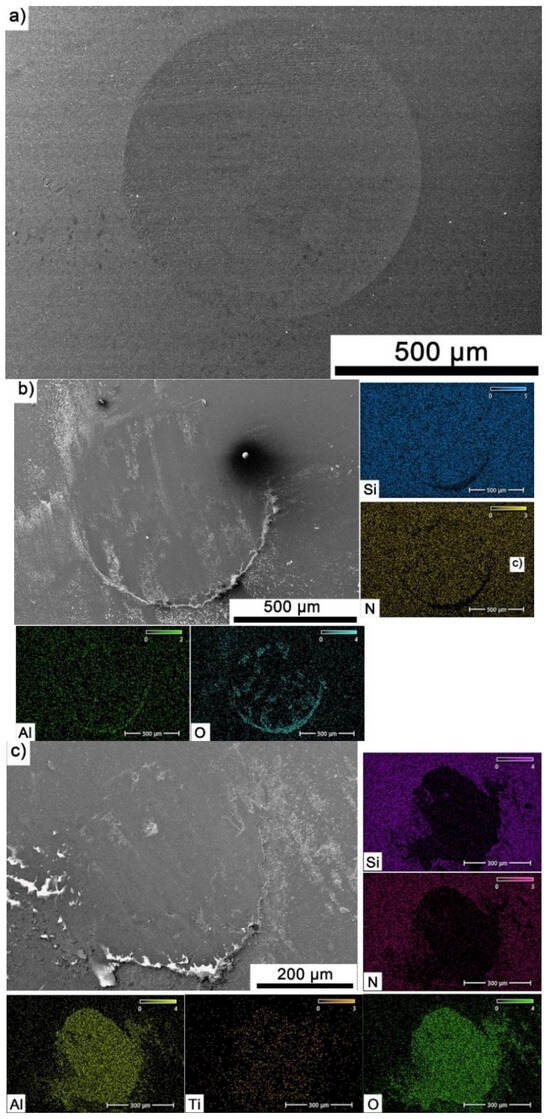
Figure 11.
Microstructure and chemical composition of the worn surface of Si3N4 balls after tribological testing, (a) Al2O3—20 °C/500 m, (b) Al2O3 + 20 wt.% Ti3AlC2—20 °C/500 m, (c) Al2O3 + 20 wt.% Ti3AlC2—700 °C/500 m.
4. Discussion
The comparison of the phase composition of the MAX phase powder, and the consolidated composite shows that the use of the SPS sintering method prevented the decomposition of the MAX phase during the consolidation process (Figure 1b and Figure 2). The XRD analysis of the composites revealed the presence of mainly Ti3AlC2 and an addition of TiC. The presence of titanium carbide was also reported by other authors [24]. It is related to the TiC residues from the MAX phase synthesis stage. Additionally, due to the lack of the Ti2AlC phase in the composites, TiC may be a product of the decomposition of this phase during the sintering process. Pang et al. [25] showed that Ti2AlC can decompose above 1400 °C in vacuum to Al and TiCx. Moreover, partial decomposition of Ti3AlC2 phases may also occur during the sintering process. All these factors cause TiC to be present in the consolidated composite. However, due to the high heating and cooling rates and the short sintering time used in the SPS method, the degradation of the strengthening phase is significantly limited [26]. The positive effect of the applied consolidation method is also visible in the density values obtained for the composites. As mentioned earlier, the relative density was not determined due to the difficulties in determining the correct theoretical density. However, based on the presented theoretical density values, calculated assuming the use of pure Ti3AlC2, it can be concluded that the addition of Ti3AlC2 does not significantly reduce the density of the obtained sinters (Figure 3). The measured values are slightly lower compared to the theoretical values, indicating low porosity and a negligible amount of the TiC phase, which has a higher density than Ti3AlC2. This is also confirmed by studies of the mechanical properties of the composites. The addition of Ti3AlC2, whose hardness is much lower (HV 380 [27]) than that of the Al2O3 matrix, did not cause a significant decrease in the hardness of the composites compared to the reference sample. The hardness value decreased by only 9%. Moreover, only a slight decrease in hardness was observed with increasing reinforcement content. Comparing the sample with the highest hardness (10% Ti3AlC2) with the sample with the lowest hardness (20% Ti3AlC2), the decrease was only 12%. As already mentioned, this decrease is related to the addition of a phase with lower hardness and the generation of pores at the matrix-reinforcing phase interface. In the case of fracture toughness, an opposite trend is observed (Figure 4). The addition of a reinforcing phase with a higher KIC value (approximately 6.4 MPa*m0.5 [28]) resulted in a 25% improvement in this parameter compared to the reference sample. This increase results from a change in the fracture mechanism of the composites. According to literature data, crack propagation in the Ti3AlC2-MAX phase is more tortuous due to its layered structure. This leads to an extended propagation path and a reduced crack tip stress [28].
As the presented research results show, the addition of Ti3AlC2 to the alumina matrix has a positive effect on the tribological properties of composites. The positive influence of this reinforcing phase is more significant for materials working at high temperatures. Similar trends of wear values are observed for the samples tested at room temperature (Figure 5). The sample containing 5% Ti3AlC2-MAX phase exhibits a slight decrease in wear, which increases with the increasing volume fraction of the reinforcing phase. Lower wear values may be caused both by changes in the mechanical properties of the sintered materials and by the formation of tribo-oxide films (Figure 9a–d). The presence of tribo-oxide films, on the one hand, improves tribological properties by reducing wear, but on the other hand, can lead to the detachment of their fragments and abrasive wear of the tribo-pair [29]. In the case of samples tested at room temperature, the tribo-oxide films in the form of a single areas were observed; worn surface analysis showed that the number of these areas is greater for samples after a longer friction period. This should translate into lower wear; however, due to the fact that the tribo-oxide film areas are single, they are more easily detached from the surface, which causes higher abrasive wear. This is confirmed by studies of the wear surface, which show a larger number of grooves for samples after 1000 m compared to 500 m (Figure 9c,d). This is also reflected in the average coefficient of friction (Figure 7). An increase in this parameter is observed with increasing volume fraction of the reinforcing phase, which is associated with the formation of tribo-oxide films and a greater contribution of abrasive wear. Analyzing the changes in the coefficient of friction as a function of the path, significant differences are visible between the composites and the reference sample (Figure 8). In the case of pure Al2O3, an increase in the friction coefficient is observed during the initial meters of the tests. This stage is attributed to the removal of roughness from the contacting surfaces. Subsequently, the friction coefficient decreases and reaches a steady-state level [14]. In the case of composites, no running-in range was observed. The coefficient of friction reaches a constant maximum value from the beginning of the friction process. This is related to the presence of Ti3AlC2 and the formation of tribo-oxide films on both the composite surface and the counter-sample surface (Figure 11a,b).
Significantly larger differences between the composites and the reference sample are visible for tests conducted at 700 °C. A very high increase in wear was observed for pure Al2O3 sintered at room temperature. The addition of MAX phase reduces wear to the level of measurements at room temperature. This is related to the formation of a tribo-oxide film that covers almost the entire wear surface (Figure 9c,f). The effect of the film formation is a significant reduction in the wear of the composites. Analysis of the chemical composition of the film (Figure 10a,b) confirms that the film consists mainly of Al2O3. Trace amounts of Si are associated with the embedding of wear products from the Si3N4 counter-sample. In addition, the presence of carbon and titanium was identified in the same places. This indicates the degradation of Ti3AlC2-MAX phase during the friction process and the formation of TiC [29]. Although Ti3AlC2 is stable at 700 °C, the low thermal conductivity of Al2O3 can lead to an increase in temperature on the surface of the samples, which in turn will lead to the degradation of the MAX phase [23]. The wear of the counter-samples also shows a significant decrease with increasing MAX phase content (Figure 6) compared to the reference sample. This is related to the formation of a thick tribo-oxide film on its surface (Figure 11c). This film is significantly thicker compared to samples tested at room temperature. Similarly to the film on the composites, it also consists mainly of Al2O3 with trace amounts of titanium from the degradation of MAX phase. The coefficient of friction also changed. Average coefficient of friction values are significantly lower than for samples tested at room temperature (Figure 7). In addition, the change in this coefficient as a function of the friction path shows a different course (Figure 8). A slower increase in its value is observed, followed by an area of significant fluctuations in its value. This is related to the processes taking place on the surface of the sample. Figure 10b shows fine cracks in the tribo-oxide film, indicating that the film cracks and crumbles during friction. This creates discontinuities in the tribo-oxide film, which are the cause of large fluctuations in the coefficient of friction. It should be noted, however, that the applied load force 1N is relatively low compared to those typically used for ceramic materials. It is not possible to definitively determine whether the same mechanisms will occur at higher loads. Further research on the friction of Al2O3-based composites reinforced with Ti3AlC2 is necessary.
A detailed analysis of the worn surfaces reveals that the dominant wear mechanisms differ significantly between room temperature and elevated temperature conditions. At 20 °C, the primary mechanism is abrasive wear, evidenced by the presence of shallow grooves and isolated tribo-oxide films that are prone to detachment. In contrast, at 700 °C, the main mechanism shifts to oxidative wear and the formation of a strong, continuous tribofilm, which covers nearly the entire surface (96%). This protective layer is responsible for the significant reduction in wear and the lower, yet fluctuating, friction coefficient observed at high temperatures. The observed fluctuations are a result of the cracking and spalling of this tribo-oxide film during the friction process.
Table 2 summarizes the obtained results for the friction coefficient and wear rate with literature data. The comparison was made for tests performed at room temperature. It is clearly visible that composites containing Ti3AlC2 exhibit significantly lower wear (by about 5 orders of magnitude) compared to multi-layered graphene (MLG), multi-layered graphene coated with Ni-P (MLG-Ni-P), or graphene oxide (GO). Smaller differences are visible for the hBN-reinforced composite. However, in this case as well, the wear value for composites reinforced with Ti3AlC2 is one order of magnitude lower. The opposite character was observed for the friction coefficient. For the composite containing Ti3AlC2, the friction coefficient is about two times higher compared to the mentioned materials. However, it should be noted that, as shown by the obtained test results, the friction coefficient decreases significantly with increasing temperature. For all the mentioned materials, the thermal stability is similar. The degradation process for friction tests conducted in air begins at a temperature of 800–900 °C. However, it should be noted that the friction test conditions for the compared materials are different, therefore comparative tests for these materials conducted under the same friction conditions are necessary.

Table 2.
Comparison of the friction coefficient and wear rate of the obtained materials with literature data (for a temperature of 25 °C).
5. Conclusions
This study successfully demonstrates that adding the Ti3AlC2-MAX phase to an Al2O3 matrix significantly improves the composite’s mechanical and tribological properties. Key findings indicate that although the reinforcement has little effect on hardness, it significantly improves fracture toughness. The most pronounced tribological benefits are observed at elevated temperatures (700 °C), where degradation of the MAX phase leads to the formation of a strong, continuous tribo-oxide layer that dramatically reduces wear and stabilizes the coefficient of friction. This tribo-oxide layer also occurs on the Si3N4 counter-sample, further reducing wear across the entire tribological pair.
This study highlights the potential of Ti3AlC2-MAX phases as effective solid lubricants in high-temperature applications. However, the study also reveals a number of limitations. The observed degradation of the MAX phase at 700 °C suggests that the stability of the composites at temperatures above this threshold requires further investigation. Furthermore, the results are limited to specific loading conditions, and future work should encompass a broader range of parameters to fully understand the material’s properties under various operating scenarios. These results provide a foundation for the development of high-performance Al2O3-based composites for applications in demanding high-temperature environments, although a more comprehensive understanding of their long-term stability and broader applicability is still needed.
Author Contributions
J.W. article concept, manuscript preparation, results analysis, SEM observations; W.P. performing tribological tests; K.B. performing hardness and fracture toughness tests; T.C. composites sintering; A.J. worn surface observations; A.O. results analysis, review, and edition; B.P. wear results analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The study was accomplished thanks to the funds allotted by the Ministry of Science and Higher Education Republic of Poland.
Data Availability Statement
The raw data and processed data required to reproduce these findings cannot be shared at this time because it is part of ongoing studies but may be available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Koli, D.K.; Agnihotri, G.; Purohit, R. A Review on Properties, Behaviour and Processing Methods for Al-Nano Al2O3 Composites. Procedia Mater. Sci. 2014, 6, 567–589. [Google Scholar] [CrossRef]
- Munro, M. Evaluated Material Properties for a Sintered Alpha-Alumina. J. Am. Ceram. Soc. 1997, 80, 1919–1928. [Google Scholar] [CrossRef]
- Sternitzke, M. Structural ceramic nanocomposites. J. Eur. Ceram. Soc. 1997, 17, 1061–1082. [Google Scholar] [CrossRef]
- Niihara, K. New Design Concept of Structural Ceramics. J. Ceram. Soc. Jpn. 1991, 99, 974–982. [Google Scholar] [CrossRef]
- Marques, A.; Suarez, M.P.; Sales, W.F.; Machado, R. Turning of Inconel 718 with whisker-reinforced ceramic tools applying vegetable-based cutting fluid mixed with solid lubricants by MQL. J. Mater. Process. Technol. 2018, 266, 530–543. [Google Scholar] [CrossRef]
- Torres, H.; Ripoll, M.R.; Prakash, B. Tribological behavior of self-lubricating materials at high temperatures. Int. Mater. Rev. 2018, 63, 309–340. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.B.; Liu, Y.F.; Luo, Y.S.; Meng, Y. Microstructure and tribological performance of Ni60-based composite coatings on Ti6Al4V alloy with different Ti3SiC2 ceramic additions by laser cladding. Ceram. Int. 2020, 46, 28996–29010. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Luster, B.; Kohli, P.; Muratore, C.; Voevodin, A.A. Progress in the development of adaptive nitride-based coatings for high temperature tribological application. Surf. Coat. Technol. 2009, 204, 962–968. [Google Scholar] [CrossRef]
- Petrus, M.; Wozniak, J.; Cygan, T.; Kostecki, M.; Cygan, S.; Jaworska, L.; Teklińska, D.; Olszyna, A. Comprehensive study on graphene-based reinforcements in Al2O3–ZrO2 and Al2O3–Ti(C,N) systems and their effect on mechanical and tribological properties. Ceram. Int. 2019, 45, 21742–21750. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Radovic, M. Elastic and mechanical properties of the MAX phases. Annu. Rev. Mater. Res. 2011, 41, 195–227. [Google Scholar] [CrossRef]
- Wozniak, J.; Jastrzębska, A.; Olszyna, A. Challenges and opportunities in tailoring MAX phases as a starting materials for MXenes development. Mater. Technol. 2022, 37, 1639–1650. [Google Scholar] [CrossRef]
- Lange, C.; Barsoum, M.W.; Schaaf, P. Towards the synthesis of MAX-phase functional coatings by pulsed laser deposition. Appl. Surf. Sci. 2007, 254, 1232–1235. [Google Scholar] [CrossRef]
- Magnus, C.; Cooper, D.; Sharp, J.; Rainforth, W.M. Microstructural evolution and wear mechanism of Ti3AlC2-Ti2AlC dual MAX phase composite consolidated by spark plasma sintering (SPS). Wear 2019, 438–439, 203013. [Google Scholar] [CrossRef]
- Wozniak, J.; Cygan, T.; Petrus, M.; Cygan, S.; Kostecki, M.; Jaworska, L.; Olszyna, A. Tribological performance of alumina matrix composites reinforced with nickel-coated graphene. Ceram. Int. 2018, 44, 9728–9732. [Google Scholar] [CrossRef]
- Prikhna, T.; Ostash, O.; Sverdun, V.; Karpets, M.; Zimych, T.; Ivasyshin, A.; Cabioc’h, T.; Chartier, P.; Dub, S.; Javorska, L.; et al. Presence of oxygen in Ti-Al-C MAX phases-based materials and their stability in oxidizing environment at elevated temperatures. Acta Phys. Pol. A 2018, 133, 789–793. [Google Scholar] [CrossRef]
- Low, I.M.; Pang, W.K.; Kennedy, S.; Smith, R.I. High-temperature thermal stability of Ti2AlN and Ti4AlN3. A comparative diffraction study. J. Eur. Ceram. Soc. 2011, 31, 159–166. [Google Scholar] [CrossRef]
- Meng, J.; Lu, J.; Wang, J.; Yang, S. Tribological behavior of TiCN-based cermets at elevated temperatures. Mater. Sci. Eng. A 2006, 418, 68–76. [Google Scholar] [CrossRef]
- Maslyuk, V.A. Tribological properties of hard alloys based on chromium carbide. Powder Metall. Met. Ceram. 2003, 42, 257–261. [Google Scholar] [CrossRef]
- Zhai, H.Z.; Huang, Z.Y.; Zhou, Y.; Zhang, Z.L.; Wang, Y.; Al, M. Oxidation layer in sliding friction surface of high-purity Ti3SiC2. J. Mater. Sci. 2004, 39, 6635–6637. [Google Scholar] [CrossRef]
- El-Raghy, T.; Blau, P.; Barsoum, M.W. Effect of grain size on friction and wear behavior of Ti3SiC2. Wear 2000, 238, 125–130. [Google Scholar] [CrossRef]
- Sun, Z.M.; Zhou, Y.C.; Li, S. Tribological behavior of Ti3SiC2-based material. J. Mater. Sci. Technol. 2002, 18, 142–145. [Google Scholar]
- Hongxiang, Z.; Zhenying, H.; Zingxing, A.; Yang, Z.; Zhilli, Z.; Shibo, L. Tribophysical properties of polycrystalline bulk Ti3AlC2. J. Am. Ceram. Soc. 2005, 88, 3270–3274. [Google Scholar] [CrossRef]
- Petrus, M.; Wozniak, J.; Cygan, T.; Pawlak, W.; Olszyna, A. Novel alumina matrix composites reinforced with MAX phases—Microstructure and mechanical properties. Materials 2022, 15, 6909. [Google Scholar] [CrossRef]
- Zheng, L.; Li, F.; Zhou, Y. Preparation, microstructure, and mechanical properties of TiB2 using Ti3AlC2 as a sintering aid. J. Am. Ceram. Soc. 2012, 95, 2028–2034. [Google Scholar] [CrossRef]
- Pang, W.K.; Low, I.M.; O’Connor, B.H.; Peterson, V.K.; Studer, A.J.; Palmquist, J.P. In situ diffraction study of thermal decomposition in Maxthal Ti2AlC. J. Alloys Compd. 2011, 509, 172–176. [Google Scholar] [CrossRef]
- Guillard, F.; Allemand, A.; Lulewicz, J.D.; Galy, J. Densification of SiC by SPS-effects of time, temperature and pressure. J. Eur. Ceram. Soc. 2007, 27, 2725–2728. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Zhai, H.; Wang, C. Preparation of High-Strength Ti3AlC2 by Spark Plasma Sintering. J. Appl. Ceram. Technol. 2015, 12, E126–E131. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, C.C.; Jiang, J.X.; Zhou, B.B. Mechanical properties of Ti3AlC2 ceramics before and after heat treatment. Rare Met. 2022, 41, 2777–2782. [Google Scholar] [CrossRef]
- Dang, W.; Ren, S.; Zhou, J.; Yu, Y.; Wang, L. The tribological properties of Ti3SiC2/Cu/Al/SiC composite at elevated temperatures. Tribol. Int. 2016, 104, 294–302. [Google Scholar] [CrossRef]
- Sun, Q.; Song, J.; Li, J.; Lin, P.; Dong, Y.; Su, Y.; Fan, H.; Hu, L.; Zhang, Y. Tribological behavior of h-BN/Al2O3 self-lubricating composites in extreme environments–Part I: Lubricating behavior and ultra-low friction coefficient mechanism in air environments from room temperature to 1200 °C. Tribol. Int. 2025, 201, 110251. [Google Scholar] [CrossRef]
- Cygan, T.; Petrus, M.; Wozniak, J.; Cygan, S.; Teklińska, D.; Kostecki, M.; Jaworska, L.; Olszyna, A. Mechanical properties and tribological performance of alumina matrix composites reinforced with graphene-family materials. Ceram. Int. 2020, 46, 7170–7177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).