Interaction Between Polycarboxylate Superplasticizer and Clay in Cement and Its Sensitivity Inhibition Mechanism: A Review
Abstract
1. Introduction
2. Polycarboxylate Superplasticizer
2.1. Main Types of PCE
- MPEG-PCE
- APEG-PCE
- HPEG-PCE
- TPEG-PCE
- EPEG-PCE
2.2. Interaction Between PCE and Clay
2.2.1. Competitive Adsorption Between Clay and Cement
2.2.2. Mechanism of Interaction Between PCE and Clay
3. Methods of Improving PCE’s Clay Sensitivity
3.1. Side Chain
3.1.1. Reduce the Length and Density of Side Chain
3.1.2. Introduce Large-Volume Groups
3.2. Main Chain
3.2.1. Introduce Anionic Groups
3.2.2. Amphoteric Polycarboxylate Superplasticizer
3.3. Change Traditional Comb Structure
3.4. Add Anti-Clay Sacrificial Agent
3.4.1. Ionic Sacrificial Agent
3.4.2. Non-Ionic Sacrificial Agent
3.4.3. Swelling Inhibitor
4. Future Prospect
5. Conclusions
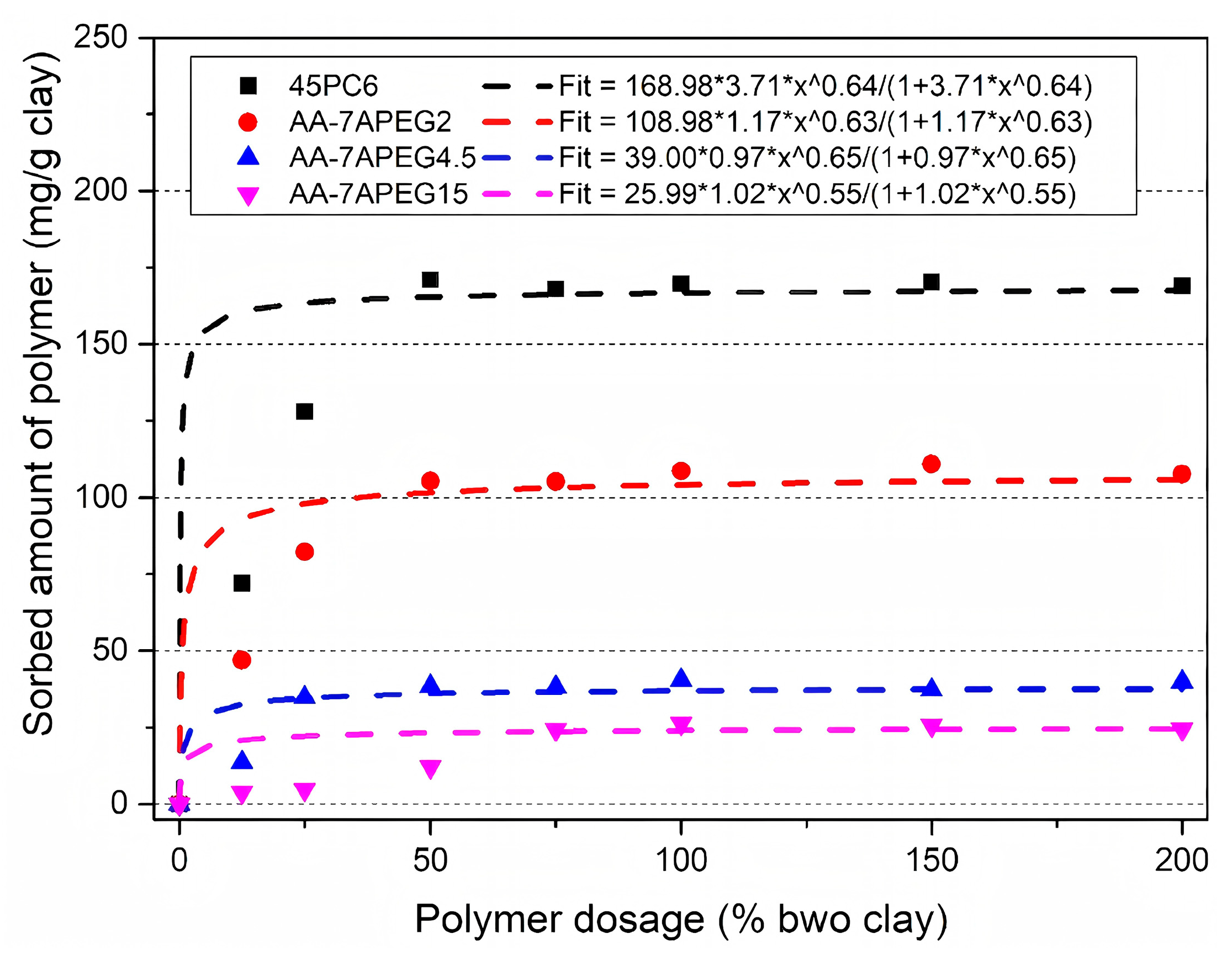
- Clay minerals exhibit competitive adsorption behavior with cement particles for PCEs through both physical adsorption and chemical intercalation mechanisms. Among common clay minerals, MMT demonstrates particularly detrimental effects on PCE performance due to its pronounced swelling characteristics and water absorption capacity, which originate from weak interlayer bonding forces and consequent interlayer expansion.
- By altering the molecular structure of PCE (adjusting the length and density of side chains, introducing bulky molecular groups, modifying the comb-shaped structure, introducing anion groups and cationic groups), the intercalation adsorption of PEO side chains can be minimized, thereby imparting clay resistance to PCE.
- Sacrificial agents primarily act by occupying the active sites on clay surfaces through ionic electrostatic adsorption or non-ionic polymer physical adsorption. Their presence prevents direct contact and reaction between clay and PCE.
- The swelling inhibitors mitigate the expansion and hydration of clay minerals by encapsulating the clay surface or intercalating into the clay interlayers. This reduces the expansion rate of clay and enhances its hydrophobicity, thereby diminishing the adverse effects of clay on PCE performance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCE | Polycarboxylate superplasticizer |
| PEO | Polyethylene oxide |
| MPEG | Methoxy polyethylene glycol |
| APEG | Allyl polyethylene glycol |
| MA | Maleic anhydride |
| AA | Acrylic acid |
| HPEG | Methyl allyl polyoxyethylene ether |
| TPEG | Isopentenyl polyoxyethylene ether |
| EPEG | Ethylene-glycol monovinyl polyethylene glycol |
| MMT | Montmorillonite |
| β-CD | β-cyclodextrin |
| AMPS | 2-acrylamyl-2-methylpropanesulfonic acid |
| S-PCE | Silane-modified polycarboxylic superplasticizer |
| PHS-PCE | Phosphonate-modified polycarboxylic superplasticizer |
| TPP | Polycarboxylic superplasticizer based on isopentenyl polyoxyethylene ether with introduced phosphate monomers |
| APC | Amphoteric polycarboxylate superplasticizer |
| DMC | Methylacryloxyethyl trimethyl ammonium chloride |
| Ct-PCE | Polycarboxylate superplasticizer with an amide cation |
| CPCE | Conventional polycarboxylate superplasticizer |
| TPCE | Polycarboxylate superplasticizer with cross-linked structure |
| HTB | Hexadecyltrimethylammonium bromide |
| AEW | Alkaline electrolyte water |
| PEG | Polyethylene glycol |
| IL | Ionic liquid |
| P(AM-DA) | Dopamine-derivative-grafted polymer |
References
- Naik, T.R. Sustainability of the Cement and Concrete Industries. In Sustainable Construction Materials and Technol Ogies; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-003-06102-1. [Google Scholar]
- Chen, C.; Xu, R.; Tong, D.; Qin, X.; Cheng, J.; Liu, J.; Zheng, B.; Yan, L.; Zhang, Q. A Striking Growth of CO2 Emissions from the Global Cement Industry Driven by New Facilities in Emerging Countries. Environ. Res. Lett. 2022, 17, 044007. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Furszyfer Del Rio, D.D.; Foley, A.M.; Bazilian, M.D.; Kim, J.; Uratani, J.M. Decarbon izing the Cement and Concrete Industry: A Systematic Review of Socio-Technical Systems, Technological Innovations, and Policy Options. Renew. Sustain. Energy Rev. 2023, 180, 113291. [Google Scholar] [CrossRef]
- Kaufmann, J. Evaluation of the Combination of Desert Sand and Calcium Sulfoaluminate Cement for the Produc tion of Concrete. Constr. Build. Mater. 2020, 243, 118281. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhu, J.; Zheng, Y.; Cui, S.; Lan, M.; Li, H. Synthesis, Characterization and Performance of a Polycarboxylate Superplasticizer with Amide Structure. Colloids Surf. Physicochem. Eng. Asp. 2014, 448, 119–129. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, L. Synthesis and Performance of a Non-Air Entraining Polycarboxylate Superplasticizer. Cem. Concr. Res. 2022, 159, 106853. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Ge, C.; Li, Q.; Guo, L.; Shu, X.; Liu, J. Mechanical Behavior and Toughening Mechanism of Polycarboxylate Superplasticizer Modified Graphene Oxide Reinforced Cement Composites. Compos. Part B Eng. 2017, 113, 308–316. [Google Scholar] [CrossRef]
- Nehdi, M.L. Clay in Cement-Based Materials: Critical Overview of State-of-the-Art. Constr. Build. Mater. 2014, 51, 372–382. [Google Scholar] [CrossRef]
- Borralleras, P.; Segura, I.; Aranda, M.A.G.; Aguado, A. Influence of the Polymer Structure of Polycarboxylate-Based Superplasticizers on the Intercalation Behaviour in Montmorillonite Clays. Constr. Build. Mater. 2019, 220, 285–296. [Google Scholar] [CrossRef]
- Borralleras, P.; Segura, I.; Aranda, M.A.G.; Aguado, A. Absorption Conformations in the Intercalation Process of Polycarboxylate Ether Based Superplasticizers into Montmorillonite Clay. Constr. Build. Mater. 2020, 236, 116657. [Google Scholar] [CrossRef]
- Tan, H.; Gu, B.; Ma, B.; Li, X.; Lin, C.; Li, X. Mechanism of Intercalation of Polycarboxylate Superplasticizer into Montmorillonite. Appl. Clay Sci. 2016, 129, 40–46. [Google Scholar] [CrossRef]
- Xu, H.; Sun, S.; Wei, J.; Yu, Q.; Shao, Q.; Lin, C. β-Cyclodextrin as Pendant Groups of a Polycarboxylate Superplasticizer for Enhancing Clay Tolerance. Ind. Eng. Chem. Res. 2015, 54, 9081–9088. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Wu, K.; Lu, M. Synthesis of Amphiphilic Polycarboxylate Copolymer and Its Notable Dispersion and Adsorption Characteristics onto Cement and Clay. Adv. Cem. Res. 2016, 28, 344–353. [Google Scholar] [CrossRef]
- Akhlaghi, O.; Aytas, T.; Tatli, B.; Sezer, D.; Hodaei, A.; Favier, A.; Scrivener, K.; Menceloglu, Y.Z.; Akbulut, O. Modified Poly(Carboxylate Ether)-Based Superplasticizer for Enhanced Flowability of Calcined Clay-Limestone-Gypsum Blended Portland Cement. Cem. Concr. Res. 2017, 101, 114–122. [Google Scholar] [CrossRef]
- Tan, H.; Guo, Y.; Ma, B.; Huang, J.; Gu, B.; Zou, F. Effect of Sodium Tripolyphosphate on Clay Tolerance of Poly carboxylate Superplasticizer. KSCE J. Civ. Eng. 2018, 22, 2934–2941. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Wang, S.; Wang, C.; Du, Q.; Yan, Y.; Yang, X.; Lv, S.; Hu, H.; Jin, Y.; et al. Maltitol-Derived Sacrificial Agent for Enhancing the Compatibility between PCE and Cement Paste. Materials 2023, 16, 7515. [Google Scholar] [CrossRef]
- Lei, L.; Palacios, M.; Plank, J.; Jeknavorian, A.A. Interaction between Polycarboxylate Superplasticizers and Non-Calcined Clays and Calcined Clays: A Review. Cem. Concr. Res. 2022, 154, 106717. [Google Scholar] [CrossRef]
- Lei, L.; Hirata, T.; Plank, J. 40 Years of PCE Superplasticizers—History, Current State-of-the-Art and an Outlook. Cem. Concr. Res. 2022, 157, 106826. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.-K.; Han, Z.; Lei, L. Why Do Conventional MAA-MPEG PCEs Not Work in Alkali-Activated Slag Systems? Cem. Concr. Res. 2024, 184, 107599. [Google Scholar] [CrossRef]
- Schmid, M.; Plank, J. Dispersing Performance of Different Kinds of Polycarboxylate (PCE) Superplasticizers in Cement Blended with a Calcined Clay. Constr. Build. Mater. 2020, 258, 119576. [Google Scholar] [CrossRef]
- Werani, M.; Lei, L. Influence of Side Chain Length of MPEG—Based Polycarboxylate Superplasticizers on Their Resistance towards Intercalation into Clay Structures. Constr. Build. Mater. 2021, 281, 122621. [Google Scholar] [CrossRef]
- Kamilov, K.K.; Abdazov, D.R. Superplasticizers for Mortars and Concrete. Cent. Asian J. Math. Theory Comput. 2023, 6, 24–27. [Google Scholar]
- Lange, A.; Hirata, T.; Plank, J. Influence of the HLB Value of Polycarboxylate Superplasticizers on the Flow Behavior of Mortar and Concrete. Cem. Concr. Res. 2014, 60, 45–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, L.; Plank, J.; Chen, L. Boosting the Performance of Low-Carbon Alkali Activated Slag with APEG PCEs: A Comparison with Ordinary Portland Cement. J. Sustain. Cem.-Based Mater. 2023, 12, 1347–1359. [Google Scholar] [CrossRef]
- Li, R.; Eisenreich, W.; Lei, L.; Plank, J. Low Carbon Alkali-Activated Slag Binder and Its Interaction with Polycarboxylate Superplasticizer: Importance of Microstructural Design of the PCEs. ACS Sustain. Chem. Eng. 2022, 10, 17241–17251. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Y.; Li, R. Specific Molecular Design of Polycarboxylate Polymers Exhibiting Optimal Compatibility with Clay Contaminants in Concrete. Cem. Concr. Res. 2021, 147, 106504. [Google Scholar] [CrossRef]
- Narayana Rao, U.V.; Kumar, N.V.S.; Kavitha, C.; Madhavi, Y.; Chowdary, P.S. Polycarboxylate Superplasticizers Used in Concrete: A Review. Int. J. Exp. Res. Rev. 2024, 38, 69–88. [Google Scholar] [CrossRef]
- Lei, L.; Chan, H.-K. Investigation into the Molecular Design and Plasticizing Effectiveness of HPEG-Based Polycarboxylate Superplasticizers in Alkali-Activated Slag. Cem. Concr. Res. 2020, 136, 106150. [Google Scholar] [CrossRef]
- Lin, Z. Synthesis and Performance Study of a Highly Dispersed HPEG-Type Polycarboxylate Superplasticizer. J. Phys. Conf. Ser. 2022, 2194, 012028. [Google Scholar] [CrossRef]
- Chen, M.; Tian, W. Preparation and Shrinkage Resistance Study of Low Activity and High Reactivity Ester-Type Monomers. J. Petrochem. Univ. 2024, 37, 58. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Fu, X.; Xie, X.; Wang, T.; Rong, Z.; Nong, Y.; Lu, Z. Enhanced Adsorption and Properties of TPEG-Type Superplasticizers Modified by Maleic Anhydride: A Perspective from Molecular Architecture and Conformation. Mater. Struct. 2024, 57, 4. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Xie, Y.; Liu, Y.; Tao, J.; Liu, R.; Li, Z.; Liu, F.; Li, M. The Effect of Alkyl Acrylate Ester Side Chain Length of Polycarboxylate Superplasticizer on the Flow Behaviour of Concrete. Constr. Build. Mater. 2024, 443, 137691. [Google Scholar] [CrossRef]
- Jiang, Z.; You, R.; Fang, Y.; Guo, Y.; Lin, T. Effect of Reaction Time on Properties of TPEG Type Polycarboxylate Superplasticizer. J. Phys. Conf. Ser. 2022, 2194, 012017. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Y.; Zhong, L. Development and Properties Research of Different Structural EPEG-Type Polycarboxylate Superplasticizers in UHPC. J. Phys. Conf. Ser. 2024, 2827, 012006. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Ren, X.; Yang, H.; Lin, S. Synthesis of Polycarboxylic Ether Superplasticizers Based on the High Conversion of EPEG in a Transition Metal Oxide Heterogeneous Catalytic System. Colloids Surf. Physicochem. Eng. Asp. 2022, 643, 128780. [Google Scholar] [CrossRef]
- Chen, H. Study on Preparation of Slow-Release Polycarboxylate Superplasticizer by Macromonomer EPEG at Room Temperature. J. Phys. Conf. Ser. 2023, 2539, 012041. [Google Scholar] [CrossRef]
- Lin, Z. Study on the Properties of Slump-Retaining Polycarboxylate Superplasticizer Synthesized by EPEG at Different pH. J. Phys. Conf. Ser. 2024, 2783, 012039. [Google Scholar] [CrossRef]
- Ma, Y.; Sha, S.; Zhou, B.; Lei, F.; Liu, Y.; Xiao, Y.; Shi, C. Adsorption and Dispersion Capability of Polycarboxylate-Based Superplasticizers: A Review. J. Sustain. Cem.-Based Mater. 2022, 11, 319–344. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, Y.; Li, Z.; Zhou, G.; Yu, X.; Xu, D.; Yong, Q.; Zhao, H.; Xie, Z. Interaction Mechanisms between Polycarboxylate Superplasticizers and Cement, and the Influence of Functional Groups on Superplasticizer Performance: A Review. Polym. Bull. 2024, 81, 10415–10438. [Google Scholar] [CrossRef]
- Lin, X.; Pang, H.; Wei, D.; Lu, M.; Liao, B. Effect of the Cross-Linker Structure of Cross-Linked Polycarboxylate Superplasticizers on the Behavior of Cementitious Mixtures. Colloids Surf. Physicochem. Eng. Asp. 2021, 608, 125437. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, L.; Li, J.; Lin, C. What Is the Mechanism of the Fiber Effect on the Rheological Behavior of Cement Paste with Polycarboxylate Superplasticizer? Constr. Build. Mater. 2021, 281, 122542. [Google Scholar] [CrossRef]
- Aïtcin, P.-C. Cements of Yesterday and Today: Concrete of Tomorrow. Cem. Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Interaction of Cement Model Systems with Superplasticizers Investigated by Atomic Force Microscopy, Zeta Potential, and Adsorption Measurements. J. Colloid Interface Sci. 2010, 347, 15–24. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Li, H.; Bai, Y. Progress in the Polycarboxylate Superplasticizer and Their Structure-Activity Relationship—A Review. Mater. Today Commun. 2023, 35, 105838. [Google Scholar] [CrossRef]
- Sha, S.; Wang, M.; Shi, C.; Xiao, Y. Influence of the Structures of Polycarboxylate Superplasticizer on Its Perfor mance in Cement-Based Materials-A Review. Constr. Build. Mater. 2020, 233, 117257. [Google Scholar] [CrossRef]
- Dong, J.; Yang, S.; Sun, D.; Liu, Z.; Xiong, Y. Preparation and Performance of Ultra-Early Strength Polycarboxylate Superplasticizer. J. Dispers. Sci. Technol. 2024, 45, 2194–2204. [Google Scholar] [CrossRef]
- Yoon, J.; Choi, B.I.; Kim, J.H. Adsorption Properties of Polycarboxylate Ether-Based Superplasticizer on Cement Particles and Their Resultant Dispersion. Front. Struct. Civ. Eng. 2022, 16, 506–514. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhang, Y.; Zheng, J.; Guo, H.; Lu, M. Study on Dispersion, Adsorption and Flow Retaining Be haviors of Cement Mortars with TPEG-Type Polyether Kind Polycarboxylate Superplasticizers. Constr. Build. Mater. 2014, 64, 324–332. [Google Scholar] [CrossRef]
- Chaparro, M.A.E.; Gnanasaravanan, S.; Rajkumar, P. Trace and Major Minerals of (Natural and Manufactured) Sand: The Importance of Manufactured Sand for Construction Purposes and the Preservation of Rivers. Innov. Infrastruct. Solut. 2021, 6, 52. [Google Scholar] [CrossRef]
- Uratani, J.M.; Griffiths, S. A Forward Looking Perspective on the Cement and Concrete Industry: Implications of Growth and Development in the Global South. Energy Res. Soc. Sci. 2023, 97, 102972. [Google Scholar] [CrossRef]
- Ng, S.; Plank, J. Interaction Mechanisms between Na Montmorillonite Clay and MPEG-Based Polycarboxylate Superplasticizers. Cem. Concr. Res. 2012, 42, 847–854. [Google Scholar] [CrossRef]
- Tan, H.; Li, X.; Liu, M.; Ma, B.; Gu, B.; Li, X. Tolerance of Clay Minerals by Cement: Effect of Side-Chain Density in Polyethylene Oxide (PEO) Superplasticizer Additives. Clays Clay Miner. 2016, 64, 732–742. [Google Scholar] [CrossRef]
- Li, Y.; Duan, C.; Meng, M.; Zhang, J.; Huang, H.; Wang, H.; Yan, M.; Tang, X.; Huang, X. Effect of Clay Minerals on Polycarboxylate Superplasticizer and Methods to Improve the Performance of Concrete Containing Clay: A Review. J. Mater. Sci. 2023, 58, 15294–15313. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Xu, Z.; Liu, Q.; Zeng, H. Effect of Polycarboxylate Ether Comb-Type Polymer on Viscosity and Interfacial Properties of Kaolinite Clay Suspensions. J. Colloid Interface Sci. 2012, 378, 222–231. [Google Scholar] [CrossRef]
- Chi, H.; Wang, C.; Tian, Y.; Xie, Z.; Yuan, Q.; Chen, Z.; Zhu, X. Unraveling Polycarboxylate Superplasticizer (PCE) Compatibility in Muscovite-Blended Cement Paste through Aggregation Mechanisms. J. Build. Eng. 2024, 95, 110133. [Google Scholar] [CrossRef]
- Schmid, M.; Plank, J. Interaction of Individual Meta Clays with Polycarboxylate (PCE) Superplasticizers in Cement Investigated via Dispersion, Zeta Potential and Sorption Measurements. Appl. Clay Sci. 2021, 207, 106092. [Google Scholar] [CrossRef]
- Xiong, L.; Zheng, G.; Bi, Y.; Fu, C. Effect of Typical Clay upon the Dispersion Performance of Polycarboxylate Superplasticizer; Atlantis Press: Dordrecht, The Netherlands, 2015; pp. 226–229. [Google Scholar]
- Aboudi Mana, S.C.; Hanafiah, M.M.; Chowdhury, A.J.K. Environmental Characteristics of Clay and Clay-Based Minerals. Geol. Ecol. Landsc. 2017, 1, 155–161. [Google Scholar] [CrossRef]
- Ewis, D.; Ba-Abbad, M.M.; Benamor, A.; El-Naas, M.H. Adsorption of Organic Water Pollutants by Clays and Clay Minerals Composites: A Comprehensive Review. Appl. Clay Sci. 2022, 229, 106686. [Google Scholar] [CrossRef]
- Sposito, R.; Beuntner, N.; Thienel, K.-C. Characteristics of Components in Calcined Clays and Their Influence on the Efficiency of Superplasticizers. Cem. Concr. Compos. 2020, 110, 103594. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Chapter 2—Structure and Mineralogy of Clay Minerals. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 21–81. [Google Scholar]
- Lei, L.; Plank, J. A Study on the Impact of Different Clay Minerals on the Dispersing Force of Conventional and Modified Vinyl Ether Based Polycarboxylate Superplasticizers. Cem. Concr. Res. 2014, 60, 1–10. [Google Scholar] [CrossRef]
- Ashraf, M.; Iqbal, M.F.; Rauf, M.; Ashraf, M.U.; Ulhaq, A.; Muhammad, H.; Liu, Q. Developing a Sustainable Concrete Incorporating Bentonite Clay and Silica Fume: Mechanical and Durability Performance. J. Clean. Prod. 2022, 337, 130315. [Google Scholar] [CrossRef]
- Chen, G.; Lei, J.; Du, Y.; Du, X.; Chen, X. A Polycarboxylate as a Superplasticizer for Montmorillonite Clay in Cement: Adsorption and Tolerance Studies. Arab. J. Chem. 2018, 11, 747–755. [Google Scholar] [CrossRef]
- Sha, S.; Lei, L.; Ma, Y.; Jiao, D.; Xiao, Z.; Shi, C. A New Insight into the Mode of Action between Cement Containing Montmorillonite and Polycarboxylate Superplasticizer. J. Sustain. Cem.-Based Mater. 2023, 12, 393–402. [Google Scholar] [CrossRef]
- Li, R.; Lei, L.; Sui, T.; Plank, J. Effectiveness of PCE Superplasticizers in Calcined Clay Blended Cements. Cem. Concr. Res. 2021, 141, 106334. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Zhou, D.; Shu, X.; Ran, Q. Molecular Dynamic Simulations of Montmorillonite Contact with Polycarboxylate Superplasticizer at Solid-Liquid Interface. Mater. Today Commun. 2021, 28, 102538. [Google Scholar] [CrossRef]
- Shakeel, A.; Ali, W.; Chassagne, C.; Kirichek, A. Tuning the Rheological Properties of Kaolin Suspensions Using Biopolymers. Colloids Surf. Physicochem. Eng. Asp. 2022, 654, 130120. [Google Scholar] [CrossRef]
- Xu, H.; Sun, S.; Yu, Q.; Wei, J. Effect of β-Cyclodextrin Pendant on the Dispersion Robustness of Polycarboxylate Superplasticizer toward Kaolin. Polym. Compos. 2018, 39, 755–761. [Google Scholar] [CrossRef]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical Admixtures—Chemistry, Applications and Their Impact on Concrete Microstructure and Durability. Cem. Concr. Res. 2015, 78, 81–99. [Google Scholar] [CrossRef]
- Bulanov, P.E.; Mavliev, L.F.; Vdovin, E.A.; Yagund, E.M. The interaction between the kaolinite or bentonite clay and plasticizing surface-active agents. Mag. Civ. Eng. 2017, 7, 171–179. [Google Scholar] [CrossRef]
- Ma, Y.; Sha, S.; Zhou, B.; Lei, F.; Liu, Y.; Xiao, Y.; Shi, C. A Study on the Interactions between Polycarboxylate Ether Superplasticizer and Montmorillonite. Cem. Concr. Res. 2022, 162, 106997. [Google Scholar] [CrossRef]
- Ma, Y.; Jiao, D.; Sha, S.; Zhou, B.; Shi, C. Effect of Molecular Structure of Polycarboxylate Ether Superplasticizer on Its Tolerance to Montmorillonite. Constr. Build. Mater. 2023, 392, 131966. [Google Scholar] [CrossRef]
- Yang, H.; Li, M.; Pan, L.; Zhang, P.; Pashameah, R.A.; Abo-Dief, H.M.; Xu, S.; Lin, C.; Algadi, H.; Li, J.; et al. Absorption Behavior of Polycarboxylate Superplasticizer with Different Molecular Structures on Montmorillonite. Environ. Res. 2023, 216, 114423. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Li, M.; Pan, L.; Li, J.; Xu, N. Synthesis of Sodium Alginate-Polycarboxylate Superplasticizer and Its Tolerance Mechanism on Montmorillonite. Cem. Concr. Compos. 2022, 133, 104638. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Jin, C.; Zhao, B.; Wu, Y. Synthesis of Polycarboxylate Superplasticizer Modified by β-cyclodextrin for Possessing Clay Tolerance. J. Appl. Polym. Sci. 2022, 139, 51918. [Google Scholar] [CrossRef]
- Guo, G.; Wu, S.; Liu, G.; Zhang, F.; Wang, L. Synthesis and Performance of HPEG-AA-AMPS-HPA Polycarboxylate Superplasticizer. J. Phys. Conf. Ser. 2024, 2737, 012007. [Google Scholar] [CrossRef]
- Wang, R.; Han, K.; Li, Y.; Jin, C. A Novel Anti-Clay Silane-Modified Polycarboxylate Superplasticizer: Preparation, Performance and Mechanism. Constr. Build. Mater. 2022, 331, 127311. [Google Scholar] [CrossRef]
- Huang, H.; Song, X.; Song, X.; Wu, J.; Liu, H.; Chen, S.; Hu, J.; Wei, J.; Yu, Q. A Migrating and Reactive Polycarboxylate Superplasticizer with Coupled Functions of New/Old Concrete Interfacial Agent and Water Reducer. Cem. Concr. Res. 2023, 172, 107218. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Zhang, J.; Cai, G.-S.; Meng, F.-J.; Liu, Y.-S.; Lu, T.-W.; Wang, L.-Y. Preparation and Application of Silane-Modified Polycarboxylate Superplasticizer in Concrete Viscosity Reduction. Sci. Adv. Mater. 2024, 16, 198–208. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Yang, X.; Sun, G. Synthesis and Characterization on Anti-Clay Polycarboxylate Superplasticizer in Concrete. Case Stud. Constr. Mater. 2024, 20, e03076. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, C.; Zhang, Q.; Pei, M. Preparation and Clay Tolerance Research of a Polycarboxylate Superplasticizer Modified by Phosphonate Group. J. Polym. Res. 2024, 31, 239. [Google Scholar] [CrossRef]
- Feng, H.; Mao, Y.; Feng, Z.; Deng, Z.; Zheng, B. Study on the Impact of Na/Ca Bentonites on the Dispersion Performance of Conventional and Modified Phosphate Polycarboxylate Superplasticizers in Cement Mortar. Langmuir 2022, 38, 5803–5811. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Miao, Z.; Shang, T. Synthesis and Performance of a Comblike Amphoteric Polycarboxylate Dispersant for Coal–Water Slurry. Colloids Surf. Physicochem. Eng. Asp. 2012, 412, 101–107. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Liu, M.; Yu, Y.; Guo, J. Synthesis, Performance and Working Mechanism of a Novel Amphoteric Polycarboxylate Dispersant without Chlorine Ion. Constr. Build. Mater. 2020, 247, 118613. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, C.; Yang, Y.; Dong, F.; Lu, X. Amphoteric Polycarboxylate Superplasticizers with Enhanced Clay Tolerance: Preparation, Performance and Mechanism. Constr. Build. Mater. 2020, 252, 119052. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, X.; Chen, X.; Guo, H.; Li, X.; Pang, L.; Yang, Y.; Dong, F. Synthesis and Performance of Anticlay Polycarboxylate Superplasticizers. J. Mater. Civ. Eng. 2024, 36, 04024251. [Google Scholar] [CrossRef]
- Ren, J.; Luo, S.; Shi, S.; Tan, H.; Wang, X.; Liu, M.; Li, X. Synthesis and Optimization of a Montmorillonite-Tolerant Zwitterionic Polycarboxylate Superplasticizer via Box-Behnken Design. Clay Miner. 2021, 56, 117–125. [Google Scholar] [CrossRef]
- Sha, S.; Lei, L.; Ma, Y.; Jiao, D.; Xiao, Z.; Shi, C. Effects of Polycarboxylate Superplasticizers with Different Functional Groups on the Adsorption Behavior and Rheology of Cement Paste Containing Montmorillonite. J. Sustain. Cem.-Based Mater. 2025, 14, 695–709. [Google Scholar] [CrossRef]
- Chang, Q.; Hu, M.; Cheng, Y.; Zeng, M.; Liu, M.; Pang, J.; Xing, Y.; Guo, J. Preparation of Amphoteric Polycarboxylate Superplasticizer at Low Temperature and Its Application in Cement-Calcined Kaolin Blended System. J. Clean. Prod. 2024, 435, 140542. [Google Scholar] [CrossRef]
- Zheng, T.; Zheng, D.; Qiu, X.; Yang, D.; Fan, L.; Zheng, J. A Novel Branched Claw-Shape Lignin-Based Polycarboxylate Superplasticizer: Preparation, Performance and Mechanism. Cem. Concr. Res. 2019, 119, 89–101. [Google Scholar] [CrossRef]
- Zhou, T.; Duan, H.; Li, Z.; Jin, Y.; Liu, H.; Pang, Y.; Lou, H.; Yang, D.; Qiu, X. Reconfiguring Molecular Conformation from Comb-Type to Y-Type for Improving Dispersion Performance of Polycarboxylate Superplasticizers. Macromolecules 2024, 57, 727–738. [Google Scholar] [CrossRef]
- Ma, Y.; Lei, L.; Sha, S.; Liu, Y.; Shi, C. Synthesis of a Cross-Linked Polycarboxylate Ether Superplasticizer and Its Effects on the Properties of Cement Paste Containing Montmorillonite. Cem. Concr. Res. 2023, 168, 107136. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Lai, G.; Wang, Z.; Zhu, J.; Cui, S.; Lan, M.; Li, H. Performances and Working Mechanism of a Novel Polycarboxylate Superplasticizer Synthesized through Changing Molecular Topological Structure. J. Colloid Interface Sci. 2017, 504, 12–24. [Google Scholar] [CrossRef]
- Lai, G.; Liu, X.; Song, X.; Guan, J.; Wang, Z.; Cui, S.; Qian, S.; Luo, Q.; Xie, H.; Xia, C. A Mechanistic Study on the Effectiveness of Star-like and Comb-like Polycarboxylate Superplasticizers in Cement Pastes. Cem. Concr. Res. 2024, 175, 107389. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Ma, X.; Zheng, Y.; Lu, L.; Bai, X. A Novel Method for Solving the Impact of Clay on Concrete Workability: Dimensional Design and Mechanism Analysis. Clay Miner. 2020, 55, 53–62. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.; Zhu, H. Synthesis of Novel Polyelectrolyte Sacrifice Agents and Their Effects on the Adaptability of Polycarboxylate Superplasticizer with Clay-Containing Cement Paste. Polym. Bull. 2024, 81, 8029–8043. [Google Scholar] [CrossRef]
- Gómez, A.; Tobón, J.I.; Orozco, C. Effect of the Molecular Weight of Sacrificial Agents on Superplasticizer Interaction with Clays on Cement-Based Materials. Constr. Build. Mater. 2023, 375, 130992. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Su, D.; Liu, C.; Yue, G.; Guo, Y.; Li, Q.; Wang, M.; Wang, L. The Adaptability of Polycarboxylate Superplasticizer in Alkaline Electrolyzed Water (AEW)-Based Cement Composites Containing Clay: Workability and Mechanical Properties. Constr. Build. Mater. 2024, 444, 137872. [Google Scholar] [CrossRef]
- Li, X.-K.; Zheng, D.-F.; Zheng, T.; Lin, X.-L.; Lou, H.; Qiu, X. Enhancement Clay Tolerance of PCE by Lignin-Based Polyoxyethylene Ether in Montmorillonite-Contained Paste. J. Ind. Eng. Chem. 2017, 49, 168–175. [Google Scholar] [CrossRef]
- He, Z.; Huang, T.; Gao, M.; Wang, E.; Kong, D.; Li, M. DFT Study on the Polyol Sacrificial Agents for Improved Clay Tolerance of Polycarboxylate Superplasticizers. Physicochem. Probl. Miner. Process. 2024, 60, 184130. [Google Scholar] [CrossRef]
- He, D.; Liang, R.; Zhao, J.; Liu, Z.; Lu, Z.; Sun, G. Effect of Ionic Liquids in Compatibility with PCE and Cement Paste Containing Clay. Constr. Build. Mater. 2020, 264, 120265. [Google Scholar] [CrossRef]
- Guan, J.; Jiang, G.; Guo, C.; He, Y.; Li, M. Inhibition of the Polymer Grafted with Dopamine Derivatives on Hydration and Swelling of Clay. J. Mol. Liq. 2024, 402, 124690. [Google Scholar] [CrossRef]
- Khandelwal, S.; Rhee, K.Y. Effect of Silane Modified Smectite Clay on the Hydration, Intercalation of PCE Superplasticizers, and Mechanical Strength of Cement Composites. Cem. Concr. Compos. 2021, 123, 104210. [Google Scholar] [CrossRef]
- He, D.; Lu, Z.; Liang, X.; Liu, R.; Sun, G. A Study to Improve the Compatibility of PCE with Cement Paste Containing Clay. Mater. Lett. 2022, 308, 131111. [Google Scholar] [CrossRef]
- Li, B.; Gao, R.; Wang, L. Synthesis and Properties of a Starch-Based Clay Tolerance Sacrificial Agent. Starch-Stärke 2021, 73, 2000223. [Google Scholar] [CrossRef]
- Ahmed Khan, R.; Murtaza, M.; Abdulraheem, A.; Kamal, M.S.; Mahmoud, M. Imidazolium-Based Ionic Liquids as Clay Swelling Inhibitors: Mechanism, Performance Evaluation, and Effect of Different Anions. ACS Omega 2020, 5, 26682–26696. [Google Scholar] [CrossRef]
- Balaban, R.D.C.; Vidal, E.L.F.; Borges, M.R. Design of Experiments to Evaluate Clay Swelling Inhibition by Different Combinations of Organic Compounds and Inorganic Salts for Application in Water Base Drilling Fluids. Appl. Clay Sci. 2015, 105–106, 124–130. [Google Scholar] [CrossRef]
















| Category | Chemical Structure | Refs |
|---|---|---|
| MPEG-PCE |  | [21] |
| APEG-PCE | 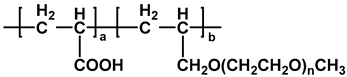 | [26] |
| HPEG-PCE |  | [28] |
| TPEG-PCE | 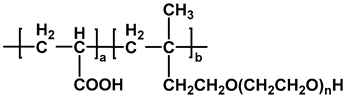 | [32] |
| EPEG-PCE | 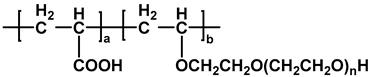 | [35] |
| Clay Minerals | Structure (SiO4:AlO6) | Interlayer Force | Mechanism | Refs |
|---|---|---|---|---|
| Kaolinite | 1:1 | Electrostatic force | Surface adsorption | [58,60] |
| Muscovite and illite | 2:1 | Electrostatic force | Electrostatic adsorption | [61,62] |
| Montmorillonite | van der Waals force | Intercalation adsorption | [51,63] |
| Molecular Structure | Factor | Functional Group/Structure | Mechanism | Refs | |
|---|---|---|---|---|---|
| Side chain | Length | Ethoxy unit | Hydrogen bonding | Shortening the PCE side chains reduces hydrogen-bond formation with clay interlayers. | [21,74] |
| Density | Carboxyl group | Charge density | A higher acid-to-ether ratio decreases side-chain density while increasing main-chain charge density, thereby enhancing surface adsorption. | [26] | |
| Bulky groups | Sodium alginate β-cyclodextrin | Steric hindrance | Bulky groups in side-chain enhance PCE steric hindrance, effectively preventing intercalation into clay interlayers. | [69,75,76] | |
| Main chain | Anionic group | Sulfonic acid group | Electrostatic attraction | Anion groups can act as anchor points and strengthen the adsorption on the clay surface through electrostatic attraction, which reduces intercalation. | [78,79,81,83] |
| Phosphate groups | |||||
| Cationic group | Quaternary ammonium | Cation exchange | Quaternary ammonium cations undergo ion exchange with clay interlayers, thereby weakening PEO side-chain interactions with clay. | [84,86] | |
| Amido | Electrostatic adsorption | Amino cations electrostatically adsorb onto clay surfaces, inhibiting PEO side-chain intercalation. | [88] | ||
| Topological structure | Cross-linking agent | Cross-linked structure | Steric hindrance | The multi-arm architecture enhances steric hindrance effect, minimizing PCE molecular depletion. | [93,94,95] |
| Star-shaped structure | |||||
| Sacrificial Agent | Chemical Substance | Mechanism | Refs | |
|---|---|---|---|---|
| Ionic sacrificial agent | Dimethylamine | Surface adsorption | Cationic groups adsorb electrostatically onto clay surfaces, while the terminal hydroxyl groups can further form hydrogen bonds with the clay. | [97] |
| Hexadecyltrimethylammonium bromide (HTB) | Synergistic effect | HTB’s extended alkyl chain synergizes with PCE, significantly boosting its clay surface adsorption capacity. | [98] | |
| Alkaline electrolyte water | Cation exchange | K+ engages in cation exchange, while OH− adsorbs onto clay particles to form an electronegative layer through surface interactions. | [99] | |
| Non-ionic sacrificial agent | Lignin-based polyoxyethylene | Intercalation adsorption | The PEO side chain intercalates into the clay interlayer and occupies the active site. | [100] |
| Small-molecule polyols | Adsorption energy | The adsorption energy between polyol and MMT exhibits an inverse correlation with the number of hydroxyl groups. | [101] | |
| Swelling inhibitor | Ionic liquid | Surface adsorption | The cations in the ionic liquid can be adsorbed on the clay surface to inhibit the clay expansion. | [102] |
| Dopamine derivatives | Stabilized clay interlayer | The protonated amine groups reduced the electrostatic repulsion between crystal layers. | [103] | |
| Silane coupling agent | Cation exchange | The silane modification improved the hydrophobicity of MMT and inhibited its expansion. | [104] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Liu, Y.; Wang, G.; Liu, J.; Cao, Z.; Yong, Q.; Zhao, H. Interaction Between Polycarboxylate Superplasticizer and Clay in Cement and Its Sensitivity Inhibition Mechanism: A Review. Materials 2025, 18, 2662. https://doi.org/10.3390/ma18112662
Gao Y, Liu Y, Wang G, Liu J, Cao Z, Yong Q, Zhao H. Interaction Between Polycarboxylate Superplasticizer and Clay in Cement and Its Sensitivity Inhibition Mechanism: A Review. Materials. 2025; 18(11):2662. https://doi.org/10.3390/ma18112662
Chicago/Turabian StyleGao, Yu, Yingying Liu, Guanqi Wang, Jiale Liu, Zijian Cao, Qiwen Yong, and Hongwei Zhao. 2025. "Interaction Between Polycarboxylate Superplasticizer and Clay in Cement and Its Sensitivity Inhibition Mechanism: A Review" Materials 18, no. 11: 2662. https://doi.org/10.3390/ma18112662
APA StyleGao, Y., Liu, Y., Wang, G., Liu, J., Cao, Z., Yong, Q., & Zhao, H. (2025). Interaction Between Polycarboxylate Superplasticizer and Clay in Cement and Its Sensitivity Inhibition Mechanism: A Review. Materials, 18(11), 2662. https://doi.org/10.3390/ma18112662







