Effect of Synthesis and Processing Conditions on the Sintering Behavior and Total Conductivity of High-Entropy Fluorite/Bixbyite Oxides (RE-HEOs)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Salian, A.; Mandal, S. Entropy stabilized multicomponent oxides with diverse functionality—A review. Crit. Rev. Solid State Mater. Sci. 2022, 47, 142–193. [Google Scholar] [CrossRef]
- Dippo, O.F.; Vecchio, K.S. A universal configurational entropy metric for high-entropy materials. Scr. Mater. 2021, 201, 113974. [Google Scholar] [CrossRef]
- McCormack, S.J.; Navrotsky, A. Thermodynamics of high entropy oxides. Acta Mater. 2021, 202, 1–21. [Google Scholar] [CrossRef]
- Kumar, A.; Bérardan, D.; Brisset, F.; Dragoe, D.; Dragoe, N. Novel entropy-stabilized fluorite oxides with multifunctional properties. J. Mater. Chem. A 2023, 11, 14320–14332. [Google Scholar] [CrossRef]
- Braun, J.L.; Rost, C.M.; Lim, M.; Giri, A.; Olson, D.H.; Kotsonis, G.N.; Stan, G.; Brenner, D.W.; Maria, J.P.; Hopkins, P.E. Charge-induced disorder controls the thermal conductivity of entropy-stabilized oxides. Adv. Mater. 2018, 30, 1805004. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-entropy oxides: Fundamental aspects and electrochemical properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef]
- Musicó, B.L.; Gilbert, D.; Ward, T.Z.; Page, K.; George, E.; Yan, J.; Mandrus, D.; Keppens, V. The emergent field of high entropy oxides: Design, prospects, challenges, and opportunities for tailoring material properties. APL Mater. 2020, 8, 040912. [Google Scholar] [CrossRef]
- Vakilifard, H.; Shahbazi, H.; Liberati, A.C.; Saraswathy, R.B.N.; Lima, R.S.; Pugh, M.D.; Moreau, C. High Entropy Oxides as Promising Materials for Thermal Barrier Topcoats: A Review. J. Therm. Spray Technol. 2024, 33, 447–470. [Google Scholar] [CrossRef]
- Banerjee, R.; Chatterjee, S.; Ranjan, M.; Bhattacharya, T.; Mukherjee, S.; Jana, S.S.; Dwivedi, A.; Maiti, T. High-entropy perovskites: An emergent class of oxide thermoelectrics with ultralow thermal conductivity. ACS Sustain. Chem. Eng. 2020, 8, 17022–17032. [Google Scholar] [CrossRef]
- Xu, L.; Su, L.; Wang, H.; Gao, H.; Lu, D.; Peng, K.; Niu, M.; Cai, Z. Tuning stoichiometry of high-entropy oxides for tailorable thermal expansion coefficients and low thermal conductivity. J. Am. Ceram. Soc. 2022, 105, 1548–1557. [Google Scholar] [CrossRef]
- Luo, X.; Huang, S.; Huang, R.; Xu, C.; Hou, S.; Jin, H. Highly anti-sintering and toughened pyrochlore (Dy0.2Nd0.2Sm0.2Eu0.2Yb0.2)2Zr2O7 high-entropy ceramic for advanced thermal barrier coatings. Ceram. Int. 2023, 49, 23410–23416. [Google Scholar] [CrossRef]
- Anand, G.; Wynn, A.P.; Handley, C.M.; Freeman, C.L. Phase stability and distortion in high-entropy oxides. Acta Mater. 2018, 146, 119–125. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Sun, M.; Zhang, Y. Physicochemical Properties of High-Entropy Oxides. Chem. Rec. 2023, 23, e202200195. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, F.; Zhao, X. Multicomponent high-entropy Zr-Y-Yb-Ta-Nb-O oxides for next-generation thermal barrier coating applications. J. Am. Ceram. Soc. 2022, 105, 35–43. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Q.; Shao, G.; Zhao, X.; Wang, Y. Multicomponent high-entropy zirconates with comprehensive properties for advanced thermal barrier coating. Scr. Mater. 2020, 178, 382–386. [Google Scholar] [CrossRef]
- Cong, L.; Li, W.; Wang, J.; Gu, S.; Zhang, S. High-entropy (Y0.2Gd0.2Dy0.2Er0.2Yb0.2)2Hf2O7 ceramic: A promising thermal barrier coating material. J. Mater. Sci. Technol. 2022, 101, 199–204. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Sang, W.; Chen, X.; Tang, A.; Zhang, H. Thermophysical performances of (La1/6Nd1/6Yb1/6Y1/6Sm1/6Lu1/6)2Ce2O7 high-entropy ceramics for thermal barrier coating applications. Ceram. Int. 2022, 48, 1512–1521. [Google Scholar] [CrossRef]
- Chen, L.B. Yttria-stabilized zirconia thermal barrier coatings—A review. Surf. Rev. Lett. 2006, 13, 535–544. [Google Scholar] [CrossRef]
- Kang, Y.X.; Bai, Y.; Fan, W.; Yuan, T.; Gao, Y.; Bao, C.G.; Li, B.Q. Thermal cycling performance of La2Ce2O7/50 vol.% YSZ composite thermal barrier coating with CMAS corrosion. J. Eur. Ceram. Soc. 2018, 38, 2851–2862. [Google Scholar] [CrossRef]
- Kumar, A.; Moledina, J.; Liu, Y.; Chen, K.; Patnaik, P.C. Nano-micro-structured 6%–8% YSZ thermal barrier coatings: A comprehensive review of comparative performance analysis. Coatings 2021, 11, 1474. [Google Scholar] [CrossRef]
- Dahl, P.; Kaus, I.; Zhao, Z.; Johnsson, M.; Nygren, M.; Wiik, K.; Grande, T.; Einarsrud, M.-A. Densification and properties of zirconia prepared by three different sintering techniques. Ceram. Int. 2007, 33, 1603–1610. [Google Scholar] [CrossRef]
- Li, C.J.; Ning, X.J.; Li, C.X. Effect of densification processes on the properties of plasma-sprayed YSZ electrolyte coatings for solid oxide fuel cells. Surf. Coat. Technol. 2005, 190, 60–64. [Google Scholar] [CrossRef]
- Pakseresht, A.; Sharifianjazi, F.; Esmaeilkhanian, A.; Bazli, L.; Nafchi, M.R.; Bazli, M.; Kirubaharan, K. Failure mechanisms and structure tailoring of YSZ and new candidates for thermal barrier coatings: A systematic review. Mater. Des. 2022, 222, 111044. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Q.; Cao, Y.; Shao, G.; Wang, Y. Multicomponent rare-earth cerate and zirconocerate ceramics for thermal barrier coating materials. J. Eur. Ceram. Soc. 2021, 41, 1720–1725. [Google Scholar] [CrossRef]
- Wu, J.; Wei, X.; Padture, N.P.; Klemens, P.G.; Gell, M.; García, E.; Miranzo, P.; Osendi, M.I. Low-thermal-conductivity rare-earth zirconates for potential thermal-barrier-coating applications. J. Am. Ceram. Soc. 2002, 85, 3031–3035. [Google Scholar] [CrossRef]
- Mathanbabu, M.; Thirumalaikumarasamy, D.; Thirumal, P.; Ashokkumar, M.J.M.T.P. Study on thermal, mechanical, microstructural properties and failure analyses of lanthanum zirconate based thermal barrier coatings: A review. Mater. Today Proc. 2021, 46, 7948–7954. [Google Scholar] [CrossRef]
- Yin, Q.; Shen, K.H.; Wang, Y.; Xing, Y.Z. Research progress of rare earth zirconate thermal barrier coating ceramic materials. Mater. Today Commun. 2025, 42, 111579. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Lai, M.; Zhao, J.; Liu, Y.; Fan, Y.; Yao, Y.; Liu, B. Influence of chemical disorder on mechanical and thermal properties of multi-component rare earth zirconate pyrochlores (nRE1/n)2Zr2O7. J. Appl. Phys. 2022, 132, 075108. [Google Scholar] [CrossRef]
- Li, F.; Zhou, L.; Liu, J.X.; Liang, Y.; Zhang, G.J. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. J. Adv. Ceram. 2019, 8, 576–582. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, C.; Sheng, Y.; Nian, H.; Li, Q.; Song, P.; Lu, W.; Yang, J.; Liu, B. Investigation of mechanical and thermal properties of rare earth pyrochlore oxides by first-principles calculations. J. Am. Ceram. Soc. 2019, 102, 2830–2840. [Google Scholar] [CrossRef]
- Zhao, M.; Pan, W.; Wan, C.; Qu, Z.; Li, Z.; Yang, J. Defect engineering in development of low thermal conductivity materials: A review. J. Eur. Ceram. Soc. 2017, 37, 1–13. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, X.; Xu, J.; Zhang, P.; Lou, Z.; Reece, M.J.; Gao, F. Ultra-low thermal conductivity and enhanced mechanical properties of high-entropy rare earth niobates (RE3NbO7, RE= Dy, Y, Ho, Er, Yb). J. Eur. Ceram. Soc. 2021, 41, 1052–1057. [Google Scholar] [CrossRef]
- Zhao, Z.; Ruan, Z.; Li, R.; Yan, S.; Sun, X.; Liu, C.; Zhang, D.; Xu, B.; Ren, Z.; Wang, M.; et al. High entropy pyrochlore (La0.3Gd0.3Ca0.4)2(Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)2O7 ceramic with amorphous-like thermal conductivity for environmental/thermal barrier coating applications. J. Mater. Sci. Technol. 2025, 205, 315–326. [Google Scholar] [CrossRef]
- Vayer, F.; Decorse, C.; Bérardan, D.; Dragoe, N. New entropy-stabilized oxide with pyrochlore structure: Dy2(Ti0.2Zr0.2Hf0.2Ge0.2Sn0.2)2O7. J. Alloys Compd. 2021, 883, 160773. [Google Scholar] [CrossRef]
- Tu, T.Z.; Liu, J.X.; Zhou, L.; Liang, Y.; Zhang, G.J. Graceful behavior during CMAS corrosion of a high-entropy rare-earth zirconate for thermal barrier coating material. J. Eur. Ceram. Soc. 2022, 42, 649–657. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Monfreda, V.; Marocco, A.; Dell’Agli, G. On the effect of kinetics on the formation of fluorite/bixbyite-structured Entropy-Stabilized Oxides. Ceram. Int. 2025; in press. [Google Scholar]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Bortolotti, M.; Dell’Agli, G. New insights in the hydrothermal synthesis of rare-earth carbonates. Materials 2019, 12, 2062. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, A.; You, L.; Song, R.; Yu, J.; Zhang, H.; Li, Q.; Liu, H. Hydrothermal synthesis of rare earth (Tb, Y) hydroxide and oxide nanotubes. Adv. Funct. Mater. 2003, 13, 955–960. [Google Scholar] [CrossRef]
- Benammar, I.; Salhi, R.; Deschanvres, J.L.; Maalej, R. The effect of rare earth (Er, Yb) element doping on the crystallization of Y2Ti2O7 pyrochlore nanoparticles developed by hydrothermal-assisted-sol-gel method. J. Solid State Chem. 2023, 320, 123856. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, F.; Zhang, X.; Shi, T.; Li, J.; Bai, Y.; Wang, C.; Wang, Z. Enhanced electrical properties of (Bi0.2Na0.2Ba0.2Ca0. 2Sr0.2)TiO3 high-entropy ceramics prepared by hydrothermal method. Ceram. Int. 2022, 48, 19492–19500. [Google Scholar] [CrossRef]
- Yang, L.; He, R.; Chai, J.; Qi, X.; Xue, Q.; Bi, X.; Yu, J.; Sun, Z.; Xia, L.; Wang, K.; et al. Synthesis Strategies for High Entropy Nanoparticles. Adv. Mater. 2025, 37, 2412337. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Ma, Z.; Liu, C.; Liu, W.; Zhao, S.; Han, Z.; Shao, T.; Gao, P.; Jiang, B.; Gu, X. Preparation of high entropy (Ba0.2Mg0.2Ca0.2Sr0.2Pb0.2) TiO3 perovskite oxide powders by a sol-hydrothermal method. Ceram. Int. 2022, 48, 15992–15999. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Dell’Agli, G. Compositional design of single-phase rare-earth based high-entropy oxides (HEOs) by using the cluster-plus-glue atom model. Ceram. Int. 2023, 49, 7662–7669. [Google Scholar] [CrossRef]
- Bourelly, C.; Vitelli, M.; Milano, F.; Molinara, M.; Fontanella, F.; Ferrigno, L. Eis-based soc estimation: A novel measurement method for optimizing accuracy and measurement time. IEEE Access 2023, 11, 91472–91484. [Google Scholar] [CrossRef]
- Wang, Y.; Marchetti, B.; Zhou, X.D. Call attention to using DRT and EIS to quantify the contributions of solid oxide cell components to the total impedance. Int. J. Hydrogen Energy 2022, 47, 35437–35448. [Google Scholar] [CrossRef]
- Mancuso, A.; Monzillo, K.; Sacco, O.; Spiridigliozzi, L.; Monfreda, V.; Muscatello, A.; Dell’Agli, G.; Esposito, S.; Vaiano, V. Engineered synthesis of a novel Bixbyite-structured High-Entropy Oxide (Ce0.2Zr0.2Yb0.2Er0.2Gd0.2)2O3.4 as a stable and high-performing visible-light-active photocatalyst for multifunctional pollutant degradation. J. Alloys Compd. 2025, 1026, 180435. [Google Scholar] [CrossRef]
- Accardo, G.; Dell’Agli, G.; Mascolo, M.C.; Spiridigliozzi, L.; Yoon, S.P. Controlled coprecipitation of amorphous cerium-based carbonates with suitable morphology as precursors of ceramic electrolytes for IT-SOFCs. Materials 2019, 12, 702. [Google Scholar] [CrossRef]
- Dell'Agli, G.; Mascolo, G.; Mascolo, M.C.; Pagliuca, C. Drying effect on thermal behavior and structural modifications of hydrous zirconia gel. J. Am. Ceram. Soc. 2008, 91, 3375–3379. [Google Scholar] [CrossRef]
- Wen, Y.; Zhou, C.; Yu, L.; Zhang, Q.; He, W.; Liu, Q. Preparation of nanometer zirconia by hydrothermal method: Influence of temperature and mechanism. Solid State Sci. 2023, 142, 107237. [Google Scholar] [CrossRef]
- Suryanarayan, C.; Norton, M.G. X-Ray Diffraction: A Practical Approach; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Rahaman, M.N. Ceramic Processing and Sintering; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zolhafizi, J.; Azmi, M.A.; Rahman, H.; Zakaria, H.; Hassan, S.; Mahzan, S.; Ismail, A.; Ariffin, A.M.T.; Tukimon, M.F.; Yusof, U.; et al. Samarium doped ceria (SDC) electrolyte modification by sintering aids addition to reducing sintering temperature: A Review. J. Kejuruter. 2023, 35, 65–76. [Google Scholar] [CrossRef]
- Yoshida, H.; Inagaki, T. Effects of additives on the sintering properties of samaria-doped ceria. J. Alloys Compd. 2006, 408, 632–636. [Google Scholar] [CrossRef]
- Abrams, H. Grain size measurement by the intercept method. Metallography 1971, 4, 59–78. [Google Scholar] [CrossRef]
- Maiti, T.K.; Majhi, J.; Maiti, S.K.; Singh, J.; Dixit, P.; Rohilla, T.; Ghosh, S.; Bhushan, S.; Chattopadhyay, S. Zirconia-and ceria-based electrolytes for fuel cell applications: Critical advancements toward sustainable and clean energy production. Environ. Sci. Pollut. Res. 2022, 29, 64489–64512. [Google Scholar] [CrossRef] [PubMed]
- Lagarias, J.C.; Reeds, J.A.; Wright, M.H.; Wright, P.E. Convergence properties of the Nelder–Mead simplex method in low dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef]
- Koettgen, J.; Grieshammer, S.; Hein, P.; Grope, B.O.; Nakayama, M.; Martin, M. Understanding the ionic conductivity maximum in doped ceria: Trapping and blocking. Phys. Chem. Chem. Phys. 2018, 20, 14291–14321. [Google Scholar] [CrossRef]
- Accardo, G.; Dell’Agli, G.; Spiridigliozzi, L.; Yoon, S.P.; Frattini, D. On the oxygen vacancies optimization through Pr co-doping of ceria-based electrolytes for electrolyte-supported solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 19707–19719. [Google Scholar] [CrossRef]
- Omar, S.; Wachsman, E.D.; Jones, J.L.; Nino, J.C. Crystal structure–ionic conductivity relationships in doped ceria systems. J. Am. Ceram. Soc. 2009, 92, 2674–2681. [Google Scholar] [CrossRef]
- Solovyev, A.; Shipilova, A.; Smolyanskiy, E.; Rabotkin, S.; Semenov, V. The properties of intermediate-temperature solid oxide fuel cells with thin film gadolinium-doped ceria electrolyte. Membranes 2022, 12, 896. [Google Scholar] [CrossRef]
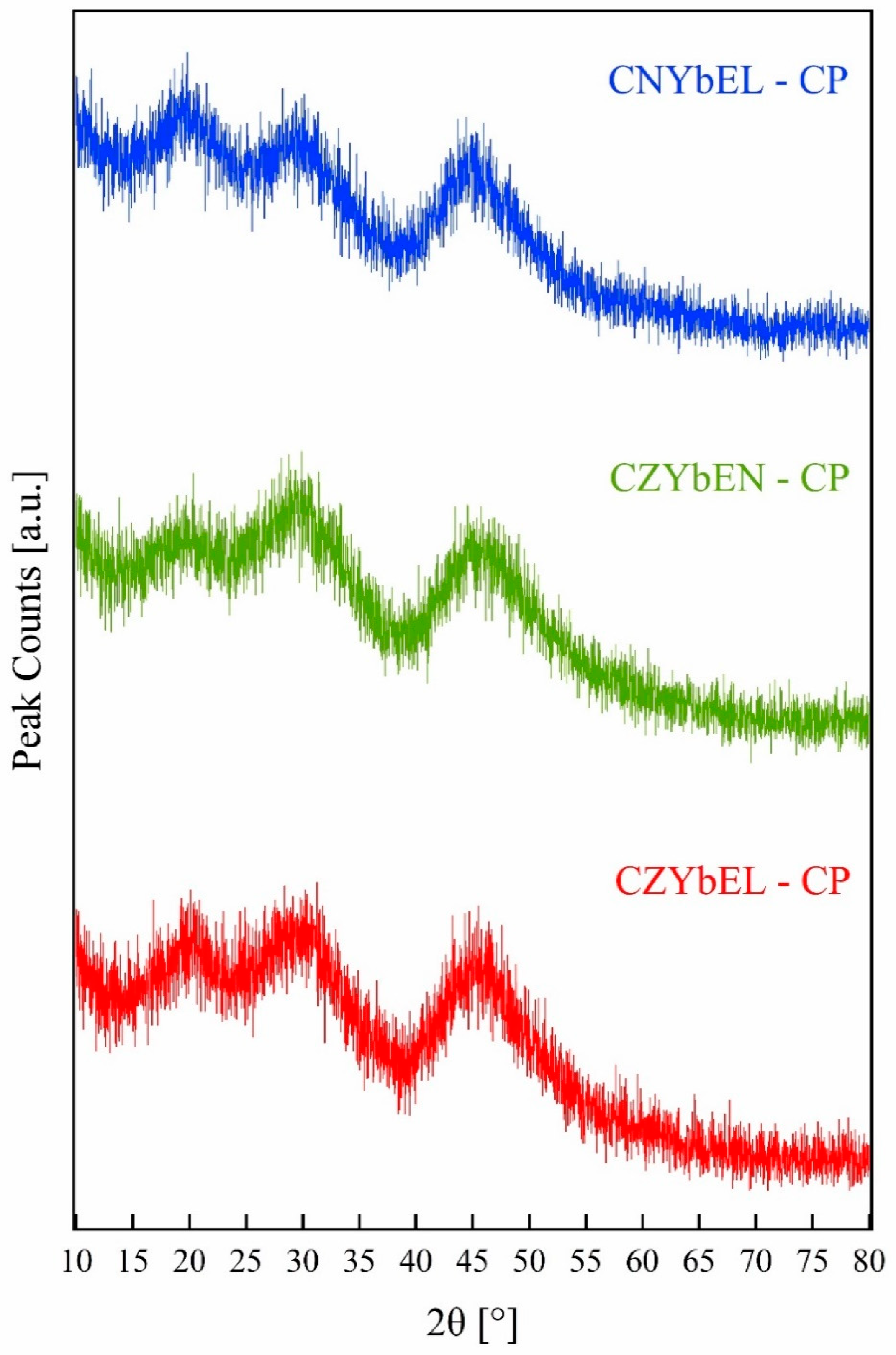
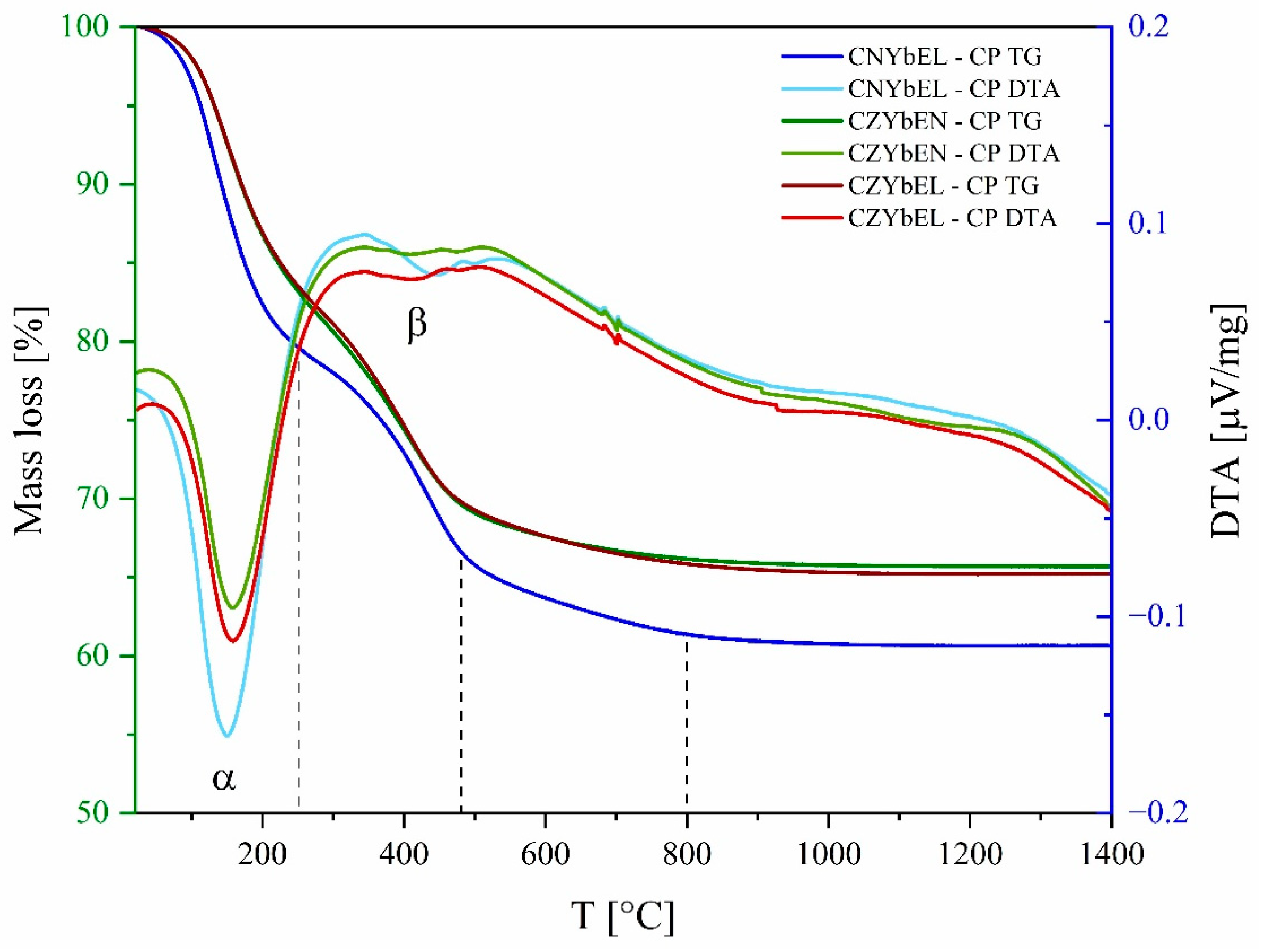
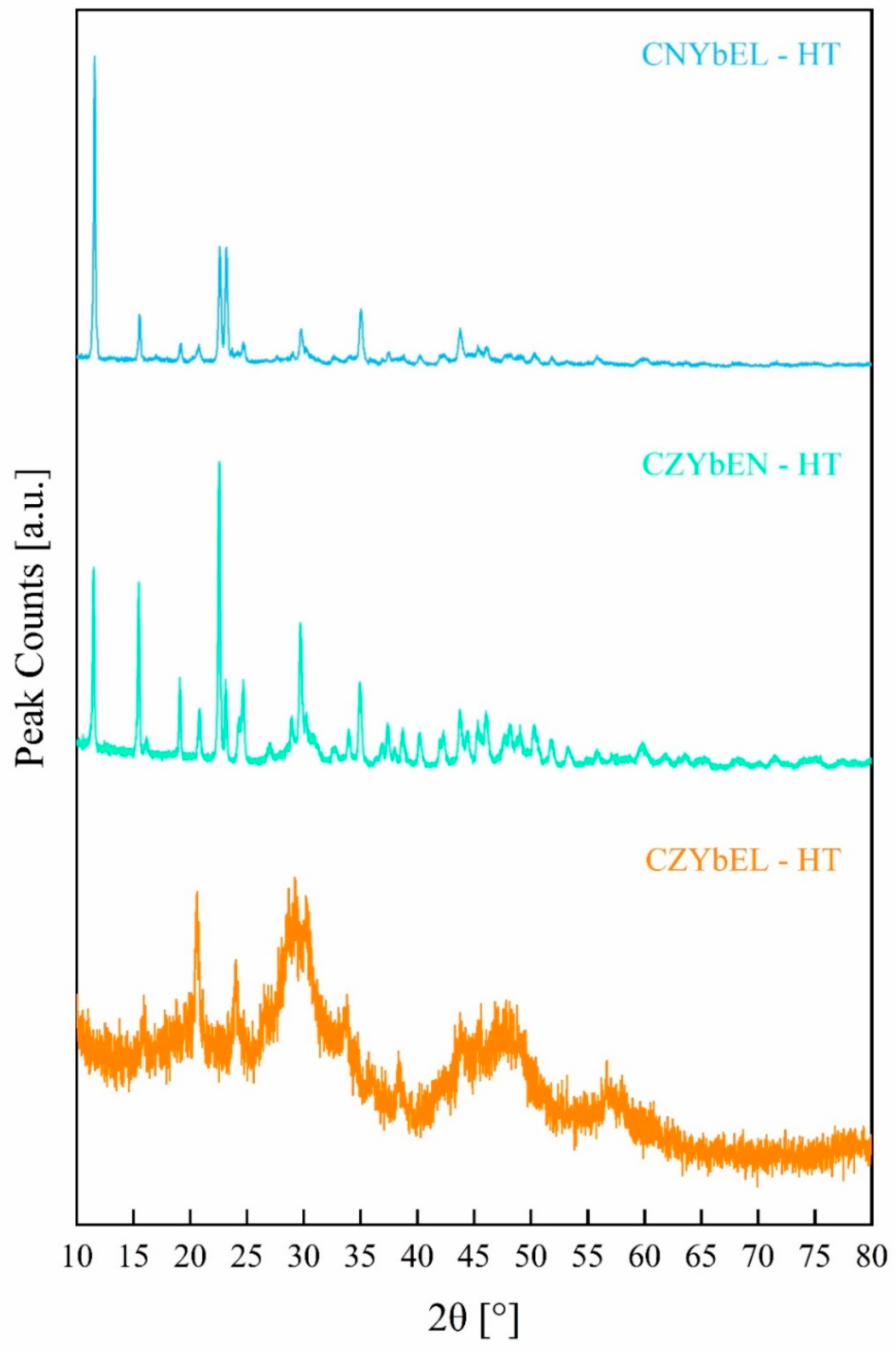
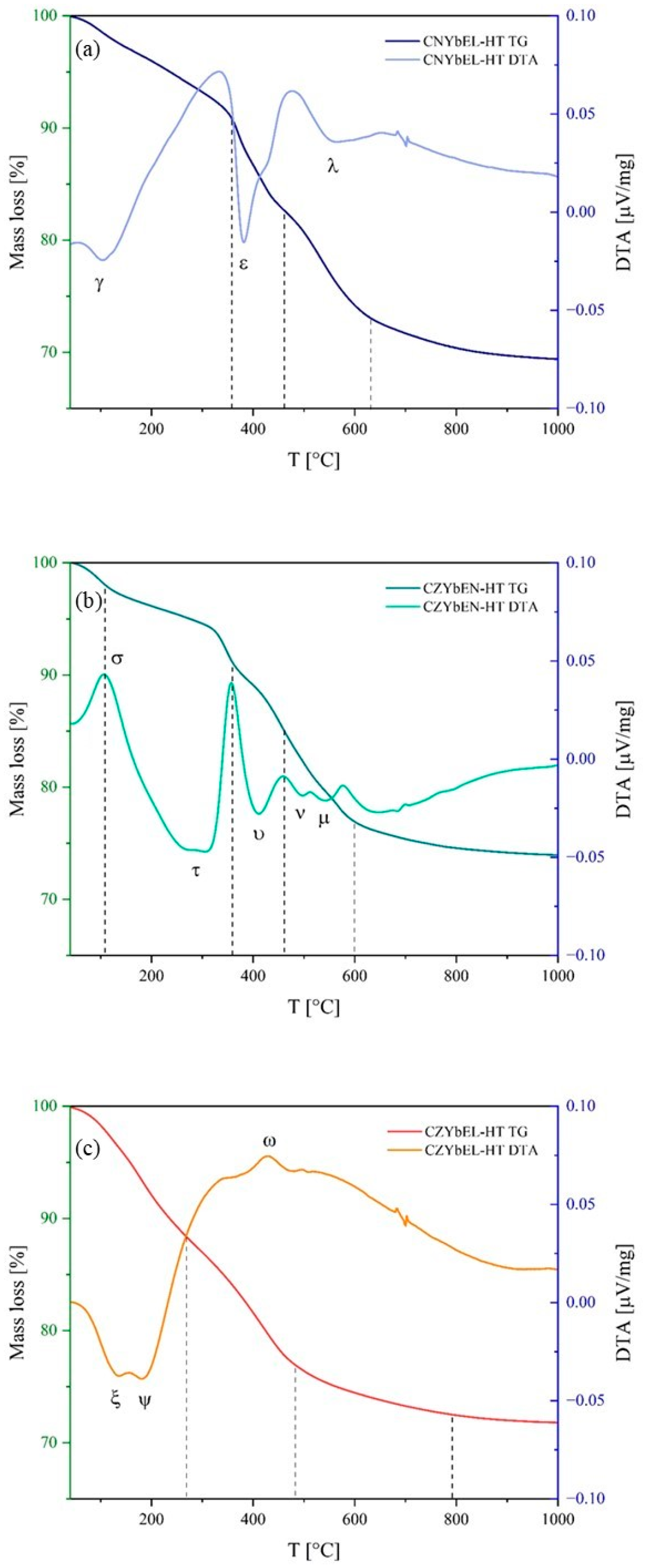
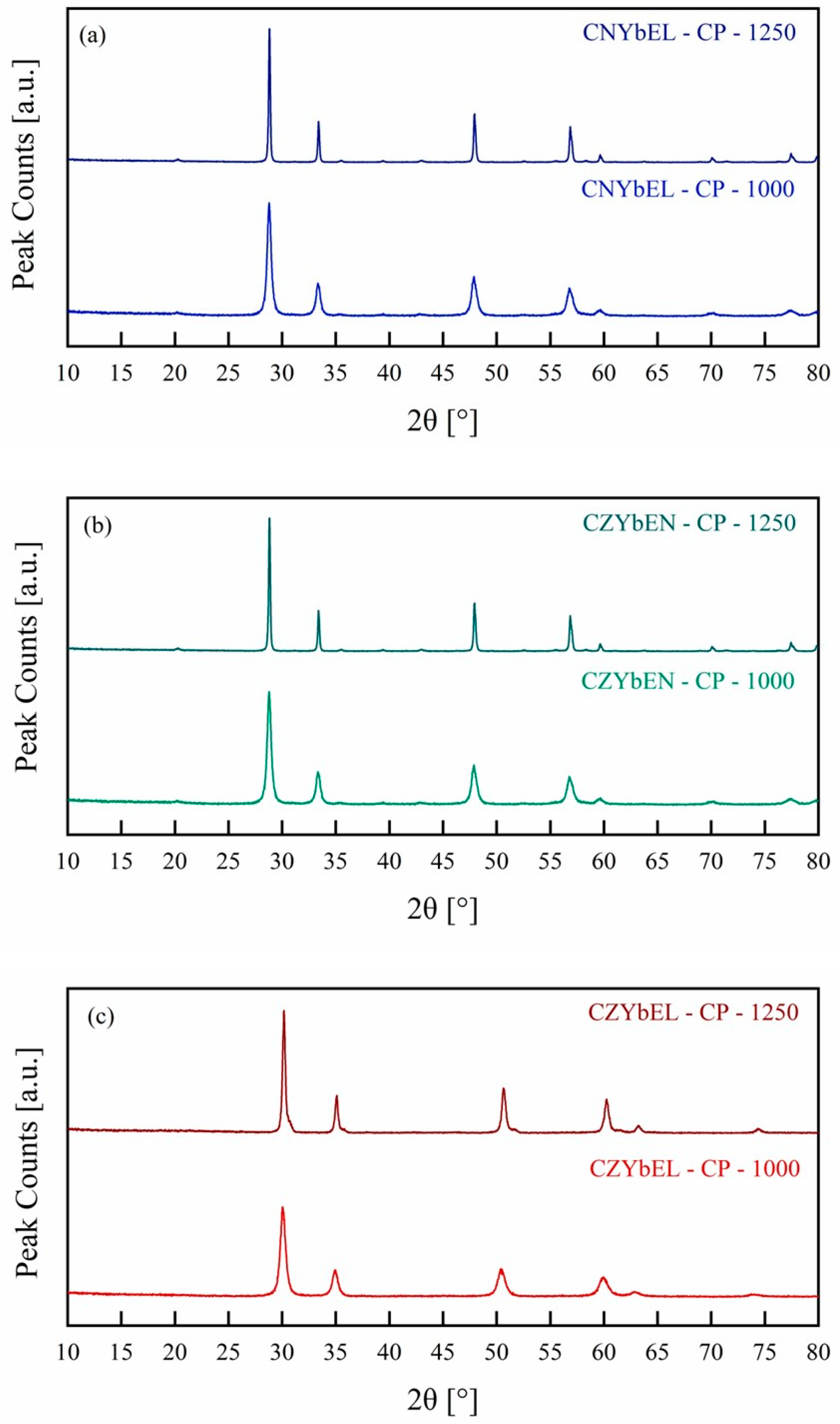
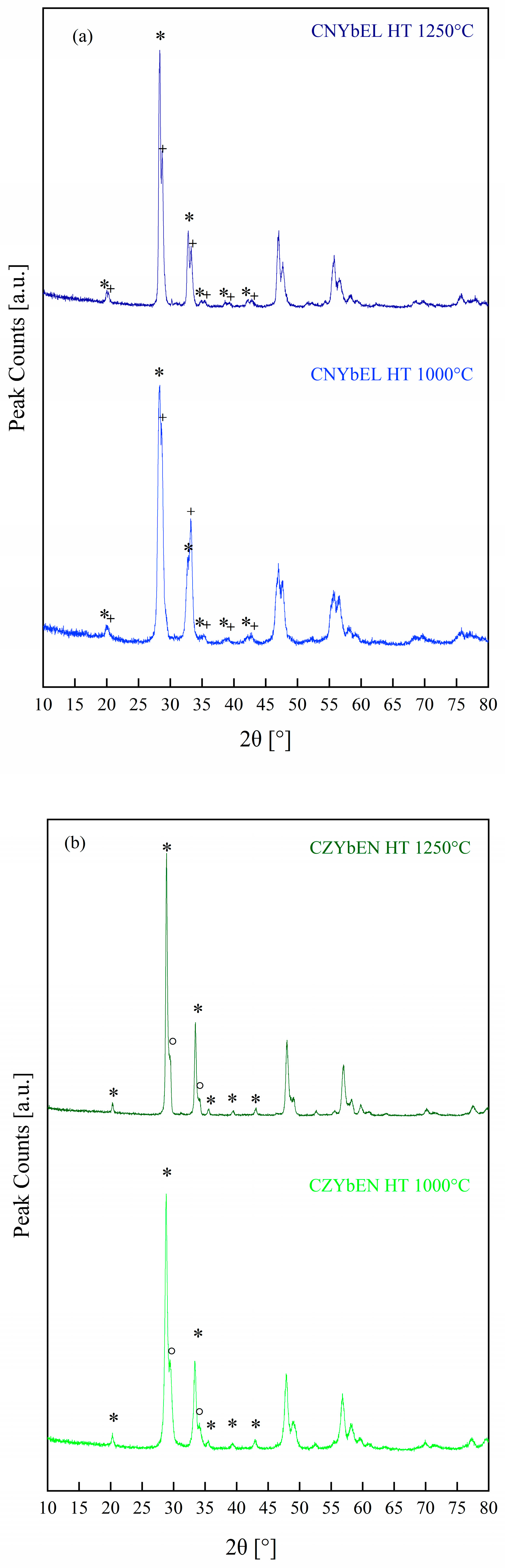
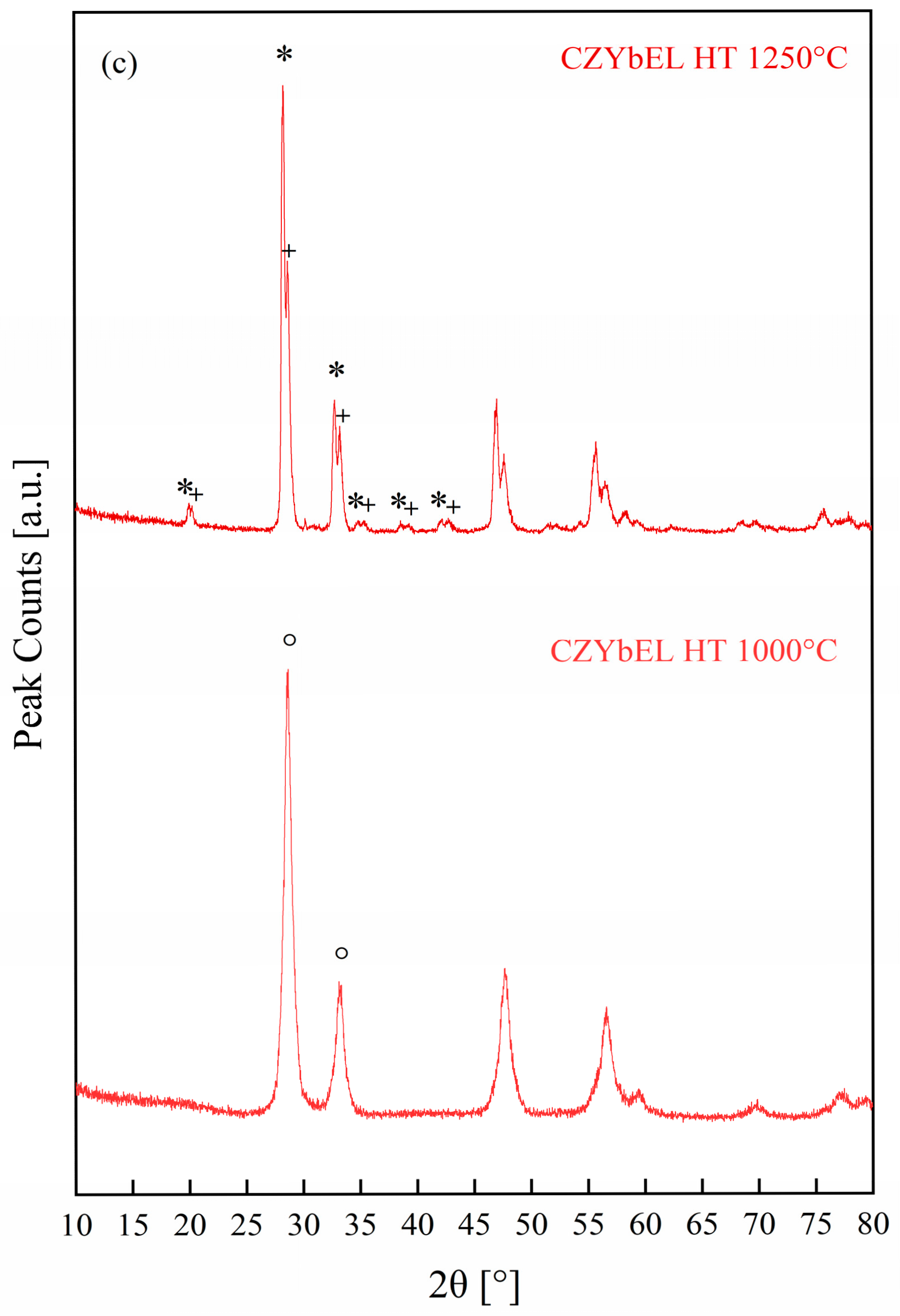
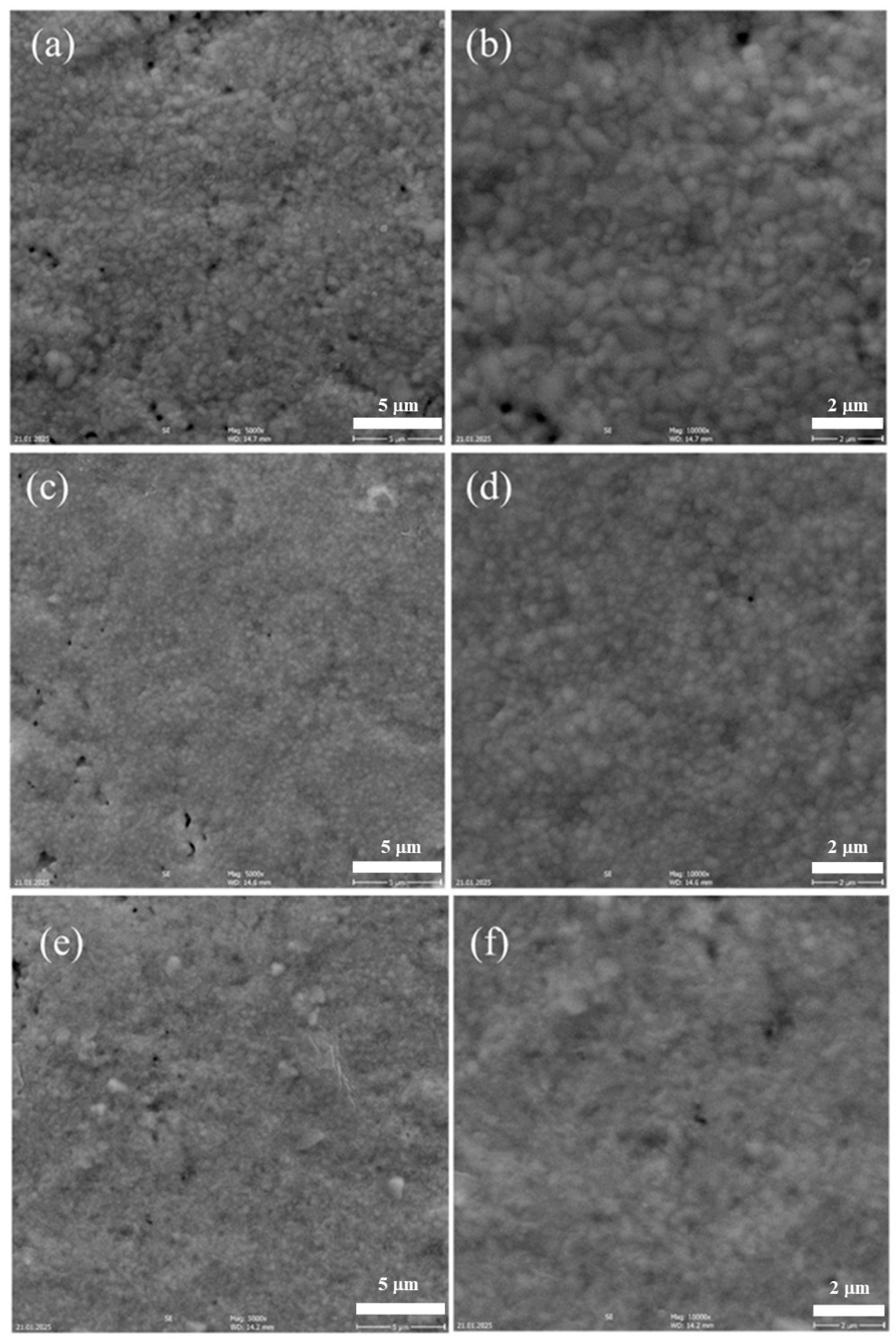
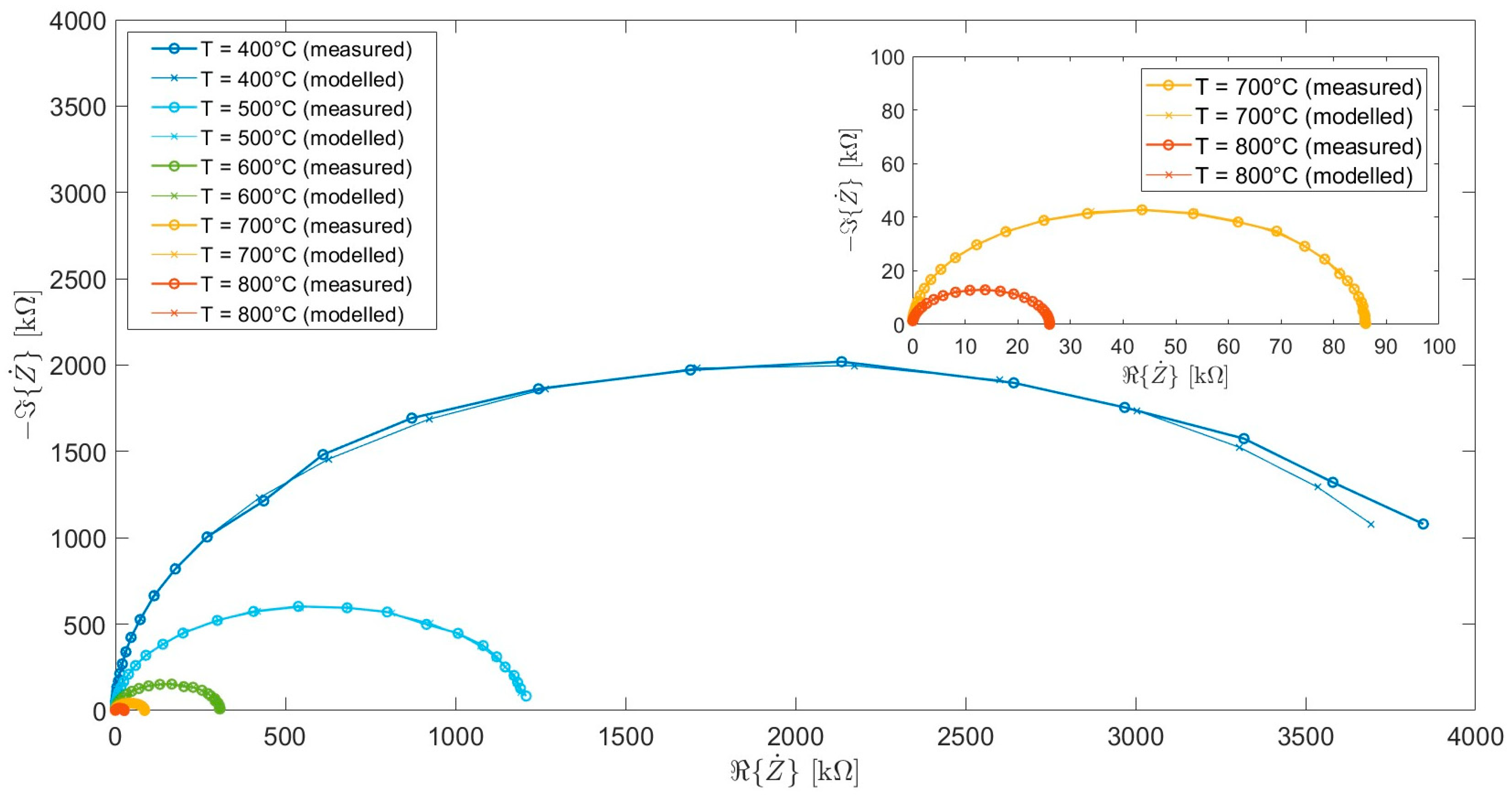
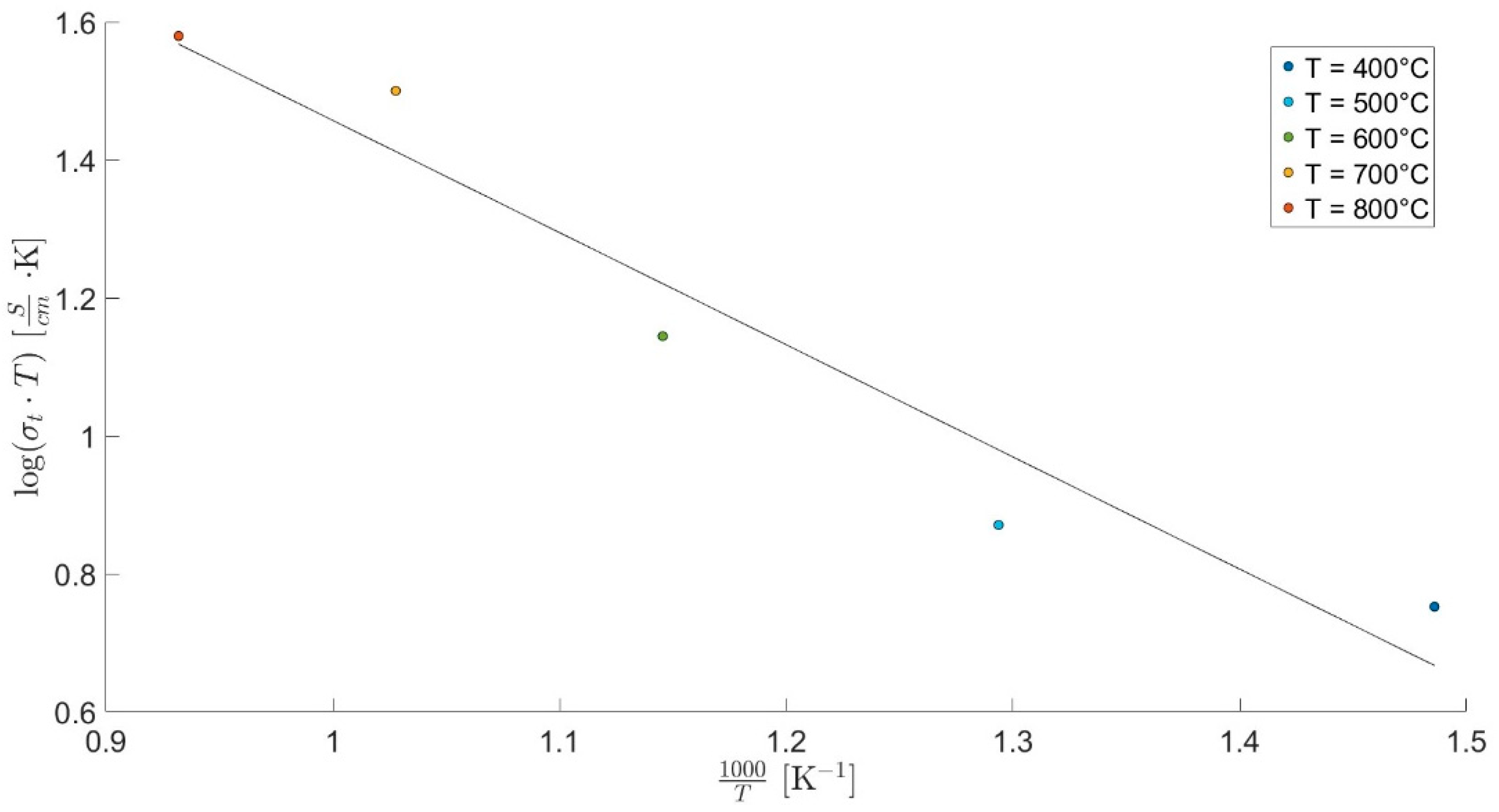
| Calcination Temperature | CNYbEL-CP | CZYbEN-CP | CZYbEL-CP |
|---|---|---|---|
| 1000 °C | 33 nm | 24 nm | 19 nm |
| 1250 °C | 60 nm | 102 nm | 63 nm |
| RE-HEO System | Theoretical Density [g/cm3] | Measured Density [g/cm3] | Relative Density |
|---|---|---|---|
| CNYbEL-CP-s1200 | 7.3548 | 7.1859 | 97.7% |
| CNYbEL-CP-s1300 | 7.3548 | 7.2001 | 97.9% |
| CZYbEN-CP-s1200 | 7.3254 | 5.1552 | 70.4% |
| CZYbEN-CP-s1300 | 7.3254 | 7.3449 | ≈100% |
| CZYbEL-CP-s1200 | 7.2231 | 4.8851 | 67.6% |
| CZYbEL-CP-s1300 | 7.2231 | 7.0406 | 97.5% |
| RE-HEO System | Average Grain Size [mm] | Residual Porosity [%] |
|---|---|---|
| CNYbEL-CP-s1300 | 0.50 | 5.0% |
| CZYbEN-CP-s1300 | 0.30 | 1.5% |
| CZYbEL-CP-s1300 | 0.25 | 3.5% |
| Circuit Parameters | Temperature [°C] | ||||
|---|---|---|---|---|---|
| 400 | 500 | 600 | 700 | 800 | |
| 8.93 | 7.81 | 4.69 | 2.31 | 2.12 | |
| 4.01 | 1.20 | 0.31 | 0.08 | 0.02 | |
| 115.67 | 114.96 | 118.97 | 114.34 | 114.11 | |
| Temperature [°C] | |||||
|---|---|---|---|---|---|
| 400 | 500 | 600 | 700 | 800 | |
| Total conductivity [S/cm] | 8.4 × 10−3 | 9.6 × 10−3 | 1.6 × 10−2 | 3.2 × 10−2 | 3.5 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiridigliozzi, L.; Monfreda, V.; Marocco, A.; Milano, F.; Vendittelli, A.; Dell’Agli, G. Effect of Synthesis and Processing Conditions on the Sintering Behavior and Total Conductivity of High-Entropy Fluorite/Bixbyite Oxides (RE-HEOs). Materials 2025, 18, 2663. https://doi.org/10.3390/ma18112663
Spiridigliozzi L, Monfreda V, Marocco A, Milano F, Vendittelli A, Dell’Agli G. Effect of Synthesis and Processing Conditions on the Sintering Behavior and Total Conductivity of High-Entropy Fluorite/Bixbyite Oxides (RE-HEOs). Materials. 2025; 18(11):2663. https://doi.org/10.3390/ma18112663
Chicago/Turabian StyleSpiridigliozzi, Luca, Viviana Monfreda, Antonello Marocco, Filippo Milano, Antonio Vendittelli, and Gianfranco Dell’Agli. 2025. "Effect of Synthesis and Processing Conditions on the Sintering Behavior and Total Conductivity of High-Entropy Fluorite/Bixbyite Oxides (RE-HEOs)" Materials 18, no. 11: 2663. https://doi.org/10.3390/ma18112663
APA StyleSpiridigliozzi, L., Monfreda, V., Marocco, A., Milano, F., Vendittelli, A., & Dell’Agli, G. (2025). Effect of Synthesis and Processing Conditions on the Sintering Behavior and Total Conductivity of High-Entropy Fluorite/Bixbyite Oxides (RE-HEOs). Materials, 18(11), 2663. https://doi.org/10.3390/ma18112663









