Abstract
Cadmium (Cd), as a type of heavy metal, can increase the incidence of many diseases, even in low concentrations. In this study, tobermorite was hydrothermally synthesized and then applied to adsorb Cd2+ from an aqueous solution. The physicochemical characteristics of the synthesized tobermorite were detected, and the results indicated that the well-crystallized tobermorite had a lot of mesopores and a large specific surface area of 140.92 m2/g. It acquired a pH self-adjustment ability via spontaneously releasing Ca2+ and OH- into the aqueous solution. The effects of different factors on Cd2+ removal were investigated. For Cd2+, the removal efficiency could reach 99.71% and the maximum adsorption capacity was 39.18 mg/g using tobermorite. The adsorption data was best fitted with the pseudo-second-order kinetic and Langmuir isotherm models. In addition, there was no strict limit on the solution pH in Cd2+ adsorption because the tobermorite could adjust the solution pH to an alkaline atmosphere spontaneously. The efficient removal of Cd2+ using tobermorite was a result of surface complexation and ion exchange.
1. Introduction
In recent decades, water demand has increased steadily due to population growth and industrial development [1]. Many parts of the world are now facing a water crisis, which may also be exacerbated by mismanagement of water resources [2,3]. Heavy metals are common in the effluents of industrial activities [4]. Excess levels of heavy metals in water resources will be a severe threat to the ecosystem and human health because they are highly toxic, bioconcentrated and nonbiodegradable [5]. In 1984, the United Nations Environment Program put forward 12 kinds of dangerous chemicals with global significance, of which Cd was ranked first [6]. Therefore, a remediation solution that can efficiently and economically remove Cd is essential for recycling and reutilizing of wastewater, which is also key to achieving sustainable management of water resources.
Numerous technologies have been employed to remove Cd, including chemical precipitation, adsorption, ion exchange, solvent extraction, reverse osmosis, coagulation/flocculation, photocatalysis and electrochemical treatment [7,8,9]. Among them, due to the simplicity, low cost and diversity of adsorbents, adsorption is regarded as promising [10,11]. However, some adsorbents still retain many disadvantages, such as poor structure, low metal selectivity and low adsorption capacity [12]. Da’na pointed out that the existence of Ca2+ and Na+ ions in adsorbent structures could enhance metal selectivity for some adsorbents [13]. Furthermore, heavy-metal removal is generally affected by solution pH [14,15]. For instance, a study by Sun and Yi indicated that concentrations of heavy-metal ions decreased when the pH was increased during the water-washing process of incineration bottom ash [14]. Therefore, it is a challenge to develop a novel, functional adsorbent that can release favorable ions (e.g., Na+ and Ca2+) and adjust solution pH simultaneously to make removal of Cd more efficient.
Tobermorite (Ca5Si6O16(OH)2·4H2O) is a type of naturally existing calcium silicate hydrate (CSH) [16]. It is widely applied in building materials (e.g., silica bricks, aerated concrete) because of its layered structure, large specific surface area, low density and thermal conductivity [17]. However, tobermorite products with low strength are easily broken during the production, transportation and application processes, and its waste usually accounts for about 15% of the total product [18]. Therefore, it is urgent to find appropriate recycling methods for tobermorite waste. In addition, depending on the hydration level, the (002) crystal plane of tobermorite can have different interplanar spacings, i.e., 0.9, 1.1 and 1.4 nm; thus, there are three types of tobermorite [18]. Numerous studies have synthesized 1.1 nm tobermorite under different hydrothermal conditions and using various raw materials [19,20,21]. A pore canal containing some water and Ca2+ ions is one of the most noticeable structural features of tobermorite [22]. Thus, tobermorite has recently been used to adsorb phosphorus at a laboratory scale [23,24,25]. The OH- released from tobermorite provides a desired alkaline environment, which can facilitate the combination of Ca2+ and PO43- to form hydroxyl calcium phosphate [25]. Considering its layered structure and ability to release Ca2+ and OH-, tobermorite has the potential to become a functional adsorbent that removes Cd2+ from wastewater efficiently and economically [1]. However, previous studies have commonly focused on synthesis and modification (mainly Al substitution) of tobermorite but rarely on its pH self-adjustment ability or Cd2+ removal mechanism.
In this study, tobermorite was hydrothermally synthesized and its physicochemical properties, including crystalline phase, morphological features, pore size distribution and pH-adjustment ability, were characterized. Then, the variables influencing removal efficiency were explored using batch adsorption experiments. Lastly, the adsorption process was modeled, and the mechanism of Cd2+ removal using tobermorite was investigated.
2. Materials and Methods
2.1. Synthesis of Tobermorite
Tobermorite was synthesized hydrothermally according to the method of Zhao et al. [26]. Smelting quartz powder, which was produced from zirconium oxychloride waste, was the silicon source. Quicklime with an effective CaO content of 93%was the calcium source. The molar masses of calcium (Ca) in quicklime and silicon (Si) in the smelting quartz powder were respectively calculated, and the Ca/Si ratio was set to 0.83. The powder and the quicklime were mixed and ground for 15 min with water (solid/water = 1/15). Then, the mixture was digested at 90 °C for 2 h in a microautoclave and further autoclaved for 24 h at 1.6 MPa and 205 °C. Finally, tobermorite was acquired after drying at 105 °C.
2.2. Static Leaching Experiments
In order to evaluate the pH self-adjustment ability of tobermorite, 10 g of tobermorite was added to a conical flask with 1 L of deionized water. After sealing, the flask was agitated at 25 °C and 120 rpm in a water bath oscillator (HZQ-F100, Huamei, Jiangsu, China). The supernatant was collected at predetermined intervals (0.5, 1, 2, 4, 6, 8, 10 h) via filtering through a 0.45 μm microfiltration membrane. Ca2+ concentration was detected with EDTA titration. All reagents used were of analytical grade.
2.3. Batch Adsorption Experiments
A total of 1.6309 g of cadmium chloride (CdCl2) was dissolved in 1 L of deionized water to prepare Cd2+ stock solution (1000 mg/L). Different desired concentrations were obtained via diluting the stock solution using deionized water. Different parameters influencing the adsorption performance of the tobermorite were comprehensively investigated through a series of batch adsorption experiments.
In total, 0.5 g of tobermorite and 50 mL of Cd2+ solution with an initial concentration of 250 mg/L were added to a 100 mL polyvinyl chloride centrifugal tube. After sealing, the tube was agitated at 25 °C and 120 rpm in a water bath oscillator. For kinetics experiments, sampling time was predetermined at 0.5, 1, 2, 4, 6, 8, 10, 12 and 14 h. Then, a sampling time of 10 h was fixed for the analysis of other adsorption factors. Isotherm experiments were conducted at different initial Cd2+ concentrations of 50, 100, 150, 250, 400 and 500 mg/L. For different initial solution pH, the desired values (2.5, 4.5, 6.5, 8.5) were regulated using 1 M NaOH and HCl solutions. Tobermorite amounts of 2.5, 5, 10 and 15 g/L were used in the dosage experiment. Specifically, except for in the adsorption experiments at different initial Cd2+ concentrations, the Cd2+ concentration used was maintained at 250 mg/L with an initial solution pH of 6.6. Likewise, with the exception of the adsorption experiments at different initial solution pH, the solution pH was not additionally adjusted and kept at 6.6 in order to avoid metal hydroxide precipitation.
The supernatant was collected via filtering through a 0.45 μm microfiltration membrane. The Cd2+ concentration in the filtrates was analyzed with an atomic absorption spectrophotometer (AAS, Model TAS-990 F, Persee, Beijing, China). Results were obtained after three repetitions. Equations (1) and (2) are defined for removal efficiency (R, %) and adsorption capacity (Qt, mg/g), respectively:
where, C0 (mg/L) is the initial Cd2+ concentration, Ct (mg/L) is the Cd2+ concentration at time t, V (L) is the solution volume and M (g) is the mass of tobermorite used.
2.4. Desorption Experiments
The adsorbed Cd2+ on tobermorite was desorbed with 0.1 M NaOH solution under 120 rpm and shaken for 1 h at 25 °C. Then, the tobermorite was washed with deionized water three times and used in the next adsorption cycle after drying. The adsorption–desorption cycle was conducted three times to investigate the reusability performance of tobermorite. In all of the adsorption experiments described here, the initial Cd2+ concentration was 250 mg/L, the contact time was 10 h and the adsorbent dosage was 0.5 g/50 mL.
2.5. Characterizations
The crystalline phase was analyzed with X-ray diffraction (XRD, D8 Advance, Bruker, Karlsruhe, Germany). Fourier transform infrared spectra (FTIR, Tensor 27, Bruker, Heidelberg, Germany) were obtained between 400 and 4000 cm−1 via adding samples in KBr pellets to observe characteristic function groups. The characteristics of pore structure were determined with a porosimeter (V-Sorb 28009, Gold APP, Beijing, China). A scanning electron microscope with energy dispersive spectroscopy (SEM-EDS, Gemini SEM 300, Zeiss, Jena, Germany) was applied to investigate morphological features and chemical composition. The zeta potential was obtained with a zeta potential meter (NanoBrook 90 Plus, Brookhaven, Suffolk County, NY, USA).
3. Results and Discussion
3.1. Characterizations of Tobermorite
The XRD pattern in Figure 1a indicates that tobermorite with good crystallinity was synthesized via the hydrothermal method. At 2θ angles of 7.9°, 29.0°, 31.8° and 49.4°, diffraction peaks for the lattice planes of (002), (110), (107) and (217) were found in orthorhombic tobermorite (PDF #83-1520, d1 = 1.1389 nm, d2 = 0.3082 nm), which could be identified as 1.1 nm tobermorite [19]. The FTIR spectrum of tobermorite is exhibited in Figure 1b. The broad band at 3460 cm−1 was attributed to O-H stretching vibration, which was generated from the adsorbed H2O molecules and the surface hydroxyls [27]. The band at 1640 cm−1 was assigned to O-H bending vibration. The small band at 1490 cm−1 was caused by H2O molecules in the interlayers of tobermorite [28]. The broad band near 974 cm−1 was attributed to Si-O symmetric stretching vibration and Si-O-Si asymmetric vibration in the silica tetrahedron. The band at 449 cm−1 was due to Si-O bending vibration in the silica tetrahedron [19]. Therefore, consistently with the above XRD analysis, tobermorite with a high degree of crystallization was synthesized successfully in this study.
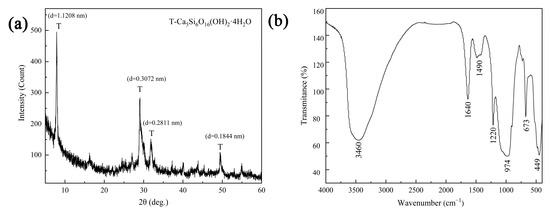
Figure 1.
XRD pattern (a) and FTIR spectrum (b) of tobermorite.
The surface morphology of tobermorite, presented in Figure 2a,b, mainly consists of micron-grade rod- and sheet-like structures. The two SEM images show different crystal growth densities in the tobermorite. In Figure 2a, most of the tobermorite crystals are small and rod-like due to limited growth space. When there was enough growth space, tobermorite crystals could grow into a sheet-like structure, as shown in Figure 2b. Regardless, numerous void spaces were still found in the tobermorite, which was beneficial for Cd2+ adsorption [26]. The adsorption–desorption isotherms of tobermorite in Figure 2c conform to the typical features of a type IV isotherm with an H3 hysteresis loop, suggesting that large and narrow slit-like pores were formed between the tobermorite crystals [29]. The pore distribution curve in Figure 2d indicated that mesopores of about 30–45 nm were predominant in the tobermorite, which could in turn explain the relatively large hysteresis loop shown in Figure 2c. In addition, the tobermorite’s surface area was 140.92 m2/g (BET method), its total pore volume was 0.60 cm3/g and its average pore width was 16.95 nm. Generally, porous materials with large surface areas can provide more adsorption and ion exchange sites, and thus, tobermorite should be a promising adsorbent [30].
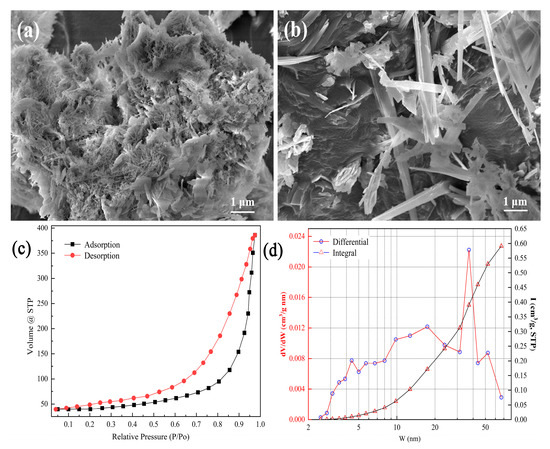
Figure 2.
(a,b) SEM images, (c) nitrogen adsorption–desorption isotherms and (d) pore size distribution of tobermorite.
The hydration of the tobermorite is described in Equation (3), showing that Ca2+ and OH− ions could be released into deionized water simultaneously [31]:
Ca5Si6O16(OH)2·4H2O → Ca2+ + OH− + CSH
Thus, the changes in Ca2+ concentration and solution pH during the static leaching experiments are described in Figure 3. An increase in Ca2+ concentration was prominent within the first 2 h and then slowed down significantly. After 10 h, 86.24 mg/L of Ca2+ was detected, accounting for 3.15% of the total Ca mass in the tobermorite used. Meanwhile, the solution pH was promptly increased to 9.8 within 0.5 h, then remained at about 9.9 after 4 h, indicating that an alkaline environment in the solution was autonomously created from the tobermorite. Thus, the tobermorite was considered to have a pH self-adjustment ability. In the structure of tobermorite, the layers consisting of SiO4 tetrahedral chain–Ca polyhedral layer–SiO4 tetrahedral chain laminates along the c-axis formed the interlayer space through shared SiO4 tetrahedrons [32]. Ca2+ ions in interlayer space are inclined to be dissolved or substituted with some suitable heavy metals [22]. Wang et al. reported that the decalcification of CSH in weak acidic environments did not affect its morphology [33]. Considering that the structures of CSH and tobermorite are similar, it was reasonable to infer that the small amount of Ca2+ ions dissolved in this study would not affect the morphology of tobermorite.
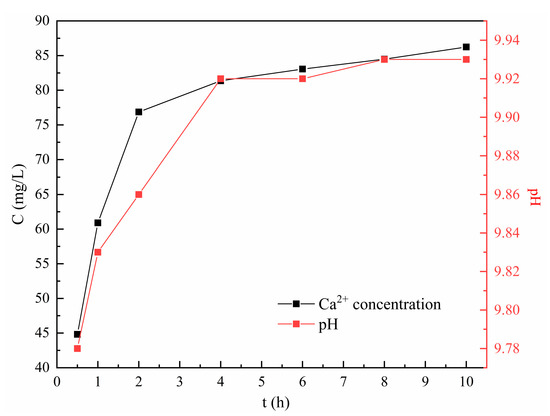
Figure 3.
Releasing ability of tobermorite.
3.2. Effects of Variables on Cd2+ Removal Using Tobermorite
3.2.1. Effect of Adsorbent Dosage
As illustrated in Figure 4a, when the tobermorite dosage increased from 2.5 to 10 g/L, the removal efficiency of Cd2+ was increased from 94.34% to 99.71%. Increased tobermorite dosages could provide more active sites to promote Cd2+ removal [34]. However, no significant improvement was observed after the dosage was increased from 10 to 15 g/L. Hence, considering economy and high efficiency, 10 g/L was selected as the optimum dosage and generalized in the following experiments.
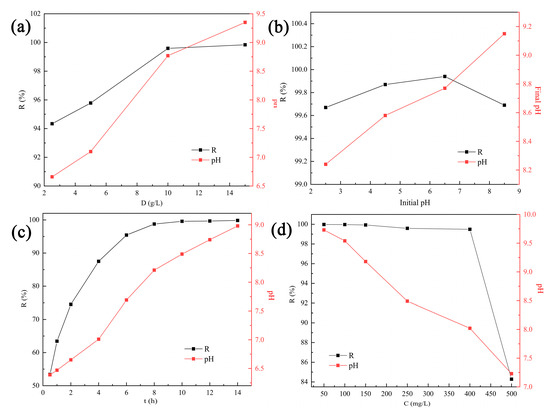
Figure 4.
Removal efficiency of Cd2+ and solution pH at different (a) dosages, (b) initial solution pH values, (c) contact times and (d) initial Cd2+ concentrations.
3.2.2. Effect of Initial Solution pH
Solution pH can affect the states of adsorbents and ions simultaneously, so it is an important factor in removal of heavy metals from wastewater [35]. Distribution of Cd2+ substances under different pH values was simulated with Visual MINTEQ 3.1 and is shown in Figure 5a. Clearly, Cd2+ was the major substance when the pH was below 8.5. Considering that Cd2+ ions are inclined to form precipitation at higher pH values, this study was carried out in an initial pH range of 2.5–8.5.
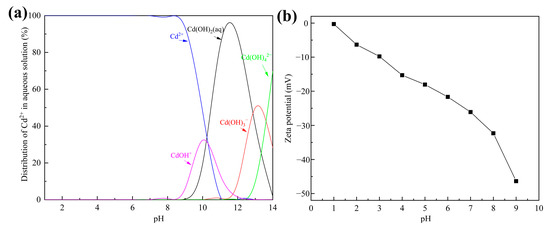
Figure 5.
(a) The distribution of Cd2+ in aqueous solution simulated with Visual MINTEQ 3.1; (b) zeta potential of tobermorite at different initial solution pH values.
The removal efficiency of Cd2+ using tobermorite and the final solution pH at different initial pH values are depicted in Figure 4b. Interestingly, the tobermorite exhibited a considerably high removal efficiency of 99.67–99.94% for Cd2+ in the whole pH range investigated. Even in an acid solution with a relatively low initial pH of 2.5, the removal efficiency still reached up to 99.67%. The obtained result was inconsistent with most of the previous research, which had indicated that initial solution pH significantly affected removal efficiency of heavy metals [36].
The zeta potential of tobermorite is displayed in Figure 5b. In the investigated pH range of 2.5–8.5, the surface charge of the tobermorite was always negative and decreased from about −9 to −40 mV. Electrostatic repulsion to positively charged Cd2+ was not produced because there was no positive charge on the surface of the tobermorite, which ensured high removal efficiency of Cd2+. Generally, the higher negative charge on the surface of tobermorite could significantly enhance electrostatic attraction to Cd2+, resulting in increased removal efficiency of Cd2+. In fact, the removal efficiency of Cd2+ increased by only 0.27% from initial pH values of 2.5 to 6.5. Compared with the corresponding high removal efficiency, this slight increase was negligible. The high removal efficiency of Cd2+ at low initial pH might be attributed to the pH self-adjustment ability of tobermorite. As shown in Figure 4b, the final solution pH was adjusted to a range of 8.2–9.2 using tobermorite. OH− ions were released from the tobermorite through the hydration process, as described in Equation (3), and captured H+ continuously, so a relatively stable alkaline environment was ensured. The ultimate result was that the tobermorite could adsorb Cd2+ efficiently over a wide pH range. In a practical application, this would mean that pH adjustment reagents are unnecessary when tobermorite is used, which could simplify the operation and reduce costs.
3.2.3. Effects of Contact Time and Adsorption Kinetics
The effect of contact time on Cd2+ removal using tobermorite is shown in Figure 4c. Removal efficiency was notoriously increased with contact time due to the presence of sufficient active sites on the surface. Then, the adsorption equilibrium was reached at 8 h with a removal efficiency of nearly 99.62%. At the same time, the solution pH increased persistently, indicating that the tobermorite could release OH− independently to regulate the pH. The pseudofirst-order (PFO) and pseudosecond-order (PSO) kinetic models, as described in Equations (4) and (5), were used to fit the kinetic data [37]. The linear plots and obtained parameters are presented in Figure 6 and Table 1, respectively. Compared with the PFO model, the correlation coefficient (R2 = 0.9993) of the PSO model was much higher. In addition, the theoretical value of Qe (26.32 mg/L) calculated from the PSO model was closer to the experimental observation (24.97 mg/L). Therefore, Cd2+ removal using tobermorite is better described with the PSO model, and the process tended to be chemical adsorption [38];
where t (h) is the contact time; Qe (mg/g) is the adsorption capacity at equilibrium; and k1 (1/h) and k1 (1/h) are the rate constants of the PFO and PSO models, respectively.
ln(Qe − Qt) = lnQe − K1t
t/Qt = 1/(K2Qe2) + t/Qe
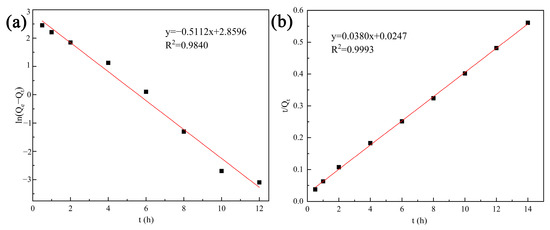
Figure 6.
Kinetic models for Cd2+ removal using tobermorite: (a) the PFO model and (b) the PSO model.

Table 1.
Kinetic model parameters for Cd2+ removal using tobermorite.
3.2.4. Effects of Initial Cd2+ Concentration and Adsorption Isotherms
The initial concentration of contaminants also exerts a critical influence during adsorption; hence, solutions with different initial Cd2+ concentrations were investigated, as exhibited in Figure 4d. The removal efficiency remained at a high level of above 99.49% within an initial concentration range of 50–400 mg/L. When the Cd2+ concentration was further increased to 500 mg/L, the removal efficiency decreased to 84.28%. However, the adsorption capacity persistently increased with the increasing Cd2+ concentration and reached a maximum value of 42.14 mg/g at the end. In addition, the solution pH after adsorption decreased from 9.7 to 7.2 with the increase in initial Cd2+ concentration. It can be inferred that the release of OH- from tobermorite might be inhibited in solutions with high Cd2+ concentrations. Isotherm data obtained in this study were analyzed using the Langmuir and Freundlich isotherm models, which are described in Equations (6) and (7), respectively [37]:
where Ce (mg/L) is the equilibrium concentration; Qe (mg/g) and Qm (mg/g) are the equilibrium and maximum adsorption capacities; KL (L/mg) and KF ((mg/g)/(mg/L)1/n) are the adsorption constants of the Langmuir and Freundlich models, respectively; and n is the empirical constant related to adsorption strength.
Ce/Qe = 1/(QmKL) + Ce/Qm
lnQe = lnKF + nlnCe
The nonlinear plots for both isotherm models are presented in Figure 7, and the calculated parameters are in Table 2. It was clear that Cd2+ removal using tobermorite was well described with the Langmuir model (R2 = 0.8983), which suggested that Cd2+ removal was a process of monolayer chemical adsorption that probably occurred through surface complexation with tobermorite [2,39]. Different studies also showed that Cd2+ removal with a range of other adsorbents was fitted better with the Langmuir model than with the Freundlich model [35,40,41]. In addition, Qm, as the maximum adsorption capacity calculated from the Langmuir model, could be used as the theoretical saturated adsorption capacity. The Qm for Cd2+ removal using tobermorite was 39.18 mg/g, which was relatively consistent with the experimental result of 42.14 mg/g. Table 3 gives a comparison of Qm values for Cd2+ adsorption for various relevant adsorbents. It could be noticed that tobermorite in this study outperformed other relevant adsorbents, since it had the highest Qm. Among them, the adsorption capacity of thermally treated limestone for Cd2+ was close to that of tobermorite. However, this method generally had the problem of high pH and Ca2+ concentration in the treated effluent, so it was usually used with other reagents or techniques in practice. In addition, tobermorite waste, such as broken silica bricks and aerated concrete, is considered to be a source of tobermorite for practical wastewater treatment applications. As a consequence, tobermorite could be considered as a more pre-eminent and economical adsorbent for Cd2+ in wastewater.
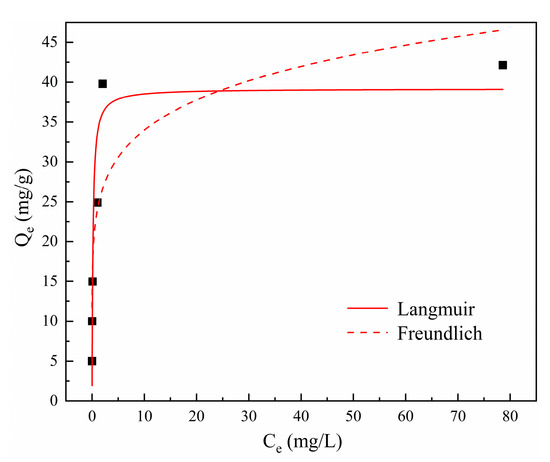
Figure 7.
Isotherm models for Cd2+ removal using tobermorite.

Table 2.
Isotherm model parameters for Cd2+ removal using tobermorite.

Table 3.
Comparison of Qm for Cd2+ using relevant adsorbents.
3.3. Mechanism of Cd2+ Removal Using Tobermorite
In order to explore the adsorption mechanism of Cd2+ removal using tobermorite, the XRD pattern of tobermorite after adsorption was detected, as shown in Figure 8a. The diffraction peaks associated with tobermorite remained, while the intensity of some peaks, especially the peak at 2θ = 7.9°, was reduced. It could be inferred that the ion exchange of Cd2+ and Ca2+ indeed occurred and affected the crystal structure of the tobermorite to some extent. Additionally, the new diffuse peak in the range of 2θ = 5–15° indicated that a certain amount of amorphous phase was generated after adsorption. Some studies reported that CSH would generate during the hydration process of tobermorite, as described in Equation (3) [31,46]. Zou et al. also revealed that the ion exchange between heavy metals and Ca2+ in tobermorite could lead to crystallinity damage, i.e., amorphization [47].
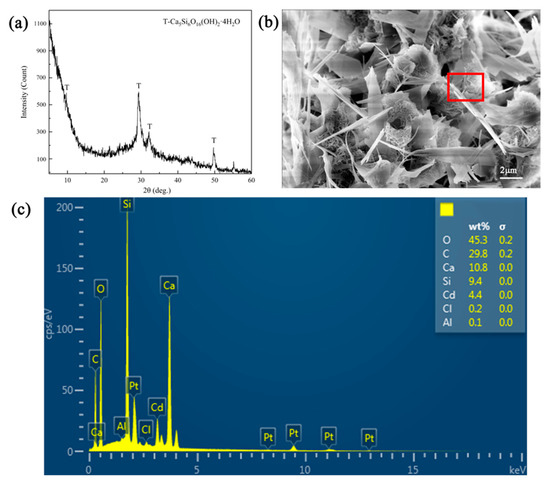
Figure 8.
(a) XRD pattern, (b) SEM and (c) EDS (the detection area was in the red box of (b)) images of tobermorite after Cd2+ adsorption.
Compared with the morphology of tobermorite before adsorption in Figure 2a,b, tobermorite after Cd2+ adsorption in Figure 8b still consisted of micron-sized rod- and sheet-like particles ascribing to its high stability. In addition, some small flocculent and clumpy structures appeared after adsorption; this might be associated with the amorphous phase. The EDS analysis in Figure 8c clearly shows that 4.40 wt% of Cd was detected on the surface of the tobermorite, demonstrating that Cd2+ was adsorbed.
XPS was employed to further compare tobermorite before and after adsorbing Cd2+. From the survey spectra in Figure 9a, signals of Ca, Si and O, which belonged to tobermorite, were observed before adsorption, and a new signal of elemental Cd appeared after adsorption, further validating the hypothesis of effective Cd2+ adsorption into tobermorite. Additionally, compared with tobermorite before adsorption, the peak intensities of Ca after adsorption decreased slightly, indicating that Ca participated in Cd2+ adsorption. The decrease was mainly due to the leaching of Ca2+ from the tobermorite through ion exchange [48,49]. The high-resolution XPS spectrum shown in Figure 9b is for Cd 3d. There were two broad and asymmetric peaks at 405.50 and 412.35 eV, which were attributable to Cd 3d5/2 and Cd 3d3/2, respectively. The subpeaks deconvoluted from Cd 3d5/2 were at 405.20 and 405.95 eV, and those from Cd 3d3/2 were at 412.00 and 412.70 eV, respectively. The two sets of subpeaks had a peak separation of ~6.8 eV, which could be attributed to CdCO3 and Cd(OH)2, respectively [50]. Therefore, it could be inferred that the monolayer of complex surface substances consisted of CdCO3 and Cd(OH)2.
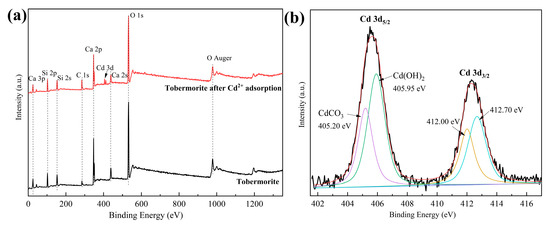
Figure 9.
(a) XPS survey scans of tobermorite before and after Cd2+ adsorption; (b) high-resolution XPS spectrum of Cd 3d.
Figure 10 presents the possible mechanism of Cd2+ removal using tobermorite. The tobermorite synthesized in this study had a high surface area and could release OH− spontaneously, which could have contributed to Cd2+ adsorption through surface complexation in the forms of Cd(OH)2 and CdCO3 first. Afterwards, when the Cd2+ concentration on the surface of the tobermorite reached a certain extent, ion exchange between Cd2+ and Ca2+ would occur on the surface. In addition, Cd2+ could diffuse into the tobermorite through the mesopores and exchange with Ca2+ in the interlayer space, which would be much easier than exchanging with Ca2+ in the bulk space of the tobermorite.
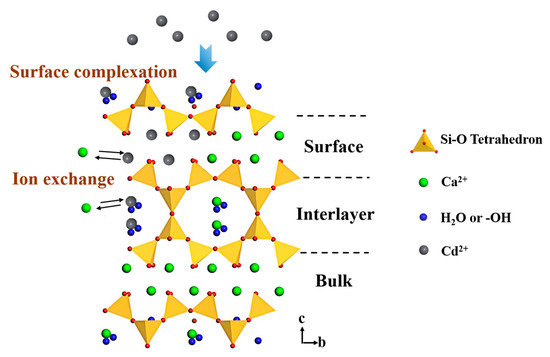
Figure 10.
Mechanism of Cd2+ removal using tobermorite.
3.4. Reusability Performance of Tobermorite
Tobermorite, as a kind of CSH, has the tendency of being dissolved in strongly acidic environments, so structure destruction may be caused by continuous use of acidic eluents. In this study, the reusability and stability of tobermorite were investigated through conducting consecutive Cd2+ adsorption–desorption experiments using 0.1 M NaOH as an eluent. As displayed in Figure 11a, the removal efficiency of Cd2+ using tobermorite decreased from 99.69% to 62.47% after three adsorption–resolution cycles. This result might be due to the strong interaction between Cd2+ and tobermorite and the gradual consumption of the active sites and Ca2+ available. From an application point of view, the reuse cycle of two to three times was more suitable for tobermorite in Cd2+ adsorption, and performing more cycles was of little economic significance due to poor removal efficiency and adsorption capacity. Nonetheless, after the last cycle, Cd2+ could be recovered with desorption and the residual tobermorite could be recycled as a raw material to prepare new, environmentally friendly materials, such as sintered silicate ceramsites and bricks, according to our previous studies [51,52]. This strategy could recycle Ca and Si resources and simultaneously immobilize the residual Cd in tobermorite via sintering at high temperatures.
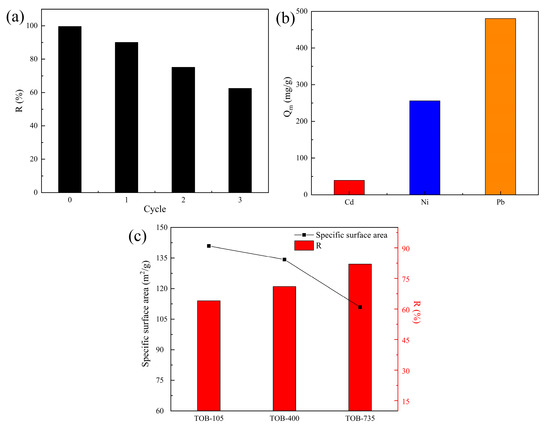
Figure 11.
(a) Reusability of tobermorite for Cd2+ adsorption. (b) Qm values of tobermorite for Cd2+, Ni2+ and Pb2+. (c) Specific surface areas and removal efficiency of Ni2+ with different modified tobermorites.
3.5. Ni2+ and Pb2+ Removal Using Tobermorite
In order to investigate the adsorption universality of tobermorite for heavy metals, two other typical divalent heavy-metal ions, Ni2+ and Pb2+, were selected to conduct batch adsorption experiments at different initial Ni2+/Pb2+ concentrations. The isotherm data obtained therein was also well fitted with the Langmuir model, and the Qm values calculated for Ni2+ and Pb2+ were 256.31 and 480.02 mg/g, respectively, as shown in Figure 11b. It can be concluded that tobermorite is an efficient and universal adsorbent for most heavy-metal ions. Moreover, the Qm values for Ni2+ and Pb2+ were clearly higher than that for Cd2+, which might be attributed to the different removal mechanisms and affinity of tobermorite for different heavy-metal ions. Further investigation and explanation of this phenomenon will be the focus of our future study.
Generally, a higher specific surface area of an adsorbent means more porosity and more abundant active sites, which may contribute to a higher adsorption capacity for contaminants [53]. However, in some specific cases, specific surface area and adsorption capacity are not necessarily proportional to each other, so more factors need to be taken into account. For example, Guo et al. found that Al substitution would decrease the specific surface area of tobermorite from 198.83 m2/g to 80.48 m2/g through refining pore structure and particle size; at the same time, it was found that the adsorption capacities of Pb2+ and Cu2+ would decrease, but the adsorption capacity of Cr6+ would increase [54]. Wang et al. synthesized tobermorite hydrothermally from fly ash at different reacting temperatures (180–260 °C) to remove Pb2+. These results indicated that tobermorite could obtain its highest specific surface area of 31.04 m2/g at 200 °C, while the optimal adsorption capacity for Pb2+ was reached at 180 °C [21]. In this study, the possible relationship between specific surface area and adsorption capacity for heavy metals was also explored using different tobermorites. The tobermorite prepared (dried at 105 °C) was further calcined at 400 and 735 °C; these samples were labeled as TOB-105, TOB-400 and TOB-735, respectively. As shown in Figure 11c, the specific surface areas of the three tobermorites were 140.92, 134.25 and 110.89 m2/g, respectively. During the calcination process, the 1.1 nm tobermorite was gradually transformed into 0.9 nm tobermorite through dehydration and dehydroxylation [53], resulting in a decrease in the number of porous channels and specific surface area. The adsorption experiments were carried out using the three tobermorites at an initial Ni2+ concentration of 400 mg/L, an adsorbent dosage of 50 mg/50 mL and a contact time of 6 h. The removal efficiency of Ni2+ for each sample was 64.11, 71.23 and 82.05% respectively, as displayed in Figure 11c. This could be attributed to the activation of Ca2+ in tobermorite at high temperatures [54], which increases the capacity of ion exchange between Ca2+ and Ni2+ during adsorption. Considering that the removal mechanism of Ni2+ (surface complexation–ion exchange) was similar to that of Cd2+ using tobermorite, an improvement in the adsorption capacity for Cd2+ could be achieved through activating Ca2+ in tobermorite in our future study. It could also be inferred that the adsorption performance of some adsorbents with considerably high specific surface areas could be optimized through enhancement of some specific functions rather than through continuous improvement of specific surface area.
4. Conclusions
In this study, with smelting quartz powder (waste material) and quicklime used as raw materials, tobermorite with pH self-adjustment ability was successfully synthesized, and its potential application for Cd2+ removal was investigated. It was found that the well-crystallized tobermorite consisted of micron-sized rod- and sheet-like structures, resulting in formation of mesopores and a large specific surface area of 140.92 m2/g. An alkaline environment in a solution could be created from tobermorite autonomously due to its hydration. Batch adsorption experiments proved that tobermorite had a high removal efficiency of 99.71% for Cd2+, and its kinetic data was best fitted with the PSO model. The Langmuir model provided the best description for its isotherm data, and the Qm was 39.18 mg/g. In addition, the removal efficiency of Cd2+ was kept at a high level, above 99.6%, in an initial pH range of 2.5–8.5. The removal mechanism of Cd2+ involved surface complexation with OH- released from tobermorite as well as ion exchange with Ca2+ from the surface and the interlayer space of the tobermorite. In addition, an adsorption–desorption cycle of two to three times was found to be more suitable for tobermorite in Cd2+ adsorption, whereas tobermorite after final desorption could be recycled as a raw material to prepare new, environmentally friendly materials, such as sintered silicate ceramsites and bricks, making it attractive from a sustainability point of view. The tobermorite also had high Qm values of 256.31 and 480.02 mg/g for Ni2+ and Pb2+, respectively, indicating that it had a good adsorption universality for additional heavy metals. Consequently, this study not only indicated that tobermorite is an effective and low-cost adsorbent for Cd2+ but also provided a feasible way to recycle torbermorite waste.
Author Contributions
Conceptualization, J.Q.; methodology, J.Q. and S.Y.; software, J.Q.; validation, J.Q., S.Y. and C.T.; formal analysis, J.Q. and S.Y.; investigation, S.Y.; resources, J.Q. and C.T.; data curation, J.Q. and S.Y.; writing—original draft preparation, J.Q.; writing—review and editing, J.Q., M.C.-U., K.O. and C.T.; visualization, J.Q. and M.C.-U.; supervision, J.Q. and C.T.; project administration, J.Q. and K.O.; funding acquisition, J.Q. and C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 51802162) and the Natural Science Foundation of Jiangsu Province (No. BK20180955).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Part of this work was performed at the Waseda Research Institute for Science and Research Organization for Open Innovation Strategy, Sustainable Energy & Environmental Society Open Innovation Research Organization, Waseda University. The authors thank Qinyi Zhao from the Nanjing University of Science and Technology and the Zhejiang Zhongjin Environmental Protection Technology Co., Ltd. for their help in the Pb and Ni adsorption experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardinale, A.M.; Carbone, C.; Fortunato, M.; Fabiano, B.; Reverberi, A.P. ZnAl-SO4 layered double hydroxide and allophane for Cr(VI), Cu(II) and Fe(III) adsorption in wastewater: Structure comparison and synergistic effects. Materials 2022, 15, 6887. [Google Scholar] [CrossRef] [PubMed]
- Takaya, Y.; Kadokura, M.; Kato, T.; Tokoro, C. Removal mechanisms of arsenite by coprecipitation with ferrihydrite. J. Environ. Chem. Eng. 2021, 9, 105819. [Google Scholar] [CrossRef]
- Sirohi, R.; Joun, J.; Lee, J.Y.; Yu, B.S.; Sim, S.J. Waste mitigation and resource recovery from food industry wastewater employing microalgae-bacterial consortium. Bioresour. Technol. 2022, 352, 127129. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Tovar, C.; Villabona-Ortiz, A.; González-Delgado, Á. Adsorption study of continuous heavy metal ions (Pb2+, Cd2+, Ni2+) removal using cocoa (Theobroma cacao L.) pod husks. Materials 2022, 15, 6937. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Mainali, B.; Angove, M.J. Manganese oxides and their application to metal ion and contaminant removal from wastewater. J. Water Process Eng. 2018, 26, 264–280. [Google Scholar] [CrossRef]
- Du, B.Y.; Zhou, J.; Lu, B.X.; Zhang, C.; Li, D.M.; Zhou, J.; Jiao, S.J.; Zhao, K.Q.; Zhang, H.H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef]
- Suzuki, K.; Kato, T.; Fuchida, S.; Tokoro, C. Removal mechanisms of cadmium by δ-MnO2 in adsorption and coprecipitation processes at pH 6. Chem. Geol. 2020, 550, 119744. [Google Scholar] [CrossRef]
- Fuchida, S.; Tajima, S.; Tokoro, C. Surface complexation modeling of Cd on Mn(III) oxyhydroxide (γ-MnOOH) for neutralizing model of acid mine drainage. Resour. Process. 2021, 67, 117–121. (In Japanese) [Google Scholar] [CrossRef]
- Qiu, B.B.; Tao, X.D.; Wang, H.; Li, W.K.; Ding, X.; Chu, H.Q. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Lu, Z.X.; Li, X.Z.; Qi, X.J. Cobalt-loaded resin can effectively remove arsenic in wastewater. Environ. Technol. Innov. 2021, 21, 101354. [Google Scholar] [CrossRef]
- Muñoz, S.V.; Martínez, M.S.; Torres, M.G.; Alcalá, S.P.; Quintanilla, F.; Rodríguez-Canto, A.; Rodríguez, J.R. Adsorption and removal of cadmium ions from simulated wastewater using commercial hydrophilic and hydrophobic silica nanoparticles: A comparison with sol-gel particles. Water Air Soil Pollut. 2014, 225, 2165. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents-a review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Da’na, E. Adsorption of Heavy Metals on Functionalized-Mesoporous Silica: A Review. Microporous Mesoporous Mat. 2017, 247, 145–157. Available online: https://elkssl0a75e822c6f3334851117f8769a30e1clib.v.ntu.edu.cn:4443/10.1016/j.micromeso.2017.03.050 (accessed on 23 October 2022).
- Sun, X.L.; Yi, Y.L. pH evolution during water washing of incineration bottom ash and its effect on removal of heavy metals. Waste Manag. 2020, 104, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.S.; Huang, L.B.; Nguyen, T.A.H.; Ok, Y.S.; Rudolph, V.; Yang, H.; Zhang, D.K. Copper and zinc adsorption by softwood and hardwood biochars under elevated sulphate-induced salinity and acidic pH conditions. Chemosphere 2016, 142, 64–71. [Google Scholar] [CrossRef]
- Komarneni, S.; Komarneni, J.S.; Newalkar, B.; Stout, S. Microwave-hydrothermal synthesis of Al-substituted tobermorite from zeolites. Mater. Res. Bull. 2002, 37, 1025–1032. [Google Scholar] [CrossRef]
- Liao, L.N.; Zhang, P. Preparation and characterization of polyaluminum titanium silicate and its performance in the treatment of low-turbidity water. Processes 2018, 6, 125. [Google Scholar] [CrossRef]
- Mitra, N.; Sarkar, P.K.; Prasad, D. Intermolecular dynamics of ultraconfined interlayer water in tobermorite: Influence on mechanical performance. Phys. Chem. Chem. Phys. 2019, 21, 11416–11423. [Google Scholar] [CrossRef]
- Liao, W.; Li, W.Q.; Fang, Z.G.; Lu, C.H.; Xu, Z.Z. Effect of different aluminum substitution rates on the structure of tobermorite. Materials 2019, 12, 3765. [Google Scholar] [CrossRef]
- Siauciunas, R.; Smalakys, G.; Eisinas, A.; Prichockiene, E. Synthesis of high crystallinity 1.13 nm tobermorite and xonotlite from natural rocks, their properties and application for heat-resistant products. Materials 2022, 15, 3474. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xu, L.H.; Wu, D.S.; Zheng, S.L. Hydrothermal synthesis of mesoporous tobermorite from fly ash with enhanced removal performance towards Pb2+ from wastewater. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 632, 127775. [Google Scholar] [CrossRef]
- Monasterio, M.; Gaitero, J.J.; Manzano, H.; Dolado, J.S.; Cerveny, S. Effect of chemical environment on the dynamics of water confined in calcium silicate minerals: Natural and synthetic tobermorite. Langmuir 2015, 31, 4964–4972. [Google Scholar] [CrossRef]
- Moriyama, K.; Kojima, T.; Minawa, Y.; Matsumoto, S.; Nakamachi, K. Development of artificial seed crystal for crystallization of calcium phosphate. Environ. Technol. 2001, 22, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.F.; He, J.T.; Zheng, H.; Huang, G.X. Experimental study on treating low phosphorus wastewater by combination of tobermorite and calcium fluoride. Environ. Sci. Technol. 2009, 32, 158–161. (In Chinese) [Google Scholar] [CrossRef]
- Tian, W.; Shui, A.; Ke, S.J.; Huang, L.L.; Xi, X.; He, C.; Chen, W.W.; Du, B. Low-temperature preparation of humidity self-regulating porous ceramics with high strength performance. Mater. Lett. 2019, 243, 128–131. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Li, T.; Cui, C.; Wang, Z.Z.; Ding, X.F.; Zhang, S.H. Preparation of porous silica powder via selective acid leaching of calcined tobermorite. Powder Technol. 2020, 375, 420–432. [Google Scholar] [CrossRef]
- Mahmoud, H.R.; El-Molla, S.A.; Saif, M. Improvement of physicochemical properties of Fe2O3/MgO nanomaterials by hydrothermal treatment for dye removal from industrial wastewater. Powder Technol. 2013, 249, 225–233. [Google Scholar] [CrossRef]
- Li, R.C.; Zheng, S.L.; Sun, Z.M.; Li, C.Q. Research on synthesis of flaky tobermorite from diatomite and its mechanism. Inorg. Chem. Ind. 2021, 53, 24–29. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, X.G.; Gou, Y.; Peng, H.; Mao, Y.T.; Wen, J. Nonthermal plasma sulfurized CuInS2/S-doped MgO nanosheets for efficient solar-light photocatalytic degradation of tetracycline. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 625, 126900. [Google Scholar] [CrossRef]
- Zhu, X.H.; Jia, X.H. Removal of chromium (III) from monoammonium phosphate solutions by a porous adsorbent of fluor (calcium silicate) composites. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2020, 35, 384–392. [Google Scholar] [CrossRef]
- Cao, P.X.; Li, G.H.; Luo, J.; Rao, M.J.; Jiang, H.; Peng, Z.W.; Jiang, T. Alkali-reinforced hydrothermal synthesis of lathy tobermorite fibers using mixture of coal fly ash and lime. Constr. Build. Mater. 2020, 238, 117655. [Google Scholar] [CrossRef]
- Kamei, S.; Ihara, T.; Ouchi, T.; Uzawa, M.; Machinaga, O. A novel synthesis of phosphorus-substituted tobermorite with calcium silicate hydrate. J. Ceram. Soc. Jpn. 2014, 122, 664–667. [Google Scholar] [CrossRef]
- Wang, X.B.; Pan, Z.H. Chemical changes and reaction mechanism of hardened cement paste-(NH4)2SO4-H2O-system. Constr. Build. Mater. 2017, 152, 434–443. [Google Scholar] [CrossRef]
- Dai, S.W.; Wen, Q.; Huang, F.; Bao, Y.Q.; Xi, X.D.; Liao, Z.P.; Shi, J.; Ou, C.J.; Qin, J. Preparation and application of MgO-loaded tobermorite to simultaneously remove nitrogen and phosphorus from wastewater. Chem. Eng. J. 2022, 446, 136809. [Google Scholar] [CrossRef]
- Shi, Q.L.; Zhang, H.; Shahab, A.; Zeng, H.H.; Zeng, H.T.; Bacha, A.U.R.; Nabi, I.; Siddique, J.; Ullah, H. Efficient performance of magnesium oxide loaded biochar for the significant removal of Pb2+ and Cd2+ from aqueous solution. Ecotox. Environ. Safe. 2021, 221, 112426. [Google Scholar] [CrossRef] [PubMed]
- Ianăşi, C.; Picioruş, M.; Nicola, R.; Ciopec, M.; Negrea, A.; Nižňanský, D.; Len, A.; Almásy, L.; Putz, A.M. Removal of cadmium from aqueous solutions using inorganic porous nanocomposites. Korean J. Chem. Eng. 2019, 36, 688–700. [Google Scholar] [CrossRef]
- Ou, C.J.; Dai, S.W.; Li, S.X.; Xu, J.; Qin, J. Adsorption performance and mechanism investigation of Mn2+ by facile synthesized ceramsites from lime mud and coal fly ash. Korean J. Chem. Eng. 2021, 38, 505–513. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Kim, K.H.; Kumar, S. Carbon nanotubes as sorbent material for removal of cadmium. J. Mol. Liq. 2017, 242, 966–970. [Google Scholar] [CrossRef]
- Tokoro, C.; Kato, T. Arsenate removal by resin-supported ferric ions: Mechanism, modeling, and column study. Adv. Powder Technol. 2021, 32, 1943–1950. [Google Scholar] [CrossRef]
- Javaheri, F.; Kheshti, Z.; Ghasemi, S.; Altaee, A. Enhancement of Cd2+ removal from aqueous solution by multifunctional mesoporous silica: Equilibrium isotherms and kinetics study. Sep. Purif. Technol. 2019, 224, 199–208. [Google Scholar] [CrossRef]
- Takdastan, A.; Samarbaf, S.; Tahmasebi, Y.; Alavi, N.; Babaei, A.A. Alkali modified oak waste residues as a cost-effective adsorbent for enhanced removal of cadmium from water: Isotherm, kinetic, thermodynamic and artificial neural network modeling. J. Ind. Eng. Chem. 2019, 78, 352–363. [Google Scholar] [CrossRef]
- Schütz, T.; Dolinská, S.; Hudec, P.; Mockovčiaková, A.; Znamenáčková, I. Cadmium adsorption on manganese modified bentonite and bentonite-quartz sand blend. Int. J. Miner. Process. 2016, 150, 32–38. [Google Scholar] [CrossRef]
- Lai, C.H.; Chen, C.Y.; Wei, B.L.; Yeh, S.H. Cadmium adsorption on goethite-coated sand in the presence of humic acid. Water Res. 2002, 36, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Gu, B.W.; Kim, Y.K.; Park, S.J. Removal of Cu2+, Cd2+ from water using thermally treated lime stone. KSWST Jour. Wat. Treat. 2016, 24, 85–94. (In Korean) [Google Scholar] [CrossRef]
- Coleman, N.J.; Brassington, D.S.; Raza, A.; Lee, W.E. Calcium silicate sorbent from secondary waste ash: Heavy metals-removal from acidic solutions. Environ. Technol. 2006, 27, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Lu, L.C.; Wang, S.D.; Zhao, P.Q.; Zhang, W.L.; Zhang, S.X. Investigation on the formation of tobermorite in calcium silicate board and its influence factors under autoclaved curing. Constr. Build. Mater. 2017, 143, 280–288. [Google Scholar] [CrossRef]
- Zou, J.J.; Guo, C.B.; Zhou, X.Q.; Sun, Y.J.; Yang, Z. Sorption capacity and mechanism of Cr3+ on tobermorite derived from fly ash acid residue and carbide slag. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 538, 825–833. [Google Scholar] [CrossRef]
- Cai, Y.C.; Li, C.L.; Wu, D.; Wang, W.; Tan, F.T.; Wang, X.Y.; Wong, P.K.; Qiao, X.L. Highly active MgO nanoparticles for simultaneous bacterial inactivation and heavy metal removal from aqueous solution. Chem. Eng. J. 2017, 312, 158–166. [Google Scholar] [CrossRef]
- Gordienko, P.S.; Yarusova, S.B.; Suponina, A.P.; Yakimenko, L.V. Sorption of Cd2+ ions by silicate materials of different origins. Russ. J. Gen. Chem+. 2013, 83, 2709–2714. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.Q.; Mao, X.M.; Li, H. Efficient removal of Cu(II) from an aqueous solution using a novel chitosan assisted EDTA-intercalated hydrotalcite-like compound composite: Preparation, characterization, and adsorption mechanism. Chem. Eng. J. 2022, 438, 135531. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.Y.; Hussain, A.; Yang, C.M.; Yang, S.H. Recycling of lime mud and fly ash for fabrication of anorthite ceramic at low sintering temperature. Ceram. Int. 2015, 41, 5648–5655. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.Y.; Hussain, A.; Yang, C.M. Preparation and characterization of ceramsites from lime mud and coal fly ash. Constr. Build. Mater. 2015, 95, 10–17. [Google Scholar] [CrossRef]
- Guo, X.L.; Shi, H.S. Microstructure and heavy metal adsorption mechanisms of hydrothermally synthesized Al-substituted tobermorite. Mater. Struct. 2017, 50, 245. [Google Scholar] [CrossRef]
- Yang, X.L. Reactivity Waster AAC and Preparation and Application of Silicate-Shell-Ceramsite. Ph.D. Thesis, Nanjing University of Science & Technology, Nanjing, China, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).