Abstract
Oxide metallurgy technology can improve the microstructure of a coarse-grained heat-affected zone (CGHAZ) but introduces extra inclusions. Local corrosion behavior of the CGHAZ of a Zr–Ti–Al–RE deoxidized steel was investigated in this work using theoretical calculations and experimental verification. The modified inclusions have a (Zr–Mg–Al–Ca–RE)Ox core claded by a CaS and TiN shell. CaS dissolves first, followed by the oxide core, leaving TiN parts. This confirms that the addition of rare earth can reduce lattice distortion and prevent a galvanic couple between the inclusions and the matrix, while the chemical dissolution of CaS causes localized acidification, resulting in the pitting corrosion initiation.
1. Introduction
High-strength low-alloy (HSLA) steels are important in the construction of offshore platforms due to their weldability and high cracking resistance [1,2]. An important method for bonding steel for large structures in industrial applications is welding. High heat input is typically utilized to improve welding efficiency and lower production cost [3]. During the welding thermal cycle, the coarse-grained heat-affected zones (CGHAZs) experience a fast heating rate and a high heating peak temperature, resulting in the abnormal growth of the grains and the development of complex microstructures such as granular bainite (GB), lath bainite (LB), acicular ferrite (AF) and martensite–austenite (M/A) constituents. HSLA steels can be classified and defined according to their morphologies and characteristics. GB consists of elongated ferrite sheaves with blocky M/A constituents. LB is formed with parallel laths at fast cooling rates. AF is staggered, interlocked and formed on non-metallic inclusions. The M/A constituent is a mixture of untempered martensite embedded in carbon-enriched retained austenite [4,5]. As a result, the CGHAZ is considered to be the weakest area in the welded joint [6]. Oxide metallurgy technology is one of the effective solutions for introducing specific fine, non-metallic inclusion particles into steels to refine the grain size and/or induce the formation of a beneficial microstructure. The introduction of fine and uniform, non-metallic inclusion can inhibit the abnormal growth of grains in the heating process and induce the nucleation of acicular ferrite in the cooling process [2,7]. Thus, the application of oxide metallurgy technology is promising for refining the microstructure and improving the mechanical properties of the CGHAZ.
Generally, the types of non-metallic inclusions—such as Al2O3, MnS, ZrO2, CaO and Al2MgO4—introduced in steels depend on the specific deoxidation techniques [8,9,10,11,12,13,14,15]. Inclusions in the steels are often considered as defects that can preferably be attacked by chloride ions in the marine environment, which induce pitting corrosion [4,16,17] that has a detrimental impact on application performance [18,19,20,21,22,23]. Numerous studies have been conducted on the local corrosion caused by inclusions in HSLA steels. Some researchers believe that galvanic corrosion could occur between the inclusions and the adjacent matrix. Hou et al. investigated the pitting behavior of Al–Ti–Mg deoxidized steel and found that MnS had a lower surface potential than Al2O3 and Al2MgO4 and acted as an anode more easily [8]. Wei et al. found that pitting corrosion behavior is related to the microgalvanic effect between MnS and the matrix [15]. However, Liu et al. found that most of the non-metallic inclusions have weak conductivity. Their research shows that ZrO2-Al2O3-Ti2O3 inclusions do not form a galvanic couple with the adjacent matrix [24]. Instead, the main factors that are assumed to induce pitting behavior are the microcracks and high dislocation density areas around the irregular Al2O3. In addition to the shape, type and conductivity of the inclusions, the distribution and size of the inclusions have a significant impact on the pitting initiation. Normally, the finer the inclusions, the higher the pitting potential and the greater the contribution to the pitting resistance of the steel [18].
Rare earth treatment is often used by researchers to effectively improve the corrosion resistance of steels because the rare earth (RE) elements have a strong affinity to oxygen and sulfur, capable of refining and spheroidizing the inclusions and densifying the corrosion layer [18,25,26,27,28]. In a simulated marine environment, Liu et al. analyzed the pitting behavior of a Q460NH steel treated with RE and found that the pitting corrosion starts from the (RE)2O2S-(RE)xSy rather than the matrix, suggesting that the matrix after RE treatment has higher corrosion resistance than the inclusions in the simulated solution [29]. Liu et al. discovered that the initiation of pitting corrosion in Zr–Ti deoxidized low-alloy steel was caused by matrix dissolution caused, in turn, by lattice distortion and microcrevices around ZrO2-Ti2O3-Al2O3 inclusions. After adding rare earth, the lattice distortion of inclusions decreased dramatically and the dissolution of the (RE)2O2S-(RE)xSy caused pitting corrosion [30]. Using the first-principles theory, Tang et al. calculated the work function of rare earth oxysulfide and confirmed that rare earth sulfide is easier to dissolve, from an atomic scale perspective, than rare earth oxide [31]. The mechanism of inclusion-induced local corrosion is still controversial. Whether the composite inclusions and the matrix constitute galvanic corrosion, crevice corrosion or the dissolution order needs to be further investigated [8,10,15,24,29,32,33,34,35].
Previous studies demonstrated that the CGHAZ exhibits a higher corrosion tendency than the base metal and weld metal and is more likely to trigger anodic dissolution (AD) behavior in the entire welded joint because of its coarse grains, complex microstructure [36,37,38], welding residual stress and crystal defects [16,39,40]. The application of oxide metallurgy technology may change the environment where corrosion occurs in the CGHAZ. For example, use of oxide metallurgy technology, such as Widmanstätten ferrite in the CGHAZ, will affect the microstructure formed in the welding thermal cycle and can improve corrosion resistance [38]. It also introduces different types of inclusions, and the difference in electrochemical information on the surface of these inclusions may affect pitting initiation behavior. In addition, oxide metallurgy techniques may lead to the distribution of aggregated inclusions in the matrix, which may cause an effective increase in the active sites of pitting initiation. Thus, to improve the mechanical properties of the CGHAZ, oxide metallurgy technology and rare earth microalloying often result in more complex inclusions, posing challenges in the study of the corrosive behavior of inclusions [11,13,41,42].
In this paper, the achievement of oxide metallurgy technology in modifying and refining inclusions and improving the microstructure of the CGHAZ in an HSLA steel were first evaluated. Next, the formation and evolution of inclusions in CGHAZ during the welding process were investigated, followed by the study of the pitting behavior of Zr–Ti–Al–RE composite inclusions in a CGHAZ, in a simulated marine environment, by means of an immersion test, use of a scanning electron microscope equipped with an energy dispersive spectroscope (SEM-EDS) and density functional theory.
2. Experimental Section
2.1. Sample Preparation and CGHAZ Simulation
Table 1 displays the chemical composition of the 12 mm-thick tested steel, which was deoxidized by Zr–Ti–Al–RE, where the RE elements refer to La and Ce. The steel was produced by thermomechanical controlled processing (TMCP) in a domestic steel factory in China. The microstructure in CGHAZ was generated using the Gleeble 3800 Thermo-Mechanical simulator (Dynamic Systems Inc., Poestenkill, NY, USA) with a heat input of 100 kJ/cm for submerged-arc welding. Machined samples with dimensions of 11 mm × 11 mm × 75 mm were reheated to peak temperature of 1320 °C at a rate of 200 °C/s, maintained for 2 s and then cooled according to Rykalin 3D models (formulation (1)) [43]:
where E is heat input, kJ/cm, λ is thermal conductivity, J/(cm·s·°C), and T0 is the initial temperature, °C. The t8/5 corresponding to 100 kJ/cm heat input was 45 s.

Table 1.
Chemical composition of the tested steel (wt, %).
2.2. Characterization of Microstructure and Complex Inclusions
To analyze the microstructure in CGHAZ, samples with dimensions of 10 mm × 10 mm × 5 mm, which were cut from the welding thermal cycle test, were ground with SiC paper from 200 to 2000 grits. The samples were then polished with diamond paste (2.5 μm), cleaned quickly with ethanol and, finally, etched with 4% nital (4 mL HNO3 + 96 mL C2H5OH). The morphology was observed using a scanning electron microscope (SEM, FEI Nova400, Lincoln, NE, USA) and transmission electron microscope (TEM, JEOL JEM-2100F, Akishima, Japan). Several inclusions were separated from the matrix by electrolysis in non-aqueous solution (pH ≈ 8) at 0~5 °C for further chemical composition and morphology observation [44]. 500 mL of the electrolyte solution was prepared, which consisted of 5.0 g of tetramethylammonium chloride, 3.0 g of anhydrous barium oxide, 50 mL of acetylacetone and 450 mL of anhydrous methanol. A scanning electron microscope (SEM, FEI Nova400) with energy dispersive spectroscopy (EDS) was used to scan random positions on the polished sample surface to characterize the chemical composition, morphology and particle size of inclusions. For the subsequent electron backscattered diffraction (EBSD) tests, the test samples were polished with diamond paste (2.5 µm) and then ionized by milling using GATAN 685.O (Pleasanton, CA, USA). The EBSD test was performed using a field emission scanning electron microscope (FE-SEM) at a voltage of 20 kV, a current of 13 nA and a step size of 0.1 µm. The data was analyzed by OIM software (Version: 5.3) to obtain the inverse pole figure (IPF) map, kernel average misorientation (KAM), image quality (IQ) diagrams to determine the crystal orientation of microstructure, and lattice distortion between the inclusions and matrix. The JMatPro software (Version: 7.0) was utilized to calculate the precipitation behavior of inclusions during the solidification process (1600–600 °C). Origin 8.0 software was used to plot the solid–solution behavior of the nanoparticles and temperature during the welding thermal cycle.
2.3. Electrochemical Tests
The potentiodynamic polarization tests, which employed a three-electrode cell composed of working electrode, counter electrode (platinum plate electrode) and reference electrode (saturated calomel electrode (SCE)) at room temperature, were conducted on electrochemical workstation (E4, Kronach Zahner, Germany). The 500 mL solution contained 3.5 wt.% NaCl (Chinese Medicine Chemical Reagent Co., Ltd., Shanghai, China). The potentiodynamic scans began at −300 mV vs. OCP and continued until the current density reached 1 mA·cm−2. The scan rate was 0.333 mVs−1. All electrochemical measurements were performed more than three times for greater reproducibility [31,45]. Electrochemical impedance spectroscopy (EIS) tests were performed at a frequency ranging from 105 Hz to 0.01 Hz with amplitude of 10 mV. ZView software (Version: 3.30d) was used to analyze impedance data of Nyquist and Bode plots.
2.4. Immersion Test
To investigate the effect of Cl− on the pitting initiation and propagation of composite inclusions by ex situ SEM observations in a simulated marine environment, a series of immersion tests (5 s, 1 min, 1 h and 2 h) were conducted. To better observe the pitting behavior of inclusions, the immersion test was conducted in 0.1 wt.% NaCl and the rust on the surface of the immersed sample was removed by using a solution of 50% HCl + 50% deionized water + 5 g/L C6H12N4 [46]. An FE-SEM with EDS was used to analyze the local corrosion morphology of the specimens before and after rust removal. For each stage of localized corrosion, the observation experiment was repeated at least three times, and at least five areas were observed in each sample.
2.5. First-Principles Calculation
The physical properties of the inclusions and body-centered cubic Fe (BCC), such as density of states, elastic constant and work function, were calculated to better elucidate the local corrosion mechanism. The conductivity of inclusions is explained by the density of states calculation. Furthermore, this investigates whether there is a microgalvanic effect between the inclusions and the matrix. The elastic constants were calculated to determine if the differences in physical properties between the inclusions and the Fe matrix might cause stress concentrations or the development of microcracks. The work function (WF), which represents the ability of electrons to escape to the metal surface, is used to assess the corrosion resistance of inclusions in tested steel. All calculations are based on density functional theory (DFT) and are performed with the Vienna ab initio simulation package (VASP) and the projector-augmented wave (PAW) method [47]. Here, the generalized gradient approximation (GGA) is adopted, and the Monkhorst–Pack grids are used in the Brillouin zone integration. All DFT models are guaranteed to have the same stoichiometric ratio as the molecular formula ratio. The setting of the K point roughly satisfies K * a (lattice constant) ≈ 45. The thickness of the vacuum layer is set to 15 Å. Dipole correction is applied along the thickest side. Depending on the type of inclusion, a cut-off energy range from 400 eV to 600 eV is selected. The convergence criteria for energy and force are set to 1.0 × 10−5 eV/atom and 0.01 eV/Å, respectively. The calculation of the density of states is conducted on the relaxed unit cell. The work function is expressed as follows (Equation (2)):
where Ve represents the vacuum level and Ef represents the Fermi level. Considering the relatively lower surface energy and more stable crystals of the low-index crystalline planes, three low-index crystalline planes, (100), (110) and (111), were selected to calculate the inclusions’ work function with the periodic slab model [31]. To simulate the bulk phase, a few atom layers at the bottom of the slab were fixed in their optimized positions. Meanwhile, at least two atomic layers were relaxed. As shown in Equations (3)–(10), the Voigt–Reuss–Hill (VRH) approximation method was used to determine the elastic mechanical properties of the inclusions and the matrix [48,49]:
where Cij represents the elastic stiffness constant and Sij represents the elastic compliance constant. BV and BR, and GV and GR are the bulk modulus and shear modulus, calculated by the Voigt and Reuss approximation methods, respectively. G, B, E and μ represent shear modulus, bulk modulus, Young ‘s modulus and Poisson ‘s ratio, respectively.
3. Results and Discussion
3.1. Formation and Evolution of the Composite Inclusions in CGHAZ
3.1.1. Microstructure and Inclusion Analysis
The microstructure was composed of GB and AF with blocky M/A constituents which were distributed discontinuously at the sub-boundary after etching with 4% nital for 8~12 s (Figure 1). At a heat input of 100 kJ/cm, the average impact energy at −20 °C of the simulated specimen reached 370 J, which indicates that Zr–Ti–Al–RE deoxidization guarantees the improved mechanical properties in the CGHAZ. Meanwhile, it was found that some inclusions prompted the formation of acicular ferrite. As shown in Figure 1b, several acicular ferrite grains emanated from a spherical inclusion, which could prevent or deviate the crack prorogation and improve the toughness [21,50]. High-strength low-alloy (HSLA) steels experience severe grain coarsening and loss of toughness as a consequence of welding heat input, particularly high heat input [5,51]. However, inclusions and nanoparticles such as TiN and Ti2O3 can effectively inhibit the austenite grain growth in the range of 1200–1350 °C and, during cooling, prompt the formation of ideal acicular ferrite, which could significantly improve the toughness of the CGHAZ [52,53].
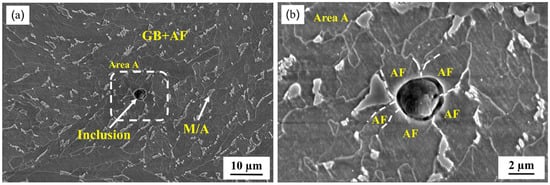
Figure 1.
(a) Scanning electron microscopy microstructure of the CGHAZ., (b) morphology of the enlarged part in the white box in (a). (GB: granular bainite, AF: acicular ferrite, M/A: martensite–austenite constituents).
To better observe and analyze the composite inclusions, they were separated by electrolysis in a non-aqueous solution (Figure 2a). In this work, the inclusions are found to be almost spherical in shape, forming a typical core–shell structure, with (RE–Mg–Al–Ca)–OxSy in the center and CaS and TiN at the periphery. The addition of Zr, Ca and RE has an obvious spheroidization effect on the inclusions [54,55,56]. The appearance of Mg is attributed to the refractory bricks used in steelmaking. However, Zr was not detected on this exposed surface. Rare earth elements could induce various types of inclusions such as (Al, Zr, RE)-Ox, (RE)2OxSy, and (RE)xSy [31,55]. According to statistical analysis, (RE–Zr–Mg–Al–Ca)–OxSy–TiN composite inclusions are formed in this tested steel. Figure 2b shows the equivalent particle size distribution of the inclusions. Nearly 85% of the inclusions are less than 3 µm in diameter, with a total average diameter of the inclusions of 2.4 µm and a particle number density of 18.27 mm−2. It should be noted that the inclusions with a particle size less than 1 µm are not included in Figure 2b.
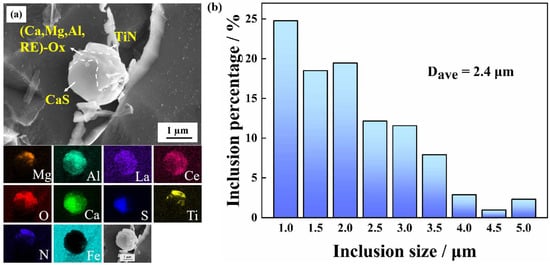
Figure 2.
(a) SEM-EDS mapping images and (b) equivalent size distribution of inclusions.
3.1.2. Thermodynamic Analysis of Inclusions and Nanoparticles’ Evolution Behavior in CGHAZ
Inclusions such as oxides, sulfides and nitrides can be formed during solidification, given the diversity of alloying elements in the tested steel. The change in Gibbs free energy of the formation reaction of the inclusions is the criterion for determining whether the reaction can proceed spontaneously at constant temperature and pressure; ΔG < 0 indicates that the reaction occurs spontaneously [25]. The chemical reaction equation and thermodynamic formula of the deoxidization reaction in molten steel are shown in Equations (11) and (12) [57]:
where ΔG and ΔGθ represent the Gibbs free energy and the standard Gibbs free energy, respectively, of the reaction, (J·mol−1), R is the gas constant, (J·mol−1·K−1), T represents the temperature, (K), and ai represents the activity of the element. Table 2 presents the corresponding free energy data for various formation reactions of the inclusions that may be involved in this work. According to Gibbs free energy change calculated at 1873 K, which is shown in Table 2, the single-metal oxide formation occurs in the following order: Al2O3 (−734.33 kJ/mol), La2O3 (−610.85 kJ/mol), Ti2O3 (−273.72 kJ/mol), ZrO2 (−156.09 kJ/mol) and CaO (−96.36 kJ/mol). Furthermore, Zr and RE can entirely or partially displace Al in Al2O3 in molten steel to form (ZrO2(−7298.12 kJ/mol) and LaAlO3(−646.15 kJ/mol)). Ca can react with solute aluminum and oxygen to form calcium aluminate (−9458.40 kJ/mol). Generally, Al2O3 dominates the initial inclusions for normal aluminum deoxidation technology used in steel making. Meanwhile, refractory bricks react easily with Al2O3 to generate Al2O3·MgO (similar to spinel) [58]. The inclusions are modified into (RE–Zr–Ti–Ca–Mg)–Ox with Zr, Ca, Ti and RE acting as deoxidizers [59]. It should be noted that the CaS (−126.03 kJ/mol) initially formed in the molten steel is easily transformed back into CaO [60,61]. The reaction for the formation of TiN (65.09 kJ/mol) has a positive Gibbs free energy change at 1873 K. Consequently, it cannot be formed spontaneously in molten steel. Figure 3a shows the precipitation curves during the cooling process, calculated using Jmatpro (Version: 7.0) thermodynamic software. It has been demonstrated that nitrides begin to precipitate and grow around 1400 °C and sulfides begin to precipitate and grow around 1300 °C; in addition, a small number of oxides would precipitate. Carbonitrides precipitate at a low temperature, about 1100 °C, and it is difficult to coarsen them into micron particles. CaS and TiN are primarily precipitated on the surface of existing inclusions at a later stage of the solidification process. The different morphologies of CaS observed in the present immersion experiments depend on the diversity of prior oxide inclusions [60].

Table 2.
Gibbs free energy of the inclusion formation reaction at 1873 K [57,58,62].
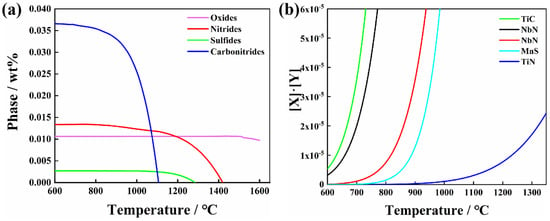
Figure 3.
(a) Precipitation curves of the sulfide and nitride during the solidification process and (b) nanoparticle precipitation in welding thermal cycle.
During the heating process of welding, small precipitations such as TiN and NbC redissolve into the matrix, which reprecipitates again during the cooling process [37,43,63]. This is well described by the expression of solid solubility product at the specific precipitations, as shown in Equation (13):
where X and Y denote the equilibrium solubility of a substance in the steels at the required temperature, (wt %), respectively. A and B are constants and T is the absolute temperature, (K). In this paper, the potential precipitations may include NbC, NbN, TiN, TiC, MnS, etc. Table 3 displays the equilibrium relationship between the solid solubility product and temperature for these precipitations. Figure 3b depicts the precipitation curve of these precipitations. According to the Ostwald aging mechanism, tiny nanoprecipitations tend to redissolve in the matrix in the welding thermal cycle, owing to the high heating temperature (peak temperature of 1320 °C), and reprecipitate during the cooling process. Meanwhile, at this peak temperature, a limited number of precipitations grow larger [64]. According to EDS analysis, the precipitated nanoparticles are mostly irregular TiN.

Table 3.
The solubility expressions for the precipitations [63,64].
During the welding thermal cycle, nanoparticles exhibit solid–solution behavior whereas inclusions, particularly oxide inclusions, have high melting points and are almost unaffected by the welding thermal cycle [37,42], which distinguishes the CGHAZ from the base metal. It should be emphasized that the role of nanoprecipitations plays a major part in precipitation strengthening and fine grain strengthening in steels; these are easily submerged by corrosion products when pitting occurs [64].
The degree of the local stress concentration or lattice distortion was characterized using kernel average misorientation (KAM) [29,65]. Figure 4 reveals that acicular ferrite grains with varied crystal orientations formed around the inclusions, while the KAM map revealed that a relatively smaller strain concentration exists around the composite inclusion, with or without formed acicular ferrite, as compared to the grain boundaries.
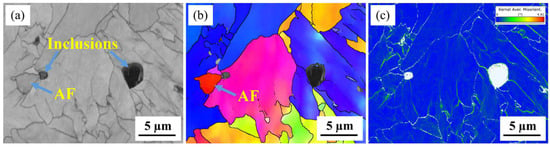
Figure 4.
EBSD characterization of the same area containing complex inclusions. (a) IQ map, (b) IPF map and (c) KAM map.
3.2. Local Corrosion Induced by the Composite Inclusions in CGHAZ
3.2.1. Potentiodynamic Polarization Tests
To compare the corrosion rates of the base metal and the CGHAZ of the tested steel, a potentiodynamic polarization test was conducted in 3.5 wt.% NaCl solution (Figure 5). The anode curves of CGHAZ and BM have almost the same shape; this may be related to the fact that there is no passive film on the surface of carbon steel when corrosion occurs. The anodic dissolution here corresponds to iron dissolution. There are two current peaks in the cathode polarization of CGHAZ, which may affect the surface state of the sample due to the larger amount of defects formed during the welding thermal cycle [38,66], the higher stress concentration caused by phase transformation and the complexity of the microstructure formed during the cooling stage [40], thereby affecting the oxygen adsorption process in the cathodic reaction. Table 4 displays the related values of corrosion current (icorr) and corrosion potential (Ecorr). The corrosion current density of the CGHAZ is one order of magnitude larger than that of the base metal. Electrochemical impedance spectroscopy (EIS) of BM and of the CGHAZ was performed to better understand the corrosion phenomenon (Figure 6). The Nyquist diagram shows that the curves of BM and the CGHAZ both presented a typical semicircle, indicating that there was only a double layer capacitance between the sample and the solution. As a result, an Rs (QdlRct) equivalent circuit diagram was chosen. Rs represents the solution resistance, Rct represents the charge transfer resistance, and Qdl represents the double capacitance. The diameter of the arc in the Nyquist diagram reflects the charge transfer resistance. In general, the larger the diameter of the arc, the better the corrosion resistance of the steel. Table 5 summarizes the results obtained from the EIS plots. It can be seen that the Rct value of BM is greater than that of the CGHAZ, indicating that BM has better corrosion resistance. In this paper, the microstructure of the base metal is a combination of ferrite and pearlite, while the microstructure of the CGHAZ is a mixture of acicular ferrite and granular bainite. As illustrated in Figure 7, for instance, the base matrix has a lower dislocation density, whereas theCGHAZ exhibits a higher dislocation density.
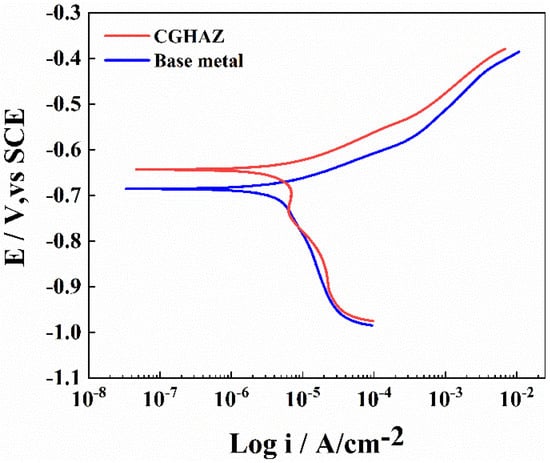
Figure 5.
Potentiodynamic polarization curves of the base metal and CGHAZ (100 kJ/cm) of the tested steels in 3.5 wt.% NaCl solution.

Table 4.
Electrochemical corrosion parameters of the tested steels.
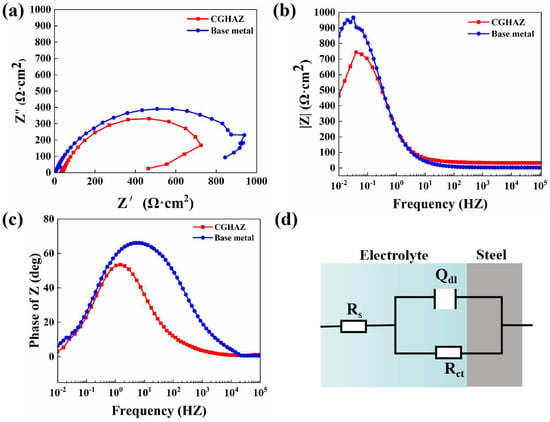
Figure 6.
The impedance diagrams for the base metal and CGHAZ in 3.5 wt.% NaCl. (a) Nyquist plots, (b,c) Bode plots and (d) proposed equivalent circuit.

Table 5.
Electrochemical parameters de1duced by EIS method in 3.5 wt.% NaCl solution.
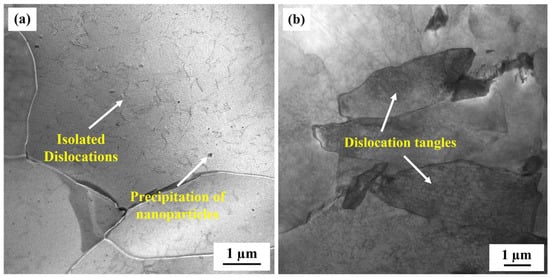
Figure 7.
TEM morphologies of the tested steel; (a) base metal and (b) CGHAZ.
3.2.2. Immersion Test
Figure 8a displays the typical morphology—before the immersion test, without any microgaps around the inclusions—of one of the inclusions of interest. As indicated in Figure 8b, a slight dissolution of the matrix occurred around the inclusions after a 5 s immersion. Meanwhile, an anisotropy was observed in the matrix dissolution rate around the inclusions. In this study, the sulfide was identified as CaS. Using SEM-EDS analysis in conjunction with the immersion test, it was shown that the matrix near the CaS at the inclusion surface exhibited a relatively larger corrosion rate than that near the rare earth complex oxides (such as LaAlO3) at the initial stage of the pitting corrosion, i.e., r1 < r2. The matrix around the inclusions further dissolved with the ongoing immersion test (Figure 8b,c) and formed ring-shaped pits due to the weakened anisotropy. Therefore, since the sulfides in the inclusions are susceptible to hydrolysis and produce acidic solutions, which accelerates the pitting occurrence, the sulfide attached to the oxide surface is easily transformed into the starting point of the pitting corrosion [9,14].
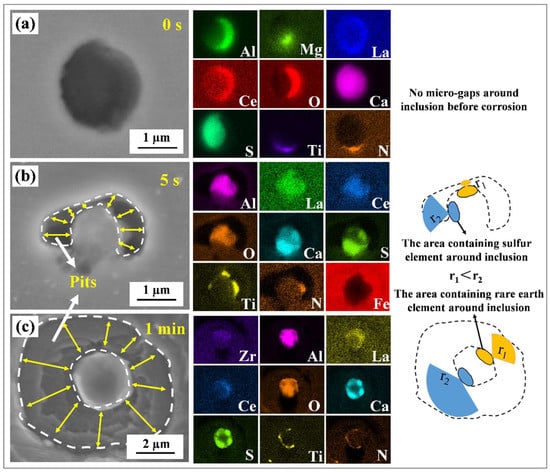
Figure 8.
SEM images and EDS results of the immersion test. (a) Immersion for 0 s, (b) immersion for 5 s and (c) immersion for 1 min.
Figure 9 displays the corrosion morphology of the composite inclusions/matrix after 1 h and 2 h of the immersion test. After the immersion test for 1 h, obvious local corrosion phenomenon occurred around the inclusion, forming a local characteristic region composed of a residual inclusion, dissolved cavity and near-circular corrosion area (Figure 9a). The enlarged area of inclusion revealed minor amounts of flocculent deposits around the inclusion, which indicated that serious localized corrosion occurred around the inclusion (Figure 9b). Combined with SEM-EDS analysis, only a small amount of sulfur element was detected on the inclusions; the residual elements may form inclusions such as (Mg–Al)–Ox, (RE–Zr–Al–Ca)–Ox and TiN. The corrosion products on the sample surface increased significantly after 2 h of immersion testing. According to the SEM results, not all the inclusions were completely covered by the corrosion products. This is attributed to the varying particle sizes of the inclusions. Figure 9c displays the representative morphology of the inclusions after de-rusting. The irregular shape of the residual inclusions and the steeper holes around the inclusions may be related to the faster expansion rate of Cl- in a vertical direction. Furthermore, the morphology of the pits exhibits behavior ranging from near-circular to anisotropic (after rust removal). EDS did not detect any sulfur elements, indicating that all sulfides were completely dissolved, while compounds such as (Mg–Al)–Ox, (RE–Zr–Al–Ca)–Ox and TiN remained undissolved.
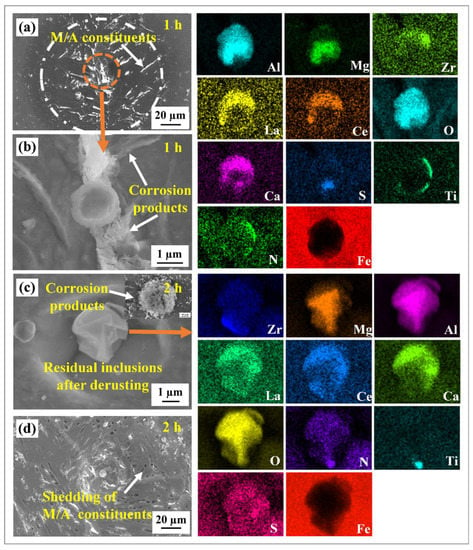
Figure 9.
SEM images and EDS results of the immersion test; (a,b) immersion for 1h and (c,d) immersion for 2 h.
Furthermore, after immersion for 1 h, a small amount of corrosion products were found on the surface of the M/A island distributed around the inclusion in the near-circular corrosion area, implying that the corrosion attack occurred in this region (Figure 9a). The micropores were observed around the inclusion after 2 h of immersion (Figure 9d). The microcouples were found to be formed between the M/A constituents and the ferrite matrix, with the ferrite matrix acting as the anode phase and the M/A constituents as the cathode phase [15,46]. The M/A island gradually fell off with the corrosion dissolution of the ferrite matrix.
3.2.3. Elastic Mechanical Properties Calculated by First-Principles Modeling
The elastic mechanical properties of the inclusions involved in this paper are shown in Table 6 and were determined using the first-principles calculation method. According to calculation results, LaAlO3 and La2O7Zr2 have lower bulk modulus than Al2O3 (249.54) and ZrO2 (271.06), and the bulk modulus of LaAlO3 (192.61) is closer to that of the iron matrix (194.76). The results indicate that LaAlO3 has excellent mechanical compatibility with the matrix. Numerous studies have demonstrated that the visible microcrevices and high-density dislocations between Al2O3 and the matrix are due to the significantly higher hardness and irregular shape of Al2O3 compared with the iron matrix [29,67]. Inclusions, such as CaO (114.11), MgO (165.84), TiN (175.02) and CaS (57.05), exhibit lower bulk modulus than the matrix. Rare earth elements can form rare earth oxides and composite inclusions, such as LaAlO3 and La2O7Zr2, due to their stronger affinity for oxygen than Zr, Ti and other deoxidizers [31]. The spheroidization and softening effects of rare earth elements on the inclusions can reduce the stress concentration around the inclusions [32,67,68]. This is consistent with the absence of microcracking that was observed between the inclusions and the matrix (Figure 4 and Figure 8a).

Table 6.
Calculated mechanical properties of the inclusions and Fe matrix.
3.2.4. Inclusion Conductivity Property Calculated by First-Principles Modeling
In the present work, density of states of the inclusions of interest were calculated to determine the electrical conductivity. It can be seen that the Fe matrix and TiN (red star) have metallic nature, whereas Al2O3, ZrO2, LaAlO3, La2O7Zr2, MgO, CaO, CaS and Al2MgO4 (blue star) exhibit the properties of insulators (Figure 10). The matrix and TiN exhibit electrical conductivity, whereas the other oxidation or sulfide inclusions exhibit the properties of insulators or semiconductors. TiN holds intact in the immersion test, which could be attributed to its insolubility in strong acids at room temperature and pressure [69,70]. In summary, the composite inclusions in the test steel will not form a microcouple effect with the matrix.
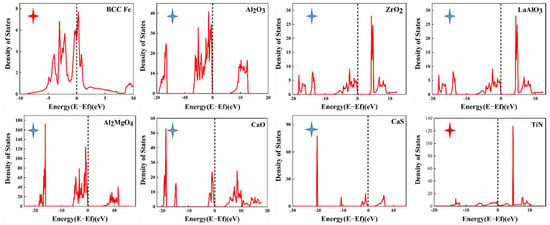
Figure 10.
Density of states (DOS) analysis of the inclusions (the black dotted line represents the Fermi energy level).
3.2.5. Work Function Calculated by First-Principles Modeling
Figure 11 presents the work functions of inclusions and the matrix involved in this paper. The work function is defined as the energy difference between the Fermi level and the vacuum level (Figure 11a) of a slab model and represents the minimum energy required to remove an electron from the sample surface [32,67]. Since Fe (110) surface has the lowest surface energy with the largest possible exposed face, the work function of the Fe (110) surface is utilized as the baseline in this paper [71]. The work functions of the low-index crystal surfaces of Al2O3, CaO, MgO, TiN and CaS are almost relatively lower, while the work functions of the low-index crystal surfaces of LaAlO3, La2O7Zr2, ZrO2 and Al2MgO4 are significantly higher than the work function of the Fe (110) surface (Figure 11b). The work function of the typical inclusion combined with the first-principles calculation results shows that the composite inclusion containing rare earth elements has a higher work function, which implies that it is less likely to act as an anode phase compared to CaS. Furthermore, the work function value corresponds to the phenomena observed in the immersion test, in which CaS was preferentially dissolved and (Mg–Al)–Ox and (RE–Zr–Al–Ca)–Ox remained undissolved (Figure 9d). The addition of rare earth elements can improve inclusion corrosion resistance [18].
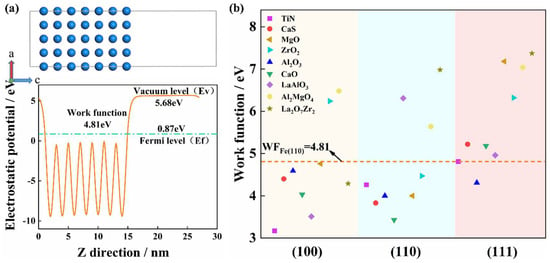
Figure 11.
Calculated work function of the inclusions and Fe matrix. (a) Electrostatic potential curve of Fe (110) slab model, (b) work function of the different crystal planes (low index) of the inclusions.
3.3. Mechanism of Local Corrosion Induced by Zr–Ti–Al–RE Composite Inclusions in CGHAZ
Figure 12I illustrates the evolution process of the inclusions involved in this paper. The addition of Zr–Fe, Ti–Fe, Ca–Fe, and RE alloys during the smelting process modified the Al2O3 inclusions into composite inclusions. CaS and TiN precipitated on the previously formed inclusions at a later stage of solidification, ultimately forming composite inclusions with a core–shell structure [12,14,72]. Figure 12II illustrates the schematic diagram of the initiation and propagation of the pitting corrosion triggered by these composite inclusions in the tested steel. As mentioned above, the mechanisms of galvanic coupling corrosion and the crevice and stress corrosion formed between the inclusions and the matrix could be excluded in this study. Since the composite inclusions are exposed to a corrosive environment with Cl- (Figure 12IIa), CaS with poor stability will preferentially hydrolyze to produce H+ and HS- by anodic reactions (Equations (14) and (15)). As the reaction progresses, the matrix begins to undergo a hydrolysis reaction (Equations (16) and (17)), resulting in further acidification in the pits and further dissolution of CaS [9,14]. The composite inclusion (RE–Zr–Al–Ca)–Ox and the surrounding matrix were gradually dissolved (Equation (18) under the attack of the increasing H+ with the continued dissolution of CaS. Meanwhile, corrosion products produced by cathodic reactions (Equations (19)–(21)) gradually accumulated at the edge of pits (Figure 12 IIb). CaS was completely dissolved, forming and propagating stable pits with the total corrosion products covering the upper layer. To maintain electrical neutrality, a large amount of Cl- will enter the pitting hole, resulting in the formation of a self-catalytic acidification-occluded corrosion cell (Figure 12IIc). Furthermore, some generated cations, such as Ca2+, Zr4+, Al3+ and RE3+, hydrolyze further, resulting in a drop in the pH value [13,30,32], causing a strong acidic environment in the pits and accelerating the dissolution of the composite inclusions ((RE–Zr–Al–Ca)–Ox and Al2MgO4) (Figure 12IId). TiN inclusion is stable, which is primarily related to its insolubility in strong acids [69]. With the dissolution of (RE–Zr–Al–Ca)–Ox, Al2MgO4 and TiN inclusions would fall to the bottom of the pits. The anodic and cathodic reactions involved during the pitting initiation and propagation of the Zr–Ti–Al–RE composite inclusions in the tested steel can be summarized as follows:
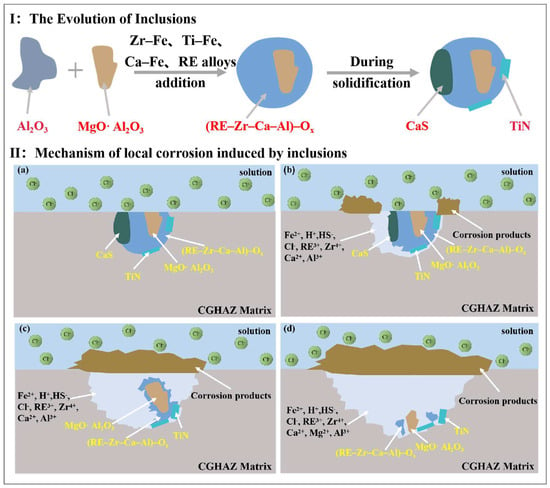
Figure 12.
(I) Schematic diagram of the inclusion evolution process and (II) pitting initiation and propagation process induced by the composite inclusions. (a) before immersion, (b) pitting initiation, (c,d) pitting propagation.
Anodic reactions:
Cathodic reactions:
The matrix around the spherical inclusions was dissolved to produce cations in the early stage of the pitting corrosion, and Cl− migrated to the anode and diffused in a horizontal direction under the concentration gradient. This resulted in the nearly circular shape of the pits, which eventually produced corrosion products covering the pits [9,13]. Following the formation of the self-catalytic acidification-occluded corrosion cell, the acidic environment accelerates the dissolution of the composite inclusions. However, the findings of the work function analyses of the inclusions in this work demonstrate that the inclusions display different corrosion resistances and the cations exhibit different chemical hydrolysis abilities. These two factors lead to asynchrony of the hydrolysis reaction, resulting in anisotropy of the pitting area around the inclusions.
In conclusion, compared with Al2O3 inclusions introduced by the traditional Al deoxidization technology, the addition of rare earth can effectively modify the characteristics of the inclusions.
4. Conclusions
- The inclusions and microstructures in the Zr–Ti–Al–RE deoxidized HSLA steels were modified. The formed spherical Zr–Ti–Al–RE composite inclusions have a core–shell structure with (Zr–Mg–Al–Ca–RE)–Ox at the center and CaS and TiN at the periphery.
- Acicular ferrite was formed at specific inclusions in the CGHAZ, which could improve the mechanical properties.
- The spheroidization and softening effects of rare earth elements on the inclusions can lower the stress concentration around the inclusions and increase the local corrosion resistance of the tested steel. Furthermore, the inclusions containing rare earth elements have a significantly higher work function than the matrix, which further reduces the corrosion tendency of the inclusions to undergo anodic dissolution.
- The initiation of pitting corrosion from composite inclusions is triggered by the chemical dissolution of CaS within the composite inclusions. Then, (RE–Zr–Al–Ca)–Ox started to dissolve until the composite inclusion Al2MgO4 region was exposed. Finally, the undissolved Al2MgO4 and TiN fell to the bottom of the pit. The composite spherical inclusions gradually dissolved and transformed into irregular shapes as the immersion duration increased. The addition of rare earth can effectively reduce the tendency for pitting corrosion in a CGHAZ in a marine environment.
Author Contributions
Conceptualization, L.C. and K.-M.W.; Methodology, C.-C.Y. and Z.-H.W.; Formal analysis, S.C., Z.-C.L. and D.L.; Investigation, C.-C.Y.; Writing—original draft, C.-C.Y.; Writing—review & editing, L.C. and T.-L.Z.; Supervision, L.C. and K.-M.W.; Validation, S.-E.H., D.-H.X., X.J. and H.-K.L. Project administration, S.-E.H., Z.-C.L., D.L., D.-H.X., X.J. and H.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. U20A20279, 52071238, 50734004), Taishan Industry Leading Talent Project (No. 2020007), Guangxi Science and Technology Program Project (No. AA22068080) and the 111 Project (No. D18018). In this work, numerical calculation is supported by High-Performance Computing Center of Wuhan University of Science and Technology.
Institutional Review Board Statement
This study does not require ethical approval.
Informed Consent Statement
Declaration of competing interest is changed to COI.
Data Availability Statement
The raw processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
Acknowledgments
We would like to thank Zhen Wang at the Analytical and Testing Center of Wuhan University of Science and Technology for the help in the SEM-EDS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buzzatti, D.T.; Kanan, L.F.; Dalpiaz, G.; Scheid, A.; Fortis Kwietniewski, C.E. Effect of heat input and heat treatment on the microstructure and toughness of pipeline girth friction welded API 5L X65 steel. Mater. Sci. Eng. A 2022, 833, 142588–142603. [Google Scholar] [CrossRef]
- Li, Y.; Wan, X.L.; Lu, W.Y.; Shirzadi, A.A.; Isayev, O.; Hress, O.; Wu, K.M. Effect of Zr-Ti combined deoxidation on the microstructure and mechanical properties of high-strength low-alloy steels. Mater. Sci. Eng. A 2016, 659, 179–187. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.F.; Xue, W.Y.; Jia, N.; Misra, R.D.K. The significant impact of introducing nanosize precipitates and decreased effective grain size on retention of high toughness of simulated heat affected zone (HAZ). Mater. Sci. Eng. A 2021, 803, 140484–140500. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wei, W.; Wan, X.; Liu, J.; Wu, K. Effects of Ti and Cu Addition on Inclusion Modification and Corrosion Behavior in Simulated Coarse-Grained Heat-Affected Zone of Low-Alloy Steels. Materials 2021, 14, 791–806. [Google Scholar] [CrossRef]
- Wang, C.; Misra, R.D.K.; Shi, M.H.; Zhang, P.Y.; Wang, Z.D.; Zhu, F.X.; Wang, G.D. Transformation behavior of a Ti–Zr deoxidized steel: Microstructure and toughness of simulated coarse grain heat affected zone. Mater. Sci. Eng. A 2014, 594, 218–228. [Google Scholar] [CrossRef]
- Kumar, S.; Nath, S.K.; Kumar, V. Continuous cooling transformation behavior in the weld coarse grained heat affected zone and mechanical properties of Nb-microalloyed and HY85 steels. Mater. Des. 2016, 90, 177–184. [Google Scholar] [CrossRef]
- Fattahi, M.; Nabhani, N.; Rafiee, E.; Nasibi, M.; Ahmadi, E.; Fattahi, Y. Effect of Ti-based inclusions and acicular ferrite on the corrosion performance of multipass weld metals. Mater. Chem. Phys. 2014, 146, 105–112. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Liu, L.; Li, G.; Zhai, D. Mechanism of pitting corrosion induced by inclusions in Al-Ti-Mg deoxidized high strength pipeline steel. Micron 2020, 138, 102898–102905. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Wu, W.; Li, Y. Role of inclusions in the pitting initiation of pipeline steel and the effect of electron irradiation in SEM. Corros. Sci. 2018, 130, 252–260. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Q.; Li, X.; Hu, J.; Cao, F. Insight into the triggering effect of (Al, Mg, Ca, Mn)-oxy-sulfide inclusions on localized corrosion of weathering steel. J. Mater. Sci. Technol. 2021, 64, 99–113. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Chen, Y.; Wang, J.; Zhao, Y. Mechanism of Surface Hole Evolution and Impact on Corrosion Resistance of High-Strength Low-Alloy Weathering Steel. J. Mater. Eng. Perform. 2021, 31, 1737–1756. [Google Scholar] [CrossRef]
- Xue, W.; Li, Z.; Xiao, K.; Yu, W.; Song, J.; Chen, J.; Dong, C.; Li, X. Initial microzonal corrosion mechanism of inclusions associated with the precipitated (Ti, Nb)N phase of Sb-containing weathering steel. Corros. Sci. 2020, 163, 108232–108240. [Google Scholar] [CrossRef]
- Wei, W.-Z.; Wu, K.-M.; Liu, J.; Cheng, L.; Zhang, X. Initiation and propagation of localized corrosion induced by (Zr, Ti, Al)-Ox inclusions in low-alloy steels in marine environment. J. Iron Steel Res. Int. 2020, 28, 453–463. [Google Scholar] [CrossRef]
- Wang, L.; Xin, J.; Cheng, L.; Zhao, K.; Sun, B.; Li, J.; Wang, X.; Cui, Z. Influence of inclusions on initiation of pitting corrosion and stress corrosion cracking of X70 steel in near-neutral pH environment. Corros. Sci. 2019, 147, 108–127. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J.H.; Ke, W.; He, X.Y. Influence of Inclusions on Early Corrosion Development of Ultra-Low Carbon Bainitic Steel in NaCl Solution. Corrosion 2015, 71, 1467–1480. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Akpanyung, K.V.; Loto, R.T. Pitting corrosion evaluation: A review. J. Phys. Conf. Ser. 2019, 1378, 022088–022102. [Google Scholar] [CrossRef]
- Zheng, W.; Yan, X.; Xiong, S.; Wang, G.; Li, G. Pitting corrosion behavior of cerium treated HSLA steel induced by sulfide inclusions in 3.5 wt% NaCl solution. J. Rare Earths 2021, 39, 348–356. [Google Scholar] [CrossRef]
- Chen, S.; Lei, H.; Hou, H.; Ding, C.; Zhang, H.; Zhao, Y. Collision-coalescence among inclusions with bubble attachment and transport in molten steel of RH. J. Mater. Res. Technol. 2021, 15, 5141–5150. [Google Scholar] [CrossRef]
- Costa e Silva, A.L.V.D. The effects of non-metallic inclusions on properties relevant to the performance of steel in structural and mechanical applications. J. Mater. Res. Technol. 2019, 8, 2408–2422. [Google Scholar] [CrossRef]
- Guo, H.; Yang, S.; Wang, T.; Yuan, H.; Zhang, Y.; Li, J. Microstructure evolution and acicular ferrite nucleation in inclusion-engineered steel with modified MgO@C nanoparticle addition. J. Mater. Sci. Technol. 2022, 99, 277–287. [Google Scholar] [CrossRef]
- Wang, P.; Wang, B.; Liu, Y.; Zhang, P.; Luan, Y.K.; Li, D.Z.; Zhang, Z.F. Effects of inclusion types on the high-cycle fatigue properties of high-strength steel. Scr. Mater. 2022, 206, 114232–114237. [Google Scholar] [CrossRef]
- Yang, W.; Peng, K.; Zhang, L.; Ren, Q. Deformation and fracture of non-metallic inclusions in steel at different temperatures. J. Mater. Res. Technol. 2020, 9, 15016–15022. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Revilla, R.I.; Sun, T.; Zhao, J.; Zhang, D.; Yang, S.; Liu, Z.; Cheng, X.; Terryn, H.; et al. Towards a better understanding of localised corrosion induced by typical non-metallic inclusions in low-alloy steels. Corros. Sci. 2021, 179, 109150–109158. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of Adding Cerium on Microstructure and Morphology of Ce-Based Inclusions Formed in Low-Carbon Steel. Sci. Rep. 2017, 7, 46503–46512. [Google Scholar] [CrossRef]
- Liu, H.; Fu, P.; Liu, H.; Cao, Y.; Sun, C.; Du, N.; Li, D. Effects of Rare Earth elements on microstructure evolution and mechanical properties of 718H pre-hardened mold steel. J. Mater. Sci. Technol. 2020, 50, 245–256. [Google Scholar] [CrossRef]
- Torkamani, H.; Raygan, S.; Mateo, C.; Rassizadehghani, J.; Vivas, J.; Palizdar, Y.; San-Martin, D. The Influence of La and Ce Addition on Inclusion Modification in Cast Niobium Microalloyed Steels. Metals 2017, 7, 377. [Google Scholar] [CrossRef]
- Wang, L.-M.; Lin, Q.; Yue, L.-J.; Liu, L.; Guo, F.; Wang, F.-M. Study of application of rare earth elements in advanced low alloy steels. J. Alloys Compd. 2008, 451, 534–537. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Zhang, D.; Liu, Z.; Lutz, A.; Zhang, F.; Zhao, T.; Ma, H.; Li, X.; Terryn, H. Role of Al2O3 inclusions on the localized corrosion of Q460NH weathering steel in marine environment. Corros. Sci. 2018, 138, 96–104. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Z.; Zhao, J.; Cheng, X.; Liu, Z.; Zhang, D.; Li, X. Influence of rare earth metals on mechanisms of localised corrosion induced by inclusions in Zr-Ti deoxidised low alloy steel. Corros. Sci. 2020, 166, 108463–108472. [Google Scholar] [CrossRef]
- Tang, M.; Wu, K.; Liu, J.; Cheng, L.; Zhang, X.; Chen, Y. Mechanism Understanding of the Role of Rare Earth Inclusions in the Initial Marine Corrosion Process of Microalloyed Steels. Materials 2019, 12, 3359–3373. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xiong, G.; Liu, L.; Li, G.; Moelans, N.; Guo, M. Effects of LaAlO3 and La2O2S inclusions on the initialization of localized corrosion of pipeline steels in NaCl solution. Scr. Mater. 2020, 177, 151–156. [Google Scholar] [CrossRef]
- Tan, J.; Wu, X.; Han, E.-H.; Ke, W.; Liu, X.; Meng, F.; Xu, X. Role of TiN inclusion on corrosion fatigue behavior of Alloy 690 steam generator tubes in borated and lithiated high temperature water. Corros. Sci. 2014, 88, 349–359. [Google Scholar] [CrossRef]
- Villavicencio, J.; Ulloa, N.; Lozada, L.; Moreno, M.; Castro, L. The role of non-metallic Al2O3 inclusions, heat treatments and microstructure on the corrosion resistance of an API 5L X42 steel. J. Mater. Res. Technol. 2020, 9, 5894–5911. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J.; Zhou, Y.; He, X.; Wang, C.; Ke, W. Influence of the secondary phase on micro galvanic corrosion of low carbon bainitic steel in NaCl solution. Mater. Charact. 2018, 139, 401–410. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Ma, Q.; Wu, T.; Liu, M.; Zhang, M.; Li, Z.; Yin, F. Selected corrosion of X80 pipeline steel welded joints induced by Desulfovibrio desulfuricans. Corros. Sci. 2022, 202, 110313–110332. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Li, J.; Wu, X.; Huang, Y.; Li, X. Effects of Niobium on the Mechanical Properties and Corrosion Behavior of Simulated Weld HAZ of HSLA Steel. Metall. Mater. Trans. A 2017, 49, 187–197. [Google Scholar] [CrossRef]
- Mohammadi, F.; Eliyan, F.F.; Alfantazi, A. Corrosion of simulated weld HAZ of API X-80 pipeline steel. Corros. Sci. 2012, 63, 323–333. [Google Scholar] [CrossRef]
- Chaves, I.A.; Melchers, R.E. Pitting corrosion in pipeline steel weld zones. Corros. Sci. 2011, 53, 4026–4032. [Google Scholar] [CrossRef]
- Lai, H.-H.; Wu, W. Practical examination of the welding residual stress in view of low-carbon steel welds. J. Mater. Res. Technol. 2020, 9, 2717–2726. [Google Scholar] [CrossRef]
- Wang, L.; Song, B.; Yang, Z.B.; Cui, X.K.; Liu, Z.; Cheng, W.S.; Mao, J.H. Effects of Mg and La on the evolution of inclusions and microstructure in Ca-Ti treated steel. Int. J. Min. Met. Mater. 2021, 28, 1940–1948. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, D.; Qiu, G.; Li, X.; Jiang, Z.; Zhang, H. Inclusion evolution in solid steel during rolling deformation: A review. J. Mater. Res. Technol. 2022, 18, 5103–5115. [Google Scholar] [CrossRef]
- Sun, L.; Li, H.; Zhu, L.; Liu, Y.; Hwang, J. Research on the evolution mechanism of pinned particles in welding HAZ of Mg treated shipbuilding steel. Mater. Des. 2020, 192, 108670–108682. [Google Scholar] [CrossRef]
- Wan, X.L.; Wang, H.H.; Cheng, L.; Wu, K.M. The formation mechanisms of interlocked microstructures in low-carbon high-strength steel weld metals. Mater. Charact. 2012, 67, 41–51. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Tang, M.; Zhang, X.; Wu, K. Role of rare earth elements on the improvement of corrosion resistance of micro-alloyed steels in 3.5 wt.% NaCl solution. J. Mater. Res. Technol. 2021, 11, 519–534. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Cheng, L.; Liu, J.; Wu, K. Role of inclusion and microstructure on corrosion initiation and propagation of weathering steels in marine environment. J. Mater. Res. Technol. 2021, 10, 306–321. [Google Scholar] [CrossRef]
- Jones, R.O. Density functional theory: Its origins, rise to prominence, and future. Rev. Mod. Phys. 2015, 87, 897–923. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Sun, X.; Zhang, X.; Liu, J.; Wu, K. First-principle study on the effects of hydrogen in combination with alloy solutes on local mechanical properties of steels. Int. J. Hydrogen Energy 2022, 47, 22243–22260. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, T.P.; Liang, X.; Zheng, P.; Zheng, Y.H.; Lin, H.F.; Wu, K.M. The structural, magnetic, electronic, and mechanical properties of orthogonal/hexagonal MC (M = Fe and Cr) carbides from first-principles calculations. Vacuum 2022, 203, 111175–111186. [Google Scholar] [CrossRef]
- Fan, H.; Shi, G.; Peng, T.; Wang, Q.; Wang, L.; Wang, Q.; Zhang, F. N-induced microstructure refinement and toughness improvement in the coarse grain heat-affected zone of a low carbon Mo–V–Ti–B steel subjected to a high heat input welding thermal cycle. Mater. Sci. Eng. A 2021, 824, 141799–141812. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Min, Y.; Liu, C.; Jiang, M. Effect of magnesium addition in low carbon steel part 2: Toughness and microstructure of the simulated coarse-grained heat-affected zone. Ironmak. Steelmak. 2017, 46, 301–311. [Google Scholar] [CrossRef]
- Ramachandran, D.C.; Moon, J.; Lee, C.-H.; Kim, S.-D.; Chung, J.-H.; Biro, E.; Park, Y.-D. Role of bainitic microstructures with M-A constituent on the toughness of an HSLA steel for seismic resistant structural applications. Mater. Sci. Eng. A 2021, 801, 140390–140400. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, C.; Yan, Z.; Li, H.; Liu, Y. Formation mechanism and control methods of acicular ferrite in HSLA steels: A review. J. Mater. Sci. Technol. 2018, 34, 737–744. [Google Scholar] [CrossRef]
- Hu, C.-y.; Dong, H.-y.; Wu, K.-m.; Misra, R.D.K.; Zhong, L.; Jin, X.; Li, Q. Effect of Zr-deoxidation on microstructure and mechanical behavior of microalloyed heavy plates with low impurity content. J. Iron Steel Res. Int. 2021, 28, 190–200. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Liu, Z.; Zhang, D.; Li, X.; Terryn, H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Nishimoto, M.; Muto, I.; Sugawara, Y.; Hara, N. Cerium addition to CaS inclusions in stainless steel: Insolubilizing water-soluble inclusions and improving pitting corrosion resistance. Corros. Sci. 2021, 180, 109222–109228. [Google Scholar] [CrossRef]
- Gollapalli, V.; Rao, M.B.V.; Karamched, P.S.; Borra, C.R.; Roy, G.G.; Srirangam, P. Modification of oxide inclusions in calcium-treated Al-killed high sulphur steels. Ironmak. Steelmak. 2018, 46, 663–670. [Google Scholar] [CrossRef]
- Zhang, T.; Min, Y.; Liu, C.; Jiang, M. Effect of Mg Addition on the Evolution of Inclusions in Al–Ca Deoxidized Melts. ISIJ Int. 2015, 55, 1541–1548. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Zhang, L. A kinetic model for Ca treatment of Al-killed steels using FactSage macro processing. Ironmak. Steelmak. 2016, 44, 497–504. [Google Scholar] [CrossRef]
- Bai, X.-F.; Sun, Y.-H.; Chen, R.-M.; Zhang, Y.-M.; Cai, Y.-F. Formation and thermodynamics of CaS-bearing inclusions during Ca treatment in oil casting steels. Int. J. Miner. Metall. Mater. 2019, 26, 573–587. [Google Scholar] [CrossRef]
- Ahmad, H.; Zhao, B.; Lyu, S.; Huang, Z.; Xu, Y.; Zhao, S.; Ma, X. Formation of Complex Inclusions in Gear Steels for Modification of Manganese Sulphide. Metals 2021, 11, 2051. [Google Scholar] [CrossRef]
- Geng, R.; Li, J.; Shi, C.; Zhi, J.; Lu, B. Effect of Ce-La on inclusion evolution in Al-killed high strength steel. Metall. Res. Technol. 2020, 117, 663–670. [Google Scholar] [CrossRef]
- Wang, H.H.; Qin, Z.P.; Wei, R.; Wan, X.L.; Wu, K.M.; Misra, R.D.K. Precipitation of carbonitrides and high-temperature strength in heat-affected zone of high-Nb containing fire-resistant steel. Sci. Technol. Weld. Join. 2016, 22, 157–165. [Google Scholar] [CrossRef]
- Fu, R.D.; Yang, Y.Q.; Wang, C.Y.; Zhang, W.H. Effects of weld thermal cycles and post-welding tempering on second phase particles in 2·25Cr–1Mo–0·25V steels. Sci. Technol. Weld. Join. 2013, 13, 349–356. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Li, X.; Jiang, Z.; Yang, S.; Cui, Z.; Zhang, D.; Terryn, H.; Li, X. New insights into the mechanism of localised corrosion induced by TiN-containing inclusions in high strength low alloy steel. J. Mater. Sci. Technol. 2022, 124, 141–149. [Google Scholar] [CrossRef]
- Khalaj, G.; Khalaj, M.-J. Investigating the corrosion of the Heat-Affected Zones (HAZs) of API-X70 pipeline steels in aerated carbonate solution by electrochemical methods. Int. J. Pres. Ves. Pip. 2016, 145, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Su, C.; Chen, X.; Liu, H.; Zhang, L. First-principles study on pitting corrosion of Al deoxidation stainless steel with rare earth element (La) treatment. Mater. Today Commun. 2021, 27, 102204–102210. [Google Scholar] [CrossRef]
- Zheng, C.; Li, X.; Mi, C. Reducing stress concentrations in unidirectionally tensioned thick-walled spheres through embedding a functionally graded reinforcement. Int. J. Mech. Sci. 2019, 152, 257–267. [Google Scholar] [CrossRef]
- Silva, F.C.; Prada Ramirez, O.M.; Tunes, M.A.; Edmondson, P.D.; Sagás, J.C.; Fontana, L.C.; de Melo, H.G.; Schön, C.G. Corrosion resistance of functionally graded TiN/Ti coatings for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2020, 45, 33993–34010. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Du, H. Electrochemical capacitance performance of titanium nitride nanoarray. Mater. Sci. Eng. B 2013, 178, 1443–1451. [Google Scholar] [CrossRef]
- Pham, H.H.; Taylor, C.D.; Henson, N.J. Metal desorption from Fe(110) and its alloyed surfaces. Chem. Phys. Lett. 2013, 585, 162–166. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Ren, Y.; Li, Z.; Slater, C.; Peng, K.; Liu, F.; Zhao, Y. Effect of compression temperature on deformation of CaO–CaS–Al2O3–MgO inclusions in pipeline steel. J. Mater. Res. Technol. 2021, 11, 1220–1231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).