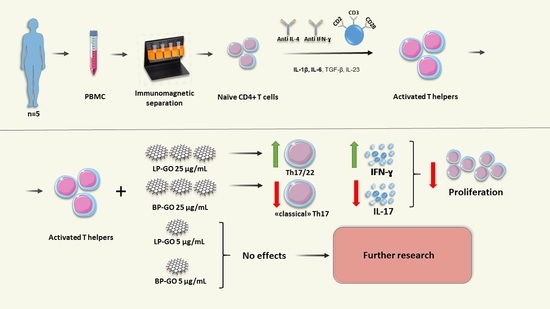
The Effect of PEGylated Graphene Oxide Nanoparticles on the Th17-Polarization of Activated T Helpers
Abstract
1. Introduction
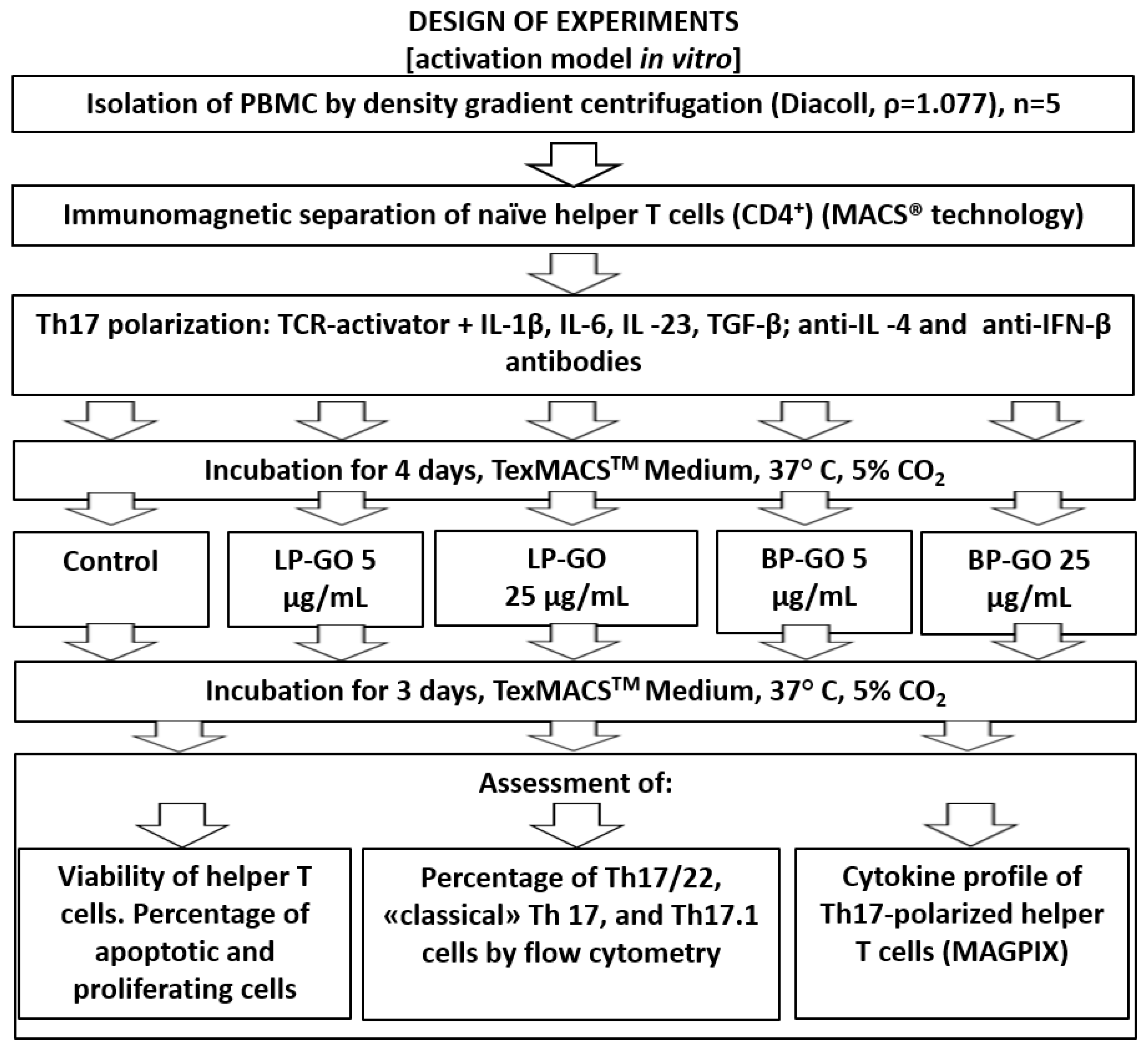
2. Materials and Methods
3. Results
3.1. Effects of P-GO on T Lymphocyte Viability
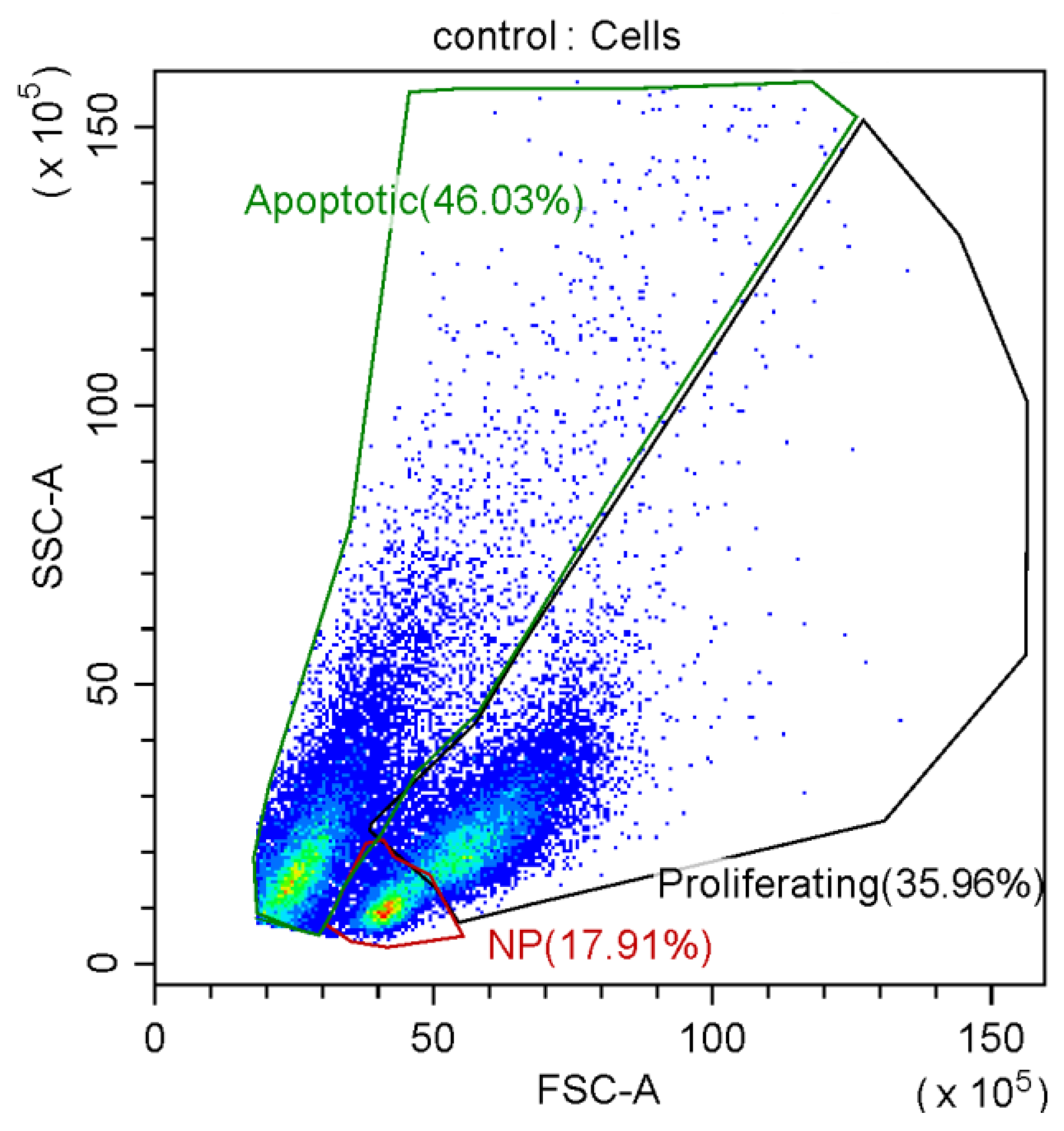
3.2. Effect of Graphene Oxide Nanoparticles on Proliferation of T-Helpers
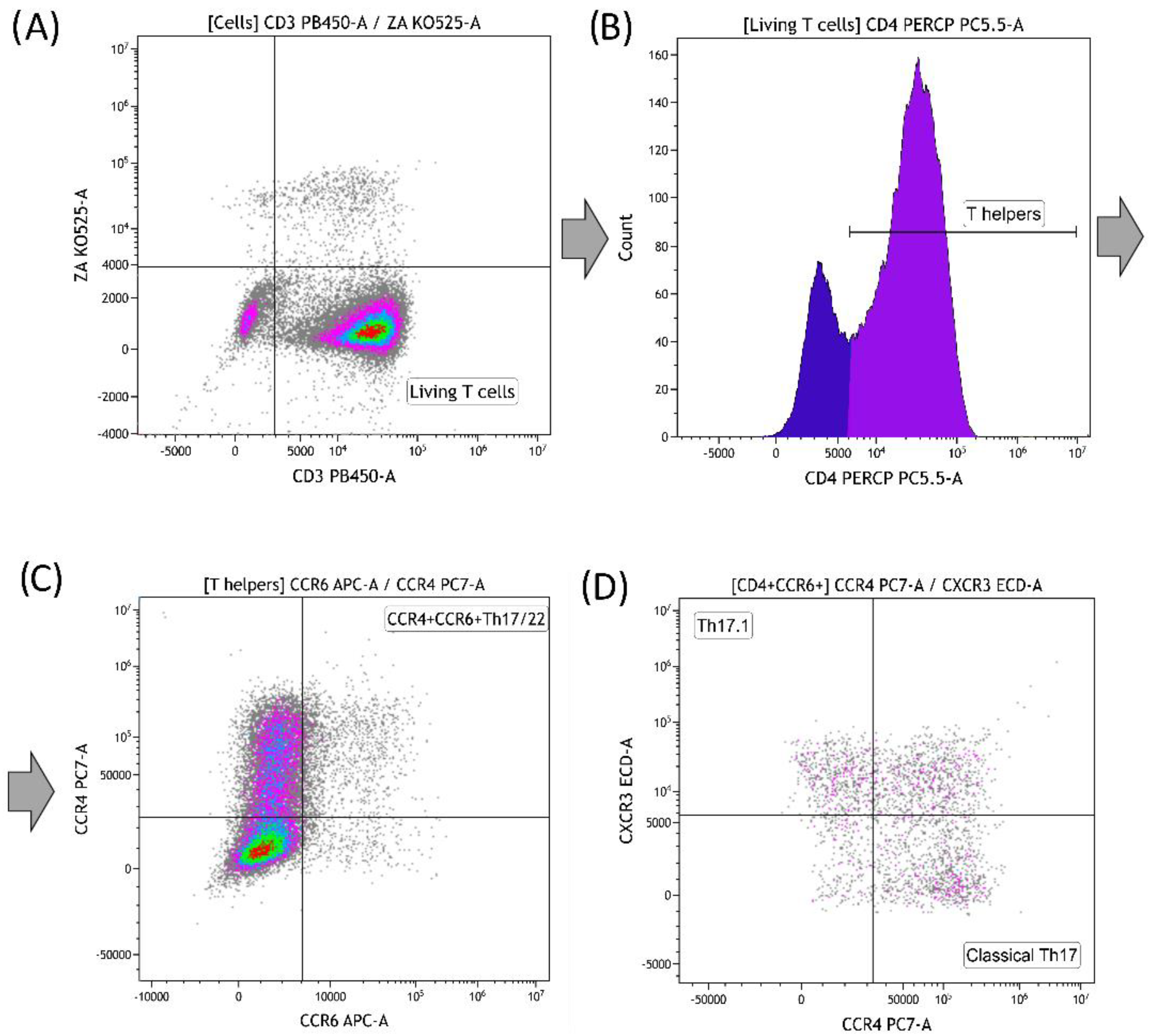
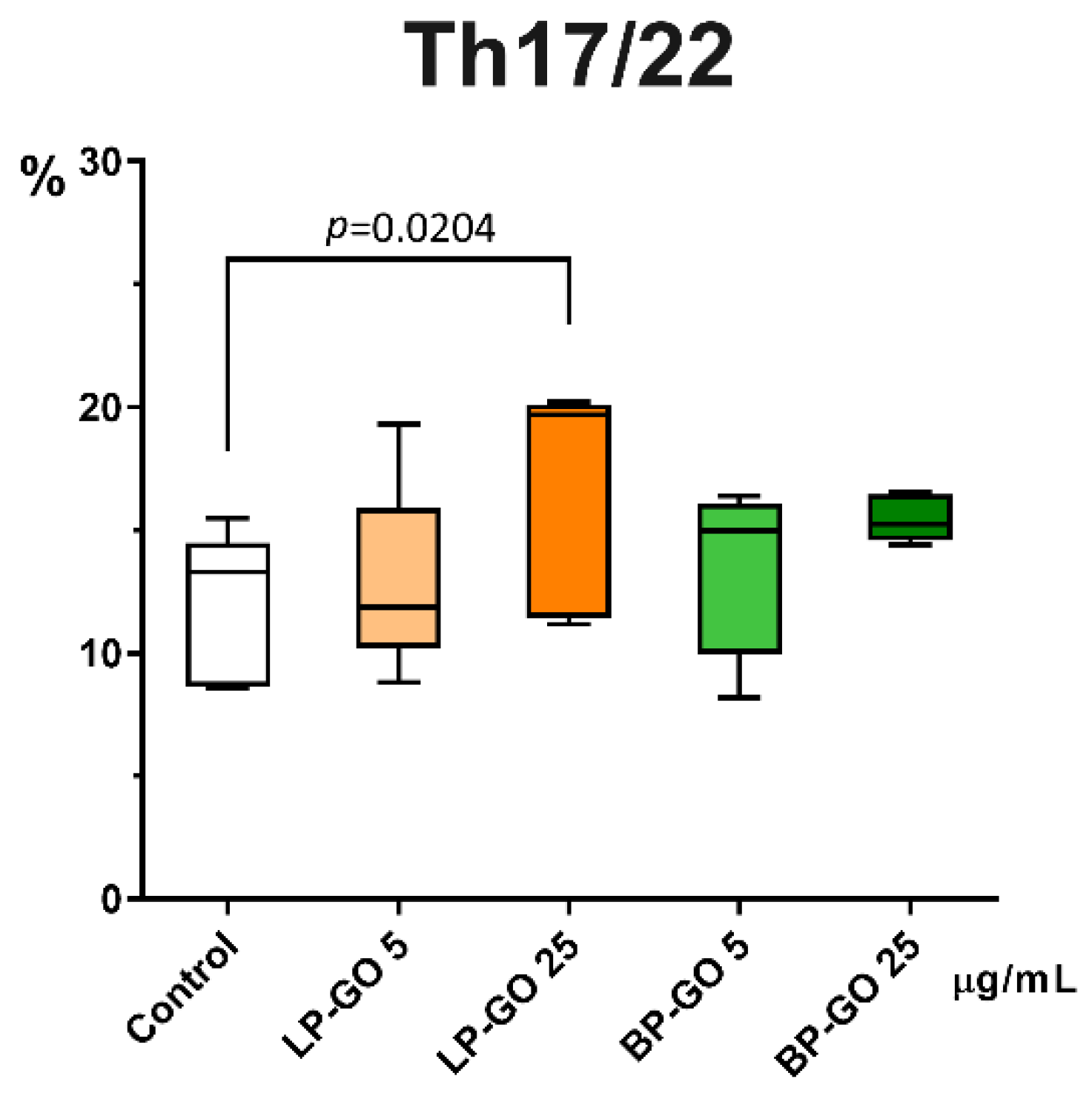
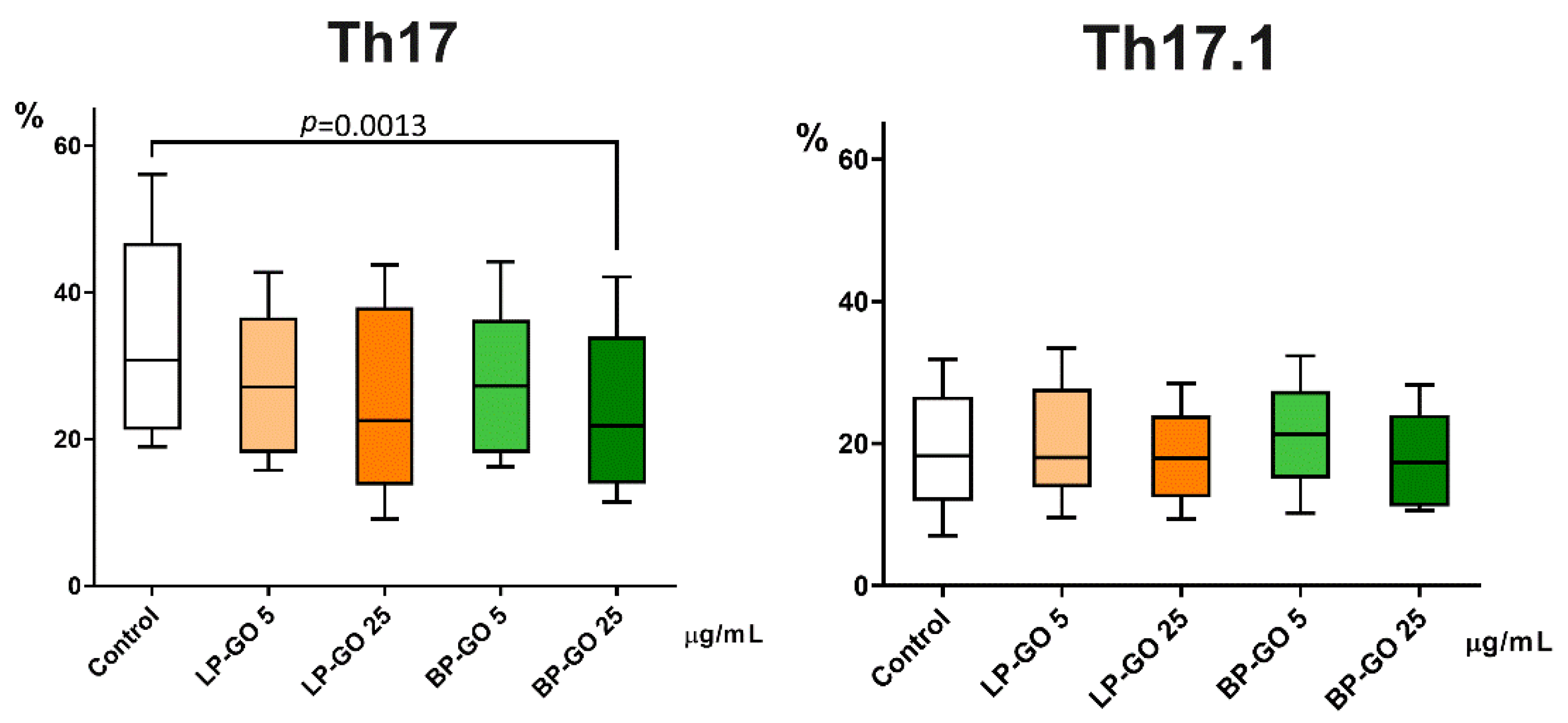
3.3. Effect of Graphene Oxide Nanoparticles on Th17 Polarization of T-Helpers
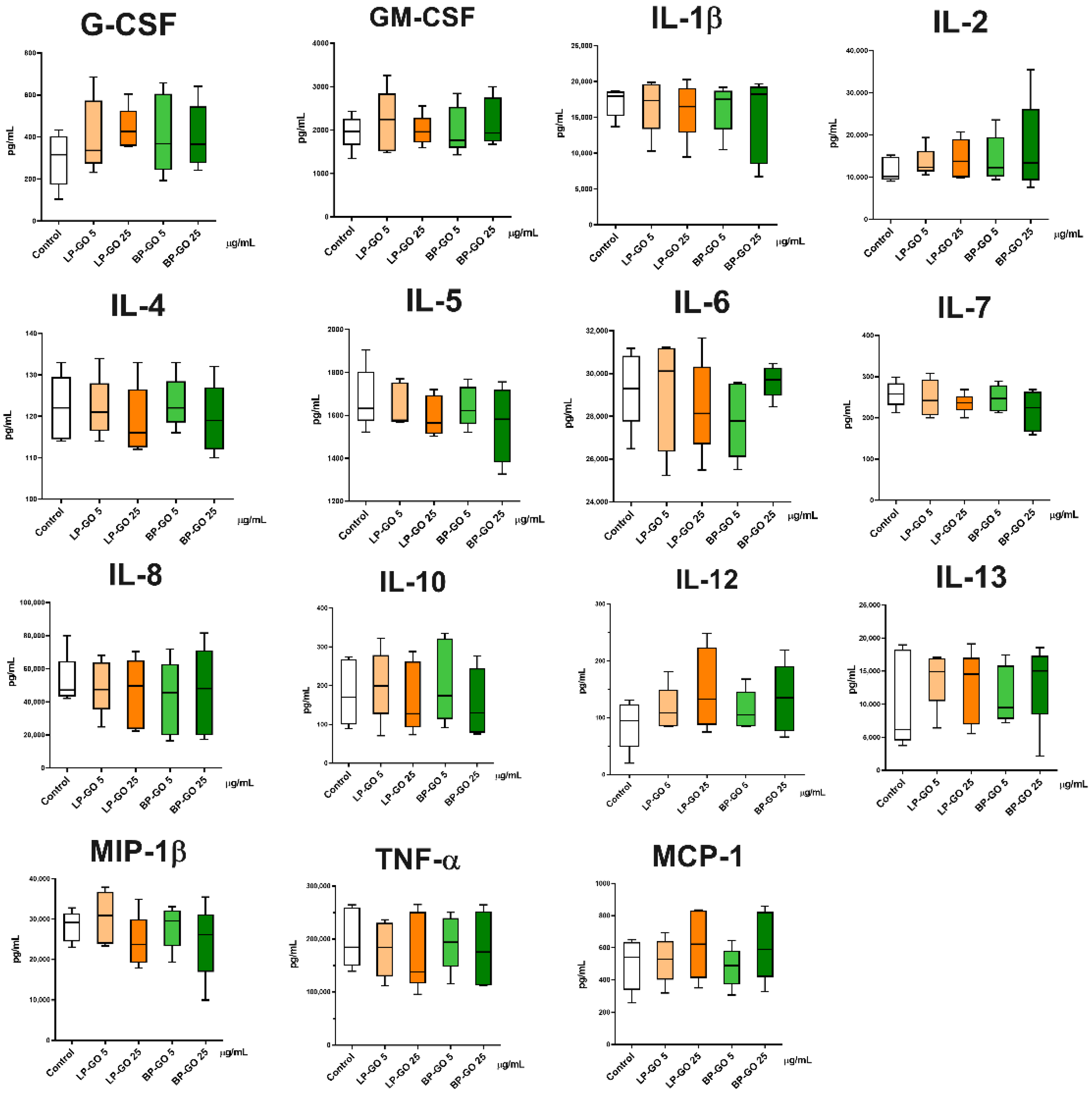
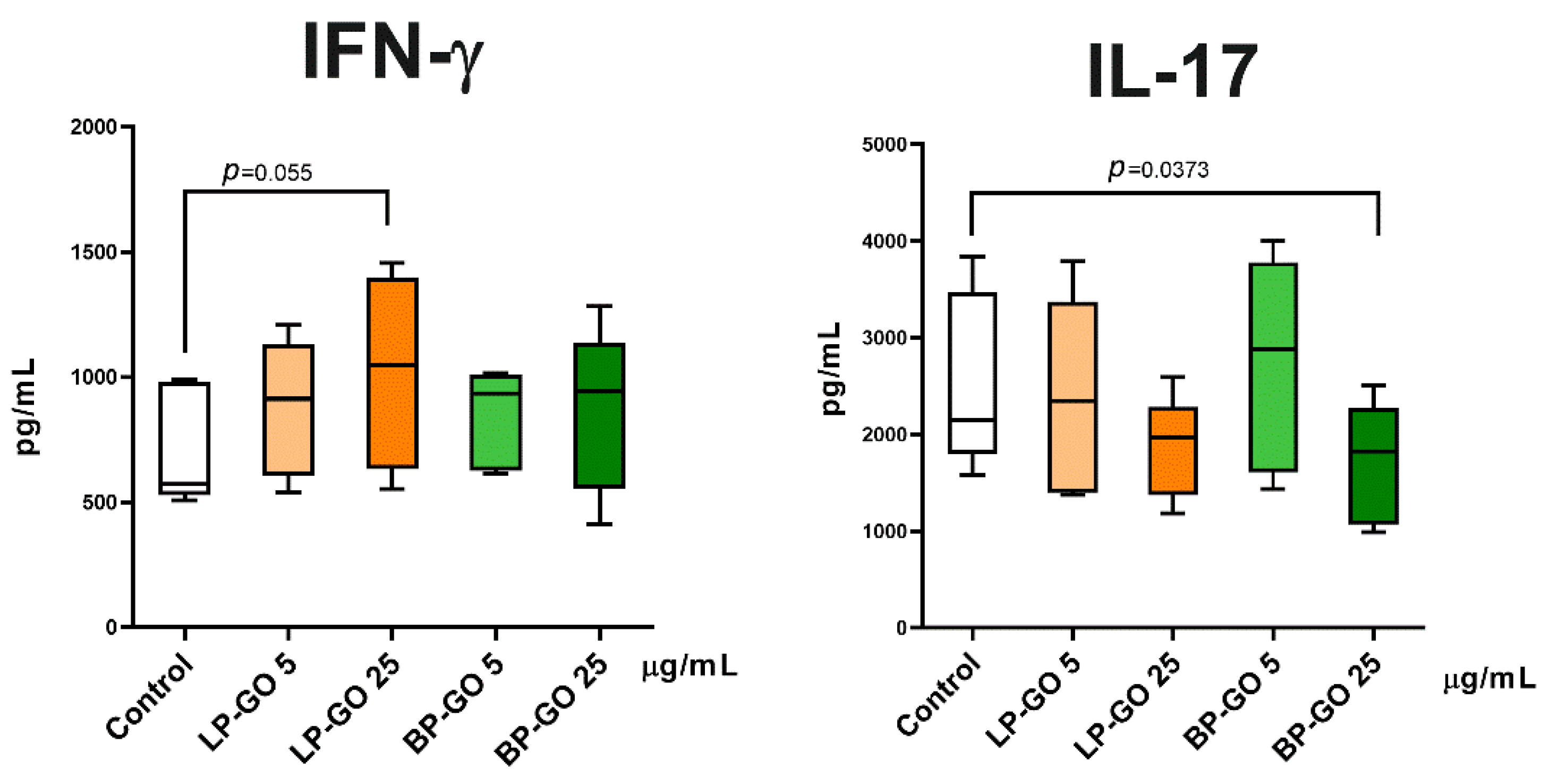
3.4. Effect of Pegylated GO Nanoparticles on the Cytokine Profile of T Helpers Polarized into the Th17 Phenotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Note for the Graphical Abstract
References
- Tang, Z.; Zhao, L.; Yang, Z.; Liu, Z.; Gu, J.; Bai, B.; Liu, J.; Xu, J.; Yang, H. Mechanisms of oxidative stress, apoptosis, and autophagy involved in graphene oxide nanomaterial antiosteosarcoma effect. Int. J. Nanomed. 2018, 13, 2907–2919. [Google Scholar] [CrossRef] [PubMed]
- Dudek, I.; Skoda, M.; Jarosz, A.; Szukiewicz, D. The molecular influence of graphene and graphene oxide on the immune system under in vitro and in vivo conditions. Arch. Immunol. Ther. Exp. 2016, 64, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Bedognetti, D.; Newman, L.; Fuoco, C.; Spada, F.; Hendrickx, W.; Marincola, F.M.; Sgarrella, F.; Rodrigues, A.F.; Ménard-Moyon, C.; et al. Single-cell mass cytometry and transcriptome profiling reveal the impact of graphene on human immune cells. Nat. Commun. 2017, 8, 1109. [Google Scholar] [CrossRef]
- Makharza, S.; Cirillo, G.; Bachmatiuk, A.; Ibrahim, I.; Loannides, N.; Trzebicka, B.; Hampel, S.; Rümmeli, M.H. Graphene oxide-based drug delivery vehicles: Functionalization, characterization, and cytotoxicity evaluation. J. Nanopart. Res. 2013, 15, 12. [Google Scholar] [CrossRef]
- Orecchioni, M.; Jasim, D.A.; Pescatori, M.; Manetti, R.; Fozza, C.; Sgarrella, F.; Bedognetti, D.; Bialco, A.; Kostarelos, K.; Delogu, L.G. Molecular and genomic impact of large and small lateral dimension graphene oxide sheets on human immune cells from healthy donors. Adv. Healthc. Mater 2016, 5, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef]
- Paino, I.M.; Santos, F.; Zucolotto, V. Biocompatibility and toxicology effects of graphene oxide in cancer, normal, and primary immune cells. J. Biomed. Mater. Res. Part A 2017, 105, 728–736. [Google Scholar] [CrossRef]
- Uzhviyuk, S.V.; Bochkova, M.S.; Timganova, V.P.; Khramtsov, P.V.; Shardina, K.Y.; Kropaneva, M.D.; Nechaev, A.I.; Rayev, M.B.; Zamorina, S.A. Graphene oxide nanoparticles in the regulation of oxidative activity of human monocytes. Med. Immunol. 2021, 23, 75–80. [Google Scholar] [CrossRef]
- Tomić, S.; Janjetović, K.; Mihajlović, D.; Milenković, M.; Kravić-Stevović, T.; Marković, Z.; Todorović-Marković, B.; Spitalsky, Z.; Micusik, M.; Vučević, D.; et al. Graphene quantum dots suppress proinflammatory T cell responses via autophagy-dependent induction of tolerogenic dendritic cells. Biomaterials 2017, 146, 13–28. [Google Scholar] [CrossRef]
- Kaneko, S.; Kondo, Y.; Yokosawa, M.; Furuyama, K.; Segawa, S.; Tsuboi, H.; Kanamori, A.; Matsumoto, I.; Yamazaki, M.; Sumida, T. The RORγt-CCR6-CCL20 axis augments Th17 cells invasion into the synovia of rheumatoid arthritis patients. Mod. Rheumatol. 2018, 28, 814–825. [Google Scholar] [CrossRef]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 2009, 119, 3573–3585. [Google Scholar] [CrossRef]
- Akdis, M.; Palomares, O.; van de Veen, W.; van Splunter, M.; Akdis, C.A. TH17 and TH22 cells: A confusion of antimicrobial response with tissue inflammation versus protection. J. Allergy Clin. Immunol. 2012, 129, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, G.; Xiao, F.; Xie, J.; Wang, S.; Lu, L.; Cui, D. Role of Th22 Cells in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2021, 12, 688066. [Google Scholar] [CrossRef] [PubMed]
- Hoe, E.; Anderson, J.; Nathanielsz, J.; Toh, Z.Q.; Marimla, R.; Balloch, A.; Licciardi, P.V. The contrasting roles of Th17 immunity in human health and disease. Microbiol. Immunol. 2017, 61, 49–56. [Google Scholar] [CrossRef]
- Peng, Z.; Hu, Y.; Ren, J.; Yu, N.; Li, Z.; Xu, Z. Circulating Th22 cells, as well as Th17 cells, are elevated in patients with renal cell carcinoma. Int. J. Med. Sci. 2021, 18, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Cui, G. TH9, TH17, and TH22 Cell Subsets and Their Main Cytokine Products in the Pathogenesis of Colorectal Cancer. Front. Oncol. 2019, 9, 1002. [Google Scholar] [CrossRef]
- Bhaumik, S.; Basu, R. Cellular and Molecular Dynamics of Th17 Differentiation and its Developmental Plasticity in the Intestinal Immune Response. Front. Immunol. 2017, 8, 254. [Google Scholar] [CrossRef]
- Kotake, S.; Yago, T.; Kobashigawa, T.; Nanke, Y. The Plasticity of Th17 Cells in the Pathogenesis of Rheumatoid Arthritis. J. Clin. Med. 2017, 6, 67. [Google Scholar] [CrossRef]
- Van Langelaar, J.; Van der Vuurst de Vries, R.M.; Janssen, M.; Wierenga-Wolf, A.F.; Spilt, I.M.; Siepman, T.A.; Dankers, W.; Verjans, G.M.G.M.; de Vries, H.E.; Lubberts, E.; et al. T helper 17.1 cells associate with multiple sclerosis disease activity: Perspectives for early intervention. Brain 2018, 141, 1334–1349. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Rivino, L.; Geginat, J.; Jarrossay, D.; Gattorno, M.; Lanzavecchia, A.; Sallusto, F.; Napolitani, G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007, 8, 639–646. [Google Scholar] [CrossRef]
- Khramtsov, P.V.; Bochkova, M.S.; Timganova, V.P.; Nechaev, A.I.; Uzhviyuk, S.V.; Shardina, K.Y.; Maslennikova, I.L.; Rayev, M.B.; Zamorina, S.A. Interaction of Graphene Oxide Modified with Linear and Branched PEG with Monocytes Isolated from Human Blood. Nanomaterials 2021, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Ganjalikhani Hakemi, M.; Ghaedi, K.; Andalib, A.; Hosseini, M.; Rezaei, A. Optimization of human Th17 cell differentiation in vitro: Evaluating different polarizing factors. In Vitro Cell Dev. Biol. Anim. 2011, 47, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Vesela, R.; Dolezalova, L.; Pytlik, R.; Rychtrmocova, H.; Mareckova, H.; Trneny, M. The evaluation of survival and proliferation of lymphocytes in autologous mixed leukocyte reaction with dendritic cells. The comparison of incorporation of 3H-thymidine and differential gating method. Cell. Immunol. 2011, 271, 78–84. [Google Scholar] [CrossRef]
- Böhmer, R.M.; Bandala-Sanchez, E.; Harrison, L.C. Forward light scatter is a simple measure of T-cell activation and proliferation but is not universally suited for doublet discrimination. Cytometry 2011, 79A, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Timganova, V.P.; Zamorina, S.A.; Litvinova, L.S.; Todosenko, N.M.; Bochkova, M.S.; Khramtsov, P.V.; Rayev, M.B. The effects of human pregnancy-specific β1-glycoprotein preparation on Th17 polarization of CD4+ cells and their cytokine profile. BMC Immunol. 2020, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Timganova, V.; Bochkova, M.; Khramtsov, P.; Kochurova, S.; Rayev, M.; Zamorina, S. Effects of pregnancy-specific β-1-glycoprotein on the helper T-cell response. Arch. Biol. Sci. 2019, 71, 369–378. [Google Scholar] [CrossRef]
- Uzhviyuk, S.V.; Bochkova, M.S.; Timganova, V.P.; Khramtsov, P.V.; Shardina, K.Y.; Kropaneva, M.D.; Nechaev, A.I.; Rayev, M.B.; Zamorina, S.A. Interaction of Human Dendritic Cells with Graphene Oxide Nanoparticles In Vitro. Bull. Exp. Biol. Med. 2022, 172, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Uzhviyuk, S.V.; Bochkova, M.S.; Timganova, V.P.; Shardina, K.Y.; Khramtsov, P.V.; Zamorina, S.A. Study of the Effect of PEG-Coated Graphene Oxide Nanoparticles on Apoptosis of NK- and NKT-cells of Human Peripheral Blood. In Science and Global Challenges of the 21st Century—Science and Technology; Rocha, A., Isaeva, E., Eds.; Perm Forum 2021; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Feito, M.J.; Cicuéndez, M.; Casarrubios, L.; Diez-Orejas, R.; Fateixa, S.; Silva, D.; Barroca, N.; Marques, P.A.A.P.; Portolés, M.T. Effects of Graphene Oxide and Reduced Graphene Oxide Nanostructures on CD4+ Th2 Lymphocytes. Int. J. Mol. Sci. 2022, 23, 10625. [Google Scholar] [CrossRef]
- Frontiñan-Rubio, J.; Gomez, M.V.; González, V.J.; Durán-Prado, M.; Vázquez, E. Sublethal exposure of small few-layer graphene promotes metabolic alterations in human skin cells. Sci. Rep. 2020, 10, 18407. [Google Scholar] [CrossRef]
- Ou, L.; Lin, S.; Song, B.; Liu, J.; Lai, R.; Shao, L. The mechanisms of graphene-based materials-induced programmed cell death: A review of apoptosis, autophagy, and programmed necrosis. Int. J. Nanomed. 2017, 12, 6633–6646. [Google Scholar] [CrossRef] [PubMed]
- Paulissen, S.M.J.; van Hamburg, J.P.; Davelaar, N.; Vroman, H.; Hazes, J.M.W.; de Jong, P.H.P.; Lubberts, E. CCR6( + ) Th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis. Arthritis Res. Therapy 2015, 17, 344. [Google Scholar] [CrossRef] [PubMed]
- Paulissen, S.M.; van Hamburg, J.P.; Dankers, W.; Lubberts, E. The role and modulation of CCR6 + Th17 cell populations in rheumatoid arthritis. Cytokine 2015, 74, 43–53. [Google Scholar] [CrossRef]
- Turner, J.E.; Paust, H.J.; Steinmetz, O.M.; Peters, A.; Riedel, J.H.; Erhardt, A.; Wegscheid, C.; Velden, J.; Fehr, S.; Mittrücker, H.W.; et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Yoshitomi, H.; Hashimoto, M.; Maeda, S.; Teradaira, S.; Sugimoto, N.; Yamaguchi, T.; Nomura, T.; Ito, H.; Nakamura, T.; et al. Preferential recruitment of CCR6−expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007, 204, 2803–2812. [Google Scholar] [CrossRef]
- Zhong, W.; Jiang, Y.; Ma, H.; Wu, J.; Jiang, Z.; Zhao, L. Elevated levels of CCR6+ T helper 22 cells correlate with skin and renal impairment in systemic lupus erythematosus. Sci. Rep. 2017, 7, 12962. [Google Scholar] [CrossRef]
- Renaude, E.; Kroemer, M.; Loyon, R.; Binda, D.; Borg, C.; Guittaut, M.; Hervouet, E.; Peixoto, P. The Fate of Th17 Cells is Shaped by Epigenetic Modifications and Remodeled by the Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 1673. [Google Scholar] [CrossRef]
- Cerboni, S.; Gehrmann, U.; Preite, S.; Mitra, S. Cytokine-regulated Th17 plasticity in human health and diseases. Immunology 2021, 163, 3–18. [Google Scholar] [CrossRef]
- Hossein-Khannazer, N.; Zian, Z.; Bakkach, J.; Kamali, A.N.; Hosseinzadeh, R.; Anka, A.U.; Yazdani, R.; Azizi, G. Features and roles of T helper 22 cells in immunological diseases and malignancies. Scand. J. Immunol. 2021, 93, e13030. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Hopkins, P.A.; Sriskandan, S. Mammalian Toll-like receptors: To immunity and behind. Clin. Exp. Immunol. 2005, 140, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 2007, 19, 39–45. [Google Scholar] [CrossRef]
- Reynolds, J.M.; Pappu, B.P.; Peng, J.; Martinez, G.J.; Zhang, Y.; Chung, Y.; Ma, L.; Yang, X.O.; Nurieva, R.I.; Tian, Q.; et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity 2010, 32, 692–702. [Google Scholar] [CrossRef]
- Orecchioni, M.; Bedognetti, D.; Sgarrella, F.; Marincola, F.M.; Bianco, A.; Delogu, L.G. Impact of carbon nanotubes and graphene on immune cells. J. Transl. Med. 2014, 12, 138. [Google Scholar] [CrossRef]
- Uto, T.; Akagi, T.; Yoshinaga, K.; Toyama, M.; Akashi, M.; Baba, M. The induction of innate and adaptive immunity by biodegradable poly(gamma-glutamic acid)nanoparticles via a TLR4 and MyD88 signaling pathway. Biomaterials 2010, 32, 5206–5212. [Google Scholar] [CrossRef]
- Chen, G.Y.; Yang, H.J.; Lu, C.H.; Chao, Y.C.; Hwang, S.M.; Chen, C.L.; Lo, K.W.; Sung, L.Y.; Luo, W.Y.; Hu, Y.C. Simultaneous induction of autophagy and toll-like receptor signaling pathways by graphene oxide. Biomaterials 2012, 33, 6559–6569. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Chen, C.L.; Tuan, H.Y.; Yuan, P.X.; Li, K.C.; Yang, H.J.; Hu, Y.C. Graphene oxide triggers toll-like receptors/autophagy responses in vitro and inhibits tumor growth in vivo. Adv. Healthc. Mater. 2014, 3, 1486–1495. [Google Scholar] [CrossRef]
- Knobloch, J.; Schild, K.; Jungck, D.; Urban, K.; Müller, K.; Schweda, E.K.; Rupp, J.; Koch, A. The T-helper cell type 1 immune response to gram-negative bacterial infections is impaired in COPD. Am. J. Respir. Crit. Care. Med. 2011, 183, 204–214. [Google Scholar] [CrossRef]
- Shive, C.L.; Jiang, W.; Anthony, D.D.; Lederman, M.M. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015, 29, 1263–1265. [Google Scholar] [CrossRef]
- Chaiwut, R.; Kasinrerk, W. Very low concentration of lipopolysaccharide can induce the production of various cytokines and chemokines in human primary monocytes. BMC. Res. Notes 2022, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Doh, K.; Kim, B.M.; Kim, K.; Chung, B.H.; Yang, C.W. Effects of resveratrol on Th17 cell-related immune responses under tacrolimus-based immunosuppression. BMC Complement Altern. Med. 2019, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Du, J.; Zhao, L.; Wang, L.; Liu, Y.; Li, D.; Yang, Y.; Zhou, R.; Zhao, Y.; Chai, Z.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 16968–16973. [Google Scholar] [CrossRef]
- Duan, G.; Kang, S.G.; Tian, X.; Garate, J.A.; Zhao, L.; Ge, C.; Zhou, R. Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane. Nanoscale 2015, 7, 15214–15224. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Ghazimoradi, M.M.; Ghorbani, M.H.; Ebadian, E.; Hassani, A.; Mirzababaei, S.; Hodjat, M.; Navaei-Nigjeh, M.; Abdollahi, M. Epigenetic effects of graphene oxide and its derivatives: A mini-review. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2022, 878, 503483. [Google Scholar] [CrossRef] [PubMed]
- Cherinka, B.; Andrews, B.H.; Sánchez-Gallego, J.; Brownstein, J.; Argudo-Fernández, M.; Blanton, M.; Bundy, K.; Jones, A.; Masters, K.; Law, D.R.; et al. Marvin: A Tool Kit for Streamlined Access and Visualization of the SDSS-IV MaNGA Data Set. Astron. J. 2019, 158, 74. [Google Scholar] [CrossRef]



| LP-GO | BP-GO | |
|---|---|---|
| Hydrodynamic diameter, nm 1 | 184 ± 73 | 287 ± 52 |
| Polydispersity index | 0.25 ± 0.02 | 0.23 ± 0.02 |
| Zeta potential, mV | −31.70 ± 1.70 | −34.28 ± 0.41 |
| PEG mass fraction, % | 17.2 ± 1.4 | 20.5 ± 1.8 |
| Control | LP-GO 5 μg/mL | LP-GO 25 μg/mL | BP-GO 5 μg/mL | BP-GO 25 μg/mL | |
|---|---|---|---|---|---|
| % of ZA-CD3+ cells | 92.12 (88.61–94.22) * | 93.53 (86.6–95.28) | 91.12 (85.23–92.51) | 92.58 (88.69–95.13) | 85.43 (80.85–93.2) |
| p value | - | >0.9999 | 0.1112 | >0.9999 | 0.182 |
| 5 μg/mL | 25 μg/mL | |
|---|---|---|
| LP-GO | 2.3 (0.23) | 11.5 (1.15) |
| BP-GO | 0.6 (0.06) | 2.8 (0.28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamorina, S.; Timganova, V.; Bochkova, M.; Shardina, K.; Uzhviyuk, S.; Khramtsov, P.; Usanina, D.; Rayev, M. The Effect of PEGylated Graphene Oxide Nanoparticles on the Th17-Polarization of Activated T Helpers. Materials 2023, 16, 877. https://doi.org/10.3390/ma16020877
Zamorina S, Timganova V, Bochkova M, Shardina K, Uzhviyuk S, Khramtsov P, Usanina D, Rayev M. The Effect of PEGylated Graphene Oxide Nanoparticles on the Th17-Polarization of Activated T Helpers. Materials. 2023; 16(2):877. https://doi.org/10.3390/ma16020877
Chicago/Turabian StyleZamorina, Svetlana, Valeria Timganova, Maria Bochkova, Kseniya Shardina, Sofya Uzhviyuk, Pavel Khramtsov, Darya Usanina, and Mikhail Rayev. 2023. "The Effect of PEGylated Graphene Oxide Nanoparticles on the Th17-Polarization of Activated T Helpers" Materials 16, no. 2: 877. https://doi.org/10.3390/ma16020877
APA StyleZamorina, S., Timganova, V., Bochkova, M., Shardina, K., Uzhviyuk, S., Khramtsov, P., Usanina, D., & Rayev, M. (2023). The Effect of PEGylated Graphene Oxide Nanoparticles on the Th17-Polarization of Activated T Helpers. Materials, 16(2), 877. https://doi.org/10.3390/ma16020877