Abstract
Glass-ceramics with the composition B2O3-Bi2O3-SrF2 were synthesized by the conventional melt-quenching technique and subsequent crystallization of the parental glasses. The temperature at which the ceramization was carried out was selected based on differential scanning calorimetry (DSC) analysis. The structure of the studied materials and the formation of SrF2 nanocrystals were confirmed by the Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) techniques. It was found that the amount of strontium fluoride introduced into the parental borate-bismuth glass has a significant impact on the growth of SrF2 nanocrystals. In particular, the influence of the crystalline SrF2 phase on luminescence intensity and kinetics was studied using Eu2O3-doped samples. An increase in luminescence intensity was observed in the samples in which SrF2 nanocrystals were formed. This is most likely related to the fact that some of the Eu3+ ions were (after annealing of the glass) located in the crystalline structure of strontium fluoride. This was confirmed both by the luminescence lifetime obtained based on the luminescence decay curves and the calculated Judd–Ofelt parameters, Ω2 and Ω4. The results achieved confirm that the glasses and glass-ceramics described in this work could be considered as a new phosphor for light-emitting diodes (LEDs).
1. Introduction
Glass as a material for applications in optics and optoelectronics has enjoyed unflagging interest for many years. In particular, a lot of research is related to glasses and glass-ceramics with optically active dopants such as rare-earth ions, which are promising materials for use in white LEDs [1,2,3]. This is because of their unique combination of properties for excellent transmission in the visible and infrared spectral range, high refractive index, and good chemical and mechanical stability [4,5]. Glasses that are the matrix for the rare-earth ions should also have low phonon energy, which decreases the risk of multi-phonon relaxation processes and, consequently, of non-radiative transitions [6]. When we consider glasses for these applications, borate glasses seem to have great potential. Boron oxide as a glass former is characterized by a wide glass formation range, high transparency, and high thermal stability [7]. Unfortunately, the addition of some network modifiers to boron oxide, usually alkali oxides, can lead to a change of coordination number of some of the boron atoms from 3 to 4, changing the properties of borate glass. Interestingly, the property change does not occur linearly with the amount of modifier. This phenomenon is known as the borate anomaly [8,9,10]. The modifier that allows very good optical properties of borate glasses to be obtained is bismuth oxide. Borate-bismuth glasses are characterized by high density and high refractive indices and, importantly due to their preparation, are glasses with a comparatively low melting point in a wide glass formation range (20–80 mol % Bi2O3) [11]. The choice of bismuth oxide over lead oxide, which has often been used in glasses in recent years, seems particularly good, as it breaks down the toxic material while leaving the glass with the desired properties. However, the addition of Bi2O3 to borate oxide also changes the coordination number of the boron atoms [10,12,13], changing the properties of the resulting glass.
Borate glasses were often used as matrices for RE3+ ions [12,14,15]. However, research devoted to this subject has shown that an interesting alternative to glasses are glass-ceramics, especially those containing metal fluoride nanocrystals. Optical transparency of glass-ceramics can be reached if the crystallite diameter does not exceed 30 nm [16]. Crystal lattice, especially when the crystalline phase is fluoride, prevents them from luminescence concentration quenching due to clusters of RE3+ ions forming [17,18,19]. Furthermore, if there are heavy atoms in the structure of the nanocrystals, an additional factor appears in the amorphous matrix that reduces the phonon energy of the matrix what consequently promotes RE3+ radiative transitions, keeping all the benefits of the glass matrix at the same time [17,20,21,22,23]. Much research has been related to the existence of PbF2 nanocrystals in the borate matrix [14,15]. However, as mentioned earlier, lead is being phased out due to its toxicity. Among various fluoride nanocrystals, which are more environmentally friendly than lead compounds, strontium fluoride SrF2 seems to be a very good choice in terms of materials for optical applications. This is because SrF2 exhibits a wide bandgap, low phonon energy, and relatively low hygroscopic properties [24]. On the other hand, to the best of our knowledge, there is no information in the literature on the possibility of crystallization of SrF2 in a borate-bismuth matrix. SrF2 nanocrystals are usually grown in glasses by annealing them at a suitable temperature above the glass transition temperature (Tg). The size of nanocrystals can be then controlled by changing heat treatment parameters, but it also depends on the type of glass structure [23,24,25,26,27]. In the case of borate-bismuth glass, the problem is more complicated because in this glass it is possible to obtain at least five stable crystalline phases of Bi2O3-B2O3 [11] and metastable bismuth orthoborate phases [28]. They can also crystallize in the matrix during annealing, leading to the crystallization of the SrF2 phase. Nevertheless, due to the optical properties of borane-bismuth glasses and the known beneficial effect of SrF2 nanocrystals on the luminescence of rare-earth ions, it is worth undertaking such research.
This work is devoted to the synthesis of borate-bismuth glasses and glass-ceramics containing SrF2 nanocrystals. We present here the results of structural studies of the above materials, as well as the impact of annealing the glasses on the luminescent properties of Eu3+ ions incorporated in them. The research was carried out to assess these materials as potential candidates for LED phosphors.
2. Materials and Methods
Borate-bismuth glasses with a nominal composition (in mol%) of 50B2O3-50Bi2O3 (BBO), 45B2O3-45Bi2O3-10SrF2 (BBO+10SrF2), and 40B2O3-40Bi2O3-20SrF2 (BBO+20SrF2) were synthesized using the conventional melt quenching technique. Moreover, glasses doped with Eu2O3 (2 mol %) were prepared: BBO+Eu, BBO+10SrF2+Eu, and BBO+20SrF2+Eu. Well-mixed starting raw materials H3BO3, Bi5OH(OH)9(NO3)4, SrF2, and Eu(NO3)3 were melted in a porcelain crucible at 1100 °C for 15 min. After that, melts were poured onto a steel hot plate (~250 °C) and immediately pressed by another plate, and then cooled down to room temperature. To investigate the effect of annealing the glasses on the crystallization of SrF2 nanocrystals, the samples were annealed in the interval of 450–590 °C for 1 h and 24 h in an air atmosphere.
Thermal properties of as-prepared glasses were studied on a Netzsch Simultaneous Thermal Analyzer, STA 449 F1, in the platinum-rhodium crucible in an air atmosphere, with a heating rate of 10 K/min. Before each measurement, a blank calibration run was conducted to account for the buoyancy effect. Differential scanning calorimetry (DSC) allowed the characteristic temperatures to be determined, such as the glass transition temperature Tg and crystallization temperature Tc.
The amorphous nature of the glasses as well as the presence of crystalline phases present in them as a result of annealing was confirmed in X-ray diffraction (XRD) studies. XRD measurements were performed on powder samples on a Philips X’PERT PLUS diffractometer with Cu-Kα radiation.
To determinate the types of structural units present in the samples, Fourier transform infrared spectroscopy (FTIR) measurements were carried out. The measurements were performed on a Perkin-Elmer Frontier MIR/FIR spectrometer with a TGS detector on pellet samples mixed with potassium bromide KBr in a weight ratio (Sample:KBr) of 1:100.
X-ray photoelectron spectroscopy (XPS) analysis confirming the valence states of ions present in the samples was carried out with an Omnicron NanoTechnology spectrometer with a 128-channel collector. XPS measurements were performed in ultra-high vacuum conditions, below 1.1 × 10−8 mBar. The photoelectrons were excited by an Mg-Kα X-ray source with X-ray anode operated at 15 keV and 300 W.
Luminescence emission and excitation spectra of the samples were collected by a SCINCO FluoroMate FS-2 fluorescence spectrometer using pellet samples mixed with KBr in a weight ratio of 1:1. The single measurement results were obtained as a quasi-three-dimensional, colorful flat image, with the wavelength in the horizontal axis, the time in the vertical axis, and the intensity expressed by a range of colors.
Time-resolved emission spectra (TRES) were acquired using a pulsed spectrofluorometer described in detail [27]. The laser system PL2251-20 with an Nd:YAG laser and a PG 401/SH optical parametric generator emitting pulses of FWHMz30 ps from EXSPLA was used as the excitation light source. The emission signal was analyzed by a Bruker Optics 2501S spectrograph and the Hammamatsu streak camera C4334-01 model. All operations were fully automated and controlled by the original Hamamatsu HPDTA software, which allows for real-time data analysis. By slicing the streak camera image at a certain time interval, the time decays were obtained.
3. Results and Discussion
3.1. DSC Analysis
To analyze the thermal properties and the thermal stability of the prepared glasses, DSC measurements were performed. The results are presented in Figure 1. One can observe that the glass transition temperature depended on the SrF2 content. Tg was located around 424 °C, 456 °C, and 459 °C for BBO, BBO+10SrF2, and BBO+20SrF2 glasses, respectively. Interestingly, the exothermic maximum associated with crystallization was only visible for the BBO sample. The onset of crystallization peak temperature (Tx) was located at 534 °C, which is well below the crystallization temperatures of the possible crystalline phases [11]. The glass stability region ∆T, defined as the difference between Tx and Tg, for BBO glass was equal to 110 °C. This is a wide range, but even a small quantity of B2O3 may have a positive effect on the thermal stability of this glass [29]. Taking into account the crystallization of the glasses, the thermal stability S parameter describing the glass resistance against devitrification was also determined [30]:
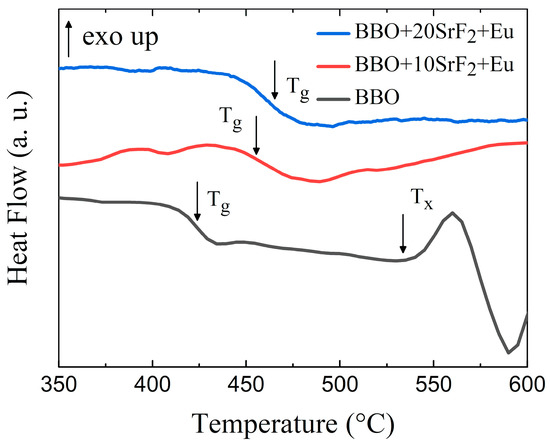
Figure 1.
DSC curves of BBO, BBO+10SrF2, and BBO+20SrF2 glasses.
In studied BBO glass, the calculated value of S is 8.72. For comparison, the values of S found in the literature for other borate glasses was even about 1 (50B2O3-50Bi2O3 [31], 50Li2O-50B2O [32]). Unfortunately, such a significant difference between these values and the value obtained for our glass was not clear to us. As can be seen, the addition of SrF2 to the base BBO composition led to the disappearance of the crystallization peak. Therefore, it can be said that it prevented the crystallization of the matrix. Unfortunately, no effect related to the crystallization of the SrF2 phase in the BBO matrix was observed in these glasses.
3.2. XRD Analysis
The amorphous character of as-prepared glasses was confirmed by XRD studies. Figure 2 shows the diffraction patterns of BBO, BBO+10SrF2, and BBO+20SrF2 glass samples. Only broad halos associated with amorphous materials can be seen.
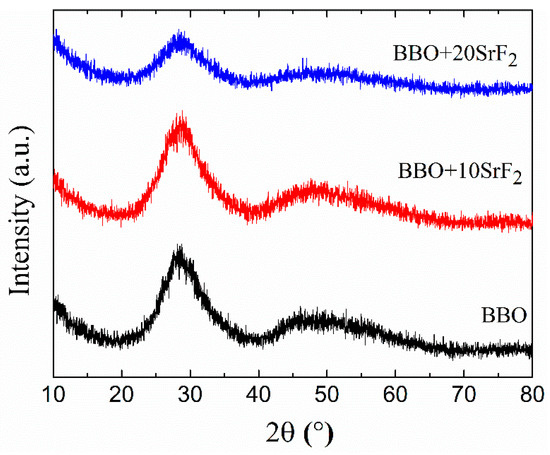
Figure 2.
XRD patterns of as-prepared BBO, BBO+10SrF2, and BBO+20SrF2 glasses.
The effect of the annealing temperature on the structure of the BBO+10SrF2 and BBO+20SrF2 glasses is shown in Figure 3 and Figure 4. In Figure 3, X-ray diffractograms for the BBO+10SrF2 and BBO+20SrF2 glasses annealed at temperatures ranging from 560 to 590 °C for 1 h are presented. Figure 4 presents XRD patterns of these glasses after heat treatment in the same temperature range, but for 24 h. The aim of the thermal treatment was the growth of SrF2 nanocrystals in the glass matrix. XRD patterns of the samples annealed at 560 °C did not differ from the patterns of the unannealed samples and showed a lack of long-range order. It should be noted, however, that in the presented XRD results a small amount of the nano-sized crystalline phase could have gone unnoticed, especially since the amorphous phase in which the nanocrystals were dispersed gives a broad halo in the range of 25–35 (2θ), in which there may be major reflections derived from the crystalline phases. Clear differences between BBO+10SrF2 and BBO+20SrF2 samples appeared after annealing at 570 °C. XRD patterns of the BBO+10SrF2 sample showed crystallization mainly in the BiBO3 phase (Ref.Code 96-720-9482), but there were also visible low-intensity reflections corresponding to the SrF2 phase (Ref.Code 96-900-9044). Unfortunately, there were also unidentified peaks in the diffractograms that were likely related to bismuth oxides, but crystalline borates sometimes have highly unusual stoichiometries [8]; therefore, it is difficult to fully characterize the presented diffraction pattern. On the other hand, BBO+20SrF2 samples showed only the peaks characteristic of the SrF2 crystalline phase (Ref.Code 96-900-9044). As can be seen, with the increase in the annealing temperature, the amount of BiBO3 crystalline phase in the BBO+10SrF2 samples decreased, whereas a weak reflection of the SrF2 phase was already present in the sample annealed at 580 °C. In the case of the annealed BBO+20SrF2 glass, the SrF2 phase crystallized regardless of the temperature increase. The obtained diffractograms confirm the conclusion drawn based on DSC studies that the presence of SrF2 in the BBO matrix prevented its crystallization. Unfortunately, annealing the samples at temperatures of 570 °C and higher caused them to begin to lose their transparency. Therefore, 560 °C was chosen as the temperature, providing a compromise between the transparency of the sample and the presence of a crystalline phase in it. At this temperature, only samples heated for 24 h became opaque.
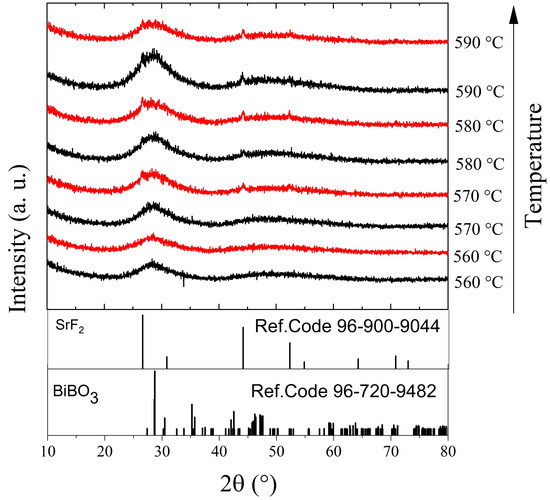
Figure 3.
XRD patterns of BBO+10SrF2 (black) and BBO+20SrF2 (red) glasses after annealing in the temperature range of 560–590 °C for 1 h.
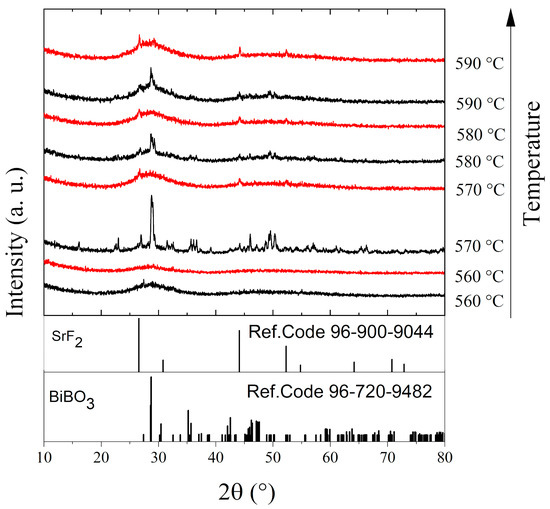
Figure 4.
XRD patterns of BBO+10SrF2 (black) and BBO+20SrF2 (red) glasses after annealing in the temperature range of 560–590 °C for 24 h.
3.3. FTIR Analysis
Changes in the structure of the studied glasses occurring due to annealing were also visible in the FTIR spectra. The FTIR spectra obtained for BBO+10SrF2 and BBO+20SrF2 glasses and glass-ceramics are shown in Figure 5a,b, respectively. For comparison, the spectrum of as-prepared BBO glass was added to each figure. It is well known that the network of borate glasses is mainly constructed from [BO3] triangular structural units, which under the influence of the modifier can transform into [BO4] tetrahedral units. Such a change can also occur under the influence of Bi2O3 [12]. The broad absorption bands in the region of 680–720 cm−1 and 1100–1250 cm−1 are usually assigned to deformation and stretching vibrations of [BO3] groups [10,13,33]. In addition, the band at about 1200–1400 cm−1 can be attributed to the stretching vibration of [BO3]. On the other hand, the presence of [BO4] groups may be indicated by the band at 900–1100 cm−1 [13,33]. However, it should be noted that these bands may shift under the influence of Bi2O3 and overlap with the Bi2O3 peaks and shoulders [33]. Looking at the spectra presented in Figure 5, it can be concluded that the annealing did not significantly affect them.
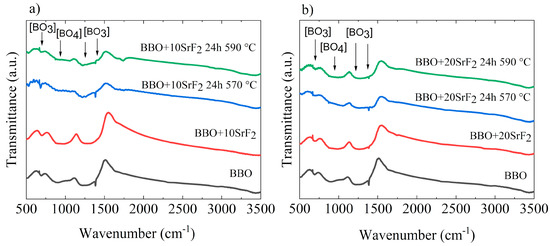
Figure 5.
FTIR spectra of BBO+10SrF2 (a) and BBO+20SrF2 (b) as-prepared glasses and glasses after annealing at 570 °C and 590 °C. For comparison, the spectrum of as-prepared BBO glass was added to each figure.
3.4. XPS Analysis
XPS analysis was performed to provide additional information about the valence states of the elements of which the samples consisted. Particular emphasis was placed on the study of the valence states of Eu and Bi ions, as well as the chemical states of Sr that could indicate Sr chemical bonds with both fluorine and oxygen [24,34,35]. As research has shown, both Eu and Bi ions are in the 3+ valence state. Exemplary spectra of the Sr region of BBO+10SrF2 and BBO+20SrF2 glasses and glass ceramics after annealing at 560 °C for 24 h are shown in Figure 6. All spectra consisted of the Sr 3d spin-orbit doublet, but a detailed analysis of these peaks suggests the presence of more than one chemical state of Sr in all samples. Peaks at 133.5 and 135.5 eV could be assigned to the Sr 3d5/2 and Sr 3d3/2 in Sr-O [34,35], whereas peaks with energies equal to 134.0 and 136.0 eV could be attributed to Sr 3d5/2 and Sr 3d3/2 in Sr-F [34,35]. The contribution of Sr-O and Sr-F doublets in the glasses was 59% and 41% (BBO+10SrF2) and 67% and 33% (BBO+20SrF2), respectively. The contribution of Sr-O and Sr-F doublets after annealing was, respectively, 80% and 20% (BBO+10SrF2) and 52% and 48% (BBO+20SrF2). Therefore, as can be seen, annealing did not change the ratio of the doublets in the same way in both samples. The increase in the contribution of the Sr-F doublet, which in turn indicated an increase in the amount of the SrF2 crystal phase in the matrix, only took place in samples containing 20 mol % SrF2. However, as the analysis of the X-ray diffractograms showed, SrF2 as the only crystalline phase was also present only in the samples containing 20 mol % SrF2. Therefore, it can be concluded from both of these methods that the formation of SrF2 nanocrystals hindered the crystallization of the matrix.
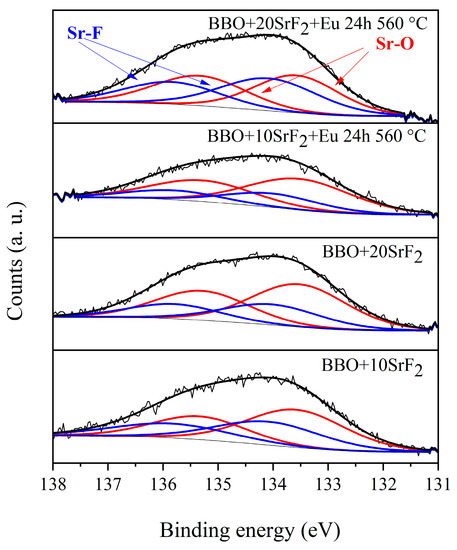
Figure 6.
Sr 3D region of XPS spectra of BBO+10SrF2 and BBO+20SrF2 as-prepared glasses and glasses after annealing at 560 °C for 24 h.
3.5. Luminescence Analysis
Luminescence properties of Eu-doped BBO, BBO+10SrF2, and BBO+20SrF2 glasses and glass-ceramics were investigated to determine the influence of SrF2 on the luminescence intensity of Eu3+ ions. Figure 7a and Figure 8a show the excitation spectra of as-prepared BBO+10SrF2+Eu and BBO+20SrF2+Eu glasses monitored at the wavelength of λem = 615 nm, which corresponds to 5D0 → 7F2 transition of Eu3+ ions [3,36]. Several characteristics for 4f-4f transition peaks were visible in the spectrum. Excitation bands at 382 and 465 nm may have been assigned to 7F0 → 5G4, 5D2 transitions, whereas bands at 396, 415, and 533 nm originated from 7F0 → 5L6, 5D3, and 5D1 transitions, respectively [3,36]. A wavelength of 465 nm was selected for the observation of the emission spectra. This is the line with the highest intensity in the excitation spectrum. Emission spectra of BBO+10SrF2+Eu glass and glass-ceramics crystallized at 560 °C (Figure 7b) consisted of several bands corresponding to Eu3+ radiative transitions at 581 nm (5D0 → 7F0), 594 nm (5D0 → 7F1), 615 nm (5D0 → 7F2), 655 nm (5D0 → 7F3), and 703 nm (5D0 → 7F4) [3,36]. As can be seen, there were no significant differences between the intensity of the bands corresponding to the BBO+Eu glass and the glass with the addition of 10 mol % of SrF2. In addition, annealing of the BBO+10SrF2+Eu samples did not change the intensity of the spectral lines. However, if we look at the results concerning the structure of the annealed samples, the crystallization of the borane-bismuth matrix, apart from the SrF2 crystal phase, cannot be ruled out. Assuming that this is the case, the emitted radiation could undergo scattering on defects. Looking at the emission spectra corresponding to the BBO+20SrF2+Eu samples (Figure 8b), it can be noticed that the emission bands were at the same wavelengths as for the samples with 10 mol % of SrF2. In addition, it is evident that annealing affected the intensity of Eu3+ ions emission. It is worth noticing that the SrF2 crystalline phase was characterized by considerably lower phonon energy compared to the oxide materials. Therefore, if Eu3+ ions are located in the SrF2 nanocrystals, this leads to a decrease in multi-phonon relaxation probability, and consequently to an increase in emission efficiency. This was also the case with other strontium fluoride-containing glass ceramics [25,37].
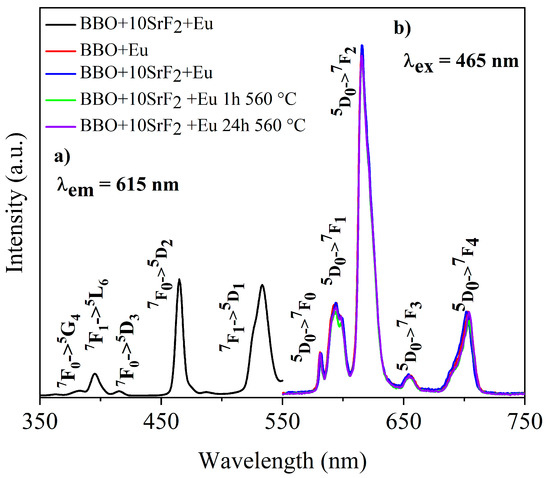
Figure 7.
The excitation spectrum of BBO+10SrF2+Eu glass (a), and the emission spectra of BBO+Eu glass, and BBO+10SrF2+Eu glass and glass-ceramics after annealing at 560 °C for 1 h and 24 h (b).
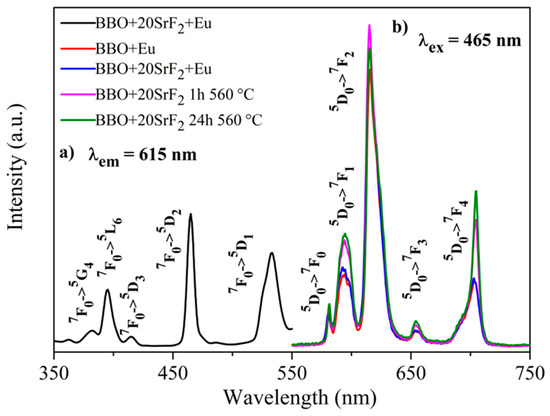
Figure 8.
The excitation spectrum of BBO+20SrF2+Eu glass (a), and the emission spectra of BBO+Eu glass, and BBO+20SrF2+Eu glass and glass-ceramics after annealing at 560 °C for 1 h and 24 h (b).
3.6. Luminescence Decay and Judd–Ofelt Analysis
Luminescence decay analysis was performed on as-prepared Eu doped BBO, BBO+10SrF2 and BBO+20SrF2 glasses and glass-ceramics. The decay curves were obtained by monitoring 5D0 →7F2 emission line (λem = 615 nm) upon excitation at λexc = 465 nm (7F0 →5D2 transition) and are shown in Figure 9a,b.
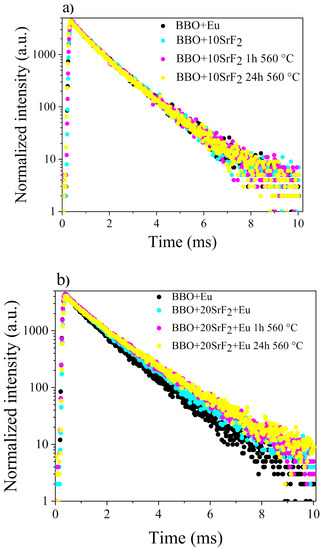
Figure 9.
Luminescence decay curves of Eu-doped BBO and BBO+10SrF2 samples (a) Eu-doped BBO and BBO+20SrF2 samples (b).
It was found that luminescence decays in both cases can be described as two-exponential decays according to the following equation [38]:
where τ1 and τ2 are long and short luminescence lifetime components contributing to the average lifetime ⟨τavg⟩, and A1 and A2 are amplitudes of respective decay components. The lifetimes τ1 and τ2, amplitudes, and average lifetime calculated for the luminescence decay curves presented in Figure 9 are collected in Table 1.

Table 1.
Fitting parameters of the luminescence decays, calculated Judd–Ofelt parameters, and luminescence intensity ratios.
This double-exponential nature of the decay curves indicates the presence of two different surroundings of Eu3+ ions. Long lifetime (τ1) is correlated with higher symmetry of the crystal field, whereas short lifetime (τ2) is associated with lower symmetry of the Eu3+ surroundings. Thus, in glasses containing SrF2 or PbF2 nanocrystals, a long lifetime is attributed to Eu3+ ions, which are located in the nanocrystals, and short lifetimes correspond to the ions incorporated into the amorphous matrix [24,25,37,39].
Concerning the tested samples, it can therefore be said that τ1 represents the lifetime of Eu3+ ions incorporated into the SrF2 nanocrystals, whereas τ2 corresponded to ions surrounded by the glass matrix. However, in the case of the studied samples, the situation seems to be more complicated. Two lifetimes were also observed in BBO+Eu glass, which did not contain strontium fluoride, and if to compare the results obtained for this glass with the values calculated for the BBO+10SrF2 sample, the lifetimes are not much different. This means that the tested glass may have contained nanocrystalline areas that were formed during the glass preparation process, which are not visible in diffraction studies. Especially in borate glasses with various dopants, single exponential decay curves are usually observed [40,41]. As can be seen, the longest τ2 lifetime was observed for the BBO+20SrF2 samples, especially those annealed for 24 h at 560 °C. It seems that in these samples the amount of Eu3+ ions incorporated into SrF2 nanocrystals increased. This result is in line with the results obtained with the XRD. They show that SrF2 as the only crystalline phase in the glass was present at 20 mol% of SrF2, whereas at 10 mol% the matrix crystallization also took place.
Changes in symmetry in the surroundings of the europium ions, as well as changes in the degree of covalence of bonds of these ions, can be observed based on Judd–Ofelt parameters. The Judd–Ofelt parameters Ω2 and Ω4 were calculated based on luminescence emission spectra using JOES application software and are presented in Table 1. Detailed information regarding software and calculations can be found in reference [42]. The Ω6 parameter was not determined in this study due to the unregistered emission band, located in the NIR range of wavelength, that corresponds to the 5D0 → 7F6 transition band. The Ω2 parameter is known to be structure sensitive and also depends on the covalence of Eu3+ bonds with the ligand [43,44,45,46]. It is determined from 5D0 → 7F2 hypersensitive transition. The high value of the Ω2 parameter (Ω2 > Ω4) suggests that Eu3+ ions occupied mostly low-symmetry sites. This corresponds to the situation where Eu3+ ions were mainly located in the glassy matrix. On the other hand, the Eu-O bond in the glasses was highly covalent, which was also reflected in the high Ω2 coefficients. In turn, the Ω4 parameter is related to the rigidity of glasses and is often attributed to the emergence of long-range effects related to crystal lattice [19]. Therefore, the processes of crystallization of glasses should consequently lead to an increase in this parameter. This behavior was observed, for example, in tellurite glass-ceramics containing SrF2 nanocrystals [25]. The parameters Ω2 and Ω4 calculated for the BBO+10SrF2 and BBO+20SrF2 glasses before and after annealing were different, but the change due to crystallization depended on the initial amount of SrF2. In the BBO+10SrF2 sample, both Ω2 and Ω4 were higher in the annealed samples. Nevertheless, it is a sample where it was difficult to say that SrF2 was the only crystalline phase in the glass matrix and it was, therefore, difficult to analyze the influence of the appearance of SrF2 nanocrystals on luminescence. On the other hand, in the case of the BBO+20SrF2 sample, after crystallization the parameter Ω2 was lower and Ω4 was higher than in as-prepared glass. However, in the BBO glass containing 20 mol% of SrF2, no additional crystallization of the matrix was observed after annealing; hence, it can be concluded that the observed change in the values of Ω2 and Ω4 parameters, as well as the increase in τ1 time, are because Eu3+ ions were located in SrF2 nanocrystals.
These results follow the luminescence intensity ratio R (asymmetry ratio), which in the case of Eu3+ ions can be calculated from the expression [45]:
The transition 5D0 → 7F1 occurs via magnetic dipole and is independent of the host matrix, whereas 5D0 → 7F2 has a pure electric dipole moment origin and is hypersensitive to changes in the crystal field around Eu3+ ions. In other words, a more intense 5D0 → 7F2 transition indicates that the Eu3+ ions mainly occupy positions without an inversion center, whereas a more intense 5D0 → 7F1 transition shows that the Eu3+ ions are located at sites with higher symmetry. Therefore, with the change in site symmetry of Eu3+ ions, when they take positions with higher symmetry, the asymmetry ratio coefficient should decrease. The intensity ratios calculated for the as-prepared BBO+20SrF2 sample and the samples after annealing at 560 °C decreased with an increase in the annealing time (resulting in the growth of SrF2 nanocrystals). This means that some of the Eu3+ ions were located in the structure of nanocrystals. Unfortunately, as shown earlier, BBO glass initially containing 10 mol % of SrF2 may behave differently, which is most likely related to the not-completely-amorphous (after annealing) borate-bismuth matrix.
4. Conclusions
In summary, borate-bismuth glass-ceramics doped with Eu3+ ions, with SrF2 nanocrystals, were obtained. The structural modifications of parental glass, leading to SrF2 nanostructure crystallization, depend strongly on the initial amount of strontium fluoride. In the case of borate-bismuth glass, it is possible to obtain at least five stable crystalline phases of Bi2O3-B2O3 [11], and 10 mol % of SrF2 introduced into the glass is insufficient to block crystallization of the matrix. This has a strong influence on the luminescent properties. The expected increase in the intensity of emission bands was not observed in such glass ceramics, which was probably related to the scattering of the emitted radiation on various types of defects occurring during the annealing of the glasses. The increase in luminescence intensity was observed after annealing in samples containing 20 mol% SrF2. The luminescence lifetimes obtained for these glass-ceramics indicate that some of the Eu3+ ions were located in SrF2 nanocrystals. This was also confirmed by the analysis of the Judd–Ofelt parameters Ω2 and Ω4, and luminescence intensity ratio R. It can therefore be concluded that the glasses and glass-ceramics described in this work could be considered as potential candidates for LED phosphors.
Author Contributions
Conceptualization, B.K. and K.M.; methodology, K.M., M.M., A.S.; M.Ł., A.M.-G., W.S., and B.K.; formal analysis, K.M. and B.K.; investigation, K.M. and B.K.; data curation, K.M. and B.K.; writing—original draft preparation, B.K. and K.M.; writing—review and editing, K.M., M.M., A.S., M.Ł., W.S., and B.K.; supervision, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study (FTIR and luminescence) are openly available at: https://mostwiedzy.pl/pl/open-research-data/luminescence-and-ftir-measurements-of-b2o3-bi2o3-srf2-glass-and-glass-ceramics-doped-with-eu3-ions,629025230630285-0 (accessed on 30 June 2020).
Acknowledgments
The authors would like to thank Leon Murawski for the fruitful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-Mallawany, R. Tellurite Glass Smart Materials, Applications in Optics and Beyond; Springer International Publishing AG, Part of Springer Nature: Cham, Switzerland, 2018; ISBN 978-3-319-76567-9. [Google Scholar] [CrossRef]
- Nagaraja Naick, B.; Damodaraiah, S.; Reddy Prasad, V.; Vijaya Lakshmi, R.P.; Ratnakaram, Y.C. Judd-Ofelt analysis and luminescence studies on Dy3+ -doped different phosphate glasses for white light emitting material applications. Opt. Int. J. Light Electron Opt. 2019, 192, 162980. [Google Scholar] [CrossRef]
- Zhao, C.; Cai, J.; Li, R.; Tie, S.; Wan, X.; Shen, J. White light emission from Eu3+/Tb3+/Tm3+ triply-doped aluminoborate glass excited by UV light. J. Non-Cryst. Solids 2012, 358, 604–608. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Santos, L.F.; Almeida, R.M. Rare-earth-doped transparent glass ceramics. C. R. Chim. 2002, 5, 845–854. [Google Scholar] [CrossRef]
- Dumbaugh, W.H.; Lapp, J.C. Heavy-metal oxide glasses. J. Am. Ceram. Soc. 1992, 75, 2315–2326. [Google Scholar] [CrossRef]
- Fan, H.; Gao, G.; Wang, G.; Hu, J.; Hu, L. Tm3+ doped Bi2O3–GeO2–Na2O glasses for 1.8 μm fluorescence. Opt. Mater. 2010, 32, 627–631. [Google Scholar] [CrossRef]
- Majérus, O.; Trégouët, H.; Caurant, D.; Pytalev, D. Comparative study of the rare earth environment in rare earth metaborate glass (REB3O6, RE = La, Nd) and in sodium borate glasses. J. Non-Cryst. Solids 2015, 425, 91–102. [Google Scholar] [CrossRef]
- Wright, A.C. My Borate Life: An Enigmatic Journey. Int. J. Appl. Glass Sci. 2015, 6, 45–63. [Google Scholar] [CrossRef]
- Wright, A.C. Borate structures: Crystalline and vitreous. Phys. Chem. Glasses 2010, 51, 1–39. [Google Scholar]
- Bobkova, N.M. Properties and Structure of Bismuth-Borate Glasses. Glass Ceram. 2016, 72, 360–365. [Google Scholar] [CrossRef]
- Bajaj, A.; Khanna, A. Crystallization of bismuth borate glasses. J. Phys. Condens. Matter 2009, 21, 035112. [Google Scholar] [CrossRef] [PubMed]
- Bobkova, N.M. Characteristic of the Structure State of Bismuth Ions in Bismuth-Borate Glasses. Glass Ceram. 2019, 75, 383–386. [Google Scholar] [CrossRef]
- Pascuta, P.; Pop, L.; Rada, S.; Bosca, M.; Culea, E. The local structure of bismuth borate glasses doped with europium ions evidenced by FTIR spectroscopy. J. Mater Sci. Mater. Electron. 2008, 19, 424–428. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Pisarska, J.; Mączka, M.; Lisiecki, R.; Grobelny, Ł.; Goryczka, T.; Dominiak-Dzik, G.; Ryba-Romanowski, W. Rare eart-doped lead borate glasses and transparent glass-ceramics: Structure-property relationship. Spectrochim. Acta Part A 2011, 27, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Pisarski, W.A.; Pisarska, J.; Ryba-Romanowski, W. Structural role of rare earth ions in lead borate glasses evidenced by infrared spectroscopy: BO3↔BO4 conversion. J. Mol. Struct. 2005, 744, 515–520. [Google Scholar] [CrossRef]
- Hopper, R.W. Stochastic theory of scattering from idealized spinodal structures: II. Scattering in general and for the basic late stage model. J. Non-Cryst. Solids 1985, 70, 111–142. [Google Scholar] [CrossRef]
- Mattarelli, M.; Tikhomirov, V.K.; Seddon, A.B.; Montagna, M.; Moserm, E.; Chiasera, A.; Chaussedent, S.; Conti, G.N.; Pelli, G.L.; Righini, C.; et al. Tm3+- activated transparent oxy-fluoride glass-ceramics: Structural and spectroscopic properties. J. Non-Cryst. Solids 2004, 346, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Boulard, B.; Péron, O.; Jestin, Y.; Ferrari, M.; Duverger-Arfuso, C. Characterization of Er3+-doped fluoride glass ceramics waveguides containing LaF3 nanocrystals. J. Lumin. 2009, 129, 1637–1640. [Google Scholar] [CrossRef]
- Rodríguez, V.D.; Lavín, V.; Rodríguez-Mendoza, U.R.; Martín, I.R. Spectroscopy of rare earth ions of fluoride glasses for laser applications. Opt. Mater. 1990, 13, 1–7. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, Y.; Zhou, D.; Qiu, J. Effect of heat treatment mechanism on upconversion luminescence in Er3+/Yb3+ co-doped NaYF4 oxyfluoride glass-ceramics. J. Alloys. Compd. 2017, 699, 303–307. [Google Scholar] [CrossRef]
- Bae, S.R.; Choi, Y.G.; Bin, W.I.; Lee, K.S.; Chung, W.J. Rare earth doped silicate-oxyfluoride glass ceramics incorporating LaF3 nano-crystals for UV-LED color conversion. Opt. Mater 2013, 35, 2034–2038. [Google Scholar] [CrossRef]
- Zur, L.; Thi, L.; Tran, N.; Meneghetti, M.; Thanh, V.T.; Righini, G.C. Tin-dioxide nanocrystals as Er3+ luminescence sensitizers: Formation of glass-ceramic thin films and their characterization. Opt. Mater. 2017, 63, 95–100. [Google Scholar] [CrossRef]
- Chen, D.; Xiang, W.; Liang, X.; Zhong, J.; Yu, H.; Ding, M.; Lu, H.; Ji, Z. Advances in transparent glass-ceramic phosphors for white light-emitting diodes—A review. J. Eur. Ceram. Soc. 2015, 35, 859–869. [Google Scholar] [CrossRef]
- Walas, M.; Lewandowski, T.; Synak, A.; Łapiński, M.; Sadowski, W.; Kościelska, B. Eu3+ doped tellurite glass ceramics containing SrF2 nanocrystals: Preparation, structure and luminescence properties. J. Alloys Compd. 2017, 696, 619–626. [Google Scholar] [CrossRef]
- Walas, M.; Lisowska, M.; Lewandowski, T.; Becerro, A.I.; Łapiński, M.; Synak, A.; Sadowski, W.; Kościelska, B. From structure to luminescence investigation of oxyfluoride transparent glasses and glass-ceramics doped with Eu3+/Dy3+ ions. J. Alloys Compd. 2019, 806, 1410–1418. [Google Scholar] [CrossRef]
- Miguel, A.; Morea, R.; Arriandiaga, M.A.; Hernandez, M.; Ferrer, F.J.; Domingo, C.; Fernandez-Navarro, J.M.; Gonzalo, J.; Fernandez, J.; Balda, R. Structural, optical, and spectroscopic properties of Er3+-doped TeO2–ZnO–ZnF2 glass-ceramics. J. Eur. Ceram. Soc. 2014, 34, 3959–3968. [Google Scholar] [CrossRef]
- Grobelna, B.; Bojarski, P.; Kuklinski, B.; Kubicki, A.A.; Synak, A. Optical properties and luminescence kinetics of Ln1.9Pr0.1(WO4)3 (where Ln = Gd, La) immobilized in silica xerogel. Opt. Mater. 2011, 34, 103–108. [Google Scholar] [CrossRef]
- Becker, P.; Fröhlich, R.Z. Crystal Growth and Crystal Structure of the Metastable Bismuth Orthoborate BiBO3. Naturforsch 2004, 59b, 256–258. [Google Scholar] [CrossRef]
- Yanmin, Y.; Yanzhou, L.; Peiqing, C.; Maalej, R.; Seo, H.J. Thermal stability and spectroscopic properties of Ho3+ doped tellurite-borate glasses. J. Rare Earths 2015, 33, 939–945. [Google Scholar] [CrossRef]
- Saad, M.; Poulain, M. Glass Forming Ability Criterion. Mater. Sci. Forum. 1987, 19–20, 1–18. [Google Scholar] [CrossRef]
- Ferreira, E.B.; Zanotto, E.D.; Feller, S.; Lodden, G.; Banerjee, J.; Edwards, T.; Affatigato, M. Critical Analysis of Glass Stability Parameters and Application to Lithium Borate Glasses. J. Am. Ceram. Soc. 2011, 94, 3833–3841. [Google Scholar] [CrossRef]
- Mary, N.; Rebours, M.; Castel, E.; Vaishnav, S.; Deng, W.; Bell, A.M.; Clegg, F.; Allsopp, B.L.; Scrimshire, A.; Bingham, P.A. Enhanced thermal stability of high-bismuth borate glasses by addition of iron. J. Non-Cryst. Solids 2018, 500, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.; Rammahb, Y.S.; El-Mallawany, R.; Souri, D. FTIR and UV spectra of pentaternary borate glasses. Measurement 2017, 105, 72–77. [Google Scholar] [CrossRef]
- Yagoub, M.Y.A.; Swart, H.C.; Noto, L.L.; O’Connel, J.H.; Lee, M.E.; Coetsee, E. The effects of Eu-concentrations on the luminescent properties of SrF2: Eu nanophosphor. J. Lumin. 2014, 156, 150–156. [Google Scholar] [CrossRef]
- Yagoub, M.Y.A.; Swart, H.C.; Coetsee, E. Concentration quenching, surface and spectral analyses of SrF2:Pr3+ prepared by different synthesis techniques. Opt. Mater. 2015, 42, 204–209. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Tarafder, A.; Biswas, K.; Annapurna, K. Sensitized red luminescence from Bi3+ co-doped Eu3+: ZnO–B2O3 glasses. Phys. B Condens. Matter 2009, 404, 3525–3529. [Google Scholar] [CrossRef]
- Luo, Q.; Qiao, X.; Fan, X.; Liu, S.; Yang, H.; Zhang, X. Reduction and luminescence of europium ions in glass ceramics containing SrF2 nanocrystals. J. Non-Cryst. Solids 2008, 354, 4691–4694. [Google Scholar] [CrossRef]
- Yu, C.; Yang, X.; Huang, A.; Chai, Z.; Qiu, J.; Song, Z.; Zhou, D. Photoluminescence properties of tellurite glasses doped Dy3 + and Eu3+ for the UV and blue converted WLEDs. J. Non-Cryst. Solids 2017, 457, 1–8. [Google Scholar] [CrossRef]
- Szpikowska-Sroka, B.; Pawlik, N.; Goryczka, T.; Pisarski, W.A. Effect of the initial reagents concentration on final crystals size and luminescence properties of PbF2:Eu3+ phosphors. J. Alloys Compd. 2008, 730, 150–160. [Google Scholar] [CrossRef]
- Jamalaiah, B.C.; Suresh Kumar, J.; Mohan Babu, A.; Rama Moorthy, L. Spectroscopic studies of Eu3+ ions in LBTAF glasses. J. Alloys Compd. 2009, 478, 63–67. [Google Scholar] [CrossRef]
- Venkatramu, V.; Babu, P.; Jayasankar, C.K. Fluorescence properties of Eu3+ ions doped borate and fluoroborate glasses containing lithium, zinc and lead. Spectrochim. Acta Part A 2006, 63, 276–281. [Google Scholar] [CrossRef]
- Ćirić, A.; Stojadinović, S.; Sekulić, M.; Dramićanin, M.D. JOES: An application software for Judd-Ofelt analysis from Eu3+ emission spectra. J. Lumin. 2019, 205, 351–356. [Google Scholar] [CrossRef]
- Haouari, M.; Ben Slimen, F.; Maaoui, A.; Gaumer, N. Structural and spectroscopic properties of Eu3+ doped tellurite glass containing silver nanoparticles. J. Alloys Compd. 2018, 743, 586–596. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Z.; Xiao, L.; Xie, F. Radiative transition propability of a europium (III) chelating polymer. Optoelecton. Adv. Mater. Rapid Commun. 2010, 4, 1396–1399. [Google Scholar]
- Bebars, S.; Gadallah, A.S.; Khedr, M.A.; Abou Kana, M.T.H. Photoluminescent properties of the europium and terbium complexes covalently bonded to functionalized mesoporous material PABA-MCM-41. J. Lumin. 2017, 192, 949–956. [Google Scholar] [CrossRef]
- Kumar, J.S.; Pavani, K.; Babu, A.M.; Giri, N.K.; Rai, S.B.; Moorthy, L.R. Fluorescence characteristics of Dy3+ ions in calcium fluoroborate glasses. J. Lumin. 2010, 130, 1916–1923. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).