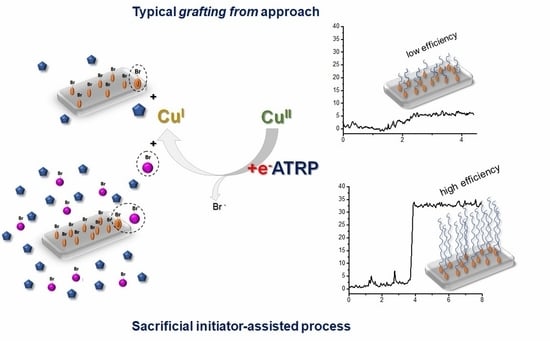
Polymer Brushes via Surface-Initiated Electrochemically Mediated ATRP: Role of a Sacrificial Initiator in Polymerization of Acrylates on Silicon Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Analysis
2.2.1. 1H NMR Spectroscopy
2.2.2. Gel Permeation Chromatography (GPC)
2.2.3. Cyclic Voltammetry (CV)
2.2.4. Atomic Force Microscopy (AFM)
2.2.5. Water Contact Angle Measurements (WCA)
2.3. Surface Functionalization of Silicon Wafers
2.4. Typical Procedure for Synthesis of the PHEA Homopolymer via seATRP under Constant Current Conditions
2.5. Synthesis of Si-g-PHEA Brushes via Surface-Initiated SI-seATRP in the Presence of a Sacrificial Initiator
2.6. Typical Procedure for PHEA Acetylation
2.7. Synthesis of Si-g-(PHEA-b-PtBA) Brushes via Chain Extension of Si-g-PHEA Brushes by Ultralow ppm Sacrificial Initiator-Assisted SI-seATRP
3. Results and Discussion
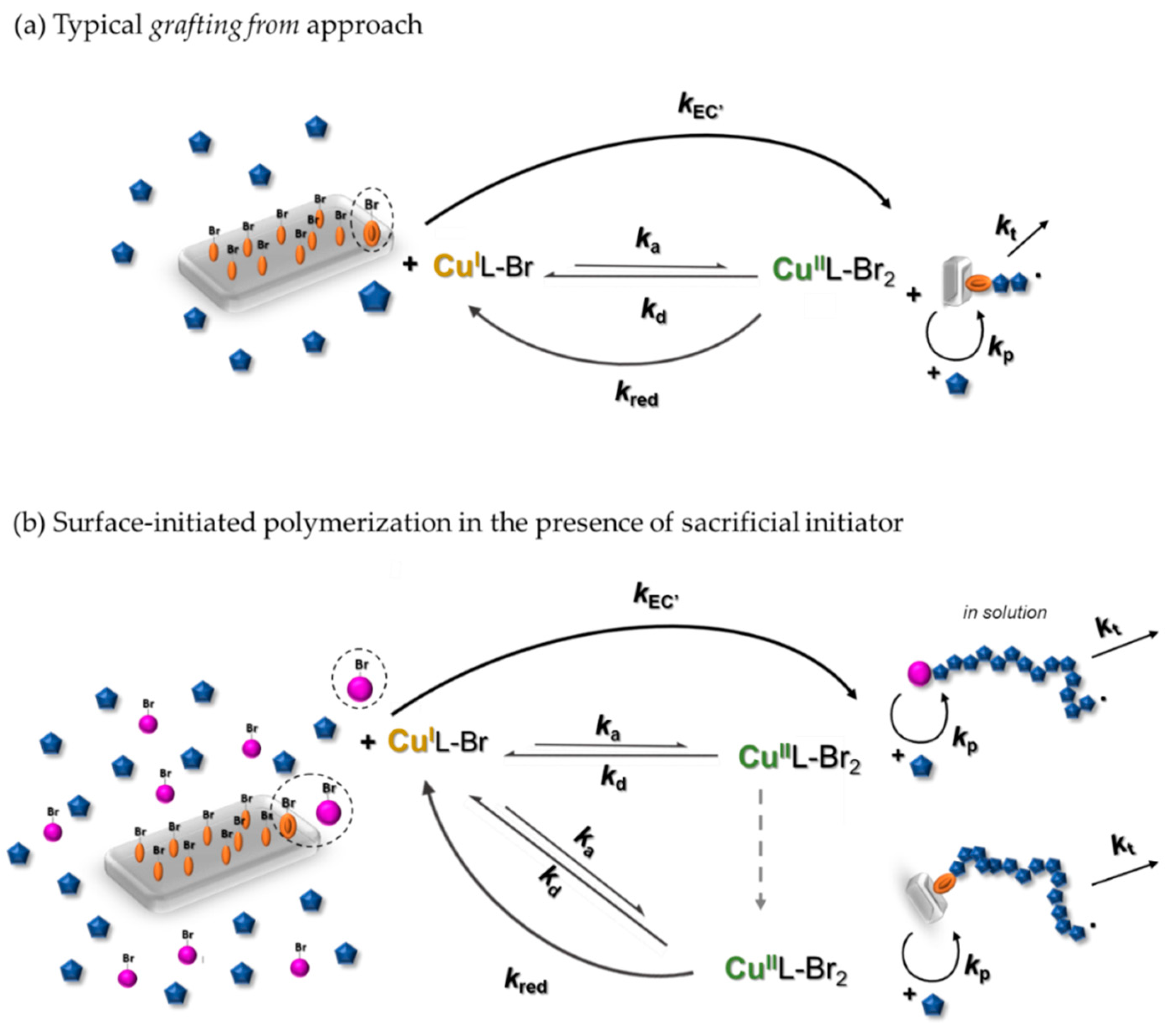
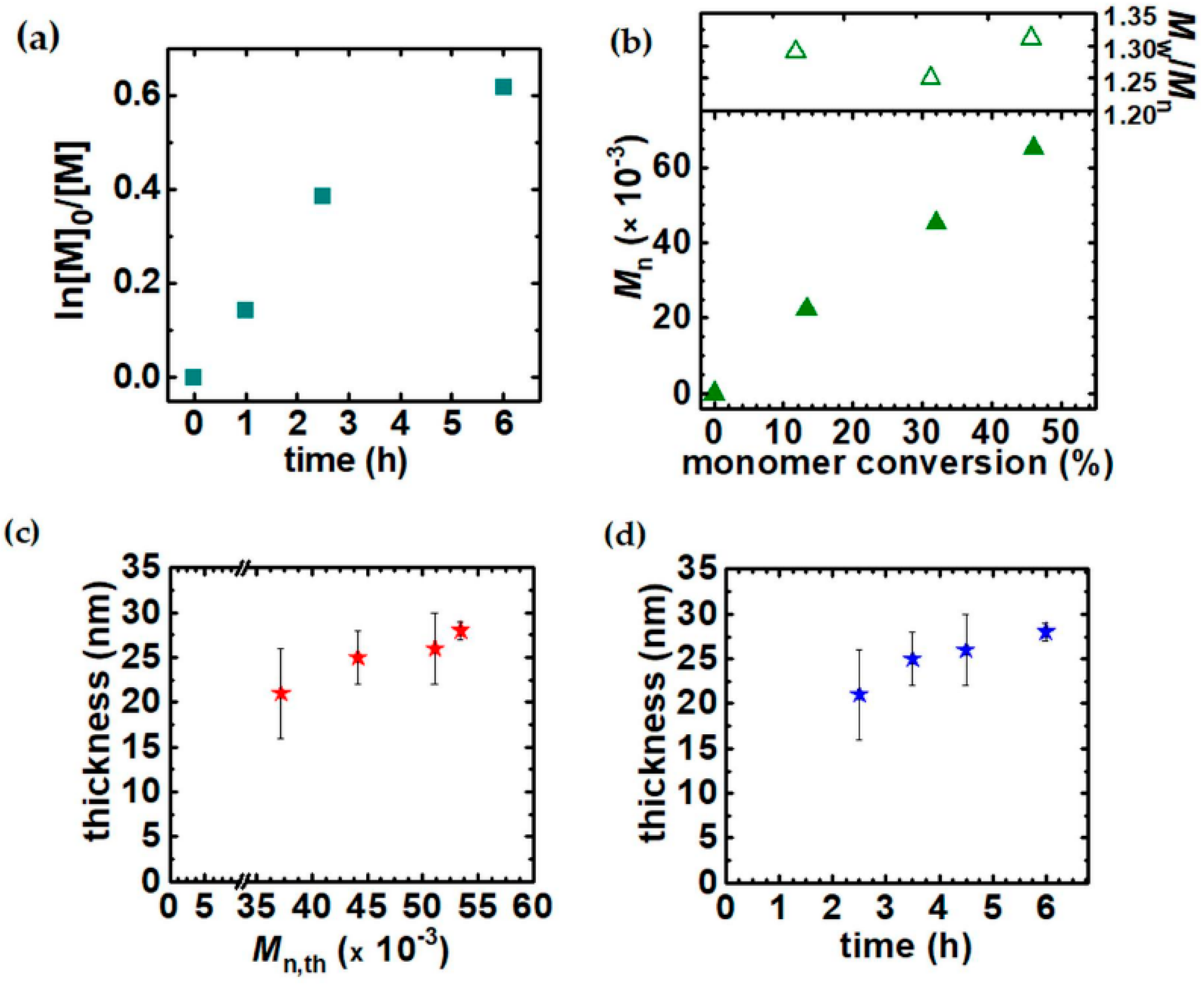
3.1. Synthesis of PHEA by Sacrificial Initiator-Assisted SI-seATRP with an Ultralow Content of Copper under Constant Potential Conditions
3.2. Synthesis of Block Copolymer Brushes Grafted from the Silicon Surface
3.3. The Impact of the Sacrificial Initiator on the Thickness of the Polyacrylate Brushes Grafted From the Silicon Surface
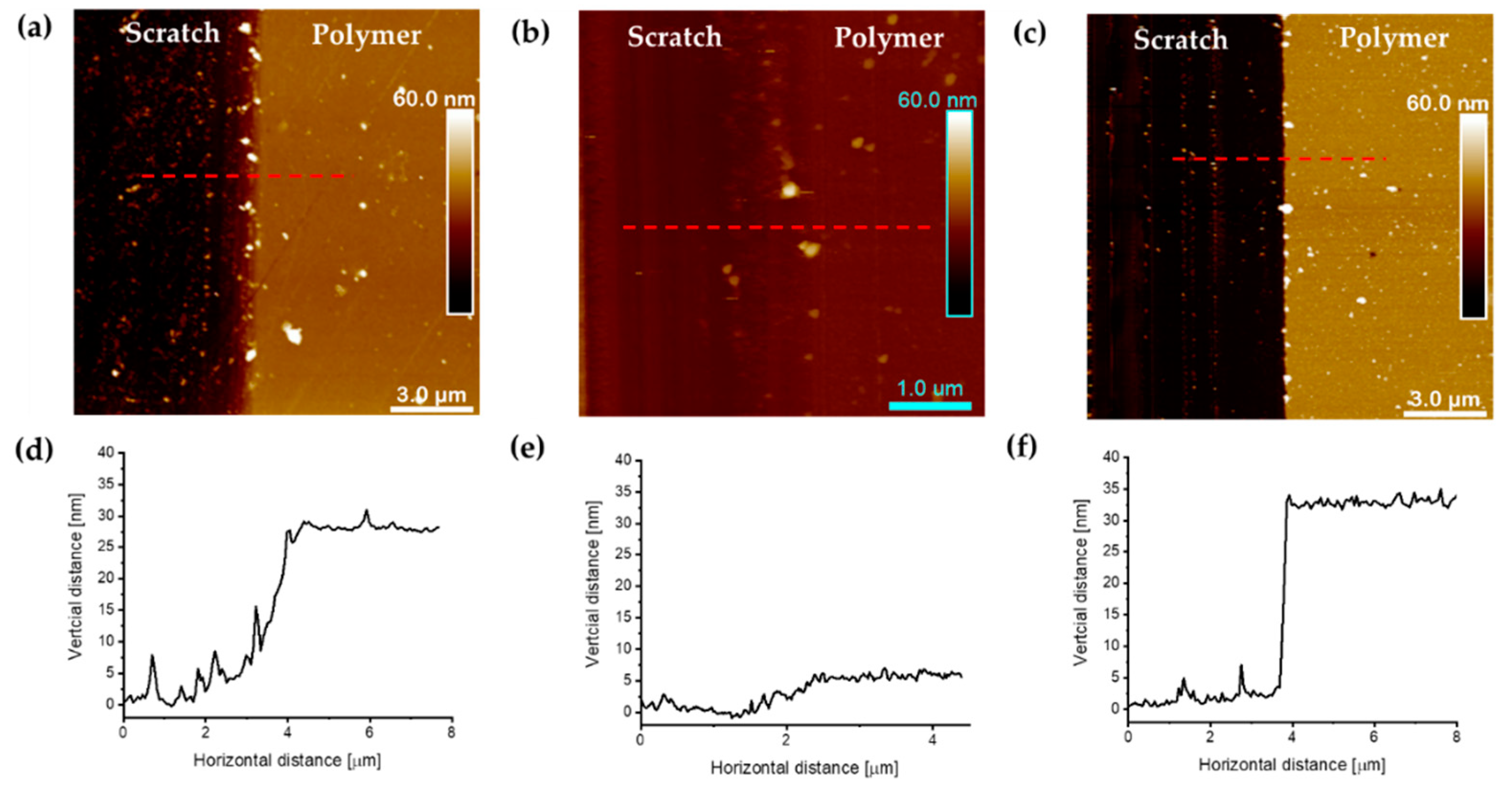
3.4. AFM Analysis of Polyacrylate Brushes Grafted from Silicon Wafers
3.5. Characteristics of Wettability of Modified Silicon Surfaces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cao, P.; Du, C.W.; He, X.Y.; Zhang, C.; Yuan, C.Q. Modification of a derived antimicrobial peptide on steel surface for marine bacterial resistance. Appl. Surf. Sci. 2020, 510, 145512. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Pan, G.Y.; Wei, X.R.; Li, Y.; Shi, H.W.; Liu, Y.Q. Preparation of high performance polyamide membrane by surface modification method for desalination. J. Membr. Sci. 2019, 573, 11–20. [Google Scholar] [CrossRef]
- Weems, A.C.; Delle Chiaie, K.R.; Yee, R.; Dove, A.P. Selective reactivity of myrcene for vat photopolymerization 3D printing and postfabrication surface modification. Biomacromolecules 2020, 21, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Flejszar, M.; Chmielarz, P. Surface modifications of poly (ether ether ketone) via polymerization methods—Current status and future prospects. Materials 2020, 13, 999. [Google Scholar] [CrossRef]
- Sharma, P.K.; Cortes, M.; Hamilton, J.W.J.; Han, Y.S.; Byrne, J.A.; Nolan, M. Surface modification of TiO2 with copper clusters for band gap narrowing. Catal. Today 2019, 321, 9–17. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Miller, P.J.; Shukla, N.; Immaraporn, B.; Gelman, A.; Luokala, B.B.; Siclovan, T.M.; Kickelbick, G.; Vallant, T.; Hoffmann, H.; et al. Polymers at interfaces: Using atom transfer radical polymerization in the controlled growth of homopolymers and block copolymers from silicon surfaces in the absence of untethered sacrificial initiator. Macromolecules 1999, 32, 8716–8724. [Google Scholar] [CrossRef]
- Xue, C.H.; Guo, X.J.; Ma, J.Z.; Jia, S.T. Fabrication of robust and antifouling superhydrophobic surfaces via surface-initiated atom transfer radical polymerization. ACS Appl. Mater. Interfaces 2015, 7, 8251–8259. [Google Scholar] [CrossRef]
- Wu, Y.P.; Guo, M.L.; Liu, G.F.; Xue, S.S.; Xia, Y.M.; Liu, D.; Lei, W.W. Surface modification of boron nitride nanosheets by polyelectrolytes via atom transfer radical polymerization. Mater. Res. Express 2018, 5, 045026. [Google Scholar] [CrossRef]
- Wang, S.S.; Song, J.X.; Li, Y.C.; Zhao, X.C.; Chen, L.; Li, G.; Wang, L.P.; Jia, Z.F.; Ge, X.C. Grafting antibacterial polymer brushes from titanium surface via polydopamine chemistry and activators regenerated by electron transfer ATRP. React. Funct. Polym. 2019, 140, 48–55. [Google Scholar] [CrossRef]
- Porter, C.J.; Werber, J.R.; Ritt, C.L.; Guan, Y.F.; Zhong, M.J.; Elimelech, M. Controlled grafting of polymer brush layers from porous cellulosic membranes. J. Membr. Sci. 2020, 596, 117719. [Google Scholar] [CrossRef]
- Pomorska, A.; Wolski, K.; Wytrwal-Sarna, M.; Bernasik, A.; Zapotoczny, S. Polymer brushes grafted from nanostructured zinc oxide layers—Spatially controlled decoration of nanorods. Eur. Polym. J. 2019, 112, 186–194. [Google Scholar] [CrossRef]
- Le Gars, M.; Bras, J.; Salmi-Mani, H.; Ji, M.; Dragoe, D.; Faraj, H.; Domenek, S.; Belgacem, N.; Roger, P. Polymerization of glycidyl methacrylate from the surface of cellulose nanocrystals for the elaboration of PLA-based nanocomposites. Carbohydr. Polym. 2020, 234, 115899. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Shimada, N.; Maruyama, A. Liposome-surface-initiated ARGET ATRP: Surface softness generated by “grafting from” polymerization. Langmuir 2019, 35, 5581–5586. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.X.; Zhang, X.H.; Huang, L.; Ma, L.F.; Liu, C.J. Diblock polymer brush (PHEAA-b-PFMA): Microphase separation behavior and anti-protein adsorption performance. Langmuir 2018, 34, 11101–11109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Jiang, X.; Lu, G.L.; Feng, C.; Huang, X.Y. The first amphiphilic graft copolymer bearing a hydrophilic poly (2-hydroxylethyl acrylate) backbone synthesized by successive RAFT and ATRP. Polym. Chem. 2014, 5, 4915–4925. [Google Scholar] [CrossRef]
- Kamada, T.; Yamazawa, Y.; Nakaji-Hirabayashi, T.; Kitano, H.; Usui, Y.; Hiroi, Y.; Kishioka, T. Patterning of photocleavable zwitterionic polymer brush fabricated on silicon wafer. Colloid Surf. B Biointerfaces 2014, 123, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.S.; Xu, J.; Gu, G.X.; Lu, G.L.; Huang, X.Y. PHEA-g-PMMA well-defined graft copolymer: ATRP synthesis, self-assembly, and synchronous encapsulation of both hydrophobic and hydrophilic guest molecules. Sci. Rep. 2017, 7, 12601. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.X.; Shen, X.R.; Gao, J.G.; Asiri, A.M.; Marwani, H.M. Grafting polyisoprene onto surfaces of nanosilica via RAFT polymerization and modification of natural rubber. Polym. Eng. Sci. 2019, 59, 1167–1174. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Liu, Y.P.; Zhang, D.H.; Lu, C.W.; Wang, C.P.; Xu, F.; Wang, J.F.; Chu, F.X. Sustainable elastomers derived from cellulose, rosin and fatty acid by a combination of “graft from” RAFT and isocyanate chemistry. Int. J. Biol. Macromol. 2019, 131, 387–395. [Google Scholar] [CrossRef]
- Messina, M.S.; Messina, K.M.M.; Bhattacharya, A.; Montgomery, H.R.; Maynard, H.D. Preparation of biomolecule-polymer conjugates by grafting-from using ATRP, RAFT, or ROMP. Prog. Polym. Sci. 2020, 100, 101186. [Google Scholar] [CrossRef]
- Cimen, D.; Caykara, T. Biofunctional oligoN-isopropylacrylamide brushes on silicon wafer surface. J. Mater. Chem. 2012, 22, 13231–13238. [Google Scholar] [CrossRef]
- Yin, Q.Y.; Charlot, A.; Portinha, D.; Beyou, E. Nitroxide-mediated polymerization of pentafluorostyrene initiated by PS-DEPN through the surface of APTMS modified fumed silica: Towards functional nanohybrids. RSC Adv. 2016, 6, 58260–58267. [Google Scholar] [CrossRef]
- Cazotti, J.C.; Fritz, A.T.; Garcia-Valdez, O.; Smeets, N.M.B.; Dube, M.A.; Cunningham, M.F. Graft modification of starch nanoparticles using nitroxide-mediated polymerization and the grafting from approach. Carbohydr. Polym. 2020, 228, 115384. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.J.; Crockett, R.M.; Spencer, N.D. Molecular-weight determination of polymer brushes generated by SI-ATRP on flat surfaces. Macromolecules 2014, 47, 269–275. [Google Scholar] [CrossRef]
- Chmielarz, P.; Krys, P.; Wang, Z.Y.; Wang, Y.; Matyjaszewski, K. Synthesis of well-defined polymer brushes from silicon wafers via surface-initiated seATRP. Macromol. Chem. Phys. 2017, 218, 1700106. [Google Scholar] [CrossRef]
- Chmielarz, P. Cellulose-based graft copolymers prepared by simplified electrochemically mediated ATRP. Express Polym. Lett. 2017, 11, 140–151. [Google Scholar] [CrossRef]
- Wolski, K.; Gruszkiewicz, A.; Wytrwal-Sarna, M.; Bernasik, A.; Zapotoczny, S. The grafting density and thickness of polythiophene-based brushes determine the orientation, conjugation length and stability of the grafted chains. Polym. Chem. 2017, 8, 6250–6262. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.S.; Tam, K.C.; Sebe, G. A comparative study on grafting polymers from cellulose nanocrystals via surface-initiated atom transfer radical polymerization (ATRP) and activator re-generated by electron transfer ATRP. Carbohydr. Polym. 2019, 205, 322–329. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Modification of wood-based materials by atom transfer radical polymerization methods. Eur. Polym. J. 2019, 120, 109253. [Google Scholar] [CrossRef]
- Lu, C.W.; Wang, C.P.; Yu, J.; Wang, J.F.; Chu, F.X. Metal-free ATRP “grafting from” technique for renewable cellulose graft copolymers. Green Chem. 2019, 21, 2759–2770. [Google Scholar] [CrossRef]
- Gruszkiewicz, A.; Slowikowska, M.; Grzes, G.; Wojcik, A.; Rokita, J.; Fiocco, A.; Wytrwal-Sarna, M.; Marzec, M.; Trzebicka, B.; Kopec, M.; et al. Enhancement of the growth of polymer brushes via ATRP initiated from ions-releasing indium tin oxide substrates. Eur. Polym. J. 2019, 112, 817–821. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem. 2009, 1, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.A.; Ribelli, T.G.; Chmielarz, P.; Park, S.; Matyjaszewski, K. A silver bullet: Elemental silver as an efficient reducing agent for atom transfer radical polymerization of acrylates. J. Am. Chem. Soc. 2015, 137, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Corrigan, N.A.; Jung, K.; Nguyen, D.; Nguyen, T.K.; Adnan, N.N.M.; Oliver, S.; Shanmugam, S.; Yeow, J. Copper-mediated living radical polymerization (atom transfer radical polymerization and copper mediated polymerization): From fundamentals to bioapplications. Chem. Rev. 2016, 116, 1803–1949. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P. Synthesis of cationic star polymers by simplified electrochemically mediated ATRP. Express Polym. Lett. 2016, 10, 810–821. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of inositol-based star polymers through low ppm ATRP methods. Polym. Adv. Technol. 2017, 28, 1804–1812. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Dually-functional riboflavin macromolecule as a supramolecular initiator and reducing agent in temporally-controlled low ppm ATRP. Express Polym. Lett. 2020, 14, 235–247. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Martinez, M.R.; Wolski, K.; Wang, Z.Y.; Matyjaszewski, K. Synthesis of high molecular weight poly (n-butyl acrylate) macromolecules via seATRP: From polymer stars to molecular bottlebrushes. Eur. Polym. J. 2020, 126, 6. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of multiarm star block copolymers via simplified electrochemically mediated ATRP. Chem. Pap. 2017, 71, 161–170. [Google Scholar] [CrossRef]
- Isse, A.A.; Lorandi, F.; Gennaro, A. Electrochemical approaches for better understanding of atom transfer radical polymerization. Curr. Opin. Electrochem. 2019, 15, 50–57. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of naringin-based polymer brushes via seATRP. Polym. Adv. Technol. 2018, 29, 470–480. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Ferreira, S.M.; Mendonca, P.V.; De Bon, F.; Serra, A.C.; Coelho, J.F.J. Guanidine as inexpensive dual function ligand and reducing agent for ATRP of methacrylates. Polym. Chem. 2019, 10, 4944–4953. [Google Scholar] [CrossRef]
- Krys, P.; Fantin, M.; Mendonca, P.V.; Abreu, C.M.R.; Guliashvili, T.; Rosa, J.; Santos, L.O.; Serra, A.C.; Matyjaszewski, K.; Coelho, J.F.J. Mechanism of supplemental activator and reducing agent atom transfer radical polymerization mediated by inorganic sulfites: Experimental measurements and kinetic simulations. Polym. Chem. 2017, 8, 6506–6519. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Synthesis of riboflavin-based macromolecules through low ppm ATRP in aqueous media. Macromol. Chem. Phys. 2020, 221, 10. [Google Scholar] [CrossRef]
- Park, S.; Chmielarz, P.; Gennaro, A.; Matyjaszewski, K. Simplified electrochemically mediated atom transfer radical polymerization using a sacrificial anode. Angew. Chem. Int. Ed. 2015, 54, 2388–2392. [Google Scholar] [CrossRef]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.D.; Sobkowiak, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- De Bon, F.; Marenzi, S.; Isse, A.A.; Durante, C.; Gennaro, A. Electrochemically mediated aqueous atom transfer radical polymerization of N,N-dimethylacrylamide. ChemElectroChem 2020, 7, 1378–1388. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of high molecular weight five-arm star polymers by improved electrochemically mediated atom transfer radical polymerization. Polimery 2017, 62, 642–649. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Wolski, K.; Grzes, G.; Isse, A.A.; Gennaro, A.; Zapotoczny, S.; Sobkowiak, A. Tannic acid-inspired star-like macromolecules via temporally controlled multi-step potential electrolysis. Macromol. Chem. Phys. 2019, 220, 190073. [Google Scholar] [CrossRef]
- Michieletto, A.; Lorandi, F.; De Bon, F.; Isse, A.A.; Gennaro, A. Biocompatible polymers via aqueous electrochemically mediated atom transfer radical polymerization. J. Polym. Sci. Polym. Chem. 2019, 58, 114–123. [Google Scholar] [CrossRef]
- Coca, S.; Jasieczek, C.B.; Beers, K.L.; Matyjaszewski, K. Polymerization of acrylates by atom transfer radical polymerization. Homopolymerization of 2-hydroxyethyl acrylate. J. Polym. Sci. Polym. Chem. 1998, 36, 1417–1424. [Google Scholar] [CrossRef]
- Leng, X.F.; Nguyen, N.H.; van Beusekom, B.; Wilson, D.A.; Percec, V. SET-LRP of 2-hydroxyethyl acrylate in protic and dipolar aprotic solvents. Polym. Chem. 2013, 4, 2995–3004. [Google Scholar] [CrossRef]
- Nicol, E.; Derouineau, T.; Puaud, F.; Zaitsev, A. Synthesis of double hydrophilic poly (ethylene oxide)-b-poly (2-hydroxyethyl acrylate) by single-electron transfer-living radical polymerization. J. Polym. Sci. Polym. Chem. 2012, 50, 3885–3894. [Google Scholar] [CrossRef]
- Nicol, E.; Nze, R.P. Supplemental activator and reducing agent atom transfer radical polymerization of 2-hydroxyethyl acrylate from high molar mass poly(ethylene oxide) macroinitiator in dilute solution. Macromol. Chem. Phys. 2015, 216, 1405–1414. [Google Scholar] [CrossRef]
- Yoshikawa, C.; Qiu, J.; Huang, C.F.; Shimizu, Y.; Suzuki, J.; van den Bosch, E. Non-biofouling property of well-defined concentrated polymer brushes. Colloid Surf. B Biointerfaces 2015, 127, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Laun, J.; Vorobii, M.; de los Santos Pereira, A.; Pop-Georgievski, O.; Trouillet, V.; Welle, A.; Barner-Kowollik, C.; Rodriguez-Emmenegger, C.; Junkers, T. Surface grafting via photo-induced copper-mediated radical polymerization at extremely low catalyst concentrations. Macromol. Rapid Commun. 2015, 36, 1681–1686. [Google Scholar] [CrossRef]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. 1997, 37, 517–524. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Popescu, D.; Steinhauer, W.; Keul, H.; Moller, M. Nitroxide-mediated copolymerization of 2-hydroxyethyl acrylate and 2-hydroxypropyl acrylate: Copolymerization kinetics and thermoresponsive properties. Macromol. Rapid Commun. 2009, 30, 2042–2048. [Google Scholar] [CrossRef]
- Chen, D.; Ping, Y.; Tang, G.P.; Li, J. Polyethyleneimine-grafted poly(N-3-hydroxypropyl) aspartamide as a biodegradable gene vector for efficient gene transfection. Soft Matter 2010, 6, 955–964. [Google Scholar] [CrossRef]
- Lin, M.; Xu, P.; Zhong, W. Preparation, characterization, and release behavior of aspirin-loaded poly (2-hydroxyethyl acrylate)/silica hydrogels. J. Biomed. Mat. Res. Part B 2012, 100B, 1114–1120. [Google Scholar] [CrossRef]
- Chmielarz, P.; Sobkowiak, A. Ultralow ppm se ATRP synthesis of PEO-b-PBA copolymers. J. Polym. Res. 2017, 24, 77. [Google Scholar] [CrossRef]
- Chmielarz, P.; Sobkowiak, A. Synthesis of poly(butyl acrylate) using an electrochemically mediated atom transfer radical polymerization. Polimery 2016, 61, 585–590. [Google Scholar] [CrossRef]
- Chmielarz, P.; Krys, P.; Park, S.; Matyjaszewski, K. PEO-b-PNIPAM copolymers via SARA ATRP and eATRP in aqueous media. Polymer 2015, 71, 143–147. [Google Scholar] [CrossRef]
- Krol, P.; Chmielarz, P. Synthesis of PMMA-b-PU-b-PMMA tri-block copolymers through ARGET ATRP in the presence of air. Express Polym. Lett. 2013, 7, 249–260. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Flejszar, M.; Surmacz, K.; Ostatek, R. Preparation of hydrophobic tannins-inspired polymer materials via low-ppm ATRP methods. Polym. Adv. Technol. 2020, 31, 913–921. [Google Scholar] [CrossRef]
- Król, P.; Król, B.; Chmielarz, P.; Wojturska, J. Assessment of susceptibility to hydrolytic degradation of different types of polyurethanes in terms of their use as biomaterials. Polimery 2013, 58, 282–291. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of α-D-glucose-based star polymers through simplified electrochemically mediated ATRP. Polymer 2016, 102, 192–198. [Google Scholar] [CrossRef]
- Chmielarz, P.; Park, S.; Sobkowiak, A.; Matyjaszewski, K. Synthesis of β-cyclodextrin-based star polymers via a simplified electrochemically mediated ATRP. Polymer 2016, 88, 36–42. [Google Scholar] [CrossRef]
- Wang, Y.; Lorandi, F.; Fantin, M.; Chmielarz, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Miniemulsion ARGET ATRP via interfacial and ion-pair catalysis: From ppm to ppb of residual copper. Macromolecules 2017, 50, 8417–8425. [Google Scholar] [CrossRef]
- Chmielarz, P.; Król, P. PSt-b-PU-b-PSt copolymers using tetraphenylethane-urethane macroinitiator through SARA ATRP. Express Polym. Lett. 2016, 10, 302–310. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Dong, H.C.; Jakubowski, W.; Pietrasik, J.; Kusumo, A. Grafting from surfaces for “everyone”: ARGET ATRP in the presence of air. Langmuir 2007, 23, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.S.; Ribelles, J.L.G.; Pradas, M.M.; Garayo, A.V.; Anton, J.S. Characterisation of macroporous poly (methyl methacrylate) coated with plasma-polymerised poly (2-hydroxyethyl acrylate). Eur. Polym. J. 2007, 43, 4552–4564. [Google Scholar] [CrossRef]
- Nomura, A.; Goto, A.; Ohno, K.; Kayahara, E.; Yamago, S.; Tsujii, Y. Controlled synthesis of hydrophilic concentrated polymer brushes and their friction/lubrication properties in aqueous solutions. J. Polym. Sci. Polym. Chem. 2011, 49, 5284–5292. [Google Scholar] [CrossRef]
- McGinty, K.M.; Brittain, W.J. Hydrophilic surface modification of poly (vinyl chloride) film and tubing using physisorbed free radical grafting technique. Polymer 2008, 49, 4350–4357. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Lai, M.; Ge, X.W.; Stanley, W.J.; Wang, B.; Wang, M.Y.; Kakinen, A.; Sani, M.A.; Whittaker, M.R.; Gurzov, E.N.; et al. Star polymers deduce islet amyloid polypeptide toxicity via accelerated amyloid aggregation. Biomacromolecules 2017, 18, 4249–4260. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Woo, S.H.; Lee, J.S.; Park, H.S.; Park, J.; Min, B.R. Improved permeate flux of PVDF ultrafiltration membrane containing PVDF-g-PHEA synthesized via ATRP. Appl. Sci. 2015, 5, 1992–2008. [Google Scholar] [CrossRef]
- Kiani, K.; Hill, D.J.T.; Rasoul, F.; Whittaker, M.; Rintoul, L. Raft mediated surface grafting of t-butyl acrylate onto an ethylene-propylene copolymer initiated by gamma radiation. J. Polym. Sci. Polym. Chem. 2007, 45, 1074–1083. [Google Scholar] [CrossRef]
- Aykac, F.S.; Yagci, Y. Simple photochemical route to block copolymers via two-step sequential type II photoinitiation. Macromol. Chem. Phys. 2018, 219, 1700589. [Google Scholar] [CrossRef]
- Yi, L.M.; Huang, C.X.; Zhou, W. Synthesis, surface properties, and morphologies of poly methyl (3,3,3-trifluoropropyl) siloxane-b-polystyrene-b-poly (tert-butyl acrylate) triblock copolymers by a combination of anionic ROP and ATRP. J. Polym. Sci. Polym. Chem. 2012, 50, 1728–1739. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 14. [Google Scholar] [CrossRef]
| Entry | M | [CuIIBr2/TPMA] [Ppm By Wt.] | Eapp(a) | DPth (b) | kpapp(c) [h−1] | kredapp(d) [s−1] | Mn,app(e) | Mn,th(f) | Ð(e) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Homopolymer | 1 | HEA | 26 | 190 mV (Epc−120 mV) | 460 | 0.107 | 0.0009 | 30,500 | 72,900 | 1.31 |
| 2 | HEA | 26 | 196 mV (Epc −120 mV) | − | − | 0.0013 | − | − | − | |
| 3 (f) | HEA | 26 | 230 mV (Epc −120 mV) | − | − | 0.0003 | − | − | − | |
| Second block of copolymer brushes | 4 | tBA | 6 | 120 mV (Epc −80 mV) | 388 | 0.058 | 0.0005 | 39,200 | 49,900 | 1.81 |
| Entry (According to Table 1) | M | Thickness (a) [nm] | Θ [°] | FSE (b) [mJ/m2] |
|---|---|---|---|---|
| 1 | HEA | 28 ± 1 | 61.9 ± 1.2 | 44.8 (±0.3)–46.4 (±0.1) |
| 2 | HEA | 4 ± 1 | 68.7 ± 1.4 | 43.1 (±0.4)–43.7 (±0.1) |
| 3 | HEA | 6 ± 1 | − | - |
| 4 | tBA | 5 ± 1 (33 ± 1) | 98.6 ± 1.4 | 36.5 (±0.2)–37.2 (±0.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flejszar, M.; Chmielarz, P.; Wolski, K.; Grześ, G.; Zapotoczny, S. Polymer Brushes via Surface-Initiated Electrochemically Mediated ATRP: Role of a Sacrificial Initiator in Polymerization of Acrylates on Silicon Substrates. Materials 2020, 13, 3559. https://doi.org/10.3390/ma13163559
Flejszar M, Chmielarz P, Wolski K, Grześ G, Zapotoczny S. Polymer Brushes via Surface-Initiated Electrochemically Mediated ATRP: Role of a Sacrificial Initiator in Polymerization of Acrylates on Silicon Substrates. Materials. 2020; 13(16):3559. https://doi.org/10.3390/ma13163559
Chicago/Turabian StyleFlejszar, Monika, Paweł Chmielarz, Karol Wolski, Gabriela Grześ, and Szczepan Zapotoczny. 2020. "Polymer Brushes via Surface-Initiated Electrochemically Mediated ATRP: Role of a Sacrificial Initiator in Polymerization of Acrylates on Silicon Substrates" Materials 13, no. 16: 3559. https://doi.org/10.3390/ma13163559
APA StyleFlejszar, M., Chmielarz, P., Wolski, K., Grześ, G., & Zapotoczny, S. (2020). Polymer Brushes via Surface-Initiated Electrochemically Mediated ATRP: Role of a Sacrificial Initiator in Polymerization of Acrylates on Silicon Substrates. Materials, 13(16), 3559. https://doi.org/10.3390/ma13163559