The Preparation of TiO2 Film by the Sol-Gel Method and Evaluation of Its Self-Cleaning Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2 Powder and TiO2 Thin Film
2.3. Characterization
3. Results and Discussion
3.1. The Effects of Different Hydrolysis Agents on the Stability and Crystalline Phase of TiO2 Sol
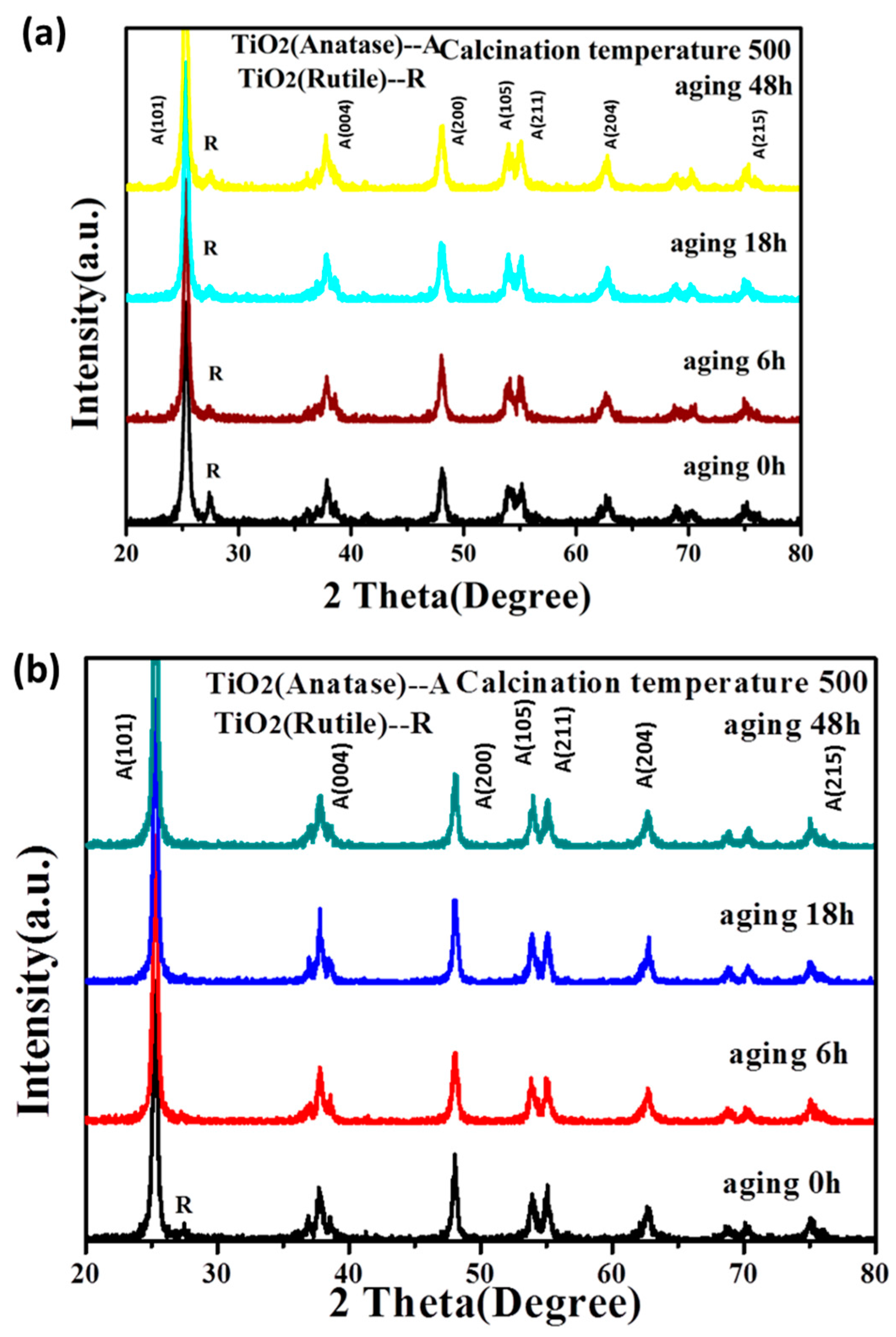
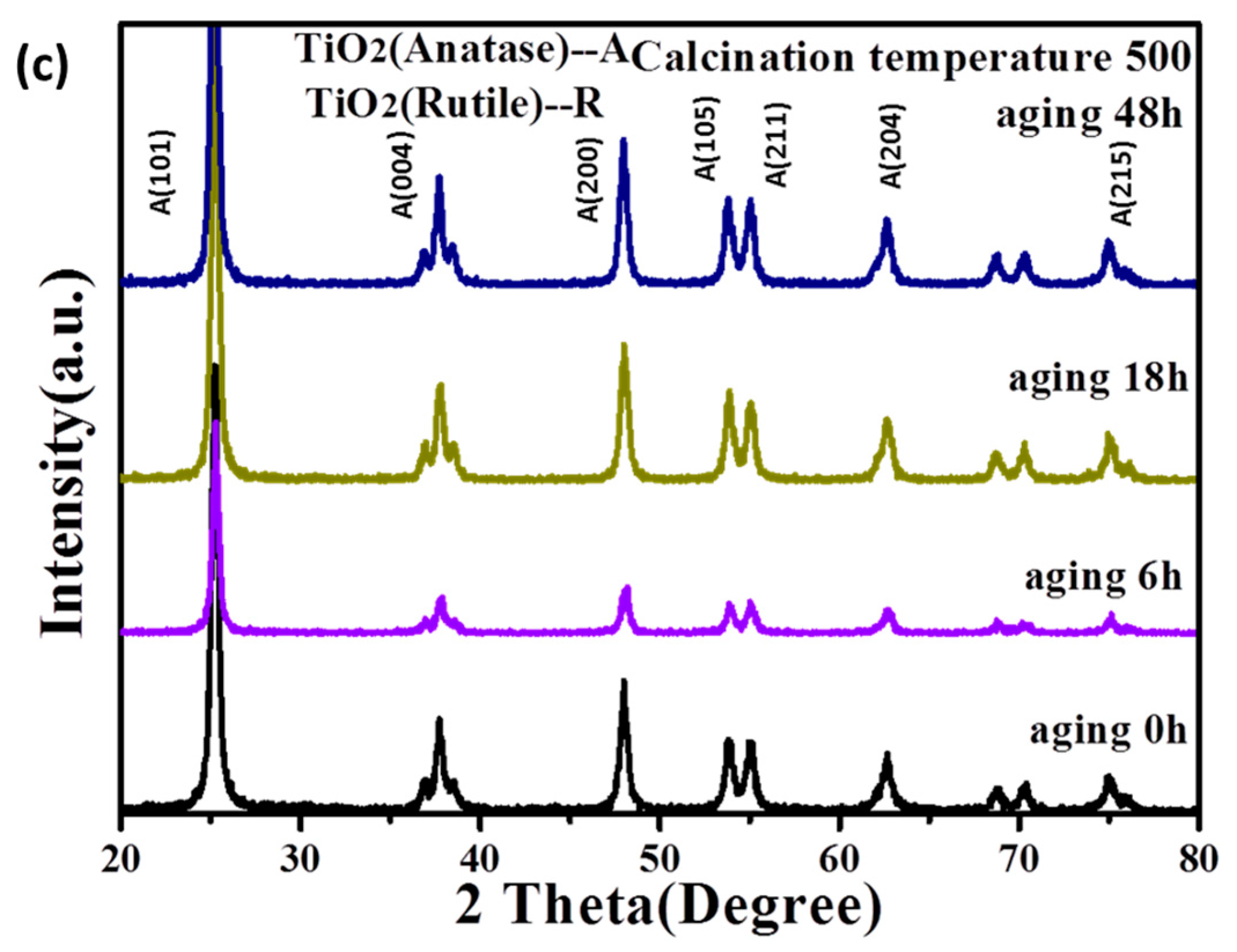
3.1.1. The Stability of TiO2 Sol
3.1.2. The Crystalline Phase of TiO2 after Calcination of the Sol
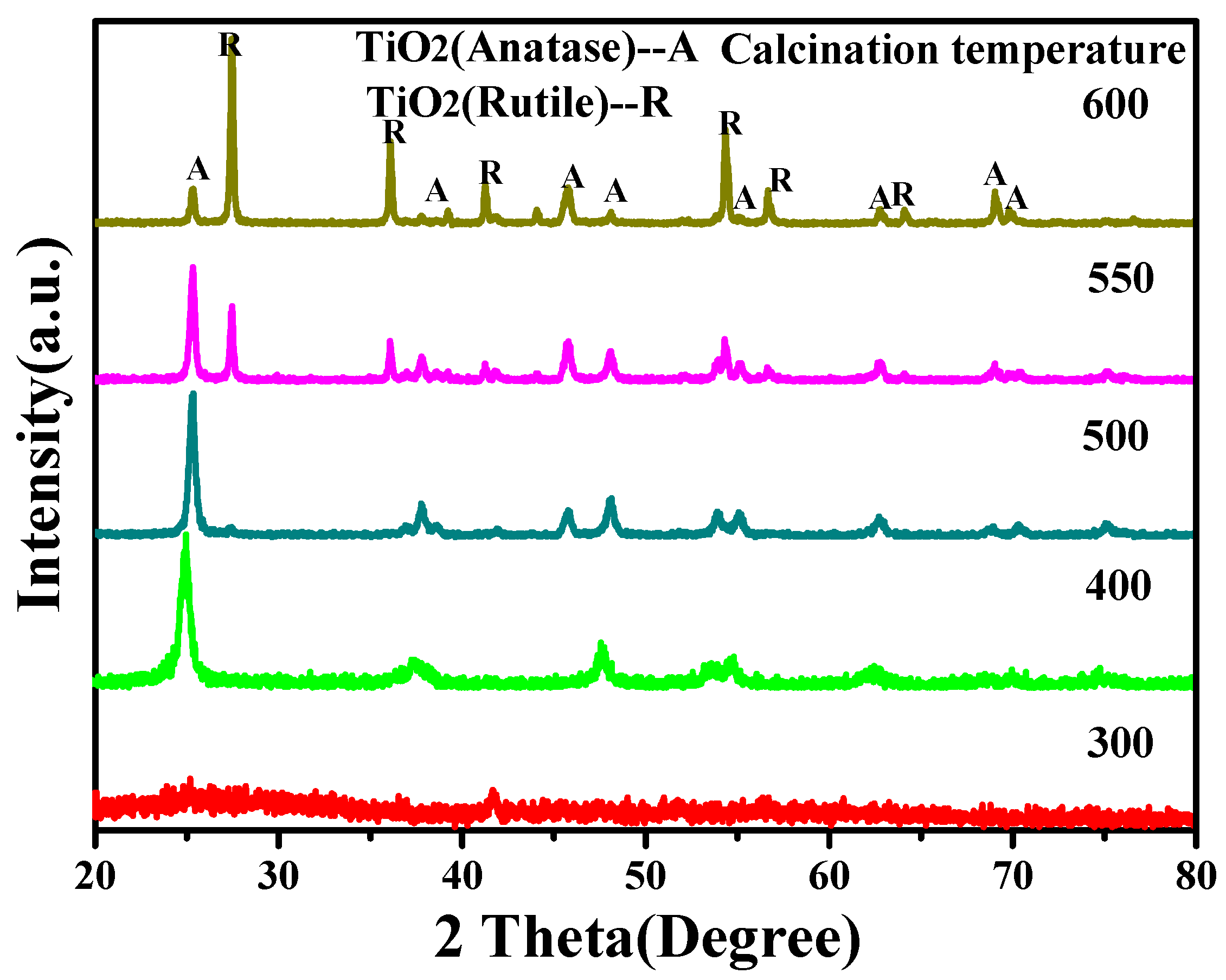
3.2. The Effect of Calcination Temperature to the Crystalline Phase and Morphology of TiO2
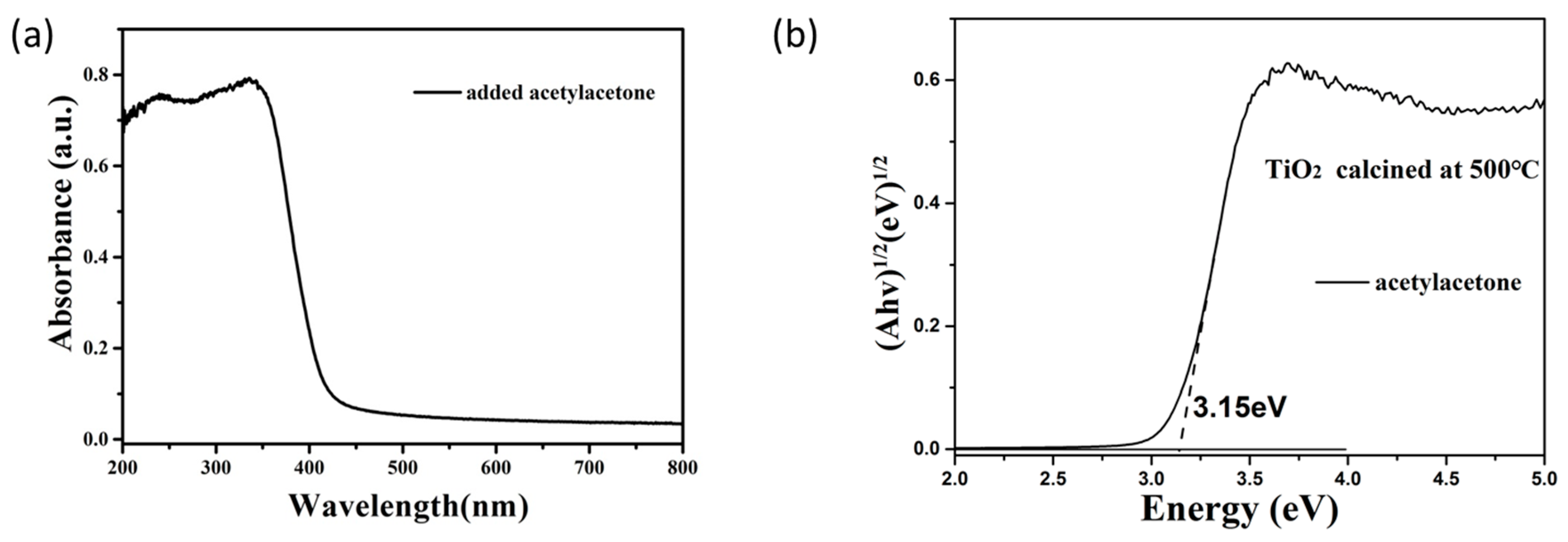
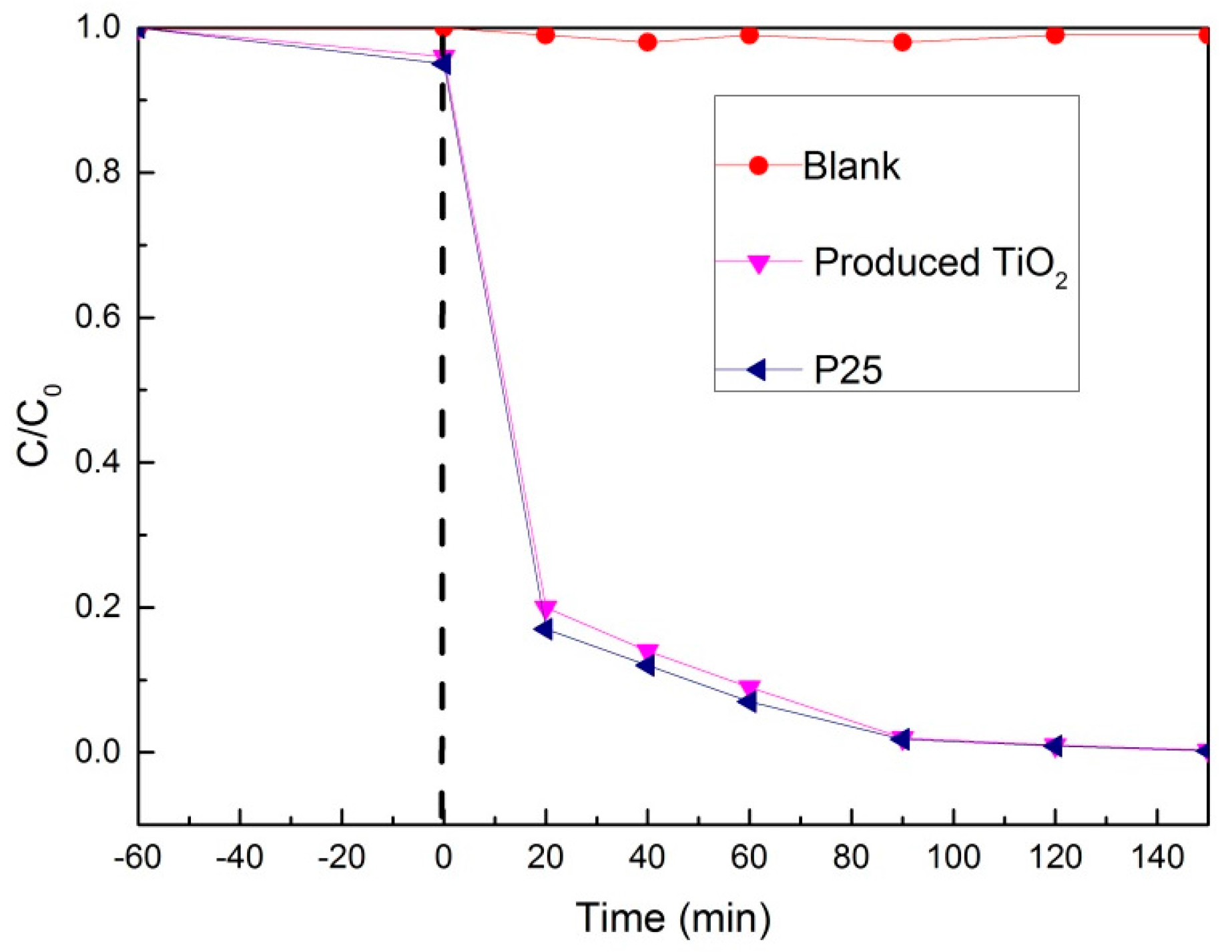
3.3. The Optical Properties and Photocatalytic Activity of TiO2 Obtained by the Calcination of TiO2 Sol
3.4. The Morphology of TiO2 Thin Film and Its Self-Cleaning Property
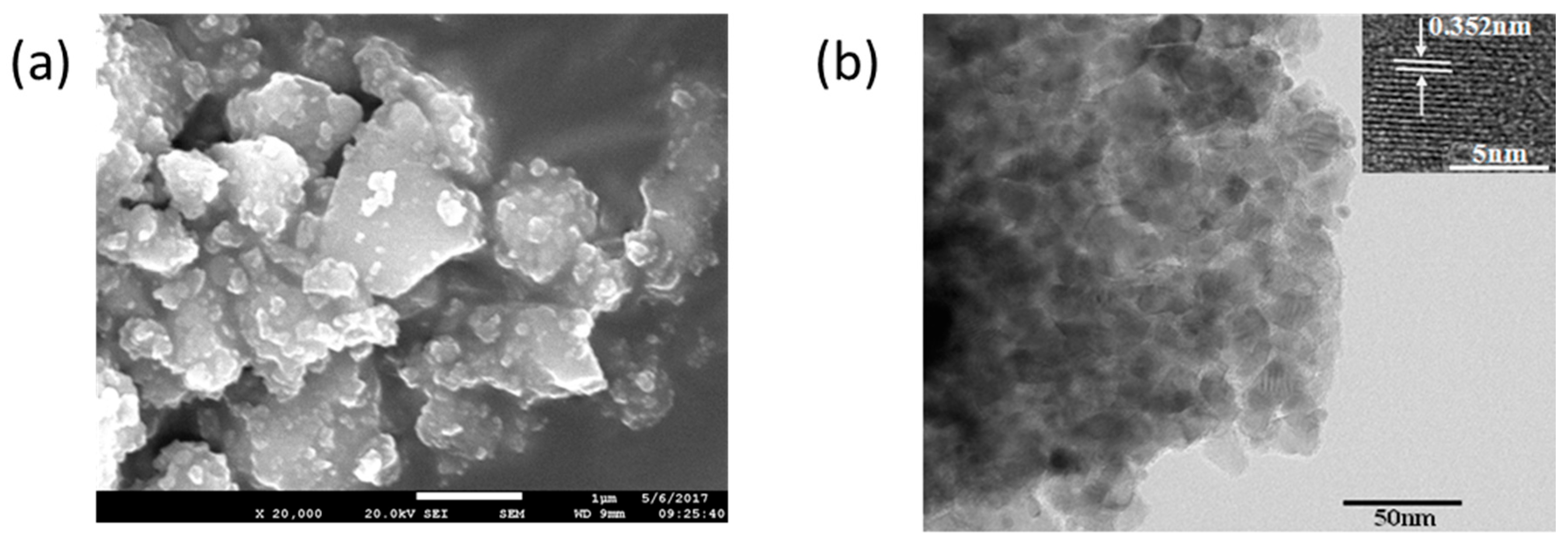
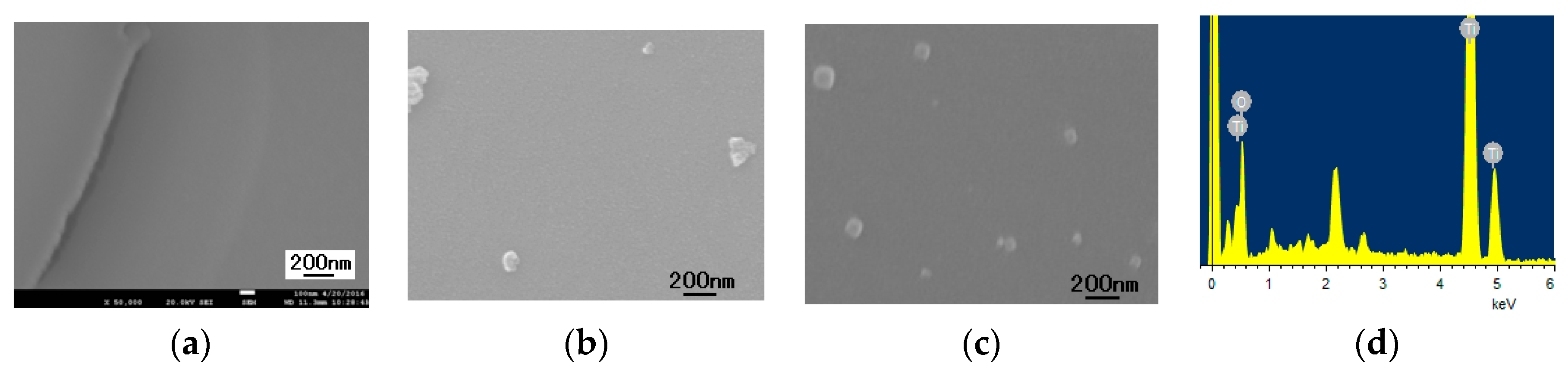
3.4.1. The Morphology of TiO2 Thin Film
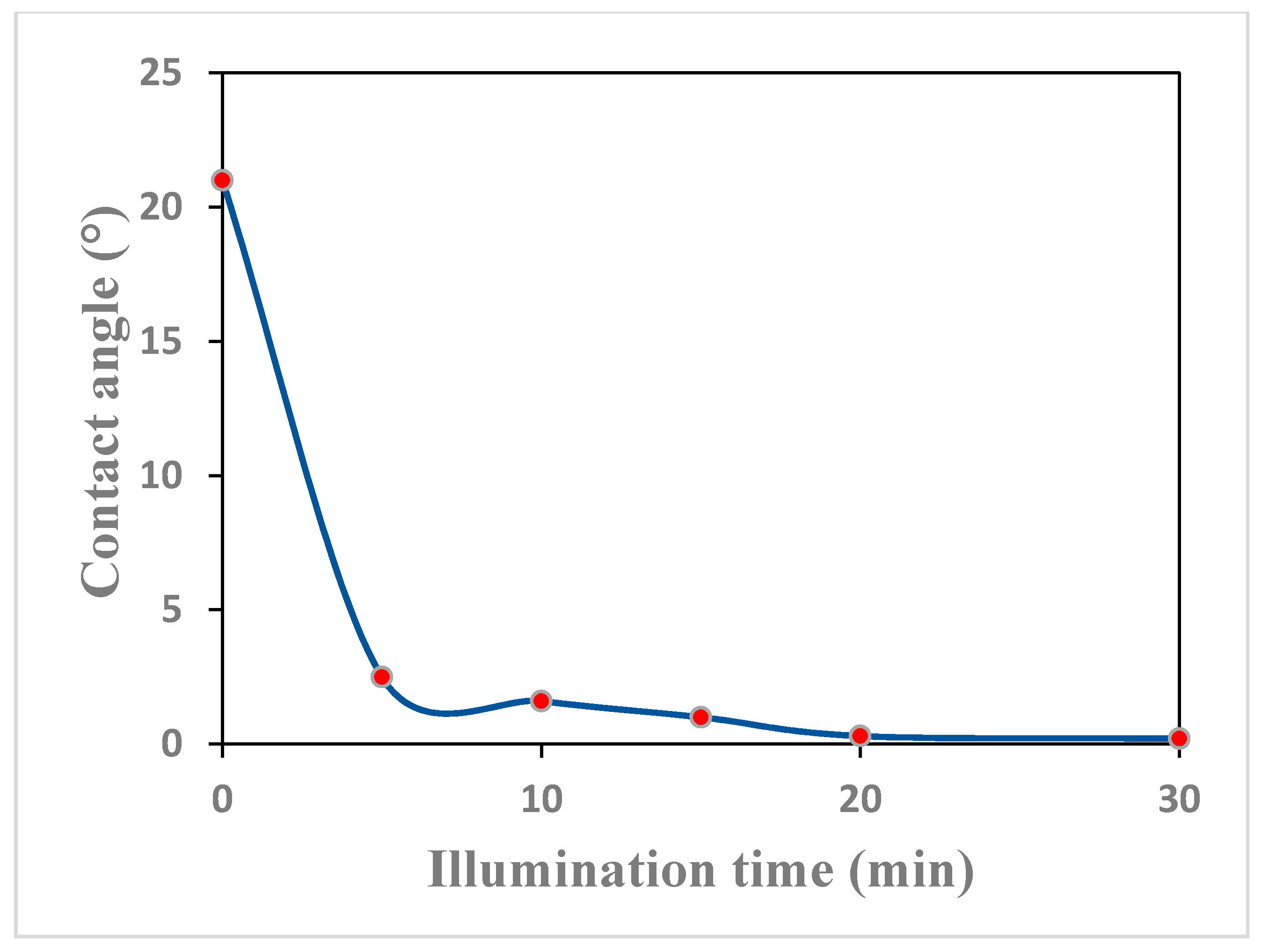
3.4.2. The Hydrophilicity and Self-Cleaning Property of the TiO2 Thin Film
3.4.3. The Antifogging Property of the TiO2 Thin Film
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Liang, Y.; Ding, H.; Xue, Q. Characterization of Brucite/TiO2 Composite Particle Material Prepared by Mechano-Chemical Method. Surf. Rev. Lett. 2018, 25, 1850085. [Google Scholar] [CrossRef]
- Adachi, M.; Murata, Y.; Takao, J.; Jiu, J.; Sakamoto, M.; Wang, F. Highly efficient dye-sensitized solar cells with a titania thin-film electrode composed of a network structure of single-crystal-like TiO2 nanowires made by the “oriented attachment” mechanism. J. Am. Chem. Soc. 2004, 126, 14943–14949. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Xiong, A.; Yoshinaga, T.; Ikeda, T.; Sakamoto, N.; Hisatomi, T.; Takashima, M.; Lu, D.; Kanehara, M.; Setoyama, T.; et al. Photocatalytic overall water splitting promoted by two different cocatalysts for hydrogen and oxygen evolution under visible light. Angew. Chem. Int. Ed. Engl. 2010, 49, 4096–4099. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, M.D.; Fresno, F.; Suareza, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Formenti, M.; Juillet, F.; Meriaudeau, P. Hetergene-ous photocatalysis for partial oxidation of paraffins. J. Chem. Technol. 1971, 4, 680–686. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 133–177. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Choina, J.; Duwensee, H.; Flechsig, G.-U.; Kosslick, H.; Morawski, A.W.; Tuan, V.A.; Schulz, A. Removal of hazardous pharmaceutical from water by photocatalytic treatment. Cent. Eur. J. Chem. 2010, 8, 1288–1297. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.T. Titanium dioxide photocatalysis: Present situation and future approaches. C. R. Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Mills, A.; Lee, S.-K. A web-based overview of semiconductor photochemistry-based current commercial applications. J. Photochem. Photobiol. A Chem. 2002, 152, 233–247. [Google Scholar] [CrossRef]
- Adesina, A.A. Industrial exploitation of photocatalysis: Progress, perspectives and prospects. Catal. Surv. Asia 2004, 8, 265–273. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.Y.L.; Tai, M.H.; Juay, J.; Liu, Z.; Sun, D. A study on the performance of self-cleaning oil–water separation membrane formed by various TiO2 nanostructures. Sep. Purif. Technol. 2015, 156, 942–951. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photolysis of Chloroform and Other Organic Molecules in Aqueous TiO2 Suspensions. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Thampi, K.R.; Reddy, T.V.; Ramakrishnan, V.; Kuriacose, J.C. Mechanism of photoelectrocatalytic dehydrogenation of 2-propanol on a polycrystalline ZnO Photoelectrode. Electrochim. Acta 1983, 28, 1869–1874. [Google Scholar] [CrossRef]
- Kandavelu, V.; Kastien, H.; Thampi, K.R. Photocatalytic degradation of isothiazolin-3-ones in water and emulsion paints containing nanocrystalline TiO2 and ZnO catalysts. Appl. Catal. B Environ. 2004, 48, 101–111. [Google Scholar] [CrossRef]
- Ge, L.; Liu, J. Efficient visible light-induced photocatalytic degradation of methyl orange by QDs sensitized CdS-Bi2WO6. Appl. Catal. B Environ. 2011, 105, 289–297. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A review on self-cleaning coatings. J. Mater. Chem. 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- Kim, S.M.; In, I.; Park, S.Y. Study of photo-induced hydrophilicity and self-cleaning property of glass surfaces immobilized with TiO2 nanoparticles using catechol chemistry. Surf. Coat. Technol. 2016, 294, 75–82. [Google Scholar] [CrossRef]
- Petica, A.; Gaidau, C.; Ignat, M.; Sendrea, C.; Anicai, L. Doped TiO nanophotocatalysts for leather surface finishing with self-cleaning properties. J. Coat. Technol. Res. 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Lucas, S.S.; Barroso de Aguiar, J.L. Multifunctional wall coating combining photocatalysis, self-cleaning and latent heat storage. Mater. Res. Express 2018, 5, 025702. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yu, R. Research on photocatalytic self-cleaning of pure cotton fabric coated with nano TiO2. Shanghai Text. Sci. Technol. 2015, 43, 54–56. [Google Scholar]
- Suyama, Y.; Kato, A. TiO2 Produced by Vapor-Phase Oxygenolysis of TiCl4. J. Am. Ceram. Soc. 1976, 59, 146–149. [Google Scholar] [CrossRef]
- Valletregi, M.; Ragel, V.; Roman, J.; Martinez, J.L.; Labeau, M.; Gonzalezcalbet, J.M. Texture evolution of SnO2 synthesized by pyrolysis of an aerosol. J. Mater. Res. 1993, 8, 138–144. [Google Scholar] [CrossRef]
- Chen, J.; Dong, X.; Xin, Y.; Zhao, M. Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure. Aquat. Toxicol. 2011, 101, 493. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wick, R.L.; Xing, B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ. Pollut. 2009, 157, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Barbana, N.; Ben Youssef, A.; Dhiflaoui, H.; Bousselmi, L. Preparation and characterization of photocatalytic TiO2 films on functionalized stainless steel. J. Mater. Sci. 2018, 53, 3341–3363. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.; Song, X.; Li, X. Effects of calcination temperature on structure of titanium dioxide photocatalyst. Inorg. Chem. Ind. 2009, 41, 37–39. [Google Scholar]
- Sang, Y.; Zhao, Z.; Zhao, M.; Hao, P.; Leng, Y.; Liu, H. From UV to Near-Infrared, WS2 Nanosheet: A Novel Photocatalyst for Full Solar Light Spectrum Photodegradation. Adv. Mater. 2015, 27, 363–369. [Google Scholar] [CrossRef] [PubMed]

| Hydrolysis Control Agent | 0 h | 6 h | 18 h | 48 h |
|---|---|---|---|---|
| acetylacetone | Brown | Brown | Brown | Brown |
| triethanolamine | Colorless | Pale white | Pale white | Some precipitation |
| acetic acid and hydrochloric acid mixture | Light brown | Light brown | Light brown | Light brown |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Sun, S.; Deng, T.; Ding, H.; Chen, W.; Chen, Y. The Preparation of TiO2 Film by the Sol-Gel Method and Evaluation of Its Self-Cleaning Property. Materials 2018, 11, 450. https://doi.org/10.3390/ma11030450
Liang Y, Sun S, Deng T, Ding H, Chen W, Chen Y. The Preparation of TiO2 Film by the Sol-Gel Method and Evaluation of Its Self-Cleaning Property. Materials. 2018; 11(3):450. https://doi.org/10.3390/ma11030450
Chicago/Turabian StyleLiang, Yu, Sijia Sun, Tongrong Deng, Hao Ding, Wanting Chen, and Ying Chen. 2018. "The Preparation of TiO2 Film by the Sol-Gel Method and Evaluation of Its Self-Cleaning Property" Materials 11, no. 3: 450. https://doi.org/10.3390/ma11030450
APA StyleLiang, Y., Sun, S., Deng, T., Ding, H., Chen, W., & Chen, Y. (2018). The Preparation of TiO2 Film by the Sol-Gel Method and Evaluation of Its Self-Cleaning Property. Materials, 11(3), 450. https://doi.org/10.3390/ma11030450





