Abstract
Hydrogen energy, with its high calorific value and zero carbon emissions, serves as a crucial solution for addressing global energy and environmental challenges while achieving carbon neutrality. The ocean offers abundant renewable energy resources including offshore wind, solar, and marine energy, along with vast seawater reserves, making it an ideal platform for green hydrogen production. This review systematically examines recent research progress in several key marine hydrogen production approaches: seawater electrolysis through both desalination-coupled and direct methods, photocatalytic seawater splitting, biological hydrogen production via algae and bacteria, and hybrid renewable energy systems, each demonstrating varying levels of technological development and industrial readiness. Despite significant advancements, challenges remain, such as reduced electrolysis efficiency caused by seawater impurities, high costs of catalysts and corrosion-resistant materials, and the intermittent nature of renewable energy sources. Future improvements require innovations in catalyst design, membrane technology, and system integration to enhance efficiency, durability, and economic feasibility. The review concludes by outlining the technological development directions for marine hydrogen energy, highlighting how hydrogen production from marine renewable energy can facilitate a sustainable blue economy through large-scale renewable energy storage and utilization.
1. Introduction
1.1. Significance of Marine Renewable Hydrogen Energy
For over two centuries since the Industrial Revolution began, the extensive use of fossil fuels has resulted in excessive greenhouse gas emissions, driving a nearly 1.5 °C increase in atmospheric temperature. This anthropogenic warming has significantly intensified global climate change, progressively threatening human survival and development. The Paris Agreement established the critical mitigation target of maintaining atmospheric CO2 concentrations below 450 ppm by the end of the 21st century [1]. In parallel, China committed in 2020 to achieve carbon neutrality by 2060 [2]. Attaining these ambitious goals necessitates urgent global restructuring of energy systems toward cleaner renewable alternatives. Hydrogen stands out as the cleanest energy carrier, producing only water as an emission byproduct during energy generation. Within the global transition toward climate change mitigation and energy system transformation, hydrogen energy—characterized by its inherent cleanliness, high energy density and zero carbon emissions—has emerged as a strategically vital secondary energy source for achieving carbon neutrality. Consequently, advancing clean hydrogen technologies, particularly “green hydrogen,” carries strategic significance for sustainable development.
Green hydrogen refers to hydrogen produced through renewable energy conversion, serving as a stable energy carrier for abundant yet intermittent natural renewable resources. Its production and utilization enable net-zero emissions of greenhouse gases and pollutants throughout the entire lifecycle, making it the most attractive hydrogen pathway compared to gray or blue hydrogen. The ocean represents Earth’s largest renewable energy reservoir, containing vast, clean, and sustainable resources including offshore wind, solar, tidal, and wave energy [3]. For instance, global offshore wind power boasts a technical potential of 27.3 TW and 66,200 TWh [4], while offshore solar energy has an annual theoretical reserve of 14 × 109 GWh [5]. Marine renewable energy offers an emerging and sustainable approach for green hydrogen production at sea. These resources can provide continuous energy for hydrogen generation, while seawater serves as an inexhaustible feedstock. This unique combination of resources positions the ocean as an ideal platform for hydrogen industry and blue economy development. The blue economy refers to the concept of sustainable utilization and management of marine resources to promote economic growth and protect the marine environment. Hydrogen production simultaneously enables flexible energy storage conversion for offshore renewables, facilitating local consumption of intermittent power supply. This approach achieves seasonal energy storage and efficient utilization, improves overall energy efficiency, and reduces costs and transmission losses associated with offshore power cables [6].
Given the promising development prospects of marine hydrogen energy, major global economies are actively advancing integrated projects focusing on offshore hydrogen production, storage, transportation and utilization. Europe currently dominates this emerging sector, accounting for 85% of existing offshore wind-to-hydrogen projects worldwide, benefiting from its stable maritime wind patterns and high-quality wind resources. France’s Sealhyfe project represents the world’s first offshore wind-powered hydrogen pilot, with a daily production capacity of 400 kg H2 [7]. The UK-led North Sea Wind Power Hub project, scheduled for commissioning in 2025 and connected to the 1.4 GW Hornsea Two wind farm (currently the world’s largest offshore wind facility), is projected to annually produce approximately 20,000 tonnes of green hydrogen. This output will supply zero-carbon hydrogen to Phillips 66’s Humber refinery, enabling annual carbon reductions exceeding 200,000 tonnes in refining processes [8]. The UK has established ambitious targets including 50 GW of offshore wind capacity with 5 GW dedicated to hydrogen production by 2030 while improving curtailment utilization for green hydrogen conversion to reach 455,000 tonnes by 2029. Concurrently, floating wind turbine-direct hydrogen production technology is being validated to achieve 82% energy conversion efficiency, paving the way for large-scale hydrogen production in deeper offshore areas with greater energy potential.
The Netherlands’ NortH2 project, currently Europe’s largest offshore wind-to-hydrogen initiative, aims to deploy 4 GW of offshore wind capacity dedicated entirely to electrolytic hydrogen production by 2030, with an annual output target of 400,000 tonnes of green hydrogen. This development will significantly contribute to the Dutch national goal of allocating one-third of its 11.5 GW offshore wind capacity for hydrogen production by 2030 [9]. The project strategically utilizes existing natural gas infrastructure in northern Netherlands, including pipeline networks and underground salt caverns for hydrogen storage, thereby minimizing new infrastructure investments. In parallel, the pioneering PosHYdon (Neptune Energy, Hague, The Netherlands) project represents the world’s first conversion of a decommissioned oil/gas platform into a green hydrogen hub, demonstrating the integration of offshore wind, legacy hydrocarbon infrastructure, and hydrogen systems [10]. This innovative approach employs offshore wind power for platform-based electrolysis, with the produced hydrogen being blended into natural gas and transported ashore via repurposed subsea pipelines. Germany has concurrently launched multiple demonstration projects encompassing offshore green hydrogen production, subsea hydrogen pipeline networks, and million-tonne-scale salt cavern storage. These initiatives include rigorous testing of critical equipment like PEM electrolyzers under simulated extreme offshore conditions (including 12-level sea states and salt spray corrosion), primarily targeting decarbonization of heavy industries (steel and chemicals) in the Ruhr region and maritime transport sectors.
Australia’s southern waters possess exceptional offshore wind potential estimated at 5000 GW, creating ideal conditions for hydrogen production. This has led the Australian government to establish ambitious targets for renewable hydrogen production and export, aiming to ship at least 200,000 tonnes annually by 2030 [11]. The Cape Hardy project in South Australia, now underway, is designed to produce over 500,000 tonnes of green hydrogen per year. Japan has initiated plans for centralized offshore hydrogen production platforms that will aggregate electricity from wind farms to semi-submersible hydrogen production units, with the produced hydrogen stored in onboard tanks and transported via specialized hydrogen carriers. This project, located off Hokkaido’s coast, targets commercialization before 2030 [12]. Japan has demonstrated leadership in maritime hydrogen infrastructure through the world’s first liquefied hydrogen carrier, Suiso Frontier, while also advancing marine applications of marine hydrogen through innovations like the Hydro Bingo, the inaugural hydrogen-powered ferry launched in 2021 [13]. China, possessing the world’s largest manufacturing capacity for offshore wind equipment and electrolyzers along with record-breaking annual installations, has established favorable industrial conditions for offshore hydrogen development, including several pilot-scale offshore wind-to-hydrogen projects [14]. Notably, China has pioneered the world’s first demonstration of direct seawater electrolysis (producing green hydrogen using untreated seawater) technology integrated with offshore wind turbines, successfully validating the system’s reliability under challenging marine conditions with wind speeds of 3–8 beaufort scale and wave heights of 0.3–0.9 m [15].
The development of hydrogen production from marine renewable sources shows considerable promise with notable economic and environmental benefits, although current technologies and applications remain at an early stage with persistent challenges. The key lies in addressing a series of specific technical issues across critical segments of the marine hydrogen value chain. These essential segments include (a) efficient conversion of marine renewable energy to hydrogen, (b) safe and reliable offshore hydrogen storage and transportation, and (c) utilization pathways and efficiency of marine hydrogen. The technical challenges encountered in these production, storage, transportation and utilization processes stem not only from inherent difficulties in hydrogen production methods, the intrinsic properties of hydrogen, and fundamental hydrogen technologies, but also from the compounded effects of unique marine environmental conditions and application scenarios.
In hydrogen production, the inherent efficiency limitations of electrolysis are exacerbated by marine conditions, where chloride-induced corrosion and biofouling significantly reduce electrolyzer lifespan, while the intermittent nature of offshore renewables further destabilizes system operation. Space constraints on turbine platforms, heavy reliance on subsea pipelines, and exposure to harsh marine weather contribute to higher failure rates. Additional investment costs arise from seawater pretreatment and hydrogen purification systems. Storage and transportation face dual challenges: hydrogen’s intrinsic low energy density (0.0899 kg/m3 at standard conditions) and marine-specific difficulties. Mechanical impacts and entrainment pose threats to desalination equipment [16]. High-pressure hydrogen storage risks material fatigue from wave impacts, cryogenic storage conflicts with humid/salty conditions due to insulation requirements, while chemical storage materials suffer performance degradation in marine climates. Utilization challenges include heightened sensitivity of fuel cell systems to vessel vibrations and tilt conditions, with hydrogen embrittlement particularly compromising equipment reliability in high-humidity, high-salinity environments. Operational constraints like limited space and difficult maintenance render land-based solutions (e.g., frequent servicing) impractical offshore. This compounding of “hydrogen characteristics” and “marine conditions” creates critical challenges across efficiency, cost-effectiveness, and safety dimensions, demanding integrated, marine-adapted solutions throughout the value chain.
1.2. Technical Framework of Marine Hydrogen Energy Systems
We have systematically outlined the technical framework of marine hydrogen energy systems, encompassing the complete technological chain from production to storage and end-use applications, along with detailed technical approaches as illustrated in Figure 1. For hydrogen production, four primary technological pathways are identified: renewable electricity-based hydrogen production, photocatalytic hydrogen production, biomass-derived hydrogen production, and ship fuel reforming. Renewable electricity-based hydrogen production involves converting offshore renewable energy into electricity to power electrolysis of either desalinated or untreated seawater. Among these approaches, offshore wind-to-hydrogen has emerged as the most mature and scalable solution, as previously discussed. Complementary to wind power, other marine energy sources including tidal, wave, and current energy can also generate electricity for electrolysis, while floating photovoltaic systems offer additional means to mitigate the intermittency of wind-based generation. Photocatalytic hydrogen production similarly utilizes solar energy and seawater as hydrogen sources, employing TiO2-based (titanium dioxide) or other catalysts to decompose seawater or surface vapor [17]. Biomass-derived hydrogen production harnesses marine microorganisms to convert seawater or biomass carbohydrates into hydrogen through controlled cultivation systems. The ship fuel reforming approach represents a transitional marine hydrogen solution, where onboard reformers catalytically process ship fuels and engine exhaust to produce hydrogen-rich gas for direct fuel use, simultaneously improving combustion efficiency and reducing maritime emissions.
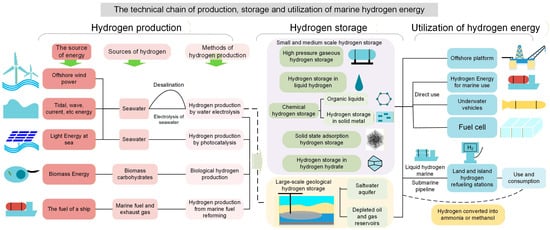
Figure 1.
The technical chain of production, storage and utilization of marine hydrogen energy.
The hydrogen storage sector primarily adopts two technical pathways: small-to-medium scale storage and large-scale geological storage. Currently, high-pressure gaseous hydrogen storage (70–80 MPa) dominates the market, despite its relatively low energy density and stringent tank requirements. Cryogenic liquid hydrogen storage, while energy-intensive, remains the preferred method for large-scale marine hydrogen transport [13]. Chemical storage approaches encompass reversible reactions using organic liquid carriers and metal hydrides for liquid-phase and solid-phase hydrogen storage/release, respectively [18]. Organic liquid carriers show promise for marine hydrogen transportation, whereas metal hydrides are better suited for modular fuel systems in hydrogen-powered vessels. H2 hydrate storage represents an emerging safe storage technology, enabling solid-state hydrogen storage under moderate temperature and pressure conditions (below 20 MPa) [19,20,21,22,23,24]. Both chemical and hydrate storage methods remain in experimental development stages. For large-scale applications, subsea geological storage leverages existing salt cavern storage and CO2 sequestration technologies, utilizing offshore saline aquifers and depleted oil/gas reservoirs with existing pipeline infrastructure. Individual storage sites can accommodate tens of thousands of metric tons of hydrogen, with stability and safety validated by petroleum industry practices [25]. This geological storage approach will serve as a primary solution for offshore wind power utilization and energy peak-shaving applications.
In the hydrogen utilization phase, offshore platforms, small islands, and marine applications including ship fuel cell systems, hydrogen-powered vessels, ROVs (remotely operated vehicle), and AUVs (autonomous underwater vehicle) can be refueled through on-site hydrogen production and storage systems. For larger islands and mainland applications, hydrogen is typically transported via subsea pipeline networks or specialized hydrogen carriers, then distributed through refueling stations before final consumption in industrial sectors such as steel manufacturing, chemical production, energy generation, and transportation [26,27]. An alternative transportation-utilization pathway involves converting the produced hydrogen into more stable liquid hydrogen carriers like ammonia or alcohols, which offer easier maritime transportation and subsequent utilization [28].
This study primarily focuses on offshore hydrogen production, systematically reviewing the latest marine renewable energy-based hydrogen generation methods and maritime-specific technologies documented in the current literature. Our comprehensive analysis encompasses direct seawater electrolysis, desalination-coupled hydrogen production, coordinated operation of intermittent renewable power with electrolysis systems, photocatalytic seawater splitting, and marine biological hydrogen production. By synthesizing cutting-edge multidisciplinary advancements, we elucidate the fundamental challenges underlying these technological bottlenecks: (1) Chloride corrosion and ion deposition in seawater electrolysis. (2) Energy consumption optimization and thermal integration requirements in desalination-coupled systems. (3) Power fluctuation management and start-stop cycling losses in renewable energy-electrolyzer coordination. (4) Charge carrier recombination limitations in photocatalysis. (5) Microbial metabolic efficiency constraints in biological approaches. Through comparative assessment of technical challenges, economic feasibility, and environmental compatibility, this review summarized and looked forward to future material innovations and system integration strategies in marine renewable hydrogen production.
The organization of this paper is structured as follows: Section 2 examines marine electrolytic hydrogen production technologies, providing a detailed analysis of the principles, characteristics, and research advancements in various marine renewable energy-to-hydrogen conversion methods. This includes electrolysis using desalinated seawater (Section 2.1), direct seawater electrolysis (Section 2.2), and the integration of renewable power generation with hydrogen production systems (Section 2.3). Section 3 focuses on photocatalytic seawater splitting. Section 4 presents marine biological hydrogen production technologies. Section 5 provides a comprehensive discussion and outlook, synthesizing the challenges across different marine renewable hydrogen production approaches and outlining future research directions. By systematically reviewing technological progress in marine renewable hydrogen production and evaluating the current status and development trajectories of various technical pathways, this study aims to offer both theoretical frameworks and practical technical insights to advance the marine hydrogen energy industry, thereby supporting global energy transition and carbon neutrality objectives.
2. Seawater Electrolysis for Hydrogen Production
2.1. Direct Hydrogen Production via Electrolysis of Desalinated Seawater
Electrochemical water splitting for hydrogen production utilizes direct current to decompose water into hydrogen and oxygen through cathodic hydrogen evolution reaction (HER) and anodic oxygen evolution reaction (OER). Catalysts are used to accelerate the two most energy-intensive reactions in water electrolysis, OER and HER, thereby increasing hydrogen production efficiency and reducing energy consumption [29]. Based on electrolyte types, the technology can be categorized into four main systems: alkaline water electrolysis (AWE), proton exchange membrane electrolysis (PEM) [30], anion exchange membrane electrolysis (AEM), and solid oxide electrolysis (SOE), with the first two currently achieving commercial viability [31]. However, in offshore applications, the additional energy conversion steps—transforming wind, solar, tidal or wave energy into electricity, then using part of this electricity for seawater desalination to obtain high-purity water before electrolysis—significantly increase production costs. Projections indicate hydrogen demand by 2030 will require approximately 21 billion cubic meters of freshwater annually [32]. Large-scale green hydrogen production still depends on sustainable and reliable freshwater supplies due to multiple adverse effects of seawater chloride content on electrolysis: competitive chlorine evolution reaction at the anode alongside OER; corrosive dissolution of non-noble metals forming metal chlorides under anodic potentials; and membrane/electrode fouling by microorganisms, minerals and contaminants, with seawater cations particularly reducing AEM conductivity [33]. Consequently, desalination remains a critical preprocessing stage for electrolytic hydrogen production from seawater.
2.1.1. Desalination
Seawater desalination technologies primarily comprise membrane-based, thermal-based, and electrical-based approaches. Among membrane processes, reverse osmosis (RO) and forward osmosis (FO) represent the most prevalent methods [34]. RO operates by selectively permitting specific solute components to pass through a semi-permeable membrane under applied pressure (make water flow from the concentrated solution side to the dilute solution side), effectively removing salts and contaminants from water. As the dominant desalination technology, RO accounts for over 70% of global desalination capacity due to its superior cost-effectiveness and lower energy requirements compared to alternative methods. Membrane-based technologies generally demonstrate significant advantages over thermal distillation systems, including reduced energy consumption, compact system design, and high modularity. Continuous advancements in membrane materials, energy recovery devices, and high-efficiency pumps have further enhanced the low-energy and operational advantages of membrane desalination. However, membrane fouling remains a critical challenge for RO systems, necessitating additional operational costs for membrane cleaning and feedwater pretreatment [35]. Thermal desalination methods fundamentally rely on multi-stage evaporation of saline water through heating or pressure reduction, followed by vapor condensation to produce fresh water. Current mainstream thermal technologies include multi-stage flash (MSF), multi-effect distillation (MED), vapor compression distillation (VC), membrane distillation (MD), and solar-powered (thermal or photovoltaic) systems [36].
Among electrical-based methods, electrodialysis (ED) employs ion-exchange membranes under an applied DC electric field to selectively transport anions and cations through respective membranes, achieving desalination or concentration. Despite its notable cost advantages [37] and direct electricity utilization benefits, ED is limited to brackish water with salinity below 3.5 wt%, making it unsuitable for direct large-scale seawater desalination and hydrogen production applications [38]. Capacitive deionization (CDI), another electrical desalination technology, typically consists of two carbon electrodes sandwiching a saline flow, operating through an electrical double-layer capacitive mechanism where salt ions are transported and adsorbed onto the electrode surfaces under an applied electric field. Both ED and CDI are restricted to low-salinity water treatment, though they may serve as secondary desalination steps for brackish water produced by mainstream RO and mechanical vapor compression (MVC) systems in marine renewable energy scenarios [39,40]. Several emerging but less mature desalination technologies are under development, including marine microalgae-based systems, freeze desalination, gas hydrate processes, and ion-exchange methods [19,37,41,42,43,44].
Notably, ED offers the distinct advantage of simultaneous seawater desalination and hydrogen production [34]. In conventional ED systems, ion migration occurs concurrently with chloride oxidation at the anode to form chlorine compounds, while water reduction at the cathode generates small amounts of hydrogen, as illustrated in Figure 2 [45]. This integrated process enables partial hydrogen production prior to electrolysis using the desalinated water, thereby enhancing overall hydrogen production efficiency and making the technology particularly suitable for offshore applications [46]. Researchers have developed various approaches to improve hydrogen yield while maintaining desalination efficiency, including reverse electrodialysis (RED), short-circuited RED (REDSC), assisted RED (ARED), and multi-stage RED systems that leverage salinity gradients for green hydrogen production, effectively reducing the levelized cost of hydrogen (LCOH) [47,48].
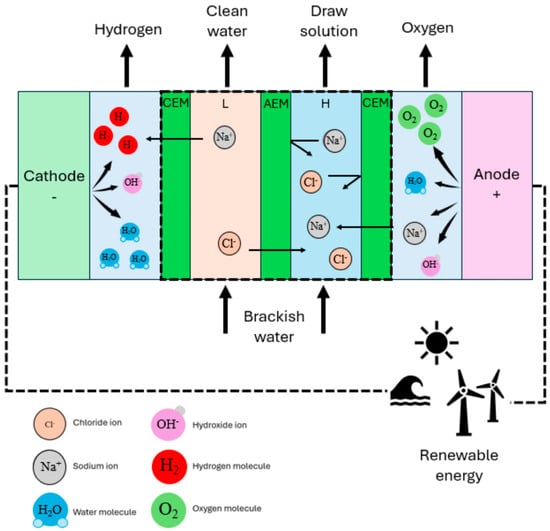
Figure 2.
Schematic diagram of green hydrogen production via ED membrane process [45].
Campisi et al. developed a novel hydrogen-electrodialysis (HED) technology by incorporating additional shared electrodes into the cell stack, enhancing hydrogen production rates without compromising freshwater output. Preliminary results demonstrated a competitive LCOH at 3.43 €/kg·H2, indicating the economic viability of upgrading conventional ED to HED systems [49]. Pellegrino et al. introduced a bipolar membrane electrodialysis (H-EDBM) approach capable of operating at current densities one order of magnitude higher than standard ED and RED processes, achieving remarkable hydrogen production efficiency (98%) and rates (18.4 mol/hm2). This configuration additionally coproduces sodium hydroxide (NaOH) for subsequent electrolysis applications [50]. Alshebli et al. investigated hydrogen recovery during Na2SO4 solution desalination via ED, where ion-exchange resin incorporation reduced system resistance, improving conductivity removal ratios beyond 95.7% and enabling maximum hydrogen evolution rates of 76.8 mg H2/h·kg Na2SO4 solution [51].
Offshore desalination systems differ significantly from coastal large-scale plants in their technological configurations and operational preferences. Marine-based units prioritize modular designs and compact footprints, while their energy-intensive nature necessitates reliance on offshore renewable energy sources [52]. Wind and marine energy (tidal and current) can power pressure-driven membrane processes like FO and RO, whereas solar energy and waste heat from hydrogen electrolysis may supply thermal-based methods including MED, MD, and MSF. Electrically powered systems such as RO, ED, and MVC can utilize offshore wind or solar generation. Currently, wind/solar-RO hybrid configurations dominate renewable-powered desalination, accounting for over 50% of such installations [53].
Researchers have investigated various configurations including displacement hydraulic wind turbines, vertical-axis wind turbine-high pressure pump systems, and direct/indirect mechanical drive systems to examine the effects of feedwater salinity, loading conditions, wind speed variations on RO desalination performance [54,55,56,57]. Amin et al. integrated wind turbines with floating desalination plant (FDP) platforms, conducting comprehensive dynamic response analyses of combined turbine-platform systems under historical Red Sea conditions through frequency-domain and time-domain motion studies [58]. Miao et al. demonstrated the feasibility of floating solar-thermal driven MD modules, where natural wave action enhanced feedwater circulation and mass transfer by agitating both the heating bags and MD units at the sea surface [36]. For island and offshore applications characterized by wind/solar intermittency, hybrid PV-wind-RO systems exhibit superior robustness against environmental variability [59,60]. Zhang et al. confirmed the technical viability of current energy-RO coupling, maintaining specific energy consumption below 3.35 kWh/m3 [61].
As mentioned in Section 1.1, all operational offshore wind-to-hydrogen projects currently employ coupled desalination-electrolyzer systems, predominantly utilizing RO technology (e.g., the UK’s Dolphyn Hydrogen project). Given RO’s dominance in offshore applications, we briefly summarize its persistent challenges and recent developments. Despite being the fastest-growing desalination sector, RO systems still exhibit relatively high energy consumption (2–5 kWh/m3) [35], particularly problematic for energy-intensive hydrogen production. The process becomes increasingly energy-demanding with higher feedwater salinity. The semipermeable membranes—RO’s core components—face limitations including short service life, mechanical fatigue from frequent start-ups under renewable power fluctuations, and permeate flux decline due to variable pressure/flow conditions [62]. Common scaling compounds like CaSO4 and CaCO3 exhibit distinct deposition patterns: particulate/organic fouling and biofilm formation primarily occur in membrane elements, while metal salt scaling concentrates in terminal elements. These accumulating deposits induce concentration polarization and progressively degrade membrane performance [63]. Such issues may be exacerbated in marine environments, necessitating novel solutions to reduce both operational costs and energy demands.
Yao et al. synthesized polymer membranes through polymerization of 3,5-dihydroxy-4-methylbenzoic acid with methanesulfonyl chloride, demonstrating remarkable resistance to hydrolytic degradation under both acidic and alkaline conditions, along with complete chlorine tolerance [64]. Wang et al. fabricated ultrathin COF membranes exhibiting exceptional desalination performance (92 kg/m2h1) through synergistic longitudinal self-healing and self-inhibition effects [65]. Liu et al. employed interfacial catalytic polymerization to produce robust polyester thin films with enhanced reaction kinetics that overcome the limited reactivity of natural monomers, achieving superior desalination performance [66]. Additional advancements include supramolecular nanocrystalline membranes [67], biomimetic artificial water channel membranes [68,69], MOF-based membranes [70,71], polymeric hollow fiber membranes, and layered carbon nitride membranes, all contributing to improved RO desalination efficiency and energy savings.
2.1.2. Electrolytic Hydrogen Production from Desalinated Water
Table 1 compares the advantages and disadvantages of electrochemical water splitting technology. AWE represents the earliest industrialized water electrolysis technology with decades of operational experience [72]. The core components of AWE electrolyzers include cathode and anode electrodes, a membrane separator, flow field plates, gas diffusion layers, and bipolar plates [73]. These systems employ highly concentrated (20–40%) KOH or NaOH aqueous solutions as electrolytes to enhance ionic conductivity. Utilizing asbestos membranes to separate hydrogen and oxygen production, AWE typically achieves 70–80% efficiency. Under direct current, water molecules undergo reduction at the cathode to produce hydrogen gas and hydroxide ions (OH−), which then migrate through the membrane to the anode where oxygen evolution occurs.

Table 1.
Comparison of electrochemical water splitting technology.
AWE offers several advantages: mature technology, relatively low capital costs, long-term operational stability, and compatibility with inexpensive non-precious metal catalysts (Ni, Co, Mn) [74]. However, limitations include the corrosive and polluting nature of strong alkaline electrolytes requiring complex maintenance, the necessity for product gas dealkalization, and environmental concerns associated with asbestos membranes. The membranes’ incomplete gas separation results in lower hydrogen purity (approximately 99.8%) [75]. Additional drawbacks comprise slow response times during startup/shutdown or load variations, poor compatibility with highly intermittent renewable power sources, relatively low current densities (0.2–0.6 A/cm2), and low output gas pressure (0.1–1.0 MPa) necessitating additional compression for storage/transport.
PEM electrolysis has experienced rapid industrial development in recent years, achieving preliminary commercialization and becoming the preferred electrolyzer technology for several operational and planned offshore wind-to-hydrogen projects worldwide [76,77]. The European Union has established a development roadmap for PEM electrolysis to progressively replace AWE. PEM electrolyzers typically employ a bipolar structure, as illustrated in Figure 3 [78], with each cell comprising seven key components: cathode/anode bipolar plates, cathode/anode gas diffusion layers, cathode/anode catalyst layers, and a proton exchange membrane [79]. The anode side utilizes pure water as the reactant, where oxidation generates oxygen and protons (H+); these H+ migrate through the membrane under the applied electric field to the cathode, undergoing reduction to form hydrogen gas [72]. The proton exchange membrane serves as a critical component of the membrane electrode assembly, exhibiting high proton conductivity, excellent gas impermeability, chemical stability, and hydrophilicity, while also providing structural support for catalyst coatings. Its performance directly determines the operational efficiency and lifespan of PEM electrolyzers. Major PEM types include sulfonated polymer membranes, composite membranes, and inorganic acid-doped membranes.
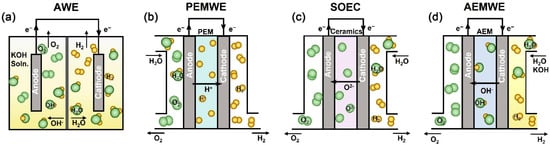
Figure 3.
Schematic illustrations of (a) AWE, (b) PEM, (c) SOE, and (d) AEM [78].
The PEM effectively isolates gases on both electrodes while facilitating proton conduction, eliminating the risk of H2 contamination by alkaline electrolytes characteristic of AWE systems. This configuration ensures hydrogen purity exceeding 99.99% and prevents formation of explosive gas mixtures. PEM electrolyzers demonstrate superior performance metrics, including higher current densities (1–4 A/cm2), broader output pressure ranges (0.1–7.0 MPa), lower operating temperatures (30–80 °C), and improved overall efficiency compared to AWE. The zero-gap cell design reduces ohmic resistance significantly while achieving more compact dimensions (approximately one-third of AWE volume) and lighter weight (Figure 3), making PEM particularly suitable for space-constrained offshore applications. These systems feature rapid response capabilities, with cold start times under 5 min and shutdown within seconds [80], enabling effective integration with highly variable wind and solar power inputs. Despite these advantages, PEM technology faces limitations including smaller single-stack production capacity compared to AWE, high material costs associated with Iridium/Ruthenium (Ir/Ru) oxides and platinum (Pt) nanoparticle catalysts [81], susceptibility to metal ion poisoning in seawater environments, and relatively shorter membrane lifespans.
SOE, also termed high-temperature steam electrolysis, remains at the preliminary demonstration stage. As the reverse process of solid oxide fuel cells (SOFC), oxygen-ion conducting SOE cells comprise dense electrolytes sandwiched between porous electrodes. High-temperature steam introduced at the cathode undergoes reduction to hydrogen and oxide ions, with the latter migrating through the electrolyte to the anode where oxygen evolution occurs. Proton-conducting SOE variants instead feed steam at the anode. These systems employ cost-effective perovskite and zirconia-based materials for both electrolytes and catalysts. Operating at 600–1000 °C, SOE benefits from accelerated reaction kinetics, substantially reducing the electrical energy requirement for water splitting while achieving superior energy efficiency (>90%) compared to AWE and PEM technologies. However, the elevated operating temperature increases thermal energy demand [82]. For offshore applications utilizing wind or solar power, this necessitates additional energy conversion from electricity to heat, while the substantial waste heat generated lacks effective utilization pathways—factors that collectively reduce overall system efficiency. Furthermore, SOE systems require specialized materials to withstand high-temperature degradation, increasing capital costs. Additional limitations include relatively short lifespans, slow start-stop cycles, operational instability, and low hydrogen output pressure (0.1 MPa).
AEM electrolysis represents an emerging water-splitting technology currently in early research stages [83]. In AEM electrolyzers, water introduced at the cathode undergoes reduction to produce OH− and hydrogen gas, with OH− migrating through the polymeric AEM to the anode where oxidation generates water and oxygen [84]. The feedwater sometimes contains low-concentration KOH as supporting electrolyte. AEM technology combines advantages of both AWE and PEM systems, achieving higher current densities, faster start-stop response times, lower catalyst costs [85], and improved energy conversion efficiency. However, the limited alkaline stability of anion exchange membranes poses a major development challenge, as localized high-pH conditions at the membrane surface during operation lead to OH−-induced degradation. This fundamental trade-off between ionic conductivity and membrane stability currently hinders practical implementation. Both SOE and AEM electrolysis technologies require breakthroughs in membrane materials, catalysts, and electrolytes before becoming viable for offshore wind and renewable energy applications, with near-term deployment remaining unlikely.
In summary, PEM technology demonstrates superior performance for marine renewable hydrogen production when considering key offshore operational metrics including compactness, startup speed, electrolysis efficiency, hydrogen output pressure and purity. To address challenges associated with PEM membrane durability, stability and high material costs, while further enhancing electrolysis efficiency, significant research efforts have been devoted to catalyst development [75,86]. Li et al. designed a Ni0.1Co0.9Se2 catalyst as a cost-effective alternative to Pt-based materials, achieving high current density (1.83 V@500 mA/cm2) and remarkable stability (500 mA/cm2@100 h) [81]. Zhang et al. employed sulfur doping to induce structural modifications in pyrite-type CoSe2, improving hydrogen diffusion and electrocatalytic performance [87]. Zeng et al. developed Mo2TiC2-MXene supported Pt nanoclusters that maintain high activity and stability while minimizing Pt loading (36 μg/cm2) [88]. Deng et al. synthesized ternary PtNiP amorphous nanoparticles (PtNiP-ANP) with optimized hydrogen adsorption Gibbs free energy (0.02 eV), surpassing crystalline Pt catalysts and demonstrating the unique electronic properties of amorphous materials [89].
Li et al. successfully oxidized Ir catalysts to the hexavalent state (IrVI) using MnO as oxidant, achieving optimal activity and stability (Figure 4). The resulting catalyst maintained a high current density of 2.3 A/cm2 over several months of operation [90]. Tran et al. developed TiO2-supported Ir@IrO(OH)x core–shell nanoparticles as a cost-effective alternative to commercial IrO2 anodes, reducing Ir usage by over 50% [89]. Zhang et al. demonstrated that tantalum (Ta) doping in RuO2 effectively mitigates dissolution during oxygen evolution, enabling its potential substitution for IrO2 while simultaneously enhancing corrosion resistance and catalytic activity. Xu et al. incorporated lattice water into IrOx·nH2O, creating a short-range ordered hollandite-like framework that exhibited both improved activity and exceptional stability (~8 months) without structural degradation [91]. Kong et al. enhanced manganese oxide catalyst durability by replacing pyramidal oxygen with planar oxygen configurations featuring stronger Mn-O bonds, suppressing Mn (manganese) dissolution and expanding the potential of earth-abundant PEM electrolysis catalysts to reduce Ir dependence [92].
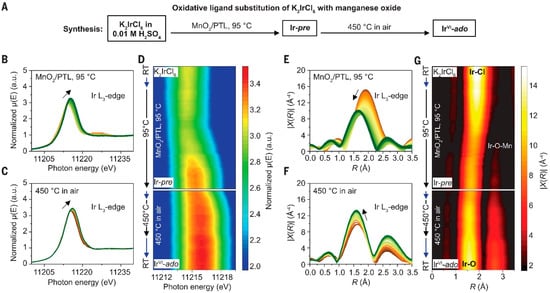
Figure 4.
In situ XAS analysis of IrVI formation: (A) Synthetic preparation of IrVI (B,C) XANES spectra for the iridium L3-edge, (E,F) EXAFS spectra for the iridium L3-edge, and (D,G) two-dimensional color maps showing the shift in the absorption edge and the coordination shell [90].
Hu et al. synthesized a high-entropy Ru-Ir oxide (M-RuIrFeCoNiO2) catalyst, where the multi-component design strategy effectively addressed the poor stability and limited activity of RuO2 under acidic oxygen evolution conditions [93]. To mitigate the continuous dissolution and oxidation of Ru-based electrocatalysts during electrolysis, Sun et al. identified ZnRuOx as exhibiting exceptional stability for acidic oxygen evolution reactions through transition metal screening [94]. Yuan et al. developed a synergistic doping approach incorporating earth-abundant Mn and Nb (Niobium) into RuO2, creating Nb0.1Mn0.1Ru0.8O2 nanoparticles that simultaneously stabilize lattice oxygen and reduce valence fluctuations at Ru sites, thereby enhancing catalytic stability [95]. Shi et al. elucidated the critical role of catalytic mechanisms and intermediate binding strength in determining the operational stability of Ru-based catalysts, leading to the development of SnRuOx solid solutions with order-of-magnitude lifespan extension [96]. Liu et al. employed a molten salt-assisted quenching strategy to induce tensile strain in Sr/Ta co-doped RuO2 nanocatalysts, spatially elongating Ru-O bonds and reducing covalency to suppress lattice oxygen participation and structural degradation, resulting in comparable stability and lower degradation rates than pristine catalysts [97].
Significant advancements in electrolyzer design have been achieved through innovative engineering approaches. Carrillo et al. integrated electrocatalytic reactions with capacitive storage mechanisms, enabling spatiotemporal separation of H2 and O2 evolution. Their system employed CoFe-EP/NF bifunctional catalysts to achieve 99% Faradaic efficiency at 100 mA/cm2, with a lower heating value efficiency of 69% [98]. Wang et al. developed a PEM electrolyzer capable of operating with impure water (containing cationic impurities) through microenvironment pH modulation, which reduced maintenance requirements while maintaining performance comparable to state-of-the-art systems using purified water [99]. Hodges et al. designed a novel electrolyzer architecture utilizing capillary-induced water transport through porous electrode separators to both hydrogen and oxygen evolution electrodes, demonstrating performance superior to conventional commercial electrolyzers [100].
2.2. Direct Seawater Electrolysis for Hydrogen Production
Direct seawater electrolysis offers significant advantages by eliminating the desalination step, thereby reducing space requirements, energy consumption, and capital costs while improving the utilization efficiency of offshore renewable electricity. This approach has emerged as a critical technology for large-scale commercialization of offshore wind-to-hydrogen systems. However, compared to conventional desalination-coupled electrolysis, direct seawater electrolysis faces substantial challenges including complex seawater composition, competitive chlorine evolution reactions, precipitate formation, electrolyzer corrosion, and catalyst deactivation. Addressing these issues requires innovations in membrane materials, catalysts, and electrolyzer design [101]. Figure 5 presents the Pourbaix diagram for OER electrodes in saline electrolytes [102], demonstrating how chloride ions (the most abundant anions in seawater) are readily oxidized to chlorine gas or hypochlorite at the anode. This process not only corrodes electrolyzer components and deactivates catalysts, but also introduces safety hazards [103]. Furthermore, cathodically generated OH− react with Mg2+ and Ca2+ in seawater to form insoluble Mg(OH)2 and Ca(OH)2 deposits that foul catalysts, membranes, and electrolyzer surfaces. These deposits increase energy consumption, impede ion transport, and degrade catalyst performance [104]. Additional operational challenges include membrane fouling, electrode deactivation, and biofouling caused by marine microorganisms and organic pollutants from domestic/industrial wastewater [33].
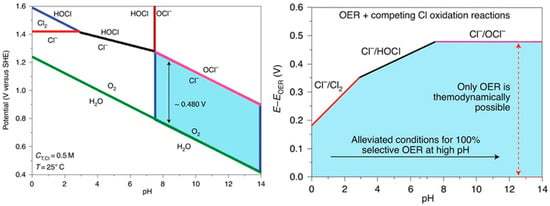
Figure 5.
Pourbaix diagram of OER electrodes in saline electrolytes with chloride species [102].
These challenges currently confine direct seawater electrolysis to laboratory-scale research. Critical solutions involve (1) developing corrosion-resistant catalysts with high activity and selectivity, (2) establishing protective strategies for electrode materials to mitigate seawater ion interference [105], and (3) designing novel ion-exchange membranes and electrolyzer configurations specifically for seawater electrolytes. Such innovations must address competitive oxidation reactions at both electrodes while overcoming corrosion and chlorine-induced degradation of catalysts, membranes, and reactor components to meet offshore hydrogen production requirements. The following discussion of recent advances follows this systematic solution framework.
Researchers have established multiple design strategies for selective OER catalysts in low-grade or seawater electrolytes. The primary approach involves incorporating highly active OER catalysts for alkaline seawater oxidation. Alternative methods include (1) selective OER catalysts with surface active sites for neutral saline electrolytes, predominantly utilizing Co- based (Cobalt) or Ru-based nanoparticles [106]; (2) chloride-blocking layers (e.g., MnO, NiFeOx, or NiCoS) at the anode periphery to prevent Cl− diffusion to active sites. Sun et al. developed a scalable hydrothermal method to synthesize spin-polarized Ni1/MoS2 single-atom catalysts, achieving 2880% OER current enhancement under 0.5 T magnetic fields with exceptional seawater electrolysis stability [107]. Xu et al. designed a Ru0.1Mn0.9Ox non-precious catalyst where Cl− adsorption on Ru sites shifts active centers to Mn, simultaneously avoiding Ru lattice oxygen consumption while creating OH−-rich local environments for OER [108]. Liu et al. demonstrated that asymmetric Fe doping in NiPS3 tunes local metal spin states, with medium-spin FeIII sites and P/S coordination synergistically enhancing OER activity and Cl− corrosion resistance [109]. Another Xu group study constructed NiMo/NiMoP (Nickel-Molybdenum-Phosphide) heterointerfaces with strong built-in electric fields that simultaneously optimize hydrogen adsorption and provide spatial shielding against Cl− attack [110].
Liu et al. revealed that Cl− specifically adsorbed at Fe sites in NiFe layered double hydroxides (NiFe-LDH) unexpectedly enhances performance by inhibiting Fe dissolution while activating additional Ni active sites, achieving 20.7% energy savings compared to conventional alkaline water electrolyzers [111]. He et al. selectively anchored phosphotungstic acid-polyoxometalates (PW12-POM) to Fe sites in CoFe hydroxides, effectively modulating the electronic structure of adjacent Co active centers and optimizing Cl−/OH− adsorption for efficient alkaline seawater oxidation [112]. Hu et al. developed a phosphate (PO43−)-induced rapid surface reconstruction strategy to fabricate Ni3FeN-based catalysts featuring conductive nitride cores and chloride-resistant hydroxide shells [113]. Dang et al. synthesized Ti3C2Tx-MXene-supported high-entropy (FeCoNiCuMn)2O3 nanoparticles that operate via an “adsorption-migration” mechanism, where strong MXene-oxygen intermediate interactions combined with coulombic repulsion of Cl− effectively prevent chloride adsorption and penetration [114].
For cathodic hydrogen evolution, preventing hydroxide deposition of seawater cations (Ca2+, Mg2+, Cu2+) is critical. Pt-based alloys and transition metal catalysts demonstrate improved corrosion resistance and reduced ion deposition, enabling higher current densities and long-term stability in seawater electrolysis [115]. While Pt-group metals enhance reaction kinetics, their high cost and instability in non-acidic seawater have motivated development of non-precious catalysts based on C, S, P, and N compounds [116]. Transition metal phosphides (TMPs) and nitrides (TMNs), with their intrinsic conductivity and corrosion resistance, show particular promise as stable HER catalysts in seawater [29]. Porous carbon nanostructures and Ni-Fe LDH-based systems also demonstrate consistent efficiency improvements [117]. Sha et al. elucidated the dynamic degradation mechanisms of cathodes under intermittent renewable power input, showing that in situ phosphate passivation layers on NiCoP-Cr2O3 surfaces effectively isolate halide corrosion and prevent active site oxidation during frequent cycling [118]. Hu et al. introduced vanadium trioxide (V2O3) protective interlayers in AEM systems to modulate local catalytic environments, where OH− trapping simultaneously inhibits Cl− corrosion and hydroxide deposition while enabling low-loading Pt/Ni3N bifunctional catalysts [119].
Gao et al. developed a two-dimensional graphdiyne-supported monolayer osmium (Os) nanosystem, where strong interfacial interactions generated high-density charge distributions that optimized the hydrogen adsorption Gibbs free energy through incomplete charge transfer effects [120]. Shao et al. designed an Ir/HfO2@C (HfO2, Hafnium(IV) oxide) catalyst exhibiting bidirectional hydrogen spillover: HfO2-mediated reverse spillover under alkaline conditions and Ir-forwarded spillover in acidic media, achieving ultralow overpotentials for seawater electrolysis [121]. Liu et al. employed yttrium (Y) doping to engineer NiMo-MoO2 heterojunctions, simultaneously inducing lattice expansion in NiMo alloys (optimizing d-band centers for hydrogen desorption) while increasing oxygen vacancy concentrations in MoO2-x (accelerating OH intermediate desorption) [122]. Li et al. constructed N-doped C-encapsulated NiCoS heterostructures (CN@NiCoS), demonstrating that dynamic S migration creates paired S-doped CN shells and S vacancies that significantly enhance HER activity [123]. Jun et al. developed Ru-nanocluster-mediated Ru-MoO2-Ni4Mo catalysts, where Ru’s high hydroxyl affinity establishes local hydroxyl-deficient environments that effectively suppress MoO2 dissolution in alkaline conditions [124].
Novel Membrane and Electrolyte Systems. Fang et al. demonstrated effective impurity ion blocking in direct seawater electrolysis through combined anion/cation exchange membrane (AEM/CEM) designs, with minimal Mg/Ca deposition observed on electrodes after 5 days of continuous operation [125]. Deng et al. synthesized robust AEM featuring poly (biphenyl piperidinium) networks, exhibiting exceptional mechanical strength (79.4 MPa), low swelling ratio (19.2%), high hydroxide conductivity (247.2 mS/cm), and stable performance over 5000 h [126]. Ren et al. developed a zero-gap electrolyzer incorporating cation exchange-bipolar membrane assemblies, utilizing in situ water dissociation to create asymmetric pH environments for direct seawater electrolysis without supporting electrolytes [127]. Wang et al. identified localized heat accumulation as the primary degradation mechanism in hydroxide exchange membranes and engineered three-dimensional thermal-conductive networks that increased membrane thermal conductivity 32-fold while reducing degradation rates by 6 times [128].
Tang et al. developed a flexible gel electrolyte with exceptional ionic conductivity and water-trapping capacity, whose concentration-gradient-driven moisture migration mechanism enables hydrogen production from seawater or even humid air while accommodating marine environmental fluctuations [129]. Chen et al. achieved 10,000 h stable seawater electrolysis by incorporating SO42− additives with NiFeBa-LDH catalysts, forming dense sulfate protective layers on anode surfaces [130]. Zhao et al. optimized proton transfer pathways at Pt electrode interfaces using theophylline derivatives to create weakly hydrogen-bonded water networks, enhancing HER activity threefold while maintaining double-layer structures [131]. Liang et al. designed a PRT ternary anode system that drives efficient chlorine oxidation while completely suppressing oxygen evolution, which combined with MnOx cathodes in membrane-free electrolyzers produced ultra-high-purity hydrogen without O2 co-production [132]. Feng et al. engineered Os-O-Co bifunctional catalysts through in situ Os single-atom modification, achieving both minimal N-H bond cleavage barriers (for hydrazine oxidation) and optimal hydrogen adsorption energies (for HER) in membrane-free flow electrolyzers, reducing energy consumption by 70.7% compared to conventional seawater electrolysis [133].
The novel electrolyzer system. As described in Section 2.1.2, while PEM electrolysis demonstrates optimal potential for offshore applications under freshwater conditions, its stringent water purity requirements pose significant challenges for direct seawater utilization, leading to rapid membrane contamination and deactivation due to seawater’s corrosive and polluting nature. Addressing these issues inevitably increases technical complexity and cost. Guo et al. proposed an effective solution by introducing a Cr2O3 Lewis acid protective layer on CoOx to create localized alkaline conditions in situ, effectively preventing chlorine corrosion and electrode precipitation while maintaining performance comparable to conventional pure-water PEM electrolyzers [134]. In contrast to PEM, AEM systems, despite being prone to hydroxide precipitation-induced blockage at the cathode, offer distinct advantages for seawater electrolysis: their anion exchange membranes are generally more cost-effective and demonstrate superior corrosion resistance. The lower operating temperatures of AEM systems additionally mitigate seawater component deposition and corrosion. Furthermore, AEM’s elimination of precious metal catalyst requirements significantly reduces material replacement costs. These combined advantages make AEM technology particularly suitable for seawater environments.
Xie et al. developed a novel in situ desalination-coupled electrolysis approach, utilizing the natural vapor pressure difference between a self-humidifying electrolyte (SDE, 30 wt% KOH) and seawater as the mass transfer driving force. This design enables spontaneous phase-change migration, where water evaporates from the seawater side, diffuses through a polytetrafluoroethylene (PTFE) membrane, and condenses into the electrolyte [135]. The porous structure of the PTFE membrane facilitates micron-scale gas diffusion for directional vapor transport while completely blocking liquid permeation and effectively suppressing seawater ion migration. The system demonstrated high efficiency, low energy consumption, and robust stability, operating continuously for over 3200 h at 250 mA/cm2 under ambient conditions. Furthermore, Xie et al. successfully implemented a wind-powered floating seawater electrolysis system (Figure 6) under dynamic marine environments (wave height: 0–0.9 m, wind speed: 0–15 m/s), achieving stable 240 h operation of a 1.2 Nm3/h hydrogen production unit, with stable impurity ion concentrations in the electrolyte over extended periods [15].
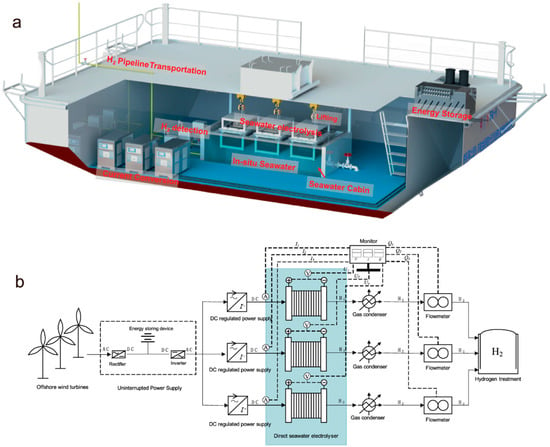
Figure 6.
Overview of the seawater direct electrolysis hydrogen production platform: (a) UPS module, current conversion module, seawater electrolysis module, H2 detection module and transportation module, (b) process diagram of the device [15].
Shen et al. proposed an anti-corrosion strategy of rare earth for kilowatt-scale alkaline seawater electrolyzers, where Eu2O3 (Europium(III) oxide) selectively adsorbs OH− to maintain a high pH anode environment while suppressing Cl− adsorption and oxidation [136]. Shi et al. designed a pH-asymmetric electrolyzer employing a Na+-exchange membrane, which leverages the chemical potential difference between different electrolytes and utilizes atomic-level dispersed Pt-anchored Ni-Fe-P nanowire catalysts to reduce voltage requirements and accelerate hydrogen evolution while simultaneously avoiding Cl− corrosion and Ca2+/Mg2+ precipitation [137]. Li et al. developed a thermodynamically favorable hybrid seawater electrolysis system featuring needle-like Co3S4 bifunctional catalysts that generate tip-enhanced electric field effects to promote sulfide ion oxidation reactions instead of chlorine evolution, achieving both energy-efficient hydrogen production and environmental remediation [138].
2.3. Coupling of Renewable Energy Generation with Hydrogen Production Systems
2.3.1. Integration of Offshore Wind Power with Hydrogen Production Systems
Offshore wind power coupled with water electrolysis presents an effective solution for renewable energy utilization by converting surplus wind electricity into storable green hydrogen during periods of low electricity prices or curtailment, simultaneously addressing wind power curtailment issues and enabling energy storage. This approach significantly reduces infrastructure costs associated with offshore power transmission while enabling large-scale green hydrogen production, as demonstrated by projects like the PosHYdon initiative. The key advantages of this integrated system include enhanced wind power utilization efficiency, improved grid flexibility, and the production of versatile clean energy carriers. Current technical challenges encompass offshore adaptability of electrolyzers (including corrosion and wave resistance), dynamic system response capabilities, offshore distance limitations, and costs related to equipment and hydrogen storage/transportation [31]. Compared to other offshore renewable energy sources, offshore wind benefits from over a century of onshore wind development experience, exhibiting the highest technological maturity, most established commercialization frameworks and policy systems, and the lowest LCOH [139], making wind-to-hydrogen integration the most extensively researched and implemented approach. Offshore wind resources offer superior quality with more stable and higher wind speeds compared to their onshore counterparts [140]. The electrolysis systems can be powered by either individual distributed or multiple centralized wind turbines, supporting simultaneous operations including system maintenance, seawater desalination, and hydrogen production.
The integration of offshore wind power with water electrolysis primarily involves two scenarios: offshore and onshore electrolysis. Currently, three typical configurations for offshore wind-to-hydrogen systems exist as illustrated in Figure 7. (a) Distributed hydrogen production employs small-scale offshore electrolyzers (installed at the base of individual wind turbines) powered by single turbines, with hydrogen subsequently collected and transported to shore via ships or pipelines, offering lower energy transmission losses. This configuration maintains continuous large-scale hydrogen production even if individual electrolyzers or turbines fail, though maintenance costs increase. (b) Centralized production utilizes larger offshore electrolyzers (mounted on dedicated platforms or repurposed oil/gas structures) powered by multiple wind turbines within a farm, requiring hydrogen transportation via ships or subsea pipelines (including repurposed oil/gas pipelines) alongside power cables. However, high-power centralized electrolyzers demonstrate inferior performance when handling frequent start-stop cycles caused by wind power fluctuations, leading to accelerated electrolyzer degradation and bubble dynamics instability [141,142]. (c) Onshore electrolysis still relies on conventional offshore substations and subsea power cables, consuming terrestrial or coastal freshwater resources, but benefits from lower capital and maintenance costs compared to offshore installations. Notably, long-distance high-voltage power transmission may incur significant energy and hydrogen losses [143]. Among these approaches, distributed hydrogen production integrating single wind turbines with individual electrolyzers demonstrates the highest efficiency and lowest costs [139,143]. Representative projects include Europe’s HOPE project featuring a 10 MW PEM electrolyzer coupled with subsea hydrogen pipelines in a single-unit distributed configuration, the UK’s Dolphyn project incorporating PEM electrolysis and desalination equipment on each floating platform for hydrogen production and subsea pipeline transportation, and the Dutch PosHYdon project utilizing a 1.25 MW PEM electrolyzer with existing oil/gas pipelines for hydrogen delivery [7].
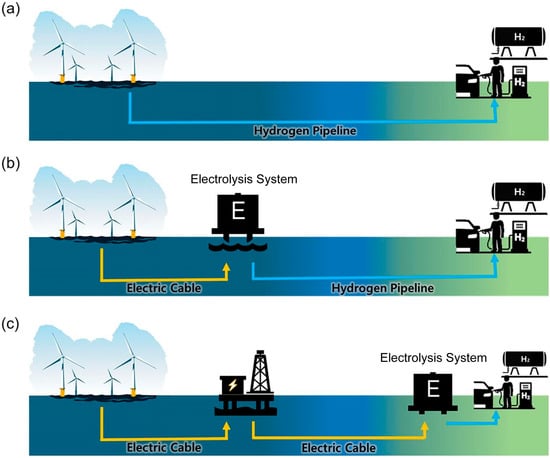
Figure 7.
Three configurations for offshore wind-powered hydrogen production are identified: (a) decentralized hydrogen production; (b) centralized hydrogen production; and (c) onshore hydrogen production [139].
Recent advances in offshore wind-to-hydrogen systems optimization demonstrate significant improvements in system configuration and operational efficiency. Gallo et al. developed a dynamic power regulation strategy for electrolyzers, revealing that variable power operation enables 168% higher optimal capacity compared to fixed-mode operation, with the key finding that switching to this mode when wind power meets minimum operational thresholds maximizes capacity utilization [144]. Castillo et al. conducted integrated simulations of a semi-submersible platform combining 15 MW wind turbines with electrolysis equipment, demonstrating negligible impacts on power generation and hydrogen production performance despite maximum pitch responses of 1° in floating platforms, while quantifying critical motion parameters for electrolyzers under typical operating conditions [145]. Dinh et al. established a differential evolution algorithm-based optimization method determining optimal electrolyzer capacity ratios, identifying 91.41% of installed wind power capacity as the ideal configuration for offshore systems [146]. Haqiqi et al. proposed coupling MED desalination with PEM electrolysis, demonstrating that waste heat from 1GW PEM systems (up to 94.12 MW) can power MED units to meet process water demands (except below 5% load conditions), thereby enhancing overall system efficiency [147]. Jiang et al. developed a Chance-Constrained Programming model incorporating electrolyzer degradation, wind power fluctuations, and stochastic equipment replacement costs for far-offshore projects, optimizing electrolyzer capacity to 336.1 MW (16.7% NPV improvement over conventional 360 MW designs) [148].
Economic analyses of offshore wind-hydrogen integration reveal critical cost determinants. Pegler et al. developed a techno-economic model for the Celtic Sea scenario incorporating eight 510 MW floating wind farms with integrated electrolyzers, demonstrating a LCOH of £ 7.25/kg·H2 (3–5 times higher than gray hydrogen), with particular sensitivity to electrolyzer efficiency and floating substructure costs, where wind power contributes 70–90% of LCOH [149]. Jin et al. proposed a co-optimization methodology for cable routing, hydrogen platform siting, and capacity allocation in offshore wind arrays, achieving total system cost minimization through efficiency improvements (1% efficiency gain reduces costs by 1.01%), highlighting the crucial role of electrolyzer efficiency and cable unit costs in economic optimization [150].
2.3.2. Integration of Marine and Solar Energy with Hydrogen Production Systems
Marine energy represents a significant renewable resource with an estimated global potential of 9.16 × 104 TWh, ranking third in generation capacity after wind and solar power [151,152]. This energy category encompasses tidal, wave, thermal gradient, and current energy (including tidal streams and ocean currents). Turbines can be deployed either as seabed-mounted installations or floating structures, typically anchored at depths of 40–100 m to harness underwater currents. While tidal energy has achieved commercial viability and tidal current energy has reached pre-commercial status, wave energy conversion remains in the research and demonstration phase. The technical approach for marine energy-based hydrogen production mirrors wind-to-hydrogen systems, utilizing tidal turbines to power electrolyzers and desalination units. However, current tidal power systems are limited to hundred-kilowatt scale generation capacity and exhibit considerable intermittency. Consequently, tidal-powered hydrogen production primarily serves offshore islands and platforms, providing sustainable hydrogen and freshwater supplies for remote communities [153].
Recent advances in marine energy-based hydrogen production systems demonstrate significant technological progress. Zhang et al. developed the first 4 kW integrated current energy-driven desalination-hydrogen production prototype, achieving stable PEM electrolyzer efficiency below 5.78 kWh/Nm3H2, while solving critical hardware integration challenges for direct-drive current turbines in multi-energy systems [61]. Driscoll et al. proposed a novel tidal phase-difference optimization method for green ammonia production around UK coastal areas, utilizing complementary tidal phases among islands to minimize low/zero-power periods through strategic turbine placement [154]. Omar et al. evaluated the performance and economics of a 10 MW hybrid ocean thermal energy conversion system coupled with alkaline/PEM electrolyzers, demonstrating a novel carbon-neutral approach for island regions through simultaneous energy-freshwater-hydrogen production without external water supply [155]. Ren et al. designed an integrated current energy-driven desalination-hydrogen production system featuring direct-drive transmission from turbine to seawater pump and permanent magnet synchronous generator, achieving 91% water-to-hydrogen conversion efficiency and implementing start-stop/MPPT cooperative control based on sinusoidal current characteristics to prevent frequent cycling [156].
Solar energy, despite its abundance and cleanliness, presents several challenges for hydrogen production applications. Although land-based photovoltaic (PV) technology has reached maturity, its conversion efficiency typically remains around 20% (lower than wind energy) and is susceptible to weather conditions, diurnal cycles, shading, soiling, and temperature variations [157]. Offshore PV systems face additional operational challenges including high humidity, saltwater corrosion, and wave impacts, without demonstrating the same offshore resource quality advantage observed in wind energy, and currently remain in the research and demonstration phase [157]. While promising for hydrogen production in arid coastal regions like the Middle East [158], offshore PV systems for water electrolysis—whether in centralized or distributed configurations—suffer from high equipment costs and significantly lower efficiency compared to offshore wind-to-hydrogen systems. The inherent intermittency and diurnal variability of solar radiation further limit its practical application. Current systems predominantly combine PV with PEM electrolyzers due to their compatible response times with solar fluctuations, though the resulting frequent start-stop cycles accelerate catalyst layer delamination and membrane thinning, ultimately reducing electrolyzer lifespan [159].
To effectively utilize offshore PV-generated electricity for hydrogen production, photovoltaic systems typically require integration with hybrid energy systems or energy storage technologies to compensate for intermittency. This approach not only enhances overall efficiency but also extends operational duration, thereby reducing curtailment losses [160]. The commercialization of offshore PV may benefit from existing offshore wind and marine energy infrastructure [161]. Hybrid energy systems offer distinct advantages by enabling complementary operation with other renewable sources, which helps mitigate output fluctuations from any single source while improving both power stability and generation capacity. Such systems allow shared utilization of electrical infrastructure, platform facilities, and maintenance resources. For offshore hydrogen production, hybrid energy systems can improve the economic viability of offshore power plants by further smoothing renewable power output fluctuations and minimizing electricity curtailment.
Recent studies have demonstrated significant advancements in optimizing hybrid marine renewable energy systems for hydrogen production. Rehman et al. applied a novel Dung Beetle Optimizer algorithm to co-design offshore wind-floating photovoltaic (FPV) hydrogen production systems, achieving a 29.2% reduction in LCOH compared to wind-only systems, with wind power equipment accounting for 45.96% of total investment [162]. Alex et al. developed a tidal-wind-grid coupled hydrogen production optimization method that eliminates 27.2 MWh/year of additional energy consumption through dynamic electrolyzer operation mode optimization and over-rating power strategies [163]. Ma et al. proposed a Hybrid Offshore Renewable Energy Harvesting System design integrating wave energy devices with monopile-steel plate composite foundations for offshore wind turbines [164]. Varotto et al. established an energy delivery and storage optimization model for hybrid wind-wave systems, demonstrating that combined cable expansion with subsea hydrogen pipelines yields optimal profitability, while ship-based hydrogen transport serves as a viable alternative when grid expansion is unfeasible [165]. Liu et al. developed a GIS-based multi-criteria evaluation framework incorporating geographical conditions, resource endowment, economics, risks, and sustainability to optimize siting of Hybrid Offshore Wind-PV-Wave-Hydrogen systems [166]. Hossain constructed a grid coordination analysis model for offshore wind-PV hydrogen systems, implementing intelligent optimization through dynamic power allocation strategies based on market prices and generation capacity [167]. Taroual employed RNN to assess Morocco’s wind-wave hybrid hydrogen potential, identifying an optimal 80% wave-20% wind energy mix [168].
3. Photocatalytic Seawater Splitting for Hydrogen Production
Photocatalytic seawater splitting represents a promising approach for direct solar-to-hydrogen conversion without requiring electricity generation. This technology utilizes transparent TiO2 photocatalytic films to decompose either seawater or atmospheric water vapor (at 70–95% relative humidity) under ambient temperature conditions when exposed to sunlight [169]. According to solid-state band theory, photon energy exceeding the semiconductor bandgap excites valence electrons to the conduction band, generating oxidative holes (h+) and reductive electrons (e−), as illustrated in Figure 8 [170]. The holes oxidize adsorbed water molecules or OH− to produce O2, while the electrons reduce H+ to form H2 [17]. The overall water splitting efficiency depends on three key factors: light absorption efficiency, charge separation capability, and surface reaction kinetics.
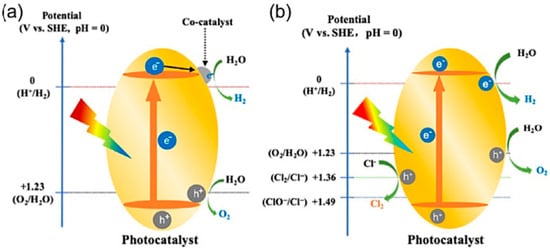
Figure 8.
Comparative analysis of photocatalytic water splitting systems: (a) pure water and (b) seawater [170].
While narrow-bandgap semiconductors (e.g., CdS (cadmium sulfide), ZnO) exhibit enhanced solar spectrum responsiveness, they suffer from photocorrosion and reduced reaction driving force. Conversely, wide-bandgap materials like TiO2 primarily absorb short-wavelength solar radiation, showing limited sensitivity to visible light and resulting in low solar-to-hydrogen efficiency (<10%) [171]. A more fundamental challenge lies in the significant timescale mismatch between nanosecond-scale charge carrier lifetimes and millisecond-scale water splitting reactions, causing substantial electron-hole recombination before they reach reaction sites. Common strategies to address this include (1) employing sacrificial agents (Na2SO3, Na2S, methanol, glycerol, or lactic acid) to rapidly consume holes and enhance charge separation and (2) utilizing cocatalysts (Pt, CuO, RhO2 (rhodium dioxide)) to selectively capture holes and improve reaction kinetics [171].
The advancement of photocatalytic seawater splitting technology primarily relies on the development and optimization of novel catalytic materials, currently dominated by TiO2-based, g-C3N4-based [172], and other oxide-type (WOx, ZnO) catalysts [173]. Current research focuses on several efficiency-enhancing approaches: (1) developing broadband photocatalysts to extend light absorption from UV to visible spectrum. (2) Employing advanced modification techniques like atomic doping and quantum dot decoration to improve both light harvesting efficiency and quantum yield. (3) Engineering nanostructured catalysts with increased surface area for enhanced hydrogen evolution rates. (4) Implementing photothermal synergistic catalysis [174]. Incorporating Ag and SiO2 nanoparticles into TiO2 photocatalysts creates nanocomposites capable of significant localized thermal energy conversion, which promotes both photocatalytic and desalination processes at the interface, resulting in a 5.2-fold hydrogen production increase compared to conventional TiO2 systems [175]. Porous Mo- or B-doped BiVO4 photoanodes demonstrate substantially improved photocurrent density and seawater stability through enhanced conductivity and hole diffusion length, while exhibiting remarkable resistance to photocorrosion [176].
Barrera et al. developed a novel equivalent circuit detailed balance model framework incorporating target and competing reactions’ electrochemical load curves, enabling the first load-line analysis for reaction selectivity prediction. Their study revealed that systems with semi-transparent light absorption layers outperform single absorbers under mass-transfer-limited conditions, with selective hydrogen production achieved by regulating mass-transfer asymmetry of redox mediators (e.g., Fe-based mediators) [177]. Wang et al. significantly enhanced g-C3N4 photocatalytic performance through thermochemical treatment and phosphorus intercalation doping, achieving a hydrogen evolution rate of 6323 μmol g−1h−1 and 5.08% quantum efficiency at 420 nm [178]. Zhang et al. engineered segregated charge centers in COFs comprising cationic frameworks and iodide anions, where the ionic polarization strategy created a strong built-in electric field enabling the CH3I-TpPa-1 material to reach 9.21 mmol g−1h−1 hydrogen production rate (42-fold enhancement) without cocatalysts [179]. Zhou et al. utilized hydrophilic 1D microporous channels in hydrogen-bonded organic framework HOF-H4TBAPy to reduce exciton transport distance to 1.88 nm, achieving exceptional hydrogen production (358 mmol g−1h−1) in sacrificial systems when microchannel length was ≤0.59 μm [180].
Recent advances in photocatalytic systems have demonstrated innovative designs for efficient hydrogen production. Collins et al. developed a Z-scheme photocatalytic reactor incorporating layered semiconductor nanoparticles to form dual light-absorption layers, which coupled hydrogen and oxygen evolution half-reactions through solution-phase redox mediators, significantly reducing both production costs and carbon emissions [181]. Lee et al. engineered a floating photocatalytic platform based on porous elastomer-hydrogel nanocomposites (featuring single-atom Cu/TiO2 systems) that achieves efficient light transmission, rapid water supply, and immediate gas separation through its unique air-water interface structure [182]. Pornrungroj et al. integrated UV-responsive RhCrOx–Al:SrTiO3 photocatalysts with visible-infrared absorbing floating porous carbon-based solar vapor generators to create an off-grid system capable of simultaneous hydrogen production and water purification [183].
Photocatalytic seawater splitting faces significant challenges due to seawater’s complex composition [184]. While low chloride concentrations can suppress electron-hole recombination (Figure 8), high ionic concentrations induce catalyst poisoning (accelerating photocorrosion) and even cause lattice distortion [185]. Cations (Na+, Mg2+, Ca2+) adsorb onto catalyst surfaces, blocking active sites and forming precipitates, while SO42− competes with H+ reduction, wasting 30% of photogenerated electrons and reducing hydrogen selectivity [186]. Several innovative solutions have emerged: Yuan et al. developed CoOx nanocages with dynamic Co2+/Co3+ reconstruction to consume holes while Pt single atoms trapped electrons, achieving 23.88 mmol g−1 h−1 hydrogen production with remarkable corrosion resistance [187]. Li et al. employed electrolyte-assisted charge polarization on N-doped TiO2, where selective ion adsorption extended carrier lifetimes 5-fold, enabling 15.9% energy conversion efficiency at 270 °C [188]. Zhang et al. fabricated ZIS/PAN nanofiber membranes via electrospinning, where surface cyano groups captured ions, boosting activity 20% over aqueous solutions while solving catalyst recovery issues [189]. Nasir et al. integrated Pd nanoparticles (as hole extractors) with GaN (gallium nitride) nanowires on silicon, simultaneously producing hydrogen and H2O2 from seawater [190]. Zhao et al. designed stainless steel-TiO2 bilayer structures where chromium-doped NiFe(oxy)hydroxides served as dual-functional protective cocatalysts for oxygen evolution and corrosion prevention [191]. Zhu et al. created self-suspending organic photocatalysts with superwetting interfaces and gradient heterojunctions for efficient solar seawater splitting [192].
4. Biological Hydrogen Production from Marine Resources
Marine biohydrogen production has emerged as a promising approach in renewable energy research, utilizing specific algae and bacteria to convert seawater and organic substrates into clean hydrogen through their inherent metabolic activities. This technology offers dual advantages by harnessing Earth’s most extensive ecosystem while combining environmental remediation with renewable energy generation. Notably, the process eliminates freshwater pretreatment requirements and can utilize various marine substrates including aquaculture sludge, carbohydrates, and algae, thereby reducing cultivation costs while enabling sustainable conversion of marine resources into green energy.
Photobiological hydrogen production in algae involves sunlight-driven water splitting catalyzed by endogenous enzymes. Hydrogenase is a key microbial enzyme that efficiently catalyzes the reversible reduction in H+ to molecular H2, serving as a central catalyst in both photobiological and fermentative marine biohydrogen production pathways. However, oxygen evolution during photosynthesis inactivates hydrogenase enzymes, thereby terminating hydrogen production [193]. Current strategies focus on suppressing oxygen evolution or creating anaerobic conditions to maintain hydrogenase activity. Two key enzyme systems facilitate algal hydrogen production: nitrogenases and reversible hydrogenases. In cyanobacteria, H+ generated from water photolysis are primarily consumed by nitrogenases for ammonia synthesis rather than hydrogen production, resulting in low hydrogen yields [194]. Notable hydrogen-producing marine cyanobacteria include Oscillatoria brevis, Oscillatoria sp. Miami BG7, and Calothrix scopulorum. In contrast, green algae employing reversible hydrogenase systems demonstrate superior catalytic efficiency and hydrogen production rates [195], with representative species including Tetraselmis, Platymonas subcordiformis, and Chlorella [196,197,198].
Marine algae offer cultivation advantages in open oceans or saline waters without competing for agricultural land. However, sulfur in seawater inhibits light-induced hydrogen production, necessitating mitigation approaches such as sulfur removal, nitrogen purging, additive supplementation, or anaerobic cultivation [199,200]. An alternative indirect biophotolysis process involves algal CO2 fixation into carbohydrates followed by bacterial fermentation to produce hydrogen [201], circumventing the oxygen sensitivity of direct biophotolysis. Additionally, certain macrophytes including water lettuce, red algae, brown algae, and kelps can produce hydrogen through fermentation of water, agar, polysaccharides, and alginates [202,203].
Marine bacteria can independently produce hydrogen through dark fermentation of organic substrates, with two primary pathways identified: dark fermentation and photofermentation [204]. Dark fermentation anaerobically decomposes carbohydrates into organic acids (acetate, butyrate, volatile fatty acids), alcohols, and hydrogen without light requirement, though with relatively low yield (<15%). This process involves various bacteria including Enterobacter, Citrobacter, Clostridium, Acetobacter, sulfate-reducing bacteria, and Lactobacillus [205]. Photofermentation employs photosynthetic bacteria containing nitrogenases to oxidize organic compounds (organic acids from dark fermentation, aquaculture sludge, marine sediments, and algae) using light energy, generating hydrogen alongside CO2 and byproducts like alcohols and acetone [206,207]. Representative photofermentative bacteria include purple non-sulfur bacteria, Escherichia coli, Rhodobacter, and Rhodopseudomonas. Combining dark-fermentative and photosynthetic bacteria enhances overall hydrogen production efficiency [208]. Several marine microorganisms demonstrate hydrogen-producing capability, including green sulfur bacteria, Vibrio spp., Staphylococcus, and E. coli through carbohydrate fermentation [209,210]. Notably, halotolerant sulfur-oxidizing bacteria isolated from marine aquaculture systems can directly utilize seawater media for hydrogen production from organic waste, with efficiency dependent on NaCl concentration, initial pH, light intensity, and carbon sources [211,212].
Appel et al. engineered a fusion system combining the NiFe hydrogenase HoxYH from cyanobacteria with photosystem I subunit PsaD, potentially utilizing both oxygenic and non-oxygenic photosynthesis pathways, which achieved 500 μM hydrogen concentration under anaerobic light conditions without hydrogen consumption [194]. Hwang et al. identified novel Chlorella strains (YSL01 and YSL16) capable of photosynthetic hydrogen production under aerobic conditions (natural water dissolved oxygen) using CO2 as the sole carbon source through upregulated hydrogenase gene expression [213]. Klinsalee et al. screened 13 microalgae species for hydrogen production potential with ethanol supplementation, with Micractinium sp. KLSc62 showing optimal performance after 7-day cultivation with 30 mM ethanol [214]. Li et al. demonstrated that nicotinic acid combined with nano zero-valent iron (nZVI) enhanced dark fermentation of algal mannitol, increasing hydrogen and ethanol production by 84% and 82%, respectively [215]. Bian et al. achieved 2.32 mol H2/mol-mannitol yield from brown algae fermentation with nZVI, which optimized the process by increasing electron supply, reducing redox potential, and providing Fe2+ to activate hydrogenase [216].
Wei et al. developed a bio-inorganic hybrid photosynthetic system featuring biomimetic silica-encapsulated Escherichia coli with in situ synthesized CdS nanoparticles, combining hydrogenase catalysis with cellular self-assembly to enable continuous hydrogen production (96 h) under aerobic conditions [217]. Gwon et al. engineered motile green algae into micro-photovoltaic stations capable of autonomous oxygen regulation in artificial photosynthesis, producing hydrogen via bioelectrogenic pathways using only CO2 and sunlight [218]. Xu et al. constructed discrete multicellular spheroids with dual photosynthetic functions using dextran-PEG biphasic emulsion droplets, where localized hypoxia in the algal core triggered hydrogen production while oxygen-consuming bacteria in the outer layer enhanced efficiency through synergistic respiration [219]. Zhang et al. established a pilot-scale continuous hydrogen production system integrating dark and photofermentation, with comprehensive sustainability assessment revealing a biomass energy consumption of 171,530 MJ per ton of hydrogen produced [220].
5. Discussion and Prospect
Marine renewable energy-based hydrogen production, as a pivotal solution for clean energy utilization in far-offshore areas and zero-carbon fuel supply, is currently undergoing a critical transition from nearshore demonstrations to far-offshore commercial applications. Table 2 summarizes and compares the energy efficiency, hydrogen production, stability, scalability, economic feasibility and, technology readiness level (TRL) of several marine renewable energy-based hydrogen production technologies.

Table 2.
Comparison of the marine renewable energy-based hydrogen production technologies.
Based on our comprehensive review in preceding sections, we identify several key developmental directions for this technology:
- 1.
- Synergistic optimization of seawater desalination and freshwater electrolysis: The advancement of low-energy desalination technologies is crucial, including solar-driven multistage membrane distillation (MD) and reverse osmosis (RO) systems, as well as ocean thermal energy conversion-powered desalination to enhance system energy efficiency and increase available energy for electrolysis, thereby reducing hydrogen production costs. Equally important is the thermal coupling between electrolysis and desalination processes, where waste heat from electrolysis can be recovered for seawater preheating to decrease desalination energy consumption. Integrated system design represents another critical direction, such as developing RO pretreatment-electrolyzer combinations (e.g., PV-RO-electrolyzer systems) or integrating forward osmosis (FO) membranes with PEM electrolyzers to utilize concentration gradients from electrolysis for water transport. Furthermore, the valorization of concentrated brine byproducts through extraction of high-value elements (e.g., lithium, uranium) from RO reject streams could potentially offset both desalination and hydrogen production costs.
- 2.
- Challenges and development directions for seawater electrolysis hydrogen production technology: The advancement of seawater electrolysis requires high-selectivity anode catalysts to suppress chlorine evolution reactions (CER) while enhancing oxygen evolution reaction (OER) efficiency. Strategies include surface phosphorization/nitridation and developing multi-metal catalysts (e.g., NiFeOx) to reduce CER selectivity to <5% and minimize noble metal dependence. Cathode design must resist Ca2+/Mg2+ scaling and passivation, with non-precious metal alternatives to IrO2/Pt. Low-resistance ion-exchange layers achieving >99% Cl− rejection, including bipolar membrane (BPM) electrolyzer optimization, are critical. Electrode geometry (e.g., porous structures) should enhance mass transfer and mitigate bubble accumulation. For marine environments, antimicrobial nanocoatings (e.g., Ag@TiO2) can prevent biofouling and current efficiency decay. Flexible electrolyzer technologies (e.g., dynamic-load electrolyzers) must address alkaline water electrolyzer (AWE) minimum load limitations, enabling grid-independent microgrid support amid wind power variability.
- 3.
- Renewable energy-hydrogen system integration: Intermittent renewables necessitate wide-power-range electrolyzers and bifunctional catalysts. Hybrid systems (offshore wind + floating PV, wave/thermal energy) can stabilize power inputs while sharing infrastructure. Concentrated solar power may reduce electrolysis temperature requirements. Dual-mode wind-hydrogen-grid systems and 15MW-scale floating turbine/GW-electrolyzer integration can lower levelized hydrogen costs (LCOH). Modular, containerized PEM units must validate reliability in marine conditions. Multipurpose infrastructure can reduce costs.
- 4.
- Photocatalytic and biological seawater hydrogen production: Photocatalysis faces low solar-to-hydrogen efficiency (STH < 9%) and scalability challenges. Narrow-bandgap materials, corrosion-resistant photoanodes, and microfluidic photoreactors could enhance performance. Floating self-cleaning units may improve marine applicability. Microbial production suffers from low yield (1.5–2.5 mol H2/mol glucose) and instability. Solutions include metabolic engineering of cyanobacterial hydrogenases, dark-photo fermentation coupling, and extremophile screening for saline/high-pressure adaptation.
- 5.
- System integration: production-storage-utilization: Vertical space utilization (wind-PV-aquaculture) can synergize oxygen byproduct use in deep-sea farming. Intermittency-adapted storage and floating hydrogen refueling stations may decarbonize shipping. Integrated desalination-hydrogen combustion can address island energy-water scarcity. Coupling coastal chemical and steel enterprises to absorb green hydrogen, achieving emission reduction and cost-sharing.
- 6.
- Marine environment and ecosystem disruption: During the development of marine renewable energy-based hydrogen production technology, multiple interferences may be imposed on the marine environment and ecosystem: Firstly, the discharge of large volumes of high-salinity brine generated during seawater desalination can alter the salinity of local marine areas, affecting marine organisms, particularly the plankton community structure. Secondly, although direct seawater electrolysis reduces brine discharge, the CER that may occur at the anode during electrolysis not only corrodes equipment but also produces toxic by-products such as chlorine gas and hypochlorites, posing threats to marine life and the environment. Additionally, noise generated by the operation of offshore wind turbines and electrolysis facilities can interfere with the communication and behavior of marine mammals. The addition and discharge of chemical agents in seawater desalination and electrolytes may also introduce pollutants. To address these challenges, a series of solutions can be adopted, including prioritizing the deployment of hydrogen production equipment in strong ocean current areas to promote rapid dispersion of concentrated brine, developing highly selective OER catalysts to suppress chlorine gas generation at the source, optimizing acoustic barrier designs to mitigate noise impact, and establishing stringent pollutant monitoring and early warning systems alongside comprehensive environmental impact standards. These measures aim to achieve a balance between large-scale green hydrogen production and marine ecological conservation.
Marine renewable hydrogen is pivotal for carbon neutrality but requires coordinated policy, technology breakthroughs, and industry collaboration. Priority areas include cost-effective, marine-adapted electrolysis and hybrid systems. While indirect seawater electrolysis (RO + electrolysis) currently dominates, direct electrolysis remains experimental. Integrated production-storage-application chains are essential for competitiveness, targeting scalable marine green hydrogen by 2040 to support global decarbonization.
6. Conclusions
The pursuit of hydrogen production from marine renewable energy represents a pivotal frontier in the global transition towards a sustainable and carbon-neutral energy system. As comprehensively reviewed, the ocean offers an unparalleled synergy of abundant renewable resources (wind, solar, and marine energy) and a inexhaustible water feedstock, positioning it as an ideal platform for large-scale green hydrogen generation. This review has systematically examined the primary technological pathways, including electrolysis (desalination and direct seawater methods), photocatalytic seawater splitting, and biological hydrogen production, each demonstrating distinct stages of maturity, unique advantages, and significant challenges. Despite promising advancements and ongoing global pilot projects, the large-scale commercialization of marine hydrogen production is hindered by persistent technical and economic barriers. The inherent intermittency of marine renewables poses integration challenges with electrolysis systems, while the corrosive and complex nature of seawater leads to catalyst degradation, membrane fouling, and competitive reactions that drastically reduce efficiency and system longevity. Consequently, the current levelized cost of hydrogen (LCOH) remains high compared to conventional alternatives. Future development must focus on strategies across several critical domains. Material science breakthroughs are essential for designing highly selective, corrosion-resistant, and non-precious metal catalysts, alongside the development of membranes and electrolyzers specifically engineered for the harsh marine environment. System-level optimization, particularly the synergistic coupling of desalination and electrolysis for waste heat recovery and the development of hybrid renewable energy systems, is crucial to enhance stability, efficiency, and overall economics. Furthermore, while photocatalytic and biological methods offer fascinating avenues for direct solar-to-hydrogen conversion, they currently face substantial hurdles in efficiency, scalability, and durability that require fundamental research. In conclusion, hydrogen production from marine renewable energy stands as a critical enabler for a future blue economy, offering a pathway for large-scale renewable energy storage and decarbonization of hard-to-abate sectors. Realizing its full potential will necessitate concerted efforts in multidisciplinary research to overcome existing technological bottlenecks, thereby unlocking the vast energy potential of the oceans for a sustainable future.
Author Contributions
Conceptualization, M.N. and Y.Y.; methodology, M.N. and Y.Y.; validation, M.N., Y.Z., F.P., Y.F. and M.S.; formal analysis, M.N. and Y.Y.; investigation, M.N., Y.Y., F.P., Y.F., D.C., M.Z. and M.S.; data curation, M.N. and Y.Y.; writing—original draft preparation, M.N.; writing—review and editing, Y.Y., D.C. and M.Z.; visualization, M.N.; supervision, Y.Y.; project administration, Y.Z. and F.P.; funding acquisition, Y.Z. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Integrated Design and Integrated Demonstration of Distributed Photovoltaic-Coupled Water Electrolysis Hydrogen Production-Hydrogen Refueling and Filling Mother Station” (ZX24013), and “Research and Development of Safety Technology and Integrated Monitoring Platform for Distributed Ammonia Decomposition Hydrogen Production and Hydrogen Refueling Integrated Station (Phase I)” (ZX24011).
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The scientific and technological projects of Sinopec Marketing Co., Ltd. in 2024: “Integrated Design and Integrated Demonstration of Distributed Photovoltaic-Coupled Water Electrolysis Hydrogen Production-Hydrogen Refueling and Filling Mother Station” (ZX24013), “Research and Development of Safety Technology and Integrated Monitoring Platform for Distributed Ammonia Decomposition Hydrogen Production and Hydrogen Refueling Integrated Station (Phase I)” (ZX24011) are gratefully acknowledged.
Conflicts of Interest
Author Min Ning was employed by the company Sinopec Sales Co., Ltd. Anhui Petroleum Branch. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Sinopec Marketing Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Horowitz, C.A. Paris Agreement. Int. Leg. Mater. 2016, 55, 740–755. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Y.; Zhao, Z.; Tan, X.; Managi, S. Carbon neutrality commitment for China: From vision to action. Sustain. Sci. 2022, 17, 1741–1755. [Google Scholar] [CrossRef]
- Weiss, C.V.C.; Guanche, R.; Ondiviela, B.; Castellanos, O.F.; Juanes, J. Marine renewable energy potential: A global perspective for offshore wind and wave exploitation. Energy Convers. Manag. 2018, 177, 43–54. [Google Scholar] [CrossRef]
- Satymov, R.; Bogdanov, D.; Breyer, C. Techno-economics of offshore wind power in global resolution. Appl. Energy 2025, 393, 125980. [Google Scholar] [CrossRef]
- Wu, Y.n.; Zhou, Q.; Wu, H.; Fang, Y.; Ding, J.; Wang, H. Characteristics Analysis and Reserve Evaluation of Offshore Solar Energy Resources in China. Acta Energiae Solaris Sin. 2023, 44, 162–169. [Google Scholar]
- Odenweller, A.; Ueckerdt, F. The green hydrogen ambition and implementation gap. Nat. Energy 2025, 10, 110–123. [Google Scholar] [CrossRef]
- Meharban, F.; Tang, X.; Yang, S.; Wu, X.; Lin, C.; Tan, L.; Hu, W.; Zhou, D.; Li, J.; Li, X. Harnessing direct seawater electrolysis for a sustainable offshore Hydrogen future: A critical review and perspective. Appl. Energy 2025, 384, 125468. [Google Scholar] [CrossRef]
- De Castro, G.; Chooyin, J.; Culhane, C.; Piracha, H.; Seaton, J.; Bagnato, G. Power to hydrogen: A geospatial and economic analysis of green hydrogen for UK high-heat industry. Fuel Process. Technol. 2025, 273, 108221. [Google Scholar] [CrossRef]
- Dute, E.F.; Fokkema, J.E.; Land, M.J.; Wortmann, J.C.; Douwes, M. Determining onshore or offshore hydrogen storage for large offshore wind parks: The North Sea Wind Power Hub case. J. Clean Prod. 2024, 472, 143395. [Google Scholar] [CrossRef]
- Marcus, N.; Graham, C. Funding awarded to PosHYdon consortium. Fuel Cells Bull. 2021, 2021, 12. [Google Scholar] [CrossRef]
- Taylor, M.; Strezov, V.; Best, R.; Pettit, J.; Cho, H.; Hammerle, M.; Beringen, E. Offshore renewable hydrogen potential in Australia: A techno-economic and legal review. Int. J. Hydrogen Energy 2025, 152, 149923. [Google Scholar] [CrossRef]
- Song, S.; Lin, H.; Sherman, P.; Yang, X.; Nielsen, C.P.; Chen, X.; McElroy, M.B. Production of hydrogen from offshore wind in China and cost-competitive supply to Japan. Nat. Commun. 2021, 12, 6953. [Google Scholar] [CrossRef]
- Simanullang, M. Liquid hydrogen storage and insulation materials for liquid hydrogen storage tanks: Trends and challenges. Int. J. Hydrogen Energy 2025, 140, 881–888. [Google Scholar] [CrossRef]
- Zhou, Z.; Cai, G.; Huang, Y.; Bai, R.; Nie, S.; Chen, X. Spatial and temporal evolution of cost-competitive offshore hydrogen in China: A techno-economic analysis. Renew. Sustain. Energy Rev. 2024, 203, 114780. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Z.; Tang, W.; Chen, Y.; Lan, C.; Zhu, L.; Jiang, W.; Wu, Y.; Wang, Y.; Yang, Z.; et al. In-situ direct seawater electrolysis using floating platform in ocean with uncontrollable wave motion. Nat. Commun. 2024, 15, 5305. [Google Scholar] [CrossRef]
- Missimer, T.M.; Maliva, R.G. Environmental issues in seawater reverse osmosis desalination: Intakes and outfalls. Desalination 2018, 434, 198–215. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Ngasse Moudio, N.D.; Bian, X.-Q.; Chinamo, D.S. Liquid hydrogen carriers for clean energy systems: A critical review of chemical hydrogen storage strategies. Fuel 2026, 404, 136329. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, L.; Zhou, X.; Li, D.; Liang, D. Phase Equilibrium of Methane Hydrate in Aqueous Solutions of Clay Stabilizers. J. Chem. Eng. Data 2021, 66, 598–608. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, M.; Zi, M.; Sun, Y.-F.; Chen, D. Coupling depressurization and flue gas flooding to enhance CH4 recovery and CO2 sequestration in hydrate-bearing sediments: Pilot-scale experimental evaluation at marine conditions. Energy 2025, 333, 137328. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, M.; Sun, Y.; Chen, D. Significance of well placement and degree of hydrate reserve recovery for synergistic CH4 recovery and CO2 storage in marine heterogeneous hydrate-bearing sediments. Geoenergy Sci. Eng. 2024, 242, 213290. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, M.; Zi, M.; Ye, H.; Chen, D. The role of phase saturation in depressurization and CO2 storage in natural gas hydrate reservoirs: Insights from a pilot-scale study. Chem. Eng. J. 2024, 499, 156444. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.-W.; Kim, D.Y.; Park, J.; Seo, Y.-T.; Zeng, H.; Moudrakovski, I.L.; Ratcliffe, C.I.; Ripmeester, J.A. Tuning clathrate hydrates for hydrogen storage. Nature 2005, 434, 743–746. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, M.; Yin, Z.; Zi, M.; Chen, D. Pilot-scale investigation of optimized fluid production in water-saturated silty hydrate sediments using dual horizontal wells via depressurization. Int. J. Heat Mass Transf. 2025, 241, 126728. [Google Scholar] [CrossRef]
- Aftab, A.; Hassanpouryouzband, A.; Martin, A.; Kendrick, J.E.; Thaysen, E.M.; Heinemann, N.; Utley, J.; Wilkinson, M.; Haszeldine, R.S.; Edlmann, K. Geochemical Integrity of Wellbore Cements during Geological Hydrogen Storage. Environ. Sci. Technol. Lett. 2023, 10, 551–556. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, R.; Zheng, S.; Lu, C.; Zhou, S. Multi-objective optimal scheduling of islands considering offshore hydrogen production. Sci. Rep. 2025, 15, 27371. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, L.; Liu, X.; Han, Z.; Wang, R. A two-level real-time energy management strategy incorporating load forecasting for hydrogen fuel cell-powered ships. Energy Convers. Manag. 2025, 341, 120032. [Google Scholar] [CrossRef]
- Vigneswaran, V.S.; Gowd, S.C.; Ravichandran, V.; Karthikeyan, M.; Ganeshan, P.; Kandasamy, S.; Lee, J.; Barathi, S.; Rajendran, K. Green ammonia as hydrogen carrier: Current status, barriers, and strategies to achieve sustainable development goals. Sci. Total Environ. 2025, 982, 179646. [Google Scholar] [CrossRef] [PubMed]
- Gomez Vidales, A.; Omanovic, S. Evaluation of nickel-molybdenum-oxides as cathodes for hydrogen evolution by water electrolysis in acidic, alkaline, and neutral media. Electrochim. Acta 2018, 262, 115–123. [Google Scholar] [CrossRef]
- Wan, L.; Wang, H.; Zeng, B.; Wang, W.; Liu, X.; Cao, L.; Hu, Y.; Cui, Z.; Dong, B. Surface-confined growth of Ru amorphous sub-nanoclusters on reductive Mn3O4: A strongly coupled interface engineering for efficient neutral hydrogen production. Energy Environ. Sci. 2025, 18, 4262–4275. [Google Scholar] [CrossRef]
- Zhang, C.; Song, P.; Hou, J.; Xiao, L.; Wang, X.; Yang, F.; Wang, X. Technical and economic analysis of hydrogen production, storage and transportation by offshore wind power in different scenarios: A Guangdong case study. Int. J. Hydrogen Energy 2024, 94, 829–837. [Google Scholar] [CrossRef]
- Kumar, P.; Date, A.; Mahmood, N.; Kumar Das, R.; Shabani, B. Freshwater supply for hydrogen production: An underestimated challenge. Int. J. Hydrogen Energy 2024, 78, 202–217. [Google Scholar] [CrossRef]
- Du, H.; Wang, X.; Song, J.; Ran, N.; Ma, J.; Wang, J.; Liu, J. Key Strategies for Continuous Seawater Splitting for Hydrogen Production: From Principles and Catalyst Materials to Electrolyzer Engineering. Adv. Funct. Mater. 2024, 34, 2407586. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Khalil, A.; Hilal, N. Emerging desalination technologies: Current status, challenges and future trends. Desalination 2021, 517, 115183. [Google Scholar] [CrossRef]
- Bartels, C.R.; Andes, K. Consideration of energy savings in SWRO. Desalination Water Treat. 2013, 51, 717–725. [Google Scholar] [CrossRef]
- Miao, Q.; Zhang, Y.; Cong, S.; Guo, F. Experimental Investigation on Floating Solar-Driven Membrane Distillation Desalination Modules. Membranes 2021, 11, 304. [Google Scholar] [CrossRef]
- Choi, J.-K.; Paudel, A.; Sapkota, S.; Alsehli, M.; Alshamrani, A.; Park, J.; Hong, Y.; Romeiko, X. Comparative assessment of conventional and emerging desalination technologies: A holistic review for sustainable water solutions. Desalination 2025, 614, 119140. [Google Scholar] [CrossRef]
- Ankoliya, D.; Mudgal, A.; Sinha, M.K.; Patel, V.; Patel, J. Techno-economic analysis of a hybrid electrodialysis–batch reverse osmosis process for brackish water desalination. AQUA Water Infrastruct. Ecosyst. Soc. 2023, 72, 593–607. [Google Scholar] [CrossRef]
- Alenezi, A.; Alabaiadly, Y. Emerging technologies in water desalination: A review and future outlook. Energy Nexus 2025, 17, 100373. [Google Scholar] [CrossRef]
- Wang, J.; Dai, J.; Jiang, Z.; Chu, B.; Chen, F. Recent progress and prospect of flow-electrode electrochemical desalination system. Desalination 2021, 504, 114964. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, M.; Zi, M.; Chen, D. Thermodynamic Stability and Component Heterogeneity of CO2/N2 Mixed Hydrates: Implications for Hydrate-based CO2 Capture and Sequestration. Sep. Purif. Technol. 2025, 357, 130151. [Google Scholar] [CrossRef]
- Yao, Y.; Yin, Z.; Kumar, R.; Gao, X.; Chen, D. Tuning effect of DIOX on the Thermodynamics and Cage Occupancy of CH4/CO2 + DIOX Mixed Hydrates. Chem. Eng. J. 2024, 482, 148984. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, M.; Zi, M.; Ye, H.; Duan, J.; Chen, D. CO2/CH4 hydrate formation from CO2/CH4 liquid mixtures: A key to CO2 sequestration in marine depleted hydrate and shallow gas reservoirs via liquid CO2 injection. Chem. Eng. J. 2025, 509, 161169. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, X.; Li, D.; Liang, D. Experiments of CO2 gas sequestration on the seabed by hydrate method. Chem. Ind. Eng. Prog. 2021, 40, 3489–3498. [Google Scholar]
- Liang, Y.Y.; Shahabuddin, M. Production of green hydrogen from desalinated water using membranes: A review. Desalination 2025, 614, 119200. [Google Scholar] [CrossRef]
- Fonseca, M.J.d.C.; Fonseca, F.V.d.; Borges, C.P. Electrodialysis feasibility for simultaneous generation of desalinated water and hydrogen as by-product. Int. J. Hydrogen Energy 2024, 53, 1396–1403. [Google Scholar] [CrossRef]
- Pellegrino, A.; Campisi, G.; Proietto, F.; Tamburini, A.; Cipollina, A.; Galia, A.; Micale, G.; Scialdone, O. Green hydrogen production via reverse electrodialysis and assisted reverse electrodialysis electrolyser: Experimental analysis and preliminary economic assessment. Int. J. Hydrogen Energy 2024, 76, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Xu, S.; Leng, Q.; Wang, S. A serial system of multi-stage reverse electrodialysis stacks for hydrogen production. Energy Convers. Manag. 2022, 251, 114932. [Google Scholar] [CrossRef]
- Campisi, G.; Pellegrino, A.; Tamburini, A.; Micale, G. Techno-economic modelling of a hydrogen-electrodialysis (HED) unit for the simultaneous production of freshwater and hydrogen. Desalination 2024, 587, 117920. [Google Scholar] [CrossRef]
- Pellegrino, A.; Campisi, G.; Proietto, F.; Tamburini, A.; Cipollina, A.; Galia, A.; Scialdone, O.; Micale, G. Hydrogen-Electrodialysis with Bipolar Membrane (H-EDBM): First experiments and preliminary economic evaluation. Desalination 2024, 592, 118169. [Google Scholar] [CrossRef]
- Alshebli, R.F.; Yuzer, B.; Bicer, Y. Experimental investigation of simultaneous hydrogen production and desalination via electrodialysis process. Int. J. Hydrogen Energy 2023, 48, 39002–39018. [Google Scholar] [CrossRef]
- Escoto, B.E.; Abundo, M.L.S. Evaluating the feasibility and sustainability of renewable energy systems for seawater reverse osmosis desalination application in small island communities. Results Eng. 2025, 27, 106015. [Google Scholar] [CrossRef]
- Mito, M.T.; Ma, X.; Albuflasa, H.; Davies, P.A. Reverse osmosis (RO) membrane desalination driven by wind and solar photovoltaic (PV) energy: State of the art and challenges for large-scale implementation. Renew. Sustain. Energy Rev. 2019, 112, 669–685. [Google Scholar] [CrossRef]
- Keisar, D.; Freger, V.; Greenblatt, D. Direct wind-powered vertical axis brackish water desalination system. Desalination 2024, 570, 117060. [Google Scholar]
- Carta, J.A.; González, J.; Cabrera, P.; Subiela, V.J. Preliminary experimental analysis of a small-scale prototype SWRO desalination plant, designed for continuous adjustment of its energy consumption to the widely varying power generated by a stand-alone wind turbine. Appl. Energy 2015, 137, 222–239. [Google Scholar]
- Esquivel-Puentes, H.A.; Vacca, A.; Chamorro, L.P.; Garcia-Bravo, J.; Warsinger, D.M.; Castillo, L. Simultaneous electricity generation and low-energy-intensive water desalination using a hydraulic wind turbine. Desalination 2025, 601, 118526. [Google Scholar]
- Amin, I.; Ali, M.E.A.; Bayoumi, S.; Balah, A.; Oterkus, S.; Shawky, H.; Oterkus, E. Numerical hydrodynamics-based design of an offshore platform to support a desalination plant and a wind turbine in Egypt. Ocean Eng. 2021, 229, 108598. [Google Scholar] [CrossRef]
- Amin, I.; Dai, S.; Day, S.; Ali, M.E.A.; Balah, A.; Shawky, H.; Oterkus, S.; Oterkus, E. Experimental study on the motion response of an integrated floating desalination plant and offshore wind turbine on a non-ship platform. Ocean Eng. 2021, 234, 109275. [Google Scholar] [CrossRef]
- Almetwally, E.M.; Elazab, M.A.; Kabeel, A.E.; Yasser, Y.; Elgebaly, A. Solar powered reverse osmosis desalination: A systematic review of technologies, integration strategies and challenges. Desalination 2025, 615, 119228. [Google Scholar] [CrossRef]
- Ghanbari, K.; Maleki, A.; Rezaei Ochbelagh, D. Optimal design of solar/wind/energy storage system-powered RO desalination unit: Single and multi-objective optimization. Energy Convers. Manag. 2024, 315, 118768. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Gu, Y.; Ren, H.; Lin, Y.; Zou, T.; Hu, W.; Huang, L. Design and test of an off-grid marine current-powered seawater desalination and hydrogen production system. Energy 2025, 332, 137241. [Google Scholar] [CrossRef]
- Freire-Gormaly, M.; Bilton, A.M. Experimental quantification of the effect of intermittent operation on membrane performance of solar powered reverse osmosis desalination systems. Desalination 2018, 435, 188–197. [Google Scholar] [CrossRef]
- Mustafa, G.; Alraqibah, O.; Alwaznani, E.; Mustakeem, M.; Asrar, N. Challenges in capital and operation cost reduction of reverse osmosis desalination process. Desalination Water Treat. 2025, 322, 101236. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, P.; Sun, F.; Zhang, W.; Li, M.; Sha, G.; Teng, L.; Wang, X.; Huo, M.; DuChanois, R.M.; et al. More resilient polyester membranes for high-performance reverse osmosis desalination. Science 2024, 384, 333–338. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Zhao, J.; Zou, J.; Liang, X.; Zhu, Z.; Zhu, J.; Wang, H.; Wang, Y.; Pan, F.; et al. Electrochemical Interfacial Polymerization toward Ultrathin COF Membranes for Brine Desalination. Angew. Chem. Int. Ed. 2023, 62, e202219084. [Google Scholar]
- Liu, Y.; Fang, W.; Yue, Z.; Wang, Y.; Zhu, Y.; Jin, J.; Jiang, L. Sustainable polyester thin films for membrane desalination developed through interfacial catalytic polymerization. Nat. Water 2025, 3, 430–438. [Google Scholar] [CrossRef]
- Lu, G.; Shang, W.; Ma, X.; Xu, H.; Hubao, A.; Sun, J.; Li, X.; Jia, M.; Lu, S.; Wu, J.; et al. Supramolecular nanocrystalline membranes with well-aligned subnanochannels for enhanced reverse osmosis desalination. Nat. Commun. 2025, 16, 6289. [Google Scholar] [CrossRef]
- Di Vincenzo, M.; Tiraferri, A.; Musteata, V.-E.; Chisca, S.; Sougrat, R.; Huang, L.-B.; Nunes, S.P.; Barboiu, M. Biomimetic artificial water channel membranes for enhanced desalination. Nat. Nanotechnol. 2021, 16, 190–196. [Google Scholar] [PubMed]
- Liu, Y.; Xu, X.; Wang, C.; Yu, H.; Wang, W.; Gong, Y.; Zhao, C.; Wang, J. Seamless incorporation of artificial water channels in defect-free polyamide membrane for desalination of brackish water. Nat. Commun. 2025, 16, 4439. [Google Scholar] [CrossRef]
- Dong, Y.; Lyu, Q.; Lin, L.-C.; Violet, C.; Lin, B.; Han, Y.; Tang, C.; Yu, H.-Q.; Elimelech, M. Ultrastable ceramic-based metal–organic framework membranes with missing linkers for robust desalination. Nat. Water 2024, 2, 464–474. [Google Scholar]
- Wen, Y.; Dai, R.; Li, X.; Zhang, X.; Cao, X.; Wu, Z.; Lin, S.; Tang, C.Y.; Wang, Z. Metal-organic framework enables ultraselective polyamide membrane for desalination and water reuse. Sci. Adv. 2022, 8, eabm4149. [Google Scholar] [CrossRef]
- Sebbahi, S.; Assila, A.; Alaoui Belghiti, A.; Laasri, S.; Kaya, S.; Hlil, E.K.; Rachidi, S.; Hajjaji, A. A comprehensive review of recent advances in alkaline water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2024, 82, 583–599. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, P.; Zhang, M.; Zhang, H.; Zhang, Y.; Han, D.; Chen, X. Exquisite restructuring of the electronic structure of cobalt phosphide via rationally controlling iron induction for water splitting under industrial conditions. J. Mater. Chem. A 2025, 13, 7263–7273. [Google Scholar] [CrossRef]
- Yang, F.; Lei, Z.; Liu, B.; Yan, R.; Dong, Y.; Sun, H. The feasible domain analysis of operating parameters considering temperature effects under fluctuating conditions and coordinated control of AWE system. Int. J. Hydrogen Energy 2025, 141, 163–175. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Kalauni, K.; Pahwa, R. A review of water electrolysis technologies with insights into optimization and numerical simulations. Int. J. Hydrogen Energy 2025, 140, 694–727. [Google Scholar] [CrossRef]
- Wang, C.R.; Stansberry, J.M.; Mukundan, R.; Chang, H.-M.J.; Kulkarni, D.; Park, A.M.; Plymill, A.B.; Firas, N.M.; Liu, C.P.; Lang, J.T.; et al. Proton Exchange Membrane (PEM) Water Electrolysis: Cell-Level Considerations for Gigawatt-Scale Deployment. Chem. Rev. 2025, 125, 1257–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, L.; Dong, Z.; Lv, F.; Guo, H.; Wang, K.; Li, M.; Qian, Z.; Ye, N.; Lin, Z.; et al. Pinning effect of lattice Pb suppressing lattice oxygen reactivity of Pb-RuO2 enables stable industrial-level electrolysis. Nat. Commun. 2024, 15, 9774. [Google Scholar] [PubMed]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and perspectives of key materials for PEM electrolyzer. Nano Res. Energy 2022, 1, 9120032. [Google Scholar] [CrossRef]
- Ham, K.; Bae, S.; Lee, J. Classification and technical target of water electrolysis for hydrogen production. J. Energy Chem. 2024, 95, 554–576. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Delpisheh, M.; Convery, C.; Niblett, D.; Vinothkannan, M.; Mamlouk, M. Offshore green hydrogen production from wind energy: Critical review and perspective. Renew. Sustain. Energy Rev. 2024, 195, 114320. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chen, X.; Wang, Z.; Wang, J.; Shi, H.; Wang, C.; Bai, Z.; Gao, Y.; Zhu, C.; et al. Hollow Bimetallic Cobalt-Based Selenide Polyhedrons for Efficient Hydrogen Production in Practical PEM Electrolysis. ACS Sustain. Chem. Eng. 2024, 12, 10020–10032. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Jensen, S.H.; Hauch, A.; Mogensen, M.B. High Temperature Electrolysis in Alkaline Cells, Solid Proton Conducting Cells, and Solid Oxide Cells. Chem. Rev. 2014, 114, 10697–10734. [Google Scholar] [CrossRef]
- Shen, H.; Gao, F.-Y.; Li, H.; Xu, J.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Durable Anion Exchange Membrane Water Electrolysis in Low-Alkaline Concentration Electrolyte. J. Am. Chem. Soc. 2025, 147, 22677–22685. [Google Scholar] [CrossRef]
- Ren, X.; Qiu, L.; Li, M.; Tian, F.; He, L.; Guo, X.; Wu, F.; Liu, Y.; Sheng, J.; Yang, W.; et al. Hierarchically interfacial sulfide/phosphides achieve industry-level water electrolyzer in alkaline conditions at 3 A cm−2. Appl. Catal. B Environ. Energy 2025, 378, 125622. [Google Scholar]
- Wu, F.; Tian, F.; Li, M.; Geng, S.; Qiu, L.; He, L.; Li, L.; Chen, Z.; Yu, Y.; Yang, W.; et al. Engineering Lattice Oxygen Regeneration of NiFe Layered Double Hydroxide Enhances Oxygen Evolution Catalysis Durability. Angew. Chem. Int. Ed. 2025, 64, e202413250. [Google Scholar]
- Lv, H.-W.; Zhao, H.-B.; Peng, X.-Y.; Ye, Z.-G.; Huang, Q.-B.; Yuan, X.-T.; Li, D.-S.; Jin, Z. Rhenium-boosted electrocatalytic activity and durability of pyrolytic IrO2 for acidic oxygen evolution. Rare Met. 2024, 43, 6758–6764. [Google Scholar]
- Zhang, X.-L.; Yu, P.-C.; Su, X.-Z.; Hu, S.-J.; Shi, L.; Wang, Y.-H.; Yang, P.-P.; Gao, F.-Y.; Wu, Z.-Z.; Chi, L.-P.; et al. Efficient acidic hydrogen evolution in proton exchange membrane electrolyzers over a sulfur-doped marcasite-type electrocatalyst. Sci. Adv. 2023, 9, eadh2885. [Google Scholar]
- Zeng, H.; Chen, Z.; Jiang, Q.; Zhong, Q.; Ji, Y.; Chen, Y.; Li, J.; Liu, C.; Zhang, R.; Tang, J.; et al. Sustainable and cost-efficient hydrogen production using platinum clusters at minimal loading. Nat. Commun. 2025, 16, 4314. [Google Scholar] [CrossRef]
- Deng, B.; Wu, Z.-Y.; Feng, E.; Ma, L.; Wang, Z.; Chen, J.; Eddy, L.; Lathem, A.; Wang, T.; Chen, W.; et al. Coupling Amorphization and Compositional Optimization of Ternary Metal Phosphides toward High-Performance Electrocatalytic Hydrogen Production. J. Am. Chem. Soc. 2025, 147, 16129–16140. [Google Scholar] [CrossRef]
- Li, A.; Kong, S.; Adachi, K.; Ooka, H.; Fushimi, K.; Jiang, Q.; Ofuchi, H.; Hamamoto, S.; Oura, M.; Higashi, K.; et al. Atomically dispersed hexavalent iridium oxide from MnO2 reduction for oxygen evolution catalysis. Science 2024, 384, 666–670. [Google Scholar] [PubMed]
- Xu, J.; Jin, H.; Lu, T.; Li, J.; Liu, Y.; Davey, K.; Zheng, Y.; Qiao, S.-Z. IrOx·H2O with lattice water-assisted oxygen exchange for high-performance proton exchange membrane water electrolyzers. Sci. Adv. 2023, 9, eadh1718. [Google Scholar]
- Kong, S.; Li, A.; Long, J.; Adachi, K.; Hashizume, D.; Jiang, Q.; Fushimi, K.; Ooka, H.; Xiao, J.; Nakamura, R. Acid-stable manganese oxides for proton exchange membrane water electrolysis. Nat. Catal. 2024, 7, 252–261. [Google Scholar] [CrossRef]
- Hu, C.; Yue, K.; Han, J.; Liu, X.; Liu, L.; Liu, Q.; Kong, Q.; Pao, C.-W.; Hu, Z.; Suenaga, K.; et al. Misoriented high-entropy iridium ruthenium oxide for acidic water splitting. Sci. Adv. 2023, 9, eadf9144. [Google Scholar] [CrossRef]
- Sun, P.; Qiao, Z.; Dong, X.; Jiang, R.; Hu, Z.-T.; Yun, J.; Cao, D. Designing 3d Transition Metal Cation-Doped MRuOx As Durable Acidic Oxygen Evolution Electrocatalysts for PEM Water Electrolyzers. J. Am. Chem. Soc. 2024, 146, 15515–15524. [Google Scholar] [PubMed]
- Yuan, B.; Dang, Q.; Liu, H.; Sendeku, M.G.; Peng, J.; Fan, Y.; Cai, L.; Cao, A.; Chen, S.; Li, H.; et al. Synergistic niobium and manganese co-doping into RuO2 nanocrystal enables PEM water splitting under high current. Nat. Commun. 2025, 16, 4583. [Google Scholar]
- Shi, Z.; Li, J.; Wang, Y.; Liu, S.; Zhu, J.; Yang, J.; Wang, X.; Ni, J.; Jiang, Z.; Zhang, L.; et al. Customized reaction route for ruthenium oxide towards stabilized water oxidation in high-performance PEM electrolyzers. Nat. Commun. 2023, 14, 843. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Li, H.; Kim, M.G.; Duan, Z.; Talat, K.; Lee, J.Y.; Wu, M.; Lee, H. Effectiveness of strain and dopants on breaking the activity-stability trade-off of RuO2 acidic oxygen evolution electrocatalysts. Nat. Commun. 2025, 16, 1717. [Google Scholar] [CrossRef]
- Toledo-Carrillo, E.A.; García-Rodríguez, M.; Sánchez-Moren, L.M.; Dutta, J. Decoupled supercapacitive electrolyzer for membrane-free water splitting. Sci. Adv. 2024, 10, eadi3180. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, Y.; Guo, J.; Zhang, Q.; Cao, F.; Wang, Y.; Han, L.; Ling, T. Cathode catalyst layers modified with Brønsted acid oxides to improve proton exchange membrane electrolysers for impure water splitting. Nat. Energy 2025, 10, 880–889. [Google Scholar] [CrossRef]
- Hodges, A.; Hoang, A.L.; Tsekouras, G.; Wagner, K.; Lee, C.-Y.; Swiegers, G.F.; Wallace, G.G. A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 2022, 13, 1304. [Google Scholar] [CrossRef]
- Jin, H.; Xu, J.; Liu, H.; Shen, H.; Yu, H.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Emerging materials and technologies for electrocatalytic seawater splitting. Sci. Adv. 2023, 9, eadi7755. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, C.; Dong, J.; Han, Y.; Huang, H.; Li, W.; Zhang, Y.; Tan, X.; Qiu, J. Seawater electrolysis for fuels and chemicals production: Fundamentals, achievements, and perspectives. Chem. Soc. Rev. 2024, 53, 7455–7488. [Google Scholar] [CrossRef]
- Cai, Z.; Liang, J.; Li, Z.; Yan, T.; Yang, C.; Sun, S.; Yue, M.; Liu, X.; Xie, T.; Wang, Y.; et al. Stabilizing NiFe sites by high-dispersity of nanosized and anionic Cr species toward durable seawater oxidation. Nat. Commun. 2024, 15, 6624. [Google Scholar] [PubMed]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Advanced membrane-based electrode engineering toward efficient and durable water electrolysis and cost-effective seawater electrolysis in membrane electrolyzers. Exploration 2024, 4, 20220112. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Long, X.; Liu, F.; Zhou, J.; Chen, N.; Feng, R.; Zhang, Y.; Fu, X.-Z.; Luo, J.-L.; Zhao, B. Interfacial engineering of defects-enriched RuO2-Co3O4-x/Cobalt Foam heterojunctions with modulated Ru-O-Co electronic bridges for long-term efficient sulfur removal and direct alkaline seawater hydrogen production. Appl. Catal. B Environ. Energy 2025, 366, 125037. [Google Scholar]
- Sun, T.; Tang, Z.; Zang, W.; Li, Z.; Li, J.; Li, Z.; Cao, L.; Dominic Rodriguez, J.S.; Mariano, C.O.M.; Xu, H.; et al. Ferromagnetic single-atom spin catalyst for boosting water splitting. Nat. Nanotechnol. 2023, 18, 763–771. [Google Scholar] [CrossRef]
- Xu, J.; Kao, C.-C.; Shen, H.; Liu, H.; Zheng, Y.; Qiao, S.-Z. Ru0.1Mn0.9Ox Electrocatalyst for Durable Oxygen Evolution in Acid Seawater. Angew. Chem. Int. Ed. 2025, 64, e202420615. [Google Scholar]
- Liu, Y.; Li, L.; Li, X.; Xu, Y.; Wu, D.; Sakthivel, T.; Guo, Z.; Zhao, X.; Dai, Z. Asymmetric tacticity navigates the localized metal spin state for sustainable alkaline/sea water oxidation. Sci. Adv. 2025, 11, eads0861. [Google Scholar] [CrossRef]
- Xu, Y.; Li, S.; Cao, J.; Ren, Z.; He, Y.; Cai, Z.; Hou, C.; Zhang, J.; Chen, Z.; Shi, R.; et al. Constructing a built-in electric field across an NiMo/NiMoP heterointerface for efficient and durable seawater electrolysis in anion exchange membrane electrolyzers. Energy Environ. Sci. 2025, 18, 4811–4820. [Google Scholar]
- Liu, H.; Shen, W.; Jin, H.; Xu, J.; Xi, P.; Dong, J.; Zheng, Y.; Qiao, S.-Z. High-Performance Alkaline Seawater Electrolysis with Anomalous Chloride Promoted Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2023, 62, e202311674. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yao, Y.; Zhang, M.; Zhou, Y.; Zhang, L.; Ren, Y.; Dong, K.; Tang, H.; Nan, J.; Zhou, X.; et al. Engineered PW12-polyoxometalate docked Fe sites on CoFe hydroxide anode for durable seawater electrolysis. Nat. Commun. 2025, 16, 5541. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Zhang, Z.; Liu, J.; Yan, X.; Wang, X.; Wang, J.; Attfield, J.P.; Yang, M. Engineered Nickel–Iron Nitride Electrocatalyst for Industrial-Scale Seawater Hydrogen Production. Adv. Mater. 2025, 37, 2415421. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Chen, C.; Li, Q.; Chen, Z.; Guo, H.; Lv, X.; Dang, J. A novel ‘Absorption-Migration’ OER mechanism and chloride repulsion effect in high-entropy (FeCoNiCuMn)2O3 nanoparticles on Ti3C2Tx MXene for efficient saline water electrolysis. Appl. Catal. B Environ. Energy 2025, 377, 125494. [Google Scholar] [CrossRef]
- Sun, X.; Shen, W.; Liu, H.; Xi, P.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Corrosion-resistant NiFe anode towards kilowatt-scale alkaline seawater electrolysis. Nat. Commun. 2024, 15, 10351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Yu, P.-C.; Sun, S.-P.; Shi, L.; Yang, P.-P.; Wu, Z.-Z.; Chi, L.-P.; Zheng, Y.-R.; Gao, M.-R. In situ ammonium formation mediates efficient hydrogen production from natural seawater splitting. Nat. Commun. 2024, 15, 9462. [Google Scholar] [CrossRef] [PubMed]
- Asghari, E.; Abdullah, M.I.; Foroughi, F.; Lamb, J.J.; Pollet, B.G. Advances, opportunities, and challenges of hydrogen and oxygen production from seawater electrolysis: An electrocatalysis perspective. Curr. Opin. Electrochem. 2022, 31, 100879. [Google Scholar] [CrossRef]
- Sha, Q.; Wang, S.; Yan, L.; Feng, Y.; Zhang, Z.; Li, S.; Guo, X.; Li, T.; Li, H.; Zhuang, Z.; et al. 10,000-h-stable intermittent alkaline seawater electrolysis. Nature 2025, 639, 360–367. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Liu, L.; Che, X.; Wang, J.; Zhu, Y.; Attfield, J.P.; Yang, M. Efficient and durable seawater electrolysis with a V2O3-protected catalyst. Sci. Adv. 2024, 10, eadn7012. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, Y.; Wu, H.; Chen, S.; Zheng, X.; Xing, C.; Li, Y. Self-Organized Gradually Single-Atom-Layer of Metal Osmium for an Unprecedented Hydrogen Production from Seawater. J. Am. Chem. Soc. 2024, 146, 10573–10580. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Xing, Z.; Xu, X.; Ye, D.; Yan, R.; Ma, T.; Wang, Y.; Zeng, Z.; Yin, B.; Cheng, C.; et al. Bioinspired Proton Pump on Ferroelectric HfO2-Coupled Ir Catalysts with Bidirectional Hydrogen Spillover for pH-Universal and Superior Hydrogen Production. J. Am. Chem. Soc. 2024, 146, 27486–27498. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Dastafkan, K.; Shen, Y.; Zhao, C.; Wang, M. Yttrium-doped NiMo-MoO2 heterostructure electrocatalysts for hydrogen production from alkaline seawater. Nat. Commun. 2025, 16, 773. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Fan, H.; Liu, Q.; Yan, Z.; Wang, A.; Yang, B.; Wang, E. Engineering interfacial sulfur migration in transition-metal sulfide enables low overpotential for durable hydrogen evolution in seawater. Nat. Commun. 2024, 15, 6154. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.E.; Myeong, S.-W.; Cho, B.-G.; Kim, J.; Park, S.J.; Kim, C.; Lee, T.H.; Lee, S.; Kim, J.Y.; Kwon, M.S.; et al. Exsolved Ru-mediated stabilization of MoO2-Ni4Mo electrocatalysts for anion exchange membrane water electrolysis and unbiased solar-driven saline water splitting. Appl. Catal. B Environ. Energy 2024, 358, 124364. [Google Scholar] [CrossRef]
- Fang, Q.-Y.; Gu, J.-J.; He, R.-X.; Ni, W.-W.; Ran, J.; Deng, N.; He, J.-B. Direct seawater electrolysis through in situ purification using dual ion exchange membranes. J. Solid State Electrochem. 2025, 29, 2051–2052. [Google Scholar] [CrossRef]
- Deng, G.; Liao, Y.; Lin, Y.; Ding, L.; Wang, H. Engineering Robust Triazine Crosslinked and Pyridine Capped Anion Exchange Membrane for Advanced Water Electrolysis. Angew. Chem. Int. Ed. 2024, 63, e202412632. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, F.; Zhang, S.; Liu, Z.; Zhang, Y.; Sun, F.; Li, J.; Chen, L.; Wang, Z.; Zhao, J.; et al. A Zero-Gap Electrolyzer Enables Supporting Electrolyte-Free Seawater Splitting for Energy-Saving Hydrogen Production. Angew. Chem. Int. Ed. 2025, 64, e202422840. [Google Scholar] [CrossRef]
- Wang, W.; Guo, R.; Zheng, A.; Jin, X.; Jia, X.; Ren, Z.; Han, Y.; Zhang, L.; Zhai, Y.; Liu, X.; et al. Promoting in-situ stability of hydroxide exchange membranes by thermally conductive network for durable water electrolysis. Nat. Commun. 2025, 16, 934. [Google Scholar] [CrossRef]
- Tang, W.; Zhao, Z.; Yang, D.; Liu, Y.; Zhu, L.; Wu, Y.; Lan, C.; Jiang, W.; Wu, Y.; Liu, T.; et al. A gel electrolyte-based direct seawater electrolysis. Energy Environ. Sci. 2025, 18, 7048–7059. [Google Scholar] [CrossRef]
- Chen, H.; Liu, P.; Li, W.; Xu, W.; Wen, Y.; Zhang, S.; Yi, L.; Dai, Y.; Chen, X.; Dai, S.; et al. Stable Seawater Electrolysis Over 10 000 H via Chemical Fixation of Sulfate on NiFeBa-LDH. Adv. Mater. 2024, 36, 2411302. [Google Scholar] [CrossRef]
- Zhao, K.; Chang, X.; Su, H.-S.; Nie, Y.; Lu, Q.; Xu, B. Enhancing Hydrogen Oxidation and Evolution Kinetics by Tuning the Interfacial Hydrogen-Bonding Environment on Functionalized Platinum Surfaces. Angew. Chem. Int. Ed. 2022, 61, e202207197. [Google Scholar] [CrossRef]
- Liang, N.-N.; Han, D.S.; Park, H. Membraneless unbuffered seawater electrolysis for pure hydrogen production using PtRuTiOx anode and MnOx cathode pairs. Appl. Catal. B Environ. 2023, 324, 122275. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, S.; Zhu, Y.; Xie, H.; Zhang, Y.; Cheng, M.; He, X.; Chen, Y.; Xiao, C.; Zhang, G.; et al. Monodisperse Os-O-Co Modules Enable Ampere-Level Hydrazine-Assisted Seawater Splitting in Membraneless Electrolyzers. Adv. Mater. 2025, 37, 2506512. [Google Scholar]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.-Z.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.; Liu, T.; Wu, Y.; Lan, C.; Jiang, W.; Zhu, L.; Wang, Y.; Yang, D.; Shao, Z. A membrane-based seawater electrolyser for hydrogen generation. Nature 2022, 612, 673–678. [Google Scholar] [CrossRef]
- Shen, W.; Ye, Y.; Hu, Y.; Wu, H.; Xia, Q.; Xie, H.; Li, Z.; Zhang, N.; An, L.; Si, R.; et al. Corrosion Protection of Rare Earth for Kilowatt-Level Alkaline Seawater Electrolyzer. J. Am. Chem. Soc. 2025, 147, 17190–17200. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, T.; Liu, J.; Chen, W.; Li, S.; Liang, J.; Liu, S.; Liu, X.; Cai, Z.; Wang, C.; et al. A sodium-ion-conducted asymmetric electrolyzer to lower the operation voltage for direct seawater electrolysis. Nat. Commun. 2023, 14, 3934. [Google Scholar] [PubMed]
- Li, T.; Wang, B.; Cao, Y.; Liu, Z.; Wang, S.; Zhang, Q.; Sun, J.; Zhou, G. Energy-saving hydrogen production by seawater electrolysis coupling tip-enhanced electric field promoted electrocatalytic sulfion oxidation. Nat. Commun. 2024, 15, 6173. [Google Scholar]
- Jang, D.; Kim, K.; Kim, K.-H.; Kang, S. Techno-economic analysis and Monte Carlo simulation for green hydrogen production using offshore wind power plant. Energy Convers. Manag. 2022, 263, 115695. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; He, Y.; Li, M.; Wang, X.; Wang, B.; Zhu, Y.; Cen, K. Comparison of onshore/offshore wind power hydrogen production through water electrolysis by life cycle assessment. Sustain. Energy Technol. Assess. 2023, 60, 103515. [Google Scholar]
- Voronova, A.; Kim, H.-J.; Jang, J.H.; Park, H.-Y.; Seo, B. Effect of low voltage limit on degradation mechanism during high-frequency dynamic load in proton exchange membrane water electrolysis. Int. J. Energy Res. 2022, 46, 11867–11878. [Google Scholar]
- Rezaei, M.; Akimov, A.; Gray, E.M.A. Techno-economics of offshore wind-based dynamic hydrogen production. Appl. Energy 2024, 374, 124030. [Google Scholar] [CrossRef]
- Rogeau, A.; Vieubled, J.; de Coatpont, M.; Affonso Nobrega, P.; Erbs, G.; Girard, R. Techno-economic evaluation and resource assessment of hydrogen production through offshore wind farms: A European perspective. Renew. Sustain. Energy Rev. 2023, 187, 113699. [Google Scholar] [CrossRef]
- Gallo, M.A.; García Clúa, J.G. Sizing and analytical optimization of an alkaline water electrolyzer powered by a grid-assisted wind turbine to minimize grid power exchange. Renew. Energy 2023, 216, 118990. [Google Scholar] [CrossRef]
- Rodríguez Castillo, C.A.; Collu, M.; Brennan, F. Design considerations and preliminary hydrodynamic analysis of an offshore decentralised floating wind-hydrogen system. Int. J. Hydrogen Energy 2024, 89, 496–506. [Google Scholar] [CrossRef]
- Dinh, Q.V.; Dinh, V.N.; Leahy, P.G. A differential evolution model for optimising the size and cost of electrolysers coupled with offshore wind farms. Int. J. Hydrogen Energy 2025, 124, 318–330. [Google Scholar] [CrossRef]
- Haqiqi, M.; Dussi, S.; Garcia-Navarro, J. Multi-effect distillation for water desalination in an offshore PEM electrolyser system. Energy Rep. 2025, 14, 1452–1466. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, W.; Yang, G. Electrolysis plant size optimization and benefit analysis of a far offshore wind-hydrogen system based on information gap decision theory and chance constraints programming. Int. J. Hydrogen Energy 2022, 47, 5720–5732. [Google Scholar] [CrossRef]
- Pegler, D.L.; Deborah, G.; Robert, R.-S.; Michele, S.; Conley, D.; Benhin, J. Techno-economic analysis for floating offshore wind and offshore green hydrogen. Int. J. Hydrogen Energy 2025, 103, 538–555. [Google Scholar] [CrossRef]
- Jin, R.; Hou, P.; Qi, Y.; Huang, Z.; Wu, T.; Cai, X. Hydrogen production efficiency: A critical factor in integrated planning of distribution and transmission system for large-scale centralized offshore wind-hydrogen system. Energy Convers. Manag. X 2025, 25, 100840. [Google Scholar] [CrossRef]
- Silalahi, D.F.; Blakers, A. Global Atlas of Marine Floating Solar PV Potential. Solar 2023, 3, 416–433. [Google Scholar] [CrossRef]
- Islam, M.M.; Hasanuzzaman, M. Chapter 1—Introduction to energy and sustainable development. In Energy for Sustainable Development; Hasanuzzaman, M.D., Rahim, N.A., Eds.; Academic Press: Amsterdam, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- Barakat, M.; Tala-Ighil, B.; Chaoui, H.; Gualous, H.; Hissel, D. Energy Management of a Hybrid Tidal Turbine-Hydrogen Micro-Grid: Losses Minimization Strategy. Fuel Cells 2020, 20, 342–350. [Google Scholar] [CrossRef]
- Driscoll, H.; Salmon, N.; Bañares-Alcántara, R. Optimal phasing of tidal stream power around the British Isles and within the English Channel for green ammonia production. Renew. Energy 2025, 241, 122335. [Google Scholar] [CrossRef]
- Omar, M.; Yakub, F.; Tasnim, R.; Zhafran, F.; Azmi, A.A.; Morisaki, T.; Thirugnana, S.T.; Jaafar, A.B.; Ikegami, Y. Techno-economic analysis of sustainable hydrogen production via 10 MW hybrid ocean thermal energy conversion offshore plant integrated alkaline and proton exchange membrane electrolyzers. Int. J. Hydrogen Energy 2025, 136, 339–357. [Google Scholar] [CrossRef]
- Ren, H.; Liu, H.; Gu, Y.; Yang, J.; Lin, Y.; Hu, W.; Li, W. Design and simulation of an off-grid marine current-powered seawater desalination and hydrogen production system. Renew. Energy 2024, 227, 120472. [Google Scholar]
- Fan, S.; Ma, Z.; Liu, T.; Zheng, C.; Wang, H. Innovations and development trends in offshore floating photovoltaic systems: A comprehensive review. Energy Rep. 2025, 13, 1950–1958. [Google Scholar] [CrossRef]
- Gharibvand, H.; Gharehpetian, G.B.; Anvari-Moghaddam, A. Feasibility studies of green hydrogen production using photovoltaic systems in Iran’s southern coastal regions. Int. J. Hydrogen Energy 2024, 94, 1212–1223. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of renewable energy power fluctuations on water electrolysis for green hydrogen production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Smith, C.; Torrente-Murciano, L. Cost efficiency versus energy utilization in green ammonia production from intermittent renewable energy. Nat. Chem. Eng. 2025, 2, 261–272. [Google Scholar] [CrossRef]
- Widén, J.; Carpman, N.; Castellucci, V.; Lingfors, D.; Olauson, J.; Remouit, F.; Bergkvist, M.; Grabbe, M.; Waters, R. Variability assessment and forecasting of renewables: A review for solar, wind, wave and tidal resources. Renew. Sustain. Energy Rev. 2015, 44, 356–375. [Google Scholar] [CrossRef]
- Rehman, S.; Menesy, A.S.; Zayed, M.E.; Zaery, M.; Al-Shaikhi, A.; Mohandes, M.A.; Irshad, K.; Kassas, M.; Abido, M.A. Synergistic sizing and energy management strategy of combined offshore wind with solar floating PV system for green hydrogen and electricity co-production using multi-objective dung beetle optimization. Results Eng. 2025, 25, 104399. [Google Scholar] [CrossRef]
- Alex, A.; Petrone, R.; Tala-Ighil, B.; Bozalakov, D.; Vandevelde, L.; Gualous, H. Optimal techno-enviro-economic analysis of a hybrid grid connected tidal-wind-hydrogen energy system. Int. J. Hydrogen Energy 2022, 47, 36448–36464. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, L.; Bhattacharya, S. A novel foundation design for the hybrid offshore renewable energy harvest system. Ocean Eng. 2025, 323, 120519. [Google Scholar] [CrossRef]
- Varotto, S.; Kazemi-Robati, E.; Silva, B. Delivering energy from hybridised offshore wind-wave parks considering electricity and hydrogen options: An optimisation approach. Sustain. Energy Grids Netw. 2025, 43, 101846. [Google Scholar] [CrossRef]
- Liu, J.; Ma, X.; Lu, C. A three-stage framework for optimal site selection of hybrid offshore wind-photovoltaic-wave-hydrogen energy system: A case study of China. Energy 2024, 313, 133723. [Google Scholar] [CrossRef]
- Hossain, M.B.; Islam, M.R.; Muttaqi, K.M.; Sutanto, D.; Agalgaonkar, A.P. A power dispatch allocation strategy to produce green hydrogen in a grid-integrated offshore hybrid energy system. Int. J. Hydrogen Energy 2024, 62, 1103–1112. [Google Scholar] [CrossRef]
- Taroual, K.; Nachtane, M.; Adeli, K.; Faik, A.; Boulzehar, A.; Saifaoui, D.; Tarfaoui, M. Hybrid marine energy and AI-driven optimization for hydrogen production in coastal regions. Int. J. Hydrogen Energy 2025, 118, 80–92. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, W.; Cao, S.; Piao, L. Recent progress for hydrogen production by photocatalytic natural or simulated seawater splitting. Nano Res. 2020, 13, 2313–2322. [Google Scholar] [CrossRef]
- Rehan, M.A.; Li, G.; Liang, H.; Ali, M. Recent advances in hybrid photocatalysts for efficient solar photocatalytic hydrogen production. Int. J. Hydrogen Energy 2025, 97, 920–949. [Google Scholar] [CrossRef]
- Antil, B.; Ranjan, R.; Gopinath, C.S.; Deka, S. Directed holey and ordered g-C3N4.5 nanosheets by a hard template nanocasting approach for sustainable visible-light hydrogen evolution with prominent quantum efficiency. J. Mater. Chem. A 2020, 8, 13328–13339. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.T.; Teo, S.H.; Mahmud, H.; Swaraz, A.M.; Rehan, A.I.; Rasee, A.I.; Kubra, K.T.; Hasan, M.M.; Salman, M.S.; et al. Progress in silicon-based materials for emerging solar-powered green hydrogen (H2) production. Adv. Colloid Interface Sci. 2025, 343, 103558. [Google Scholar] [CrossRef]
- Xiao, Y.; Yao, B.; Cao, M.; Wang, Y. Super-Photothermal Effect-Mediated Fast Reaction Kinetic in S-Scheme Organic/Inorganic Heterojunction Hollow Spheres Toward Optimized Photocatalytic Performance. Small 2023, 19, 2207499. [Google Scholar] [CrossRef]
- Gao, M.; Connor, P.K.N.; Ho, G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016, 9, 3151–3160. [Google Scholar] [CrossRef]
- Luo, W.; Yang, Z.; Li, Z.; Zhang, J.; Liu, J.; Zhao, Z.; Wang, Z.; Yan, S.; Yu, T.; Zou, Z. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ. Sci. 2011, 4, 4046–4051. [Google Scholar] [CrossRef]
- Barrera, L.; Layne, B.W.; Chen, Z.; Watanabe, K.; Kudo, A.; Esposito, D.V.; Ardo, S.; Bala Chandran, R. Revealing the role of redox reaction selectivity and mass transfer in current–voltage predictions for ensembles of photocatalysts. Energy Environ. Sci. 2024, 17, 8254–8273. [Google Scholar] [CrossRef]
- Wang, W.; Du, L.; Xia, R.; Liang, R.; Zhou, T.; Lee, H.K.; Yan, Z.; Luo, H.; Shang, C.; Phillips, D.L.; et al. In situ protonated-phosphorus interstitial doping induces long-lived shallow charge trapping in porous C3−xN4 photocatalysts for highly efficient H2 generation. Energy Environ. Sci. 2023, 16, 460–472. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Hu, H.; Huang, H.; Li, H.; Sun, X.; Ma, T. Enhancing photocatalytic performance of covalent organic frameworks via ionic polarization. Nat. Commun. 2024, 15, 9576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Guo, Y.; Zhu, Y. Photocatalytic sacrificial H2 evolution dominated by micropore-confined exciton transfer in hydrogen-bonded organic frameworks. Nat. Catal. 2023, 6, 574–584. [Google Scholar] [CrossRef]
- Collins, S.; Acevedo, Y.; Esposito, D.V.; Bala Chandran, R.; Ardo, S.; James, B.D.; Breunig, H. Levelized cost and carbon intensity of solar hydrogen production via water splitting using a scalable and intrinsically safe photocatalytic Z-scheme raceway system. Energy Environ. Sci. 2025, 18, 6690–6700. [Google Scholar] [CrossRef]
- Lee, W.H.; Lee, C.W.; Cha, G.D.; Lee, B.-H.; Jeong, J.H.; Park, H.; Heo, J.; Bootharaju, M.S.; Sunwoo, S.-H.; Kim, J.H.; et al. Floatable photocatalytic hydrogel nanocomposites for large-scale solar hydrogen production. Nat. Nanotechnol. 2023, 18, 754–762. [Google Scholar] [CrossRef]
- Pornrungroj, C.; Mohamad Annuar, A.B.; Wang, Q.; Rahaman, M.; Bhattacharjee, S.; Andrei, V.; Reisner, E. Hybrid photothermal–photocatalyst sheets for solar-driven overall water splitting coupled to water purification. Nat. Water 2023, 1, 952–960. [Google Scholar] [CrossRef]
- Jiang, L.; Luan, J.; Zhang, H.; Bai, Y.; Zhang, Y.; Liu, W.; Yan, Z.; Zhao, H. Interference mechanism of electrolyte cations on vanadium-oxygen binary doped carbon nitride for hydrogen evolution from artificial seawater splitting: Coupling experiments, DFT calculations and machine learning. Appl. Catal. B Environ. Energy 2025, 362, 124781. [Google Scholar] [CrossRef]
- Kumari, S.; Turner White, R.; Kumar, B.; Spurgeon, J.M. Solar hydrogen production from seawater vapor electrolysis. Energy Environ. Sci. 2016, 9, 1725–1733. [Google Scholar] [CrossRef]
- Dang, V.-H.; Nguyen, T.-A.; Le, M.-V.; Nguyen, D.Q.; Wang, Y.H.; Wu, J.C.S. Photocatalytic hydrogen production from seawater splitting: Current status, challenges, strategies and prospective applications. Chem. Eng. J. 2024, 484, 149213. [Google Scholar] [CrossRef]
- Yuan, C.; Yin, H.; Li, J.; Zhang, Y.; Chen, H.; Xiao, D.; Wang, Q.; Zhang, Y.; Xue, Q.-K. Light-induced CoOX surface reconstruction in hollow heterostructure for durable photocatalytic seawater splitting. Nat. Commun. 2025, 16, 6607. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, H.; Cai, S.; Prabhakaran, D.; Niu, W.; Large, A.; Held, G.; Taylor, R.A.; Wu, X.-P.; Tsang, S.C.E. Electrolyte-assisted polarization leading to enhanced charge separation and solar-to-hydrogen conversion efficiency of seawater splitting. Nat. Catal. 2024, 7, 77–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, L.; Li, Z.; Yang, T.; Liu, Y.; Kang, Z. A recyclable ZnIn2S4/PAN photocatalytic nanofiber membrane for boosting visible light hydrogen evolution in seawater without cocatalyst. Appl. Catal. B Environ. Energy 2024, 357, 124300. [Google Scholar] [CrossRef]
- Salman Nasir, M.; Zhao, Y.; Ye, H.; Wang, T.; Sheng, B.; Song, J.; Li, J.; Wang, P.; Wang, X.; Huang, Z.; et al. Efficient Hole Extraction and *OH Alleviation by Pd Nanoparticles on GaN Nanowires in Seawater for Solar-Driven H2 and H2O2 Generation. Angew. Chem. Int. Ed. 2025, 64, e202420796. [Google Scholar]
- Zhao, S.; Liu, B.; Li, K.; Wang, S.; Zhang, G.; Zhao, Z.-J.; Wang, T.; Gong, J. A silicon photoanode protected with TiO2/stainless steel bilayer stack for solar seawater splitting. Nat. Commun. 2024, 15, 2970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Dang, J.; Xiao, H.; Wang, Y.; Ding, L.; Zheng, J.; Chen, J.; Zhang, J.; Wang, X.; Xin, J.H.; et al. Multi-Scale Hierarchical Organic Photocatalytic Platform for Self-Suspending Sacrificial Hydrogen Production from Seawater. Angew. Chem. Int. Ed. 2025, 64, e202412794. [Google Scholar] [CrossRef]
- Milrad, Y.; Nagy, V.; Elman, T.; Fadeeva, M.; Tóth, S.Z.; Yacoby, I. A PSII photosynthetic control is activated in anoxic cultures of green algae following illumination. Commun. Biol. 2023, 6, 514. [Google Scholar] [CrossRef]
- Appel, J.; Hueren, V.; Boehm, M.; Gutekunst, K. Cyanobacterial in vivo solar hydrogen production using a photosystem I–hydrogenase (PsaD-HoxYH) fusion complex. Nat. Energy 2020, 5, 458–467. [Google Scholar] [CrossRef]
- Sheng, X.; Watanabe, A.; Li, A.; Kim, E.; Song, C.; Murata, K.; Song, D.; Minagawa, J.; Liu, Z. Structural insight into light harvesting for photosystem II in green algae. Nat. Plants 2019, 5, 1320–1330. [Google Scholar] [CrossRef]
- Batyrova, K.; Gavrisheva, A.; Ivanova, E.; Liu, J.; Tsygankov, A. Sustainable Hydrogen Photoproduction by Phosphorus-Deprived Marine Green Microalgae Chlorella sp. Int. J. Mol. Sci. 2015, 16, 2705–2716. [Google Scholar] [CrossRef]
- Ji, C.-F.; Yu, X.-J.; Chen, Z.-A.; Xue, S.; Legrand, J.; Zhang, W. Effects of nutrient deprivation on biochemical compositions and photo-hydrogen production of Tetraselmis subcordiformis. Int. J. Hydrogen Energy 2011, 36, 5817–5821. [Google Scholar] [CrossRef]
- Lambert, G.R.; Smith, G.D. Hydrogen formation by marine blue—Green algae. FEBS Lett. 1977, 83, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Liu, J. The enhancement of hydrogen photoproduction in marine Chlorella pyrenoidosa under nitrogen deprivation. Int. J. Hydrogen Energy 2015, 40, 14784–14789. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, X.; Yang, Z.; Wang, H.; Yang, D.; Guo, R. Characterization of H2 photoproduction by a new marine green alga, Platymonas helgolandica var. tsingtaoensis. Appl. Energy 2012, 92, 38–43. [Google Scholar] [CrossRef]
- Guan, Y.; Deng, M.; Yu, X.; Zhang, W. Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem. Eng. J. 2004, 19, 69–73. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Shin, H.-S. Fermentative hydrogen production from Laminaria japonica and optimization of thermal pretreatment conditions. Bioresour. Technol. 2011, 102, 2745–2750. [Google Scholar] [CrossRef] [PubMed]
- Corneli, E.; Adessi, A.; Olguín, E.J.; Ragaglini, G.; García-López, D.A.; De Philippis, R. Biotransformation of water lettuce (Pistia stratiotes) to biohydrogen by Rhodopseudomonas palustris. J. Appl. Microbiol. 2017, 123, 1438–1446. [Google Scholar]
- Ariful Islam, M.; Chowdhury, A.; Jahan, I.; Farrok, O. Mitigation of environmental impacts and challenges during hydrogen production. Bioresour. Technol. 2025, 415, 131666. [Google Scholar]
- Anwar, M.; Lou, S.; Chen, L.; Li, H.; Hu, Z. Recent advancement and strategy on bio-hydrogen production from photosynthetic microalgae. Bioresour. Technol. 2019, 292, 121972. [Google Scholar] [CrossRef] [PubMed]
- Enquist-Newman, M.; Faust, A.M.E.; Bravo, D.D.; Santos, C.N.S.; Raisner, R.M.; Hanel, A.; Sarvabhowman, P.; Le, C.; Regitsky, D.D.; Cooper, S.R.; et al. Efficient ethanol production from brown macroalgae sugars by a synthetic yeast platform. Nature 2014, 505, 239–243. [Google Scholar]
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Nejat Veziroglu, T.; Allakhverdiev, S.I. Review: Biofuel production from plant and algal biomass. Int. J. Hydrogen Energy 2016, 41, 17257–17273. [Google Scholar] [CrossRef]
- Yang, H.; Guo, L.; Liu, F. Enhanced bio-hydrogen production from corncob by a two-step process: Dark- and photo-fermentation. Bioresour. Technol. 2010, 101, 2049–2052. [Google Scholar]
- Matsumura, Y.; Sato, K.; Al-saari, N.; Nakagawa, S.; Sawabe, T. Enhanced hydrogen production by a newly described heterotrophic marine bacterium, Vibrio tritonius strain AM2, using seaweed as the feedstock. Int. J. Hydrogen Energy 2014, 39, 7270–7277. [Google Scholar] [CrossRef]
- Frigaard, N.-U.; Bryant, D.A. Genomic Insights into the Sulfur Metabolism of Phototrophic Green Sulfur Bacteria. In Sulfur Metabolism in Phototrophic Organisms; Hell, R., Dahl, C., Knaff, D., Leustek, T., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 337–355. [Google Scholar]
- Cai, J.; Wang, G. Hydrogen production by a marine photosynthetic bacterium, Rhodovulum sulfidophilum P5, isolated from a shrimp pond. Int. J. Hydrogen Energy 2012, 37, 15070–15080. [Google Scholar] [CrossRef]
- Freyria, N.J.; de Oliveira, T.C.; Chovatia, M.; Johnson, J.; Kuo, A.; Lipzen, A.; Barry, K.W.; Grigoriev, I.V.; Lovejoy, C. Stress responses in an Arctic microalga (Pelagophyceae) following sudden salinity change revealed by gene expression analysis. Commun. Biol. 2024, 7, 1084. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Kim, H.-C.; Choi, J.-A.; Abou-Shanab, R.A.I.; Dempsey, B.A.; Regan, J.M.; Kim, J.R.; Song, H.; Nam, I.-H.; Kim, S.-N.; et al. Photoautotrophic hydrogen production by eukaryotic microalgae under aerobic conditions. Nat. Commun. 2014, 5, 3234. [Google Scholar] [CrossRef]
- Klinsalee, R.; Laokua, N.; Rittiyan, N.; Kornrawudaphikasama, Y.; Tonawut, Y.; Preechaphonkul, N.; Khetkorn, W.; Lakshmikandan, M.; Maneeruttanarungroj, C. Ethanol-enhanced biohydrogen production and metabolomic response in the green microalga Micractinium sp. KLSc62. Int. J. Hydrogen Energy 2025, 103, 901–910. [Google Scholar] [CrossRef]
- Li, W.; Bian, M.; Jin, C.; Zeng, X.; Jia, Y. Unlocking synergistic hydrogen-ethanol co-production from brown algae through nicotinic acid-mediated NAD pool expansion. Int. J. Hydrogen Energy 2025, 159, 150575. [Google Scholar] [CrossRef]
- Bian, M.; Li, W.; Yu, Y.; Yao, S.; Zeng, X.; Ren, N.; Jia, Y. Enhanced co-production of hydrogen and ethanol from brown algae fermentation by supplementing nano zero-valent iron (nZVI). J. Clean Prod. 2024, 467, 143035. [Google Scholar] [CrossRef]
- Wei, W.; Sun, P.; Li, Z.; Song, K.; Su, W.; Wang, B.; Liu, Y.; Zhao, J. A surface-display biohybrid approach to light-driven hydrogen production in air. Sci. Adv. 2018, 4, eaap9253. [Google Scholar] [CrossRef]
- Gwon, H.J.; Park, G.; Yun, J.; Ryu, W.; Ahn, H.S. Prolonged hydrogen production by engineered green algae photovoltaic power stations. Nat. Commun. 2023, 14, 6768. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, S.; Zhao, C.; Li, S.; Liu, X.; Wang, L.; Li, M.; Huang, X.; Mann, S. Photosynthetic hydrogen production by droplet-based microbial micro-reactors under aerobic conditions. Nat. Commun. 2020, 11, 5985. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiao, Y.; He, C.; Ruan, R.; Hu, J.; Ren, J.; Toniolo, S.; Jiang, D.; Lu, C.; Li, Y.; et al. Biological fermentation pilot-scale systems and evaluation for commercial viability towards sustainable biohydrogen production. Nat. Commun. 2024, 15, 4539. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).