Abstract
The massive arrival of pelagic sargassum on the Gulf of Mexico coast has become an environmental and socioeconomic challenge, generating high management costs and affecting tourism, fisheries, and coastal ecosystems. In this context, its valorization through anaerobic digestion represents a sustainable alternative for renewable energy production. This study assessed its valorization through anaerobic digestion as a renewable energy route. Pelagic sargassum (Sargassum natans/Sargassum fluitans) was collected, mechanically pretreated, and digested in batch mode using ruminal fluid as inoculum. Two inoculum:substrate ratios (2:1 and 3:1, v/v) were operated for 7 days, and daily cumulative biogas production was recorded. The 3:1 ratio reached 10.6 mL of cumulative biogas, approximately twice the 5.0 mL obtained at 2:1, and its production curve did not plateau by day 7, suggesting ongoing activity. Elemental analysis of the sargassum showed a low C/N ratio (6.9:1) and high moisture (~95%), both of which constrain performance. Boyle’s model was used to estimate theoretical CH4 and CO2 yields and as expected, largely overpredicted the experimental volumes because it assumes ideal conversion. These results indicate that ruminal fluid enhances early-stage biogas formation but also highlight process limitations associated with biomass quality and short retention time. Future work should include extended digestion, co-digestion strategies to adjust the C/N ratio, and full monitoring of pH, soluble COD, VFAs, and volatile solids consumption.
1. Introduction
The use of fossil fuels has underpinned the development of modern societies; however, their intensive exploitation has caused considerable environmental impacts. Chief among these is the high emission of greenhouse gases, which has accelerated climate change []. Biofuels are considered a viable and sustainable alternative to fossil fuels; because they derive from renewable resources such as crops and agro-industrial residues, they hold the potential to substantially reduce greenhouse gas emissions. Their implementation in industry and in the transport sector can lower oil dependence and contribute to climate change mitigation [].
In this context, the diversification of renewable energy sources has gained importance as a means to satisfy energy demand while reducing environmental pressure. Renewable energy sources exhibit substantial potential to support energy demand and are characterized by diversity and sustainability []. Biofuels can be incorporated with relative ease into existing energy systems []. Among the different types, bioethanol, biodiesel, and biogas are especially prominent []. Biogas, in particular, has attracted growing attention as a versatile energy carrier within the circular economy framework, and it is recognized as one of the most relevant renewable energy sources for many countries, given the wide availability of biomass as feedstock. It is produced primarily through anaerobic digestion of diverse substrates, although other recovery routes such as sanitary landfilling, aerobic composting, and incineration are also used []. The main feedstocks include crops, manures, and agro-industrial by-products, which account for roughly 75% of production; municipal wastewater and industrial effluents contribute about 17%, and wastes generated in manure-processing facilities contribute around 8% [].
Once generated, biogas can be upgraded to biomethane for injection into natural gas grids. In crude form, biogas is a microbial metabolic by-product that can be used directly for heat and power or purified to biomethane and employed to synthesize higher-value compounds for energy and industrial applications []. Its composition is dominated by methane (CH4), typically 40 to 70%, and carbon dioxide (CO2), typically 15 to 60%. Minor components include ammonia (NH3), nitrogen (N2), hydrogen sulfide (H2S), oxygen (O2), hydrogen (H2), carbon monoxide (CO), and siloxanes []. Despite its advantages, the efficiency of anaerobic digestion strongly depends on the characteristics of the substrate employed.
This technology has been implemented worldwide as a strategy for waste management; however, substrates rich in lignin, such as wood residues, show limitations because microbial access to available carbon is hindered by the recalcitrant structure of this polymer. Energy crops have also been used, although the practice is considered less sustainable because it diverts land from food production to energy purposes []. For this reason, recent studies have increasingly focused on alternative biomasses such as algae, which do not compete with agricultural land and can be cultivated or collected in aquatic environments.
Microalgae stand out as biogas feedstocks due to their low cost, renewability, and integration potential with biohydrogen systems []. In this regard, brown macroalgae of the genus Sargassum have emerged as promising substrates for biogas production.
It has been reported that Sargassum particles contain lower levels of cellulose, hemicellulose, and lignin than other agro-industrial residues commonly used to produce non-conventional materials []. This characteristic is of particular interest for energy valorization. The genus Sargassum, which belongs to brown algae, comprises close to 360 recorded species distributed widely across the world’s oceans. In the Atlantic, especially in pelagic zones, Sargassum fluitans and Sargassum natans predominate [].
Since 2011, substantial accumulations of Sargassum have been recorded in the tropical Atlantic, with a distribution that spans from the Gulf of Mexico to the Gulf of Guinea. In the open ocean these rafts support high biodiversity; once ashore they can exceed ten million tons in a single season, which leads to significant ecological alterations and socioeconomic impacts that affect public health, fisheries, coastal communities, and tourism [,]. Johns et al. (2020) [] indicated that the origin of these proliferations is linked to an extreme North Atlantic Oscillation event during the winter of 2009–2010, when anomalous winds dispersed biomass toward the eastern Atlantic and promoted new accumulations in tropical waters.
In Mexico, the massive accumulation of Sargassum on Caribbean coasts has had a marked impact over the past decade. Since 2011 there have been recurrent landings, with notable peaks in September 2015 and May 2018, and persistence through 2021, reaching in some cases about 10,000 metric tons per day of wet biomass []. This phenomenon has produced economic, social, and environmental consequences. In 2019, for example, the city of Cancún spent 36.7 million US dollars on beach cleaning. During these events, Sargassum fluitans III represented approximately 65% of the biomass, while Sargassum natans I and VIII occurred in lower proportions. The scale of the problem has motivated the search for valorization pathways in sectors such as agriculture, cosmetics, and pharmaceuticals, as well as clean energy production []. Hence, the conversion of Sargassum biomass into bioenergy represents an opportunity to mitigate coastal accumulation while contributing to renewable energy goals.
These species present moisture contents near 7 to 8%, although the organic fraction differs, being higher in S. natans VIII (33.73 ± 1.47%) and lower in S. fluitans (28.86 ± 0.40%). Calcium carbonate is more abundant in S. fluitans (10.75 ± 1.10%) than in S. natans VIII (6.30 ± 0.47%). Arsenic concentrations exceed 58 µg g−1 in all three morphotypes, which constrains food or agricultural uses. In terms of compounds of interest, S. natans VIII has higher phenols, phlorotannins, and mannitol, while alginate yields are relatively low in all cases (9 to 12% of dry weight), with a predominance of mannuronic and guluronic acids [].
Among studies that have used Sargassum for biogas, Thompson et al. (2021) [] evaluated S. natans and S. fluitans combined with food waste under mesophilic anaerobic digestion. The algae were hydrothermally pretreated and various mixing ratios were tested. The highest yield occurred at a 25:75 ratio (algae:food waste), reaching 292 mL CH4/gVS with a methane content of 69.4%. Thompson et al. (2020) [] assessed anaerobic digestion of S. natans and S. fluitans with hydrothermal pretreatment at different severities. Untreated algae showed low yields, while higher severity (severity factor 2.65) achieved 116.7 mL CH4/gVS, with methane near 50% and H2S reduced from 3% to 1%.
Zaidi et al. (2019) [] examined anaerobic digestion of the green alga Enteromorpha, adding nickel nanoparticles as an enhancement strategy, and reported an accumulated biogas production of 346 mL. As well Castro et al. (2022) [] studied co-digestion of S. natans and S. fluitans with food waste in Punta Cana. A 45% Sargassum and 55% food waste mixture produced six times more biogas than 100% Sargassum. These studies demonstrate the technical feasibility of biogas production from Sargassum, although the yields strongly depend on pretreatment and co-digestion strategies.
In this context, S. natans and S. fluitans are viable for biogas production. The use of ruminal fluid as an additive presents significant potential to optimize the process. Ruminal fluid is characterized by a high microbial density that includes 10 to 50 billion bacteria per milliliter, about one million protozoa, and variable amounts of yeasts and fungi []. Orhorhoro and Oghoghorie (2024) [] evaluated anaerobic co-digestion of Sargassum spp. with cow, pig, goat, and chicken manure. Mixtures with manure significantly improved biogas production, with a maximum of 3.51 m3 for the combination of chicken manure and Sargassum. Given these findings, introducing ruminal inoculum could provide a natural consortium of anaerobic microorganisms that accelerates hydrolysis and fermentation, particularly for substrates with low C/N ratios such as Sargassum.
Therefore, the objective of this study was to evaluate the feasibility of Sargassum collected on the coast of Yucatán, Mexico, as a substrate for biogas production by anaerobic digestion, using ruminal fluid as inoculum due to its high microbial density. A laboratory-scale batch experiment was designed that included collection, preparation, and characterization of the algae, as well as evaluation of different mixing ratios in batch biodigesters over seven days. The results were analyzed statistically and complemented with Boyle’s model to estimate biogas composition, providing evidence of the energetic potential of Sargassum and the feasibility of its valorization as a renewable resource when combined with ruminal fluid as an additive.
2. Materials and Methods
2.1. Sample Collection and Preparation
Sargassum sampling was conducted during the winter season on the coastline of the municipality of Progreso, Yucatán, Mexico (21°17′10.1″ N, 89°40′03.7″ W), coast of the Gulf of Mexico. Figure 1a presents a regional satellite image of the sampling area, whereas Figure 1b shows a local-scale satellite view. In addition, Figure 2 includes a photograph taken on the collection date that documents the shoreline conditions and the accumulation of Sargassum on the swash zone at the time of sampling.
Figure 1.
(a) Regional satellite image of the sampling area. (b) Local-scale satellite image of the sampling area.

Figure 2.
Shoreline conditions and the accumulation of Sargassum on the swash zone at the time of sampling.
The pelagic Sargassum sample (S. natans and S. fluitans) was manually collected and transported to the Environmental Laboratory of the Faculty of Engineering at the Autonomous University of Yucatán. The seaweed was subjected to manual cleaning to remove sand particles, shells, and solid debris, followed by washing with potable water; subsequently, a mechanical pretreatment was applied, consisting of blending 300 g of biomass with 300 mL of water in a blender for 5 min. It is worth noting that this liquefaction-type pretreatment has been previously reported in Sargassum valorization studies for the production of bioethanol and biogas [,,]. The product of this procedure was a homogeneous pulp, which was stored at approximately 5 °C.
The substrate used in anaerobic digestion corresponded to the collected Sargassum, whereas bovine ruminal fluid was employed as the inoculum to favor process development. The rumen sample was obtained in Mérida, Yucatán, on the same day as the Sargassum collection. The donor cattle were maintained on a controlled diet consisting of grass hay and supplements. According to Mekonnen et al. (2025) [], ruminal fluid contains a high content of organic matter and volatile solids (VS), making it a valuable source of carbon and of microorganisms capable of enhancing biogas production.
2.2. Characterization of Substrate and Inoculum
The basic physicochemical properties of both the substrate (pelagic Sargassum spp.) and the inoculum (ruminal fluid) were characterized prior to digestion. The analyses included total solids (TS), volatile solids (VS), moisture content, and elemental composition, which are key indicators of substrate suitability and microbial activity in anaerobic digestion.
Elemental analysis of the algal biomass was conducted using a FLASH 2000 CHNS/O elemental analyzer (Thermo Scientific, Waltham, MA, USA), determining the percent content of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S). These determinations were performed in triplicate. The same procedure was applied to the ruminal fluid to quantify its elemental composition and compare it with that of the algal substrate. This allowed the identification of differences in organic matter content and C/N ratio between both materials. This type of characterization by elemental analysis has previously been documented in studies on Sargassum, such as that of Olguin-Maciel et al. (2022) [].
TS, VS, and moisture content were determined in the substrate and in the inoculum following Standard Methods 2540 B and 2540 D []. All analyses were performed in triplicate to ensure the reliability of the results.
2.3. Experimental Setup
Biogas quantification was performed using ten 120 mL serum bottles sealed with headspace septum caps. Each bottle was connected via intravenous (IV) tubing coupled to a hypodermic needle, which was inserted into a second inverted serum bottle held on a universal stand. This second bottle, also sealed with a headspace septum, contained potable water, and had an additional outlet through a hypodermic needle that allowed displacement of the liquid into a 50 mL graduated cylinder. The volume of displaced water was considered equivalent to the volume of biogas produced in the reaction bottle. Figure 3 shows a diagram illustrating the experimental design.
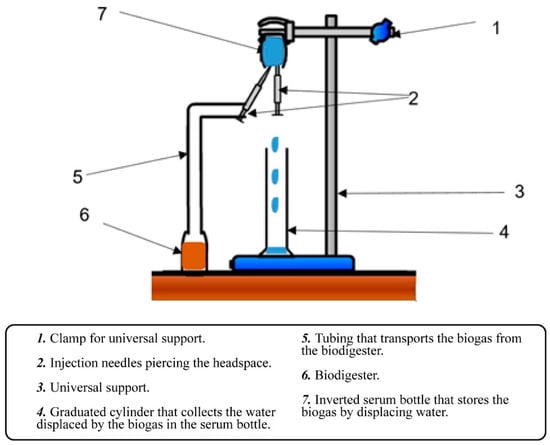
Figure 3.
Experimental design.
2.4. Validation of the Study
The positive control is an experimental group to which a substrate with a known methane potential is added, with the purpose of verifying both the experimental setup and the performance of the inoculum, since its biogas production must fall within an expected range to validate the assay. In contrast, the negative control or blank consists of bottles containing only the inoculum; its function is to quantify the methane generated from residual substrates and the existing microbial biomass, so that this volume can be subtracted from the total production to obtain the net production attributable to the evaluated substrate [].
Studies such as those by Koch et al. (2020) [] and Qiu et al. (2025) [] have employed these controls in biochemical methane potential (BMP) tests using various substrates and digested sludge as inoculum, to ensure the reliability of biogas production results.
Positive and Negative Control
Two types of controls were employed. On the one hand, the positive control consisted of five experimental reactors (P1–P5) to which 30 mL of ruminal fluid, 15 mL of a sucrose solution (0.111 M), and distilled water were added up to a final volume of 100 mL. Likewise, the negative control or blank consisted of five reactors (N1–N5) containing only 30 mL of ruminal fluid. Inclusion of the negative control was essential to exclude biogas generated exclusively by the inoculum, whereas the positive control allowed comparison of a readily biodegradable substrate with Sargassum.
2.5. Experiment Design
Two inoculum:substrate ratios (3:1 and 2:1), were evaluated. Reactor preparation followed the same procedure described for the positive control, adding the corresponding amounts of inoculum and substrate for each ratio and bringing the volume to 100 mL with distilled water. The incubation time was 7 days, and the response variable considered was the volume of biogas produced. For the 3:1 ratio, 10 mL of substrate and 30 mL of inoculum were used, whereas for the 2:1 ratio, 15 mL of substrate and 30 mL of inoculum were used. This experiment was conceived as a short-term batch screening rather than a full BMP test. The 7-day period was established to capture the initial stage of biogas generation and to compare the short-term effect of inoculum concentration under identical conditions. Each condition (2:1 and 3:1) was tested using five independent batch reactors. Likewise, five positive controls and five negative controls were operated simultaneously. The biogas volume of each reactor was corrected by subtracting the mean value from the negative controls on the same day to obtain the net biogas attributable to the substrate.
All reactors were maintained at room temperature without active stirring, under static conditions typical of batch assays.
2.6. Statistical Analysis
Biogas production data were processed using descriptive statistics. For each inoculum:substrate ratio (2:1 and 3:1), five independent batch reactors were operated. The daily biogas volume of each reactor was corrected by subtracting the mean volume measured in the negative controls to obtain the net biogas attributable to the substrate. The corrected data were then averaged, and results are expressed as mean ± standard deviation (SD). Error bars representing ± 1 SD are shown in Figure 4. All calculations were performed in Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
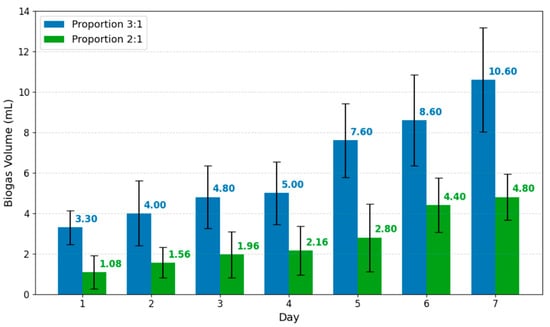
Figure 4.
Daily cumulative biogas production for inoculum:substrate ratios 2:1 and 3:1 over 7 days. Values represent mean ± standard deviation after correction with the negative control.
2.7. Data Analysis
Boyle’s model is a stoichiometric formulation used in anaerobic digestion studies to predict biogas generation under ideal conditions and subsequently compare those values with experimental yields [].
This model has been used to estimate the theoretical methane potential in investigations [,,]. In this context, the model was used to analyze the results and to estimate CH4 and CO2 production, considering Equation (1) [].
where:
: Number of moles of carbon introduced into the digester.
: Number of moles of hydrogen introduced into the digester.
: Number of moles of oxygen introduced into the digester.
: Number of moles of nitrogen introduced into the digester.
: Number of moles of sulfur introduced into the digester.
In this study, the parameters used in Boyle’s model were obtained from both the laboratory experimental results and theoretical values derived from the substrate’s elemental composition. In this way, the model not only enabled estimation of the theoretical production potential of CH4 and CO2 but also allowed those predictions to be contrasted with the experimentally observed values, thereby highlighting the limitations associated with the ideal conditions assumed by the formulation.
3. Results and Discussion
3.1. Results of the Characterization of the Substrate and the Inoculum
Elemental analyses of the sargassum were performed in triplicate from the same field-collected sample; the determinations (S1, S2, and S3) correspond to independent replicates obtained with the elemental analyzer. The mean values are presented in Table 1.

Table 1.
Percentages of C, H, N, and S in the Sargassum samples.
Based on the data in Table 1, the carbon to nitrogen (C/N) ratio is calculated as 6.9:1. This is a determining factor in anaerobic digestion, since a ratio between 16:1 and 45:1 is considered optimal; higher values lead to rapid nitrogen depletion and lower biogas production, whereas ratios below 15:1 favor the accumulation of inhibitors such as ammonia and volatile fatty acids. In marine algae, the C/N ratio can vary widely, and values between 8.72 and 58 have been reported [].
In this regard, Table 2 provides a comparative summary of the percent C and N composition of pelagic Sargassum, as well as the resulting C/N ratio.

Table 2.
Comparison of the C/N ratio of Sargassum with the literature.
The value resulting from the elemental analysis was lower than the optimal range reported for anaerobic digestion (16:1–45:1) and lower than those recorded in previous studies with Sargassum spp. The elevated nitrogen fraction observed in the samples may be attributed to the presence of nitrogenous compounds intrinsic to marine algae, such as proteins and amino acids, as well as to environmental factors associated with the collection site and the state of biomass decomposition. This marked deviation from the C/N ratios reported for fresh pelagic Sargassum indicates that the collected material was likely in an advanced state of degradation, which can enrich the nitrogen fraction and reduce the carbon content.
Additionally, the elemental composition of the ruminal fluid was determined to establish a direct comparison with the algal substrate. The solid fraction of the ruminal contents presented 58% C, 7.5% N, 6.5% H, and 0.85% S, corresponding to a C/N ratio of approximately 7.7:1. This value is similar to that of the sargassum sample, indicating that both materials possess relatively high nitrogen levels. However, the ruminal fluid also contains active microbial consortia and degradable organic compounds that can accelerate hydrolysis and fermentation processes when used as inoculum.
On the other hand, the results of the TS, VS, and moisture analysis for the Sargassum and the ruminal fluid are presented in Table 3.

Table 3.
Percentages of TS, VS and moisture of the substrate and inoculum.
The results in Table 3 show that the Sargassum had low TS (5.3%) and VS (4%) contents, together with moisture close to 95%. These characteristics confirm the highly aqueous nature of this substrate, which represents a limitation for anaerobic digestion, since a high moisture fraction reduces the effective organic loading and makes it difficult to maintain adequate solids concentration in batch digesters.
In contrast, the ruminal fluid exhibited slightly higher TS (8.4%) and VS (6.6%) values, which suggests a greater density of available organic matter and, therefore, a higher potential for energy contribution. The comparison between the two materials indicates that Sargassum alone offers limited performance for biogas production, and that, when combined with ruminal fluid, it could introduce microorganisms capable of accelerating organic matter degradation and increasing the VS fraction in the system.
According to the literature, batch biodigesters can operate under two schemes: dry digestion (≥15% TS) or wet digestion (<15% TS), depending on the type of substrate and the technology employed [,]. To contrast the experimental values, a theoretical scenario with a target concentration of 40% TS was considered; however, when that criterion was applied to the values obtained for Sargassum (5.3% TS) and ruminal fluid (8.4% TS), the calculations yielded negative results, evidencing the impossibility of reaching that concentration due to the high moisture content of the algae.
In practice, the biodigesters operated under wet-digestion conditions, with values close to 5–8% TS. This interval is consistent with reports for anaerobic digestion in liquid systems, although it entails a lower effective organic loading and, therefore, a limited biogas yield. The discrepancy between the theoretical scenario and actual operation highlights the advisability of applying drying pretreatments or co-digestion with higher-solids residues in order to improve process efficiency.
3.2. Anaerobic Digestion of Sargassum
Figure 4 shows the biogas volume produced for the inoculum:substrate ratios 3:1 and 2:1. The values were corrected using the negative control and were recorded daily over the seven-day experiment at the same time each day. The mean masses of the algal samples (wet weight) were 15.42 g for the 2:1 ratio and 10.28 g for the 3:1 ratio.
Figure 4 shows that the 3:1 ratio exhibited a more pronounced upward trend over the seven days, reaching a cumulative volume of 10.6 mL at the end of the experiment. In contrast, the 2:1 ratio showed a more moderate increase, stabilizing from day 6 onward with a final volume of 5.0 mL.
The comparative analysis indicates that the 3:1 ratio doubled the production achieved by the 2:1 ratio, suggesting that a greater inoculum input favored microbial activity and, consequently, substrate degradation. In addition, a plateau is evident from day 6 in the 2:1 treatment, whereas in the 3:1 treatment the curve maintained a positive slope through day 7, indicating a more sustained process.
When normalized to the VS content, cumulative biogas yields were 153 mL/g VS and 486 mL/g VS for the 2:1 and 3:1 ratios, respectively, confirming that the higher inoculum concentration enhanced production under the high-moisture conditions of this assay.
These results confirm that, under the conditions of the present study, the 3:1 ratio was more efficient for biogas production, likely due to the additional microorganisms from the ruminal fluid that improved the biomass degradation kinetics.
3.3. Estimation of Biogas Composition
Biogas is considered a mixture of gases, primarily CH4 (50–70% of total volume), CO2 (30–50%), and traces of other gases such as hydrogen sulfide (H2S) and water vapor []. In this study, Boyle’s model was used exclusively to calculate the theoretical yields of CH4 and CO2 under ideal stoichiometric conversion of the substrate. These values are theoretical predictions and do not represent measured gas composition, since methane content was not directly analyzed.
For the estimation based on Boyle’s model (Equation (1)), the oxygen content on a dry basis was calculated by elemental difference, considering the percentages of C, H, N, S, and ash; in this way, a value of 1.6% O was obtained, which was used in the stoichiometry for the theoretical estimation of CH4 and CO2.
It was also necessary to first calculate the number of moles of each element present in the samples. Based on the mean sample masses (15.42 g for the 2:1 ratio and 10.28 g for the 3:1 ratio, both on a wet-weight basis) and considering the TS content (5.3%), the mass of TS corresponded to 0.82 g for the 2:1 ratio and 0.55 g for the 3:1 ratio.
Using the atomic weights of the elements (C = 12 g/mol, H = 1 g/mol, N = 14 g/mol, S = 32 g/mol and O = 16 g/mol) and the mass of TS for each sample, the molar quantities were calculated as mass divided by atomic weight. The results obtained for the 2:1 and 3:1 ratios are summarized in Table 4, which presents the number of moles of each element calculated for the specified ratios.

Table 4.
Theoretical quantities of moles of C, H, N, S and O calculated for the inoculum:substrate ratios 2:1 and 3:1.
Accordingly, the results from applying the model are presented in Table 5; it should be noted that a density of 0.656 g/L for CH4 and 1.976 g/L for CO2 was considered.

Table 5.
Theoretical yields of CH4 and CO2 calculated using Boyle’s model for each inoculum:substrate ratio.
Table 5 presents only the theoretical stoichiometric yields of methane and carbon dioxide estimated with Boyle’s model. Experimental gas volumes are shown separately in Figure 4.
The quantities calculated with Boyle’s model markedly exceeded the experimental values, confirming that this model operates under idealized theoretical conditions. Nevertheless, it is important to note that, in the case of the 3:1 ratio, biogas production still showed an upward trend on day seven, which suggests that anaerobic digestion had not fully concluded and that cumulative production could have been higher with a longer incubation period: likewise, the model does not incorporate critical variables for real digester performance, such as TS and VS content, the C/N ratio, or the accumulation of inhibitory compounds; these factors constrain microbial activity and help explain the gap between theoretical and experimental yields.
To contextualize the results obtained in this study relative to recent investigations, Table 6 provides a comparative summary of theoretical and experimental biogas yields reported for Sargassum spp., calculated using Boyle’s model or related stoichiometric formulations. Table 6 summarizes a comparative analysis between the theoretical yields obtained in this work and experimental data reported in the literature.

Table 6.
Boyle Comparison of theoretical and experimental biogas yields from Sargassum spp. using the Boyle model.
Table 6 shows that the experimental productions of biogas and methane were considerably lower than the theoretical estimates from Boyle’s model. In this study, the measured volumes were far below the calculated values, which is consistent with Salgado-Hernández et al. (2023) [], who found that Sargassum fractions reached experimental values well below the estimated potential. Similarly, Salgado-Hernández et al. (2023) [] observed that, even with pretreatments, practical yields remained far from the theoretical predictions.
Finally, Bueno et al. (2023) [] confirmed this trend in a life-cycle analysis, where methane emissions calculated with Boyle’s model were markedly reduced after correction with degradability factors. Taken together, these results confirm the usefulness of Boyle’s model as a theoretical reference, while showing that experimental values can be lower due to the conditions of anaerobic digestion of Sargassum.
3.4. Prospects for the Use of Sargassum in Co-Digestion
Authors such as Canul-Ku et al. (2025) [] have highlighted improvements in the fermentative process when Sargassum is inoculated with ruminal fluid, which suggests its potential as an additive for biogas production. Likewise, Rivera-Hernández et al. (2022) [] reported the feasibility of co-digesting Sargassum with pig manure. In the study by Thompson et al. (2021) [], co-digestion of Sargassum with food waste using wastewater as inoculum was reported. The results indicate that this approach improved process stability and facilitated compound degradation. In addition, a considerable increase in biogas production was observed in mixtures with higher proportions of food waste.
Similarly, Hütter et al. (2023) [] reported that co-digestion of Sargassum muticum with wheat straw allowed adjustment of the system C/N ratio and improved process performance, supporting the view that incorporating carbon-rich lignocellulosic materials could represent an alternative to overcome the limitations associated with Sargassum as a sole substrate.
Consistent with these reports, in this study Sargassum used as the only substrate showed limited performance, whereas the addition of ruminal fluid enhanced production, with the 3:1 inoculum:substrate ratio achieving the highest biogas volumes. These results, together with the literature, support the conclusion that Sargassum alone is not an adequate substrate due to its low C/N ratio and high moisture content; however, its co-digestion with carbon-richer substrates, as well as inoculation with active microorganisms, can enhance its energy valorization. In this way, integrating agro-industrial residues, manure, carbon-rich lignocellulosic materials, or food waste with Sargassum can be considered a promising alternative to consolidate its valorization for biogas production.
4. Conclusions
The results of this study confirm that pelagic sargassum collected on the Gulf of Mexico coast can be used as a substrate for biogas production; however, its physico-chemical characteristics, particularly the low C/N ratio, suggest a state of prior fermentation at the time of sampling, which poses a challenge for scale-up given that its valorization seeks to address the problem arising from its substantial accumulations in recent years. The addition of ruminal fluid was essential to increase the volumes obtained, with the 3:1 ratio emerging as the most efficient, underscoring the importance of the inoculum for microbial activity and degradation kinetics. Boyle’s model enabled estimation of the theoretical biogas production potential and comparison with the experimental values, confirming its usefulness as a reference even though actual yields were lower than predicted. Although gas composition was not experimentally determined, the theoretical estimation based on Boyle’s model provided a useful reference for evaluating process performance.
The 3:1 ratio reached 10.6 ± 2.58 mL of cumulative biogas, approximately twice the 4.8 ± 1.14 mL obtained at 2:1, confirming that higher inoculum concentration enhanced production under identical conditions. The purpose of this test was to capture the initial phase of hydrolysis and fermentation for comparative screening of inoculum ratios under identical conditions. When normalized to VS, cumulative yields were 153 mL/g for the 2:1 ratio and 486 mL/g for the 3:1 ratio, corroborating the positive effect of the inoculum under high-moisture conditions. The observed upward trend at 3:1 on day 7 supports that extended operation could further increase cumulative yields, which is proposed for future work.
The repeatability of the measurements was confirmed by the low variation among replicates, with coefficients of variation below 25% for most sampling days. Reported results correspond to mean ± SD from five independent reactors per condition, corrected with the blank to represent net biogas generation.
In future work, it is recommended to extend the operation period and evaluate alternatives to improve the C/N ratio. Potential strategies such as co-digestion with food waste, manure, carbon-rich lignocellulosic materials, or agro-industrial residues are suggested as prospective approaches to enhance process stability and methane yield.
Author Contributions
All authors contributed to the elaboration of this study, the contributions are distinguished as follows: Conceptualization, L.S.-P. and L.A.L.-G.; Methodology, L.A.L.-G. and J.E.Á.-L.; Formal analysis, L.A.L.-G.; Supervision, A.M.A. and G.G.-V. The first draft of the manuscript was written by L.S.-P.; Investigation and Writing—Review and Editing, J.E.Á.-L. and L.S.-P.; Data curation, A.B. and G.G.-V.; Validation, A.M.A. and A.B.; Visualization, J.E.Á.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are not publicly available due to file size and practical limitations, but they are available from the corresponding author upon reasonable request.
Acknowledgments
The collaboration of the Environmental Engineering laboratories of the Faculty of Engineering of the Autonomous University of Yucatan, Mexico.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Álvarez-Ley, J.E.; Méndez-Novelo, R.I.; Giácoman-Vallejos, G.; Paniagua Solar, L.A.; San-Pedro, L. Microbial Fuel Cells for Power Generation and Wastewater Treatment: A Review of Components, Performance and Sustainability. Int. J. Hydrogen Energy 2025, 137, 429–447. [Google Scholar] [CrossRef]
- Rial, R.C. Biofuels versus Climate Change: Exploring Potentials and Challenges in the Energy Transition. Renew. Sustain. Energy Rev. 2024, 196, 114369. [Google Scholar] [CrossRef]
- San-Pedro, L.; Coronado-Cauich, M.J.; Hernández-Núñez, E.; González-Díaz, M.O.; Álvarez-Ley, J.E.; Flota-Bañuelos, M.I. Electrochemical Characterization of a Testaceous Membrane (Chicken Eggshell) For Use in Fuel Cells. J. Membr. Sci. Res. 2024, 10, 2004886. [Google Scholar] [CrossRef]
- Dalbanjan, N.P.; Korgaonkar, K.; Kadapure, A.J.; Halladamani, S.B.; Ramangouda, G.; Kumar, S.K.P. Green Energy from Waste: Evaluating the Sustainability of Anaerobic Biofuel Technologies. Microbe 2025, 7, 100410. [Google Scholar] [CrossRef]
- Asaad, S.M.; Tawalbeh, M.; Ali, A.; Al Kindi, S.R.; Al-Othman, A. Definition of Bioenergy. In Renewable Energy—Volume 2: Wave, Geothermal, and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 215–243. [Google Scholar]
- Sher, F.; Smječanin, N.; Hrnjić, H.; Karadža, A.; Omanović, R.; Šehović, E.; Sulejmanović, J. Emerging Technologies for Biogas Production: A Critical Review on Recent Progress, Challenges and Future Perspectives. Process Saf. Environ. Prot. 2024, 188, 834–859. [Google Scholar] [CrossRef]
- Ngabala, F.J.; Emmanuel, J.K. Potential Substrates for Biogas Production through Anaerobic Digestion—An Alternative Energy Source. Heliyon 2024, 10, e40632. [Google Scholar] [CrossRef] [PubMed]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Biogas Production and Applications in the Sustainable Energy Transition. J. Energy 2022, 2022, 8750221. [Google Scholar] [CrossRef]
- Biswas, R.; Ahmadi, V.; Ummethala, R.; Mozumder, M.S.I.; Aryal, N. Recent Advances in Electrochemical Carbon Dioxide Reduction Strategies in Biogas Upgrading and Biomethane Production. Chem. Eng. J. Adv. 2025, 22, 100722. [Google Scholar] [CrossRef]
- Tjutju, N.A.S.; Ammenberg, J.; Lindfors, A. Biogas Potential Studies: A Review of Their Scope, Approach, and Relevance. Renew. Sustain. Energy Rev. 2024, 201, 114631. [Google Scholar] [CrossRef]
- Wang, K.; Khoo, K.S.; Chew, K.W.; Selvarajoo, A.; Chen, W.H.; Chang, J.S.; Show, P.L. Microalgae: The Future Supply House of Biohydrogen and Biogas. Front. Energy Res. 2021, 9, 660399. [Google Scholar] [CrossRef]
- Duran, A.J.F.P.; Lyra, G.P.; Campos Filho, L.E.; Bueno, C.; Rossignolo, J.A.; Alves-Lima, C.; Fiorelli, J. The Use of Sargasso Seaweed as Lignocellulosic Material for Particleboards: Technical Viability and Life Cycle Assessment. Buildings 2024, 14, 1403. [Google Scholar] [CrossRef]
- Debue, M.; Guinaldo, T.; Jouanno, J.; Chami, M.; Barbier, S.; Berline, L.; Chevalier, C.; Daniel, P.; Daniel, W.; Descloitres, J.; et al. Understanding the Sargassum Phenomenon in the Tropical Atlantic Ocean: From Satellite Monitoring to Stranding Forecast. Mar. Pollut. Bull. 2025, 216, 117923. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Advances in the Pretreatment of Brown Macroalgae for Biogas Production. Fuel Process. Technol. 2019, 195, 106151. [Google Scholar] [CrossRef]
- Johns, E.M.; Lumpkin, R.; Putman, N.F.; Smith, R.H.; Muller-Karger, F.E.; Rueda-Roa, D.T.; Hu, C.; Wang, M.; Brooks, M.T.; Gramer, L.J.; et al. The Establishment of a Pelagic Sargassum Population in the Tropical Atlantic: Biological Consequences of a Basin-Scale Long Distance Dispersal Event. Prog. Ocean. 2020, 182, 102269. [Google Scholar] [CrossRef]
- Orozco-González, J.G.; Amador-Castro, F.; Gordillo-Sierra, A.R.; García-Cayuela, T.; Alper, H.S.; Carrillo-Nieves, D. Opportunities Surrounding the Use of Sargassum Biomass as Precursor of Biogas, Bioethanol, and Biodiesel Production. Front. Mar. Sci. 2022, 8, 791054. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass Composition of the Golden Tide Pelagic Seaweeds Sargassum fluitans and S. Natans (Morphotypes I and VIII) to Inform Valorisation Pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Enhancing Biogas Production from Caribbean Pelagic Sargassum Utilising Hydrothermal Pretreatment and Anaerobic Co-Digestion with Food Waste. Chemosphere 2021, 275, 130035. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Efficiency of Hydrothermal Pretreatment on the Anaerobic Digestion of Pelagic Sargassum for Biogas and Fertiliser Recovery. Fuel 2020, 279, 118527. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Khan, S.Z.; Shi, Y. Optimization of Nickel Nanoparticles Concentration for Biogas Enhancement from Green Algae Anaerobic Digestion. Mater. Today Proc. 2019, 39, 1025–1028. [Google Scholar] [CrossRef]
- Castro, Y.A.; Rodríguez, A.; Rivera, E. Biomethane Production Kinetics during the Anaerobic Co-Digestion of Sargassum spp. and Food Waste Using Batch and Fed-Batch Systems in Punta Cana, Dominican Republic. Mater. Renew. Sustain. Energy 2022, 11, 287–297. [Google Scholar] [CrossRef]
- Chellapandi, P.; Bharathi, M.; Sangavai, C.; Prathiviraj, R. Methanobacterium formicicum as a Target Rumen Methanogen for the Development of New Methane Mitigation Interventions: A Review. Vet. Anim. Sci. 2018, 6, 86–94. [Google Scholar] [CrossRef]
- Orhorhoro, E.K.; Oghoghorie, O. Enhancing Biogas Yield through Anaerobic Co-Digestion of Animal Manure and Seaweed. Prog. Energy Environ. 2024, 28, 1–22. [Google Scholar] [CrossRef]
- Aparicio, E.; Rodríguez-Jasso, R.M.; Pinales-Márquez, C.D.; Loredo-Treviño, A.; Robledo-Olivo, A.; Aguilar, C.N.; Kostas, E.T.; Ruiz, H.A. High-Pressure Technology for Sargassum spp. Biomass Pretreatment and Fractionation in the Third Generation of Bioethanol Production. Bioresour. Technol. 2021, 329, 124935. [Google Scholar] [CrossRef] [PubMed]
- Chikani-Cabrera, K.D.; Fernandes, P.M.B.; Tapia-Tussell, R.; Parra-Ortiz, D.L.; Hernández-Zárate, G.; Valdez-Ojeda, R.; Alzate-Gaviria, L. Improvement in Methane Production from Pelagic Sargassum Using Combined Pretreatments. Life 2022, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Paletta, R.; Girimonte, R.; Castro, Y.A.; De Frias, J.A.; Calabrò, V. Effect of Particle Size on the Biomethanation Kinetics of Mechanically Pretreated Sargassum spp. Biomass. Methane 2024, 3, 160–171. [Google Scholar] [CrossRef]
- Mekonnen, A.M.; Sendekie, Z.B.; Ebissa, D.T.; Bezie, Y. Optimizing Rumen Fluid Inoculation for Enhanced Biogas Production Using Organic Waste Codigestion. Int. J. Chem. Eng. 2025, 2025, 2463014. [Google Scholar] [CrossRef]
- Olguin-Maciel, E.; Leal-Bautista, R.M.; Alzate-Gaviria, L.; Domínguez-Maldonado, J.; Tapia-Tussell, R. Environmental Impact of Sargassum spp. Landings: An Evaluation of Leachate Released from Natural Decomposition at Mexican Caribbean Coast. Environ. Sci. Pollut. Res. 2022, 29, 91071–91080. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- Koch, K.; Hafner, S.D.; Astals, S.; Weinrich, S. Evaluation of Common Supermarket Products as Positive Controls in Biochemical Methane Potential (BMP) Tests. Water 2020, 12, 1223. [Google Scholar] [CrossRef]
- Qiu, Y.; Lower, L.; Rondon Berrio, V.; Cunniffe, J.; Kolar, P.; Cheng, J.; Sagues, W.J. Impacts of Municipal and Industrial Organic Waste Components on the Kinetics and Potentials of Biomethane Production via Anaerobic Digestion. Waste Biomass Valorization 2025, 16, 5019–5035. [Google Scholar] [CrossRef]
- Kusi, J.Y.; Empl, F.; Müller, R.; Pelz, S.; Poetsch, J.; Sailer, G.; Kirchhof, R.; Derkyi, N.S.A.; Attiogbe, F. An Evaluation of Biogas Potential of Cassava, Yam and Plantain Peel Mixtures Using Theoretical Models and Hohenheim Biogas Yield Test-Based Experiments. Energies 2025, 18, 947. [Google Scholar] [CrossRef]
- Orangun, A.; Kaur, H.; Kommalapati, R.R. Batch Anaerobic Co-Digestion and Biochemical Methane Potential Analysis of Goat Manure and Food Waste. Energies 2021, 14, 1952. [Google Scholar] [CrossRef]
- Kusi, J.Y.; Empl, F.; Müller, R.; Pelz, S.; Poetsch, J.; Sailer, G.; Kirchhof, R.; Agyemang Derkyi, N.S.; Attiogbe, F.; Siabi, S.E. Evaluation of Energetic Potential of Slaughterhouse Waste and Its Press Water Obtained by Pressure-Induced Separation via Anaerobic Digestion. Energies 2024, 17, 5522. [Google Scholar] [CrossRef]
- López-Aguilar, H.A.; Morales-Durán, B.; Quiroz-Cardoza, D.; Pérez-Hernández, A. Lag Phase in the Anaerobic Co-Digestion of Sargassum spp. and Organic Domestic Waste. Energies 2023, 16, 5462. [Google Scholar] [CrossRef]
- Bauta, J.; Calbrix, E.; Capblancq, S.; Cecutti, C.; Peydecastaing, J.; Delgado Raynaud, C.; Rouilly, A.; Simon, V.; Vaca-Medina, G.; Vandenbossche, V.; et al. Global Chemical Characterization of Sargassum spp. Seaweeds from Different Locations on Caribbean Islands: A Screening of Organic Compounds and Heavy Metals Contents. Phycology 2024, 4, 190–212. [Google Scholar] [CrossRef]
- Milledge, J.J.; Maneein, S.; López, E.A.; Bartlett, D. Sargassum Inundations in Turks and Caicos: Methane Potential and Proximate, Ultimate, Lipid, Amino Acid, Metal and Metalloid Analyses. Energies 2020, 13, 1523. [Google Scholar] [CrossRef]
- Hatt, D.C.; Bally, N.K.; Iporac, L.A.R.; Olszak, S.; Campbell, J.E.; Collado-Vides, L. Comprehensive Analysis of Biomass, Nutrient, and Heavy Metal Contributions of Pelagic Sargassum Species (Phaeophyceae) Inundations in South Florida. Phycology 2024, 4, 235–255. [Google Scholar] [CrossRef]
- Li, W.; Gupta, R.; Zhang, Z.; Cao, L.; Li, Y.; Show, P.L.; Gupta, V.K.; Kumar, S.; Lin, K.Y.A.; Varjani, S.; et al. A Review of High-Solid Anaerobic Digestion (HSAD): From Transport Phenomena to Process Design. Renew. Sustain. Energy Rev. 2023, 180, 113305. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry Anaerobic Digestion of Organic Waste: A Review of Operational Parameters and Their Impact on Process Performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.K.; Mustafa, M.A.; Ahmed, H.S.; Jassim Mohammed, A.; Ghazy, H.; Shakir, M.N.; Lawas, A.M.; Khudhur Mohammed, S.; Idan, A.H.; Mahmoud, Z.H.; et al. Biogas: Production, Properties, Applications, Economic and Challenges: A Review. Results Chem. 2024, 7, 101549. [Google Scholar] [CrossRef]
- Salgado-Hernández, E.; Ortiz-Ceballos, Á.I.; Martínez-Hernández, S.; Rosas-Mendoza, E.S.; Dorantes-Acosta, A.E.; Alvarado-Vallejo, A.; Alvarado-Lassman, A. Methane Production of Sargassum spp. Biomass from the Mexican Caribbean: Solid–Liquid Separation and Component Distribution. Int. J. Envrion. Res. Public Health 2023, 20, 219. [Google Scholar] [CrossRef]
- Salgado-Hernández, E.; Ortiz-Ceballos, Á.I.; Alvarado-Lassman, A.; Martínez-Hernández, S.; Rosas-Mendoza, E.S.; Velázquez-Fernández, J.B.; Dorantes-Acosta, A.E. Energy-Saving Pretreatments Affect Pelagic Sargassum Composition and DNA Metabarcoding Reveals the Microbial Community Involved in Methane Yield. PLoS ONE 2023, 18, e0289972. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.; Rossignolo, J.A.; Gavioli, L.M.; Sposito, C.C.A.; Tonin, F.G.; Veras, M.M.; Moraes, M.J.B.; de Lyra, G.P. Life Cycle Assessment Applied to End-of-Life Scenarios of Sargassum spp. for Application in Civil Construction. Sustainability 2023, 15, 6254. [Google Scholar] [CrossRef]
- Canul-Ku, L.A.; Casanova-Lugo, F.; Aguilar-Urquizo, E.; Valdivieso-Pérez, I.; Arcos-Álvarez, D.; Canul-Solís, J.; Castillo-Sánchez, L.; Chay-Canul, A.; Dzib-Castillo, B.; Piñeiro-Vázquez, A. In Vitro Fermentation Characteristics of Pelagic Sargassum for Inclusion in Integral Diets for Ruminants. Fermentation 2025, 11, 390. [Google Scholar] [CrossRef]
- Rivera-Hernández, Y.; Hernández-Eugenio, G.; Balagurusamy, N.; Espinosa-Solares, T. Sargassum-Pig Manure Co-Digestion: An Alternative for Bioenergy Production and Treating a Polluting Coastal Waste. Renew. Energy 2022, 199, 1336–1344. [Google Scholar] [CrossRef]
- Hütter, M.; Sailer, G.; Hülsemann, B.; Müller, J.; Poetsch, J. Impact of Thermo-Mechanical Pretreatment of Sargassum muticum on Anaerobic Co-Digestion with Wheat Straw. Fermentation 2023, 9, 820. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).