Abstract
In this study, a rough-surfaced LiFePO4 (RS-LFP) cathode material with a well-defined porous architecture was successfully synthesized via a scalable, template-assisted spray drying method. The resulting RS-LFP exhibited a high specific surface area of 41.2 m2 g−1, significantly enhancing electrode–electrolyte contact. This tailored microstructure, combined with an in-situ-formed carbon network, reduced the charge-transfer resistance and facilitated efficient ion/electron transport. Consequently, the RS-LFP demonstrated outstanding electrochemical performance, including a high initial capacity of ~140 mAh g−1 at 0.2 C, excellent cycling stability with over 95% capacity retention after 30 cycles, and superior rate capability. The RS-LFP also exhibited a remarkable capacity recovery of ~99% when the current returned to 0.2 C. These findings highlight that engineering porous architectures through template-assisted spray drying is a promising and scalable strategy for developing high-performance phosphate-based cathodes for advanced energy storage applications.
1. Introduction
Lithium-ion batteries (LIBs) have become essential for modern energy storage applications, particularly in electric vehicles (EVs). For the past decade, nickel–cobalt–manganese (NCM) cathodes dominated the EV battery market due to their superior energy density [1,2]. However, recent advances have significantly narrowed this energy density gap while lithium iron phosphate (LiFePO4) offers superior thermal stability, safety, cost-effectiveness (15–30% cheaper), and environmental sustainability by eliminating toxic and expensive heavy metals such as cobalt and nickel [1,3,4,5,6]. This paradigm shift has resulted in LiFePO4 accounting for over 40% of global EV battery production by 2024. LiFePO4 exhibits a long cycle life owing to its robust olivine crystal structure that maintains its integrity during repeated charge–discharge cycles, and possesses a good theoretical specific capacity of 170 mAh g−1 [7,8,9]. Despite its compelling advantages, the widespread adoption of LiFePO4 is hindered by its intrinsically low electronic conductivity and one-dimensional Li-ion diffusion kinetics [10,11,12]. While numerous advanced strategies, such as elemental doping and complex nanostructuring, have been reported to address these issues, the key challenge for commercial viability lies in developing a synthesis route that is not only effective but also scalable, cost-efficient, and reproducible [13,14,15,16,17]. Therefore, the field has increasingly recognized that focus should shift from merely achieving the highest performance metrics to establishing practical, industrially viable pathways for mass production. In this context, recent innovations in LiFePO4 cathode engineering have demonstrated complementary approaches to performance enhancement across multiple length scales. Manufacturing process optimization through dry electrode technology has achieved unprecedented material loading and cycle stability while eliminating solvent-dependent processing steps [18]. Simultaneously, sophisticated interfacial engineering using coordination chemistry strategies has unlocked near-theoretical LiFePO4 capacities through carefully designed binder–cathode interactions [19]. Additionally, heteroatom-doped carbon surface coatings—particularly nitrogen and fluorine co-doping—have shown substantial improvements in charge-transfer kinetics and performance under demanding operational conditions [20]. These multifaceted recent advances underscore the importance of optimizing LiFePO4 at multiple hierarchical levels: synthesis methodology, interfacial chemistry, and surface functionalization. In this context, the spray drying method has emerged as a promising candidate. It is a continuous and industrially scalable process for producing spherical secondary particles with a uniform size distribution, which is highly beneficial for improving the tap density and slurry rheology of electrodes [21,22,23,24]. However, the success of this method is critically dependent on the design of the homogeneous aqueous precursor solution. To achieve this, Fe(NO3)3·9H2O and NH4H2PO4 were specifically selected as the iron and phosphate sources due to their high aqueous solubility compared with other common precursors, ensuring a clear and stable solution for atomization [25,26,27]. Our approach utilizes a precursor system in which citric acid plays a dual role: it acts not only as an in situ carbon source for the conductive network, but also as a crucial chelating agent. This chelating function prevents the premature precipitation of metal ions, ensuring molecular-level mixing and leading to a highly uniform final product [28,29,30,31]. Furthermore, this strategy, in which an organic acid acts as the sacrificial material, streamlines the overall process. This avoids the complex, multistep procedures required to remove a hard template in conventional template-assisted methods, offering a more efficient and direct route to synthesize the final composite material [30,32,33,34]. Therefore, in this study, we synthesized a rough-surface LiFePO4 composite via a scalable spray drying route using a carefully designed cost-effective precursor system. We then systematically compared its physicochemical and electrochemical properties with those of a commercial LiFePO4 cathode material to validate the efficacy and practical potential of this synthetic strategy.
2. Materials and Methods
2.1. Material Characterization
The morphologies and elemental distributions of the synthesized rough-surfaced LiFePO4 (RS-LFP) and commercial LiFePO4 (C-LFP) powders were investigated by field-emission scanning electron microscopy (FE-SEM) and energy-dispersive X-ray spectroscopy (EDS) using an S-4800 instrument (Hitachi, Tokyo, Japan). The phase purity and crystal structure of the samples were determined by X-ray diffraction (XRD) using a MiniFlex 600 diffractometer (Rigaku, Tokyo, Japan). The porosity and specific surface area of the RS-LFP powder were determined by nitrogen adsorption–desorption isotherm measurements at 77 K using a BELSORP-max II analyzer (MicrotracBEL, Osaka, Japan). The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method, and the pore size distribution was analyzed using the Barrett–Joyner–Halenda (BJH) method. For the analysis of micropore-width distribution, the Horváth–Kawazoe (HK) method was additionally applied using the same nitrogen adsorption data. The detailed analyses, including XRD, SEM, BET, and other advanced characterizations, were performed at the central laboratory of Hankyong National University. The static contact-angle measurements were carried out using a Phoenix 10 instrument (SEO, Suwon, Republic of Korea), and deionized (DI) water was used as the probe liquid.
2.2. Electrochemical Characterization
To evaluate their electrochemical properties, coin-type half-cells were assembled using the prepared RS-LFP and commercial LFP (C-LFP) as working electrodes. The galvanostatic charge–discharge profiles of the cells were recorded using a MACCOR battery testing system (USA) at a constant current density of 0.2 C. These tests were performed at 25 °C within a voltage window of 2.5 to 4.0 V. To investigate the redox reactions of the electrodes, cyclic voltammetry (CV) was conducted between 2.5 and 4.0 V at a scan rate of 1.0 mV s−1. Electrochemical impedance spectroscopy (EIS) measurements were also performed. CV and EIS analyses were performed using a BioLogic VSP-300 potentiostat (BioLogic, Seyssinet-Pariset, France).
2.3. Fabrication of the RS-LFP Composite
A rough-surfaced LiFePO4 composite, hereafter referred to as RS-LFP, was successfully synthesized via spray drying. First, a homogeneous precursor solution was prepared. Stoichiometric amounts of lithium carbonate (Li2CO3, Duksan, Ansan, Republic of Korea), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, Duksan), ammonium phosphate monobasic (NH4H2PO4, Duksan), and anhydrous citric acid (C3H4(OH)(COOH)3, Duksan) were dissolved in 250 mL of DI water to maintain a molar ratio of Li:Fe:P:acid of 1:1:1:1. The final concentrations were 25.36 mM for Li2CO3 and 50.72 mM for the other reagents. The resulting mixture was stirred at 400 RPM overnight at room temperature to ensure maximum dissolution of the reagents and homogeneous distribution. The final precursor was a mustard yellow solution.
After continuous stirring at 400 RPM to prevent precipitation, the precursor solution was atomized using a laboratory-scale spray dryer (ADL311S, Yamato Scientific, Tokyo, Japan). The operational parameters were set as follows: inlet temperature of 160 °C, feeding pump rate of 4.5 mL min−1, and atomizing air pressure of 0.15 MPa. During the drying process, the outlet temperature was observed to be in the range of 93–103 °C. Finally, the as-sprayed precursor powder was collected and subjected to a two-step heat treatment. It was first dried in a muffle furnace at 120 °C for 5 h to remove any residual moisture. Subsequently, the dried powder was calcined in a box furnace under a flowing nitrogen (N2) atmosphere. The furnace was heated from room temperature (approx. 25 °C) to 700 °C over 1 h (a ramp rate of approx. 11.25 °C min−1), held at this temperature for 12 h, and then allowed to cool naturally to room temperature. The final product was the RS-LFP composite powder.
2.4. Preparation of the Cathodes and Test Cell
The working electrodes were fabricated by casting the slurry onto an Al foil. The slurry was prepared by mixing the active material (RS-LFP), a conductive agent (Super-P carbon black), and a binder (polyvinylidene fluoride, PVDF) in a weight ratio of 8:1:1 in N-methyl-2-pyrrolidone (NMP). The resulting homogeneous slurry was then cast onto the Al foil with a uniform thickness of 90 μm using a doctor blade. The prepared electrodes were subsequently dried in a muffle furnace at 60 °C for 12 h to completely evaporate the solvent.
For electrochemical evaluation, CR2032-type coin cells were assembled in an Ar-filled glove box. Cells were constructed using the prepared RS-LFP electrode as the cathode, Li metal as the anode, and a polyethylene (PE) film as the separator. The electrolyte used was 1 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI, Acros Organics, Geel, Belgium) dissolved in a mixture of 1,2-dimethoxyethane (DME) and 1,3-dioxolane (DOL) (1:1, v/v) with a 1 wt% lithium nitrate (LiNO3) additive.
3. Results and Discussion
The morphology and elemental composition of the synthesized rough-surfaced LiFePO4 (RS-LFP) and commercial LiFePO4 (C-LFP) were systematically investigated by FE-SEM and EDS, as presented in Figure 1. The C-LFP powder, observed at 15.0 k magnification (Figure 1a), consisted of discrete, submicron primary particles. This observation is consistent with the manufacturer’s description of a pulverized powder with a smooth surface morphology. In contrast, the RS-LFP exhibited a distinct hierarchical structure. The low-magnification image (Figure 1c, 1.0 k) revealed large secondary agglomerates of approximately 60 µm in diameter. These microspheres were composed of smaller, spherical primary particles (~2–4 µm), as shown at higher magnification (Figure 1b, 15.0 k). This morphology is consistent with the droplet-to-particle transformation mechanism inherent to spray drying, and the formation of such large secondary agglomerates can be attributed to the increased viscosity of the precursor solution containing citric acid, which promotes droplet coalescence during atomization [32,35,36,37]. A key feature of the RS-LFP was its surface texture. As shown in Figure 1b, the primary particles presented a distinctly roughened shell with protrusions and shallow pits rather than a smooth surface. This surface texture was attributed to the citric acid acting as a sacrificial template [38,39]. After post-annealing the precursor, the thermal decomposition of citric acid created a porous carbon matrix that served as a soft template and generated a mesoporous architecture [40,41]. The resulting pore architecture enhanced electrolyte wettability and reduced Li+ transport distances within the secondary spheres, thereby justifying the designation of our material as a “rough-surfaced” LFP [42]. The EDS elemental mapping (Figure 1d) indicated the successful synthesis of a homogeneous composite. The P, O, and Fe maps showed a uniform distribution across the entire secondary particle, with the P signal appearing more distinct owing to its characteristic X-ray response under the present conditions. However, the C signal appeared diffuse and less distinct within the core of the particles. This could be partly attributed to the decomposition of the citric acid template at high temperatures, which generates gaseous products (e.g., CO, CO2). The pressure from these evolving gases may lead to a final carbon content that is lower than that stoichiometrically expected. For instance, a study by Feng et al. using a similar molar ratio reported a carbon content of 4.3%, which was asserted to be sufficient to enhance the electronic conductivity [32]. More significantly, it is a well-known limitation that the sensitivity of EDS to light elements like carbon is significantly lower, especially when present as an amorphous phase within a matrix of heavier elements like Fe and P. Therefore, although not clearly resolved by EDS, the carbon derived from citric acid was considered to be finely distributed within the porous matrix, providing the necessary electronic pathways for enhanced electrochemical performance [43].
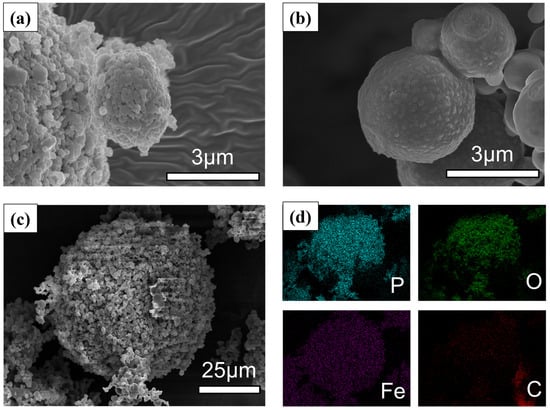
Figure 1.
SEM morphology and EDS elemental analysis of LFP samples. SEM images of (a) C-LFP and (b) RS-LFP. (c) Low-magnification SEM image of RS-LFP and (d) corresponding EDS elemental mapping of the distribution of P, O, Fe, C.
Figure 2a shows the Fourier-transform infrared (FT-IR) spectra of the RS-LFP (red line) and C-LFP (black line) samples synthesized via spray drying. LiFePO4 crystallizes in an orthorhombic olivine structure, where layers composed of LiO6 and FeO6 octahedra are formed in the bc-plane [44,45]. These adjacent layers are interconnected by PO43− polyanions, creating one-dimensional channels for lithium-ion movement along the a-axis [45]. Through heat treatment of the spray-dried precursor, the RS-LFP sample achieved a notable degree of crystallinity, comparable to that of hydrothermally synthesized samples commonly reported in the literature [46]. The observed vibrational bands, located primarily in the 372–1139 cm−1 range, correspond to the internal vibration modes of the PO43− group, which are derived from its four fundamental modes (ν1–ν4) [44,46]. Similar to the C-LFP, the regions corresponding to the stretching and bending vibrations in the FT-IR spectrum of the RS-LFP were distinctly separated from each other. It is well-established that the fundamental vibration modes of the PO43− polyanion split into multiple components; this splitting is attributed to a correlation effect that arises from the coupling with Fe-O units within the crystal structure. The internal vibrational modes originating from the phosphate anions of the RS-LFP sample were clearly observed in the high-wavenumber region (above 500 cm−1). The absorption peaks at 1137, 1095, and 1048 cm−1 were attributed to the antisymmetric stretching vibrations (ν3) of the P–O bonds, whereas the peak at 966 cm−1 mainly corresponded to the symmetric stretching vibration (ν1) of the P–O bond. At lower wavenumbers, the absorption peaks around 636, 577, and 549 cm−1 originated from the antisymmetric bending vibrations (ν4) of the O–P–O bonds, with a minor contribution from phosphorus-related lattice vibrations [47]. The absorption peak at 502 cm−1 corresponded to the symmetric bending vibration (ν2) of the O–P–O bond, and according to Christopher and Roger, this peak is also associated with the translational vibrations of Li+ ions adjacent to neighboring oxygen atoms in the olivine LiFePO4 structure [48]. Notably, the characteristic peak for lithium phosphate (Li3PO4) impurity, which typically appears as a single sharp band around 1000 cm−1, was not observed in the spectrum of the RS-LFP sample [49].
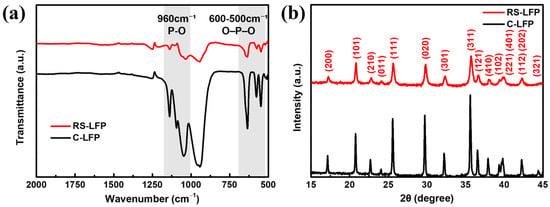
Figure 2.
(a) Fourier transform infrared (FT-IR) spectra and (b) XRD patterns of RS-LFP and C- LFP.
Furthermore, consistent with previous findings for samples containing ~5 wt% of carbon prepared via heat treatment at 700 °C under an inert atmosphere, no additional spectral adsorption lines were detected [50]. Salah et al. stated that such a small amount of carbon is too low to be detected using FTIR spectroscopy. This finding implies that although the incorporation of carbon using citric acid may influence the lithium environment, the interaction of carbon atoms cannot be clearly detected in the FT-IR spectrum of RS-LFP [51].
The crystal structure of the synthesized RS-LFP was investigated using XRD and compared with that of the C-LFP. As shown in Figure 2b, both the C-LFP (black line) and RS-LFP (red line) exhibited distinct characteristic diffraction peaks of the orthorhombic olivine-structured LiFePO4 [52]. All the intense peaks in the diffraction pattern confirmed the single-phase formation of the olivine structure, and the pattern was well indexed to an orthorhombic system with the Pnma space group (JCPDS Card No. 40-1499), and no impurity phases were detected [53]. The reflections at 2θ = 17.24°, 20.82°, 22.78°, 23.48°, 25.62°, 29.82°, 32.36°, 35.70°, 36.60°, 38.02°, 39.36°, 39.86°, 42.28°, and 44.68° correspond to the (200), (101), (210), (011), (111), (020), (301), (311), (121), (410), (102), (221)/(401), (112)/(202), and (321) planes, respectively [45,46,54]. Compared with the C-LFP, the diffraction peaks of the RS-LFP were slightly shifted towards higher 2θ values, a progressive shift characteristic of samples prepared with citric acid [54]. This well-defined olivine structure is critical for the electrochemical performance. In LiFePO4 crystals, Li+ diffusion during charge–discharge mainly proceeds along the [010] direction (b-axis), and charge transfer predominantly occurs on the (010) facet. This is because the [010] direction is free from obstacles, whereas diffusion along [100] (a-axis) and [001] (c-axis) is hindered by other ions such as Fe2+/3+, P5+, and O2− [11,45,52,55,56].
Notably, no peaks corresponding to impurity components were observed in the RS-LFP pattern. Impurity phases such as Li3PO4, characterized by intense peaks at 2θ = 20–25°, and Fe3(PO4)2·8H2O, prominent at 2θ = 18–20°, were absent [52,57]. Furthermore, while LiFePO4 synthesized above 320 °C typically forms an ordered olivine structure, the inhomogeneity of precursors during synthesis below 700 °C can lead to the formation of stable Fe3+ compounds like Fe2O3 or Li3Fe2(PO4)3 [58]. The absence of these impurity peaks indicates that citric acid acted effectively as a complexing agent and that the precursors were thoroughly homogenized during stirring before spray drying. The citric acid served not only as a complexing agent but also as a carbon source, improving composite conductivity while hindering the excessive growth of LiFePO4 particles [59]. However, no distinct carbon peak was observed in the XRD pattern, indicating that the amount of carbon was below the XRD detection limit or that it was primarily amorphous [54].
Figure 3a displays the N2 adsorption–desorption isotherms of the synthesized RS-LFP. According to the IUPAC classification, the isotherm exhibited a typical Type IV(a) behavior with a distinct H3-type hysteresis loop in the relative pressure range of 0.5–1.0. This Type IV(a) isotherm with an H3 hysteresis is typically observed in spray-dried materials, where rapid solvent evaporation and particle agglomeration create a slit-like mesoporous structure between loosely packed plate-like particles. This porous architecture is highly beneficial for efficient electrolyte infiltration and shortened Li+ diffusion pathways. The hysteresis itself can be attributed to capillary condensation within these slit-like mesopores, providing interconnected channels favorable for comparison (13 ± 2 m2 g−1) and represents an approximately 3–6-fold enhancement over typical commercial LiFePO4 materials (7–15 m2 g−1), providing abundant electrochemically active sites for Li+ insertion/extraction reactions [60,61,62]. The applicability of the BET model was confirmed by the excellent linear correlation (R2 > 0.99) in the relative pressure range of 0.05–0.20 [63]. The corresponding pore size distribution derived from the desorption branch of the isotherm using the BJH model is shown in Figure 3b. The distribution exhibited a prominent peak centered in the 5–20 nm range, with a maximum at 12.24 nm (inset of Figure 3b), confirming that the porosity was dominated by mesopores.
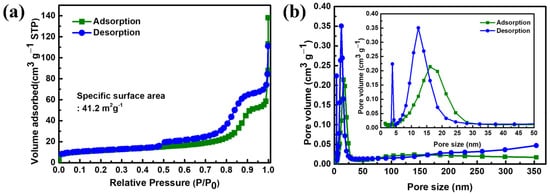
Figure 3.
(a) N2 adsorption/desorption isotherm and (b) BJH pore size distribution (inset: mesopores range) of the RS-LFP and C-LFP.
To further investigate the pore structure, the Horváth–Kawazoe (HK) method was employed to analyze the microporous range (<2 nm). The resulting HK plot revealed a sharp peak at a pore width of 0.74 nm, indicating the presence of distinct and uniform micropores. The coexistence of these micropores with the previously discussed mesopores confirmed that RS-LFP possessed a hierarchical porous architecture. This hierarchical architecture is highly advantageous; while a structure composed solely of micropores could restrict electrolyte access, the well-developed mesopores in the RS-LFP could act as efficient “highways” for rapid Li+ transport. This allowed the micropores to serve their primary function, providing numerous additional active sites for Li storage, thereby enhancing the overall Li storage capacity [64].
The calculated total pore volume of 0.09767 cm3 g−1 is particularly noteworthy when compared with those of typical LiFePO4 materials (typically below 0.05 cm3 g−1), further verifying the well-developed porous network [63]. Moreover, the average pore diameter of 13.675 nm falls within the optimal range for lithium-ion transport, as pores smaller than 2 nm may restrict electrolyte access, whereas larger pores (>50 nm) provide a limited contribution to the electrochemical activity [65]. This synergy of properties (high surface area, optimal pore size, and interconnected architecture) is critical for the performance. As evidenced by the H3-type hysteresis, the slit-like geometry provides multiple pathways for stress relaxation during the ~6.8% volume expansion of LiFePO4 during the lithiation/delithiation processes. This ability to effectively alleviate internal stress is the key to maintaining structural integrity and achieving superior rate capability and long-term cycling stability [66].
Static contact angle analysis was performed to evaluate the surface properties and wettability. The RS-LFP electrode exhibited an average contact angle of 56.13°, a significant reduction of 12.44° from 68.57° observed for the conventional C-LFP electrode (Figure 4). This statistically significant decrease indicated a marked improvement in wettability, suggesting a more favorable interaction with the electrolyte. This enhancement was attributed to a combination of distinct physical and chemical factors.
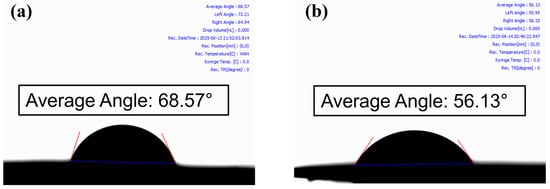
Figure 4.
Results of contact angle measurement for (a) C-LFP and (b) RS-LFP cathode samples.
The primary physical origin of the enhanced wettability was the unique porous architecture of the RS-LFP material. This structure, formed during the spray-drying process, provided a significantly higher BET surface area compared with that of the C-LFP, thereby maximizing the physical contact area for the electrolyte. In addition to the increased surface area, the internal architecture offered a crucial advantage for ion transport. As observed from the SEM analysis (Figure 1b), the RS-LFP consisted of uniform spherical particles. This morphology promoted a more ordered packing structure, which was expected to form a highly interconnected pore network. This network provided fewer tortuous pathways for Li-ion diffusion, thus lowering the overall tortuosity of the electrode. This structural advantage was strongly supported by the electrochemical data, where the superior rate capability and capacity retention of the RS-LFP electrode) were consistent with the reduced ion transport resistance, which is a direct consequence of low tortuosity [67,68,69,70]. Furthermore, this well-defined and interconnected porous network can mitigate the detrimental effects of a polymer binder (e.g., PVDF), ensuring that sufficient channels remain available for electrolyte impregnation, thereby maximizing the electrochemically active surface area [71,72].
Chemically, the improved wettability can be partly attributed to the potential surface modification resulting from the use of citric acid as a precursor. It is hypothesized that a thin carbonaceous layer or new surface functionalities may have formed during synthesis. Such a modification would alter the native surface chemistry of the LFP, likely increasing its surface free energy. A higher surface energy creates a more energetically favorable interface for the electrolyte, thereby lowering the solid–liquid interfacial tension (γsl) and promoting spontaneous wetting, as reflected in the lower contact angle measured in Figure 4 [73,74,75].
EIS was conducted to compare the interfacial characteristics of the C-LFP and RS-LFP electrodes before and after cycling. Figure 5 shows the Nyquist plots for the (a) C-LFP and (b) RS-LFP electrodes after 30 cycles in the fully discharged state, which were measured in the frequency range of 100 mHz to 1 MHz. Both plots exhibited fundamentally similar characteristics, as they were composed of two partially overlapping semicircles in the high-to-medium-frequency region and a straight line in the low-frequency region. The high-frequency intercept with the real axis (Z’) corresponds to the bulk ohmic resistance of the electrolyte (Rs). The two semicircles correspond to the resistive and capacitive responses at the electrode/electrolyte interface, whereas the low-frequency line, known as the Warburg impedance (Zw), is associated with the solid-state diffusion of lithium ions within the bulk electrode. All these features are represented in the equivalent circuit model shown in Figure 5c, which was used to fit the experimental data. The first semicircle in the high-frequency range indicates the interfacial resistance (Rint), which includes contributions from the particle-to-particle contact, particle-to-current collector contact, and surface films. The second semicircle in the medium-frequency range represents the charge transfer resistance (Rct) at the interface between the electrolyte and active material, and its diameter corresponds to the Rct value. Although the first semicircle is often associated with the solid electrolyte interphase (SEI), its magnitude in the present cathodes (~77–563 Ω) was significantly larger than that of a typical SEI layer alone (~10–50 Ω) [76,77]. This suggests that the semicircle represents the combined interfacial resistance (Rint), which is dominated by factors such as the particle and contact resistances in addition to the SEI. The impedance behavior of each electrode could be analyzed further based on this information [11,78].
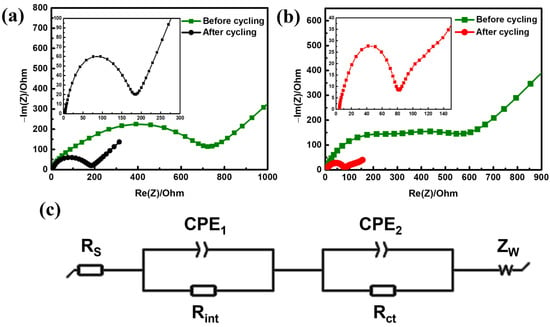
Figure 5.
Electrochemical impedance spectra (EIS) of (a) C-LFP and (b) RS-LFP (inset plot: spectra after 30 cycling); (c) equivalent circuit for fitting experimental EIS data.
For the C-LFP electrode (Figure 5a), the first semicircle for Rint was dominant, whereas the second semicircle for Rct was not well defined. Before cycling, the Rint and Rct values were 563 and 114 Ω, respectively. After 30 cycles, these values decreased significantly to 140 and 34 Ω, though Rint remained the larger contributor to the overall impedance. In contrast, the RS-LFP electrode (Figure 5b) displayed a distinct second semicircle corresponding to Rct. Before cycling, the Rs, Rint, and Rct values were 4.4, 271.1, and 242.9 Ω, respectively. Unlike C-LFP, the initial Rint and Rct values were comparable. This high initial resistance was likely due to the incomplete wetting of the rough and porous surface by the electrolyte. However, after an initial activation process of over 30 cycles, a stable interface was formed, and the electrolyte wettability improved. This resulted in a remarkable decrease in resistance, with Rs, Rint, and Rct values dropping to 4.1, 77, and 42 Ω, respectively, which are substantially lower than those of the cycled C-LFP.
For both materials, the interfacial resistance was initially greater than the charge transfer resistance (Rint > Rct), indicating that interfacial processes, rather than the electrochemical charge transfer reaction, are the rate-limiting steps in the electrode performance. The dominant interfacial resistance in the RS-LFP can be attributed to its unique morphology. The numerous particle–particle interfaces within the secondary agglomerates formed during the spray-drying synthesis create additional resistance pathways for electron transport. However, despite this inherent structural limitation, the ionic transport efficiency was significantly enhanced during the initial cycling. Further improvements in Rint can potentially be achieved by optimizing the network between the active material, conductive agent, and binder and by applying a more uniform carbon coating [79,80,81].
The CV curves of the C-LFP and RS-LFP electrodes prepared in this study were measured at a scan rate of 1.0 mV/s, as shown in Figure 6a. Both electrodes exhibited distinct oxidation and reduction peaks corresponding to the Li+ intercalation and deintercalation reactions, indicating the inherent quasi-reversible electrochemical behavior of LiFePO4. The C-LFP electrode exhibited clear oxidation/reduction peaks at 3.797 V (1.368 mA) and 3.078 V (−1.252 mA). In contrast, the RS-LFP electrode exhibited lower peak currents and broader peaks, with the scan reversing before fully reaching the potential limits (2.5–4.0 V). Furthermore, in the non-faradaic region outside the redox peaks, the RS-LFP electrode exhibited a slightly sloped and fluctuating baseline, unlike the flat baseline observed for the C-LFP electrode. These results collectively suggest that the RS-LFP electrode still exhibited relatively high internal resistance (polarization), indicating that electron and ion transport remained partially hindered despite the improved conductive network and porous architecture [82,83].
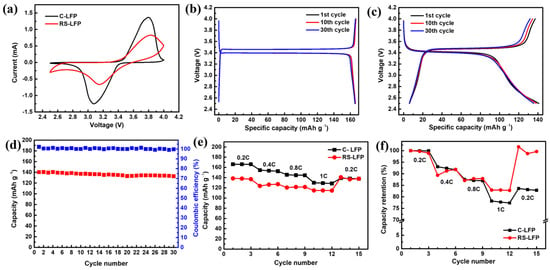
Figure 6.
(a) Cyclic voltammetry (CV) curves at and (b) galvanostatic charge and discharge (GCD) curves of (c) C-LFP and (d) RS-LFP at the 1st, 10th, and 30th cycles at 0.2 C. (d) Cycling performance and coulombic efficiency, (e) rate performance at different current rates from 0.2 to 1 C, and (f) discharge capacity retention (%) at different C-rates of C-LFP and RS-LFP.
To more clearly evaluate the ion-transport behavior, the apparent diffusion coefficient (Dapp) was calculated from the anodic peak current using the Randles–Ševčík equation. The calculated diffusion coefficient for the C-LFP electrode was 2.58 × 10−8 cm2 s−1, approximately 2.7 times higher than that of the RS-LFP electrode (9.45 × 10−9 cm2 s−1). At first glance, this seems to indicate restricted macroscopic Li+ transport in the RS-LFP electrode. However, it should be emphasized that the CV-derived Dapp primarily reflects surface-controlled kinetics rather than bulk diffusion. The mesoporous and roughened morphology of RS-LFP increases the electrochemical interfacial area, which enhances surface polarization and broadens the CV peaks, thereby lowering the apparent diffusion coefficient. This behavior is also consistent with the presence of a thin in-situ carbon layer, which improves the conductivity compared with bare LiFePO4 but remains less effective than the optimized carbon network typically found in commercial materials. Importantly, within the secondary particles, the hierarchical porous architecture of RS-LFP provides shortened Li+ diffusion pathways and efficient electrolyte penetration, which support the favorable cycling and rate performance observed in the GCD measurements. Therefore, the lower Dapp value does not contradict the structural advantages of RS-LFP but rather highlights the different kinetic regimes probed by CV and GCD [84].
To further verify the behavior observed in the CV analysis, galvanostatic charge–discharge (GCD) tests were performed at 0.2 C, and the results for the 1st, 10th, and 30th cycles are presented in Figure 6b,c. The C-LFP electrode exhibited a distinct and stable voltage plateau around 3.4 V and a high initial discharge capacity of approximately 160 mAh g−1, demonstrating excellent stability with negligible capacity decay after 30 cycles. The RS-LFP electrode delivered an initial discharge capacity of approximately 140 mAh g−1 and retained approximately 135 mAh g−1 after 30 cycles, indicating good overall cycling stability. This quantitatively demonstrates that both samples underwent a highly stable one-step phase transition reaction during the Li+ intercalation/deintercalation process. Although the RS-LFP electrode exhibited a slightly higher polarization than the C-LFP electrode, the difference was small, suggesting that the RS-LFP electrode achieved voltage stability comparable to that of a commercial electrode [85]. In conclusion, the RS-LFP electrode exhibited an electrochemical behavior comparable to that of the C-LFP electrode.
Figure 6d shows the charge–discharge capacity and Coulombic efficiency of the prepared RS-LFP electrode over 30 cycles at 0.2 C. The initial discharge capacity was approximately 140 mAh g−1, and the electrode retained over 95% of its capacity after 30 cycles, indicating excellent cycling stability without significant capacity decay. A slight capacity increase was observed during the first few cycles, which was attributed to the gradual activation process in which electrolyte penetration into the porous structure increased the electrochemically active surface area. This excellent stability was attributed to two main factors. First, the hierarchical porous structure formed within the microsized secondary particles simultaneously provides short Li+ diffusion paths for lithium-ion transport and effective channels for electrolyte penetration, enabling excellent cycling characteristics [86]. Second, the strong P–O covalent bonds within the PO4 tetrahedral units of the olivine structure maintain the structural integrity, ensuring long-term cycling stability [87]. Additionally, the Coulombic efficiency remained above 99% throughout the cycles, which is consistent with the stable electrochemical behavior observed in the preceding CV and GCD analyses.
Figure 6e shows the change in discharge capacity for the C-LFP and the prepared RS-LFP electrodes cycled three times at various C-rates (0.2, 0.4, 0.8, 1 C) and then returned to 0.2 C. Figure 6f illustrates the capacity retention (%) at each current density, setting the first-cycle capacity as the 100% reference. Both electrodes exhibited the typical behavior of decreasing capacity with increasing C-rate, but C-LFP maintained a higher capacity across all rates. At the initial 0.2 C rate, the RS-LFP electrode exhibited a capacity approximately 20 mAh g−1 lower than that of C-LFP. This lower initial capacity was attributed to the residual impurities and lower crystallinity resulting from the simple spray-drying process, which compromised the interfacial contact with the electrolyte and led to suboptimal electron/ion transport pathways. As the current density increased, the capacities of both samples decreased; however, the rate of performance degradation for RS-LFP was relatively more gradual. Notably, when the rate was returned from 1 C to 0.2 C, the C-LFP recovered to approximately 140 mAh g−1 (recovery: 80–85%), whereas the RS-LFP restored its initial capacity (~140 mAh g−1, recovery: 98–100%). The excellent capacity recovery stemmed from two key structural characteristics. First, the conductive carbon is believed to reduce the internal resistance by providing an efficient electronic pathway, thereby suppressing polarization and maintaining voltage efficiency during high-rate cycling. Second, the porous hierarchical architecture shortens the Li+ diffusion path, ensuring effective ion transport even at high current densities.
4. Conclusions
In this study, a LiFePO4 (LFP) cathode material with well-defined porous architecture (RS-LFP) was successfully synthesized using a template-assisted spray-drying method. BET analysis revealed that the RS-LFP exhibited a high specific surface area of 41.2 m2 g−1, which was significantly larger than that of the C-LFP. This structural feature enhanced the electrode–electrolyte contact area, thereby facilitating more efficient lithium-ion transport. This tailored microstructure led to improved electrochemical performance. Although the initial capacity of RS-LFP (~140 mAh g−1 at 0.2 C) is slightly lower than that of commercial C-LFP, this value remains high given that RS-LFP was produced through a much simpler spray-drying process without optimized high-temperature calcination or carbon-coating steps. This simplified one-pot route not only reduces processing time and energy consumption but also offers clear advantages in terms of scalability and overall techno-economic feasibility. Additionally, its enlarged surface area, hierarchical porosity, and uniformly distributed in-situ carbon collectively contribute to fast Li+ transport and stable cycling behavior. EIS analysis confirmed that the RS-LFP maintained lower interfacial and charge-transfer resistances than the C-LFP even after cycling. This improvement was attributed to the enhanced electronic conductivity provided by the in-situ formed carbon network. In galvanostatic charge–discharge tests, the RS-LFP delivered an initial reversible capacity of approximately 140 mAh g−1 at 0.2 C and retained over 95% of this capacity after 30 cycles with a Coulombic efficiency consistently above 99%. Moreover, the rate capability tests demonstrated excellent performance at high current densities and a superior capacity recovery of ~99% when the current returned to 0.2 C. In conclusion, the proposed synthesis strategy effectively imparted a high surface area and optimized porous structure to the LFP particles, which improved both the ionic and electronic transport pathways. As a result, the RS-LFP exhibited notably enhanced cycling stability and rate performance compared with its commercial counterpart. These findings demonstrate that template-assisted spray drying is a promising and scalable approach for developing high-performance phosphate-based cathode materials and other advanced lithium-ion electrodes.
Author Contributions
J.K.: Conceptualization, formal analysis, investigation, and writing—original draft preparation; S.A.: Conceptualization and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hasan, M.; Haque, R.; Jahirul, M.; Rasul, M.G.; Fattah, I.; Hassan, N.; Mofijur, M. Advancing energy storage: The future trajectory of lithium-ion battery technologies. J. Energy Storage 2025, 120, 116511. [Google Scholar] [CrossRef]
- Yang, J.; Guan, N.; Xu, C.; Si, L.; Wen, B.; Yuan, J.; Yang, H.; Zhong, H.; Lin, X.; Wu, Y. Synthesis and modification of LiFePO4 lithium-ion battery cathodes: A mini review. CrystEngComm 2024, 26, 3441–3454. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Mei, W.; Jiang, L.; Jia, Z.; Cheng, Z.; Sun, J.; Wang, Q. Understanding of thermal runaway mechanism of LiFePO4 battery in-depth by three-level analysis. Appl. Energy 2023, 336, 120695. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Liu, F.; Fang, Z.; Gu, N.; Chen, B.; Yang, N.; Jia, Y. A comparative study of overcharge thermal runaway force-electrical-thermal characteristics and safety assessment of lithium batteries with different cathode materials. Appl. Therm. Eng. 2024, 256, 124092. [Google Scholar] [CrossRef]
- Quan, J.; Zhao, S.; Song, D.; Wang, T.; He, W.; Li, G. Comparative life cycle assessment of LFP and NCM batteries including the secondary use and different recycling technologies. Sci. Total Environ. 2022, 819, 153105. [Google Scholar] [CrossRef]
- Visone, B.; Senneca, O.; Prosini, P.; Apicella, B. Recovery of LiFePO4 cathodes: Criticalities and prospect towards a long-term eco-friendly solution. J. Power Sources Adv. 2025, 31, 100168. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, J.; Wang, X.; Chen, L.; Cao, G.; Wang, J.; Zhang, H.; He, X. Insights for understanding multiscale degradation of LiFePO4 cathodes. eScience 2022, 2, 125–137. [Google Scholar] [CrossRef]
- Na, S.; Park, C.; Park, K. LiFePO4 (LFP) coating and blending on Li1.05 (Ni0.88Co0.08Mn0.04)O2 (Ni-rich NCM): A strategy for reduced gas generation. J. Alloys Compd. 2025, 1031, 180771. [Google Scholar] [CrossRef]
- Xia, D.; Huang, W.; Shi, C.; Promi, A.; Hou, D.; Sun, C.; Hwang, S.; Kwon, G.; Huang, H.; Lin, F. Stable-Cycling Sustainable Na-Ion Batteries with Olivine Iron Phosphate Cathode in an Ether Electrolyte. ACS Sustain. Chem. Eng. 2025, 13, 241–250. [Google Scholar] [CrossRef]
- Jiao, L.; Li, Z.; Zhu, Y.; Wei, Z.; Liang, Y.; Wang, X.; Cui, Y.; Zhang, Z.; He, M.; Song, B. Enhanced electrical conductivity and lithium ion diffusion rate of LiFePO4 by Fe site and P site doping. AIP Adv. 2023, 13, 075306. [Google Scholar] [CrossRef]
- Kim, C.; Yang, Y.; Ha, D.; Kim, D.H.; Kim, H. Crystal alignment of a LiFePO4 cathode material for lithium ion batteries using its magnetic properties. RSC Adv. 2019, 9, 31936–31942. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tse, J.S. Li ion diffusion mechanisms in LiFePO4: An ab initio molecular dynamics study. J. Phys. Chem. A 2011, 115, 13045–13049. [Google Scholar] [CrossRef] [PubMed]
- Akhmetova, K.; Sultanov, F.; Mentbayeva, A.; Umirov, N.; Bakenov, Z.; Tatykayev, B. Advances in multi-element doping of LiFePO4 cathode material for capacity enhancement in Li-ion batteries. J. Power Sources 2024, 624, 235531. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, X.; Gridley, B.; Golden, W.; Ji, Y.; Johnson, S.; Lu, D.; Lin, F.; Liu, J.; Liu, Y. From Mining to Manufacturing: Scientific Challenges and Opportunities behind Battery Production. Chem. Rev. 2025, 125, 6397–6431. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, X.; Xiang, H.; Xie, J.; Li, J.; Zhou, Y. Mechanism for hydrothermal synthesis of LiFePO4 platelets as cathode material for lithium-ion batteries. J. Phys. Chem. C 2010, 114, 16806–16812. [Google Scholar] [CrossRef]
- Lan, T.; Guo, X.; Li, D.; Chen, Y. Preparation of LiFePO4 powders by ultrasonic spray drying method and their memory effect. Materials 2021, 14, 3193. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, L.; Zhao, S.; Hai, C.; Chen, T.; Zhang, J.; Zhao, Y.; Sun, Y.; Dong, S.; He, X. Cost-effective and efficient preparation of FePO4 electrodes from spent LiFePO4 batteries for enhanced lithium extraction from salt lakes. Mater. Today Energy 2025, 50, 101848. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, J.; Han, S.; Lee, J.; Lee, H.; Kwon, J.; Lee, J.; Seo, J.; Kim, P.J.; Song, T. Low-Resistance LiFePO4 Thick Film Electrode Processed with Dry Electrode Technology for High-Energy-Density Lithium-Ion Batteries. Small Sci. 2024, 4, 2300302. [Google Scholar] [CrossRef]
- Ma, Z.; Zuo, Z.; Li, L.; Li, Y. Unleash the Capacity Potential of LiFePO4 through Rocking-Chair Coordination Chemistry. Adv. Funct. Mater. 2022, 32, 2108692. [Google Scholar] [CrossRef]
- Pan, X.; Liang, Q.; Zhu, B.; He, R.; Jiang, S.; Ren, Y.; Lu, M.; Zhang, W.; Zhuang, S. Accelerating charge-transfer kinetics in LiFePO4 cathode through a construction of multifunctional layer for advanced lithium-ion batteries. Appl. Energy 2025, 382, 125229. [Google Scholar] [CrossRef]
- Rigamonti, M.G.; Chavalle, M.; Li, H.; Antitomaso, P.; Hadidi, L.; Stucchi, M.; Galli, F.; Khan, H.; Dolle, M.; Boffito, D.C. LiFePO4 spray drying scale-up and carbon-cage for improved cyclability. J. Power Sources 2020, 462, 228103. [Google Scholar] [CrossRef]
- Chen, C.; Luo, C.; Jin, Y.; Li, J.; Zhao, Q.; Yang, W. Short-process spray-drying synthesis of lithium iron phosphate@ carbon composite for lithium-ion batteries. ACS Sustain. Chem. Eng. 2024, 12, 14077–14086. [Google Scholar] [CrossRef]
- Styuf, E.A.; Othman, M.; Savina, A.A.; Komayko, A.I.; Filimonov, D.S.; Nikitina, V.A.; Abakumov, A.M. The microwave-assisted spray drying synthesis: Application to the LiFePO4/C cathode material for Li-ion batteries. J. Power Sources 2025, 642, 236951. [Google Scholar] [CrossRef]
- Rajasekar, K.; Raja, B. Heat and mass transfer characteristics during spray drying of Na2Fe0.6Mn0.4PO4F/C cathode material for Na-ion batteries. Appl. Therm. Eng. 2023, 221, 119838. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, S.; Shi, H.; Hu, H. Synthesis of FePO4·xH2O for fabricating submicrometer structured LiFePO4/C by a co-precipitation method. Ceram. Int. 2014, 40, 2685–2690. [Google Scholar] [CrossRef]
- Chairunnisa, A. Synthesis of LiFePO4 (lithium iron phosphate) with several methods: A review. RHAZES Green Appl. Chem. 2020, 10, 49–81. [Google Scholar]
- Lim, J.; Seo, S.; Kim, C. Synthesis of Iron Phosphate Via Coprecipitation Method for LiFePO4 Cathode. Korean J. Mater. Res. 2024, 34, 482–490. [Google Scholar] [CrossRef]
- Chen, L.; Feng, W.; Pu, Z.; Wang, X.; Su, W.; Li, M.; Song, C.; Shi, Z.; Zheng, Y. Effects of citric acid on the preparation of a LiFePO4@C cathode material assisted by biomineralization. Int. J. Electrochem. Sci. 2019, 14, 8048–8057. [Google Scholar] [CrossRef]
- Xie, G.; Zhu, H.-J.; Liu, X.-M.; Yang, H. A core–shell LiFePO4/C nanocomposite prepared via a sol–gel method assisted by citric acid. J. Alloys Compd. 2013, 574, 155–160. [Google Scholar] [CrossRef]
- Rostami, H.; Valio, J.; Tynjälä, P.; Lassi, U.; Suominen, P. Life cycle of LiFePO4 batteries: Production, recycling, and market trends. ChemPhysChem 2024, 25, e202400459. [Google Scholar] [CrossRef]
- Islam, M.; Ahmed, M.S.; Faizan, M.; Ali, B.; Bhuyan, M.M.; Bari, G.A.R.; Nam, K.-W. Review on the Polymeric and Chelate Gel Precursor for Li-Ion Battery Cathode Material Synthesis. Gels 2024, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, J.; Yang, Y.; Song, G. Preparation and characterization of mesoporous LiFePO4/C microsphere by spray drying assisted template method. J. Power Sources 2009, 189, 794–797. [Google Scholar] [CrossRef]
- Liang, F.; Yao, Y.; Dai, Y.; Yang, B.; Ma, W.; Watanabe, T. Preparation of porous structure LiFePO4/C composite by template method for lithium-ion batteries. Solid State Ion. 2012, 214, 31–36. [Google Scholar] [CrossRef]
- Deng, H.; Jin, S.; Zhan, L.; Qiao, W.; Ling, L. Nest-like LiFePO4/C architectures for high performance lithium ion batteries. Electrochim. Acta 2012, 78, 633–637. [Google Scholar] [CrossRef]
- Boel, E.; Koekoekx, R.; Dedroog, S.; Babkin, I.; Vetrano, M.R.; Clasen, C.; Van den Mooter, G. Unraveling particle formation: From single droplet drying to spray drying and electrospraying. Pharmaceutics 2020, 12, 625. [Google Scholar] [CrossRef]
- Sloth, J.; Jørgensen, K.; Bach, P.; Jensen, A.D.; Kiil, S.; Dam-Johansen, K. Spray drying of suspensions for pharma and bio products: Drying kinetics and morphology. Ind. Eng. Chem. Res. 2009, 48, 3657–3664. [Google Scholar] [CrossRef]
- Finotello, G. Droplet Collision Dynamics in a Spray Dryer: Experiments and Simulations. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2019. [Google Scholar]
- Liu, L.; Deng, Q.-F.; Ma, T.-Y.; Lin, X.-Z.; Hou, X.-X.; Liu, Y.-P.; Yuan, Z.-Y. Ordered mesoporous carbons: Citric acid-catalyzed synthesis, nitrogen doping and CO2 capture. J. Mater. Chem. 2011, 21, 16001–16009. [Google Scholar] [CrossRef]
- Pavlenko, V.; Żółtowska, S.; Haruna, A.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.; Abbas, Q.; Jesionowski, T. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Yang, L.H.; Takeuchi, M.; Chen, Y.; Ng, N.L. Characterization of thermal decomposition of oxygenated organic compounds in FIGAERO-CIMS. Aerosol Sci. Technol. 2021, 55, 1321–1342. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Li, C.; Kong, L.; Zhang, S.; Hu, X. In-situ decomposition of citric acid for eliminating coking in steam reforming of acetic acid through enhanced gasification of coke precursors. J. Environ. Chem. Eng. 2024, 12, 114332. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Miao, Z.; Huang, K.; Liao, Y.; Xu, X.; Meng, J.; Li, Z.; Huang, Y. Hierarchical electrode architecture enabling ultrahigh-capacity LiFePO4 cathodes with low tortuosity. ACS Appl. Mater. Interfaces 2023, 15, 26824–26833. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, M.; Cao, Y.; Ai, X.; Yang, H. Template-free hydrothermal synthesis of nanoembossed mesoporous LiFePO4 microspheres for high-performance lithium-ion batteries. J. Phys. Chem. C 2010, 114, 3477–3482. [Google Scholar] [CrossRef]
- Ait Salah, A.; Jozwiak, P.; Zaghib, K.; Garbarczyk, J.; Gendron, F.; Mauger, A.; Julien, C. FTIR features of lithium-iron phosphates as electrode materials for rechargeable lithium batteries. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 1007–1013. [Google Scholar] [CrossRef]
- Abhilash, K.; Selvin, P.C.; Nalini, B.; Xia, H.; Adams, S.; Reddy, M. Electrochemical analysis of the carbon-encapsulated lithium iron phosphate nanochains and their high-temperature conductivity profiles. ACS Omega 2018, 3, 6446–6455. [Google Scholar] [CrossRef]
- Miao, C.; Bai, P.; Jiang, Q.; Sun, S.; Wang, X. A novel synthesis and characterization of LiFePO4 and LiFePO4/C as a cathode material for lithium-ion battery. J. Power Sources 2014, 246, 232–238. [Google Scholar] [CrossRef]
- Lin, Q.; Su, K.; Huang, Y.; He, Y.; Zhang, J.; Yang, X.; Xu, H. Molecular Crystal Structure Simulations and Structure-Magnetic Properties of LiFePO4 Composite Particles Optimized by La. Molecules 2024, 29, 3933. [Google Scholar] [CrossRef]
- Burba, C.M.; Frech, R. Raman and FTIR spectroscopic study of LixFePO4 (0 ≤ x ≤ 1). J. Electrochem. Soc. 2004, 151, A1032. [Google Scholar] [CrossRef]
- Benoy, S.M.; Singh, S.; Pandey, M. Characterization of nanocarbon based electrode material derived from anthracite coal. Mater. Res. Express 2020, 6, 125624. [Google Scholar] [CrossRef]
- Julien, C.M.; Zaghib, K.; Mauger, A.; Groult, H. Enhanced electrochemical properties of LiFePO4 as positive electrode of Li-ion batteries for HEV application. Adv. Chem. Eng. Sci. 2012, 2, 321–329. [Google Scholar] [CrossRef]
- Ait-Salah, A.; Zaghib, K.; Mauger, A.; Gendron, F.; Julien, C. Magnetic studies of the carbothermal effect on LiFePO4. Phys. Status Solidi A 2006, 203, R1–R3. [Google Scholar] [CrossRef]
- Kroff, M.; Hevia, S.A.; O’Shea, J.N.; Muro, I.G.d.; Palomares, V.; Rojo, T.; Del Río, R. Lithium iron phosphate/carbon (LFP/C) Composite using nanocellulose as a reducing agent and carbon source. Polymers 2023, 15, 2628. [Google Scholar] [CrossRef]
- Lin, L.; Wen, Y.; Guo, Y.; Xiao, D. X-ray diffraction study of LiFePO4 synthesized by hydrothermal method. RSC Adv. 2013, 3, 14652–14660. [Google Scholar] [CrossRef]
- Naik, A.; Zhou, J.; Gao, C.; Wang, L. Microwave Assisted Solid State Synthesis of LiFePO4/C Using Two Different Carbon Sources. Int. J. Electrochem. Sci. 2014, 9, 6124–6133. [Google Scholar] [CrossRef]
- Guo, B.; Ruan, H.; Zheng, C.; Fei, H.; Wei, M. Hierarchical LiFePO4 with a controllable growth of the (010) facet for lithium-ion batteries. Sci. Rep. 2013, 3, 2788. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, Z.; Pang, W.; Tong, G.; Liang, F. High electrochemical performance of highly (020) preferred orientation LiFePO4 for lithium ion battery. J. Power Sources 2025, 644, 237131. [Google Scholar] [CrossRef]
- Shiraishi, K.; Dokko, K.; Kanamura, K. Formation of impurities on phospho-olivine LiFePO4 during hydrothermal synthesis. J. Power Sources 2005, 146, 555–558. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.-C.; Hinokuma, K. Optimized LiFePO4 for lithium battery cathodes. J. Electrochem. Soc. 2001, 148, A224. [Google Scholar] [CrossRef]
- Hsu, K.-F.; Tsay, S.-Y.; Hwang, B.-J. Synthesis and characterization of nano-sized LiFePO4 cathode materials prepared by a citric acid-based sol–gel route. J. Mater. Chem. 2004, 14, 2690–2695. [Google Scholar] [CrossRef]
- Cabán-Huertas, Z.; Ayyad, O.; Dubal, D.P.; Gómez-Romero, P. Aqueous synthesis of LiFePO4 with fractal granularity. Sci. Rep. 2016, 6, 27024. [Google Scholar] [CrossRef]
- Yim, C.-H.; Baranova, E.A.; Abu-Lebdeh, Y.; Davidson, I. Highly ordered LiFePO4 cathode material for Li-ion batteries templated by surfactant-modified polystyrene colloidal crystals. J. Power Sources 2012, 205, 414–419. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Huang, S.-Z.; Jin, J.; Liu, J.; Li, Y.; Wang, H.-E.; Chen, L.-H.; Wang, B.-J.; Su, B.-L. Engineering 3D bicontinuous hierarchically macro-mesoporous LiFePO4/C nanocomposite for lithium storage with high rate capability and long cycle stability. Sci. Rep. 2016, 6, 25942. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Liu, J.; Qiao, S.; Lu, G.M.; Munroe, P.; Ahn, H. Mesoporous LiFePO4/C nanocomposite cathode materials for high power lithium ion batteries with superior performance. Adv. Mater. 2010, 22, 4944–4948. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Sun, D.; Tang, Y.; He, K.; Ren, Y.; Liu, S.; Wang, H. Long-lived aqueous rechargeable lithium batteries using mesoporous LiTi2 (PO4)3@C anode. Sci. Rep. 2015, 5, 17452. [Google Scholar] [CrossRef]
- Dai, F.; Yi, R.; Yang, H.; Zhao, Y.; Luo, L.; Gordin, M.L.; Sohn, H.; Chen, S.; Wang, C.; Zhang, S. Minimized volume expansion in hierarchical porous silicon upon lithiation. ACS Appl. Mater. Interfaces 2019, 11, 13257–13263. [Google Scholar] [CrossRef]
- Meng, Z.; Ma, X.; Azhari, L.; Hou, J.; Wang, Y. Morphology controlled performance of ternary layered oxide cathodes. Commun. Mater. 2023, 4, 90. [Google Scholar] [CrossRef]
- Kim, J.; Lesmana, L.A.; Aziz, M. Impact analysis of particle sphericity on the properties of porous materials via particle packing method for hydrogen fuel and electrolysis cells. Comput. Chem. Eng. 2025, 192, 108907. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Demortière, A.; Fleutot, B.; Delobel, B.; Delacourt, C.; Cooper, S.J. The electrode tortuosity factor: Why the conventional tortuosity factor is not well suited for quantifying transport in porous Li-ion battery electrodes and what to use instead. Npj Comput. Mater. 2020, 6, 123. [Google Scholar] [CrossRef]
- Müller, M.; Schneider, L.; Bohn, N.; Binder, J.R.; Bauer, W. Effect of nanostructured and open-porous particle morphology on electrode processing and electrochemical performance of Li-ion batteries. ACS Appl. Energy Mater. 2021, 4, 1993–2003. [Google Scholar] [CrossRef]
- Qin, T.; Yang, H.; Li, Q.; Yu, X.; Li, H. Design of functional binders for high-specific-energy lithium-ion batteries: From molecular structure to electrode properties. Ind. Chem. Mater. 2024, 2, 191–225. [Google Scholar] [CrossRef]
- Chang, J.H.; Pin, M.W.; Kim, I.; Kim, S.; Kim, S.; Moon, S.; Cho, J.; Choi, S.; Heo, B.; Chandio, Z.A. Binder migration: Frequently observed yet overlooked phenomena in electrode processing for lithium-ion batteries. J. Energy Storage 2024, 83, 110729. [Google Scholar] [CrossRef]
- Wang, S.-H.; Yue, J.; Dong, W.; Zuo, T.-T.; Li, J.-Y.; Liu, X.; Zhang, X.-D.; Liu, L.; Shi, J.-L.; Yin, Y.-X. Tuning wettability of molten lithium via a chemical strategy for lithium metal anodes. Nat. Commun. 2019, 10, 4930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012, 5, 5163–5185. [Google Scholar] [CrossRef]
- Youn, J.W.; Park, G.H.; Kim, M.; Kang, S.K.; Jang, D.; Kim, W.B. Surface modification with F-doped carbon layer coating on natural graphite anode for improving interface compatibility and electrochemical performance of lithium-ion capacitors. ACS Appl. Electron. Mater. 2023, 5, 4344–4353. [Google Scholar] [CrossRef]
- Zabara, M.A.; Katırcı, G.k.; Ülgüt, B. Operando investigations of the interfacial electrochemical kinetics of metallic lithium anodes via temperature-dependent electrochemical impedance spectroscopy. J. Phys. Chem. C 2022, 126, 10968–10976. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and applications of electrochemical impedance spectroscopy (EIS) for lithium-ion batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Nara, H.; Mukoyama, D.; Shimizu, R.; Momma, T.; Osaka, T. Systematic analysis of interfacial resistance between the cathode layer and the current collector in lithium-ion batteries by electrochemical impedance spectroscopy. J. Power Sources 2019, 409, 139–147. [Google Scholar] [CrossRef]
- Peng, Y.; Zeng, L.; Dai, S.; Liu, F.; Rao, X.; Zhang, Y. LiFePO4/C twin microspheres as cathode materials with enhanced electrochemical performance. RSC Adv. 2023, 13, 6983–6992. [Google Scholar] [CrossRef]
- Ma, Z.-P.; Shao, G.-J.; Wang, W.; Zhang, C.-Y. Preparation and Study on Electrochemical Performance of High-Rate LiFePO4/C Cathode Material. Asian J. Chem. 2013, 25, 3579. [Google Scholar] [CrossRef]
- Syed, M.A.; Salehabadi, M.; Obrovac, M. High energy density large particle LiFePO4. Chem. Mater. 2024, 36, 803–814. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Y.; Zhao, X.; Zhi, X.; Liang, G. Effect of particle size and purity on the low temperature electrochemical performance of LiFePO4/C cathode material. J. Alloys Compd. 2016, 683, 123–132. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, M.; Liu, F.; Yang, J.; Feng, T. Variation of carbon coatings on the electrochemical performance of LiFePO4 cathodes for lithium ionic batteries. RSC Adv. 2017, 7, 44296–44302. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Safari, M.; Delacourt, C. Aging of a commercial graphite/LiFePO4 cell. J. Electrochem. Soc. 2011, 158, A1123. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, Z.; Li, J. Hierarchical carbon-coated LiFePO4 nanoplate microspheres with high electrochemical performance for Li-ion batteries. Adv. Mater. 2011, 23, 1126–1129. [Google Scholar] [CrossRef]
- Zaghib, K.; Dubé, J.; Dallaire, A.; Galoustov, K.; Guerfi, A.; Ramanathan, M.; Benmayza, A.; Prakash, J.; Mauger, A.; Julien, C. Enhanced thermal safety and high power performance of carbon-coated LiFePO4 olivine cathode for Li-ion batteries. J. Power Sources 2012, 219, 36–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).