Abstract
Organic chlorine (Org-Cl) in crude oil poses continuous operational and environmental risks during production, trading, and refining processes. This article reviews the management of Org-Cl from its origin assumptions to analysis and mitigation measures and proposes a practical closed-loop framework. Quantitative merit value indicators (typical detection limit/quantitative limit, accuracy, and repeatability) and greenness indicators are used to compare standard methods and advanced methods, and to guide the selection of applicable methods. Corresponding technical maturity levels (TRLs) are assigned to mitigation measures (protective beds/adsorption, HDC, and emerging electrochemical/photochemical routes). Technical economic indicators with reference values (relative capital expenditure/operating expenditure levels) are summarized to assist decision-making. The main findings are as follows: (i) Evidence of secondary formation of organic chlorine under distillation-related conditions still relies on the matrix and requires independent verification; (ii) MWDXRF can achieve rapid screening (usually only 5 to 10 min), while CIC/D5808 supports quality balance arbitration; (iii) adsorption can remove a considerable portion of organic chlorine in light fractions under laboratory conditions, while the survival ability of HDC related to crude oil depends on the durability of the catalyst and the tail gas treatment capacity; and (iv) minimum viable implementation (MVI) combined with online total-chlorine monitoring and a physical principle-based digital twin technology can provide auditable closed-loop control. The limitations of this review include partial reliance on laboratory-scale data, inconsistent reports among studies, and the lack of standardized public datasets for model benchmarking. Prioritization should be given to analysis quality control, process durability indicators, and data governance to achieve reliable digital deployment.
1. Introduction
1.1. Industrial Challenge and Research Significance
Trace organic chlorine in crude oil represents a concealed yet highly destructive risk source throughout the refining process. Under high-temperature conditions in units such as distillation, coking, and hydrotreating, its C-Cl bonds crack to form hydrogen chloride (HCl), which subsequently reacts with ammonia (NH3) in the system to form ammonium chloride (NH4Cl) salts. These salts deposit in the cold sections of equipment, like heat exchangers and air coolers, leading to severe under-deposit corrosion, decreased heat transfer efficiency, and increased energy consumption [,,]. Furthermore, chlorine species poison expensive hydrotreating catalysts, shorten operation cycles, and increase maintenance frequency and carbon emissions. The organic chlorine contamination incident involving Azeri BTC crude in the European market in July 2025, while not causing unit shutdowns due to timely early warning, still triggered significant market volatility and supply chain disruptions, underscoring the critical importance of systematic whole-chain governance from “source control → process monitoring → engineering removal”. Therefore, a comprehensive and rigorous review focusing on the three core aspects of organic chlorine—sources, detection, and removal—holds significant industrial value and aligns perfectly with Energies’ focus on “energy sustainability, reliability, and decarbonization” [].
1.2. Concept Definition and Terminology
The industry commonly uses the related concepts of total chlorine (Total Cl), inorganic chlorine (Inorganic Cl−), and organic chlorine (Organochlorine Cl). From an operational perspective, “total parameter” indicators such as AOX/EOX/TOX (adsorbable/extractable/total organic halogens) are often introduced to assess the halogen load in complex matrices and aid material balance calculations []. In crude oil and heavy fractions, organic chlorine can exist as halogenated alkanes, alkenes, aromatics, and polar chlorinated compounds; its boiling range distribution and speciation directly influence laboratory determination strategies and the interpretation of corrosion mechanisms []. Furthermore, recent mechanistic studies indicate that some inorganic chlorine can undergo secondary transformation under process conditions like distillation to form organic chlorine, a phenomenon that further exposes the blind spot of “removing salt without controlling chlorine” in traditional processes [].
1.3. Scope, Structure, and Contributions of This Review
To overcome the limitations of traditional fragmented discussions that often focus solely on detection, this review centers on the core logical thread of “Source → Detection → Removal and Risk Control”, systematically covering the following: (i) Multiple source mechanisms, including geological origins, introduction during production and transportation, and contamination from chemical additives; (ii) performance comparison and standardization challenges of analytical methods such as combustion-ion chromatography (CIC), gas chromatography–mass spectrometry (GC-MS/GC × GC-MS), and compound-specific chlorine isotope analysis (CSIA-Cl); (iii) techno-economic feasibility and low-carbon potential of engineering removal pathways, including enhanced desalting, adsorption, membrane separation, hydrodechlorination (HDC), and emerging methods like electrochemical/photocatalytic degradation; (iv) a multi-dimensional evaluation system encompassing corrosion mechanisms, compliance requirements, and life cycle assessment (LCA); and (v) intelligent management strategies based on data-driven spectral deconvolution, machine learning, and online monitoring.
Compared with the existing reviews, the contributions of this article lie in the following: (i) a unified metric diagram that aligns ASTM and advanced analyses (GC × GC-MS, CSIA-Cl, MWDXRF/CIC) with clear indicators and greenness scores; (ii) a contextualized technology maturity level matrix that distinguishes implementations related to the petrochemical industry from those related to crude oil; (iii) a practical and auditable “multivariate industrial process” (MVI) that connects online total-chlorine monitoring with physical-based digital twin models and operational key performance indicators (KPIs); and (iv) a reference-based “technical and economic” comparison that matches the credibility of the analysis with deployment costs/complexity. We also discussed the open limitations in the previous framework (such as insufficient quality balance closure, lack of long-term sustainable durability data, and limited dataset standardization) and explained how this review addresses these issues or defines them as key verification matters (As shown in Figure 1).
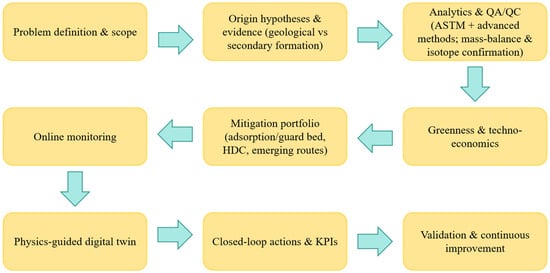
Figure 1.
Review scope and closed-loop framework.
1.4. Methodology for Literature Selection and Appraisal
We searched Web of Science, Scopus, and Google Scholar (January 2000 to June 2025) using combinations of “organic chlorine”, “crude oil”, “hydrodechlorination”, “adsorption”, “MWDXRF”, “CIC”, “GC × GC”, and “CSIA-Cl”. Inclusion criteria: (i) Crude-relevant matrices or well-specified model systems; (ii) quantitative metrics (LOD/LOQ, accuracy/recovery, runtime, and removal efficiency); (iii) clear protocols and QA/QC; and (iv) peer-reviewed or international standards (e.g., ASTM). Exclusion criteria: Ambiguous matrices, lacking controls/blanks, or unverifiable claims. When multiple studies conflicted, we prioritized mass-balance-consistent evidence and cross-validated with orthogonal methods. Risk-of-bias was appraised qualitatively (sampling, blanks, matrix effects, and durability), and figures are reported as typical ranges rather than single-point values unless CRMs or inter-lab trials were available.
2. Sources and Formation Mechanisms of Organic Chlorine in Crude Oil
2.1. Geological and Genetic Sources: Controversies and New Insights
The debate over whether organic chlorine exists naturally in crude oil persists in academia and industry. The traditional view holds that most commercial crudes do not naturally contain significant levels of organic chlorine, and any chlorine detected is primarily attributed to post-generation contamination or transformation during processing. However, fractionation studies of crudes like those from the Shengli Oilfield have detected organochlorine compounds across different boiling point fractions, with notably higher levels in light fractions, providing some phenomenological support for a “natural origin”.
Currently, the “secondary origin” mechanism is gaining more evidential support. Under distillation-relevant conditions, hydrochlorination of olefinic species can proceed via Lewis-acid catalysis (e.g., FeCl3/AlCl3) with operating windows commonly reported in the 120–180 °C range for alkyl chloride synthesis. Metal surfaces exposed to HCl may develop FeCl3-like acidic sites that catalyze electrophilic addition. Bench- and pilot-scale distillation studies further indicate that a fraction of inorganic chloride can convert to organically bound chlorine during heating and phase transitions, underscoring the need for paired mass balance and isotope/GC × GC confirmation in plant investigations.
Critical appraisal of evidence for “secondary formation”. While several laboratory simulations and plant observations support the hypothesis that inorganic chlorides (e.g., HCl from MgCl2/NaCl hydrolysis) can generate organochlorines during thermal treatment (distillation/pyrolysis), the overall evidential base remains constrained by (i) small sample sizes and model oils not representative of real crudes; (ii) poorly controlled confounders such as solvent carry-over, halogenated cleaning agents, and memory effects in glassware and combustion lines; and (iii) limited isotopic constraints, meaning that conversion pathways inferred from speciation alone may be non-unique. Thermodynamic/kinetic models describing electrophilic addition to olefins typically assume complete phase separation and constant acidity; however, acidity and micro-heterogeneity evolve dynamically across trays and reboilers, which can bias reaction extent estimates. A more rigorous chain-of-evidence should combine the following: (a) paired “unwashed vs. washed” TBP runs with spiked recovery and process blanks; (b) orthogonal total-chlorine mass balance by combustion–coulometry/CIC; (c) compound-specific chlorine isotope analysis (CSIA-Cl) to differentiate in situ formation from exogenous blending; and (d) GC × GC fingerprint alignment across batches to rule out mixing artifacts. Under this framework, the most parsimonious explanation for many industrial anomalies is still exogenous contamination plus limited in-unit transformation, rather than a pervasive geological origin. We therefore reposition “secondary formation” as a conditional, matrix-dependent risk and explicitly state its current limitations and validation needs in industry practice.
2.2. Introduction During Production and Gathering
The upstream sector is a high-risk area for the introduction of exogenous organic chlorine, primarily through the following pathways.
Chemical Additive Contamination: Improper use of organochlorine solvents (e.g., 1,2-dichloroethane) and chlorine-containing additives during wellbore cleaning and downhole operations has been identified as a root cause in multiple refinery corrosion incidents. Regional case studies and refinery failure records published by NACE/AMPP indicate that abnormal organic chlorine in piped crude oil is not an isolated phenomenon and is showing an increasing trend.
Oil–Water Emulsions and Demulsifier Systems: Demulsifiers (e.g., resin alkoxylates, cationic polyamines/surfactants), while improving dehydration efficiency, should not contain organochlorine functional groups that can crack and release HCl. However, improper chemical selection, formulation incompatibility, or blending operations can introduce a detectable organic chlorine background, exacerbating risks for downstream process units [].
Brine and Gathering Water Systems: High-chloride environments themselves do not directly introduce organic chlorine. However, under subsequent thermal processing or reaction conditions with olefins, inorganic chloride can be converted to organic chlorine. This reveals the process blind spot of focusing solely on desalting while ignoring chlorine control.
2.3. Cross-Contamination in the Pre-Refining Sector (Storage, Transportation, and Trading)
Quality-related black swan events frequently occur during crude oil storage, transportation, blending, and cross-border trade. The 2019 “Druzhba pipeline organic chlorine contamination” incident led to reduced loads, or even shutdowns, at refineries in multiple European countries. Subsequent investigations clearly identified the blending of exogenous organic chlorine as a direct trigger, and it was an event that had a profound impact on the supply chain and price system. In July 2025, several refiners detected organic chlorine anomalies in Azeri BTC crude before port arrival. Although it did not enter the units, it still caused price fluctuations and shipment delays, again highlighting the critical importance of pre-refining checks and supply-side compliance. Such cases indicate that blending operations, cleaning procedures, and the management of recycled solvents in the trade and logistics chain are indispensable links in the source control chain.
2.4. Chemical Speciation and Distribution Characteristics
In terms of characterizable chemical forms, low molecular weight organochlorides, such as dichloropropane, dichloromethane, chloroform, and carbon tetrachloride, are more readily detected in light fractions, particularly in the naphtha fraction. These compound signals are commonly found in the ASTM D4929/D8150 determination process after inorganic chlorine is removed by water or alkaline washing [,]. Fractionation studies of Shengli crude show that organic chlorine is “widespread but unevenly” distributed from light to heavy fractions, with typically higher concentrations in the light fractions. Recent applications of CIC for elemental chlorine determination coupled with GC/GC × GC qualification, as well as high-resolution mass spectrometry and chlorine isotope analysis (CSIA-Cl), have significantly enhanced the ability to identify and trace organochlorine species in complex matrices [].
2.5. Process and Environmental Risk Pathways
Once organic chlorine enters the refinery system, it can crack under high-temperature conditions (e.g., distillation, cracking, and hydrotreating) to release HCl. This HCl then reacts with NH3 in the system to form NH4Cl, leading to salt deposition in cold sections, causing overhead corrosion, reduced heat exchange efficiency, and chlorine poisoning of hydrotreating catalysts. This shortens the run lengths and increases energy consumption and carbon emissions. Numerous engineering case studies have documented overhead failures in crude distillation units (CDUs) and problems in naphtha hydrotreating (NHT) units due to “unconventional high chlorine”. From a lifecycle perspective, the coupled pathway of “source → form → process” can be summarized as follows: (i) Exogenous blending or chemical input → (ii) enrichment in light fractions and detection → (iii) thermal cracking releasing HCl and forming NH4Cl → (iv) causing corrosion, salt plugging, and catalyst poisoning → (v) leading to frequent maintenance, increased energy consumption, and emissions (As shown in Figure 2).
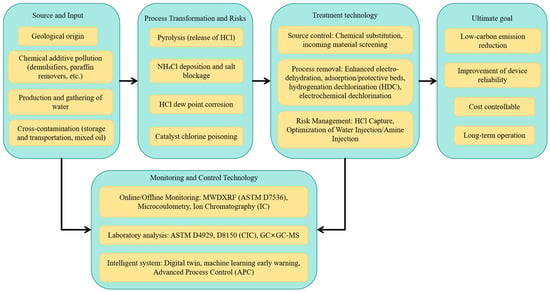
Figure 2.
Schematic diagram of the sources, formation pathways, and risk impacts of organochlorine compounds in crude oil.
Therefore, subsequent chapters of this review will focus on the closed loop of “detection → source apportionment → removal” to form a systematic solution applicable in engineering practice (As shown in Figure 3).
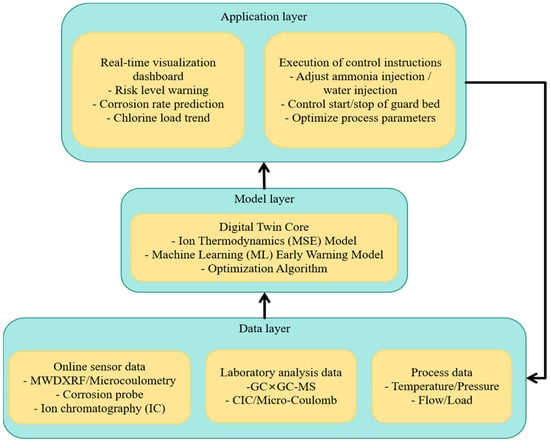
Figure 3.
Roadmap of the lifecycle risks and integrated management strategy for organochlorine compounds in crude oil.
3. Analytical Methods and Quality Control
3.1. Overview of Total and Organic Chlorine Determination Methods
Industrial and laboratory methods for determining organic chlorine in crude oil and petroleum products mainly include the following:
(a) Distillation combined with washing (ASTM D4929): Inorganic chlorine and H2S are removed by alkaline or water washing, followed by quantitative determination of organically bound chlorine using sodium biphenyl reduction titration (Test Method A), combustion-microcoulometry (Test Method B), or X-ray fluorescence spectrometry (Test Method C, XRF). This standard explicitly states its applicability for determining organic chlorine ≥ 1 mg·kg−1 and emphasizes that its abnormal presence is often linked to contamination and operational risk []. We recommend D4929 for organic chlorine arbitration in light fractions because it is widely standardized and auditable; however, we explicitly acknowledge its limitations in heavy/complex matrices, including the risk of artifactual formation during distillation/washing. Therefore, arbitration should pair D4929 with D5808 (pyrolysis-microcoulometry) or CIC for total-chlorine mass balance, enforce strict blanks/cleaning controls, and, where feasible, confirm with GC × GC speciation and CSIA-Cl [].
(b) Combustion-Ion Chromatography (CIC, ASTM D8150): Suitable for the highly selective quantification of organic chlorine in naphtha fractions. The sample is combusted, generating HCl, which is absorbed into a solution and determined as chloride ions using ion chromatography (IC).
(c) High-Temperature Pyrolysis-Microcoulometry and MWDXRF: ASTM D5808 (high-temperature pyrolysis-microcoulometry) is suitable for determining ultra-trace chlorine (0.2–25 mg·kg−1) in aromatics and chemicals, with reported LOD and LOQ of 0.2 and 0.7 mg·kg−1, respectively []. ASTM D7536 (mono-chromatic wavelength dispersive X-ray fluorescence, MWDXRF) is suited for rapid (5–10 min), non-destructive analysis of total chlorine in liquid samples like aromatics, with a regular range of 0.66–10 mg·kg−1, extendable by dilution [].
Practical Recommendation: For trade dispute arbitration, ASTM D4929 should be the primary method, and its procedures, B/C, can be cross-validated with methods like D8150, D5808, or D7536. For high-sulfur or complex matrix samples, combustion-coulometric or combustion-ion chromatographic methods are recommended to minimize matrix interference [].
3.2. Key Analytical Techniques and Advances
3.2.1. Combustion-Microcoulometry and Combustion-Ion Chromatography (CIC)
Microcoulometry is based on the complete pyrolysis of the sample at 900–1050 °C. The generated hydrogen chloride reacts with silver ions in the electrolytic cell, and the chlorine content is quantified by coulometric consumption, offering absolute quantification and broad matrix adaptability []. ASTM D5808 details instrument parameters and operating conditions, ensuring method reproducibility. As the core technology for determining AOX/EOX/TOX, the blank control, gas purity, and absorption efficiency of CIC systematically impact the LOD and repeatability, for which recent studies have proposed clear optimization strategies [].
3.2.2. High-Temperature Pyrolysis-Coulometry and Portable Analysis
This type of method is easily portable or online, and is suitable for rapid total-chlorine screening and incident investigation at receiving points or desalters. Compared to XRF, microcoulometry is less affected by matrix heterogeneity or high sulfur content, making it particularly suitable for heavy fraction analysis. Note: it measures total chlorine; for specific organic chlorine quantification, consistency must be verified against standard methods like ASTM D4929 through blank experiments and washing controls.
3.2.3. AOX/EOX-like Systems and Online Combustion–Extraction Coupling
The strategy of adsorption/extraction coupled with online combustion (AOX/EOX) provides a crucial perspective for assessing halogen mass balance in complex matrices. Recent research focuses on error sources such as adsorbent cleanliness, memory effects in combustion units, and residual halogens in carrier gas, proposing full-process blank control and sequential analysis quality control recommendations [,]. For crude oil/naphtha analysis, bidirectional extraction and online combustion-IC/GC coupling can effectively achieve species identification and trace the total amount [].
3.3. Speciation and Molecular Fingerprinting Analysis
3.3.1. GC-MS and Comprehensive Two-Dimensional Gas Chromatography–Mass Spectrometry (GC × GC-TOFMS)
Based on the D4929/D8150 framework, light fractions can be further subjected to species identification and fingerprint analysis using GC-MS or GC × GC-TOFMS. Halogen-selective detectors (e.g., XSD, ELCD, and AED) can effectively highlight halocarbon signals in the hydrocarbon background, which is suitable for targeted confirmation and semi-quantification of anomalous peaks []. For the high-dimensional data generated by comprehensive two-dimensional chromatography, automated scripting tools are available for rapid screening and identification of halogenated compounds, significantly reducing manual interpretation costs. High-boiling fractions may suffer column bleed and co-elution, leading to false positives for chlorinated species. Mitigations include the following: high-temperature, low-bleed stationary phases; periodic blank and carry-over checks; optimized modulation periods to separate late-eluters; deconvolution with retention-index constraints; and orthogonal confirmation by element-selective detectors (AED/XSD) or CSIA-Cl. Reported identifications should be flagged with confidence levels and, where possible, linked to total-chlorine mass balance. An analytical workflow of “GC × GC broad-spectrum screening → GC-MS/XSD targeted confirmation → total amount method (CIC/microcoulometry) backtracking verification” is recommended to avoid false positives being potentially introduced by derivatization or distillation processes [].
3.3.2. High-Resolution Mass Spectrometry and Chlorine Isotope Analysis
High-resolution mass spectrometers like Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) or Orbitrap can provide precise molecular formulas and 35Cl/37Cl isotopic fine structure information, which is suitable for non-targeted identification and source comparison of unknown polar chlorinated compounds or high-boiling components [,]. Compound-specific chlorine isotope analysis (CSIA-Cl) combined with gas chromatography–isotope ratio mass spectrometry (GC-IRMS) or GC-MS can effectively differentiate contamination sources and transformation pathways (e.g., pyrolysis, hydrolysis) through 37Cl values, showing significant value in arbitration of major disputed samples [,,].
3.4. Sample Preparation and Matrix Effect Control
Reliable determination heavily depends on sample preparation.
Sampling and Storage: Must follow ASTM D4057 and D5854 protocols to ensure sample representativeness and prevent volatilization or cross-contamination [,]. Container material, mixing method, and process blanks must match the target detection limit. NIST and industry guidelines emphasize that sampling and preservation are major sources of experimental error and should be explicitly documented [].
Pretreatment Strategies: Volatile halocarbons in light fractions can be enriched and cleaned up using headspace-solid phase microextraction (HS-SPME), liquid–liquid extraction (LLE), or solid phase extraction (SPE); heavy fractions and residue are recommended to use gel permeation chromatography (GPC), silica/alumina fractionation, or direct matrix introduction (DMI) techniques to mitigate contamination of the chromatographic column and ion source by asphaltenes and resins [].
Inorganic Chlorine Removal and Interference Control: ASTM D4929 and D8150 require the removal of H2S and inorganic halogens by alkaline or water washing to avoid misjudgment of organic chlorine. High-salt or high-asphaltene samples require additional steps such as desalting, settling, or membrane filtration, and it is advised to set up “unwashed” and “washed” parallel samples to assess the risk of conversion or loss.
3.5. Method Validation and Quality Control
3.5.1. Analytical Performance Parameters
Each standard method specifies its linear range, LOD, LOQ, and applicable scenarios: D5808 has LOD and LOQ of 0.2 and 0.7 mg·kg−1, respectively, with an upper quantification limit of 25 mg·kg−1; D7536 is applicable in the range of 0.66–10 mg·kg−1; D4929 states its applicability for determining organic chlorine ≥ 1 mg·kg−1. Method comparison should use certified reference materials (CRMs), spiked recovery experiments, and Passing–Bablok regression analysis to assess systematic bias. Proficiency testing program (PTP) data show that MWDXRF (D7536) and microcoulometry (D5808) results are consistent for most aromatic samples, but systematic deviations may occur in high-aromatic or high-impurity samples, requiring matrix-matched calibration.
3.5.2. Matrix Tolerance, Throughput, and Cost
Microcoulometry and CIC exhibit strong anti-interference capability against high-sulfur or heavy oil matrices but require high-temperature combustion systems and strict blank management; MWDXRF offers the advantages of being non-destructive, high-throughput, and easily online, making it highly suitable for process monitoring and incoming material inspection. GC × GC-MS is information-rich but has low throughput and high data-processing costs, recommended for tracing of disputed samples or method development scenarios.
3.5.3. Reference Materials and Inter-Laboratory Comparison
The use of ISO 17034-ccredited certified reference materials (CRMs) is recommended to establish metrological traceability []. Regular participation in proficiency testing (PTP) is essential to ensure inter-laboratory result comparability []. For the CIC/AOX/EOX analysis chain, a triple strategy of “process blank–method spike–sequence quality control” should be implemented as recommended in recent reviews, and cross-platform comparisons should be actively conducted [].
3.6. Online Analysis and Process Monitoring
3.6.1. Online Total Chlorine Analysis at Desalting and Process Nodes
Online analyzers based on the MWDXRF principle (consistent with ASTM D7536) enable continuous monitoring of total chlorine in desalted crude or process streams (typical cycle 5 min, supports 15 sec fast scanning), suitable for catching blending operation switches and abnormalities. Their results require closed-loop verification with laboratory standard methods (D4929/D5808). Coupled with periodic monitoring of chloride ions in the aqueous phase by ion chromatography (ASTM D4327), a “oil-water” two-phase chlorine mass balance closure can be further achieved [].
3.6.2. Corrosion and Deposition Risk Correlation Monitoring
In the overhead system of crude distillation units (CDUs), it is recommended to combine continuous total chloride/chloride monitoring with process modeling based on ion thermodynamics (salt dew point/water dew-point calculation) to identify NH4Cl deposition risk and implement closed-loop control measures such as water washing, injection, or load adjustment. This strategy can complement online thickness monitoring and corrosion probe data, providing support for early warning and chemical optimization.
3.7. Green Analysis and ESG Considerations
3.7.1. Method Greenness Assessment
In the method selection and development phase, it is advisable to use assessment tools like GAPI (Green Analytical Procedure Index) or AGREE/AGREEprep to visually score sample consumption, energy consumption, reagent toxicity, waste generation, and automation level [,,]. To move beyond qualitative statements, we benchmarked representative procedures using ComplexGAPI v0.2 beta (2021) and AGREE metric (2020)/AGREEprep (released in 2022). Considering sample/reagent consumption, energy use, waste, and automation, MWDXRF (D7536) achieved the highest greenness among routine methods due to non-destructive operation and short cycle time; CIC (D8150) and micro-coulometry (D5808) scored mid-level, mainly penalized by combustion absorbents and gas demands; GC × GC-MS scored lowest because of solvent-intensive preparation and data-processing load (As shown in Table 1). These results can guide method selection when environmental performance is a co-objective with metrology.

Table 1.
GAPI/AGREE summary (representative lab conditions).
3.7.2. Treatment of Chlorinated By-Products
The generation amount and treatment pathways of by-products such as combustion absorption liquid, extraction residues, and halogen-containing adsorbents should be quantitatively assessed []. Their environmental burden should be incorporated into a comprehensive evaluation system considering greenness (GAC) and whiteness (practicality), avoiding the transfer of pollution from the detection step to the environment (As shown in Table 2) [,].

Table 2.
Comparison of major standard test methods for organic/total chlorine in crude oil and its fractions.
4. Removal and Control Technologies and Engineering Applications
4.1. Source Control and Pre-Refining Management
The fundamental approach to effectively managing organic chlorine is to prevent its entry into the refining system at the source. Key strategies include the following:
Chemical Substitution and Process Optimization: In production and gathering operations, prioritize the use of demulsifiers and additives free of functional groups that easily crack to release chlorine. Research indicates that alternative demulsifiers such as novel non-ionic surfactants, ionic liquids (ILs), and deep eutectic solvents (DESs) can achieve efficient dehydration at lower dosage rates and wider operating windows, thereby reducing the introduction of organic chlorine [,,]. Furthermore, chemicals used for water treatment, corrosion inhibition, and biocides, such as quaternary ammonium compounds (QACs), must be evaluated for their chlorine content and degradation products, with preference given to biodegradable, chlorine-free alternatives [].
Feedstock Qualification and Trade Specifications: Establishing a dual-threshold detection mechanism based on ASTM D4929 (organic chlorine) and D8150/CIC (total organic halogens) at crude receiving, blending, and terminal transfer points can effectively intercept abnormal batches. Analysis of historical incidents shows that implementing online/at-line total-chlorine monitoring upstream of atmospheric and vacuum distillation and pre-hydrotreating units, alongside designing bypass treatment strategies for high-chlorine crudes, can significantly reduce the risk of equipment corrosion and catalyst poisoning [].
Digital Compliance and Traceability Systems: Implement batch retention sampling and full-chain tracking for key suppliers. Establish equivalence between at-line monitoring (e.g., MWDXRF/microcoulometry) and standard methods (D4929), and rely on ISO 17034-accredited certified reference materials (CRMs) for metrological traceability, providing technical assurance for contract compliance.
4.2. Physicochemical Removal Technologies
4.2.1. Enhanced Electrostatic Desalting and Multi-Process Coupling
Practical and simulation studies indicate that optimizing operational parameters such as electric field strength, water content, viscosity, and demulsifier formulation can significantly improve desalting efficiency, effectively removing inorganic chlorine and reducing its risk of secondary conversion in subsequent thermal processes [,,]. Analysis based on actual unit data further clarifies the sensitivity of various operational variables, providing a basis for low-cost revamps and efficiency improvements of existing units []. Coupling electrostatic desalting with new technologies, such as ultrasonic or microwave demulsification or membrane separation, represents an important direction for future improvements in dechlorination efficiency [].
4.2.2. Adsorption and Ion Exchange
Molecular Sieve and Modified Oxide Adsorption: Adsorbents such as Zn/Hβ, Na-LSX, modified ZSM-5, and SAPO-34 show good selectivity for organic chlorine in naphtha []. For instance, Zn/Hβ achieved a dechlorination rate of 73% in ambient batch experiments, and Ni/SAPO-34 maintained approximately 70% dechlorination efficiency under dynamic conditions at 150 °C, demonstrating potential as guard bed materials [].
Surface Modification of Activated Carbon: Recent studies show that introducing nitrogen/oxygen-containing functional groups and finely tuning the pore structure can significantly enhance the adsorption capacity and regeneration stability of activated carbon for various organochlorine compounds, offering the potential for developing low-carbon, low-investment-cost pretreatment options [].
HCl By-product Capture: Molecular sieves like NaY and Y-type can be used for gaseous or liquid-phase absorption of HCl released during processing, reducing its corrosion risk to downstream sections and offering the possibility for resource recovery of chlorine [,].
4.2.3. Membrane Separation and Integrated Processes
Although membrane technology still faces challenges like fouling and wettability when directly treating high-viscosity crude oil, in desalting wastewater and produced water treatment, nanofiltration (NF), reverse osmosis (RO), and membrane distillation (MD) show good potential for desalting, dechlorination, and water reuse []. They can serve as an effective complement to electrostatic desalting units, forming a closed-loop oil–water chlorine management process [,,]. Current research focuses on optimizing energy consumption and footprint, developing antifouling membrane materials, and designing integrated processes [].
Engineering Recommendation: For scenarios with enriched organic chlorine in light fractions, low-temperature adsorption/reactive adsorption can be prioritized as a bypass polishing unit. For full-process chlorine risk, an integrated oil–water chlorine management strategy combining “enhanced electrostatic desalting + membrane water treatment + HCl capture” should be constructed.
4.3. Catalytic and Electrochemical Dechlorination
4.3.1. Hydrodechlorination (HDC)
HDC cleaves the C-Cl bond via R-Cl + H2 → R-H + HCl and is a core technology for deep dechlorination. Common catalysts are noble metal materials like Pd/Al2O3. Its primary challenge is the strong adsorption and irreversible chlorination of active sites by product HCl and Cl−, leading to rapid catalyst deactivation. Introducing promoters like Ni and Fe or constructing alloy structures (e.g., Pd-Ni) are effective strategies to enhance chlorine poisoning resistance [,,]. While hydrodechlorination (HDC) is TRL 8–9 in several petrochemical chloride-removal applications, for crude oil feeds containing a broad mix of organochlorines, the demonstrated readiness is more conservatively TRL 6–7, pending long-duration data on catalyst stability, chloride mass-balance closure, and off-gas handling under representative slates. Performing HDC in aqueous or alkaline environments can buffer HCl and improve reaction selectivity, but excess Cl− can cause active site masking, indicating that HDC needs to be combined with front-end dechlorination and tail gas treatment [].
4.3.2. Process Coupling and Guard Beds
In industrial practice, guard beds/adsorptive reactors are often combined with mild HDC as a pretreatment section for hydrotreating units: the guard bed (typically adsorptive or reactive material) removes the majority of chlorine species; the subsequent HDC unit is responsible for deep purification. This combination can significantly reduce the risk of chlorine poisoning for the main hydrotreating catalyst, lower operational severity, and inhibit undesirable side reactions like over-hydrogenation and cracking [,,].
4.3.3. Electrochemical and Photochemical Dechlorination
Recent studies show that electrochemical reduction/oxidation synergy and photocatalysis can achieve highly selective C-Cl bond cleavage under mild conditions using non-hydrogen sources like formic acid or indirect electron donors, demonstrating low-carbon, low-energy-consumption potential [,,]. Electrochemical and photocatalytic dechlorination show promise for niche, low-throughput polishing after primary chloride removal. However, their current TRL remains 1–2 for crude-relevant matrices; pilot TRL 3–4 will require durability, selectivity, and energy-intensity benchmarks under authentic streams. Given current low TRLs and engineering hurdles (e.g., interfacial transport, electrode/catalyst deactivation, and chloride management), these routes are presented as exploratory options for niche polishing rather than baseline solutions, pending rigorous cradle-to-gate assessments and pilot demonstrations.
4.3.4. Phase-Transfer Catalysis (PTC) Dechlorination
Recent studies report oil-phase dechlorination methods based on phase-transfer catalysis (PTC), achieving efficient and selective dechlorination in model oil, providing a new approach for non-hydrogenation low-temperature dechlorination []. However, its engineering prospects remain unclear; issues such as by-product salt handling, phase separation efficiency, and the safety and cost of solvents require comprehensive evaluation [].
4.4. Biological and Hybrid Treatment Strategies
Biological dechlorination (including reductive dechlorination and co-metabolism) has been applied in groundwater remediation but faces challenges like significant mass transfer limitations, poor substrate tolerance, and difficult nutrient regulation in the multiphase, high-salinity, and potentially biotoxic environment of crude oil systems, resulting in low engineering feasibility [,]. A more realistic strategy is to use it in wastewater treatment units or combine it with electrochemical, adsorption, and other technologies into hybrid processes for treating specific recalcitrant chlorinated compounds.
4.5. Scale-Up and Performance Assessment
The following key performance indicators (KPIs) are recommended for technology evaluation and process selection:
Technical Performance: Dechlorination efficiency (%), system chlorine mass closure, HCl load (ppm, as Cl), corrosion rate (mpy), and catalyst lifetime (h).
Economic and Environmental: Specific energy consumption (kW·h·t−1), specific treatment cost (USD·t−1), and carbon intensity (kg CO2e·t−1).
Integrated Strategy and Decision Recommendation:
For intermittent high-chlorine crude, proceed as follows: Prioritize bypass adsorption/reactive adsorption, combined with online total-chlorine monitoring.
For persistent organic chlorine in light fractions, proceed as follows: Add a combination process of guard bed + mild HDC.
For high inorganic chlorine and corrosion-sensitive systems, proceed as follows: Implement enhanced electrostatic desalting + HCl capture + overhead corrosion prediction linkage.
To support decision-making, we summarize indicative techno-economics (relative CAPEX/OPEX tiers and dominant cost drivers) for alternative chloride-control strategies, including bypass adsorption, full HDC, and hybrid guard-bed + mild HDC. Values are site-specific and should be refined with unit hydrogen pricing, adsorbent working capacity, replacement intervals, and planned runtime between turnarounds (As shown in Table 3).

Table 3.
Evaluation of Dechlorination Treatment Scenarios and Cost Implications.
TRL ratings in Table 4 follow the EU-H2020/ISO 16290 mapping: TRL 1–3 (proof-of-concept, lab bench with model oils), TRL 4–5 (bench/pilot with relevant matrices, closed mass balance, and ≥100 h stability), TRL 6–7 (pilot/demo in relevant units with integrated HCl capture and KPI tracking), and TRL 8–9 (qualified in continuous industrial service with documented MTBF/availability and QA/QC) []. For adsorption/guard beds and HDC, TRL ≥ 7 requires material balance closure (Cl-in/Cl-out) and catalyst deactivation rates reported under real feeds; for electrochemical/photocatalytic/PTC routes, current demonstrations remain TRL 2–4 due to scale, mass-transfer, and durability gaps (As shown in Table 4) [].

Table 4.
Comprehensive comparison of organic chlorine removal technologies for crude oil and its fractions.
5. Risk, Standards, and Regulatory Framework
5.1. Quality Indicators and Internal Control Limits
The detection and assessment of organic chlorine in crude oil and its fractions primarily rely on ASTM standard methods. Among these, ASTM D4929 is widely used as the reference method. It involves the removal of inorganic chlorine and H2S by alkaline or water washing, followed by quantification of organic chlorine using microcoulometry (Procedure B) or XRF (Procedure C). It explicitly applies to samples with concentrations ≥1 mg·kg−1 and notes that organic chlorine anomalies are typically associated with contamination or operational risk. The U.S. Strategic Petroleum Reserve (SPR) routinely employs D4929 B/C procedures as part of its quality compliance basis for receipt and exchange operations.
There is currently no unified statutory upper limit for organic chlorine in crude oil internationally. However, limit requirements are commonly stipulated in trade contracts and pipeline transportation terms. For instance, following the 2019 “Druzhba contamination incident”, Global Platts introduced a 3 mg·kg−1 upper limit of organic chlorine for OSN naphtha as a market norm. Based on unit tolerance and historical operational experience, many refineries set their crude intake thresholds between 1 and 5 mg·kg−1, often using ASTM D8150 (CIC) or online total-chlorine monitoring for cross-verification. To ensure the comparability and traceability of results, the use of ISO 17034-accredited certified reference materials (CRMs) and regular participation in proficiency testing (PTP) is strongly advised.
5.2. Corrosion Mechanisms and Safety Impacts
The cracking of organic chlorine releases HCl in high-temperature processes like distillation and cracking. This HCl reacts with NH3 in the system to form NH4Cl, triggering three main failure modes:
Salt Deposition: NH4Cl precipitates in the cold sections of heat exchangers and distillation column overheads, causing reduced flow area and increased pressure drop.
Under-Deposit Corrosion: A local confined environment forms beneath the deposits, promoting pitting and crevice corrosion.
Dew-Point Corrosion: HCl condenses with water vapor in low-temperature areas, forming a highly acidic environment that accelerates equipment corrosion [,].
Recent studies, through failure analysis and electrochemical testing, have further quantified the coupling mechanism between deposition- and dissolution-localized acidification. They identify a high-risk window for NH4Cl-HCl complex corrosion around 98–101 °C and pH ≈ 3 []. To improve the accuracy of corrosion prediction and control, ion dew point/salt point models based on multi-scale electrolyte (MSE) thermodynamics are gradually replacing traditional empirical margin methods, enabling the online optimization of ammonia injection, water-washing, and blending strategies. Integrating this predictive capability with online total-chlorine monitoring, aqueous chloride ion analysis (by IC), and spot thickness measurements can significantly enhance the early identification of salt plugging and corrosion.
5.3. Compliance Requirements and Trade Practices
Recent organic chlorine contamination incidents demonstrate that contractual constraints and market mechanisms have become the primary enforcement tools for quality control. The following procedural measures are recommended to mitigate trade and operational risk.
Explicitly define organic chlorine content limits and detection methods in contracts (preferring ASTM D4929 as primary, with CIC as an arbitration supplement).
Implement third-party independent sampling, dual-laboratory testing, and full-chain sample retention.
Execute isolation, re-blending, or bypass treatment for abnormal batches, assessing the risk of gaseous HCl and NH4Cl deposition.
Establish a regular proficiency testing and traceability mechanism, referencing the SPR quality manual, to reduce methodological bias and dispute cost.
In summary, establishing a comprehensive chlorine management framework from “source → trade → refinery”, combined with standardized detection methods and contractual accountability mechanisms, is key to controlling organic chlorine risk and ensuring long-term unit operation.
6. Data-Driven Source Apportionment and Process Optimization
6.1. Fingerprint Analysis and Multivariate Statistical Methods
The identification of organic chlorine in complex petroleum matrices can leverage the high-dimensional fingerprint information generated by comprehensive two-dimensional gas chromatography (GC × GC). Its two-dimensional retention behavior can be converted into characteristic images and processed using pixel or peak cluster-level statistical methods. Recent studies have systematically reviewed reproducible workflows for peak alignment, signal normalization, batch correction, and fingerprint similarity evaluation, significantly improving identification efficiency and interpretability []. The latest research further applies imaging fingerprint technology to the source discrimination of crude oil and its fractions, greatly reducing the cost of manual spectrum interpretation. This is suitable for the engineering practice path of “rapid screening first, targeted confirmation later” []. Multivariate statistical methods such as principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) remain highly valuable for such high-dimensional data: PCA can be used for unsupervised clustering of sources or batches, while PLS-DA is suitable for supervised classification scenarios like “normal vs. abnormal” or “geological source vs. exogenous contamination” []. Recent studies demonstrate end-to-end ML pipelines for petroleum fingerprints: GC × GC imaging with pixel-based chemometrics for source/batch discrimination; hybrid models that combine retention-time modeling with CNN/ViT regressors to predict bulk properties and trace contaminants; and domain adaptation to transfer models across batches and instruments. Published applications in fuels compliance and pre-salt crude characterization show that such pipelines reduce manual review and improve recall for “abnormal halogenated clusters”. When combined with CSIA-Cl, models gain orthogonal features for transformation-pathway discrimination, improving robustness in arbitration and root-cause analysis. To enhance discrimination reliability, it is recommended to unify data preprocessing and peak alignment rules, and incorporate total indicator data (from CIC/microcoulometry) into statistical models, constructing a “total amount-species-fingerprint” triple verification framework [].
6.2. Application of Machine Learning in Spectral Deconvolution and Concentration Prediction
Facing overlapping chromatographic peaks and strong background interference in complex matrices, machine learning (ML) and deep learning techniques offer new pathways for high-throughput, automated parsing. Research shows that deep learning models trained jointly on real and synthetic GC × GC data can efficiently identify suspected exogenous contaminants (e.g., chlorinated compounds), significantly reducing the need for manual review []. For quantitative prediction, convolutional neural networks (CNNs) or vision transformer (ViT) models trained on two-dimensional chromatographic fingerprints can act as regressors, directly outputting predictions for organic chlorine concentration or dechlorination efficiency. Their cross-batch generalization capability can be improved by incorporating retention time modeling []. To achieve robust modeling under small sample conditions, it is recommended to employ synthetic data augmentation, domain adaptive transfer learning, and feature attribution analysis based on Shapley values. A workflow of “Total amount calibration (CIC) → GC × GC fingerprint acquisition → End-to-end ML prediction” is proposed: Use the traceable concentration provided by CIC or microcoulometry as the supervised label to train the model to output concentration and uncertainty estimates, and automatically screen highly suspicious peak clusters for manual review [,].
Compound-specific chlorine isotope analysis (CSIA-Cl) provides orthogonal dimensional information for source apportionment and transformation pathway discrimination. Integrating multi-isotope features like 37Cl and 13C into machine learning models can significantly enhance the ability to distinguish between exogenous contamination and process-transformed chlorine [].
6.3. Digital Twin and Online Control Loop
Integrating mechanistic models (e.g., ion dew point/salt point prediction models based on multi-scale electrolyte (MSE) thermodynamics) with process big data (online chlorine monitoring, process parameters) to construct a corrosion digital twin system is a cutting-edge direction for achieving predictive control and intelligent management. Such systems can dynamically predict NH4Cl deposition windows and corrosion rates, providing decision support for optimizing water washing, ammonia injection, and blending strategies. This replaces traditional fixed safety margin methods, thereby achieving energy savings and consumption reduction. Industry practice shows that combining first-principles models with process historical data to build a corrosion digital twin system enables closed-loop management of early warning, strategy recommendation, and action tracking. Typical applications include the dynamic coupling prediction of NH4Cl deposition, acid dew-point corrosion, and local pH changes. Integrating this system with online total chlorine (e.g., MWDXRF/microcoulometry) and aqueous chloride ion (IC) monitoring can further refine the “oil-water” chlorine mass balance and model self-calibration.
Minimum Viable Implementation (MVI) Framework:
Data Foundation: Build a sample–process–equipment correlation database; achieve automatic collection of online total chlorine/ion concentration data (1–5 min granularity).
Model Construction: Fuse MSE mechanistic models with machine learning for error correction (e.g., using quantile loss functions to output uncertainty).
Control Linkage: Integrate with advanced process control (APC) systems to achieve automatic adjustment of ammonia injection, water washing, and load switching; link the start–stop logic of guard bed/adsorption units.
Performance Evaluation: Use key performance indicators (KPIs) such as corrosion warning lead time, abnormal batch interception rate, specific energy consumption, and chemical consumption to regularly evaluate and iteratively optimize the model.
Regarding the “digital twin core” architecture and verification for integrating ion thermodynamic models with machine learning, we have designed a simple architecture consisting of five levels: (1) data collection and quality control; (2) physical engine (ion thermodynamics and mass balance); (3) features and soft sensors; (4) machine learning, including uncertainty and drift detection; and (5) actions and audits. The verification process combines cross-validation of historical activities, forward-looking backtesting, and acceptance criteria related to safety and quality assurance/quality control checkpoints.
At the unit level, refineries have begun coupling online total-chlorine analyzers (MWDXRF/microcoulometry) at desalter outlets with ion-thermodynamic dew/salt-point models (MSE) to form corrosion digital twins for CDU overheads (As shown in Figure 4). Reported benefits include earlier identification of NH4Cl deposition windows, tighter ammonia/water-wash control, and better alignment between oil–water chloride mass balance and maintenance planning. We position our MVI as a minimal, auditable instantiation of these elements, emphasizing data quality (calibration to standard methods), model interpretability (salt/acid dew-point physics), and action traceability (APC linkage).
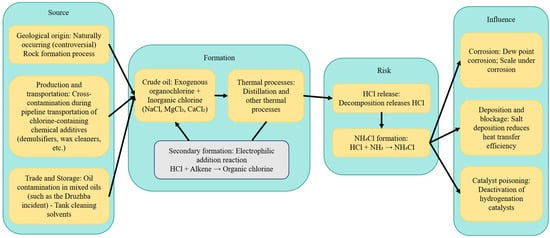
Figure 4.
Schematic diagram of the data-driven intelligent management and control loop for organochlorine compounds.
7. Research Gaps and Future Directions
7.1. Challenges in Chlorine Analysis of Highly Complex Matrices
For heavy crude oils, residues, and extra-heavy oils, mainstream total analysis methods like combustion-ion chromatography (CIC) and adsorbable organic halogen (AOX) still face significant matrix interference issues. Recent critical studies point out that factors such as gas path contamination, absorbent impurities, inorganic chlorine residues, and incomplete combustion can introduce systematic positive biases, which are particularly pronounced in high-salinity or high-chlorine backgrounds [,]. It is recommended to establish a four-step quality control procedure of “unwashed-washed-spiked recovery-process blank” and introduce steps like gel permeation chromatography (GPC) or membrane pretreatment for extra-heavy oils to remove asphaltene interference []. Notably, the distillation-fractional determination strategy employed by ASTM D4929 is inherently indirect; its applicability and reproducibility in complex matrices still require more inter-laboratory validation.
7.2. Method Comparability and Metrological Traceability
There is still a lack of systematic comparative studies between different standard methods (e.g., ASTM D4929 B/C, D8150, D5808, D7536, and GC × GC-MS). Large-scale cross-laboratory comparison studies (involving ≥20 laboratories) are needed, utilizing methods like Passing–Bablok regression and bias–variance analysis to establish equivalence criteria and arbitration rules applicable to multiple matrices and concentration ranges. ASTM emphasizes the need for sufficient statements of precision and bias in research reports; however, large-sample collaborative studies focusing on organic chlorine in crude oil remain scarce.
The shortage of reference materials is another critical issue. Existing standard reference materials (e.g., NIST SRM 1818a) provide certified chlorine content but are primarily based on lubricant matrices. Commonly used crude oil reference materials (e.g., SRM 2722) mostly focus on sulfur, mercury, and water content, lacking certified values for chlorine, creating a gap in the metrological traceability chain. Although some ISO 17034 producers supply oil-based chlorine standards, they are often limited to solutions or simulated matrices, failing to represent the complexity of real crude oil. It is recommended to collaborate with standardizing bodies to develop matrix-matched certified reference materials (CRMs) for real crude oil and to establish a three-step arbitration and confirmation process centered on “D4929 → CIC/Microcoulometry → GC × GC/CSIA-Cl”.
7.3. Low-Carbon and Anti-Poisoning Dechlorination Technologies
In hydro dechlorination (HDC), the strong adsorption and irreversible chlorination of active sites by HCl and Cl− remain the primary causes of catalyst deactivation. Although alloy systems like Pd-Ni/Pd-Fe show potential for improving chlorine tolerance, their long-term stability and regenerability in continuous processing of real oil feeds require further validation. Recent research indicates the feasibility of non-noble metal-based materials in continuous dichlorination–regeneration cycles; if integrated with HCl capture and resource recovery technologies, a closed-loop chlorine management system could be formed [].
Non-hydrogen routes such as electrocatalytic dechlorination (ECH/ECHD) and hydrogen supply via formic acid decomposition demonstrate significant low-carbon potential, as they can achieve C-Cl bond cleavage under mild conditions and couple with renewable energy. However, major bottlenecks for engineering applications remain, including mass transfer efficiency in multiphase organic systems, electrode corrosion resistance, and long-term stability [,]. Pilot-scale validation along the path of “Guard Bed → Mild HDC → HCl Capture → Resource Recovery” is recommended. Energy consumption, hydrogen consumption, carbon intensity, and chlorine recovery efficiency should be incorporated into life cycle assessment (LCA) for an objective evaluation of techno-economic viability.
7.4. Data Sharing and Algorithm Interpretability
The field currently lacks high-quality, openly shared benchmark datasets, limiting the generalization capability and performance evaluation of machine learning algorithms in tasks like chromatographic fingerprint parsing, concentration prediction, and source discrimination. Existing GC × GC open data platforms and chemometric methods can be partially migrated to crude oil organic chlorine research, but a comprehensive data resource covering light/heavy/high-salinity crudes and containing intentional spiking—total amount calibration—cross-batch alignment is still missing [,,]. It is advised to promote the establishment of open-source datasets and benchmark frameworks through multi-institutional collaboration, unifying data formats, peak alignment algorithms, validation standards, and providing unified evaluation metrics for feature importance, transfer robustness, and uncertainty quantification.
7.5. Evolution of Process Modeling and Control
Corrosion control strategies are shifting from traditional “empirical safety margins” to “model-predictive decision-making”. Integrating ion thermodynamic models (e.g., OLI systems) with online chlorine monitoring and anion analysis data enables dynamic prediction of dew/salt points and optimized control of ammonia injection/water washing. Industry practice shows that such digital twin systems can already provide early warning and strategy recommendations in crude distillation unit (CDU) overheads and hydrotreating units. However, their model transferability and generalization ability for material–process condition coupling effects still require more cross-unit validation. It is recommended to publish a minimum viable guideline (MVG) covering process modeling, data specifications, and KPI evaluation, clarifying the equivalence conditions and scope of application for online/at-line sensing technologies and standard methods (e.g., D4929).
8. Conclusions and Outlook
8.1. Conclusions
This review synthesizes the end-to-end management of organically bound chlorine (Org-Cl) in crude oil—from origin hypotheses and analytical fidelity to mitigation and plant-level control—and consolidates scattered evidence into an actionable framework. We reconcile standard and advanced analyses via a quantitative metrology map (typical LOD/LOQ ranges, accuracy, and repeatability) and greenness indicators, enabling fit-for-purpose method selection rather than single-method prescriptions. Mitigation options are positioned with contextual TRLs, explicitly distinguishing petrochemical settings from crude-relevant implementations. Indicative techno-economics (relative CAPEX/OPEX tiers and dominant cost drivers) connect analytical confidence to deployment practicality. Building on these elements, we define a practical, auditable minimum viable implementation (MVI) that couples online total-chlorine monitoring with a physics-guided digital-twin core and actionable KPIs to support closed-loop decisions []. Together, these contributions align laboratory evidence, process constraints, and operations governance to deliver transparent and scalable Org-Cl control across variable crude slates.
8.2. Limitations
The verification of MVI is divided into three levels: (i) measurement equivalence—comparison tests are conducted between the online total-chlorine analyzer and laboratory standards (D4929/D5808/CIC), using the Parsons–Babcock analysis method and the Brand–Oltramann analysis method, and detection is carried out using standard reference materials and spiked recovery rates; (ii) physical consistency—ion thermodynamic models (such as MSE) are compared with the observed dew point/salinity points and the chloride mass balance in each test cycle; and (iii) operational key performance indicators—prospective trials record the early warning lead time, abnormal batch interception rate, corrosion alarm accuracy/recall rate, and the chemicals/energy saved by avoiding incidents.
The framework presumes (i) stable method equivalence between online total-Cl analyzers and laboratory standards (D4929/D8150/D5808), (ii) reliable oil–water phase sampling, and (iii) transferability of MSE-based dew/salt-point predictions across crude slates. Current limitations include the following: (a) measurement bias/uncertainty (e.g., combustion blanks, matrix effects) that propagate into model calibration; (b) incomplete Cl-in/Cl-out closure at low-ppm levels; (c) limited >1000 h continuous runtime data for adsorption/HDC guard trains under real feeds; (d) uneven data quality and heterogeneous reporting across studies; (e) the lack of standardized open datasets (e.g., aligned GC × GC fingerprints with CIC/pyrolysis-coulometry or CSIA-Cl labels) for reproducible benchmarking; and (f) site-specific uncertainty in the indicative CAPEX/OPEX tiers. These factors constrain the generality of quantitative ranges and motivate the validation priorities below.
8.3. Outlook
Develop matrix-matched CRMs for Org-Cl in real crudes; standardize a four-step QC workflow (“unwashed-washed-spike-blank”) for heavy/high-salinity matrices; report figures of merit with uncertainty and embed GAPI/AGREE greenness scoring into method selection.
Run crude-relevant pilots with ≥1000 h operation, documented Cl-in/Cl-out closure, off-gas management, and catalyst/adsorbent durability metrics; scale guard bed + mild HDC + HCl capture as a balanced baseline; evaluate electrochemical/PTC routes only on niche high-risk streams with durability metrics before pilot.
Deploy physics-guided features (salt/acid dew-point predictors, mass-balance residuals), uncertainty quantification, and drift monitoring; publish open GC × GC datasets with aligned fingerprints and CIC labels; and validate twins with prospective back-tests using lead time, interception rate, precision/recall, and chemicals/energy per avoided event.
Execute the MVI validation plan in three layers: (i) measurement equivalence via Passing–Bablok/Bland–Altman against D4929/D5808/CIC with CRMs and spikes; (ii) physics conformance by back-testing the MSE model against dew/salt points and chloride mass balance; and (iii) operational KPIs tracked in prospective trials, complemented by inter-lab proficiency tests and periodic mass-balance audits. Meeting these gates will convert the MVI from a validated concept into a low-risk, scalable pillar of refinery chloride control [].
Author Contributions
Z.C. (corresponding author) was responsible for the preparation, creation, and presentation of the published work, specifically writing the initial draft (including substantive translation); Methodology was developed or designed, and models were created by W.L. and Y.S.; Q.C. and K.W. applied statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the C2 Technology Project of China National Petroleum Corporation: Key Technology Research and Application on Quality Risk Management of Crude Oil and Oil Additives. The project number is 2024YQX201. And the APC was funded by Weidong Liu.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AED | Atomic emission detector (element-selective GC detection). |
| AGREE | Analytical GREEnness metric. |
| AGREEprep | Greenness metric for sample preparation. |
| AOX | Adsorbable organic halogens; halogenated organics retained on activated carbon and quantified after combustion. |
| APC | Advanced process control. |
| ASTM D4929 | Organic chlorides in light petroleum distillates (Procedures A/B/C: titration/microcoulometry/XRF). |
| ASTM D5808 | High-temperature pyrolysis microcoulometry; total chlorine determination. |
| ASTM D8150 | Combustion-ion chromatography; total organic halogens in light fractions. |
| Bland–Altman | Agreement analysis for two measurement methods (bias and limits of agreement). |
| CAPEX | Capital expenditure (equipment and installation). |
| CDU | Crude distillation unit. |
| CIC | Combustion-ion chromatography; ASTM D8150 for total organic halogens in light fractions. |
| CRM | Certified reference material (ISO 17034). |
| CSIA-Cl | Compound-specific chlorine isotope analysis. |
| DESs | Deep eutectic solvents. |
| Dew point/Salt point | Onset of acid/water dew or ammonium-salt deposition; predicted by thermodynamic models. |
| DMI | Direct matrix introduction (chromatography inlet). |
| ECH/ECHD | Electrocatalytic (hydro)dehalogenation. |
| ELCD | Electrolytic conductivity detector (halogen-selective) for GC. |
| EOX | Extractable organic halogens; solvent-extractable halogenated organics quantified after combustion. |
| FT-ICR MS | Fourier transform ion cyclotron resonance mass spectrometry. |
| GAC | Green analytical chemistry (greenness). |
| GAPI | Green analytical procedure index. |
| GC × GC-MS | Comprehensive two-dimensional gas chromatography–mass spectrometry (often TOFMS). |
| GC-MS | Gas chromatography–mass spectrometry. |
| GPC | Gel permeation chromatography. |
| HCl | Hydrogen chloride. |
| HDC | Hydrodechlorination (catalytic C-Cl cleavage with H2). |
| HS-SPME | Headspace solid-phase microextraction. |
| ILs | Ionic liquids. |
| Inorganic Cl− | Inorganic chloride ion. |
| KPI | Key performance indicator. |
| LLE | Liquid–liquid extraction. |
| LOD | Limit of detection. |
| LOQ | Limit of quantitation. |
| Mass-balance closure | Reconciliation of Cl-in vs. Cl-out across phases/units. |
| MD | Membrane distillation. |
| MSE | Multi-scale electrolyte thermodynamic model for ion properties and dew/salt-point prediction. |
| MVI | Minimum viable implementation (auditable closed-loop deployment bundle). |
| MWDXRF | Monochromatic wavelength dispersive X-ray fluorescence; ASTM D7536 for total chlorine. |
| NF | Nanofiltration (membrane). |
| NHT | Naphtha hydrotreating unit. |
| OPEX | Operating expenditure (consumables, energy, labor). |
| Orbitrap MS | High-resolution Orbitrap mass spectrometry. |
| Org-Cl | Organically bound chlorine in crude oil. |
| Passing–Bablok | Non-parametric regression for method comparison/equivalence assessment. |
| ppm (as Cl) | Parts per million expressed as chlorine. |
| Precision/Recall | Classification metrics used for ML model evaluation (PPV and sensitivity). |
| PT/PTP | Proficiency testing/proficiency testing program. |
| PTC | Phase-transfer catalysis (biphasic nucleophilic substitution). |
| QA/QC | Quality assurance/quality control. |
| QACs | Quaternary ammonium compounds (biocides/corrosion control). |
| RI | Retention index (chromatography). |
| RO | Reverse osmosis (membrane). |
| SPE | Solid-phase extraction. |
| Total Cl | Total chlorine (organic + inorganic) in a sample. |
| TOX | Total organic halogens; AOX/EOX-like or combustion-based total halogen content. |
| TRL | Technology readiness level (ISO 16290/EU-H2020 mapping). |
| XSD | Halogen-specific detector for GC. |
References
- Mitra, S.; Sulakhe, S.; Shown, B.; Mandal, S.; Das, A.K. Organic Chlorides in Petroleum Crude Oil: Challenges for Refinery and Mitigations. ChemBioEng Rev. 2022, 9, 319–332. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Obot, I.B. Corrosion Challenges in Petroleum Refinery Operations: Sources, Mechanisms, Mitigation, and Future Outlook. J. Saudi Chem. Soc. 2021, 25, 101370. [Google Scholar] [CrossRef]
- Chis, T.; Sterpu, A.E.; Săpunaru, O.V. The Effect of Corrosion on Crude Oil Distillation Plants. ChemEngineering 2022, 6, 41. [Google Scholar] [CrossRef]
- Li, R.; Huang, H.; Wang, X.; Wang, Y. Effect of Ammonium Salt on Corrosion of Pipelines and Components in a Crude Oil Distillation Column: Electrochemical and AIMD Studies. Corros. Sci. 2022, 203, 110362. [Google Scholar] [CrossRef]
- Spaan, K.M.; Yuan, B.; Plassmann, M.M.; Benskin, J.P.; de Wit, C.A. Characterizing the Organohalogen Iceberg: Extractable, Multihalogen Mass Balance Determination in Municipal Wastewater Treatment Plant Sludge. Environ. Sci. Technol. 2023, 57, 9309–9320. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Li, X.; Zhu, J. Distribution and Identification of Chlorides in Distillates from YS Crude Oil. Energy Fuels 2015, 29, 1391–1396. [Google Scholar] [CrossRef]
- Pagliano, E.; Gajdosechova, Z.; Lopez-Linares, F.; Mester, Z. Conversion of Inorganic Chlorides into Organochlorine Compounds during Crude Oil Distillation: Myth or Reality? Energy Fuels 2021, 35, 894–897. [Google Scholar] [CrossRef]
- Hassanshahi, N.; Hu, G.; Li, J. Application of Ionic Liquids for Chemical Demulsification: A Review. Molecules 2020, 25, 4915. [Google Scholar] [CrossRef] [PubMed]
- ASTM D8150-22; Standard Test Method for Determination of Organic Chloride Content in Crude Oil by Distillation Followed by Detection Using Combustion Ion Chromatography. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- ASTM D4929-24; Standard Test Method for Determination of Organic Chloride Content in Crude Oil. ASTM International: West Conshohocken, PA, USA, 2025. [CrossRef]
- Souchon, V.; Maleval, M.; Chainet, F.; Lienemann, C.-P. Chlorine Speciation in Complex Hydrocarbon Matrices Using GC-ICP-MS/MS. J. Anal. At. Spectrom. 2023, 38, 1634–1642. [Google Scholar] [CrossRef]
- Melder, J.; Zinsmeister, J.; Grein, T.; Jürgens, S.; Köhler, M.; Oßwald, P. Comprehensive Two-Dimensional Gas Chromatography: A Universal Method for Composition-Based Prediction of Emission Characteristics of Complex Fuels. Energy Fuels 2023, 37, 4580–4595. [Google Scholar] [CrossRef]
- ASTM D5808-23; Standard Test Method for Determining Chloride in Aromatic Hydrocarbons and Related Chemicals by Microcoulometry. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Horst, A.; Renpenning, J.; Richnow, H.-H.; Gehre, M. Compound Specific Stable Chlorine Isotopic Analysis of Volatile Aliphatic Compounds Using Gas Chromatography Hyphenated with Multiple Collector Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2017, 89, 9131–9138. [Google Scholar] [CrossRef]
- ASTM D7536-24; Standard Test Method for Chlorine in Aromatics by Monochromatic Wavelength Dispersive X-Ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2024. [CrossRef]
- Zimmermann, J.; Halloran, L.J.S.; Hunkeler, D. Tracking Chlorinated Contaminants in the Subsurface Using Compound-Specific Chlorine Isotope Analysis: A Review of Principles, Current Challenges and Applications. Chemosphere 2020, 244, 125476. [Google Scholar] [CrossRef]
- Kowalewska, Z.; Brzezińska, K.; Zieliński, J.; Pilarczyk, J. Method Development for Determination of Organic Fluorine in Gasoline and Its Components Using High-Resolution Continuum Source Flame Molecular Absorption Spectrometry with Gallium Fluoride as a Target Molecule. Talanta 2021, 227, 122205. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, J.; Tan, X.; Li, J.; Croué, J.-P.; Chen, B. Insights into Adsorbable Organic Halogen Analysis: Two Overlooked Factors Impacting Water Quality Assessment. Sci. Total Environ. 2024, 928, 172429. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Gong, X.; He, M.; Wang, X.; Zhao, R.; Hao, Z.; Liu, J. Total Organic Halogen (TOX) Analysis in Waters: A Short Review. Environ. Pollut. Bioavailab. 2023, 35, 2203350. [Google Scholar] [CrossRef]
- Gröger, T.M.; Käfer, U.; Zimmermann, R. Gas Chromatography in Combination with Fast High-Resolution Time-of-Flight Mass Spectrometry: Technical Overview and Perspectives for Data Visualization. TrAC Trends Anal. Chem. 2020, 122, 115677. [Google Scholar] [CrossRef]
- Andersson, A.; Ashiq, M.J.; Shoeb, M.; Karlsson, S.; Bastviken, D.; Kylin, H. Evaluating Gas Chromatography with a Halogen-Specific Detector for the Determination of Disinfection by-Products in Drinking Water. Env. Sci. Pollut. Res. 2019, 26, 7305–7314. [Google Scholar] [CrossRef]
- Nicolas, M.; Theurillat, X.; Redeuil, K.; Nagy, K. Monochromatic Wavelength Dispersive X-Ray Fluorescence as a Tool for Monitoring the Source of Chlorine in the Formation of Monochloropropane-Diol Esters during Vegetable Oil Refining. J. Agric. Food Res. 2022, 8, 100297. [Google Scholar] [CrossRef]
- Ajaero, C.; Vander Meulen, I.; Heshka, N.E.; Xin, Q.; McMartin, D.W.; Peru, K.M.; Chen, H.; McKenna, A.M.; Reed, K.; Headley, J.V. Evaluations of Weathering of Polar and Nonpolar Petroleum Components in a Simulated Freshwater–Oil Spill by Orbitrap and Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2024, 38, 6753–6763. [Google Scholar] [CrossRef]
- Schmidt, E.M.; Pudenzi, M.A.; Santos, J.M.; Angolini, C.F.F.; Pereira, R.C.L.; Rocha, Y.S.; Denisov, E.; Damoc, E.; Makarov, A.; Eberlin, M.N. Petroleomics via Orbitrap Mass Spectrometry with Resolving Power above 1,000,000 at m/z 200. RSC Adv. 2018, 8, 6183–6191. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Gui, J.; Li, X.; Qi, G. Compound-Specific Chlorine Isotope Analysis of Organochlorine Pesticides by Gas Chromatography-Negative Chemical Ionization Mass Spectrometry. J. Anal. Methods Chem. 2021, 2021, 8874679. [Google Scholar] [CrossRef] [PubMed]
- Ponsin, V.; Torrentó, C.; Lihl, C.; Elsner, M.; Hunkeler, D. Compound-Specific Chlorine Isotope Analysis of the Herbicides Atrazine, Acetochlor, and Metolachlor. Anal. Chem. 2019, 91, 14290–14298. [Google Scholar] [CrossRef]
- Vinyes-Nadal, M.; Masbou, J.; Kümmel, S.; Gehre, M.; Imfeld, G.; Otero, N.; Torrentó, C. Novel Extraction Methods and Compound-Specific Isotope Analysis of Methoxychlor in Environmental Water and Aquifer Slurry Samples. Sci. Total Environ. 2024, 931, 172858. [Google Scholar] [CrossRef]
- ASTM D5854-25; Standard Practice for Mixing and Handling of Liquid Samples of Petroleum and Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2025. [CrossRef]
- ASTM D4057-22; Standard Practice for Manual Sampling of Petroleum and Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Marguí, E.; Eichert, D.; Jablan, J.; Bilo, F.; Depero, L.E.; Pejović-Milić, A.; Gross, A.; Stosnach, H.; Kubala-Kukuś, A.; Banaś, D.; et al. An Overview of the Applications of Total Reflection X-Ray Fluorescence Spectrometry in Food, Cosmetics, and Pharmaceutical Research. J. Anal. At. Spectrom. 2024, 39, 1700–1719. [Google Scholar] [CrossRef]
- Bulsink, P.; de Miguel Mercader, F.; Sandström, L.; van de Beld, B.; Preto, F.; Zacher, A.; Oasmaa, A.; Dahmen, N.; Funke, A.; Bronson, B. Results of the International Energy Agency Bioenergy Round Robin on the Analysis of Heteroatoms in Biomass Liquefaction Oils. Energy Fuels 2020, 34, 11123–11133. [Google Scholar] [CrossRef]
- Holkem, A.P.; Agostini, G.; Costa, A.B.; Barin, J.S.; Mello, P.A. A Simple and Green Analytical Alternative for Chloride Determination in High-Salt-Content Crude Oil: Combining Miniaturized Extraction with Portable Colorimetric Analysis. Processes 2024, 12, 2425. [Google Scholar] [CrossRef]
- ISO 17034:2016; General Requirements for the Competence of Reference Material Producers. International Organization for Standardization (ISO): Beijing, China, 2016.
- D4327-17R25; Standard Test Method for Anions in Water by Suppressed Ion Chromatography. ASTM International: West Conshohocken, PA, USA, 2025. [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Tobiszewski, M.; Wojnowski, W.; Psillakis, E. A Tutorial on AGREEprep an Analytical Greenness Metric for Sample Preparation. Adv. Sample Prep. 2022, 3, 100025. [Google Scholar] [CrossRef]
- Romero, A.; Moreno, I.; Escudero, L.; Yuste, R.; Pizarro, P.; Moreno, J.M.; Serrano, D.P. Dechlorination of a Real Plastic Waste Pyrolysis Oil by Adsorption with Zeolites. J. Environ. Chem. Eng. 2024, 12, 112638. [Google Scholar] [CrossRef]
- Yin, L.; Yu, L.; Guo, Y.; Wang, C.; Ge, Y.; Zheng, X.; Zhang, N.; You, J.; Zhang, Y.; Shi, M. Green Analytical Chemistry Metrics for Evaluating the Greenness of Analytical Procedures. J. Pharm. Anal. 2024, 14, 101013. [Google Scholar] [CrossRef]
- Hammad, S.F.; Hamid, M.A.A.; Adly, L.; Elagamy, S.H. Comprehensive Review of Greenness, Whiteness, and Blueness Assessments of Analytical Methods. Green Anal. Chem. 2025, 12, 100209. [Google Scholar] [CrossRef]
- Husain, A.; Adewunmi, A.A.; Kamal, M.S.; Mahmoud, M.; Al-Harthi, M.A. Demulsification of Heavy Petroleum Emulsion Using Pyridinium Ionic Liquids with Distinct Anion Branching. Energy Fuels 2021, 35, 16527–16533. [Google Scholar] [CrossRef]
- Tang, L.; Wang, T.; Xu, Y.; He, X.; Yan, A.; Zhang, Z.; Li, Y.; Chen, G. Research and Application Progress of Crude Oil Demulsification Technology. Processes 2024, 12, 2292. [Google Scholar] [CrossRef]
- Low, J.Y.; Khe, C.S.; Usman, F.; Hassan, Y.M.; Lai, C.W.; You, K.Y.; Lim, J.W.; Khoo, K.S. Review on Demulsification Techniques for Oil/Water Emulsion: Comparison of Recyclable and Irretrievable Approaches. Environ. Res. 2024, 243, 117840. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, R.; Sand, W.; Mathivanan, K.; Zhang, Y.; Wang, N.; Duan, J.; Hou, B. Comprehensive Review on the Use of Biocides in Microbiologically Influenced Corrosion. Microorganisms 2023, 11, 2194. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, P.; Jasra, R.V. Sorption of HCl from an Aromatic Hydrocarbon Mixture Using Modified Molecular Sieve Zeolite 13X. ACS Omega 2021, 6, 28742–28751. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Argaez, M.A.; Abreú-López, D.; Gracia-Fadrique, J.; Dutta, A. Numerical Study of Electrostatic Desalting: A Detailed Parametric Study. Processes 2022, 10, 2118. [Google Scholar] [CrossRef]
- Kooti, G.; Dabir, B.; Taherdangkoo, R.; Butscher, C. Modelling Droplet Size Distribution in Inline Electrostatic Coalescers for Improved Crude Oil Processing. Sci. Rep. 2023, 13, 20209. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Sun, Z.; Wang, X.; Chen, Q.; Liu, W.; Qi, Z.; Wei, L.; Li, B. Molecular Dynamics Study of Droplet Electrocoalescence in the Oil Phase and the Gas Phase. Sep. Purif. Technol. 2021, 278, 119622. [Google Scholar] [CrossRef]
- Ranaee, E.; Ghorbani, H.; Keshavarzian, S.; Ghazaeipour Abarghoei, P.; Riva, M.; Inzoli, F.; Guadagnini, A. Analysis of the Performance of a Crude-Oil Desalting System Based on Historical Data. Fuel 2021, 291, 120046. [Google Scholar] [CrossRef]
- Liu, S.; Otero, J.A.; Martin-Martinez, M.; Rodriguez-Franco, D.; Rodriguez, J.J.; Gómez-Sainero, L.M. Understanding Hydrodechlorination of Chloromethanes. Past and Future of the Technology. Catalysts 2020, 10, 1462. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Wang, Z. Electrocatalytic Hydro-Dehalogenation of Halogenated Organic Pollutants from Wastewater: A Critical Review. Water Res. 2023, 234, 119810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, R.; Zhang, G.; Dong, L.; Zhang, D.; Wang, G.; Li, T. Zn-Modified Hβ Zeolites Used in the Adsorptive Removal of Organic Chloride from Model Naphtha. ACS Omega 2020, 5, 11987–11997. [Google Scholar] [CrossRef]
- Huang, B.; Sun, Q.; Bing, L.; Han, D.; Wang, G.; Wang, F. Adsorptive Removal of Organochlorine from Oils by Modified Activated Carbon: Effect of Surface Groups and Kinetic and Thermodynamic Studies. Ind. Eng. Chem. Res. 2024, 63, 10955–10964. [Google Scholar] [CrossRef]
- Sharma, R.; Segato, T.; Delplancke, M.-P.; Terryn, H.; Baron, G.V.; Denayer, J.F.M.; Cousin-Saint-Remi, J. Hydrogen Chloride Removal from Hydrogen Gas by Adsorption on Hydrated Ion-Exchanged Zeolites. Chem. Eng. J. 2020, 381, 122512. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Tarach, K.A.; Góra-Marek, K. Top-down Engineering of Zeolite Porosity. Chem. Soc. Rev. 2025, 54, 7484–7560. [Google Scholar] [CrossRef] [PubMed]
- Dadari, S.; Rahimi, M.; Zinadini, S. Crude Oil Desalter Effluent Treatment Using High Flux Synthetic Nanocomposite NF Membrane-Optimization by Response Surface Methodology. Desalination 2016, 377, 34–46. [Google Scholar] [CrossRef]
- Norouzbahari, S.; Roostaazad, R.; Hesampour, M. Crude Oil Desalter Effluent Treatment by a Hybrid UF/RO Membrane Separation Process. Desalination 2009, 238, 174–182. [Google Scholar] [CrossRef]
- Huang, J.; Ran, X.; Sun, L.; Bi, H.; Wu, X. Recent Advances in Membrane Technologies Applied in Oil–Water Separation. Discov. Nano 2024, 19, 66. [Google Scholar] [CrossRef]
- Li, S.; Dong, R.; Musteata, V.-E.; Kim, J.; Rangnekar, N.D.; Johnson, J.R.; Marshall, B.D.; Chisca, S.; Xu, J.; Hoy, S.; et al. Hydrophobic Polyamide Nanofilms Provide Rapid Transport for Crude Oil Separation. Science 2022, 377, 1555–1561. [Google Scholar] [CrossRef]
- Jeon, J.; Park, Y.; Hwang, Y. Catalytic Hydrodechlorination of 4-Chlorophenol by Palladium-Based Catalyst Supported on Alumina and Graphene Materials. Nanomaterials 2023, 13, 1564. [Google Scholar] [CrossRef] [PubMed]
- Mishakov, I.V.; Vedyagin, A.A.; Bauman, Y.I.; Potylitsyna, A.R.; Kadtsyna, A.S.; Chesnokov, V.V.; Nalivaiko, A.Y.; Gromov, A.A.; Buyanov, R.A. Two Scenarios of Dechlorination of the Chlorinated Hydrocarbons over Nickel-Alumina Catalyst. Catalysts 2020, 10, 1446. [Google Scholar] [CrossRef]
- Mahy, J.G.; Delbeuck, T.; Tran, K.Y.; Heinrichs, B.; Lambert, S.D. Green Chemistry for the Transformation of Chlorinated Wastes: Catalytic Hydrodechlorination on Pd-Ni and Pd-Fe Bimetallic Catalysts Supported on SiO2. Gels 2023, 9, 275. [Google Scholar] [CrossRef]
- Beil, S.B.; Bonnet, S.; Casadevall, C.; Detz, R.J.; Eisenreich, F.; Glover, S.D.; Kerzig, C.; Næsborg, L.; Pullen, S.; Storch, G.; et al. Challenges and Future Perspectives in Photocatalysis: Conclusions from an Interdisciplinary Workshop. JACS Au 2024, 4, 2746–2766. [Google Scholar] [CrossRef]
- Niu, H.; Zhao, D.; Xie, G.; Yuan, Y.; Zhang, W.; Zhang, C.; Li, C.; Cui, L. Deep Dechlorination of Model Oil by Reactive Adsorption on Porous Oxides. Fuel 2021, 304, 121410. [Google Scholar] [CrossRef]
- Zhang, R.; Ding, L.; Cai, B.; Zhao, P.; Ding, M.; Wang, C.; Qu, C. Recent Developments in Electrochemical Reduction for Remediation of Chlorinated Hydrocarbons Contaminated Groundwater. J. Environ. Chem. Eng. 2025, 13, 115125. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, X.; Tian, K.; Tan, D.; Song, X.; Wang, P.; Jiang, Q.; Lu, J. Electrochemical Reduction and Oxidation of Chlorinated Aromatic Compounds Enhanced by the Fe-ZSM-5 Catalyst: Kinetics and Mechanisms. ACS Omega 2022, 7, 33500–33510. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, G.; Song, Z.; He, Z.; Zhong, A.; Cui, M. Catalytic Dechlorination of Three Organochlorides by Recyclable Nano-Palladium-Engineered Natural Sponge with Formic Acid. Catalysts 2024, 14, 424. [Google Scholar] [CrossRef]
- Hu, B.; Wu, B.; Du, Y.; Zhu, J. The Removal of Organochlorine Compounds from Model Oil by Nucleophilic Substitution. ACS Omega 2025, 10, 24089–24096. [Google Scholar] [CrossRef]
- Milani, M.; Mazzanti, M.; Stevanin, C.; Chenet, T.; Magnacca, G.; Pasti, L.; Molinari, A. CdS-Based Hydrothermal Photocatalysts for Complete Reductive Dehalogenation of a Chlorinated Propionic Acid in Water by Visible Light. Nanomaterials 2024, 14, 579. [Google Scholar] [CrossRef]
- Čičáková, C.; Tóth, R.; Horváthová, H.; Jurkovič, Ľ.; Špirová, V.; Drábik, A.; Kravchenko, D. Integrated Method of Electrochemical Dechlorination of Chlorinated Aliphatic Hydrocarbons in Combination with Groundwater Pumping in Highly Contaminated Groundwater–Field Application. Electrochim. Acta 2025, 525, 146125. [Google Scholar] [CrossRef]
- ISO 16290:2013; Space Systems—Definition of the Technology Readiness Levels (TRLs) and Their Criteria of Assessment. BSI British Standards Institution: Reston, VA, USA, 2013.
- Pereira Nicacio, J.A.; Castro Oliveira, F.; Rosa Dumont, M. Failure Analysis and Electrochemical Testing of Ammonium Chloride Corrosion in a Heat Exchanger in a Diesel Hydrotreating Unit of a Petroleum Refinery. Eng. Fail. Anal. 2024, 156, 107758. [Google Scholar] [CrossRef]
- Jin, H.; Yin, H.; Xu, S.; Chen, W.; Liu, X.; Jiang, Y. Experimental Study on the Deposition Characteristics of Ammonium Chloride Particles in a Hydrogenation Air Cooler. Powder Technol. 2025, 452, 120533. [Google Scholar] [CrossRef]
- Li, R.; Jin, H.; Sun, J.; Liu, X.; Wang, C.; Zhang, L. Numerical and Experimental Study on Corrosion Mechanism of the Water-Oil Two-Phase Flow in the Atmospheric Tower Top System. Eng. Fail. Anal. 2025, 167, 108981. [Google Scholar] [CrossRef]
- Sudol, P.E.; Pierce, K.M.; Prebihalo, S.E.; Skogerboe, K.J.; Wright, B.W.; Synovec, R.E. Development of Gas Chromatographic Pattern Recognition and Classification Tools for Compliance and Forensic Analyses of Fuels: A Review. Anal. Chim. Acta 2020, 1132, 157–186. [Google Scholar] [CrossRef]
- Santos, M.C.; Coutinho, D.M.; Torres, C.L.; Barra, T.A.; Cardoso, V.G.K.; Silva, R.V.S.; Dubois, D.S.; Lopes, J.P.; Neto, F.R.A.; Azevedo, D.A. Pixel-Based Chemometric Analysis of Pre-Salt Crude Oils: Advancing GC×GC-TOFMS for Reservoir Characterization. Energy Fuels 2025, 39, 7204–7213. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, D.; Sun, J. Application of GC-IMS Coupled with Chemometric Analysis for the Classification and Authentication of Geographical Indication Agricultural Products and Food. Front. Nutr. 2023, 10, 1247695. [Google Scholar] [CrossRef] [PubMed]
- Devers, J.; Pattison, D.I.; Hansen, A.B.; Christensen, J.H. Comprehensive Two-Dimensional Gas Chromatography as a Tool for Targeted and Non-Targeted Analysis of Contaminants of Emerging Concern in Wastewater. Talanta 2025, 282, 127032. [Google Scholar] [CrossRef] [PubMed]
- Kaspi, O.; Avissar, Y.Y.; Grafit, A.; Chibel, R.; Girshevitz, O.; Senderowitz, H. Machine Learning-Based Identification of Petroleum Distillates and Gasoline Traces Using Measured and Synthetic GC Spectra from Collected Samples. Mol. Inf. 2025, 44, e202400371. [Google Scholar] [CrossRef]
- Arey, J.S.; Martin Aparicio, A.; Vaiopoulou, E.; Forbes, S.; Lyon, D. Modeling the GCxGC Elution Patterns of a Hydrocarbon Structure Library To Innovate Environmental Risk Assessments of Petroleum Substances. Environ. Sci. Technol. 2022, 56, 17913–17923. [Google Scholar] [CrossRef]
- Zweigle, J.; Tisler, S.; Bevilacqua, M.; Tomasi, G.; Nielsen, N.J.; Gawlitta, N.; Lübeck, J.S.; Smilde, A.K.; Christensen, J.H. Prioritization Strategies for Non-Target Screening in Environmental Samples by Chromatography—High-Resolution Mass Spectrometry: A Tutorial. J. Chromatogr. A 2025, 1751, 465944. [Google Scholar] [CrossRef]
- Badea, S.-L.; Niculescu, V.-C.; Popescu (Stegarus), D.-I.; Geana, E.-I.; Ciucure, C.-T.; Botoran, O.-R.; Ionete, R.-E. Recent Progresses in Compound Specific Isotope Analysis of Halogenated Persistent Organic Pollutants. Assessing the Transformation of Halogenated Persistent Organic Pollutants at Contaminated Sites. Sci. Total Environ. 2023, 899, 165344. [Google Scholar] [CrossRef] [PubMed]
- Shelor, C.P.; Warren, C.; Odinaka, C.V.; Dumre, K. Comprehensive Review of Combustion Ion Chromatography for the Analysis of Total, Adsorbable, and Extractable Organic Fluorine. J. Sep. Sci. 2024, 47, 2400235. [Google Scholar] [CrossRef] [PubMed]
- Katona, R.; Krójer, A.; Locskai, R.; Bátor, G.; Kovács, T. Comparison of Analytical Methods for Measuring Chloride Content in Crude Oil. Appl. Radiat. Isot. 2021, 170, 109594. [Google Scholar] [CrossRef] [PubMed]
- Mitländer, K.; Henseler, J.; Rullo, F.; Nathrath, P.; Geißelbrecht, M.; Wasserscheid, P.; Schühle, P. Continuous (Hydro-)Dechlorination of Aromatic Chloride Compounds in Benzyltoluene. Int. J. Hydrog. Energy 2025, 128, 674–683. [Google Scholar] [CrossRef]
- Gkatziouras, C.; Solakidou, M.; Louloudi, M. Formic Acid Dehydrogenation over a Recyclable and Self-Reconstructing Fe/Activated Carbon Catalyst. Energy Fuels 2024, 38, 17914–17926. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, J.; Wang, P.; Jin, H.; Zheng, Y.; Qiao, S.-Z. Recent Advances in Electrocatalytic Hydrogenation Reactions on Copper-Based Catalysts. Adv. Mater. 2024, 36, 2307913. [Google Scholar] [CrossRef]
- Weggler, B.A.; Dubois, L.M.; Gawlitta, N.; Gröger, T.; Moncur, J.; Mondello, L.; Reichenbach, S.; Tranchida, P.; Zhao, Z.; Zimmermann, R.; et al. A Unique Data Analysis Framework and Open Source Benchmark Data Set for the Analysis of Comprehensive Two-Dimensional Gas Chromatography Software. J. Chromatogr. A 2021, 1635, 461721. [Google Scholar] [CrossRef]
- Moreira de Oliveira, A.; Teixeira, C.A.; Castiblanco, J.E.B.; Medeiros Júnior, Í.; Hantao, L.W. Multiparameter Modeling for 10 Crude Oil Properties Using Comprehensive Two-Dimensional Gas Chromatography and Pixel-Based Chemometrics. Energy Fuels 2025, 39, 12882–12892. [Google Scholar] [CrossRef]
- Morales-Medina, G.; Berbesí, E. Analysis of the Environmental Impact of Fuel Hydrotreating through Life Cycle Assessment and Process Data. Energy Convers. Manag. 2025, 345, 120333. [Google Scholar] [CrossRef]
- Ilyushin, Y.; Nosova, V.; Krauze, A. Application of Systems Analysis Methods to Construct a Virtual Model of the Field. Energies 2025, 18, 1012. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).