Numerical Simulation of Double-Pore Bubble Coalescence Behavior in Direct Methanol Fuel Cells
Abstract
1. Introduction
2. Mathematical Modeling Theory
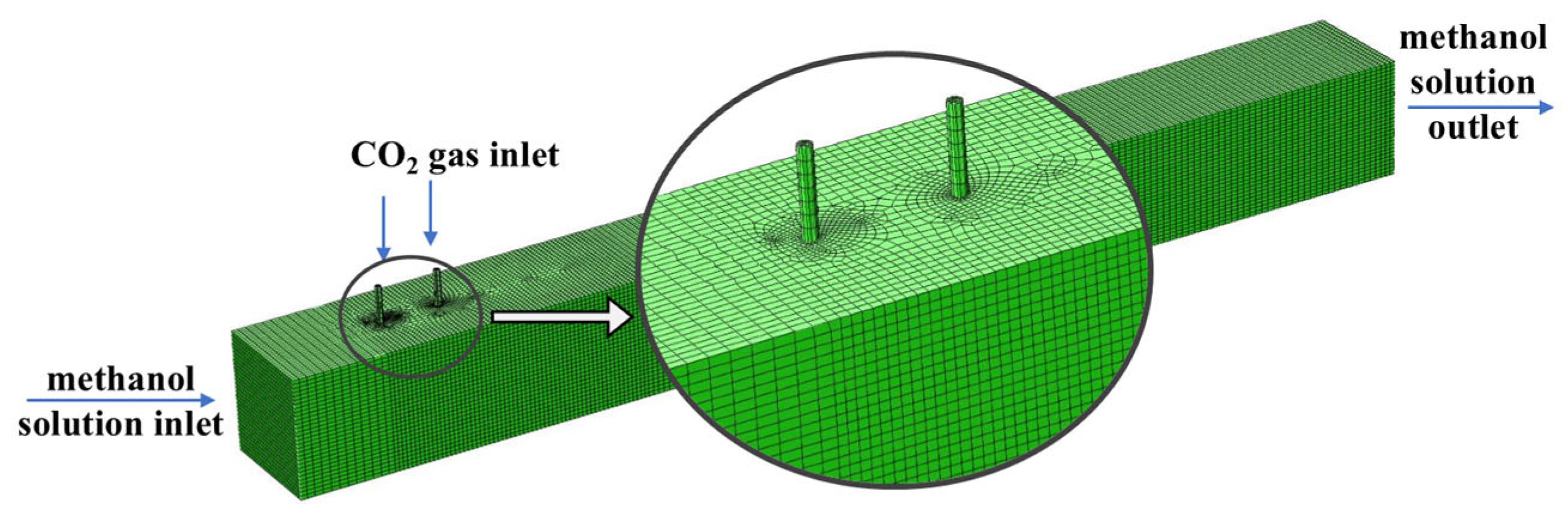
2.1. Model Computational Domain and Assumptions
- Based on Re < 1055, the methanol–water solution is in laminar flow;
- Based on Bond number [24] calculation < 0.1, the influence of gravity is neglected;
- No-slip conditions are assumed for the walls;
- The solution is in a CO2-saturated state; any CO2 gas introduced during the calculation process does not dissolve in the solution.
| Pore Size dN (μm) Parameters | Values |
|---|---|
| methanol solution density (kg/m3) | 970.86 |
| CO2 density (kg/m3) | 1.5729 |
| methanol solution dynamic viscosity (Pa/s) | 4.6 × 10−4 |
| CO2 dynamic viscosity (Pa/s) | 1.63 × 10−5 |
| Surface tension coefficient (N/m) | 0.06548 |
2.2. Control Equations
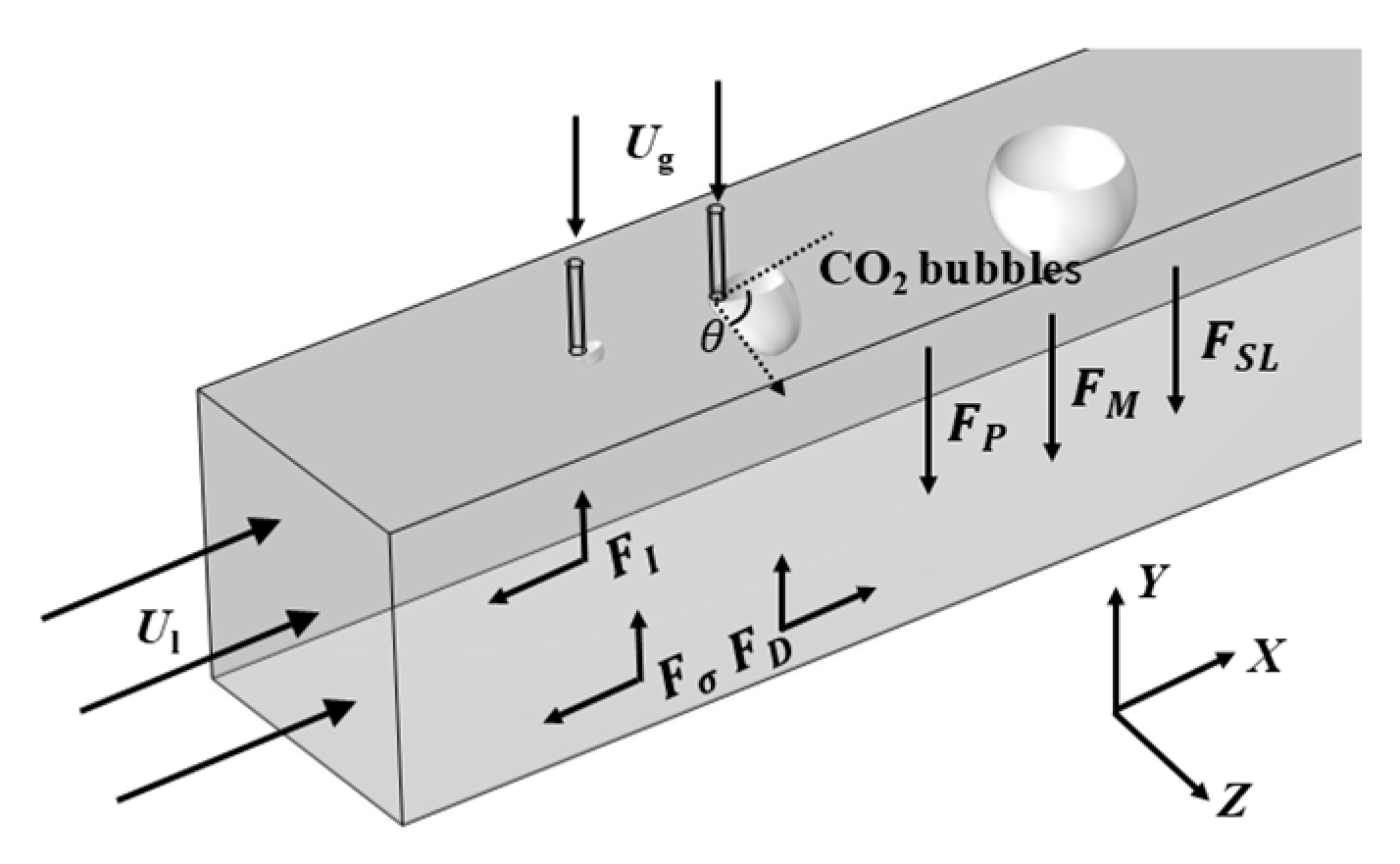
2.3. Force Analysis
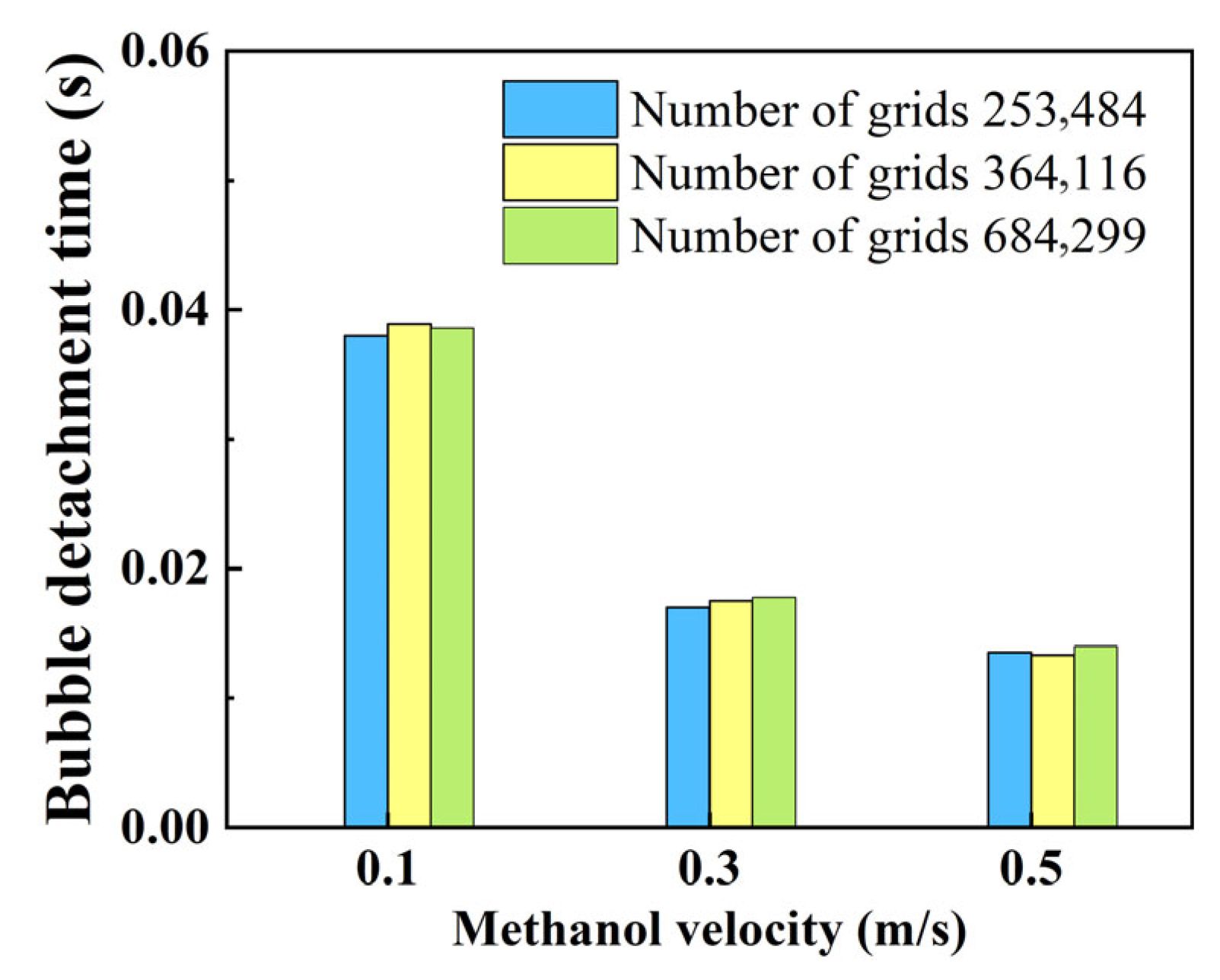
2.4. Model Validation and Mesh Independence Validation
3. Results and Discussion
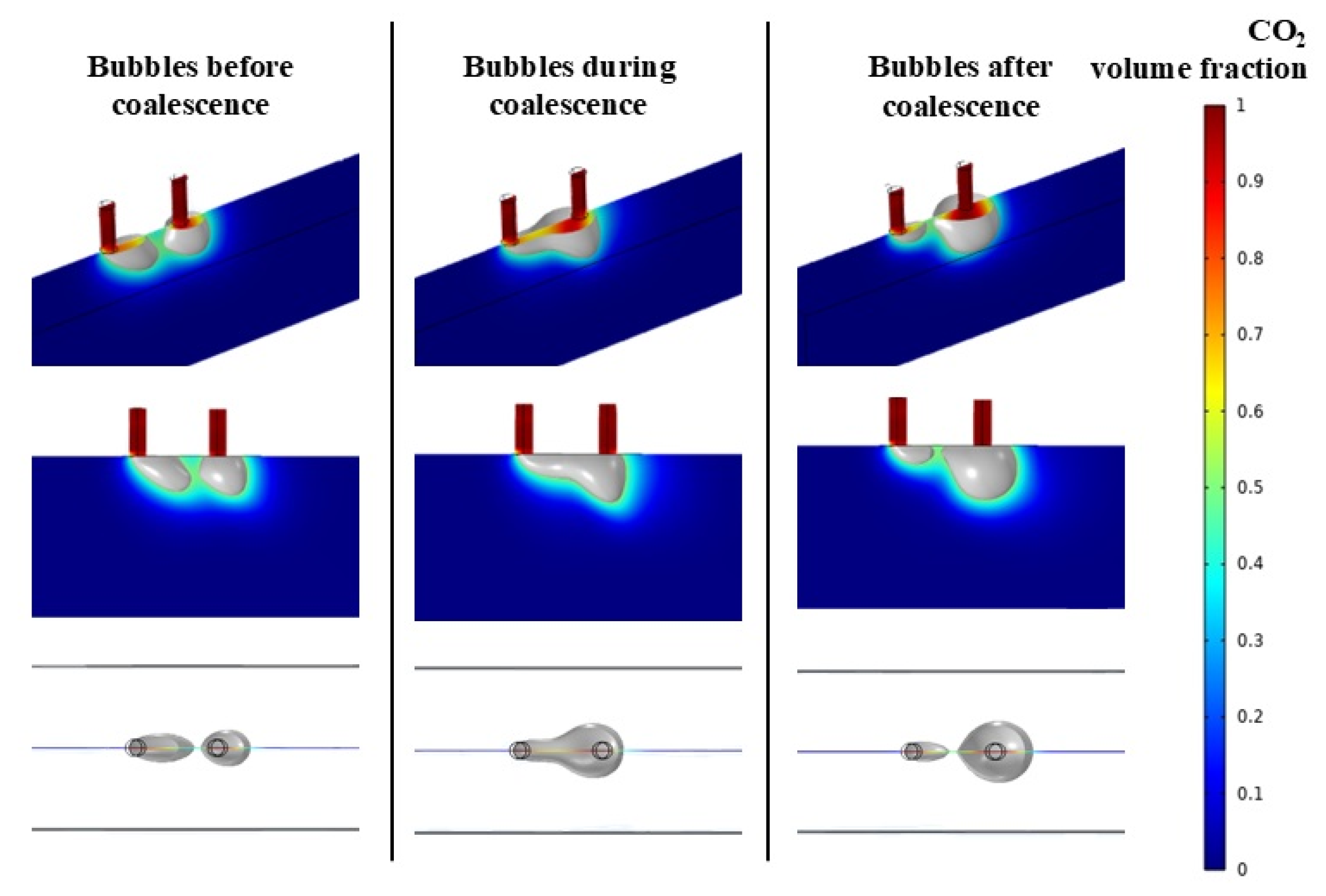
3.1. Analysis of Bubble Coalescence Behavior
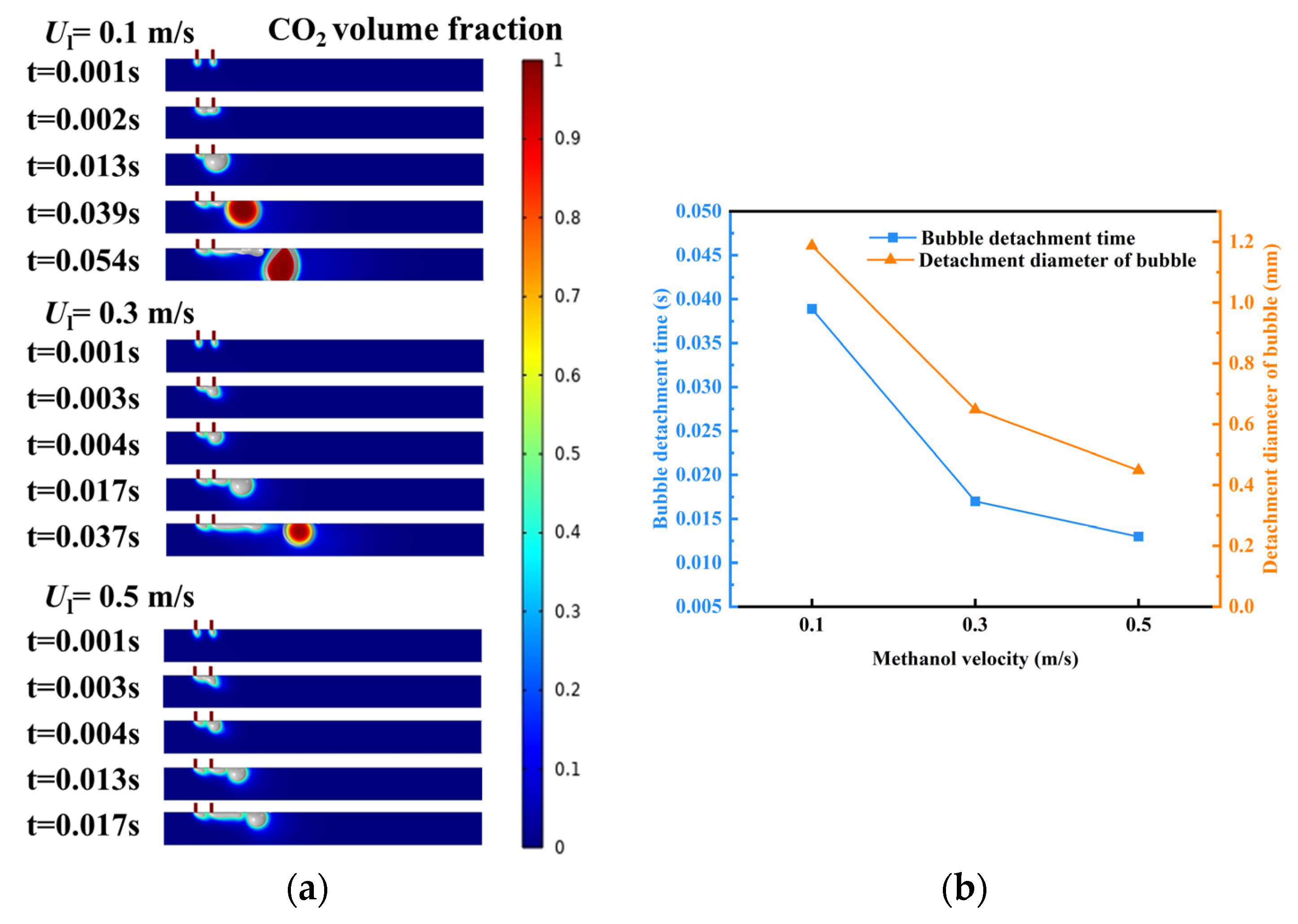
3.2. Effect of Methanol Inlet Velocity on Bubble Coalescence
3.3. Effect of Pore Size on Bubble Coalescence
3.4. Effect of Pore Spacing on Bubble Coalescence
3.5. Effect of Contact Angle on Bubble Coalescence
4. Conclusions
- (1)
- Bubble coalescence behavior: Bubbles typically coalesce at the interface between the flow channel and the diffusion layer. During coalescence, differing local velocity field distributions upstream and downstream of the bubbles, coupled with uneven shear and pressure distributions, result in size discrepancies between the two bubbles. The resulting coalesced bubble exhibits a larger detachment diameter.
- (2)
- Effect of methanol inlet flow velocity on bubble coalescence behavior: At lower flow velocities, the drag force and the shear-lift force are weaker, allowing bubbles to linger longer at the orifice and facilitating coalescence into larger bubbles. As flow velocity increases, the drag force and the shear-lift force intensify, causing bubbles to be detached before complete coalescence occurs. This shortens the coalescence time and may even prevent coalescence, resulting in bubbles rapidly detaching at smaller volumes.
- (3)
- Effect of pore size on bubble coalescence behavior: At a pore size of 50 μm, bubbles do not form. At a pore size of 80 μm, due to the surface tension force being lower than the drag force, the gas cannot gather on the pore surface. The surface tension increases with the increase in pore size, forming a bubble on the pore surface. The adjacent bubbles coalesce when the pore size is larger than 100 μm.
- (4)
- Effect of pore spacing on bubble coalescence behavior: pore spacing directly influences the frequency and intensity of coalescence by altering the spatial distribution of bubble nucleation sites. When the hole spacing is 0.3 mm, adjacent bubbles coalesce before detachment due to their proximity, forming large bubbles that detach rapidly. When the pore spacing increases to 1.0 mm, the detachment time and detachment diameter of bubbles decrease, while the distance traveled after detachment increases. Bubbles contact and merge with others in the later stages of the flow channel, but their distribution becomes more uniform. Increasing the pore spacing helps suppress bubble coalescence.
- (5)
- Effect of contact angle on bubble coalescence behavior: Under hydrophilic walls, bubble coalescence occurs more rapidly. Bubbles experience greater shear forces during growth, resulting in shorter detachment times and smaller detachment diameters. However, after detachment, these bubbles readily coalesce with others while moving through the flow channel. Under hydrophobic walls, bubble coalescence time is longer. Bubbles grow close to the wall surface, experiencing lower shear-lift forces and higher wall viscous forces, making separation difficult and time-consuming. Separated bubbles have larger volumes but maintain greater spacing afterward, reducing their tendency to coalesce with others.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct Liquid Fuel Cells: A Review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Souza, F.M.; Pinheiro, V.S.; Gentil, T.C.; Lucchetti, L.E.B.; Silva, J.C.M.; Santos, M.; De Oliveira, I.; Dourado, W.M.C.; Amaral-Labat, G.; Okamoto, S.; et al. Alkaline Direct Liquid Fuel Cells: Advances, Challenges and Perspectives. J. Electroanal. Chem. 2022, 922, 116712. [Google Scholar] [CrossRef]
- Kulikovsky, A.A. Model of the Flow with Bubbles in the Anode Channel and Performance of a Direct Methanol Fuel Cell. Electrochem. Commun. 2005, 7, 237–243. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Active Direct Methanol Fuel Cell: An Overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Balzer, R.J.; Vogt, H. Effect of Electrolyte Flow on the Bubble Coverage of Vertical Gas-Evolving Electrodes. J. Electrochem. Soc. 2003, 150, E11. [Google Scholar] [CrossRef]
- Vogt, H.; Balzer, R.J. The Bubble Coverage of Gas-Evolving Electrodes in Stagnant Electrolytes. Electrochim. Acta 2005, 50, 2073–2079. [Google Scholar] [CrossRef]
- Tavakoli, B.; Roshandel, R. The Effect of Fuel Cell Operational Conditions on the Water Content Distribution in the Polymer Electrolyte Membrane. Renew. Energy 2011, 36, 3319–3331. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, A.; Ye, G.; Pan, B.; Tang, K.; Chen, H. Dynamic Relationship between the CO2 Gas Bubble Behavior and the Pressure Drop Characteristics in the Anode Flow Field of an Active Liquid-Feed Direct Methanol Fuel Cell. Appl. Energy 2017, 188, 431–443. [Google Scholar] [CrossRef]
- Luo, W.-J.; Kuan, Y.-D.; Sung, M.-F.; Huang, M.-S.; Hsu, Y.-W. Direct Methanol Fuel Cell Bubble Removal Mechanism Using Magnetic Actuation. Int. J. Green Energy 2017, 14, 1172–1177. [Google Scholar] [CrossRef]
- Kang, S.; Zhou, B. Numerical Study of Bubble Generation and Transport in a Serpentine Channel with a T-Junction. Int. J. Hydrogen Energy 2014, 39, 2325–2333. [Google Scholar] [CrossRef]
- Zheng, W.; Suominen, A.; Lagercrantz, H.; Tuominen, A. A Two-Dimensional Model for CO2/Fuel Multiphase Flow on the Anode Side of a DMFC. J. Fuel Cell Sci. Technol. 2012, 9, 011009. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Zhu, X.; Ye, D.; Chen, R.; Liao, Q. Bubble-Trap Layer for Effective Removing Gas Bubbles and Stabilizing Power Generation in Direct Liquid Fuel Cell. J. Power Sources 2021, 507, 230260. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Y.; Fu, W.; Li, Z.; Liu, X. Investigation of Corner Effect on Micro-Scale Flow of μDMFC. Microelectron. Eng. 2013, 111, 96–100. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Y.; Leng, J.; Gao, Y.; Liu, X. Development of a 4-Cell Air-Breathing Micro Direct Methanol Fuel Cell Stack. J. Power Sources 2012, 202, 134–142. [Google Scholar] [CrossRef]
- Su, X.; Yuan, W.; Lu, B.; Zheng, T.; Ke, Y.; Zhuang, Z.; Zhao, Y.; Tang, Y.; Zhang, S. CO2 Bubble Behaviors and Two-Phase Flow Characteristics in Single-Serpentine Sinusoidal Corrugated Channels of Direct Methanol Fuel Cell. J. Power Sources 2020, 450, 227621. [Google Scholar] [CrossRef]
- Falcão, D.S.; Pereira, J.P.; Pinto, A.M.F.R. Numerical Simulations of Anode Two-Phase Flow in Micro-DMFC Using the Volume of Fluid Method. Int. J. Hydrogen Energy 2016, 41, 19724–19730. [Google Scholar] [CrossRef]
- Jain, D.; Kuipers, J.A.M.; Deen, N.G. Numerical Study of Coalescence and Breakup in a Bubble Column Using a Hybrid Volume of Fluid and Discrete Bubble Model Approach. Chem. Eng. Sci. 2014, 119, 134–146. [Google Scholar] [CrossRef]
- Colin, C.; Riou, X.; Fabre, J. Bubble Coalescence in Gas–Liquid Flow at Microgravity Conditions. Microgravity Sci. Technol. 2008, 20, 243–246. [Google Scholar] [CrossRef]
- Wen, J.; Sun, Q.; Sun, Z.; Gu, H. Bubble Coalescence Efficiency near Multi-Orifice Plate. Chin. J. Chem. Eng. 2019, 27, 1765–1776. [Google Scholar] [CrossRef]
- Yonemoto, Y.; Kunugi, T. Fundamental Numerical Simulation of Microbubble Interaction Using Multi-Scale Multiphase Flow Equation. Microgravity Sci. Technol. 2010, 22, 397–405. [Google Scholar] [CrossRef]
- Zheng, S.; Xu, K.; Zhang, D. Reactive Precipitation during Overlaying CO2 Dissolution into Brine: The Role of Porous Structure. Adv. Water Resour. 2025, 196, 104880. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, A.; Yan, Z.; Tan, Z.; Tang, Y.; Xia, H. Visualization of Two-Phase Flow and Temperature Characteristics of an Active Liquid-Feed Direct Methanol Fuel Cell with Diverse Flow Fields. Appl. Energy 2016, 179, 85–98. [Google Scholar] [CrossRef]
- Wong, C.W.; Zhao, T.S.; Ye, Q.; Liu, J.G. Transient Capillary Blocking in the Flow Field of a Micro-DMFC and Its Effect on Cell Performance. J. Electrochem. Soc. 2005, 152, A1600. [Google Scholar] [CrossRef]
- Paust, N.; Litterst, C.; Metz, T.; Eck, M.; Ziegler, C.; Zengerle, R.; Koltay, P. Capillary-Driven Pumping for Passive Degassing and Fuel Supply in Direct Methanol Fuel Cells. Microfluid. Nanofluid. 2009, 7, 531–543. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, H.; Tian, Z.; Zhu, J.; Zhu, J. Analysis of CO2 Bubble Growth Detachment Kinetics in Direct Methanol Fuel Cell Flow Channels. J. Power Sources 2025, 628, 235880. [Google Scholar] [CrossRef]
- Nahra, H.K.; Kamotani, Y. Prediction of Bubble Diameter at Detachment from a Wall Orifice in Liquid Cross-Flow under Reduced Andnormal Gravity Conditions. Chem. Eng. Sci. 2003, 58, 55–69. [Google Scholar] [CrossRef]
- Liu, C.; Liang, B.; Tang, S.; Zhang, H.; Min, E. A Theoretical Model for the Size Prediction of Single Bubbles Formed under Liquid Cross-Flow. Chin. J. Chem. Eng. 2010, 18, 770–776. [Google Scholar] [CrossRef]
- Eskin, D.; Meretskaya, E.; Vikhansky, A. A Model of Breakup of a Rising Bubble in a Turbulent Flow. Chem. Eng. Sci. 2020, 226, 115846. [Google Scholar] [CrossRef]
- Gnyloskurenko, S.V.; Byakova, A.V.; Raychenko, O.I.; Nakamura, T. Influence of Wetting Conditions on Bubble Formation at Orifice in an Inviscid Liquid. Transformation of Bubble Shape and Size. Colloids Surf. A Physicochem. Eng. Asp. 2003, 218, 73–87. [Google Scholar] [CrossRef]
- Zhang, H. Bubble Behavior in Anode Channel of Direct Methanol Fuel Cells. Ph.D. Thesis, Zhejiang University of Technology, Hangzhou, China, 2011. [Google Scholar] [CrossRef]
- Lu, G.Q.; Wang, C.Y. Electrochemical and Flow Characterization of a Direct Methanol Fuel Cell. J. Power Sources 2004, 134, 33–40. [Google Scholar] [CrossRef]
- Yuan, W.; Tang, Y.; Yang, X.; Liu, B.; Wan, Z. Structural Diversity and Orientation Dependence of a Liquid-Fed Passive Air-Breathing Direct Methanol Fuel Cell. Int. J. Hydrogen Energy 2012, 37, 9298–9313. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Zhou, H.; Wang, J.; Tong, Y.; Zhu, J. Numerical Simulation of Double-Pore Bubble Coalescence Behavior in Direct Methanol Fuel Cells. Energies 2025, 18, 6041. https://doi.org/10.3390/en18226041
Tian Z, Zhou H, Wang J, Tong Y, Zhu J. Numerical Simulation of Double-Pore Bubble Coalescence Behavior in Direct Methanol Fuel Cells. Energies. 2025; 18(22):6041. https://doi.org/10.3390/en18226041
Chicago/Turabian StyleTian, Zemu, Hongxiu Zhou, Jiahao Wang, Yu Tong, and Junhao Zhu. 2025. "Numerical Simulation of Double-Pore Bubble Coalescence Behavior in Direct Methanol Fuel Cells" Energies 18, no. 22: 6041. https://doi.org/10.3390/en18226041
APA StyleTian, Z., Zhou, H., Wang, J., Tong, Y., & Zhu, J. (2025). Numerical Simulation of Double-Pore Bubble Coalescence Behavior in Direct Methanol Fuel Cells. Energies, 18(22), 6041. https://doi.org/10.3390/en18226041





