Scalable Hybrid Arrays Overcome Electrode Scaling Limitations in Micro-Photosynthetic Power Cells
Abstract
1. Introduction
2. Materials and Methods
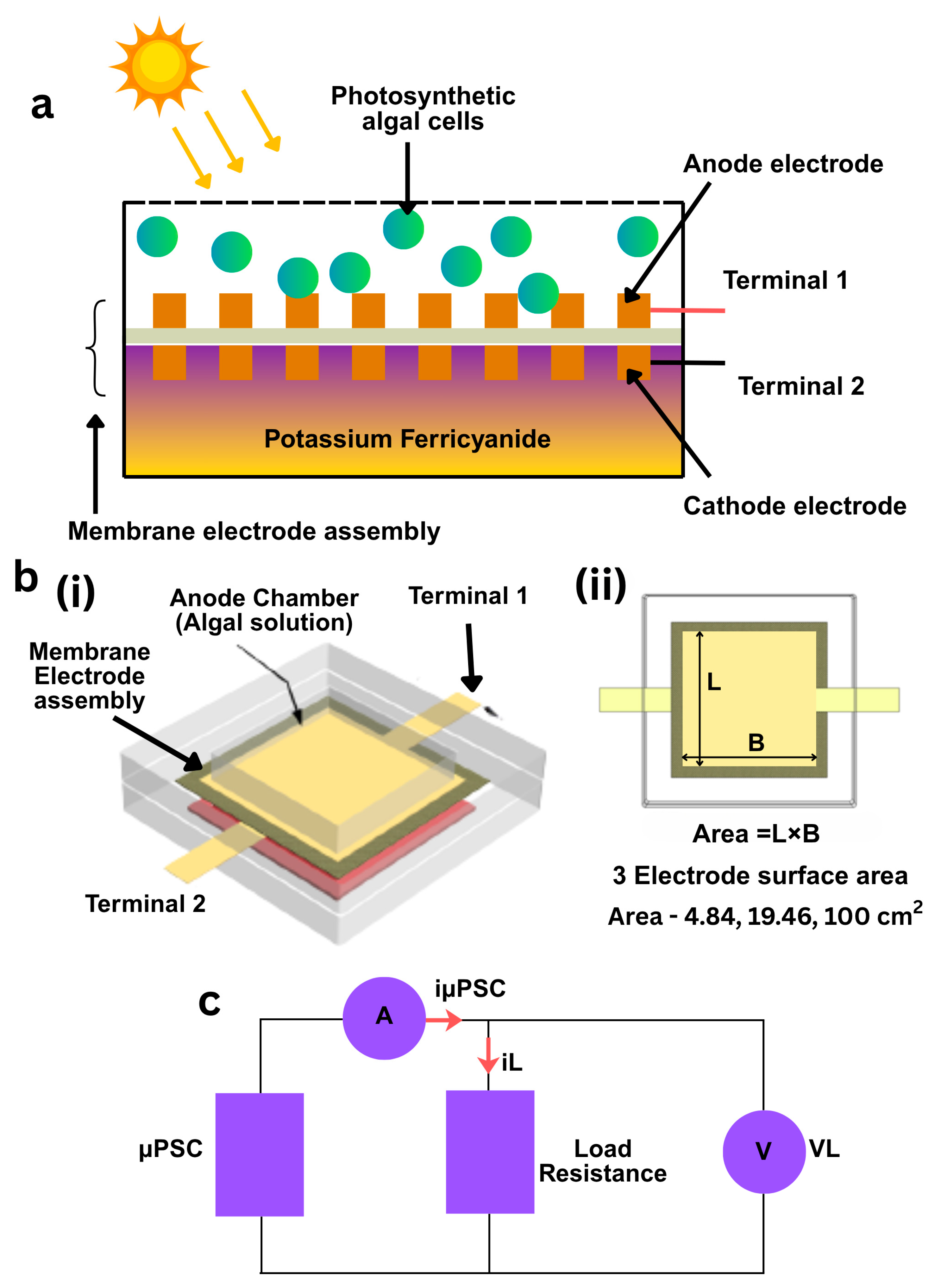
2.1. µPSC Fabrication
2.2. Preparation of Electrolytes and Photosynthetic Microorganisms
2.3. Terminal Connections
2.4. Light Condition
2.5. Loading Tests and Measurements
2.6. Generative AI for Image Generation
3. Results
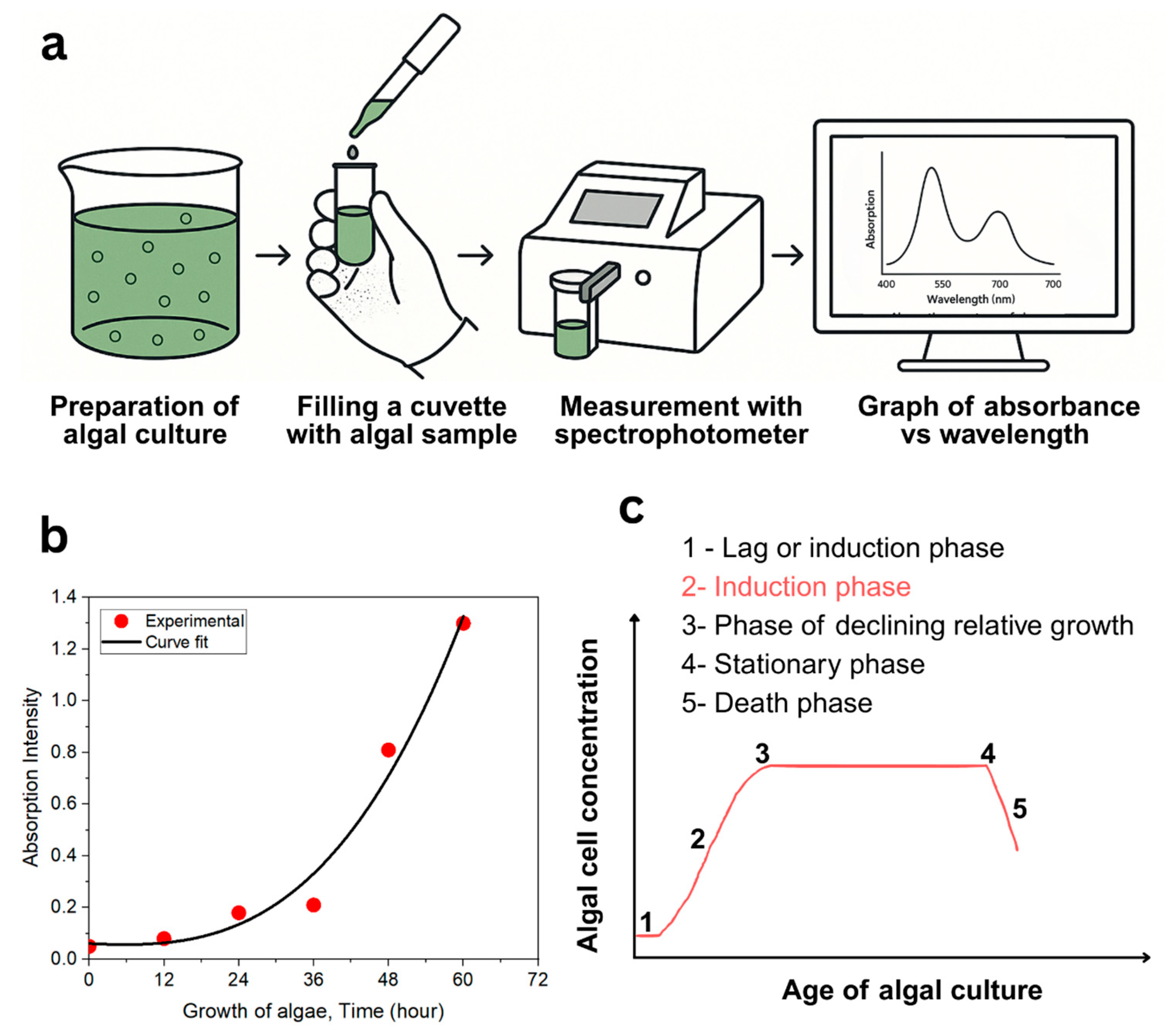
3.1. Optimized Growth of Algal Culture for the µPSC Testing Subsection
3.2. Effect of Electrode Surface Area (ESA) on µPSC Performance
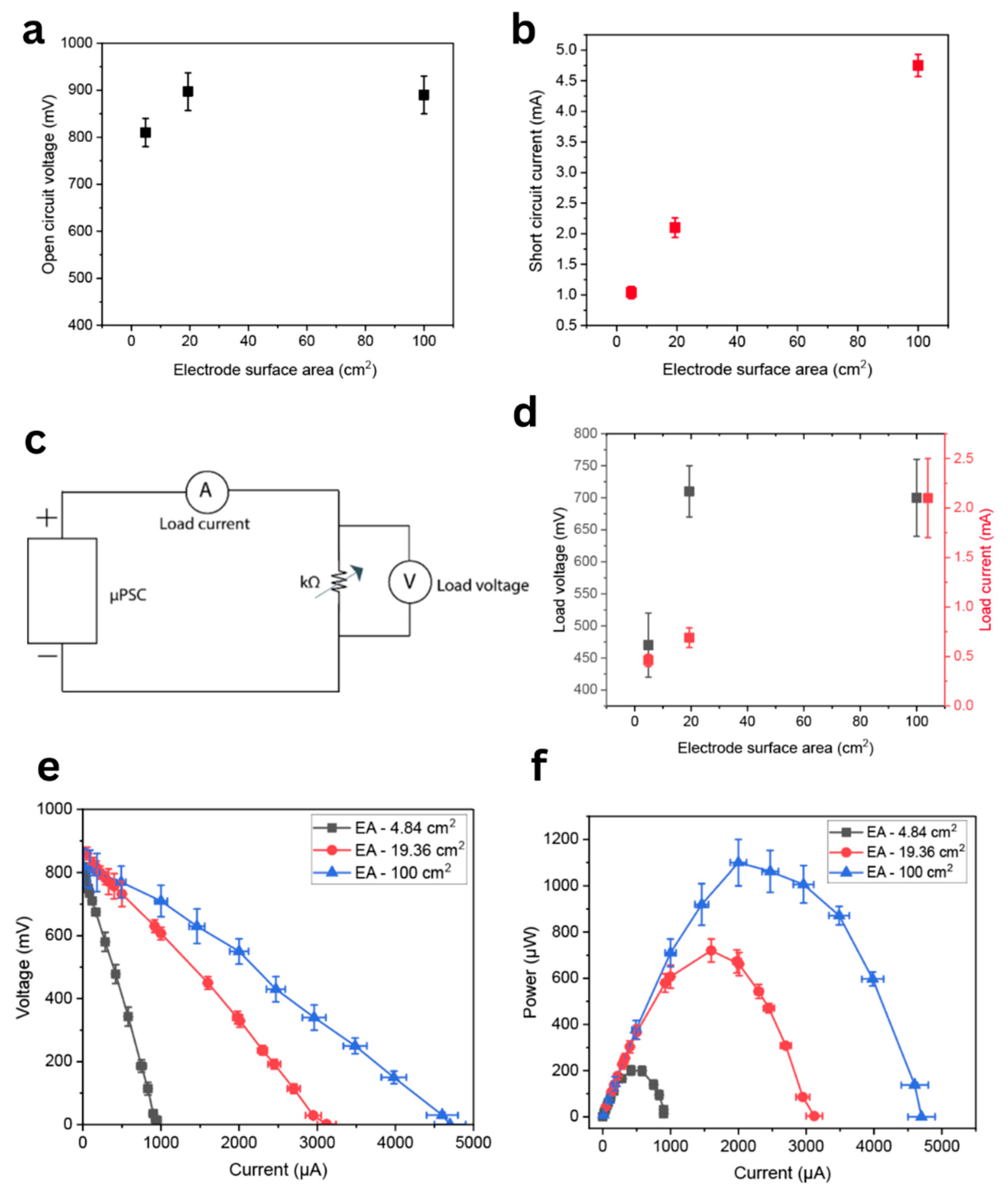
3.2.1. Open Circuit Voltage (Voc)
3.2.2. Short Circuit Current (Isc)
3.2.3. Load Voltage (VL) and Load Current (IL) at 1 kΩ Resistance
3.2.4. Polarization Characteristics
3.2.5. Current-Power (I-P) Characteristics
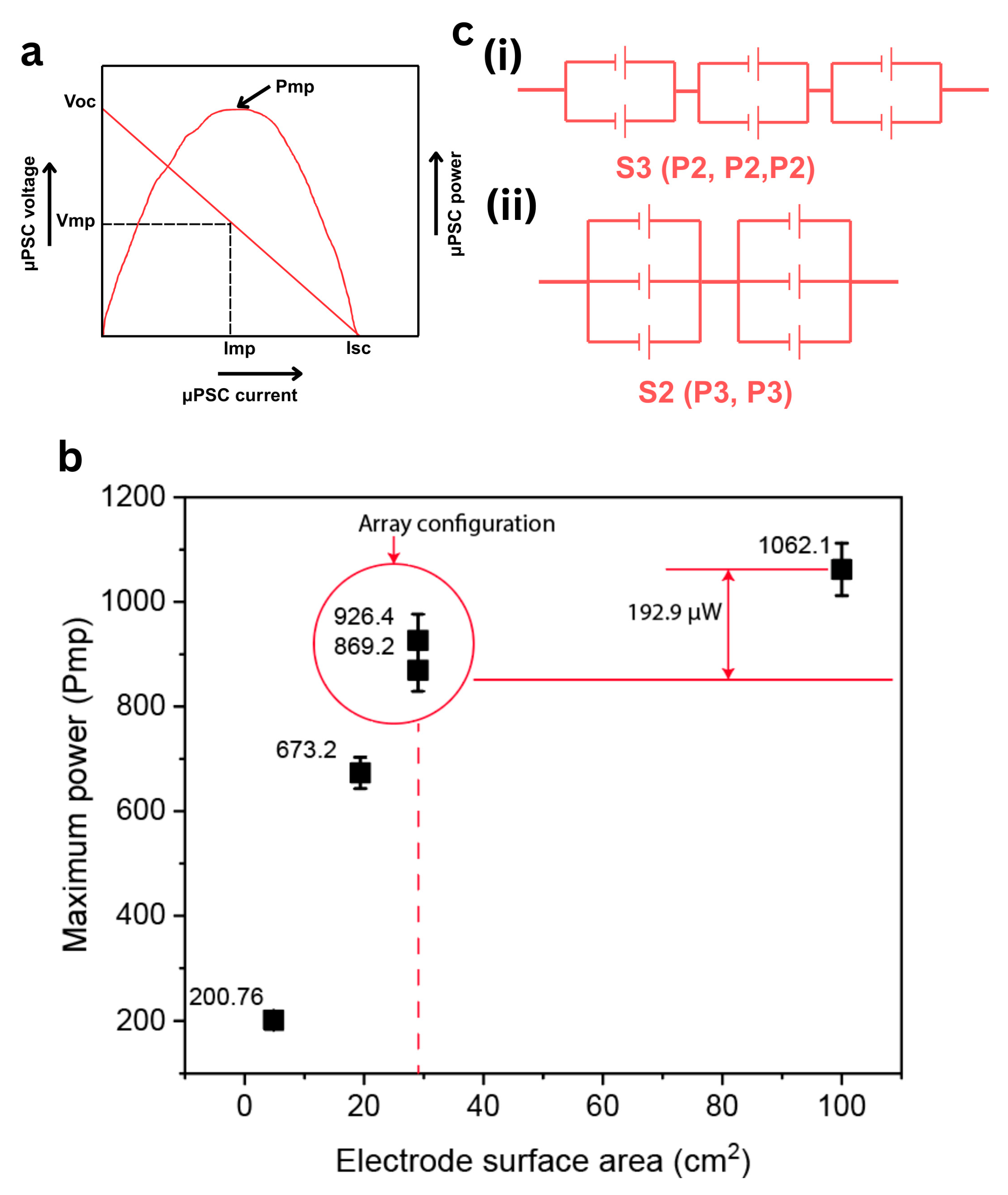
3.2.6. Practical Application and System Integration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saar, K.L.; Bombelli, P.; Lea-Smith, D.J.; Call, T.; Aro, E.-M.; Müller, T.; Howe, C.J.; Knowles, T.P.J. Enhancing Power Density of Biophotovoltaics by Decoupling Storage and Power Delivery. Nat. Energy 2018, 3, 75–81. [Google Scholar] [CrossRef]
- Bombelli, P.; Savanth, A.; Scarampi, A.; Rowden, S.J.L.; Green, D.H.; Erbe, A.; Årstøl, E.; Jevremovic, I.; Hohmann-Marriott, M.F.; Trasatti, S.P.; et al. Powering a Microprocessor by Photosynthesis. Energy Environ. Sci. 2022, 15, 2529–2536. [Google Scholar] [CrossRef]
- Sawa, M.; Fantuzzi, A.; Bombelli, P.; Howe, C.J.; Hellgardt, K.; Nixon, P.J. Electricity Generation from Digitally Printed Cyanobacteria. Nat. Commun. 2017, 8, 1327. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, P.; Bradley, R.W.; Scott, A.M.; Philips, A.J.; McCormick, A.J.; Cruz, S.M.; Anderson, A.; Yunus, K.; Bendall, D.S.; Cameron, P.J.; et al. Quantitative Analysis of the Factors Limiting Solar Power Transduction by Synechocystis sp. PCC 6803 in Biological Photovoltaic Devices. Energy Environ. Sci. 2011, 4, 4690–4698. [Google Scholar] [CrossRef]
- McCormick, A.J.; Bombelli, P.; Scott, A.M.; Philips, A.J.; Smith, A.G.; Fisher, A.C.; Howe, C.J. Photosynthetic Biofilms in Pure Culture Harness Solar Energy in a Mediatorless Bio-Photovoltaic Cell (BPV) System. Energy Environ. Sci. 2011, 4, 4699–4709. [Google Scholar] [CrossRef]
- Bradley, R.W.; Bombelli, P.; Rowden, S.J.L.; Howe, C.J. Biological Photovoltaics: Intra- and Extra-Cellular Electron Transport by Cyanobacteria. Biochem. Soc. Trans. 2012, 40, 1302–1307. [Google Scholar] [CrossRef]
- Howe, C.J.; Bombelli, P. Electricity Production by Photosynthetic Microorganisms. Joule 2020, 4, 2065–2069. [Google Scholar] [CrossRef]
- McCormick, A.J.; Bombelli, P.; Bradley, R.W.; Thorne, R.; Wenzel, T.; Howe, C.J. Biophotovoltaics: Oxygenic Photosynthetic Organisms in the World of Bioelectrochemical Systems. Energy Environ. Sci. 2015, 8, 1092–1109. [Google Scholar] [CrossRef]
- Chiao, M.; Lam, K.B.; Lin, L. Micromachined Microbial and Photosynthetic Fuel Cells. J. Micromech. Microeng. 2006, 16, 2547. [Google Scholar] [CrossRef]
- Fan, L.P.; Li, J.J. Overviews on Internal Resistance and Its Detection of Microbial Fuel Cells. Int. J. Circuits Syst. Signal Process. 2016, 10, 316–320. [Google Scholar]
- Oseyemi, A.E.; Kuruvinashetti, K.; Packirisamy, M. Perspective—Trends in the Miniaturization of Photosynthetic Power Cell towards Improved Power Density. J. Electrochem. Soc. 2022, 169, 126501. [Google Scholar] [CrossRef]
- Ali, A.O.; Elgohr, A.T.; El-Mahdy, M.H.; Zohir, H.M.; Emam, A.Z.; Mostafa, M.G.; Al-Razgan, M.; Kasem, H.M.; Elhadidy, M.S. Advancements in Photovoltaic Technology: A Comprehensive Review of Recent Advances and Future Prospects. Energy Convers. Manag. X 2025, 26, 100952. [Google Scholar] [CrossRef]
- Chen, X.; Lawrence, J.M.; Wey, L.T.; Schertel, L.; Jing, Q.; Vignolini, S.; Howe, C.J.; Kar-Narayan, S.; Zhang, J.Z. 3D-Printed Hierarchical Pillar Array Electrodes for High-Performance Semi-Artificial Photosynthesis. Nat. Mater. 2022, 21, 811–818. [Google Scholar] [CrossRef]
- Krieg, T.; Wood, J.A.; Mangold, K.-M.; Holtmann, D. Mass Transport Limitations in Microbial Fuel Cells: Impact of Flow Configurations. Biochem. Eng. J. 2018, 138, 172–178. [Google Scholar] [CrossRef]
- Bozan, M.; Berreth, H.; Lindberg, P.; Bühler, K. Cyanobacterial Biofilms: From Natural Systems to Applications. Trends Biotechnol. 2025, 43, 318–332. [Google Scholar] [CrossRef]
- Ng, F.-L.; Phang, S.-M.; Periasamy, V.; Beardall, J.; Yunus, K.; Fisher, A.C. Algal Biophotovoltaic (BPV) Device for Generation of Bioelectricity Using Synechococcus Elongatus (Cyanophyta). J. Appl. Phycol. 2018, 30, 2981–2988. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, H.; Fraiwan, A.; Dai, C.; Choi, S. A Microsized Microbial Solar Cell: A Demonstration of Photosynthetic Bacterial Electrogenic Capabilities. IEEE Nanotechnol. Mag. 2014, 8, 24–29. [Google Scholar] [CrossRef]
- Siu, C.-P.-B.; Chiao, M. A Microfabricated PDMS Microbial Fuel Cell. J. Microelectromech. Syst. 2008, 17, 1329–1341. [Google Scholar] [CrossRef]
- Kuruvinashetti, K.; Packirisamy, M. Arraying of Microphotosynthetic Power Cells for Enhanced Power Output. Microsyst. Nanoeng. 2022, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Sundareswaran, K.; Kuruvinashetti, K.; Hariprasad, B.; Sankar, P.; Nayak, P.S.; Vigneshkumar, V. Optimization of Dual Input Buck Converter Control through Genetic Algorithm. IFAC Proc. Vol. 2014, 47, 142–146. [Google Scholar] [CrossRef]
- Sundareswaran, K.; Kuruvinashetti, K.; Gangadhar, I.; Sankar, P.; Nayak, P.S.; Vigneshkumar, V. Output Voltage Control and Power Management of a Dual Input Buck–Boost Converter Employing P&O Algorithm. IFAC Proc. Vol. 2014, 47, 1039–1043. [Google Scholar] [CrossRef]
- Bombelli, P.; Müller, T.; Herling, T.W.; Howe, C.J.; Knowles, T.P.J. A High Power-Density, Mediator-Free, Microfluidic Biophotovoltaic Device for Cyanobacterial Cells. Adv. Energy Mater. 2015, 5, 1401299. [Google Scholar] [CrossRef]
- Torabi, N.; Qiu, X.; López-Ortiz, M.; Loznik, M.; Herrmann, A.; Kermanpur, A.; Ashrafi, A.; Chiechi, R.C. Fullerenes Enhance Self-Assembly and Electron Injection of Photosystem I in Biophotovoltaic Devices. Langmuir 2021, 37, 11465–11473. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifar, M.; Tahernia, M.; Choi, S. A Miniaturized, Self-Sustaining, and Integrable Bio-Solar Power System. Nano Energy 2020, 72, 104668. [Google Scholar] [CrossRef]
- Sekar, N.; Umasankar, Y.; Ramasamy, R.P. Photocurrent Generation by Immobilized Cyanobacteria via Direct Electron Transport in Photo-Bioelectrochemical Cells. Phys. Chem. Chem. Phys. 2014, 16, 7862–7871. [Google Scholar] [CrossRef]
- Masi, M.; Bollella, P.; Riedel, M.; Lisdat, F.; Katz, E. Photobiofuel Cell with Sustainable Energy Generation Based on Micro/Nanostructured Electrode Materials. ACS Appl. Energy Mater. 2020, 3, 9543–9549. [Google Scholar] [CrossRef]
- Liu, L.; Choi, S. Self-Sustainable, High-Power-Density Bio-Solar Cells for Lab-on-a-Chip Applications. Lab Chip 2017, 17, 3817–3825. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Choi, S. A Paper-Based Biological Solar Cell. SLAS Technol. 2020, 25, 75–81. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Sheet, S.; Sathishkumar, Y.; Lee, Y.S.; Phang, S.-M.; Periasamy, V.; Gnana Kumar, G. Titania/Reduced Graphene Oxide Composite Nanofibers for the Direct Extraction of Photosynthetic Electrons from Microalgae for Biophotovoltaic Cell Applications. Appl. Phys. A 2018, 124, 769. [Google Scholar] [CrossRef]
- Thong, C.-H.; Priyanga, N.; Ng, F.-L.; Pappathi, M.; Periasamy, V.; Phang, S.-M.; Kumar, G.G. Metal Organic Frameworks (MOFs) as Potential Anode Materials for Improving Power Generation from Algal Biophotovoltaic (BPV) Platforms. Catal. Today 2022, 397–399, 419–427. [Google Scholar] [CrossRef]
- Shahparnia, M.; Packirisamy, M.; Juneau, P.; Zazubovich, V. Micro Photosynthetic Power Cell for Power Generation from Photosynthesis of Algae. Technology 2015, 03, 119–126. [Google Scholar] [CrossRef]
- Ramanan, A.V.; Pakirisamy, M.; Williamson, S.S. Advanced Fabrication, Modeling, and Testing of a Microphotosynthetic Electrochemical Cell for Energy Harvesting Applications. IEEE Trans. Power Electron. 2015, 30, 1275–1285. [Google Scholar] [CrossRef]
- Thorne, R.; Hu, H.; Schneider, K.; Bombelli, P.; Fisher, A.; Peter, L.M.; Dent, A.; Cameron, P.J. Porous Ceramic Anode Materials for Photo-Microbial Fuel Cells. J. Mater. Chem. 2011, 21, 18055–18060. [Google Scholar] [CrossRef]
- Saper, G.; Kallmann, D.; Conzuelo, F.; Zhao, F.; Tóth, T.N.; Liveanu, V.; Meir, S.; Szymanski, J.; Aharoni, A.; Schuhmann, W.; et al. Live Cyanobacteria Produce Photocurrent and Hydrogen Using Both the Respiratory and Photosynthetic Systems. Nat. Commun. 2018, 9, 2168. [Google Scholar] [CrossRef]
- Schneider, K.; Thorne, R.J.; Cameron, P.J. An Investigation of Anode and Cathode Materials in Photomicrobial Fuel Cells. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150080. [Google Scholar] [CrossRef] [PubMed]
- Inglesby, A.E.; Yunus, K.; Fisher, A.C. In Situ Fluorescence and Electrochemical Monitoring of a Photosynthetic Microbial Fuel Cell. Phys. Chem. Chem. Phys. 2013, 15, 6903–6911. [Google Scholar] [CrossRef]
- Firoozabadi, H.; Mardanpour, M.M.; Motamedian, E. A System-Oriented Strategy to Enhance Electron Production of Synechocystis sp. PCC6803 in Bio-Photovoltaic Devices: Experimental and Modeling Insights. Sci. Rep. 2021, 11, 12294. [Google Scholar] [CrossRef]
- Cevik, E.; Tombuloglu, H.; Anıl, I.; Senel, M.; Sabit, H.; AbdulAzeez, S.; Borgio, J.F.; Barghouthi, M. Direct Electricity Production from Microalgae Choricystis sp. and Investigation of the Boron to Enhance the Electrogenic Activity. Int. J. Hydrogen Energy 2020, 45, 11330–11340. [Google Scholar] [CrossRef]
- Zhu, H.; Meng, H.; Zhang, W.; Gao, H.; Zhou, J.; Zhang, Y.; Li, Y. Development of a Longevous Two-Species Biophotovoltaics with Constrained Electron Flow. Nat. Commun. 2019, 10, 4282. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez del Campo, A.; Perez, J.F.; Cañizares, P.; Rodrigo, M.A.; Fernandez, F.J.; Lobato, J. Characterization of Light/Dark Cycle and Long-Term Performance Test in a Photosynthetic Microbial Fuel Cell. Fuel 2015, 140, 209–216. [Google Scholar] [CrossRef]
- Ng, F.-L.; Jaafar, M.M.; Phang, S.-M.; Chan, Z.; Salleh, N.A.; Azmi, S.Z.; Yunus, K.; Fisher, A.C.; Periasamy, V. Reduced Graphene Oxide Anodes for Potential Application in Algae Biophotovoltaic Platforms. Sci. Rep. 2014, 4, 7562. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Neves, C.; Sebastião, D.; Nobre, B.P.; Matos, C.T. Effect of Light on the Production of Bioelectricity and Added-Value Microalgae Biomass in a Photosynthetic Alga Microbial Fuel Cell. Bioresour. Technol. 2014, 154, 171–177. [Google Scholar] [CrossRef]
- Bradley, R.W.; Bombelli, P.; Lea-Smith, D.J.; Howe, C.J. Terminal Oxidase Mutants of the Cyanobacterium Synechocystis sp. PCC 6803 Show Increased Electrogenic Activity in Biological Photo-Voltaic Systems. Phys. Chem. Chem. Phys. 2013, 15, 13611–13618. [Google Scholar] [CrossRef] [PubMed]
- Luimstra, V.M.; Kennedy, S.-J.; Güttler, J.; Wood, S.A.; Williams, D.E.; Packer, M.A. A Cost-Effective Microbial Fuel Cell to Detect and Select for Photosynthetic Electrogenic Activity in Algae and Cyanobacteria. J. Appl. Phycol. 2014, 26, 15–23. [Google Scholar] [CrossRef]
- Madiraju, K.S.; Lyew, D.; Kok, R.; Raghavan, V. Carbon Neutral Electricity Production by Synechocystis sp. PCC6803 in a Microbial Fuel Cell. Bioresour. Technol. 2012, 110, 214–218. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Zheng, Y.; Xiao, Y.; Yang, Z.; Zhao, F. Light Intensity Affects the Performance of Photo Microbial Fuel Cells with Desmodesmus sp. A8 as Cathodic Microorganism. Appl. Energy 2014, 116, 86–90. [Google Scholar] [CrossRef]
- Bombelli, P.; Zarrouati, M.; Thorne, R.J.; Schneider, K.; Rowden, S.J.L.; Ali, A.; Yunus, K.; Cameron, P.J.; Fisher, A.C.; Ian Wilson, D.; et al. Surface Morphology and Surface Energy of Anode Materials Influence Power Outputs in a Multi-Channel Mediatorless Bio-Photovoltaic (BPV) System. Phys. Chem. Chem. Phys. 2012, 14, 12221. [Google Scholar] [CrossRef]
- Nishio, K.; Hashimoto, K.; Watanabe, K. Light/Electricity Conversion by a Self-Organized Photosynthetic Biofilm in a Single-Chamber Reactor. Appl. Microbiol. Biotechnol. 2010, 86, 957–964. [Google Scholar] [CrossRef]
- Gunaseelan, K.; Jadhav, D.A.; Gajalakshmi, S.; Pant, D. Blending of Microbial Inocula: An Effective Strategy for Performance Enhancement of Clayware Biophotovoltaics Microbial Fuel Cells. Bioresour. Technol. 2021, 323, 124564. [Google Scholar] [CrossRef]
| S. No | ESA (cm2) | Vmp (mV) | Imp (µA) | Pmp (µW) |
|---|---|---|---|---|
| 1 | 4.84 | 478 | 420 | 200 |
| 2 | 19.36 | 495 | 1172 | 580 |
| 3 | 100 | 510 | 2350 | 1200 |
| S. No | ESA (cm2) | Pmp (µW) | Vmp (mV) |
|---|---|---|---|
| 1 | 4.84 | 200.76 | 0.81 |
| 2 | 19.36 | 673.2 | 0.86 |
| 3 | 29.04 [S3 (P2, P2, P2)] | 869.2 | 2.4 |
| 4 | 29.04 [S2 (P3, P3)] | 926.4 | 1.6 |
| 5 | 100 | 1062.1 | 0.88 |
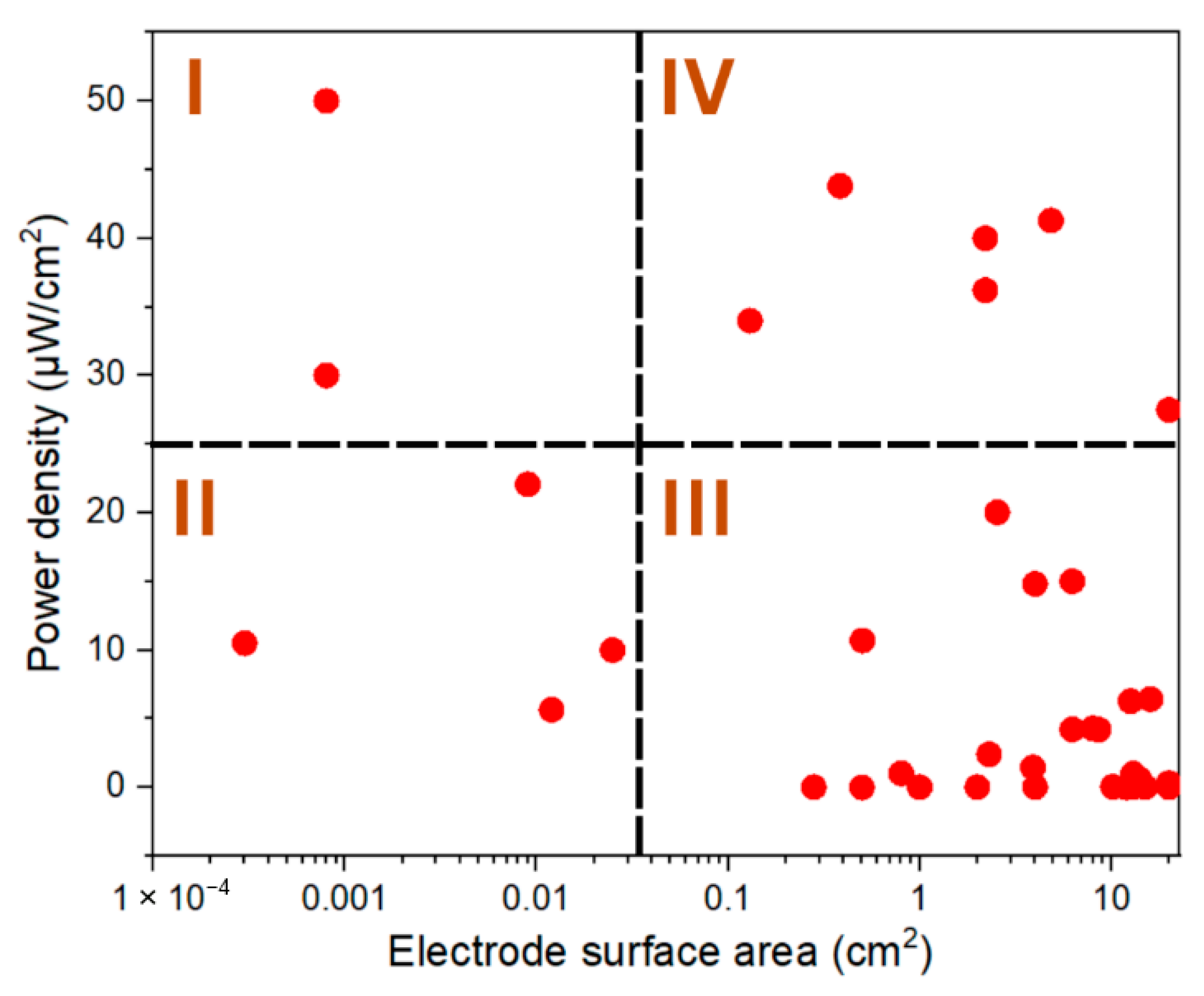
| S. No | Device Name | ESA (cm2) | PD (mW/m2) | Reference |
|---|---|---|---|---|
| 1 | BPV | 0.0003 | 10.5 | [22] |
| 2 | BPV | 0.0008 | 30.0 | [1] |
| 3 | BPV | 0.0008 | 50.0 | [1] |
| 4 | BPV | 0.009 | 22.07 | [23] |
| 5 | Bio solar cell | 0.012 | 5.64 | [24] |
| 6 | Photo-bioelectrochemical cells | 0.025 | 10.0 | [25] |
| 7 | Biofuel cell | 0.13 | 34.0 | [26] |
| 8 | Microbial solar cell | 0.28 | 0.00709 | [17] |
| 9 | Microbial solar cell | 0.385 | 43.8 | [27] |
| 10 | Photosynthetic electrochemical cell | 0.5 | 0.00004 | [9] |
| 11 | Biosolar cell | 0.503 | 10.7 | [28] |
| 12 | BPV | 0.8 | 1.02 | [4] |
| 13 | BPV | 1 | 0.0036 | [29] |
| 14 | PSC | 2 | 0.004 | [30] |
| 15 | PSC | 2.2 | 36.22 | [31] |
| 16 | PSC | 2.2 | 40.0 | [32] |
| 17 | Bio-electrophotochemical cell | 2.3 | 2.4 | [33] |
| 18 | Photo-MFC | 2.54 | 20.04 | [34] |
| 19 | Photosynthetic-MFC | 3.9 | 1.45 | [35] |
| 20 | BPV | 4 | 0.00248 | [36] |
| 21 | Bio-electrochemical fuel cell | 4 | 14.82 | [37] |
| 22 | Bio-electrochemical fuel cell | 6.25 | 4.22 | [38] |
| 23 | BPV | 6.25 | 15.0 | [39] |
| 24 | Photosynthetic-MFC | 8 | 4.29 | [40] |
| 25 | Aluminum biophotovoltaic system | 8.6 | 4.2 | [2] |
| 26 | Aluminum biophotovoltaic system | 10.2 | 0.04 | [2] |
| 27 | Photosynthetic electrochemical cell | 12 | 0.00004 | [18] |
| 28 | BPV | 12.25 | 0.027 | [41] |
| 29 | BPV | 12.25 | 0.032 | [16] |
| 30 | BPV | 12.25 | 0.0538 | [16] |
| 31 | BPV | 12.25 | 0.038 | [3] |
| 32 | Photosynthetic alga microbial fuel cell | 12.6 | 6.27 | [42] |
| 33 | BPV | 13 | 1.0 | [5] |
| 34 | BPV | 13 | 0.02 | [43] |
| 35 | Photosynthetic microbial fuel cell | 14 | 0.6 | [44] |
| 36 | MFC | 15 | 0.03 | [45] |
| 37 | Photo-MFC | 16 | 6.42 | [46] |
| 38 | BPV | 20 | 0.002 | [47] |
| 39 | Photo-bioreactor | 20 | 0.32 | [48] |
| 40 | BPV | 20 | 27.5 | [49] |
| 41 | µPSC | 4.84 | 413 | This work |
| 42 | µPSC | 19.36 | 299.6 | This work |
| 43 | µPSC | 100 | 120 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuruvinashetti, K.; Packirisamy, M. Scalable Hybrid Arrays Overcome Electrode Scaling Limitations in Micro-Photosynthetic Power Cells. Energies 2025, 18, 5644. https://doi.org/10.3390/en18215644
Kuruvinashetti K, Packirisamy M. Scalable Hybrid Arrays Overcome Electrode Scaling Limitations in Micro-Photosynthetic Power Cells. Energies. 2025; 18(21):5644. https://doi.org/10.3390/en18215644
Chicago/Turabian StyleKuruvinashetti, Kirankumar, and Muthukumaran Packirisamy. 2025. "Scalable Hybrid Arrays Overcome Electrode Scaling Limitations in Micro-Photosynthetic Power Cells" Energies 18, no. 21: 5644. https://doi.org/10.3390/en18215644
APA StyleKuruvinashetti, K., & Packirisamy, M. (2025). Scalable Hybrid Arrays Overcome Electrode Scaling Limitations in Micro-Photosynthetic Power Cells. Energies, 18(21), 5644. https://doi.org/10.3390/en18215644






