Abstract
Spent coffee grounds (SCG) constitute a significant organic waste stream with considerable potential for bioenergy recovery. This review critically examines the viability of anaerobic digestion (AD) as a sustainable valorization pathway for SCG, addressing both technical and environmental challenges. Due to their elevated lignin levels, lipid content, and inhibitory substances, SCG exhibit strong recalcitrance that limits their direct digestibility in anaerobic systems. Therefore, a range of pretreatment methods, including oil extraction, alkaline hydrolysis, thermo-alkaline processes, oxidative treatments, and hydrothermal techniques, are evaluated for their effectiveness in enhancing biodegradability and methane yields. Co-digestion with nutrient-rich substrates is explored as a strategy to improve process stability, mitigate inhibitory effects, and optimize nutrient balance. Furthermore, techno-economic and life cycle assessments underscore the feasibility of SCG-based AD compared to conventional waste management practices. The integration of SCG digestion into biorefinery models offers a promising approach to energy recovery, resource efficiency, and waste minimization within a circular bioeconomy framework. This review highlights the need for continued optimization and scale-up to fully harness the potential of SCG in renewable energy systems.
1. Introduction
Anaerobic digestion (AD) has become a key technology in sustainable waste management and renewable energy production [], addressing global challenges of organic waste disposal and carbon-neutral energy generation []. During AD, organic residues are transformed into biogas and digestate, supporting the circular economy through renewable energy production and nutrient recycling [].
The global coffee industry produces approximately 6 to 8 million tons of spent coffee grounds (SCG) annually [], though recent estimates suggest this figure may reach up to 18 million tons []. Traditionally, SCG has been either landfilled [] or used in low-value applications such as composting [] or direct combustion []. These conventional disposal methods are considered less sustainable than AD. Landfilling leads to an uncontrolled release of methane and a loss of organic nutrients, while composting can lead to ammonia volatilization and offers no energy recovery. Direct incineration is less energy efficient and can release harmful pollutants if not handled properly []. In contrast, AD enables the production of renewable energy and the recycling of nutrients, which aligns with the principles of the circular economy []. While early research in the 1980s and 1990s explored alternative uses, significant advancements have occurred over the past two decades, driven by the emergence of circular bioeconomy models and the need for sustainable waste-to-energy solutions [].
The potential to valorize SCG through biorefinery processes, especially AD, presents a sustainable alternative to landfilling. AD is particularly attractive due to its capacity to convert organic substrates into CH4-rich biogas, with a CH4 content typically between 55% and 61%, and to use digestate as a biofertilizer [,]. However, several challenges limit the efficiency of SCG digestion, including high lignin content [], the presence of inhibitory compounds (e.g., polyphenols) [], and inherently low biodegradability []. Despite this potential, SCG remains an underutilized raw material, mainly due to the complexity and variability of its chemical composition [].
Furthermore, the economic viability and long-term sustainability of large-scale AD based on SCG continues to be questioned. Successful implementation depends on optimized processing techniques and integration into broader biorefinery models []. Policy instruments, such as carbon credit incentives [], renewable energy mandates, and waste-management regulations, also play a crucial role in promoting the uptake and scalability of AD systems [].
This review provides a comprehensive and critical evaluation of processes for the AD of SCG, covering key aspects such as feedstock composition, pre-treatment strategies, process optimization techniques, and environmental impact metrics. It also compares alternative recovery routes to contextualize the benefits and trade-offs of AD. By integrating these multidisciplinary perspectives, the review supports the development of resilient, resource-efficient, and circular waste-to-energy systems that are in line with the principles of the bioeconomy.
To ensure a comprehensive and up-to-date review, a literature search was performed using renowned scientific databases such as Web of Science, Scopus, ScienceDirect, and Google Scholar, as well as in relevant publications on ResearchGate. The search strategy focused on peer-reviewed articles published mainly in the last 10 years. It used combinations of keywords such as coffee grounds, AD, biogas, pretreatment, co-digestion, and biorefinery. Filters were used to highlight recent studies, high-impact journals, and those offering experimental or techno-economic insights into the use of coffee grounds. The reference lists of key papers were also screened to identify further relevant studies.
2. Physicochemical Properties and Composition of Spent Coffee Grounds
SCG are the solid residue left after brewing coffee, generated across the entire coffee supply chain—from household kitchens to coffee shops and industrial processing plants. For every ton of green coffee beans processed, approximately 650 kg of SCG are produced. In the production of soluble coffee, roughly 2 kg of wet SCG are generated per kilogram of product [].
2.1. Factors Influencing SCG Composition
The chemical composition of SCG is determined by multiple upstream variables, including coffee species, cultivation conditions, post-harvest processing, roasting, and brewing methods []. For instance, arabica and robusta, the dominant coffee species, exhibit notable compositional differences; robusta contains more caffeine and chlorogenic acids, whereas arabica is richer in lipids and sucrose (Table 1).
Environmental factors like climate, soil composition, and altitude influence bean chemistry, which is subsequently reflected in SCG composition [].
Coffee bean composition is influenced by environmental conditions such as climate, soil, and altitude. For example, higher altitudes (>1200 m) have been associated with lower lipid content in arabica beans due to slower maturation and metabolic shifts during growth []. These variations in bean chemistry can influence the composition of SCG, particularly regarding digestibility and biogas potential. Post-harvest processing methods also significantly affect SCG composition. The wet process generates various residues, including pulp, mucilage, and processing wastewater, rich in organic compounds. For instance, coffee pulp contains carbohydrates (21–32%), protein (7.5–15%), and fat (2–7%), while mucilage is rich in protein (8.9%), sugar (4.1%), and peptic substances (0.91%) []. In contrast, the dry process involves drying the entire coffee cherries without removing the pulp [].
Roasting plays a decisive role in coffee processing, as roasting at high temperatures is the most important factor in the formation of melanoidins. This process, which mainly occurs through the Maillard reaction, usually starts at around 160 °C and continues through the caramelization phase (often between 170 °C and 200 °C), which is within the general roasting range of 180–240 °C for green coffee beans []. Melanoidins are brown polymers with a high molecular weight, which are largely absent in green coffee beans, but become an important product at higher roasting degrees and increase significantly. However, it should be noted that very intensive roasting conditions can lead to the degradation of these polymers. In SCG, which comprise 13–25% of the composition, these melanoidins change important properties []. They contribute significantly to antioxidant capacity and have binding capabilities, such as biochelation with metals []. In addition, they are associated with humic-like plant-stimulating activities, indicating a link between the Maillard reaction and humification, which enables SCGs to promote plant growth [].
Finally, brewing methods like espresso, French press, or drip coffee influence the degree of compound extraction and therefore determine the residual matrix in SCG [].

Table 1.
Key factors affecting spent coffee grounds’ composition.
Table 1.
Key factors affecting spent coffee grounds’ composition.
| Factor | Influence on Spent Coffee Grounds Composition | Ref. |
|---|---|---|
| Coffee species | Arabica (higher lipids, sucrose); Robusta (higher caffeine, chlorogenics) | [] |
| Cultivation conditions | Soil (fatty acid composition), altitude (lipid content), climate (fatty acids, elements, aromatic compounds), and organic farming practices (fatty acids and quinic acid derivatives, lower levels of trigonelline and chlorogenic isomers) | [] |
| Post-harvest processing | Wet method reduces some carbohydrates and enhances polyphenol retention | [] |
| Roasting degree | Formation of melanoidins, alteration of antioxidants | [] |
| Brewing method | Varies residual lipid, protein, and sugar content | [] |
2.2. Chemical Composition of SCG
The chemical composition of SCG is heterogeneous and influenced by factors such as coffee species, roasting degree, brewing method, and extraction conditions. On a dry-weight basis, SCG typically contains between 64 and 89% carbohydrates, 10 to 17% proteins, and 7 to 21% lipids, with an ash fraction generally below 1% []. This profile highlights its potential as a lignocellulosic feedstock for both energy generation and the recovery of value-added bioproducts [].
2.2.1. Elemental Composition
Elemental analysis shows that SCG is particularly rich in carbon, which typically accounts for 47 to 56% of dry matter, while hydrogen makes up about 7%, nitrogen 1.5 to 2.4%, and sulfur around 0.1%, with oxygen constituting the remainder [].
Proximate analyses report volatile matter contents of 50 to 60%, and fixed carbon between 10 and 15% []. Freshly collected SCG is characterized by a very high moisture content, often exceeding 80%, which makes it unstable and prone to rapid microbial degradation during storage. To ensure stability and facilitate handling, drying to a moisture content below 10% is generally recommended []. Such stabilization not only improves shelf-life but also reduces logistical and processing challenges when SCG is used in large-scale valorization pathways [].
2.2.2. Organic Compounds
The organic fraction of SCG is dominated by structural carbohydrates, lipids, proteins, and a range of phenolic compounds. Among polysaccharides, hemicellulose represents 35 to 39% of dry matter, cellulose contributes around 9 to 12%, and lignin accounts for 19 to 24% []. This lignocellulosic matrix defines SCG’s character as a renewable resource, but also its recalcitrance to microbial degradation. Hemicellulose and cellulose are potential substrates for enzymatic hydrolysis, which can release fermentable sugars for bioethanol or bioplastic production, whereas lignin, though difficult to degrade biologically, enhances the calorific value of SCG and is important in thermochemical conversion processes [].
Lipids are another significant fraction, ranging from 7 to 21% of dry matter [], with some reports extending up to 22–27% depending on brewing conditions []. The lipid profile is rich in fatty acids, particularly linoleic acid (43–50%), palmitic acid (32–43%), and oleic acid (6–10%), with minor contributions from stearic, linolenic, and arachidic acids []. This composition makes SCG an attractive substrate for biodiesel production [], and the residual oil has been investigated for use in biopolymers [], surfactants [], and specialty chemicals [].
Proteins account for approximately 13 to 17% of SCG dry matter []. The amino acid composition makes them suitable as fermentation substrates and potential additives in animal feed. However, their digestibility can be affected by phenolic interactions and Maillard reaction products formed during roasting. Alongside these macronutrients, SCG is particularly rich in bioactive molecules []. Chlorogenic acids are typically present in the range of 0.2 to 0.8%, while flavonoids may reach up to 29 mg/g of dry matter []. Melanoidins, formed during roasting, also contribute to the strong antioxidant and antimicrobial properties of SCG [].
2.2.3. Mineral Profile
The mineral fraction of SCG, though relatively small, contains a variety of macro- and micronutrients of agronomic and biotechnological importance. Potassium is the most abundant element, with concentrations close to 11.7 g/kg dry matter, followed by phosphorus (1.8 g/kg), magnesium (1.3–1.9 g/kg), and calcium (0.8 g/kg) []. Trace elements such as iron are also present in meaningful amounts, supporting their potential role in soil amendment and plant nutrition [].
Nitrogen levels of around 2% on a dry weight basis result in an average carbon-to-nitrogen (C/N) ratio of approximately 17:1. While this ratio is less than ideal for AD as a standalone substrate, it is highly advantageous for co-digestion with nitrogen-rich feedstocks such as animal manures or food waste. SCG contributes carbon and energy while balancing nutrient availability, thereby improving biogas production and process stability [].
2.3. Physical Properties of SCG
The physical properties of SCG play a critical role in determining their suitability for various applications, including adsorption, composting, biochar production, and soil amendment. These properties (color, texture, particle size, bulk density, and water retention capacity) affect handling, processing, and end-use performance in both energy and environmental technologies (Table 2).

Table 2.
Physical properties of spent coffee grounds and their impact on anaerobic digestion.
Table 2.
Physical properties of spent coffee grounds and their impact on anaerobic digestion.
| Property | Characteristics | Relevance | Impact on AD |
|---|---|---|---|
| Color | Dark brown to black; changes with roast and treatment | Indicator of chemical changes during processing | Reduces biodegradability (melanoidins) and increases toxicity (polyphenols, furfurals) |
| Texture | Fine and granular | Facilitates mixing with soil and substrates | Provides temporary adsorption of VFAs or inhibitory compounds |
| Particle Size | 125–425 μm (post-extraction) | Impacts adsorption, biochar reactivity, and reactor efficiency | Enhances microbial access but can lead to rapid acidification |
| Bulk Density | Increases after defatting (not consistently reported) | Improves transport and reactor design | Affects mixing and influencing mass transfer |
| Water Holding Capacity | High | Enhances soil moisture, supports compost aeration | Affects reactor stability |
| Natural Moisture Content | 55–80% | Supports hydrothermal processes without drying | Moderate moisture improves hydrolysis and dilutes inhibitors; excessively high moisture content reduces storage stability |
2.3.1. Color and Texture
SCG typically exhibits a dark brown to black hue and a fine, granular texture. This color is largely influenced by the degree of roasting of the original coffee beans and the brewing method employed []. Treatments such as hydrothermal or hydrogen peroxide pretreatment can significantly change the color of SCG, transforming it from dark brown to lighter shades such as yellow. This transformation reflects the degradation of melanoidins, nitrogenous, brown-colored macromolecules formed via Maillard reactions, as well as the degradation of lignin and phenolic compounds, all of which contribute significantly to the dark appearance of SCG [].
2.3.2. Particle Size and Density
Particle size influences SCG performance in processes such as adsorption, soil amendment, and biochar production. SCG particle sizes typically range between 125 and 425 μm, with the majority falling within this interval after oil extraction via Soxhlet methods []. Particle size reduction has also been reported following direct transesterification, enhancing its reactivity in bioconversion and sorption systems []. While bulk density is not consistently reported, SCG through oil extraction leads to notable changes in particle size distribution, where the majority of particles are in the 250–425 µm range, which can improve packing efficiency in reactors []. Although a direct increase in bulk density after degreasing could not be confirmed, this finer particle size, combined with a lower lignin content (from 4.21% to 1.70%) and better biodegradability, suggests better handling properties []. In addition, densification techniques such as palletization, which are often applied after degreasing, are crucial for improving transport and storage logistics, especially given the challenges associated with the high moisture content and dispersed formation of SCG [].
2.3.3. Water Holding and Absorption Capacity
SCG possesses a high water-holding capacity due to its fibrous and porous structure []. This property enhances its value as a soil improver, particularly in arid or semi-arid regions []. Studies show that SCG amendment in Mediterranean soils can enhance moisture retention and improve soil aggregate structure. The natural moisture content of SCG, ranging from 55% to 80%, is particularly advantageous for hydrothermal processing since it minimizes the need for pre-drying, reducing energy input [].
In addition to soil-related benefits, SCG’s water absorption capability allows it to serve as a desiccant in the treatment of sewage sludge. Its ability to retain water and air spaces also makes it an effective bulking agent in composting operations, contributing to the regulation of moisture and aeration during microbial degradation [].
3. Pretreatment Methods
Effective pretreatment is essential for enhancing the AD performance of SCG, particularly by improving their biodegradability and increasing the resulting methane yield, even up to 24% []. As outlined in Section 2.2.2, SCG contains significant amounts of lignin, lipids, and polyphenolic compounds that reduce biodegradability and hinder microbial activity. Pretreatment methods are therefore employed to overcome these compositional barriers, enhance substrate solubilization, and improve conversion efficiency in processes such as AD and biofuel production []. Pretreatment aims to remove or modify these recalcitrant components [], improve solubilization [], and increase the bioavailability of carbohydrates and proteins [].
3.1. Oil Extraction (Defatting)
Oil extraction is often the first step in SCG valorization. It reduces the lipid and lignin content and removes inhibitory compounds such as caffeine and diterpenes []. Although the process may slightly decrease carbohydrate content, cellulose and hemicellulose structures typically remain intact. The defatted SCG (DSCG) becomes more suitable for AD due to reduced long-chain fatty acid (LCFA) toxicity, which can otherwise inhibit methanogenic activity.
3.1.1. Solvent Extraction
Solvent extraction is a widely adopted method for defatting SCG. Among these, Soxhlet extraction is particularly prevalent in laboratory-scale studies. In this method, dried SCG is placed in a thimble inside a Soxhlet apparatus []. A solvent, commonly hexane, is heated, vaporized, condensed, and cycled over the sample. As the solvent dissolves oils and lipophilic compounds, it accumulates in the boiling flask, and the cycle continues for hours, typically ranging from 3 to 8 h depending on experimental conditions [].
In addition to hexane, various other solvents have been tested, including isopropanol, ethanol, diethyl ether, chloroform, and mixtures such as hexane–isopropanol or methanol–hexane []. The choice of solvent has a considerable influence on the efficiency of extraction and subsequent digestibility. For example, refluxing hexane at 80 °C has been shown to achieve high oil recovery yields, up to 30.4% w/w, and is widely regarded as one of the most effective and commonly used methods for lipid extraction from SCG, also enhancing the substrate’s suitability for AD []. The solvent-to-solid ratio (e.g., 1:3 w/v) and temperature control are crucial parameters that influence oil yield and selectivity. Furthermore, the use of hexane for oil extraction at 70 °C was found to not only remove the oil but also improve the quality of the starting material for AD, as the lignin content was reduced from 4.21% (w/w) in SCG to 1.70% (w/w) in DSCG [].
In post-extraction, the solvent–oil mixture is separated via rotary evaporation, leaving behind purified coffee oil. This oil can be converted into biodiesel, while the remaining DSCG is used in AD or other applications []. In addition to traditional solvent-based extraction, advanced technologies such as ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) have been explored to enhance process efficiency. UAE has been shown to achieve up to 98% oil yield within just 30 min [] and is effective in recovering phenolic compounds (e.g., 33.84 ± 0.59 mg GAE/g in 40 min) []. MAE, on the other hand, offers significant time and energy savings, reducing processing times for compounds like levulinic acid from 8 h to just 30 min [], and achieving high antioxidant yields (e.g., 398.95 mg GAE/g TPC in 40 s at 80 W) []. Despite their benefits, these methods are currently more feasible at the laboratory scale and require further development for industrial application [].
3.1.2. Supercritical Fluid Extraction (SFE)
Supercritical fluid extraction, particularly using supercritical CO2 (scCO2), is gaining popularity as a “green” and efficient alternative to traditional solvent extraction []. In SFE, CO2 is brought above its critical point (around 31 °C and 74 bar), exhibiting gas-like diffusion and liquid-like solvating power. This allows it to efficiently extract lipophilic compounds, including triglycerides, diterpenes (e.g., cafestol and kahweol), and tocopherols from SCG [].
The condition is a pressure of 250 bar, although optimum settings may vary depending on the target compound. Unlike conventional solvents, CO2 is non-toxic, non-flammable and can be easily separated from the extract by depressurization, eliminating the need for costly solvent recovery []. By using co-solvents (e.g., ethanol), the polarity can be increased and the range of extractable compounds expanded [].
Supercritical fluid extraction not only improves oil recovery from SCG but also results in defatted SCG (DSCG) with increased biochemical methane potential (BMP) []. This improvement is largely due to the removal of inhibitory compounds such as phenolic compounds [], caffeine [], tannins, and LCFAs [], which are known to impair microbial activity, retard methane production, and destabilize AD systems []. By eliminating or reducing these compounds and partially degrading lignocellulosic structures, SFE improves microbial accessibility to the substrate, which ultimately improves CH4 yield and process stability []. Table 3 summarizes various solvent-based lipid extraction methods applied to SCG and their respective effects on AD performance.

Table 3.
Solvent-based lipid extraction from spent coffee grounds and impacts on anaerobic digestion.
Table 3.
Solvent-based lipid extraction from spent coffee grounds and impacts on anaerobic digestion.
| Extraction Method | Conditions | Target Compound(s) | BMP/CH4 Yield (mL/g VS) | Impact on AD Performance | Reference |
|---|---|---|---|---|---|
| Soxhlet (Hexane extraction) | 2 h, n-hexane | Lipids (15–20% w/w) | Untreated: 310; Defatted: 336 | Lipid removal reduced LCFA inhibition, leading to +8.4% BMP increase | [,] |
| Organosolv extraction (MeOH/n-hexane + H2SO4 catalyst) | 160 °C, 60 min | Lignin, Fatty Acid Methyl Esters | Residue BMP: 36.0 vs. theoretical 248.5 | Strong reduction in BMP due to removal of major organics; valuable co-products recovered | [] |
| Supercritical CO2 extraction | 20 MPa, 40–80 °C; neat CO2 or CO2 + 15% ethanol | Oils, diterpenes, sterols | Not directly measured; Oil yield 0.1–12.2% (neat CO2), up to 15.9% (CO2 + ethanol) | Lipid removal reduces LCFA toxicity; BMP improvement expected though not quantified | [] |
| Ethanolic extraction (Ultrasound-assisted) | 60% EtOH, 60 °C, 30 min | Polyphenols, caffeine | BMP not reported | Removal of inhibitors (polyphenols, caffeine) potentially enhances AD stability, especially in co-digestion | [] |
3.2. Alkaline Pretreatment
Alkaline pretreatment, specifically with NaOH, is effective in breaking the ester bonds between lignin, hemicellulose, and cellulose []. Increasing NaOH concentration generally resulted in a slightly increased lignin removal and increased the soluble organic material, measured as dissolved organic carbon (DOC). The DOC value increased significantly with increased NaOH loading, indicating increased bioavailability of organics []. However, for small-particle-size SCG, substantial variation in fiber composition might not be as evident compared to biomasses with wider particle dimensions. Pretreatment using NaOH is an effective method to remove lignin and lipid contents and increase the exposure of carbohydrates in downstream degradation steps [].
The key mechanism is that alkali causes swelling of the cellulose, which increases the surface area of the fibers that is accessible to the hydrolytic enzymes involved in AD []. Alkaline hydrolysis leads to partial solubilization of the lignin and can effectively remove the lignin content []. By breaking up the complex structure, NaOH pretreatment increases the exposure of the carbohydrates []. The treatment also contributes to the solubilization of complex organics, possibly including proteins and oil fractions, which are transferred from the solid to the liquid phase []. This leads to a very high increase in DOC, which correlates with the biogas potential of the feedstock and effectively demonstrates the effect of NaOH in increasing the bioavailability of the organics to the microorganisms in the subsequent biogas plant [].
Alkaline pretreatments significantly alter the surface morphology and structural properties of SCG, resulting in increased porosity, impaired structural integrity, and increased surface area. Untreated SCG is characterized by a dense, compact, and relatively smooth outer structure, with limited visible porosity and minimal accessible surface area. According to the studies by Tsafrakidou et al. [], SEM and CLSM images confirmed that raw SCG has a rigid cell wall matrix with a relatively closed surface morphology, that limits enzymatic attack and microbial colonization. After soaking in aqueous ammonia solution (AAS), there was a pronounced morphological disruption of the surface. This pretreatment selectively dissolved and redistributed the lignin, while hemicellulose and cellulose are retained, resulting in partial delamination of the lignocellulose structure. Under optimized AAS conditions (28–30% v/v ammonia, 60 °C, 120 min, 10% w/v solids), a significant increase in porosity and the exposure of a well-defined honeycomb structure was observed. These changes result from the swelling of the cell wall matrix, the partial cleavage of lignin–carbohydrate complexes, and the weakening of non-covalent interactions within the biomass matrix []. The resulting microstructure facilitates the infiltration of enzymes by increasing the specific surface area and exposing internal polysaccharide domains, thereby improving saccharification efficiency.
In parallel, NaHCO3-assisted SCG torrefaction acts through a different but complementary mechanism. During torrefaction at 300 °C for 60 min in the presence of 8.3 wt% NaHCO3, the thermal decomposition of the additive releases CO2 gas, which acts as a pore-forming agent in situ. The CO2 bubbles penetrate the SCG matrix, destroying its structure and forming a network of mesopores (2–50 nm). The resulting biochar shows a drastic increase in specific surface area (141%) and total pore volume (76%), compared to non-activated torrefied SCG, as confirmed by a BET analysis [].
Alkaline pretreatment has a positive effect on increasing the CH4 yield from SCG []. A study in which alkaline pretreatment with NaOH was performed at room temperature (20 ± 0.5 °C) for 24 h showed that increasing the NaOH loading (from 2% to 8% w/w) in the mixture with SCG resulted in a linear increase in DOC concentration and a generally strong increase in CH4 production. The highest NaOH concentration tested (8% w/w) gave the best AD performance and achieved a CH4 yield of 392 mL CH4/g volatile solids (VS), an increase of 24% compared to untreated SCG (316 mL CH4/g VS), with the difference reported as statistically significant (p < 0.05). It is noteworthy that AD was not inhibited in any of the pretreated substrates within the tested range of 2–8% NaOH at this ambient temperature. The pH after pretreatment and addition of the inoculum remained within the acceptable range for methanogens (up to 8.5). This method was also successful in increasing the degradation of total solids and VS and improving digestate stability. Alkaline pretreatment with NaOH resulting in 350 NmL CH4/g VS from DSCG at a substrate-to-inoculum ratio (SI) of 1 was also reported, demonstrating the advantage of alkaline hydrolysis [].
Alkaline pretreatment of SCG significantly modifies the chemical profile of both inhibitory and bioactive components, such as caffeine, cafestol, and kahweol. Caffeine, although naturally occurring, poses environmental and process-related challenges due to its phytotoxic and antimicrobial properties. Its persistence in SCG has been associated with instability in AD and reduced microbial efficiency in hydrolysis and fermentation systems. Alkaline oxidative treatments have proven effective in mitigating these effects. For example, treating SCG with a 1% NaOH solution followed by hydrogen peroxide (H2O2) bleaching at 60 °C for 90 min results in a marked reduction in caffeine content, as evidenced by the diminished caffeine-related bands in FTIR spectra and reduced UV-absorbance at 273 nm. This treatment promotes caffeine solubilization through pH-driven hydrolysis and oxidation, reducing its environmental impact and improving substrate compatibility for biotechnological processes [].
In parallel, alkaline saponification techniques are employed for the extraction and quantification of valuable diterpenes such as cafestol and kahweol, which are predominantly present in esterified forms with fatty acids like oleic and linoleic acids. The standard laboratory procedure involves dissolving lipid extracts of SCG in 1 M potassium hydroxide (KOH) in ethanol, followed by incubation at 80 °C for 60 min under reflux conditions. This process cleaves ester bonds, liberating the diterpene alcohols for downstream quantification. However, the thermal and alkaline environment can also induce partial degradation of diterpenes, leading to by-products such as dehydrocafestol- and dehydrokahweol-like oxidized derivatives that have been used as chemical markers of roasting or saponification severity [].
While saponification is effective for analytical purposes, industrial-scale valorization favors supercritical CO2 (SC-CO2) extraction, often modified with 10–15% ethanol as a co-solvent []. Under conditions of 300 bar and 40–60 °C for 60–120 min, SC-CO2 enables selective extraction of diterpenes with minimal degradation, increasing yield by up to 15-fold and concentration by 200–400% compared to conventional Soxhlet extraction with n-hexane. These combined strategies demonstrate how alkaline conditions, when precisely controlled, can either eliminate undesirable compounds or facilitate the selective recovery of high-value biomolecules from SCG [].
Thermo-Alkaline Pretreatment of Spent Coffee Grounds
The method combines heat and NaOH and has proven effective for the solubilization of SCG, particularly at higher NaOH concentrations and elevated temperatures. This effectiveness is attributed to the synergistic interaction between heat and NaOH, which enhances lignocellulose hydrolysis []. In contrast, thermal pretreatment alone resulted in significantly lower solubilization compared to pretreatment with NaOH [].
Kim et al. [] examined thermo-alkaline pretreatment of SCG using NaOH (0–0.2 M) at elevated temperatures (60–90 °C) for 6 h. This combined approach significantly enhanced solubilization (SD), with the maximum value (36.4%) achieved at 0.18 M NaOH and 90 °C. However, higher solubilization did not translate directly into higher methane yield (Ym). Instead, methane production followed an optimum curve; the highest experimental Ym (254.0 mL CH4/g COD added) occurred at 0.1 M NaOH and 75 °C, while modeling predicted the true optimum at 0.13 M NaOH and 70.5 °C (263.3 mL CH4/g COD added). By contrast, the most severe condition (0.2 M NaOH and 90 °C) led to the lowest Ym (151.9 mL CH4/g COD added), even lower than untreated SCG. This negative effect of the high NaOH concentration on Ym could be related to the inhibition of methanogenic activity and the formation of inhibitory compounds. Na+ concentrations reached up to 2.2 g/L, a value consistent with inhibition thresholds for methanogenic archaea, as inhibition often starts at 2 g/L and is pronounced above 3–5 g/L [,]. The main mechanism of sodium inhibition is osmotic stress. Elevated Na+ concentrations increase the osmotic pressure of the medium, and force methanogenic cells to expend additional energy to maintain intracellular ionic balance. This disrupts the proton gradient and reduces the efficiency of ATP formation, which ultimately impairs cell metabolism [].
In addition, high sodium levels can interfere with enzyme systems important for methanogenesis. Sodium ions can displace critical cofactors such as K+ or Mg2+ from the active sites of enzymes, which alters their conformation and reduces catalytic activity. This effect is particularly evident in acetoclastic methanogens such as Methanosaeta and Methanosarcina, which are more sensitive to ionic imbalance than hydrogenotrophic genera such as Methanoculleus or Methanobacterium [,].
Elevated sodium concentrations can also change the structure of the microbial community. Sensitive methanogens may be suppressed, while halotolerant or halophilic species (including certain hydrogenotrophic archaea) may proliferate. Such shifts often lead to changes in the predominant metabolic pathways, the accumulation of intermediates such as volatile fatty acids, and a loss of process stability [].
Finally, harsh alkaline–thermal pretreatment can also generate secondary inhibitory compounds, including furfural, hydroxymethylfurfural (HMF), phenols, and aldehydes, which are known to impair methanogenic activity [].
3.3. Oxidative Pretreatment (Using Hydrogen Peroxide)
Oxidative pretreatment, in particular the use of hydrogen peroxide (H2O2), is presented in the sources as a chemical treatment method aimed at improving the degradability of lignocellulosic biomass, such as coffee grounds, especially for processes such as AD []. The main advantage of H2O2 as an oxidizing agent in this context is that it degrades to oxygen and water, leaving no solid residues in the biomass and hardly any unwanted by-products. The mechanism by which H2O2 pretreatment operates involves its nature as a strong oxidant. H2O2 has a known delignifying action on lignocellulosic materials. The sources indicate that the formation of reactive free radicals, including hydroxyl (HO•) and hydroperoxyl (HO2•), upon the breakdown of oxygen bonds in H2O2, promotes the decomposition of organic matter. This action breaks down the lignocellulose structure. Beyond chemical breakdown, H2O2 is also recognized as an active bleaching agent. Studies on H2O2-pretreated SCG observed a dramatic color change from dark brown to light yellow, indicative of bleaching, and noted that this change, along with changes in surface morphology, resulting in a porous surface, may be due to the removal of lignin and phenolic compounds [].
The main objective of hydrogen peroxide (H2O2) pretreatment prior to AD is to improve the biodegradability of SCG and thereby increase biogas and methane production. H2O2 acts as a strong oxidizing agent, which breaks down the lignin structures and promotes the solubilization of organic matter. In the study by Sayoud et al. [], four H2O2 concentrations (0.5%, 1%, 2%, and 4% w/w) were tested under mesophilic conditions (37 ± 2 °C) with 24 h exposure at ambient pressure. Pretreatment resulted in a large increase in soluble chemical oxygen demand (sCOD) and total sugar release, with sCOD increasing by 556–713% and sugars by 748–818% compared to untreated SCG. However, these gains in solubilization were not directly reflected in proportional increases in biogas or methane yields. The highest performance was achieved with 4% H2O2, which resulted in 642.69 mL biogas/gVS and 345.53 mL CH4/gVS, corresponding to a yield increase of 16.28% and 16.93% over untreated SCG, respectively. In contrast, the 2% H2O2 pretreatment produced only 520.37 mL biogas/gVS and 263.61 mL CH4/gVS, which were 5.9% and 10.8% lower than the control, probably due to the reduced alkalinity (257 mg CaCO3/L) and increased concentrations of ammonium nitrogen (N–NH4+) (1.31 mg/L), both of which can inhibit methanogenic activity. These results confirm that while higher solubilization is consistently achieved at higher H2O2 loadings, methane formation does not follow the same trend, highlighting the importance of a balance between oxidation strength and reactor stability. It is also noteworthy that the maximum methane yields achieved with H2O2 (4% w/w) were still lower than those obtained with ultrasonic pretreatment or alkaline NaOH pretreatment at 6–8% concentrations, suggesting that oxidative pretreatment alone is less efficient for methane enhancement. Nevertheless, the use of H2O2 at ambient temperature and pressure offers practical advantages, as it avoids the energy-intensive requirements of thermal or pressure-based processes, making it a more cost-effective and scalable option for SCG valorization.
3.4. Dilute Acid–Thermal Pretreatment for Enhanced SCG Solubilization
The Dilute Acid–Thermal Pretreatment (DATP) is a combination of dilute acid and heat treatment. When optimized, the acid pretreatment can cause almost complete saccharification of the hemicellulose, although the crystalline cellulose fraction is only partially dissolved []. In combination with dilute acids, such as sulfuric or hydrochloric acid, hydrothermal treatment allows the penetration of water into the lignocellulosic structure, enabling the hydrolysis of the cellulose and the dissolution of parts of the lignin and hemicellulose []. FTIR analysis showed a decrease in the peaks associated with hydrogen bonds in cellulose, hemicellulose, and phenolic lignin after pretreatment, indicating acid-induced hydrolysis. It was also found that the content of hemicellulose and cellulose in the pretreated SCG decreased, although a large proportion remained in the solid []. The decrease in the intensity of certain bands also indicates the degradation of tannins, lignin, caffeine, and carotenoids, even though qualitatively a large proportion may remain []. A higher sCOD value is associated with better pretreatment efficiency [].
In studies on the use of DATP in SCG, various parameters have been investigated to optimize efficacy. These include factors such as dilute acid concentration (e.g., HCl at 1.5–2.5% v/v), pretreatment contact time (e.g., 15–45 min), and liquid-to-solid (LS) ratio (e.g., 10–20% w/v) []. Specific temperature and pressure conditions were also tested, such as autoclaving at 120 °C and 1.15 bar or 135 °C and 2.4 bar for 3 h with 0.5% v/v HCl and LS 10 v/w. After DATP, the pH of the pre-treated material usually needs to be brought back into a neutral range suitable for AD, which can be achieved with agents such as NaOH, potassium hydroxide (KOH), or monopotassium phosphate (KH2PO4). Vanyan et al. [] pointed out that pH adjustment with K2HPO4 is inefficient for biogas and biohydrogen production. The strong buffering capacity of phosphate can suppress acidogenesis by stabilizing the pH at suboptimal levels for fermentative bacteria. In addition, a high phosphate content increases the ionic strength of the medium, which can lead to osmotic stress and inhibit microbial activity. Lackner et al. [] reported that phosphate concentrations above 25 mM led to a significant inhibition of CH4 production in the early stages of batch AD. At 100 mM, methanogenesis was significantly impaired, accompanied by acetate accumulation and a shift in the composition of the methanogenic community from a mixed Methanosarcina–Methanoculleus community to a Methanoculleus-dominated community.
In terms of performance, DATP has shown significant positive effects on the solubilization of organic material, often measured by sCOD []. The increase in sCOD after DATP reflects the hydrolysis of complex lignocellulosic structures and the transfer of soluble sugars, organic acids, and phenolic derivatives to the liquid phase [], improving the accessibility of the substrate for subsequent AD [].
The objective of using DATP is to increase the methane yield by improving the bioavailability of the substrate. Comparative BMP tests show that hydrolysates treated with DATP consistently perform better than untreated or hydrothermally treated SCG (water only). For example, Semaan et al. [] reported methane yields of 93.1–119.7 mL CH4/g VS from DATP hydrolysates, an increase of over 200% compared to hydrothermal controls.
Further studies confirm the broader applicability of DATP. Nava-Valente et al. [] showed that the combination of mild thermal pretreatment (70–90 °C, 1 h) with dilute organic acids (2.5–10% acetic acid) improved the solubilization of coffee pulp and increased biogas production, reducing the required hydraulic retention time from 21 to 15 d. Similarly, Pereira et al. [] demonstrated that dilute sulfuric acid hydrolysis (5–10% H2SO4, 121 °C, 1 h) increased sugar release and short-chain organic acid (SCOA) production in the acidogenic fermentation of SCG, with a 185% increase in SCOAs compared to untreated biomass. These results emphasize that dilute acid pretreatment is versatile and supports both biomethane and bioproduct intermediates.
However, DATP performance is not only dependent on solubilization. Vanyan et al. [] reported that while 0.4% H2SO4 at 121 °C effectively increased the solubilization of the hydrolysate, the methane yield was lower than untreated SCG at neutral pH, suggesting that the accumulation of inhibitors such as furfural, hydroxymethylfurfural (HMF), and phenols may negate the gains from hydrolysis. Similarly, Kim et al. [] emphasized that strong chemical or thermal pretreatments can enhance solubilization but do not always correlate with methane yield, as inhibitory compounds or excessive solubilization can disrupt methanogenic communities.
Overall, DATP is a promising strategy to improve SCG digestibility and biomethane potential, but its success depends on careful optimization of acid concentration, LS ratio, and retention time to maximize sugar release while minimizing inhibitor formation [].
3.5. Hydrothermal Pretreatment of Spent Coffee Grounds
Hydrothermal pretreatment is a thermochemical process in which a biomass, such as SCG, is treated at elevated temperatures in an aqueous environment. It is considered a simple, cost-effective and environmentally friendly process that is water-based and chemical-free in some applications, such as carbohydrate hydrolysis []. This method is used to improve the processing of lignocellulosic materials, in particular to increase their biodegradability and accessibility for downstream processes such as AD. There are several variants within this category, including hydrothermal carbonization (HTC), which produces a solid fuel (hydrochar) and process water [], and hydrothermal liquefaction (HTL), which is typically carried out at higher temperatures and pressure to produce biocrude oil, biochar, and an aqueous effluent [].
The core mechanism of hydrothermal pretreatment involves the action of heat and water on the complex lignocellulosic structure. Heat facilitates the penetration of water into the biomass matrix. In this hot-water environment, the complex organic compounds within the biomass are broken down through processes like hydrolysis. This leads to the hydrolysis of cellulose and the solubilization of portions of the lignin and hemicellulose fractions. This disruption effectively breaks down the rigid plant cell wall structure, making the material more available for microbial degradation. Studies suggest that hydrothermal treatments at temperatures below 160–180 °C may not significantly alter the macro-constituent composition of biomass. Conversely, very high temperatures, such as those above 220 °C in subcritical water liquefaction, can potentially lead to the degradation of desirable compounds like proteins, phenolics, flavonoids, and reducing sugars [].
One of the effects of hydrothermal pretreatment is a considerable solubilization of the organic material. A notable result, particularly in processes such as HTC and HTL, is the generation of a solid residue (hydrochar or biochar) and a liquid phase, often referred to as process water or post hydrothermal liquefaction wastewater (PHWW). This aqueous phase usually has a high COD and is often acidic due to the formation of organic acids from carbohydrate degradation []. The PHWW from HTL can contain high concentrations of nutrients and organic matter. For instance, PHWW from SCG HTL had an average COD content of 15.4 ± 1.4 g COD/L. Also, recalcitrant compounds such as polyphenols and heterocyclic nitrogen compounds, as well as potentially inhibitory substances such as furfural, hydroxymethylfurfural, and phenolic acids, are formed during the degradation of hemicellulose and lignin at elevated temperatures []. Hydrothermal pretreatment can also lead to the degradation of proteins and other compounds at sufficient temperatures. Gas is also produced, especially CO2, during HTC [].
Hydrothermal pre-treatment is mainly used before AD to improve the energy recovery of biomass. By breaking up the complex structure and dissolving the organic substances, the substrate becomes more available to the microorganisms, which leads to increased biogas and CH4 production. Studies have shown that the CH4 yield is significantly improved compared to untreated biomass. For example, the CH4 yield from the liquid hydrolysate after a combined pre-treatment with dilute acid and thermal treatment was at least 200% higher than in a control with hydrothermal pre-treatment []. In the case of coffee husks, hydrothermal pretreatment significantly increased CH4 production compared to the raw material []. Importantly, hydrothermal pretreatment is particularly advantageous for biomass with inherently high moisture content, such as SCG, which typically contains 55–85% water []. Under such conditions, hydrothermal pretreatment uses the intrinsic moisture of the feedstock as a reaction medium, making energy-intensive drying unnecessary []. In contrast, conventional thermochemical processes such as pyrolysis require prior drying of the wet biomass, which significantly increases the energy requirement []. This makes hydrothermal pretreatment a more energy efficient and sustainable option for the valorization of SCG [].
Adsorption (For HTC Process Water)
The need to treat HTC process water arises from the fact that it usually has a high COD due to the presence of DOC. This water is not only rich in organic matter but can also contain heat-resistant and inhibitory substances that can hinder microbial activity and lead to instabilities in downstream processes such as AD. Adsorption can be used to selectively remove these problematic compounds. In particular, the sources mention the removal of hydrophobic organic material. Adsorption is used as a pre-treatment method for process water produced during the HTC of biomass, such as SCG. This treatment is applied before the subsequent processes of AD. The main purpose of adsorption is to change the properties of the HTC process water to improve its suitability for further biological treatment and energy recovery [].
By removing these refractory and inhibitory organic compounds through adsorption, the process water becomes more amenable to AD. The studies of Campbell et al. [] have shown that this pretreatment can lead to significantly higher COD degradation efficiency and increased specific CH4 yields compared to untreated process water. For instance, AD of treated process water resulted in COD degradation efficiencies up to 93.5% and specific methane CH4 of 0.21 L CH4/g COD, compared to 69.6% COD degradation and 0.16 L CH4/gCOD for untreated process water. The removal of these compounds is crucial for sustaining long-term high COD degradation efficiencies and consistently elevated methane yields.
3.6. Subcritical Water Liquefaction
Subcritical water liquefaction is a thermochemical process that has been explored for treating lignocellulosic biomass like SCG. It specifically utilizes water at elevated temperatures and pressures to process the material [].
An important application of subcritical water liquefaction is the extraction of valuable compounds, such as phenols, from SCG. This process is often investigated after an initial pretreatment step. Temperature plays a decisive role in subcritical water liquefaction, particularly with regard to the degradation of certain components. Kourmentza et al. [] investigated different temperatures (180–240 °C) and pressures (20–60 bars) of the process. Increasing the temperature above 220 °C during subcritical water liquefaction of SCG can potentially lead to the degradation of useful compounds, including proteins, phenols, flavonoids, and reducing sugars. Conversely, the recovery of phenols by subcritical water extraction was also investigated at lower temperatures, e.g., between 160 °C and 180 °C, in combination with different extraction times and solid–liquid ratios.
As shown in Table 4, each pretreatment method has different disadvantages for SCG. Alkaline and thermo-alkaline processes offer the highest CH4 yield improvements and good scalability, but require chemical management []. Oxidative and hydrothermal processes are cost-efficient and environmentally friendly, but offer only minor performance improvements []. Solvent-based and supercritical CO2 extraction methods offer valuable oil recovery, but their higher cost and process complexity may limit wide application []. Dilute acid thermal pretreatment has high methane potential but can generate inhibitory by-products. While adsorption does not directly increase methane yield, it effectively improves downstream digestion by removing inhibitory compounds from the process water []. Table 4 serves as a comparative tool to assist in the selection of appropriate strategies based on performance, feasibility, and environmental considerations.

Table 4.
Comparison of spent coffee grounds pretreatment methods.
Table 4.
Comparison of spent coffee grounds pretreatment methods.
| Pretreatment Method | CH4 Yield Improvement | Cost | Scalability | Environmental Impact | Ref. |
|---|---|---|---|---|---|
| Oil Extraction (Solvent) | Moderate to High (up to 24%) | Moderate | Medium (solvent recovery required) | Solvent use poses recovery/disposal challenges | [] |
| Supercritical CO2 Extraction | Moderate to High | High | Low to Medium (complex setup) | Green solvent; low residue | [,,] |
| NaOH Alkaline Pretreatment | High (up to 24%) | Low to Moderate | High | Chemical handling and neutralization needed | [,] |
| Thermo-Alkaline | Moderate to High | Moderate | Medium | Risk of inhibition at high NaOH/temp; energy use | [,] |
| H2O2 Oxidative Pretreatment | Moderate (~17% increase in CH4 yield) | Low | High | Low toxicity; no harmful residues | [,] |
| Dilute Acid + Thermal (DATP) | High (up to 200% CH4 increase in hydrolysate) | Moderate to High | Medium | Acid handling; potential inhibitor formation | [,] |
| Hydrothermal Treatment | Moderate | Low to Moderate | High | Environmentally friendly; water-only option | [,,] |
| Subcritical Water Liquefaction | Low to Moderate | High | Low | High energy input; degradation of bioactives | [,] |
| Adsorption (HTC process water) | Indirect CH4 yield improvement (up to 31%) | Low | High | Selective removal of inhibitors; minimal waste | [] |
4. Optimizing SCG Biogas Production Through Co-Digestion
Co-digestion is a strategy used in AD where different organic waste streams are mixed in the digester. The main advantage of this process is that it can increase CH4 production under controlled conditions. Co-digestion can also help to improve the balance of macronutrients (such as carbon, nitrogen, and phosphorus) and micronutrients, increase buffering capacity, and dilute toxic compounds, all of which contribute to better stability of the anaerobic digester [].
This strategy is particularly relevant for the AD of SCG, as the mono-digestion (sole digestion) of SCG is often considered disadvantageous []. The challenges of mono-digestion of SCG include slow degradation, nutrient imbalance, lack of trace elements, high levels of lignocellulosic materials with poor bioavailability, instability, and potential accumulation of volatile fatty acids (VFA). Co-digestion offers a viable way to overcome these limitations [].
Various organic materials have been investigated as co-substrates for SCG. These include cow manure (CM) [], pig manure (PM), spent tea waste (STW), food waste (FW), Ulva biomass (UB), waste activated sludge (WAS), whey, pure glycerine, macroalgae, milk waste, coffee processing wastewater, brewery wastewater [], coffee pulp, and coffee mucilage []. The feasibility and overall success of co-digestion largely depend on several interrelated parameters. A crucial factor is the selection of suitable co-substrates, as their physico-chemical properties (e.g., carbon–nitrogen ratio [], biodegradability [], and moisture content []) and chemical composition determine the balance of nutrients available to the microorganisms []. The presence of essential micronutrients and trace elements, such as cobalt [], nickel [], and iron [], is equally important as they serve as cofactors in the enzymatic pathways of methanogenesis and can prevent metabolic limitations. The mixing ratio between SCG and the chosen co-substrate has a major impact on the process outcome an optimal ratio ensures sufficient nutrient balance and dilution of potential inhibitors (e.g., phenols, caffeine, or LCFAs from SCG), while avoiding excessive organic loading []. Successful co-digestion also relies on synergistic effects between microbial populations, where hydrolytic, acidogenic, and methanogenic communities complement each other’s activities to accelerate the degradation of complex substrates []. Finally, these combined factors contribute to overall process stabilization by maintaining pH in the optimal range, reducing the risk of volatile fatty acid accumulation, and maintaining robust methane production [].
Studies have investigated the effectiveness of various co-substrates and mixing ratios. For example, the co-digestion of DSCG with STW at a ratio of 50% STW/50% DSCG resulted in a good biogas yield of 318 mL CH4/g VS. Co-digestion of SCG with FW also resulted in comparable or higher CH4 production (between 0.308 and 0.360 L CH4/g VSin) compared to SCG mono-digestion []. The use of UB as a co-substrate (25% COD basis) has been shown to be beneficial for SCG biomethanization performance and stability, with a positive correlation observed between UB use and CH4 productivity []. Co-digestion of SCG liquid fraction with CM resulted in an average increase of 8% in CH4 yield compared to CM mono-digestion []. Co-digestion of SCG with liquid PM significantly increased the CH4 production rate and increased biogas production by almost 340% compared to liquid PM alone in a pilot-scale study []. Among the coffee processing residues, the high-mucilage feed proved to be the most productive, producing 50% more CH4 than the low-mucilage feed, due to its lower cellulose/xylan content, higher C/N ratio (16.5%), and resulting favorable microbial communities, especially methanogens []. Brewery wastewater proved to be an excellent co-substrate in co-digestion with residues from the coffee industry, as it reduced the acidity of coffee wastewater, diluted toxic components such as phenol and provided a balanced concentration of macro- and micronutrients [].
However, the choice of co-substrate and mixing ratio is crucial, as some combinations can lead to antagonistic effects or even inhibition. These negative interactions can be due to various biochemical and operational factors. For example, unbalanced nutrient ratios, especially too low or too high a carbon–nitrogen (C/N) ratio, can disrupt microbial metabolism and lead to ammonia accumulation or nutrient deficiency []. High lipid content from SCG or co-substrates can lead to the formation of LCFAs, which are known to inhibit methanogenic archaea by adsorbing to microbial cell membranes and reducing mass transfer []. In addition, excessive accumulation of volatile fatty acids (VFAs) due to rapid hydrolysis or insufficient methanogenic activity can lower the pH, creating an unfavorable environment for methane-producing microbes. Operational factors such as improper substrate loading rates, poor mixing, or temperature fluctuations can exacerbate these problems, leading to process instability or microbial community imbalance []. In some cases, toxic compounds (from manure or protein-rich substrates) can also contribute to microbial inhibition if they are not diluted or removed by pre-treatment []. For example, some combinations of SCG and STW with DSCG negatively affected CH4 yield when the DSCG content decreased, with efficacy only achieved at STW levels below 60% []. Low co-digestion performance was also observed when co-digesting SCG with pure glycerol. Co-digestion of SCG with cow dung and FW has been reported to have negative or antagonistic effects on biogas production [], although acceptable results were obtained with a three-substrate mixture (SCG–anaerobic sludge (AS)–CM) as long as the SCG content was kept low (~8% vs. basis). The lignin content in co-substrates such as STW and CM can pose problems. This recalcitrant fraction slows down hydrolysis, leading to asynchronous degradation rates and accumulation of partially degraded compounds. The accumulation of VFAs and organic acids can lead to instability in digestion, and although phenol can be released during lignin degradation, the amounts observed were not inhibitory []. However, the simultaneous presence of phenolic compounds, caffeine, and polyphenols from SCG may exert cumulative stress on microbial consortia, further amplifying the inhibitory effect. WAS had a detrimental effect on CH4 yield during co-digestion with SCG, which is attributed to an antagonistic interaction despite the provision of trace elements. This may be linked to nutrient imbalances, as a suboptimal C/N ratio or excess nitrogen release from protein-rich substrates can cause ammonia accumulation, which is toxic for sensitive methanogens. Achieving stable, long-term, and continuous fermentation of some co-digestion mixtures, such as HTC process water with other materials, can be challenging and has been shown to be unstable in some cases []. Proper analysis of substrate proportions is important as the C/N ratio varies with these proportions and the composition of the medium, especially in terms of lipids, which can affect VFA production and the risk of inhibition []. In addition, the rapid hydrolysis of soluble substrates such as glycerol can lead to a sudden increase in VFAs that overwhelm the buffering capacity of the system [], while the lipid fraction of SCG contributes to the formation of LCFAs, which are known to adsorb to microbial cell membranes and interfere with methanogenic activities [].
Key Factors Influencing Biogas and CH4 Yield from SCG Co-Digestion
The sources show that several key factors influence the biogas and CH4 yield from the co-digestion of SCG:
- Type of co-substrate and mixing ratio: The type and mixing proportion of the co-substrate with SCG are crucial for successful AD. Co-digestion is often necessary because SCG alone does not contain sufficient nutrients and trace elements and contains poorly bioavailable lignocellulosic material. Mixing SCG with other organic wastes can improve nutrient balance, dilute inhibitory substances, increase buffering capacity, and enhance overall digestibility [].
AS or WAS: Mixing SCG with AS or WAS can have a positive or synergistic effect on biogas generation []. AS provides acclimatized biomass; contributes to the dilution of toxic components such as caffeine, tannins, and phenols; and supplies trace elements []. It also provides sufficient iron, which can reduce sulfur toxicity []. WAS can provide the nitrogen and mineral elements necessary for the growth and stability of microorganisms []. Maximum biogas was achieved when AS and SCG were mixed at about 80% AS and 20% SCG on a VS basis, with yields reaching up to 231 mL/gVS in batch systems operated at 37 °C for 30 d. This mixture showed a strong increase in biogas–methane production during the first week of digestion, indicating rapid acclimatization and strong synergistic effects between the AS-derived microbial communities and the SCG substrates [].
FW: Co-digestion of SCG with FW has shown positive effects on CH4 generation. Optimal ratios often involve a higher proportion of FW than SCG, which resulted in high CH4 yields. Teixeira et al. [] reported that mixing 75% FW with 25% SCG at mesophilic conditions (35 ± 2 °C) produced methane yields up to 0.345 Nm3 CH4/kg VS, considerably higher than SCG mono-digestion (0.188 Nm3 CH4/kg VS). Even small additions of SCG (1–4% on a VS basis) have been shown to stimulate methane yields compared to FW mono-digestion, reflecting the contribution of lipids and structural carbon from SCG. Kim et al. [] tested it in mesophilic CSTRs (35 ± 2 °C, 40 d HRT, 2 L working volume) over 791 d, with daily feeding and supplementation of trace elements (100 mg Fe, 2 mg Co, and 1 mg Ni per L). The reactors were first operated on FW alone, then with increasing SCG ratios of 1–10%. Results showed that methane yield increased progressively with SCG addition up to 4%, reaching 0.47–0.48 L CH4/g VS fed compared to 0.43 L CH4/g VS fed for FW alone. Residual VFA concentrations remained very low (<200 mg COD/L), and pH was stable near neutrality, indicating that small SCG additions did not disrupt process stability. However, when the SCG content was increased to 10% without trace element supplementation, both reactors failed within three HRT turnovers. This failure was linked to sudden VFA accumulation, primarily acetate and propionate, followed by acidification.
CM: The interaction between SCG and CM in co-digestion can be complex. A negative (antagonistic) effect on biogas generation when SCG was mixed with CM, leading to reduced biogas production compared to CM alone. CM has a low C/N ratio and high nitrogen content, which can lead to inhibiting ammonia production and may contain H2S, toxic to methanogens. Biogas generation with SCG and CM occurred when CFW content was kept below 59% (VS basis) [].
Liquid Swine Manure (LSM) and SCG: LSM is considered essential for process stability due to its high ammonia concentration providing buffering capacity []. When LSM was used as a nitrogen-rich substrate in combination with carbon-rich coffee wastewater (CFW), a balanced system was created in which LSM provides alkalinity and micronutrients, while CFW provides readily available organic matter. Batch tests at 37 °C and pH 7 with 10% inoculum have shown that the proportion of CFW in the liquid substrate mixture and the concentration of dissolved organic matter (OMD) are decisive factors for the gas composition. At low CFW contents (7–13% of the mixture) and/or low OMD concentrations (3–10 g COD/L), the biogas was rich in methane, with CH4 contents of 65–70% and negligible hydrogen detection. The maximum methane yield was observed at 6% CFW and 3 g COD/L, with a COD/N ratio of 32, resulting in 423.04 mL CH4/gVS, a cumulative production of 866.9 mL CH4 over 25 d, and a methane content of 66.8%. In contrast, at higher CFW fractions (30–54%) and increased OMD concentrations (10–17 g COD/L), the process shifted towards acidogenesis, which favored hydrogen production. Under these conditions, the methane yield decreased to 3–9%, while the hydrogen content increased up to 34%, especially at COD/N ratios ≥ 50.
- 2.
- Substrate-to-inoculum ratio (SI): The SI (VS basis) significantly impacts the degree of methanization and digester health []. A high SI ratio can overload the system, leading to VFA accumulation and inhibited bio-CH4 yields []. A low SI can lead to washout []. Decreasing the SI can improve process kinetics and microbial activity. Studies suggest that an optimal SI ratio for SCG is likely between 1 and 0.25 [].
- 3.
- pH: pH is a crucial factor for the stability and efficiency of the AD process. Methanogenic archaea, responsible for CH4 production, function optimally in a near-neutral pH range, generally between 6.5 and 8.2, with an ideal pH of around 7.0 []. The pH values below 5.0 or above 8 can be highly toxic or lethal to methanogens []. Co-substrates like WAS or LSM can provide buffering capacity to help maintain a stable, favorable pH [].
- 4.
- Nutrient balance (C/N ratio) and organic matter (OM) concentration: An optimal C/N ratio, usually 25–35, is important for efficient AD []. Co-digestion plays a critical role in achieving this balance, as single substrates like SCG often have a suboptimal C/N ratio (around 20:1), which can limit microbial activity and lead to ammonia or VFA imbalances []. The OM concentration in the feed also influences the result. Lower OM concentrations (3–10 g COD/L) favored CH4 production during co-digestion of SCG and LSM, while higher concentrations (10–17 g COD/L) favored H2 production. High organic loads can lead to VFA accumulation and process inhibition [].
- 5.
- Presence of inhibitory substances: SCG naturally contains compounds like caffeine, tannins, and phenols that can be toxic to anaerobic microorganisms. High lipid content in SCG can also inhibit AD by producing LCFAs that interfere with methanogenesis []. Pretreatment processes or certain co-substrates (like CM with H2S or FW with rapid VFA accumulation) can also introduce or generate inhibitory substances. Co-digestion can help dilute these inhibitors [].
- 6.
- Trace elements: SCG mono-digestion is hindered by a lack of trace elements. Supplementation or co-digestion with substrates providing necessary trace elements like Fe, Co, and Ni is important for stable operation and enhanced methanation. WAS is noted as a source of these elements [].
5. Microbial Community Dynamics in AD of SCG
The AD process occurs in stages, driven by different functional groups of microorganisms. The initial stages, hydrolysis and acidogenesis, are primarily carried out by bacteria. These bacteria break down complex organic polymers like carbohydrates, proteins, and lipids into simpler molecules and VFAs []. In SCG co-digestion with swine manure, specific bacterial phyla observed include Firmicutes and Bacteroidota/Bacteroideta. Firmicutes are associated with hydrolytic and acidogenic processes, capable of degrading cellulose and lipids and producing acetic acid. Bacteroidota/Bacteroideta are involved in the degradation of lignocellulosic materials and the hydrolysis/acidogenesis of proteins. Genera from Porphyromonadaceae can ferment carbohydrates and proteins into organic acids [], while Olsenella species are primarily saccharolytic bacteria, that break down carbohydrates and produce lactic acid []. In contrast, cellulolytic groups such as Candidatus Cloacamonas, members of the Ruminococcaceae family, and Atopobium play an important role in fiber degradation in SCG []. Some of these microorganisms, including Candidatus Cloacamonas and Clostridium spp., are also involved in hydrogen production under certain co-digestion conditions []. However, the rapid activity of acidogenic and hydrogen-producing bacteria can lead to excessive accumulation of VFA, which can inhibit downstream acetogenesis and methanogenesis if not properly controlled [].
The final stage, methanogenesis, is performed by methanogenic archaea, which convert VFAs, hydrogen, and CO2 into CH4. These archaea are highly sensitive to environmental conditions, particularly pH, and function optimally in a near-neutral range []. Inhibitory substances contained in SCG such as caffeine, phenols, and tannins can negatively influence the activity of these microorganisms []. A high lipid content in SCG can also be problematic, as the resulting LCFA can be toxic for methanogenic archaea and acetoclastic bacteria, as they impair nutrient uptake. Trace elements are also crucial for methanogenic activity []. Co-digestion with substrates such as WAS or AS can provide essential trace elements such as Fe, Co, and Ni, which are crucial for methanogens and may not be sufficiently present in SCG []. The addition of anaerobic sludge (AS) to SCG provides acclimatized biomass, dilutes toxic components such as phenols and caffeine, and increases buffering capacity, thereby promoting methanogenic activity. Community analyses of SCG co-digestion systems have consistently identified Methanothrix (formerly Methanosaeta) as the dominant acetoclastic genus under stable conditions [], while hydrogenotrophic taxa such as Methanospirillum often proliferate in VFA-rich or organically overloaded environments []. In co-digesters with pig manure and coffee wastewater, the methanogenic community consisted largely of Methanothrix and Methanolinea, with additional contributions from hydrogenotrophic genera such as Methanoculleus and Methanoregula []. These shifts in archaeal community structure, particularly at different SCG feeding ratios, illustrate how the composition of the starting material directly influences the balance between acetoclastic and hydrogenotrophic pathways and, thus, the overall stability of the AD process [].
6. SCG Valorization and Biorefinery Strategies
SCG valorization refers to the process of treating SCG to recover or convert its components into valuable products, energy, or materials []. This approach is seen as a crucial strategy for sustainable waste management and for moving towards a green circular bioeconomy [].
Valorization pathways enable the recovery of SCG components for transformation into diverse value-added products and energy carriers, with applications spanning the energy, chemical, agricultural, and industrial sectors (Figure 1).
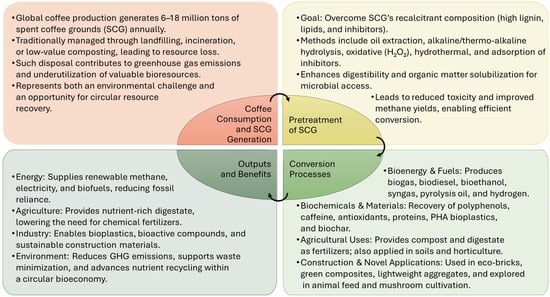
Figure 1.
Valorization pathways of spent coffee grounds including pretreatment, conversion processes, outputs, and benefits.
In the field of bioenergy and biofuels, SCG can be converted into biogas (mainly CH4) by AD either in mono- or co-digestion systems. The lipid fraction of SCG is a promising source for biodiesel and bio-oil, while the sugar content can be fermented to produce bioethanol. SCG can also be used to generate bio-syngas and electricity through thermochemical processes such as gasification, combustion or incineration []. In addition, it can be processed into fuel pellets for heating purposes [], pyrolysis oil [], and biohydrogen [].
In terms of chemicals and materials, biochar derived from SCG serves as an effective biosorbent to remove pollutants from the environment []. Valuable compounds such as antioxidants, caffeine, and polyphenols or tannins can be recovered for use in the pharmaceutical, cosmetic, or food industries. SCG also shows promise for the production of biopolymers and bioplastics, especially polyhydroxyalkanoates (PHA) (Figure 1). In addition, SCG can be a source of monosaccharides such as mannose, galactose, and glucose as well as oligosaccharides. It also contains lipids, proteins, fatty acids, and other bioactive compounds with various applications [].
In agriculture, compost and digestate from the AD of SCG can be used as fertilizer or soil conditioner. Alternatively, SCG can also be applied directly to the soil or used in horticulture to improve soil quality [].
SCG also has the potential to be used in construction and material development. It can be incorporated into construction aggregates or bricks and used in the development of environmentally friendly composites or innovative building materials [].
Finally, several novel applications are emerging. The use of SCG or its components in animal feed has been explored, though there are concerns related to digestibility and the presence of potentially toxic substances. Interestingly, the use of SCG in mushroom cultivation is also being investigated []. Increasingly, the valorization of SCG is conceptualized within the framework of a biorefinery. The biorefinery concept for SCG valorization relies on integrated, multi-stage processes that aim to recover or transform all major components of the feedstock, rather than targeting a single product []. In such systems, lipids can be directed to biodiesel production, polysaccharides to fermentation for bioethanol or bioplastics, and lignocellulosic residues to biogas or biochar []. By generating multiple high-, medium-, and low-value products within the same platform, overall resource efficiency is maximized, and economic feasibility is improved compared to single-route utilization. Biorefineries typically combine chemical, thermochemical, and biological conversion methods in a sequential or cascading manner to ensure complete use of the biomass. Increasingly, attention is also being given to simplified, small-scale biorefinery models tailored for local or community applications, which reduce transport costs and promote decentralized circular economy solutions [].
Despite the promising potential, several challenges remain for widespread SCG valorization. Many technologies are still at the laboratory stage and require piloting and industrial scaling up. Technoeconomic viability is a significant factor, as the energy costs of processes like drying (necessary due to SCG’s high moisture content, causing rapid spoilage) can be limiting []. Mata et al. [] reported biogas yields of 0.55–0.60 m3/kg VS with methane concentrations of 55–61% during SCG digestion, noting that co-digestion improved process stability and produced positive energy balances, with operating costs (OPEX) largely linked to heating requirements. Similarly, Orfanoudaki et al. [] demonstrated at the pilot scale that co-digestion of SCG with pig manure achieved up to a 340% increase in methane yield compared to manure alone, with COD removal efficiency nearly doubling and biogas production costs reduced by 20–25% relative to mono-digestion. More detailed techno-economic analysis was provided by Kisiga [], who modeled a mid-scale AD plant processing 2000 kg SCG per hour. The study estimated a capital expenditure (CAPEX) of approximately USD 1.03 million and a project-lifetime OPEX of about USD 1.8 million. Under baseline assumptions (25-year project life, 10% annual depreciation, 12% discount rate, 28% tax), the process achieved a net present value (NPV) of USD 3.44 million, an internal rate of return (IRR) of 34%, and a discounted payback period (DPBP) of 5.7 years. Together, these findings demonstrate that SCG anaerobic digestion not only delivers environmental benefits but also shows strong economic viability, particularly when integrated into co-digestion or biorefinery frameworks.
Detailed techno-economic analyses are essential to evaluate the feasibility of SCG valorization pathways. Inhibitory or toxic compounds such as caffeine, tannins, and polyphenols can hinder biological processes like AD or fermentation, highlighting the need for effective pretreatment or detoxification strategies []. Moreover, the variability in SCG composition, influenced by factors such as bean origin and processing methods, necessitates feedstock-specific optimization.
Equally important is the assessment of environmental performance using Life Cycle Assessment (LCA) []. Kisiga et al. [] compared six valorization routes for SCG, including AD, biodiesel, bioethanol, pyrolysis, hydrothermal liquefaction, and gasification. AD was identified as one of the most environmentally favorable pathways, due to comparatively low electricity and water requirements and additional benefits associated with nutrient recycling from digestate. However, the interpretation of LCA results is not always straightforward, as outcomes can diverge from qualitative waste hierarchy or value chain rankings. For example, in one comparative LCA of SCG valorization routes, incineration with energy recovery emerged as the most environmentally favorable option in several impact categories, outperforming approaches such as composting or biodiesel production that are usually prioritized by waste hierarchy models []. Similar contradictions have been observed in broader assessments of bioresource utilization, where local conditions, system boundaries, and methodological choices strongly influence results. These inconsistencies highlight that while LCA is an essential tool for evaluating sustainability, the absence of harmonized and standardized assessment methods can complicate decision-making []. Developing consistent frameworks is therefore crucial to ensure that LCA provides reliable guidance for policy and industrial practice in SCG valorization.
SCG Digestion: Digestate Valorization as Fertilizer
The primary use of digestate from AD, including SCG, is as a natural fertilizer or soil conditioner []. Digestate is considered a very good fertilizer due to its high nutrient content, especially nitrogen, phosphorus, and potassium, which are present in plant-available form []. This makes digestate an excellent candidate for replacing inorganic fertilizers and contributes to more sustainable agricultural practices and a short-term turnover of organic material in the soil. Digestate can consist of both a liquid and a solid fraction, and both are considered good natural fertilizers. In addition to direct use as a fertilizer, the sources also suggest combining digestate with other materials such as biochar or hydrochar, which could ultimately help improve soil fertility and quality [].
7. Conclusions
This review highlights the considerable potential of SCG as a versatile and underutilized feedstock for AD in the context of a circular economy. The chemical and physical complexity of SCG—characterized by a high content of lignocellulose, lipid fractions, polyphenols, and a variable composition—requires robust pretreatment strategies to improve biodegradability and biogas yield. Of the methods investigated, alkaline, thermo-alkaline, oxidative, and combined acid–thermal pretreatments show promise for dissolving organic matter and mitigating inhibitory effects, although their optimization remains critical to avoid microbial inhibition and environmental pollution.
Oil extraction (defatting), especially using solvents or supercritical CO2 methods, has been shown to be an important pretreatment step that reduces lipid-related toxicity while providing additional utilization opportunities through biodiesel production. Pretreatments such as alkaline hydrolysis with NaOH and oxidative treatment with H2O2 have shown improved CH4 yields, although their effectiveness depends on tight control of chemical concentrations and reaction conditions to minimize the formation of inhibitory compounds.
Co-digestion further improves the feasibility of SCG-based AD by balancing nutrient composition, improving microbial stability, and diluting toxic substances. Optimal performance has been observed with substrates such as FW, LSM, and brewery effluents that provide missing trace elements and buffering capacity. However, the success of co-digestion is highly dependent on substrate conditions, organic load, and the presence of inhibitory elements, emphasizing the need for integrated feedstock analysis and process control.
From a system perspective, the utilization of SCG through AD offers better sustainability and economic performance than conventional disposal methods such as landfilling or incineration. The production of biogas and digestate not only contributes to the generation of renewable energy and the recycling of nutrients but is also in line with policy frameworks that promote waste minimization and the reduction of carbon emissions. LCA and techno-economic analyses confirm the environmental and financial benefits of SCG-based AD, especially when integrated into broader biorefinery models that maximize resource recovery.
While AD of SCG holds great potential for biogas production and resource recovery, the transition from a laboratory or pilot scale to a commercial scale presents a number of technical and logistical challenges. These include the consistent collection of feedstocks from dispersed sources, handling substrates with high moisture and oil contents, optimizing reactor design to avoid inhibition, and integration with existing waste management infrastructure. Future research should focus on overcoming these bottlenecks through improved pretreatment, co-digestion strategies, and decentralized biogas systems that can accommodate variable SCG supply chains.
Author Contributions
Conceptualization, K.B.; investigation, K.B.; resources, K.B. and M.Z.; writing—review and editing, K.B. and M.Z.; visualization, K.B.; supervision, K.B. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Obileke, K.C.; Nwokolo, N.; Makaka, G.; Mukumba, P.; Onyeaka, H. Anaerobic Digestion: Technology for Biogas Production as a Source of Renewable Energy—A Review. Energy Environ. 2021, 32, 191–225. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Circular Economy Is Game-Changing Municipal Wastewater Treatment Technology towards Energy and Carbon Neutrality. Chem. Eng. J. 2022, 429, 132114. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Karthigadevi, G.; Bharathiraja, B.; Praveen Kumar, R.; Abo, L.D.; Venkatesa Prabhu, S.; Balachandar, R.; Jayakumar, M. Current and Prognostic Overview on the Strategic Exploitation of Anaerobic Digestion and Digestate: A Review. Environ. Res. 2023, 216, 114526. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, E.; Cruzat, V.; Singh, I.; Rose’Meyer, R.B.; Panchal, S.K.; Brown, L. The Potential of Spent Coffee Grounds in Functional Food Development. Nutrients 2023, 15, 994. [Google Scholar] [CrossRef] [PubMed]
- Horgan, F.G.; Floyd, D.; Mundaca, E.A.; Crisol-Martínez, E. Spent Coffee Grounds Applied as a Top-Dressing or Incorporated into the Soil Can Improve Plant Growth While Reducing Slug Herbivory. Agriculture 2023, 13, 257. [Google Scholar] [CrossRef]
- Roychand, R.; Kilmartin-Lynch, S.; Saberian, M.; Li, J.; Zhang, G.; Li, C.Q. Transforming Spent Coffee Grounds into a Valuable Resource for the Enhancement of Concrete Strength. J. Clean. Prod. 2023, 419, 138205. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of Different Rates of Spent Coffee Grounds (SCG) on Composting Process, Gaseous Emissions and Quality of End-Product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef]
- Brunerová, A.; Roubík, H.; Brožek, M.; Haryanto, A.; Hasanudin, U.; Iryani, D.A.; Herák, D. Valorization of Bio-Briquette Fuel by Using Spent Coffee Ground as an External Additive. Energies 2019, 13, 54. [Google Scholar] [CrossRef]
- Hashemi, S.E.; Wijnsma, S.; Hillestad, M.; Austbø, B. Direct vs. Indirect Biogas Methanation for Liquefied Biomethane Production: A Concept Evaluation. Fuel 2024, 371, 131835. [Google Scholar] [CrossRef]
- Mahmoud, E.; Atabani, A.E.; Badruddin, I.A. Valorization of Spent Coffee Grounds for Biogas Production: A Circular Bioeconomy Approach for a Biorefinery. Fuel 2022, 328, 125296. [Google Scholar] [CrossRef]
- Dorrego-Viera, J.I.; Urbinati, A.; Lazzarotti, V. Transition towards Circular Economy: Exploiting Open Innovation for Circular Product Development. J. Innov. Knowl. 2025, 10, 100668. [Google Scholar] [CrossRef]
- Kim, D.; Cha, J.; Lee, C. Enhanced Methane Production with Co-Feeding Spent Coffee Grounds Using Spare Capacity of Existing Anaerobic Food Waste Digesters. Sci. Rep. 2024, 14, 4472. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Tan, L.C.; Papirio, S.; Esposito, G.; Lens, P.N.L. Effect of Methanol-Organosolv Pretreatment on Anaerobic Digestion of Lignocellulosic Materials. Renew. Energy 2021, 169, 1000–1012. [Google Scholar] [CrossRef]
- Oliva, A.; Tan, L.C.; Papirio, S.; Esposito, G.; Lens, P.N.L. Use of N-Methylmorpholine N-Oxide (NMMO) Pretreatment to Enhance the Bioconversion of Lignocellulosic Residues to Methane. Biomass Convers. Biorefin. 2024, 14, 11113. [Google Scholar] [CrossRef]
- Ahmed, H.; Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Toward Circular Economy: Potentials of Spent Coffee Grounds in Bioproducts and Chemical Production. Biomass 2024, 4, 286–312. [Google Scholar] [CrossRef]
- Yeoh, L.; Ng, K.S. Future Prospects of Spent Coffee Ground Valorisation Using a Biorefinery Approach. Resour. Conserv. Recycl. 2022, 179, 106123. [Google Scholar] [CrossRef]
- O’Hara, J.K. U.S. Climate-Smart Agricultural and Forestry Incentives: From Offsets to Clean Energy Credits. Agric. Resour. Econ. Rev. 2024, 53, 371–386. [Google Scholar] [CrossRef]
- Syropoulos, S.; Sparkman, G.; Constantino, S.M. The Expressive Function of Public Policy: Renewable Energy Mandates Signal Social Norms. Philos. Trans. R. Soc. B 2024, 379, 20230038. [Google Scholar] [CrossRef]
- Kibret, H.A.; Kuo, Y.L.; Ke, T.Y.; Tseng, Y.H. Gasification of Spent Coffee Grounds in a Semi-Fluidized Bed Reactor Using Steam and CO2 Gasification Medium. J. Taiwan Inst. Chem. Eng. 2021, 119, 115–127. [Google Scholar] [CrossRef]
- Sayoud, S.; Derbal, K.; Panico, A.; Pontoni, L.; Fabbricino, M.; Pirozzi, F.; Benalia, A. The Effect of Hydrogen Peroxide on Biogas and Methane Produced from Batch Mesophilic Anaerobic Digestion of Spent Coffee Grounds. Fermentation 2025, 11, 60. [Google Scholar] [CrossRef]
- Getachew, M.; Tolassa, K.; De Frenne, P.; Verheyen, K.; Tack, A.J.M.; Hylander, K.; Ayalew, B.; Boeckx, P. The Relationship between Elevation, Soil Temperatures, Soil Chemical Characteristics, and Green Coffee Bean Quality and Biochemistry in Southwest Ethiopia. Agron. Sustain. Dev. 2022, 42, 61. [Google Scholar] [CrossRef]
- Tsegay, G.; Redi-Abshiro, M.; Chandravanshi, B.S.; Ele, E.; Mohammed, A.M.; Mamo, H. Effect of Altitude of Coffee Plants on the Composition of Fatty Acids of Green Coffee Beans. BMC Chem. 2020, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Sossa, J.P.; Murillo-Roos, M.; Uribe, L.; Uribe-Lorio, L.; Marsh, T.; Larsen, N.; Chen, R.; Miranda, A.; Solís, K.; Rodriguez, W.; et al. Effects of Coffee Processing Residues on Anaerobic Microorganisms and Corresponding Digestion Performance. Bioresour. Technol. 2017, 245, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Dinesh Kumar, M.; Preethi; Atabani, A.E.; Kumar, G. Biorefinery of Spent Coffee Grounds Waste: Viable Pathway towards Circular Bioeconomy. Bioresour. Technol. 2020, 302, 122821. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Rodríguez Casas, A.; del Castillo, M.D. Interest of Coffee Melanoidins as Sustainable Healthier Food Ingredients. Front. Nutr. 2021, 8, 730343. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Fernández-Arteaga, A.; Navarro-Alarcón, M.; Hinojosa, D.; Pastoriza, S.; Delgado, G.; Rufián-Henares, J.Á. Spent Coffee Grounds as a Source of Smart Biochelates to Increase Fe and Zn Levels in Lettuces. J. Clean. Prod. 2021, 328, 129548. [Google Scholar] [CrossRef]
- Kwon, S.; Young Yoon, H.; Thanh Phong, N.; Young Lee, G.; Jang, K.S.; Joe, E.N.; Lee, Y.; Jeon, J.R. Humic-like Crop Stimulatory Activities of Coffee Waste Induced by Incorporation of Phytotoxic Phenols in Melanoidins during Coffee Roasting: Linking the Maillard Reaction to Humification. Food Res. Int. 2022, 162, 112013. [Google Scholar] [CrossRef]
- Song, W.; Yang, Y.; Cheng, X.; Jiang, M.; Zhang, R.; Militky, J.; Cai, Y. Utilization of Spent Coffee Grounds as Fillers to Prepare Polypropylene Composites for Food Packaging Applications. Microsc. Res. Tech. 2023, 86, 1475–1483. [Google Scholar] [CrossRef]
- Purwoko, T.; Suranto; Setyaningsih, R.; Marliyana, S.D. Chlorogenic Acid and Caffeine Content of Fermented Robusta Bean. Biodiversitas 2022, 23, 902–906. [Google Scholar] [CrossRef]
- Grüter, R.; Trachsel, T.; Laube, P.; Jaisli, I. Expected Global Suitability of Coffee, Cashew and Avocado Due to Climate Change. PLoS ONE 2022, 17, e0261976. [Google Scholar] [CrossRef]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Contreras, G.F.; Cai, Z.; Moccand, C.; Weckx, S.; De Vuyst, L. Influence of Various Processing Parameters on the Microbial Community Dynamics, Metabolomic Profiles, and Cup Quality During Wet Coffee Processing. Front. Microbiol. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; de Toledo Benassi, M. Roasting Process Affects Differently the Bioactive Compounds and the Antioxidant Activity of Arabica and Robusta Coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Nerurkar, P.V.; Yokoyama, J.; Ichimura, K.; Kutscher, S.; Wong, J.; Bittenbender, H.C.; Deng, Y. Medium Roasting and Brewing Methods Differentially Modulate Global Metabolites, Lipids, Biogenic Amines, Minerals, and Antioxidant Capacity of Hawai‘i-Grown Coffee (Coffea arabica). Metabolites 2023, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef]
- Jin Cho, E.; Gyo Lee, Y.; Song, Y.; Nguyen, D.T.; Bae, H.J. An Integrated Process for Conversion of Spent Coffee Grounds into Value-Added Materials. Bioresour. Technol. 2022, 346, 126618. [Google Scholar] [CrossRef]
- Dari, D.N.; da Silva, L.F.; Júnior, A.M.B.L.; Freitas, I.S.; da Silva Aires, F.I.; dos Santos, J.C.S. Spent Coffee Grounds: Insights and Future Prospects for Bioenergy and Circular Economy Applications. Green Technol. Sustain. 2025, 3, 100213. [Google Scholar] [CrossRef]
- Solomakou, N.; Tsafrakidou, P.; Goula, A.M. Valorization of SCG through Extraction of Phenolic Compounds and Synthesis of New Biosorbent. Sustainability 2022, 14, 9358. [Google Scholar] [CrossRef]
- Stylianou, M.; Agapiou, A.; Omirou, M.; Vyrides, I.; Ioannides, I.M.; Maratheftis, G.; Fasoula, D. Converting Environmental Risks to Benefits by Using Spent Coffee Grounds (SCG) as a Valuable Resource. Environ. Sci. Pollut. Res. 2018, 25, 35776–35790. [Google Scholar] [CrossRef]
- Copik, P.; Korus, A.; Szlęk, A.; Ditaranto, M. A Comparative Study on Thermochemical Decomposition of Lignocellulosic Materials for Energy Recovery from Waste: Monitoring of Evolved Gases, Thermogravimetric, Kinetic and Surface Analyses of Produced Chars. Energy 2023, 285, 129328. [Google Scholar] [CrossRef]
- Thoppil, Y.; Zein, S.H. Techno-Economic Analysis and Feasibility of Industrial-Scale Biodiesel Production from Spent Coffee Grounds. J. Clean. Prod. 2021, 307, 127113. [Google Scholar] [CrossRef]
- Mäder, G.; Rüegg, N.; Tschichold, T.; Yildirim, S. Utilizing Spent Coffee Grounds as Sustainable Fillers in Biopolymer Composites: Influence of Particle Size and Content. Sustain. Food Technol. 2025, 3, 1151–1163. [Google Scholar] [CrossRef]
- Padma Ishwarya, S.; Nisha, P. Insights into the Composition, Structure-Function Relationship, and Molecular Organization of Surfactants from Spent Coffee Grounds. Food Hydrocoll. 2022, 124, 107204. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Valorization of Spent Coffee Grounds as the Specialty Material for Dullness and Aging of Skin Treatments. Chem. Biol. Technol. Agric. 2021, 8, 55. [Google Scholar] [CrossRef]
- Singh, T.A.; Pal, N.; Sharma, P.; Passari, A.K. Spent Coffee Ground: Transformation from Environmental Burden into Valuable Bioactive Metabolites. Rev. Environ. Sci. Biotechnol. 2023, 22, 887–898. [Google Scholar] [CrossRef]
- Gebreeyessus, G.D. Towards the Sustainable and Circular Bioeconomy: Insights on Spent Coffee Grounds Valorization. Sci. Total Environ. 2022, 833, 155113. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioproc. Tech. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Pigozzi, M.T.; Passos, F.R.; Mendes, F.Q. Quality of Commercial Coffees: Heavy Metal and Ash Contents. J. Food Qual. 2018, 2018, 5908463. [Google Scholar] [CrossRef]
- Atabani, A.E.; Al-Rubaye, O.K. Valorization of Spent Coffee Grounds for Biodiesel Production: Blending with Higher Alcohols, FT-IR, TGA, DSC, and NMR Characterizations. Biomass Convers. Biorefin. 2022, 12, 577–596. [Google Scholar] [CrossRef]
- Krinski, I.M.; Leite, V.R.; Moura, L.M.; Mariani, V.C. Critical Extraction Parameters for Maximizing Oil Yield from Spent Coffee Grounds. Energies 2025, 18, 1346. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Son, J.; Lee, J.W. Solvo-Thermal in Situ Transesterification of Wet Spent Coffee Grounds for the Production of Biodiesel. Bioresour. Technol. 2018, 249, 494–500. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Abut, S.; Kaya, M.; Eskicioglu, C.; Semaan, G.; Lee, C.; Yildiz, Y.; Unalan, S.; Mohanasundaram, R.; et al. Anaerobic Co-Digestion of Oil-Extracted Spent Coffee Grounds with Various Wastes: Experimental and Kinetic Modeling Studies. Bioresour. Technol. 2021, 322, 124470. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Bio-Refinery Approach for Spent Coffee Grounds Valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Comino, F.; Aranda, V.; Domínguez-Vidal, A.; Ayora-Cañada, M.J. Thermal Destruction of Organic Waste Hydrophobicity for Agricultural Soils Application. J. Environ. Manag. 2017, 202, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.S.; Thorpe, R.B.; Peus, D.; Lee, J. Anaerobic Digestion of Untreated and Treated Process Water from the Hydrothermal Carbonisation of Spent Coffee Grounds. Chemosphere 2022, 293, 133529. [Google Scholar] [CrossRef] [PubMed]
- Shayene Campos de Bomfim, A.; Magalhães de Oliveira, D.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2022, 1, 2–20. [Google Scholar] [CrossRef]
- Girotto, F.; Lavagnolo, M.C.; Pivato, A. Spent Coffee Grounds Alkaline Pre-Treatment as Biorefinery Option to Enhance Their Anaerobic Digestion Yield. Waste Biomass Valorizat. 2018, 9, 2565–2570. [Google Scholar] [CrossRef]
- Shaikh-Ibrahim, A.; Curci, N.; De Lise, F.; Sacco, O.; Di Fenza, M.; Castaldi, S.; Isticato, R.; Oliveira, A.; Aniceto, J.P.S.; Silva, C.M.; et al. Carbohydrate Conversion in Spent Coffee Grounds: Pretreatment Strategies and Novel Enzymatic Cocktail to Produce Value-Added Saccharides and Prebiotic Mannooligosaccharides. Biotechnol. Biofuels Bioprod. 2025, 18, 2. [Google Scholar] [CrossRef]
- Hernandez-Hosaka, C.; Park, B.-r.; Zhao, Y.; Jung, J. Effect of Pretreatment and Peracetic Acid Pulping on Cellulosic Materials Converted from Spent Coffee Grounds. J. Food Sci. 2024, 89, 9407–9419. [Google Scholar] [CrossRef]
- Xin, D.; Yin, H.; Jiao, Z.; Ran, G. Integrated Alkaline Pretreatment and Surfactant-Assisted Hydrolysis for High-Yield Manno-Oligosaccharides Production from Spent Coffee Grounds. Food Chem. X 2025, 29, 102892. [Google Scholar] [CrossRef]
- Pereira, J.; de Melo, M.M.R.; Silva, C.M.; Lemos, P.C.; Serafim, L.S. Impact of a Pretreatment Step on the Acidogenic Fermentation of Spent Coffee Grounds. Bioengineering 2022, 9, 362. [Google Scholar] [CrossRef]
- Somnuk, K.; Eawlex, P.; Prateepchaikul, G. Optimization of Coffee Oil Extraction from Spent Coffee Grounds Using Four Solvents and Prototype-Scale Extraction Using Circulation Process. Agric. Nat. Res. 2017, 51, 181–189. [Google Scholar] [CrossRef]
- Karmee, S.K. A Spent Coffee Grounds Based Biorefinery for the Production of Biofuels, Biopolymers, Antioxidants and Biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Spent Coffee Grounds Make Much More than Waste: Exploring Recent Advances and Future Exploitation Strategies for the Valorization of an Emerging Food Waste Stream. J. Clean. Prod. 2018, 172, 980–992. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Effect of Solvent Extraction Parameters on the Recovery of Oil From Spent Coffee Grounds for Biofuel Production. Waste Biomass Valorizat. 2019, 10, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Kafková, V.; Kubinec, R.; Mikulec, J.; Variny, M.; Ondrejíčková, P.; Ház, A.; Brisudová, A. Integrated Approach to Spent Coffee Grounds Valorization in Biodiesel Biorefinery. Sustainability 2023, 15, 5612. [Google Scholar] [CrossRef]
- Abdullah, M.; Bulent Koc, A. Oil Removal from Waste Coffee Grounds Using Two-Phase Solvent Extraction Enhanced with Ultrasonication. Renew. Energy 2013, 50, 965–970. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and Validation of Ultrasound-Assisted Solid-Liquid Extraction of Phenolic Compounds from Waste Spent Coffee Grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Holló, A.T.; Rétfalvi, N.; Cséfalvay, E.; Dibó, G.; Havasi, D.; Mika, L.T. Microwave-Assisted Valorization of Biowastes to Levulinic Acid. ChemistrySelect 2017, 2, 1375–1380. [Google Scholar] [CrossRef]
- Pavlović, M.D.; Buntić, A.V.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Ethanol Influenced Fast Microwave-Assisted Extraction for Natural Antioxidants Obtaining from Spent Filter Coffee. Sep. Purif. Technol. 2013, 118, 503–510. [Google Scholar] [CrossRef]
- Beaudor, M.; Vauchel, P.; Pradal, D.; Aljawish, A.; Phalip, V. Comparing the Efficiency of Extracting Antioxidant Polyphenols from Spent Coffee Grounds Using an Innovative Ultrasound-Assisted Extraction Equipment versus Conventional Method. Chem. Eng. Process. Process. Intensif. 2023, 188, 109358. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Paula Robalo, M.; Boyadzhieva, S.; Cholakov, G.S.; Stateva, R.P. Supercritical CO2 Extraction of Spent Coffee Grounds. Influence of Co-Solvents and Characterization of the Extracts. J. Supercrit. Fluids 2020, 161, 104825. [Google Scholar] [CrossRef]
- Couto, R.M.; Fernandes, J.; da Silva, M.D.R.G.; Simões, P.C. Supercritical Fluid Extraction of Lipids from Spent Coffee Grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Muangrat, R.; Pongsirikul, I. Recovery of Spent Coffee Grounds Oil Using Supercritical CO2: Extraction Optimisation and Physicochemical Properties of Oil. CyTA J. Food 2019, 17, 334–346. [Google Scholar] [CrossRef]
- Burniol-Figols, A.; Cenian, K.; Skiadas, I.V.; Gavala, H.N. Integration of Chlorogenic Acid Recovery and Bioethanol Production from Spent Coffee Grounds. Biochem. Eng. J. 2016, 116, 54–64. [Google Scholar] [CrossRef]
- Scully, D.S.; Jaiswal, A.K.; Abu-Ghannam, N. An Investigation into Spent Coffee Waste as a Renewable Source of Bioactive Compounds and Industrially Important Sugars. Bioengineering 2016, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Prabhudessai, V.; Ganguly, A.; Mutnuri, S. Effect of Caffeine and Saponin on Anaerobic Digestion of Food Waste. Ann. Microbiol. 2009, 59, 643–648. [Google Scholar] [CrossRef]
- Qiao, W.; Takayanagi, K.; Shofie, M.; Niu, Q.; Yu, H.Q.; Li, Y.Y. Thermophilic Anaerobic Digestion of Coffee Grounds with and without Waste Activated Sludge as Co-Substrate Using a Submerged AnMBR: System Amendments and Membrane Performance. Bioresour. Technol. 2013, 150, 249–258. [Google Scholar] [CrossRef]
- Jomnonkhaow, U.; Plangklang, P.; Reungsang, A.; Peng, C.Y.; Chu, C.Y. Valorization of Spent Coffee Grounds through Integrated Bioprocess of Fermentable Sugars, Volatile Fatty Acids, Yeast-Based Single-Cell Protein and Biofuels Production. Bioresour. Technol. 2024, 393, 130107. [Google Scholar] [CrossRef]
- Lee, M.; Yang, M.; Choi, S.; Shin, J.; Park, C.; Cho, S.K.; Kim, Y.M. Sequential Production of Lignin, Fatty Acid Methyl Esters and Biogas from Spent Coffee Grounds via an Integrated Physicochemical and Biological Process. Energies 2019, 12, 2360. [Google Scholar] [CrossRef]
- Araújo, M.N.; Azevedo, A.Q.P.L.; Hamerski, F.; Voll, F.A.P.; Corazza, M.L. Enhanced Extraction of Spent Coffee Grounds Oil Using High-Pressure CO2 plus Ethanol Solvents. Ind. Crops Prod. 2019, 141, 111723. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Lee, C. Effect of Mild-Temperature Thermo-Alkaline Pretreatment on the Solubilization and Anaerobic Digestion of Spent Coffee Grounds. Energies 2018, 11, 865. [Google Scholar] [CrossRef]
- Ribeiro, G.M.; Martins, P.L.; Oliveira, A.C.; Carvalheiro, F.; Fragoso, R.; Duarte, L.C. The Role of Mild Alkaline Pretreatment in the Biorefinery Upgrade of Spent Coffee Grounds. Energies 2023, 16, 3907. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Lee, J.; Kim, S.; Park, C.; Yoo, H.Y. Statistical Optimization of Alkali Pretreatment to Improve Sugars Recovery from Spent Coffee Grounds and Utilization in Lactic Acid Fermentation. Processes 2021, 9, 494. [Google Scholar] [CrossRef]
- da Fonseca, Y.A.; Gurgel, L.V.A.; Baêta, B.E.L.; Dragone, G.; Mussatto, S.I. Reduced-Pressure Alkaline Pretreatment as an Innovative and Sustainable Technology to Extract Protein from Brewer’s Spent Grain. J. Clean. Prod. 2023, 416, 137966. [Google Scholar] [CrossRef]
- Nava-Valente, N.; Del Ángel-Coronel, O.A.; Atenodoro-Alonso, J.; López-Escobar, L.A. Effect of Thermal and Acid Pre-Treatment on Increasing Organic Loading Rate of Anaerobic Digestion of Coffee Pulp for Biogas Production. Biomass Convers. Biorefin. 2023, 13, 4817–4830. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Moutsoglou, A.; Prodromidis, P.; Moschakis, T.; Goula, A.; Biliaderis, C.G.; Michaelidou, A.M. Aqueous Ammonia Soaking Pretreatment of Spent Coffee Grounds for Enhanced Enzymatic Hydrolysis: A Bacterial Cellulose Production Application. Sustain. Chem. Pharm. 2023, 33, 101121. [Google Scholar] [CrossRef]
- Chen, W.H.; Du, J.T.; Lee, K.T.; Ong, H.C.; Park, Y.K.; Huang, C.C. Pore Volume Upgrade of Biochar from Spent Coffee Grounds by Sodium Bicarbonate during Torrefaction. Chemosphere 2021, 275, 129999. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.A.; Bueno, B.A.; Borges, R.M.; Bringhenti, J.R. Biochemical Methane Potential of Spent Coffee Grounds Via Co-Digestion with Food Waste. Bioenergy Res. 2024, 17, 1133–1144. [Google Scholar] [CrossRef]
- Caño-Carrillo, I.; Batteau, M.; Bruna, L.; Gilbert-López, B.; García-Reyes, J.F.; Chatel, G.; Faure, K. Determination of Free and Derivative Terpenoids and Sterols in Spent Coffee Grounds by Two-Dimensional Liquid Chromatography—Supercritical Fluid Chromatography Coupled to High-Resolution Mass Spectrometry. Food Chem. 2025, 488, 144853. [Google Scholar] [CrossRef]
- Zhang, Y.; Diono, W.; Rujiravanit, R.; Kanda, H.; Goto, M. Extraction of Diterpenes from Spent Coffee Grounds and Encapsulation into Polyvinylpyrrolidone Particles Using Supercritical Carbon Dioxide. Sep. Sci. Technol. 2022, 57, 1081–1096. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, H.; Du, L.; Liang, J.; Lu, X.; Li, N.; Zhang, K. Influence of NaOH and Thermal Pretreatment on Dewatered Activated Sludge Solubilisation and Subsequent Anaerobic Digestion: Focused on High-Solid State. Bioresour. Technol. 2015, 185, 171–177. [Google Scholar] [CrossRef]
- Feijoo, G.; Soto, M.; Méndez, R.; Lema, J.M. Sodium Inhibition in the Anaerobic Digestion Process: Antagonism and Adaptation Phenomena. Enzyme Microb. Technol. 1995, 17, 180–188. [Google Scholar] [CrossRef]
- Lee, J.; Kim, E.; Hwang, S. Effects of Inhibitions by Sodium Ion and Ammonia and Different Inocula on Acetate-Utilizing Methanogenesis: Methanogenic Activity and Succession of Methanogens. Bioresour. Technol. 2021, 334, 125202. [Google Scholar] [CrossRef]
- Schönheit, P.; Beimborn, D.B. Presence of a Na+/H+ Antiporter in Methanobacterium Thermoautotrophicum and Its Role in Na+ Dependent Methanogenesis. Arch. Microbiol. 1985, 142, 354–361. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Chen, Z.; Ye, Z.; Lyu, R. Multi-Omics Analysis Unravels Effects of Salt and Oil on Substance Transformation, Microbial Community, and Transcriptional Activity in Food Waste Anaerobic Digestion. Bioresour. Technol. 2023, 387, 129684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Wang, D.; Chen, F.; Li, X.; Zeng, G.; Yang, Q. Potential Impact of Salinity on Methane Production from Food Waste Anaerobic Digestion. Waste Manag. 2017, 67, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.C.; Li, X.K.; Wang, X.W.; Liu, G.G.; Zuo, J.L.; Wang, S.T.; Wang, K. Impact of Salinity on Anaerobic Microbial Community Structure in High Organic Loading Purified Terephthalic Acid Wastewater Treatment System. J. Hazard. Mater. 2020, 383, 121132. [Google Scholar] [CrossRef]
- Semaan, G.; Atelge, M.R.; Dune Cayetano, R.; Kumar, G.; Kommedal, R. Spent Coffee Grounds Anaerobic Digestion: Investigating Substrate to Inoculum Ratio and Dilute Acid Thermal Pretreatment. Fuel 2022, 331, 125598. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- da Silva, F.E.; Rodríguez, D.M.; Duarte, N.A.; Barbosa, C.K.; Figueiredo, P.A. Valorization of Spent Coffee Grounds as a Substrate for Fungal Laccase Production and Biosorbents for Textile Dye Decolorization. Fermentation 2025, 11, 396. [Google Scholar] [CrossRef]
- Vanyan, L.; Cenian, A.; Trchounian, K. Biogas and Biohydrogen Production Using Spent Coffee Grounds and Alcohol Production Waste. Energies 2022, 15, 5935. [Google Scholar] [CrossRef]
- Lackner, N.; Wagner, A.O.; Markt, R.; Illmer, P. PH and Phosphate Induced Shifts in Carbon Flow and Microbial Community during Thermophilic Anaerobic Digestion. Microorganisms 2020, 8, 286. [Google Scholar] [CrossRef]
- Satari Baboukani, B.; Vossoughi, M.; Alemzadeh, I. Optimisation of Dilute-Acid Pretreatment Conditions for Enhancement Sugar Recovery and Enzymatic Hydrolysis of Wheat Straw. Biosyst. Eng. 2012, 111, 166–174. [Google Scholar] [CrossRef]
- Banu, J.R.; Sugitha, S.; Kavitha, S.; Kannah, R.Y.; Merrylin, J.; Kumar, G. Lignocellulosic Biomass Pretreatment for Enhanced Bioenergy Recovery: Effect of Lignocelluloses Recalcitrance and Enhancement Strategies. Front. Energy Res. 2021, 9, 646057. [Google Scholar] [CrossRef]
- Massaya, J.; Chan, K.H.; Mills-Lamptey, B.; Chuck, C.J. Developing a Biorefinery from Spent Coffee Grounds Using Subcritical Water and Hydrothermal Carbonisation. Biomass Convers. Biorefin. 2023, 13, 1279–1295. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, G.B.; Jeong, C.J.; Kim, H.; Kim, C.G. Determination of Relationship between Higher Heating Value and Atomic Ratio of Hydrogen to Carbon in Spent Coffee Grounds by Hydrothermal Carbonization. Energies 2021, 14, 6551. [Google Scholar] [CrossRef]
- Yang, L.; Nazari, L.; Yuan, Z.; Corscadden, K.; Xu, C.C.; He, Q.S. Hydrothermal Liquefaction of Spent Coffee Grounds in Water Medium for Bio-Oil Production. Biomass Bioenergy 2016, 86, 191–198. [Google Scholar] [CrossRef]
- Dias, M.E.; Oliveira, G.H.D.; Couto, P.T.; Dussán, K.J.; Zaiat, M.; Ribeiro, R.; Stablein, M.J.; Watson, J.T.; Zhang, Y.; Tommaso, G. Anaerobic Digestion of Hydrothermal Liquefaction Wastewater from Spent Coffee Grounds. Biomass Bioenergy 2021, 148, 106030. [Google Scholar] [CrossRef]
- Massaya, J.; Pickens, G.; Mills-Lamptey, B.; Chuck, C.J. Enhanced Hydrothermal Carbonization of Spent Coffee Grounds for the Efficient Production of Solid Fuel with Lower Nitrogen Content. Energy Fuels 2021, 35, 9462–9473. [Google Scholar] [CrossRef]
- de Oliveira Fernandes, M.A.; Baêta, B.E.L.; Adarme, O.F.H.; Fonseca, A. LCA-Based Carbon Footprint Analysis of Anaerobic Digestion of Coffee Husk Waste. Renew. Sustain. Energy Rev. 2025, 207, 114993. [Google Scholar] [CrossRef]
- Gómez-De La Cruz, F.J.; Cruz-Peragón, F.; Casanova-Peláez, P.J.; Palomar-Carnicero, J.M. A Vital Stage in the Large-Scale Production of Biofuels from Spent Coffee Grounds: The Drying Kinetics. Fuel Process Technol. 2015, 130, 188–196. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Katerla, J.; Janus, R.; Lewandowski, M.; Plata, M.; Korzeniowski, Ł. High-Energy-Density Hydrochar and Bio-Oil from Hydrothermal Processing of Spent Coffee Grounds—Experimental Investigation. Energies 2024, 17, 6446. [Google Scholar] [CrossRef]
- Lee, K.T.; Tsai, J.Y.; Hoang, A.T.; Chen, W.H.; Gunarathne, D.S.; Tran, K.Q.; Selvarajoo, A.; Goodarzi, V. Energy-Saving Drying Strategy of Spent Coffee Grounds for Co-Firing Fuel by Adding Biochar for Carbon Sequestration to Approach Net Zero. Fuel 2022, 326, 124984. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Niu, H.; McNutt, J.; He, Q. A Comparative Study on Thermochemical Valorization Routes for Spent Coffee Grounds. Energies 2021, 14, 3840. [Google Scholar] [CrossRef]
- Getachew, A.T.; Chun, B.S. Influence of Pretreatment and Modifiers on Subcritical Water Liquefaction of Spent Coffee Grounds: A Green Waste Valorization Approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Amna, R.; Faheem, A.F. Green Solvent Extraction of Lipids from Spent Coffee Grounds: A Computational Fluid Dynamics Approach. Ind. Crops Prod. 2025, 232, 121271. [Google Scholar] [CrossRef]
- Rodrigues, C.V.; Camargo, F.P.; Lourenço, V.A.; Sakamoto, I.K.; Maintinguer, S.I.; Silva, E.L.; Amâncio Varesche, M.B. Towards a Circular Bioeconomy to Produce Methane by Co-Digestion of Coffee and Brewery Waste Using a Mixture of Anaerobic Granular Sludge and Cattle Manure as Inoculum. Chemosphere 2024, 357, 142062. [Google Scholar] [CrossRef]
- Villa Montoya, A.C.; Cristina da Silva Mazareli, R.; Delforno, T.P.; Centurion, V.B.; Sakamoto, I.K.; Maia de Oliveira, V.; Silva, E.L.; Amâncio Varesche, M.B. Hydrogen, Alcohols and Volatile Fatty Acids from the Co-Digestion of Coffee Waste (Coffee Pulp, Husk, and Processing Wastewater) by Applying Autochthonous Microorganisms. Int. J. Hydrogen Energy 2019, 44, 21434–21450. [Google Scholar] [CrossRef]
- Dias, M.E.S.; Takeda, P.Y.; Fuess, L.T.; Tommaso, G. Inoculum-to-Substrate Ratio and Solid Content Effects over in Natura Spent Coffee Grounds Anaerobic Digestion. J. Environ. Manag. 2023, 325, 116486. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Baek, G.; Lee, C. Anaerobic Co-Digestion of Spent Coffee Grounds with Different Waste Feedstocks for Biogas Production. Waste Manag. 2017, 60, 322–328. [Google Scholar] [CrossRef]
- Karakaş, D.E.; Akdemir, M.; Atelge, M.R.; Kaya, M.; Atabani, A.E. Defatted Spent Coffee Grounds-Supported Cobalt Catalyst as a Promising Supercapacitor Electrode for Hydrogen Production and Energy Storage. Clean. Technol. Environ. Policy 2023, 25, 483–493. [Google Scholar] [CrossRef]
- Qiao, W.; Mohammad, S.; Takayanagi, K.; Li, Y.Y. Thermophilic Anaerobic Co-Digestion of Coffee Grounds and Excess Sludge: Long Term Process Stability and Energy Production. RSC Adv. 2015, 5, 26452–26460. [Google Scholar] [CrossRef]
- Al bkoor Alrawashdeh, K.; Al-Samrraie, L.A.; Damseh, R.A.; Al Bsoul, A.; Gul, E. Improving Biogas Production and Organic Matter Degradation in Anaerobic Co-Digestion Using Spent Coffee Grounds: A Kinetic and Operational Study. Fermentation 2025, 11, 295. [Google Scholar] [CrossRef]
- Passos, F.; Cordeiro, P.H.M.; Baeta, B.E.L.; de Aquino, S.F.; Perez-Elvira, S.I. Anaerobic Co-Digestion of Coffee Husks and Microalgal Biomass after Thermal Hydrolysis. Bioresour. Technol. 2018, 253, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ayodele, O.O.; Adekunle, A.E.; Adesina, A.O.; Pourianejad, S.; Zentner, A.; Dornack, C. Stabilization of Anaerobic Co-Digestion of Biowaste Using Activated Carbon of Coffee Ground Biomass. Bioresour. Technol. 2021, 319, 124247. [Google Scholar] [CrossRef]
- Orfanoudaki, A.; Makridakis, G.; Maragkaki, A.; Fountoulakis, M.S.; Kallithrakas-Kontos, N.G.; Manios, T. Anaerobic Co-Digestion of Pig Manure and Spent Coffee Grounds for Enhanced Biogas Production. Waste Biomass Valorizat. 2020, 11, 4613–4620. [Google Scholar] [CrossRef]
- Zhang, T.; Tonouchi, K.; Kong, Z.; Li, Y.; Cheng, H.; Qin, Y.; Li, Y.Y. Improvement of Coffee Grounds High Solid Thermophilic Methane Fermentation by Co-Digestion with in-Situ Produced Waste Activated Sludge: Performance and Stability. Sci. Total Environ. 2021, 765, 142551. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, X.; Qi, L.; Gao, F.; Wu, G.; Li, H.; Guo, W.; Ngo, H.H. Enhancement of Volatile Fatty Acids Degradation and Rapid Methanogenesis in a Biochar-Assisted Anaerobic Membrane Bioreactor via Enhancing Direct Interspecies Electron Transfer. J. Environ. Manag. 2025, 380, 125045. [Google Scholar] [CrossRef]
- Akyol, Ç. In Search of the Optimal Inoculum to Substrate Ratio during Anaerobic Co-Digestion of Spent Coffee Grounds and Cow Manure. Waste Manag. Res. 2020, 38, 1278–1283. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, Y.; Zhou, Z.; Liu, R.; Qiu, Z.; Qiu, Y.; Li, J.; Wang, W.; Li, X.; Yin, L.; et al. Are Microplastics in Livestock and Poultry Manure an Emerging Threat to Agricultural Soil Safety? Environ. Sci. Pollut. Res. 2024, 31, 11543–11558. [Google Scholar] [CrossRef]
- Kampioti, A.; Komilis, D. Anaerobic Co-Digestion of Coffee Waste with Other Organic Substrates: A Mixture Experimental Design. Chemosphere 2022, 297, 134124. [Google Scholar] [CrossRef] [PubMed]
- Beschkov, V.N.; Angelov, I.K. Volatile Fatty Acid Production vs. Methane and Hydrogen in Anaerobic Digestion. Fermentation 2025, 11, 172. [Google Scholar] [CrossRef]
- Alhraishawi, A.; Aslan, S. Effect of Lipid Content on Anaerobic Digestion Process and Microbial Community: Review Study. Eur. Sci. J. 2022, 8, 197. [Google Scholar] [CrossRef]
- Alves Lourenço, V.; Pereira Camargo, F.; Kimiko Sakamoto, I.; Silva, E.L.; Amâncio Varesche, M.B. Waste Valorization through Anaerobic Co-Digestion in Coffee and Swine Farms: CH4 Yield Optimization and Farm-Scale Viability. Bioresour. Technol. 2025, 415, 131667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Q.; Li, Y. A Review on Sulfur Transformation during Anaerobic Digestion of Organic Solid Waste: Mechanisms, Influencing Factors and Resource Recovery. Sci. Total Environ. 2023, 865, 161193. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kaushik, P.; Singh, S.; Agrahari, P.; Kumar, B.; Kumar, P.; Arya, V.P. Potential Use of Sewage Sludge as Fertilizer in Organic Farming. Clean. Waste Syst. 2025, 10, 100245. [Google Scholar] [CrossRef]
- Lourenço, V.A.; Camargo, F.P.; Sakamoto, I.K.; Silva, E.L.; Varesche, M.B.A. Influence of Substrates Proportion and Concentration on Biogas Composition and Yield in the Co-Digestion of Solid and Liquid Waste from Coffee and Swine Farms. Renew. Energy 2025, 249, 123191. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhen, F.; Liu, H.; Xiao, F.; Sun, Y.; Peng, X.; Li, Y.; Wang, X. Thermodynamics of Volatile Fatty Acid Degradation during Anaerobic Digestion under Organic Overload Stress: The Potential to Better Identify Process Stability. Water Res. 2022, 214, 118187. [Google Scholar] [CrossRef]
- Sri Bala Kameswari, K.; Kalyanaraman, C.; Porselvam, S.; Thanasekaran, K. Optimization of Inoculum to Substrate Ratio for Bio-Energy Generation in Co-Digestion of Tannery Solid Wastes. Clean. Technol. Environ. Policy 2012, 14, 241–250. [Google Scholar] [CrossRef]
- Sumantri, I.; Kusnadi, P.; Handoyo, I.G.R.; Kumoro, A.C. Biodigestion of Mixed Substrates of Cow Manure-Delignified Spent Coffee Ground (DSCG) Using Microorganism Enhancer for Biogas Production and Its Kinetic Study. Environ. Res. Eng. Manag. 2022, 78, 96–109. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, X.; Xia, W.; Li, Z.; Wang, L.; Chen, Z.; Ge, S. Effect of Extreme PH Conditions on Methanogenesis: Methanogen Metabolism and Community Structure. Sci. Total Environ. 2023, 877, 162702. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Zhou, M.; Pan, X.; Li, C.; Lv, N.; Wang, T.; Cai, G.; Wang, R.; Li, J.; Zhu, G. Simultaneous Biogas and Biogas Slurry Production from Co-Digestion of Pig Manure and Corn Straw: Performance Optimization and Microbial Community Shift. Bioresour. Technol. 2019, 282, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioproc. Tech. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Ouafa, A.; Kerroum, D.; Antonio, P.; Lamis, R.; Chaima, B.; Rania, Z.Z.; Mossaab, B.L. Coffee Grounds and Fruit and Vegetable Waste Co-Digestion in Dark Fermentation: Evaluation of Mixing Ratio and Hybrid Pretreatments Impact on Bio-Hydrogen Production. Biomass Bioenergy 2025, 199, 107896. [Google Scholar] [CrossRef]
- Paek, J.; Bai, L.; Shin, Y.; Kim, H.; Kim, S.H.; Shin, J.H.; Kook, J.K.; Chang, Y.H. Description of Olsenella Absiana Sp. Nov., an Anaerobic Bacterium Isolated from Pig Faeces. Int. J. Syst. Evol. Microbiol. 2024, 74, 006579. [Google Scholar] [CrossRef]
- Callaway, R.; Weimer, P.J. Degradation of Cellulose and Hemicellulose by Ruminal Microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef]
- Rui, J.; Li, J.; Zhang, S.; Yan, X.; Wang, Y.; Li, X. The Core Populations and Co-Occurrence Patterns of Prokaryotic Communities in Household Biogas Digesters. Biotechnol. Biofuels 2015, 8, 158. [Google Scholar] [CrossRef]
- Vasmara, C.; Marchetti, R. Spent Coffee Grounds from Coffee Vending Machines as Feedstock for Biogas Production. Environ. Eng. Manag. J. 2018, 17, 2401–2408. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Dave Oomah, B. Spent Coffee Grounds: A Review on Current Research and Future Prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Forcina, A.; Petrillo, A.; Travaglioni, M.; di Chiara, S.; De Felice, F. A Comparative Life Cycle Assessment of Different Spent Coffee Ground Reuse Strategies and a Sensitivity Analysis for Verifying the Environmental Convenience Based on the Location of Sites. J. Clean. Prod. 2023, 385, 135727. [Google Scholar] [CrossRef]
- Hao, Z.; Yang, B.; Jahng, D. Spent Coffee Ground as a New Bulking Agent for Accelerated Biodrying of Dewatered Sludge. Water Res. 2018, 138, 250–263. [Google Scholar] [CrossRef]
- AlMallahi, M.N.; Maen Asaad, S.; Rocha-Meneses, L.; Inayat, A.; Said, Z.; El Haj Assad, M.; Elgendi, M. A Case Study on Bio-Oil Extraction from Spent Coffee Grounds Using Fast Pyrolysis in a Fluidized Bed Reactor. Case Stud. Chem. Environ. Eng. 2023, 8, 100529. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.G.; Kwak, J.; Kim, S.; Lee, S.H.; Park, Y.; Lee, S.D.; Chon, K. Adsorption of Radioactive Strontium by Pristine and Magnetic Biochars Derived from Spent Coffee Grounds. J. Environ. Chem. Eng. 2021, 9, 105119. [Google Scholar] [CrossRef]
- Chung, L.L.P.; Wong, Y.C.; Arulrajah, A. The Application of Spent Coffee Grounds and Tea Wastes as Additives in Alkali-Activated Bricks. Waste Biomass Valorizat. 2021, 12, 6273–6291. [Google Scholar] [CrossRef]
- Leifa, F.; Pandey, A.; Soccol, C.R. Production of Flammulina Velutipes on Coffee Husk and Coffee Spent-Ground. Braz. Arch. Biol. Technol. 2001, 44, 205–212. [Google Scholar] [CrossRef]
- Bomfim, A.S.C.D.; Oliveira, D.M.D.; Voorwald, H.J.C.; Benini, K.C.C.D.C.; Dumont, M.J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Kisiga, W. Techno-Economic Analysis and Life Cycle Assessment for Production of Biofuels from Spent Coffee Grounds; Durban University of Technology: Durban, South Africa, 2023. [Google Scholar]
- Janissen, B.; Huynh, T. Chemical Composition and Value-Adding Applications of Coffee Industry by-Products: A Review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Kisiga, W.; Chetty, M.; Rathilal, S. Environmental Impact Assessment of Alternative Technologies for Production of Biofuels from Spent Coffee Grounds. Energy Sci. Eng. 2024, 12, 4823–4842. [Google Scholar] [CrossRef]
- Schmidt Rivera, X.C.; Gallego-Schmid, A.; Najdanovic-Visak, V.; Azapagic, A. Life Cycle Environmental Sustainability of Valorisation Routes for Spent Coffee Grounds: From Waste to Resources. Resour. Conserv. Recycl. 2020, 157, 104751. [Google Scholar] [CrossRef]
- Panepinto, D.; Riggio, V.A.; Campo, G.; Cerutti, A.; Comoglio, C.; Zanetti, M.C. Analysis of Two Treatment Technologies for Coffee Roasting Matrixes: Combustion and Anaerobic Digestion. Clean. Technol. Environ. Policy 2019, 21, 685–694. [Google Scholar] [CrossRef]
- Greenberg, I.; Kaiser, M.; Gunina, A.; Ledesma, P.; Polifka, S.; Wiedner, K.; Mueller, C.W.; Glaser, B.; Ludwig, B. Substitution of Mineral Fertilizers with Biogas Digestate plus Biochar Increases Physically Stabilized Soil Carbon but Not Crop Biomass in a Field Trial. Sci. Total Environ. 2019, 680, 181–189. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).