Abstract
This study presents a detailed analysis of the combustion dynamics of stoichiometric H2–air and CH4–air mixtures in a 20 L closed vessel over an initial temperature range of 298–423 K. We integrate experimental pressure–time P(t) measurements with numerical analysis to extract laminar burning velocity (LBV) and deflagration index (KG) values, and we assess three independent kinetic mechanisms (KiBo_MU, University of San Diego, Lund University) via simulations. For H2–air, LBV increases from 0.50 m/s at 298 K to 0.94 m/s at 423 K (temperature exponent α ≈ 1.79), while for CH4–air, LBV rises from 0.36 m/s to 0.96 m/s (α ≈ 2.82). In contrast, the deflagration index KG decreases by ca. 20% for H2–air and ca. 30% for CH4–air over the same temperature span. The maximum explosion pressure (Pmax) and peak pressure rise rate ((dP/dt)max) also exhibit systematic increases with temperature. A comparison with model predictions shows agreement within experiments, providing data for safety modeling and kinetic mechanism validation in H2- and CH4-based energy systems.
1. Introduction
H2–air and CH4–air mixtures are gaining traction across multiple sectors of the process industry, including natural gas distribution networks, compressed natural gas (CNG) vehicle fueling stations, gas storage facilities, and combined cycle power plants, due to the fact that they can be applied as low-emission technologies while maintaining high energy density and combustion efficiency [1,2,3]. However, the safe handling of these light gases throughout their entire lifecycle (production, storage, transport, and end use) remains a critical challenge [4,5]. This work investigates the deflagration dynamics for these mixtures at elevated temperatures (298–423 K) and provides measurements of key safety parameters, including laminar burning velocity (LBV), maximum explosion pressure (Pₘₐₓ), and deflagration index (KG), that underpin explosion risk assessments and inform the design of mitigation measures such as the use of vent sizing, flame arrestors, and safe separation distances in process industries [6,7]. Building on this safety imperative, LBV emerges as a key fundamental parameter: it governs flames, influences deflagration indices, and underpins both CFD and kinetic mechanism validation [8]. A clear, quantitative understanding of LBV as a function of temperature and fuel type is therefore essential for accurate risk assessment and model development in H2- and CH4-based systems.
The laminar burning velocity (LBV) of hydrogen–air as well as methane–air mixtures has been extensively studied, with a particular focus on the influence of various conditions, including equivalence ratios, initial pressures and temperatures [9,10] and the H2–CH4–air ratio [11,12]. Another investigation, employing both experimental and numerical approaches, has analyzed the LBV of mixtures of CH4, NH3, and air using various kinetic models [13]. Other researchers have also explored the laminar burning velocity and burned gas Markstein length of CH4–air mixtures diluted with N2, H2O, and CO2 [14]. Also, the effects of H2 addition and CO2 attenuation on LBV for CH4–air mixtures have been studied experimentally and numerically [15], highlighting the significant impacts of these additives [16,17]. There are also several studies that obtained experimental LBV results using the Bunsen flame method at 373 K, with kinetic models accurately predicting LBV for methane–air premixed flames containing DMMP [18]. The correlation between the LBV and burned gas Markstein length of CH4–air mixtures shows that LBV can increase by 23–29% with a temperature rise from 373 K to 423 K and by 22–34% from 423 K to 473 K [19]. A comprehensive review highlighted the use of polynomial regression curves to fit LBV data from various publications, including those focused on CH4–air mixtures [6,20,21]. Akram et al. [22] demonstrated that slightly richer CH4–air mixtures exhibit a minimal temperature exponent and an increased adiabatic flame temperature. This behavior arises because the normalized sensitivity to methane in the dominant reactions increases with temperature for mixtures near the lean flammability limit, while it slightly decreases for stoichiometric mixtures. It is recommended to avoid assuming a linear change in the temperature exponent as a function of the equivalence ratio. The maximum flame speed has been observed in slightly richer methane–air mixtures, aligning with computational results. However, this contrasts with experimental findings, which indicate peak flame speeds for either lean or very rich mixtures at high temperatures. This discrepancy is attributed to the inaccuracy of and variability in the temperature exponent across different equivalence ratios. Finally, Ghosh et al. [23] measured the LBV of H2–air mixtures under stoichiometric conditions across a temperature range of 160 to 295 K. Their findings revealed that LBV decreased by 50% when the unburned mixture temperature was reduced by 100 K. This behavior followed a power law relationship with an exponent of 1.571, determined for temperatures above ambient conditions. In this study, the temperature dependence on the LBV of H2–air mixtures as well as CH4–air mixtures was up to 423.15 K [24]. However, all of these above studies typically adhere to the following:
- They cover only a narrow temperature range, where most focus on near-ambient conditions (298–323 K) and do not report LBV trends above 350 K, leaving high-temperature behavior underexplored.
- They examine a single fuel in isolation, precluding direct cross-fuel comparisons under identical conditions.
- They evaluate at most one or two kinetic mechanisms, limiting the ability to identify strengths and weaknesses across modern detailed and skeletal models.
Our work fills this gap by performing pressure-based LBV and deflagration index measurements for both fuels over 298–423 K in a 20 L bomb, coupled with kinetic modeling using the KiBo_MU, San Diego, and Lund mechanisms and providing the unified dataset for both H2 and CH4 combustion under elevated temperatures.
2. Experiments and Kinetic Modeling
The experimental measurements reported in this work were collected by means of the 20 L combustion vessel shown in Figure 1. Also, Table 1 shows a detailed description of the experimental set-up, which comprises the following components. The combustion chamber was designed as a spherical stainless steel container with a volume of 20 dm3 (D = 340 mm), including two piezoelectric pressure transducers (±0.1% FS) and a type-K thermocouple (±1 K), water-jacketed with PID control (±0.5 K). Stainless steel was chosen for its corrosion resistance against reactive substances and combustion by-products, as well as its high-temperature durability, both crucial for combustion testing. The equipment was carefully selected to withstand pressures of up to 15 bar, ensuring durability and reliability under demanding experimental conditions. The spherical chamber is equipped with specialized ports for introducing and removing test gases and injecting or extracting the methane–air mixture. For mixture preparation we used partial pressure filling to φ = 1.00 ± 0.005, evacuation to 50 mbar, and 120 s mechanical stirring for homogeneity. For ignition and data acquisition, we applied a 0.2 mm Ni–Cr wire at 1 kV for 20 µs, and pressure was sampled at 150 kHz. For a repetition and error analysis, we used five independent runs per condition; the reported Pmax, (dP/dt)max, LBV, and KG are means ± one standard deviation. Instrumental uncertainties (pressure ±0.1%, temperature ±0.3 K, timing ±0.02 ms) were propagated through the model, yielding combined uncertainties of ±3% for LBV and ±5% for KG. This set-up allows for the precise control of the gas composition within the chamber, which is essential for accurate and reproducible experimental results. Such control ensures that the experimental conditions replicate real-world scenarios, facilitating the transfer of the findings to practical engineering applications. Temperature within the chamber is monitored using a thermocouple positioned to avoid interference with the flame path. Temperature readings are displayed on a monitor located at the base of the apparatus for immediate observation.

Figure 1.
An overview of the 20 L deflagration vessel used for testing.

Table 1.
Description of experimental set-up.
To minimize heat loss and maintain stable conditions, the chamber is equipped with a water jacket, which ensures uniform temperature distribution and enhances experimental precision and reliability. The water jacket also facilitates rapid cooling after experiments, expediting the research cycle. The ignition system, centrally located in the test volume, uses a 0.2 mm nickel–chromium (Ni-Cr) wire ignited by an electric arc to initiate combustion. Ni-Cr was chosen for its high heat resistance and chemical stability. The chamber also includes a mechanical mixer, which uses a partial pressure technique to prepare a homogeneous test mixture. This ensures an even distribution of components, critical for achieving consistent and reliable results. A heat-resistant quartz glass observation window allows for the direct inspection of the combustion process. Quartz glass was selected for its ability to withstand high temperatures, enabling long-term observation without the risk of damage. This visual access is invaluable for the qualitative analysis and verification of the combustion process. An advanced data acquisition system is integrated to collect key parameters during combustion. This system includes a calibrated pressure transducer, a signal amplifier, and a recording unit, ensuring precise measurements across a wide pressure range. The collected data is transferred to a connected computer with specialized software for comprehensive analysis. This software enables accurate data processing, identification of key trends, and modeling of combustion dynamics [6].
This advanced system facilitates a detailed study of combustion dynamics, enabling an in-depth analysis of the experimental results and a more profound understanding of combustion processes. By leveraging these insights, predictive models can be developed to optimize real-world combustion systems, improve efficiency, and minimize environmental impact. The data collected also serves as a valuable resource for validating numerical models, enhancing their reliability and applicability in engineering practice. In this study, we meticulously adhered to experimental procedures using the described equipment to ensure accurate and reliable data collection. These efforts established a robust foundation for observations and subsequent analyses [19].
The numerical investigations performed in this work include different strategies devoted to the analysis of the fuel reactivity in the closed vessel, based on empirical correlations and detailed chemical kinetic mechanisms. LBV was calculated as a function of the unburned properties of the mixtures, as described below. The operative conditions (intended as fuel composition, fuel-to-oxidant ratios, initial temperatures and pressures) included in the experimental procedure were tested numerically, as well. Kinetic calculations for the evaluation of LBV for H2 and CH4 were performed using 3 kinetic models: the first was developed by the University of Bologna (KiBo_MU) [25], the second was from the University of San Diego (C.R. Group, 2016), and the third was from Lund University developed by Konnov et al. [26]. Each of them derives from diverse assumptions and target species, resulting in a significantly different number of species and reactions, as reported in Table 2.

Table 2.
The details of the kinetic mechanisms used in this study.
Simulations of laminar burning velocity were performed using an open-source Cantera suite with appropriate reactor modules using a transient condition as a first-attempt solution for the steady-state conditions. In addition, acceptance criteria for residuals were considered once steady-state (ss) and transient-state (ts) problems were solved. More specifically, the following values were imposed: absolute tolerance(ss) = 1.0 × 10−8; relative tolerance(ss) = 1.0 × 10−15; absolute tolerance(ts) = 1.0 × 10−4; relative tolerance(ts) = 1.0 × 10−13. An adaptive grid was determined by using the following criteria: a maximum acceptable ratio among adjacent solutions (ratio) equal to 3, maximum first derivative for adjacent solutions (slope) equal to 0.06, and a maximum acceptable second derivative for adjacent solutions (curve) equal to 0.12. Additional details on the reported numerical methodology can be found elsewhere.
Our data were compared with results combining experimental ones collected in this work, following the procedure described in the previous section, with empirical correlations. For both fuel mixtures, LBV was calculated from the experimental P-t history using equations provided by Dahoe, Zevenbergen, Lemkowitz, and Scarlett (commonly written as the DZLS model, [27] (Equation (1)).
where is the flame radius defined in Equation (2), V is vessel volume, is the initial pressure, P is the actual pressure, Pmax is the maximum explosion pressure, and is the adiabatic coefficient of the unburned gas. Considering the nature of the experiments, only the data related to the reactive phase were selected. Namely, pre-ignition and post-combustion data were neglected for the evaluation of LBV. In addition, since fuel reactivity is also a function of fuel composition in the fuel mixtures, LBV was also computed using a power law expression of fuel composition, using both temperature and pressure as reported by Dahoe et al. [14], as shown in Equation (3).
where LBV0 is the velocity at the reference pressure (i.e., P0) and temperature (i.e., T0), is the coefficient for temperature dependence, is the coefficient for pressure dependence, and is the equivalence ratio.
Other than the reactivity expressed as LBV, the kinetic mechanism considered so far was also selected to assess the pressure profile, too. To this aim, a constant-volume zero-dimensional reactor simulation with adiabatic and chemically inert walls in Cantera [23], using input pressures from experimental readings in closed vessels, was modeled. The investigation was performed at low initial temperatures (i.e., 298–423 K), which are lower than the auto-ignition temperature of the investigated mixtures. Hence, an ignition source needs to be considered in the numerical study, as well, to overcome the activation barrier and initiate the chemistry. To this end, in Cantera, spark ignition can be mimicked by artificially adding a pulsed flow of atomic hydrogen into the reaction mixtures. Once the reaction starts, it releases heat and produces highly reactive intermediates, apparently accelerating the reaction to produce ignition. Then, the igniter turns off once the system approaches steady burning. A pressure controller with a fixed volume is used for the simulation. Simulations were performed using the KiBo_MU detailed chemical kinetic model.
From the measured pressure–time P(t) histories, the deflagration index (KG) was quantified according to the classical cubic root equation:
where V is the vessel volume, and (dP/dt)max is the maximum rate of pressure rise. As can be seen from Equation (5), the rate of pressure rise monotonically increases with explosion pressure, and hence the maximum rate of pressure rise is attained when P = Pex (explosion pressure). So, by multiplying both sides of Equation (4) by V1/3, (dP/dt)max can be related to the laminar burning velocity as reported by Dahoe et al. [28], considering the dependency of the parameter on the size and shape of the vessel in addition to the maximum pressure rise and volume. In the same way, as reported in the literature [13,24,29,30,31], flame shape, structure, and flame speed are influenced by the size and shape of the testing chamber, eventually affecting the deflagration index KG.
where Pexp is the instantaneous explosion pressure at which the value of (dP/dt)max is reached. P0 is the initial pressure, and γ is the adiabatic coefficient of the unburned gas. Equation (5) was used to calculate the deflagration index for H2–air and CH4–air under the operative conditions tested experimentally in this work.
3. Results
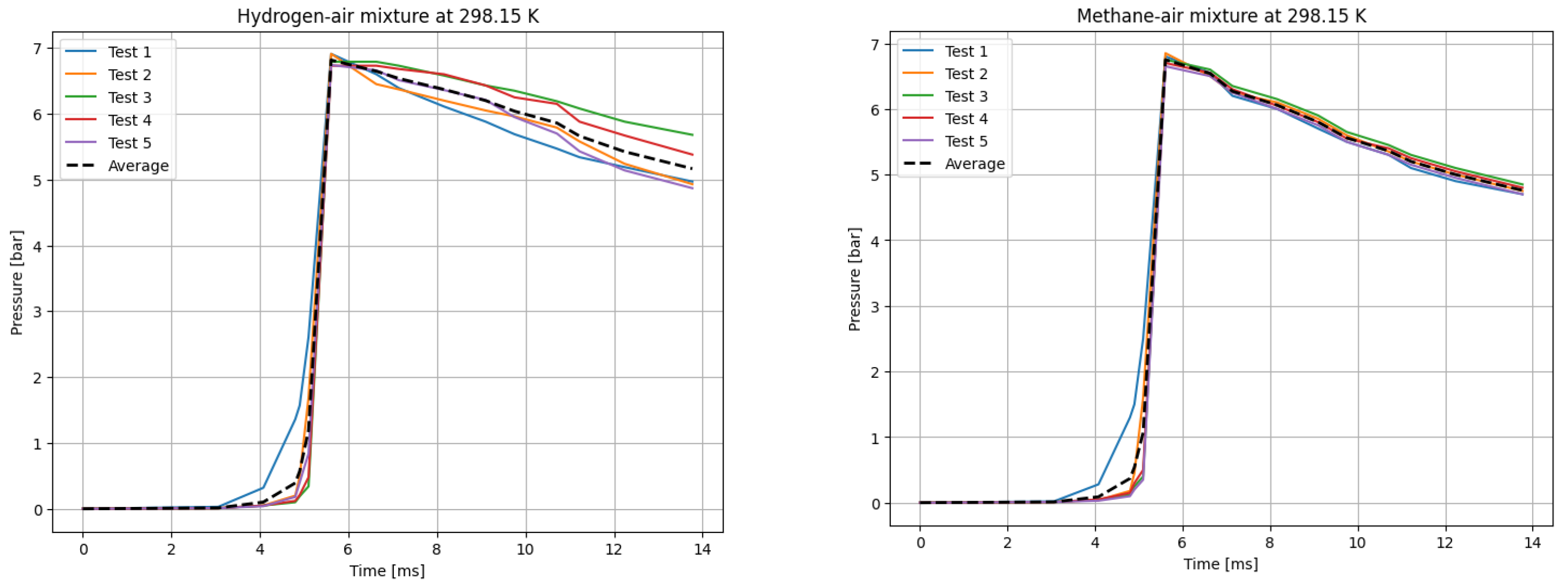
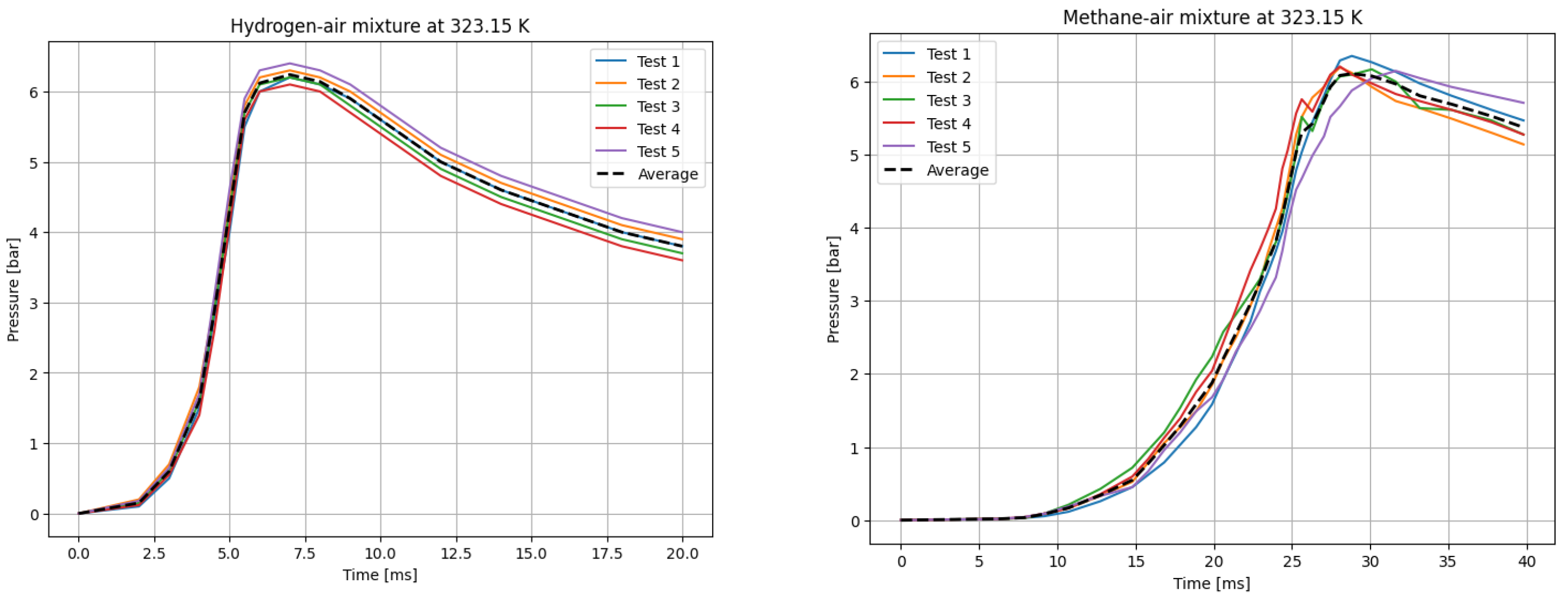
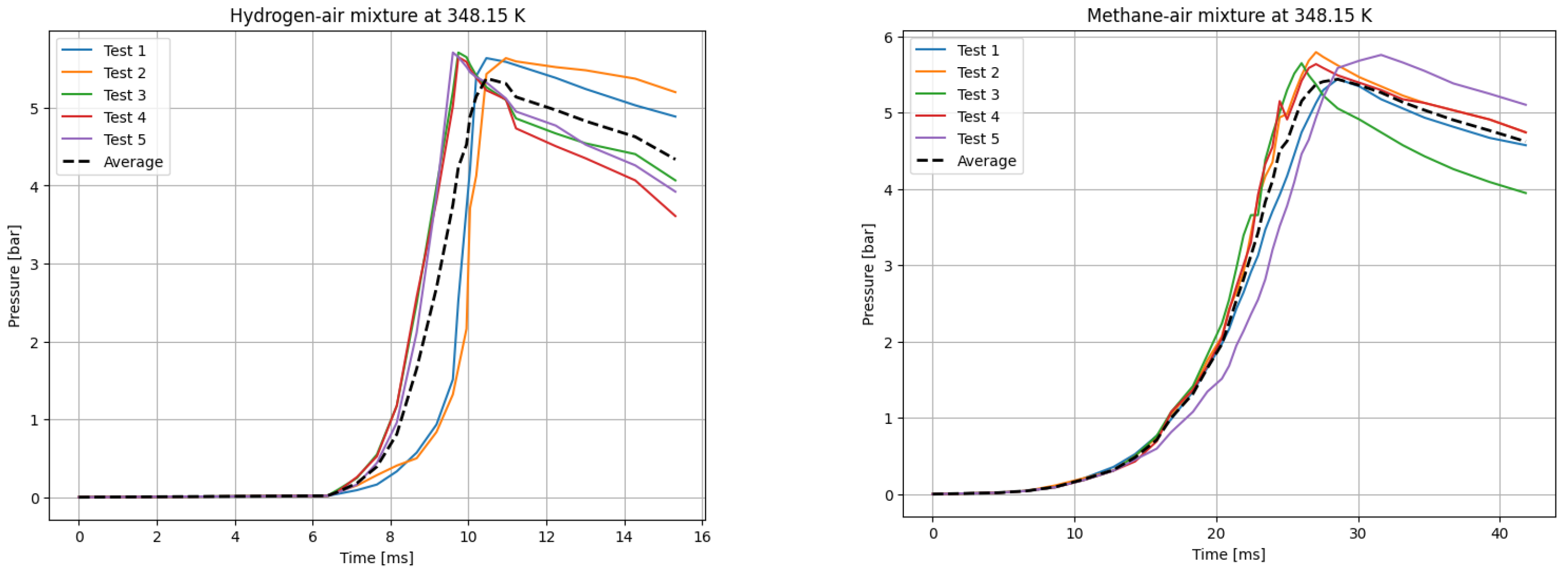
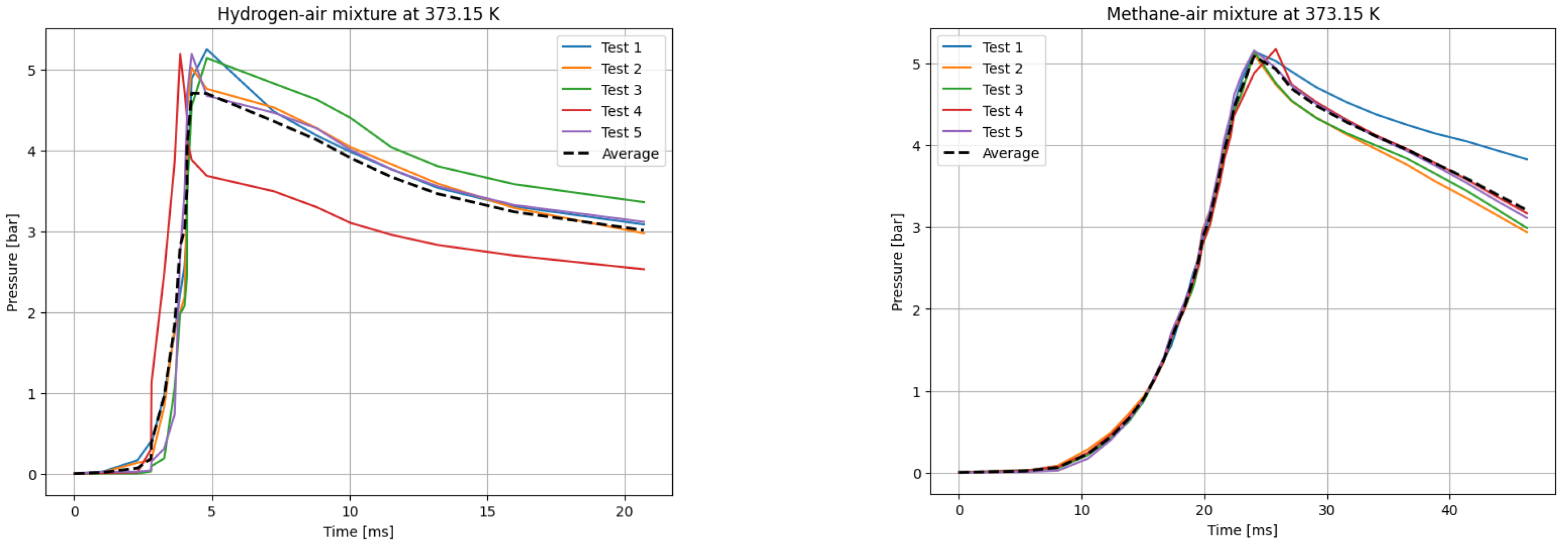
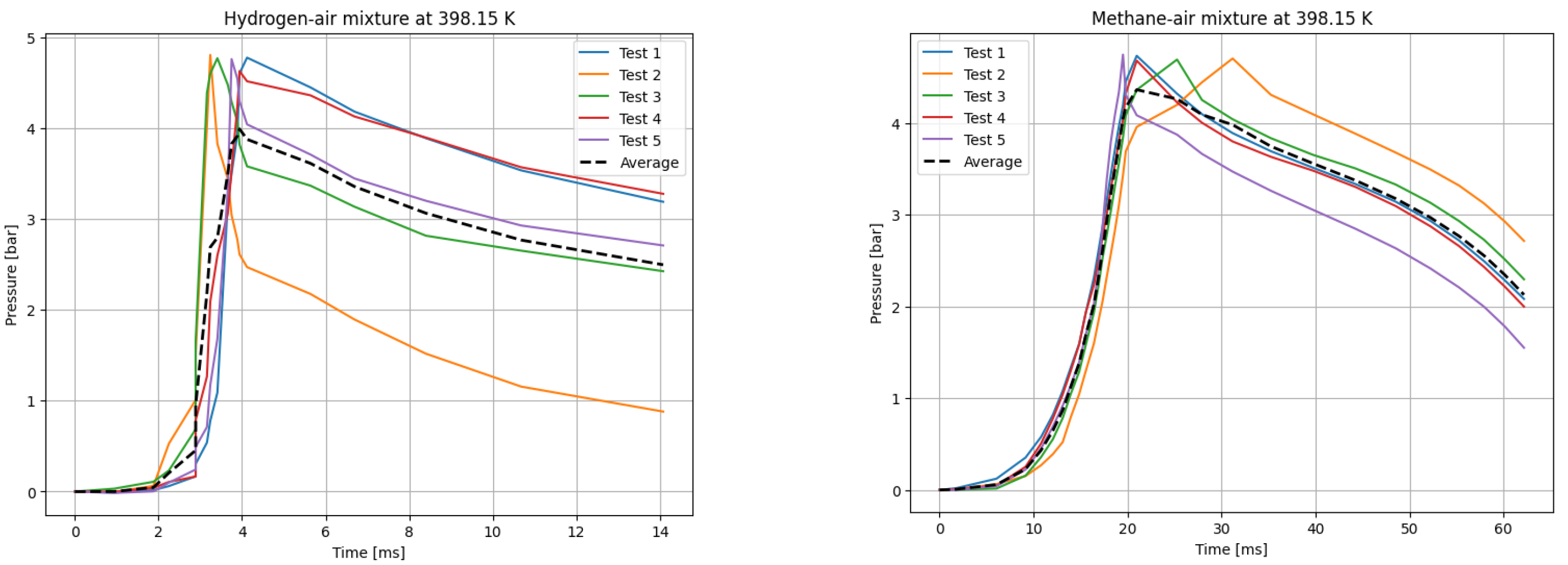
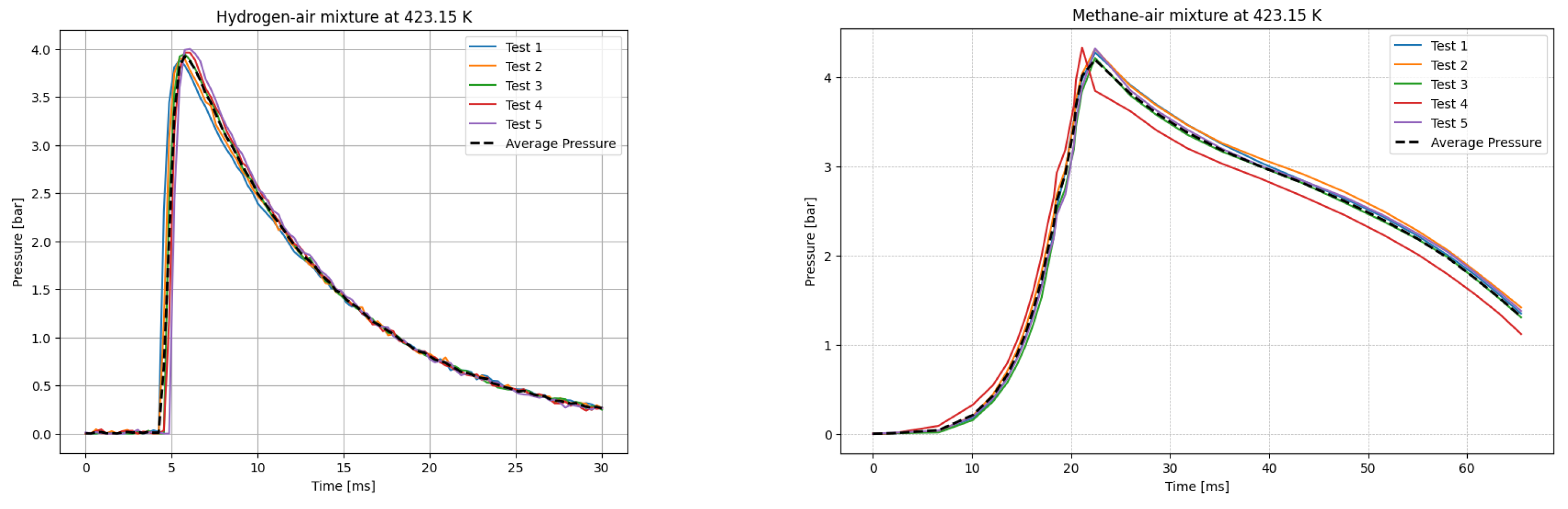
The deflagrations of H2 and CH4 with air at the stoichiometric ratio ( were carried out for six different initial temperatures of the mixtures. Five measurements were taken for each initial temperature T0, each according to the test procedure presented. The recording frequency of the reaction explosion parameters was maintained at 150 kHz. The initial pressure for each trial was P0 = 1 bar. The results of a single trial are plotted as a curve of explosion pressure increase over time. The summary curves of the five measurements for T0 = 298.15 K are shown in Figure 2, for T0 = 323.15 K in Figure 3, for T0 = 348.15 K in Figure 4, for T0 = 373.15 K in Figure 5, for T0 = 398.15 K in Figure 6, and for T0 = 423.15 K in Figure 7. The temperatures were chosen for the LBV test because they represent typical operating temperatures for many hydrogen- or methane-fueled systems. These temperatures are also easy to achieve and precisely controlled, making them ideal for laboratory testing.
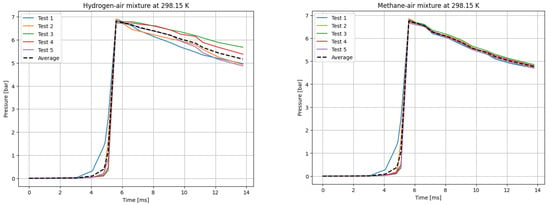
Figure 2.
P(t) curves for H2 (left) and CH4 (right) mixtures with air at T0 = 298.15 K.
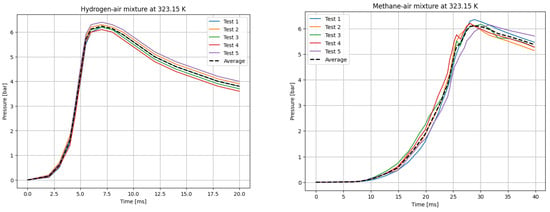
Figure 3.
P(t) curves for H2 (left) and CH4 (right) mixtures with air at T0 = 323.15 K.
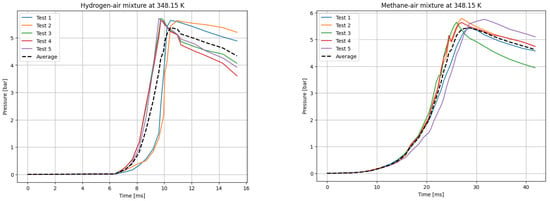
Figure 4.
P(t) curves for H2 (left) and CH4 (right) mixtures with air at T0 = 348.15 K.
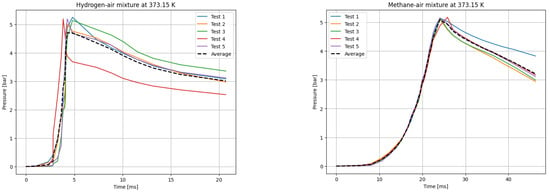
Figure 5.
P(t) curves for H2 (left) and CH4 (right) mixtures with air at T0 = 373.15 K.
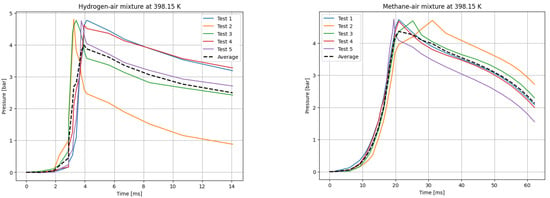
Figure 6.
P(t) curves for H2 (left) and CH4 (right) mixtures with air at T0 = 398.15 K.
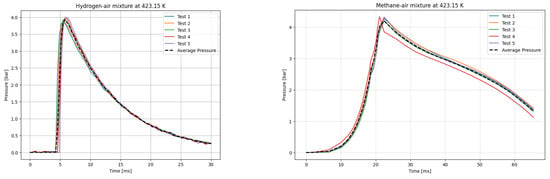
Figure 7.
P(t) curves for H2 (left) and CH4 (right) mixtures with air at T0 = 423.15 K.
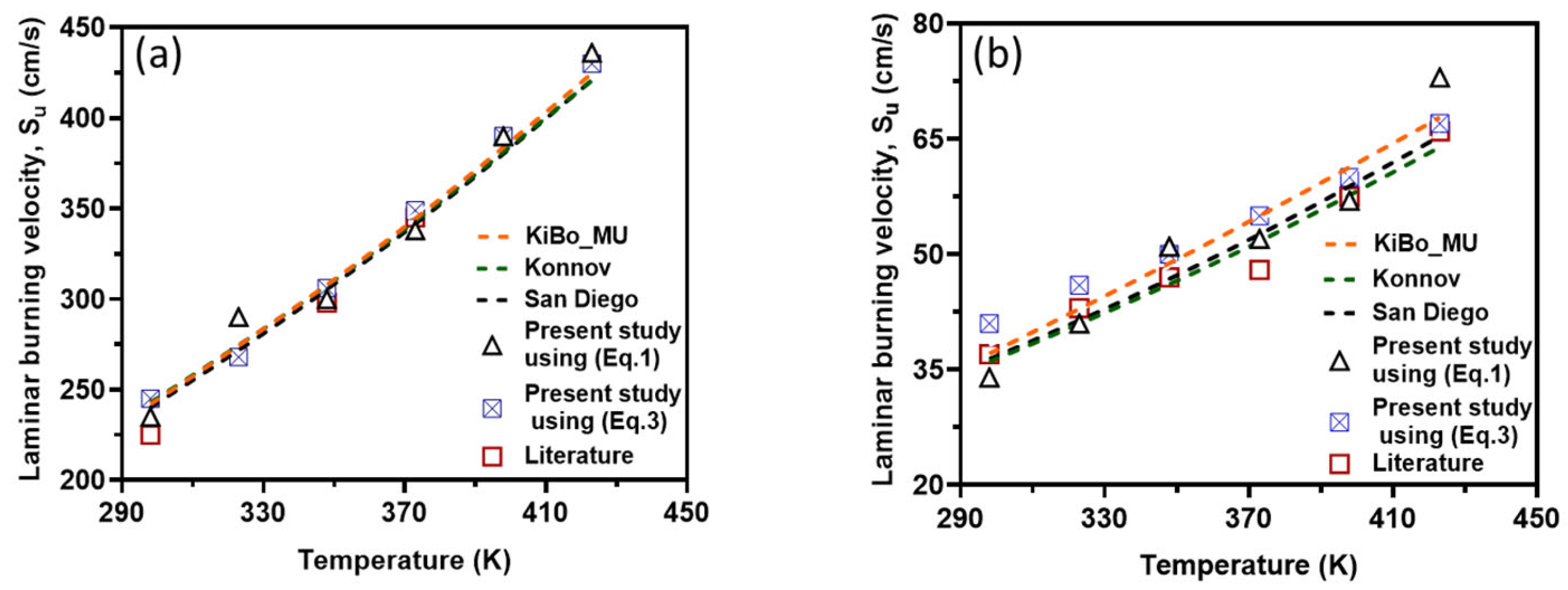
Figure 8 presents the variation in LBV at elevated reaction temperatures ranging from 298 to 423 K, as obtained from newly collected experimental data, numerical calculations using three kinetic mechanisms, and values reported in the literature.
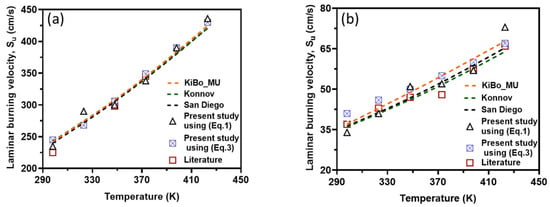
Figure 8.
The LBV of H2 (a) and CH4 (b) air mixtures obtained by different approaches versus the initial temperature. Symbols: Experimental data from the present work and the literature [6]. Broken lines: The simulation results from detailed mechanisms.
As depicted in the figure, the results obtained using both approaches (i.e., correlations based on experimental data and numerical simulations) exhibit strong agreement for both fuel components. This consistency validates the accuracy of both methods for computing LBV. Additionally, the experimental pressure–time history recorded in this study aligns well with both the literature-reported experimental results and simulation outcomes.
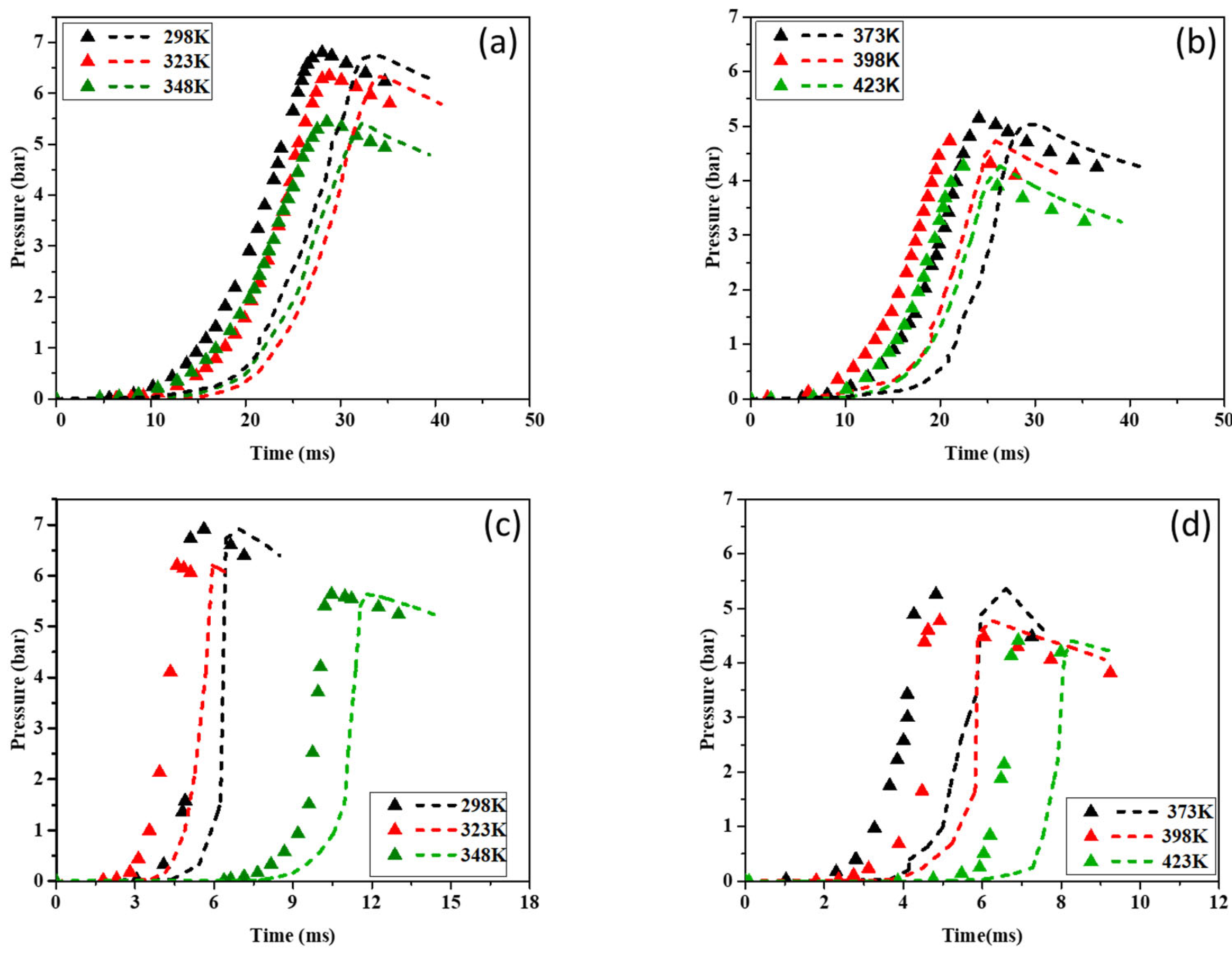
Figure 9 illustrates the P-t curves for CH4–air and H2–air mixtures over the studied temperature range (298–423 K). The simulated pressure–time histories generally follow the same trend as the experimental results across all tested temperatures, although minor deviations are observed. Notably, in graphs (a) and (b), ignition occurs later in the simulated cases compared to the experimental conditions.
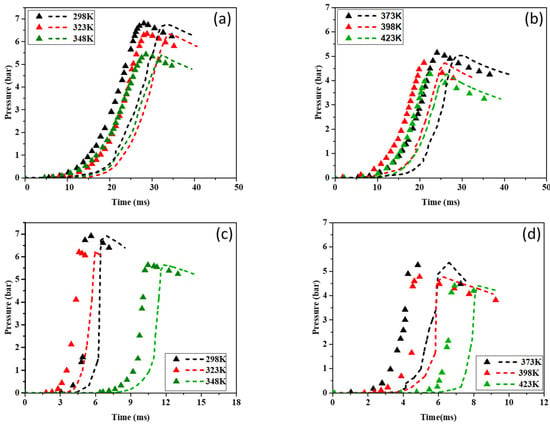
Figure 9.
Pressure rise in a closed vessel as a function of time for CH4–air (a,b) and H2–air (c,d) mixtures. Symbols: Experimental data. Broken lines: The simulation results.
In Figure 9 and Figure 10, the simulated ignition delays provided by all three mechanisms (KiBo_MU, San Diego, Lund) are systematically longer by approx. 5–15% than the experimental delays across 298–373 K. Several factors contribute to this offset:
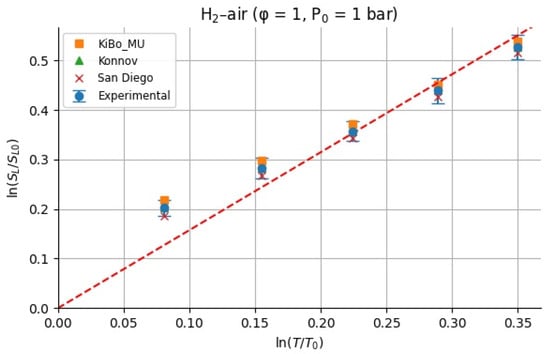
Figure 10.
Calculated for hydrogen–air mixture based on experiments and kinetics.
- The mechanisms differ in their third-body efficiencies and rate constants for key low-temperature branching reactions (e.g., H + O2 + M → HO2 + M). Underpredicting these rates leads to slower radical build-up and thus longer modeled delays, particularly below 323 K.
- The closed-vessel configuration may induce slight residual motion after spark initiation, enhancing mixing and effectively seeding the flame with radicals. Our one-dimensional reactor cannot capture these hydrodynamic effects.
- While our pressure transducers sample at 150 kHz, there remains a finite sensor lag (≈10–20 μs) that can marginally shift the experimental delay. We estimate that this contributes <2% uncertainty.
These factors explain most of the 5–15% ignition delay excess in the simulations. Future work could employ detailed ignition models and updated low temperature rate coefficients to reduce this discrepancy, improving the fidelity of ignition delay predictions in safety critical applications.
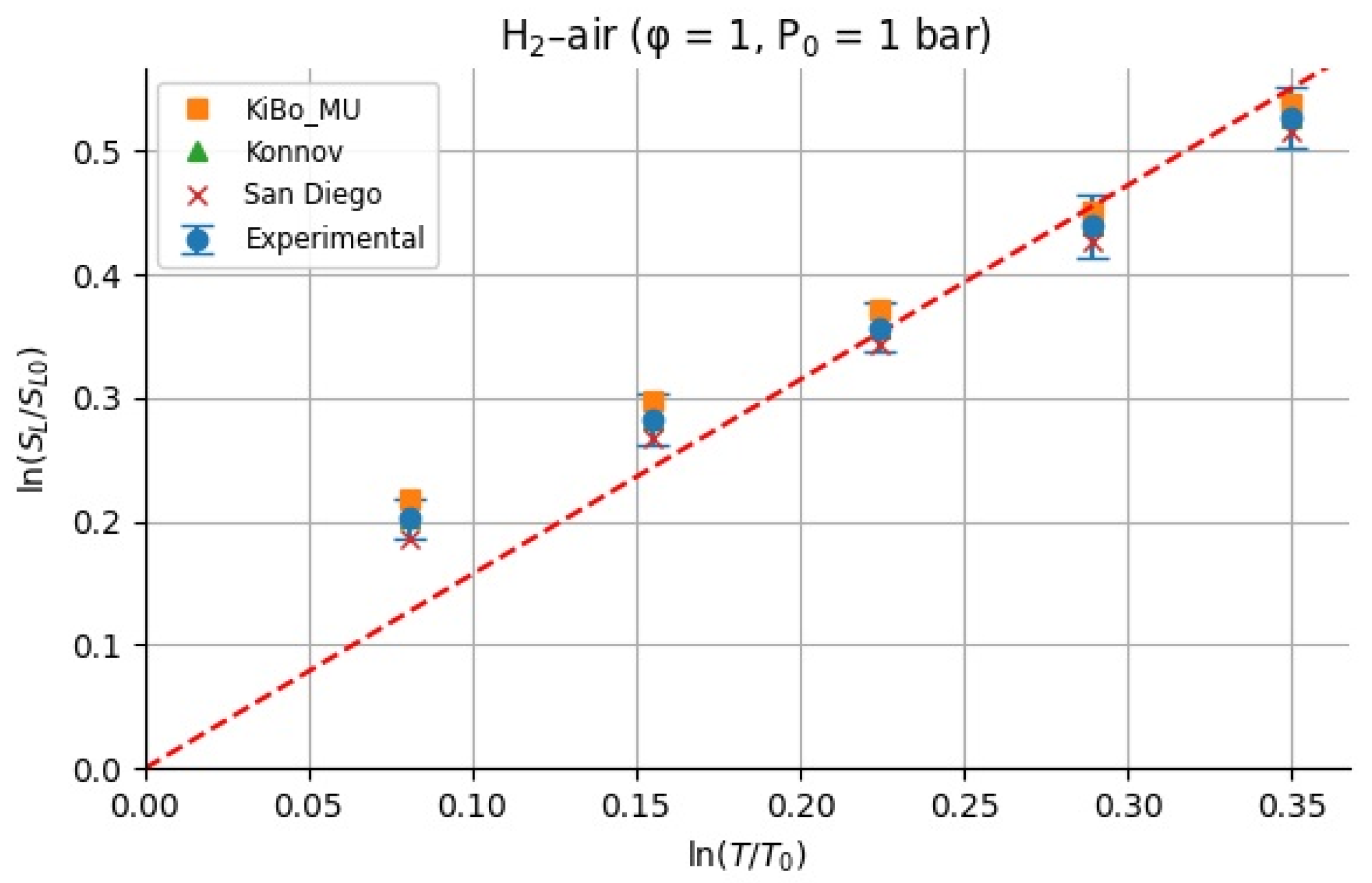
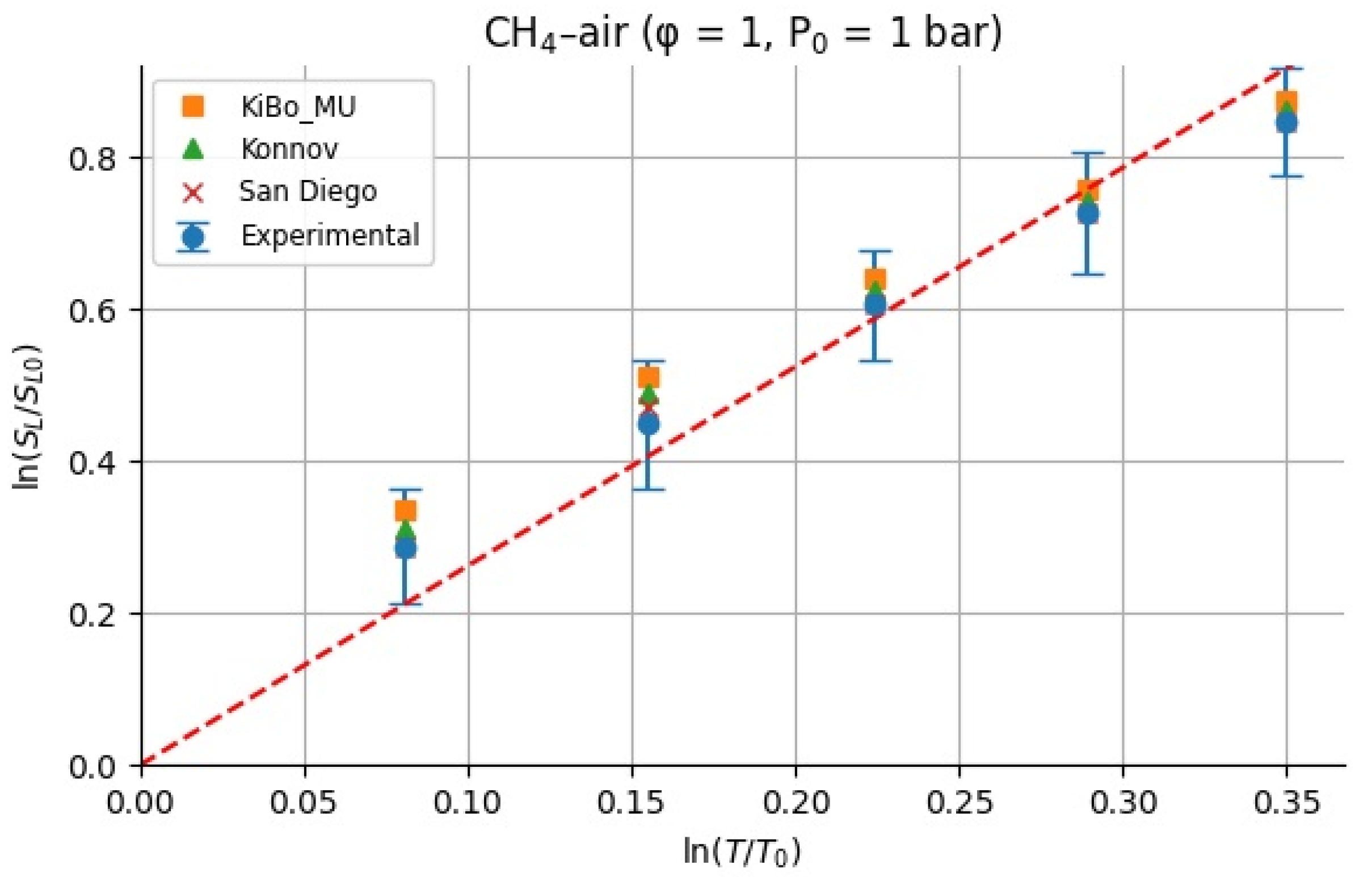
Because we only study the temperature dependence, the values of were calculated having considered the unity for the equivalence ratio. For each fuel (H2 and CH4), we assembled LBV data as a function of the initial temperature from the measured experimental pressures at φ = 1 and p = 1 atm [9] and computed LBV from three kinetic mechanisms (KiBo_MU, Konnov, San Diego) under the same conditions. We then chose a reference state at T0 = 298.15 K with reference LBV0 = LBV(T0) from the experimental dataset. For each higher temperature Ti in the set {323.15, 348.15, 373.15, 398.15, 423.15 K}, the local exponent αi was computed via the log–log definition:
Figure 10 and Figure 11 show the calculated for H2–air and CH4–air mixtures at the same range of initial temperatures as that for experiments and numerical simulations.
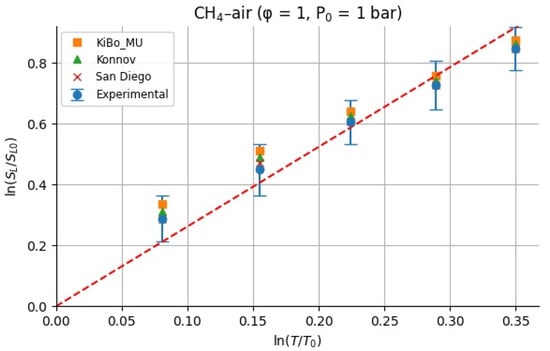
Figure 11.
Calculated for methane–air mixture based on experiments and kinetics.
All α values are shown in Table 3 and Table 4, where the average α and standard deviation σ over the temperature range are reported separately for the experiments and each mechanism. This provides insight into how the sensitivity exponent α varies (or remains approximately constant) with temperature.

Table 3.
Details of for H2–air mixture ( = 1; P0 = 1 bar).

Table 4.
Details of for CH4–air mixture ( = 1; P0 = 1 bar).
For stoichiometric H2–air mixtures at φ = 1 and 1 atm, ref. [32] compiled reported α values around 1.54–1.72. Although some studies fit data over different temperature intervals and methods, a mid-range near ≈1.6 is common. In our study, from experimental data, α may appear higher at lower T increments (e.g., 2.5 at 323 K) but declines toward 1.5 at 423 K; the resulting average over 323–423 K is 1.79 ± 0.38. Kinetic mechanisms (KiBo_MU, Konnov, San Diego) yield similar local averages, indicating consistency with experimental trends. A global ln–ln regression through zero over all combined points gives an effective exponent αfit = 1.66, in line with the literature mid-range around 1.6. For stoichiometric CH4–air mixtures at φ = 1 and 1 bar, ref. [32,33] showed α values between roughly 1.58 and 2.0, with many well-controlled studies clustering near 1.7–1.9. Our calculated α, derived from experimental data, decreases from higher values at moderate T to 1.5–1.6 at 423 K, yielding an average over 323–423 K around 1.7–1.8 with moderate scatter. Simulation mechanisms agree closely with the experiments at each temperature. In both fuels, the agreement between the literature-reported α ranges [32] and our combined experimental/simulation exponents confirms that detailed kinetic models capture the temperature sensitivity of LBV well in 298–423 K. Using an average exponent (e.g., 1.66 for H2–air, 1.75 for CH4–air) is acceptable for moderate temperature spans, but one should be cautious when extrapolating beyond ~450 K, where fewer data exist, and α may differ a lot. That means that more experimental studies are needed for higher initial temperatures.
A larger α implies that small temperature excursions (e.g., hot-day ambient conditions) can substantially accelerate flame spread and increase deflagration severity, underscoring the importance of using temperature-resolved LBV data when sizing vents or modeling accidental releases.
4. Discussion
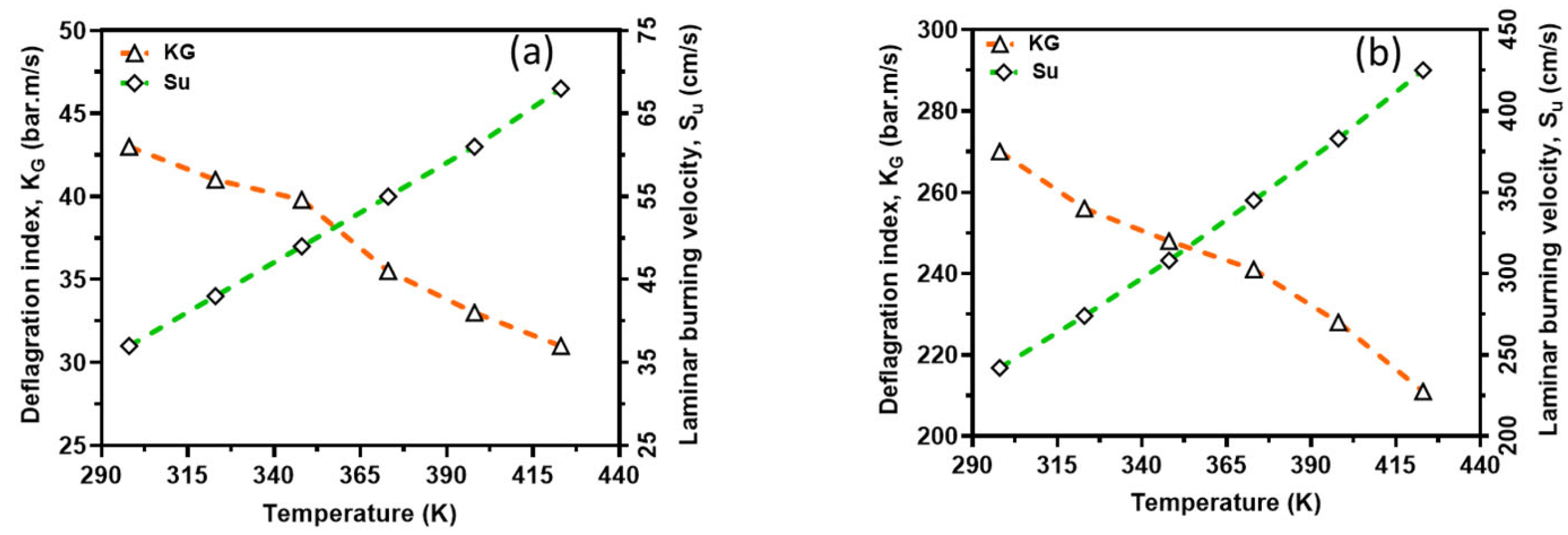
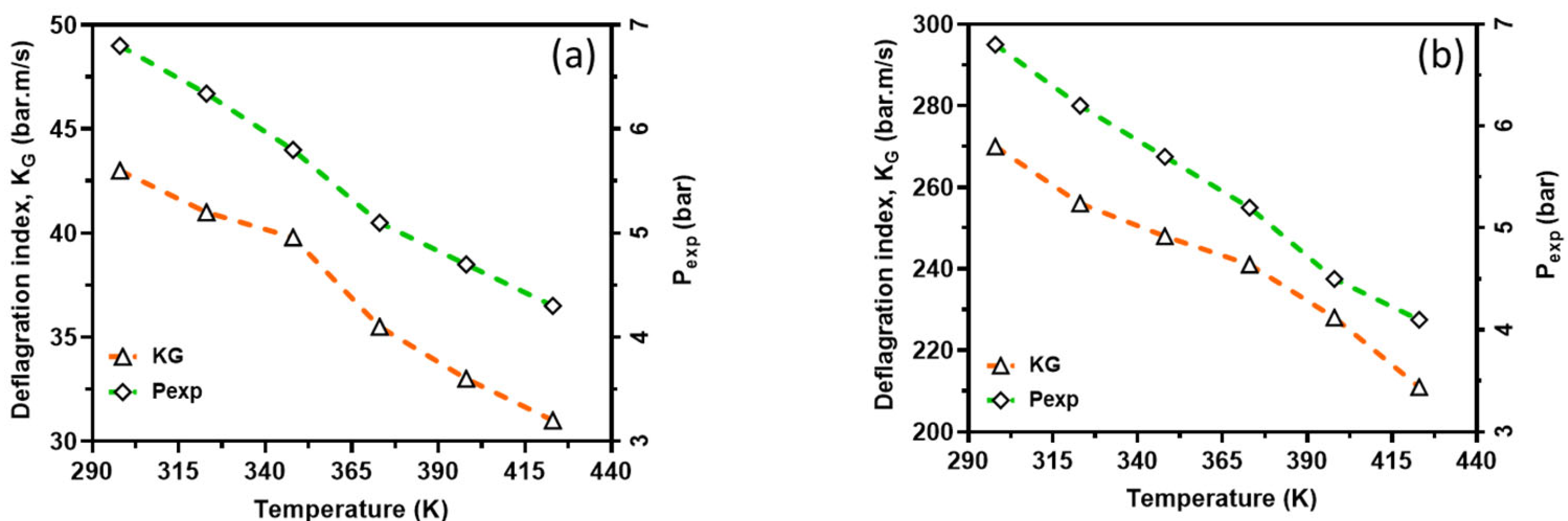
In order to capture the intrinsic reactivity of CH4–air and H2–air mixtures, the deflagration index was obtained from the P(t) history of a 20 L closed explosion vessel together with LBV and is shown in Figure 12. From this figure, the deflagration index showed an inverse relationship with the reaction temperatures, whereas laminar burning velocity showed a direct relationship with the reaction temperatures. This is because the initial temperature has a great influence on the explosion pressure, consequently dictating the maximum rate of pressure rise in the vessel and, therefore, the deflagration index. In other words, an increasing initial temperature has an inverse relationship with explosion pressure and thus KG. However, there is too much inconsistency in the measured deflagration index, as reported by several authors [34,35,36,37], ranging from 30 to 86 bar*m/s for CH4–air and 215 to 1100 bar*m/s for H2–air mixtures under standard conditions. The present study’s index lies in the range experimentally reported in the literature. For instance, for methane, the closest experimental results reported in the literature to those calculated are from the project SAFEKINEX [37]. From this observation, it can be said that the deflagration index and LBV are sensitive to initial temperatures, which are consistent with the literature. Overall, the deflagration index showed an inverse relationship with flame propagation. Furthermore, the relationship between the explosion pressure and the deflagration index is shown in Figure 13 within the studied temperature ranges. It can be seen from this figure that the deflagration index has a direct relationship with the explosion pressure and the maximum pressure rise rate, because the maximum rate for pressure rise has a similar relationship with the maximum explosion pressure.
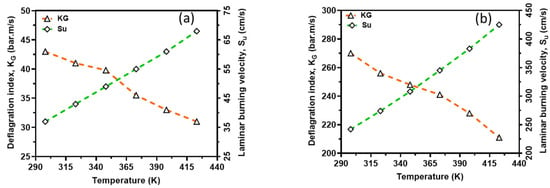
Figure 12.
Comparison of deflagration index and laminar burning velocity; CH4–air (a) and H2–air (b). Symbols: Experimental data. Broken lines: Simulation results.
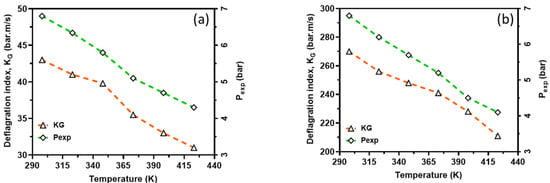
Figure 13.
Deflagration index as function of actual explosion pressure; CH4–air (a) and H2–air (b). Symbols: Experimental data. Broken lines: Simulation results.
The investigation into the deflagration dynamics of H2 and CH4 mixtures provides valuable insights into the combustion characteristics and safety implications of using these gases as alternative energy sources. The results of this study highlight several critical aspects that can influence the efficiency, safety, and environmental impact of combustion processes involving H2 and CH4. One significant finding is the variation in LBV with temperature for both H2–air and CH4–air mixtures. The experimental data, supported by numerical calculations, demonstrate that LBV increases exponentially with temperature. This increase in LBV at higher temperatures suggests the enhanced reactivity of the fuel mixtures, which is crucial for optimizing combustion processes in practical applications. The agreement between the experimental results and numerical simulations further validates the reliability of the kinetic models used in this study.
The deflagration index was observed to have an inverse relationship with temperature, which contrasts with the direct relationship between LBV and temperature. This inverse relationship indicates that higher initial temperatures lead to a lower maximum rate of pressure rise during combustion, which could influence the design and safety measures of combustion systems. The deflagration index values obtained in this study are consistent with those reported in the literature, reinforcing the validity of the experimental approach and findings.
The P(t) histories for both H2–air and CH4–air mixtures provide a detailed understanding of the combustion dynamics. The observed trends in pressure rise and the time to ignition underscore the importance of precise control over the initial conditions, such as temperature and mixture composition, to achieve desired combustion outcomes. The discrepancies between experimental and numerical ignition times suggest potential areas for improving the accuracy of kinetic models, particularly in simulating the initial stages of combustion.
Moreover, Konnov et al. [32] reported an α value in the range 1.54–1.72 for stoichiometric H2–air over 298–423 K, obtained via planar flame heat flux burners. Our slightly higher value (α = 1.79) can be attributed to differences because of the following:
- Pressure-based extraction in a closed vessel may sample a slightly earlier, more stretched flame regime, enhancing the apparent T sensitivity;
- Our mixtures at elevated T and P corrections can increase m in DT ∝ Tm;
- Small discrepancies in radical pool modeling (e.g., third-body efficiencies) can shift the local activation energy.
Taken together, these factors account for the modest 5–15% difference between our α and Konnov’s range and confirm overall consistency within experimental uncertainty.
Also, the LBV and deflagration index (KG) data reported here carry value for industrial safety practice. In particular, the following can be found:
- Current standards (e.g., NFPA 68, EN 14491) require knowledge of KG to calculate the necessary vent area and panel design. Our temperature-resolved KG(T) curves (Figure 13) allow engineers to select conservative vent dimensions for conditions up to 423 K, rather than relying on ambient-only data.
- By providing LBV(T, ϕ = 1) for H2–air and CH4–air, safety engineers can more accurately assign equipment and facility zoning requirements under worst-case hot-day scenarios.
- Quantitative LBV versus temperature functions enhance the fidelity of CFD-based deflagration models, improving the predictions of overpressure loads and flame acceleration phenomena in complex geometries.
- An agreement of three independent mechanisms against our data builds confidence in using these models within process safety simulations.
5. Conclusions
This study about the temperature dependence on the deflagration dynamics of H2 and CH4 mixtures offers several key conclusions that are critical for advancing the use of these gases in applications of energy and fuel technologies.
Overall, this study contributes to the foundational understanding of the combustion dynamics of H2 and CH4 mixtures at elevated temperatures, providing a basis for further research and development in the field of process industries.
Finally, our combined dataset of temperature-dependent LBV and KG not only advances the scientific understanding of mixture reactivity but also provides data for explosion safety engineers. The adoption of these values in vent sizing calculations, hazardous area classification, and CFD-based models will yield more accurate and temperature-resilient safety margins in industrial applications.
Author Contributions
Conceptualization, R.P., E.S. and G.P.; methodology, R.P. and E.S.; software, F.M.W. and T.G.; validation, T.G., F.M.W. and R.K.; formal analysis, R.K., V.J. and T.G.; investigation, R.P., G.P., F.M.W., T.G. and V.J.; resources, R.P., E.S. and V.J.; data curation, E.S., R.P. and V.J.; writing—original draft preparation, R.K., R.P., F.M.W. and T.G.; writing—review and editing, E.S., G.P. and V.J.; visualization, R.K., F.M.W. and T.G.; supervision, E.S.; project administration, R.P.; funding acquisition, E.S. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a subsidy from the Polish Ministry of Science and Higher Education for Jan Kochanowski University of Kielce, under research grant no. SUPB.RN.25.057.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This work was supported by the European Union under the Horizon Europe project CESAR (Centre of Excellence for Safety Research, GA No 101186946).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brusca, S.; Lanzafame, R.; Garrano, A.M.C.; Messina, M. Effects of Pressure, Temperature and Dilution on Fuels/Air Mixture Laminar Flame Burning Velocity. Energy Procedia 2015, 82, 125–132. [Google Scholar] [CrossRef]
- Luo, C.; Yu, Z.; Wang, Y.; Ai, Y. Experimental Investigation of Lean Methane–Air Laminar Premixed Flames at Engine-Relevant Temperatures. ACS Omega 2021, 6, 17977–17987. [Google Scholar] [CrossRef]
- Milton, B.E.; Keck, J.C. Laminar burning velocities in stoichiometric hydrogen and hydrogen-hydrocarbon gas mixtures. Combust. Flame 1984, 58, 13–22. [Google Scholar] [CrossRef]
- Salzano, E.; Cammarota, F. A Di Benedetto, V Di Sarli Explosion behavior of hydrogen–methane/air mixtures. J. Loss Prev. Process Ind. 2012, 25, 443–447. [Google Scholar] [CrossRef]
- Skrinsky, J. Calculation and Experimental Validation of Pressure and Temperature Effects on COG-Air Fuel Mixtures. ITM Web Conf. 2018, 16, 03003. [Google Scholar] [CrossRef]
- Porowski, R.; Kowalik, R.; Nagy, S.; Gorzelnik, T.; Szurlej, A.; Grzmiączka, M.; Zielińska, K.; Dahoe, A. Deflagration Dynamics of Methane–Air Mixtures in Closed Vessels at Elevated Temperatures. Energies 2024, 17, 2855. [Google Scholar] [CrossRef]
- Wako, F.M.; Pio, G.; Salzano, E. The effect of hydrogen addition on low-temperature combustion of light hydrocarbons and alcohols. Energies 2020, 13, 3808. [Google Scholar] [CrossRef]
- Movileanu, C.; Gosa, V.; Razus, D. Explosion of gaseous ethylene–air mixtures in closed cylindrical vessels with central ignition. J. Hazard. Mater. 2012, 235–236, 108–115. [Google Scholar] [CrossRef]
- Cammarota, F.; Di Benedetto, A.; Di Sarli, V.; Salzano, E.; Russo, G. Combined effects of initial pressure and turbulence on explosions of hydrogen-enriched methane/air mixtures. J. Loss Prev. Process Ind. 2009, 22, 607–613. [Google Scholar] [CrossRef]
- Pio, G.; Salzano, E. Laminar burning velocity of methane, hydrogen, and their mixtures at extremely low-temperature conditions. Energy Fuels 2018, 32, 8830–8836. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Jin, C.; Zheng, J. Experimental and numerical study on laminar burning characteristics of premixed methane–hydrogen–air flames. Int. J. Hydrogen Energy 2009, 34, 4876–4888. [Google Scholar] [CrossRef]
- Nilsson, E.J.K.; van Sprang, A.; Larfeldt, J.; Konnov, A.A. The comparative and combined effects of hydrogen addition on the laminar burning velocities of methane and its blends with ethane and propane. Fuel 2017, 189, 369–376. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Duva, B.C.; Chance, L.E.; Toulson, E. Dilution effect of different combustion residuals on laminar burning velocities and burned gas Markstein lengths of premixed methane/air mixtures at elevated temperature. Fuel 2020, 267, 117153. [Google Scholar] [CrossRef]
- Ueda, A.; Nisida, K.; Matsumura, Y.; Ichikawa, T.; Nakashimada, Y.; Endo, T.; Kim, W. Effects of hydrogen and carbon dioxide on the laminar burning velocities of methane–air mixtures. J. Energy Inst. 2021, 99, 178–185. [Google Scholar] [CrossRef]
- AL-Khafaji, M.; Yang, J.; Tomlin, A.S.; Thompson, H.M.; de Boer, G.; Liu, K.; Morsy, M.E. Laminar burning velocities and Markstein numbers for pure hydrogen and methane/hydrogen/air mixtures at elevated pressures. Fuel 2023, 354, 129331. [Google Scholar] [CrossRef]
- Varghese, R.J.; Kolekar, H.; Kishore, V.R.; Kumar, S. Measurement of laminar burning velocities of methane-air mixtures simultaneously at elevated pressures and elevated temperatures. Fuel 2019, 257, 116120. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.; Jin, Y.; Zhu, X. Investigation of the influence of DMMP on the laminar burning velocity of methane/air premixed flames. Fuel 2019, 235, 1294–1300. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Fu, J.; Liu, J. Numerical study of the effect of CO2 H2O dilution on the laminar burning velocity of methane/air flames under elevated initial temperature and pressure. Can. J. Chem. Eng. 2023, 101, 4092–4105. [Google Scholar] [CrossRef]
- Halter, F.; Tahtouh, T.; Mounaïm-Rousselle, C. Nonlinear effects of stretch on the flame front propagation. Combust. Flame 2010, 157, 1825–1832. [Google Scholar] [CrossRef]
- Ihme, M.; Chung, W.T.; Mishra, A.A. Combustion machine learning: Principles, progress and prospects. Prog. Energy Combust. Sci. 2022, 91, 101010. [Google Scholar] [CrossRef]
- Akram, M.; Saxena, P.; Kumar, S. Laminar Burning Velocity of Methane–Air Mixtures at Elevated Temperatures. Energy Fuels 2013, 27, 3460–3466. [Google Scholar] [CrossRef]
- Ghosh, A.; Munoz-Munoz, N.M.; Chatelain, K.P.; Lacoste, D.A. Laminar burning velocity of hydrogen, methane, ethane, ethylene, and propane flames at near-cryogenic temperatures. Appl. Energy Combust. Sci. 2022, 12, 100094. [Google Scholar] [CrossRef]
- Mitu, M.; Razus, D.; Schroeder, V. Laminar Burning Velocities of Hydrogen-Blended Methane–Air and Natural Gas–Air Mixtures, Calculated from the Early Stage of p(t) Records in a Spherical Vessel. Energies 2021, 14, 7556. [Google Scholar] [CrossRef]
- Wako, F.M.; Pio, G.; Salzano, E. Modeling Formic Acid Combustion. Energy Fuels 2022, 36, 14382–14392. [Google Scholar] [CrossRef]
- Konnov, A.A.; Mohammad, A.; Kishore, V.R.; Kim, N.; Prathap, C.; Kumar, S. A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel+air mixtures. Prog. Energy Combust. Sci. 2018, 68, 197–267. [Google Scholar] [CrossRef]
- Dahoe, A.E.; Zevenbergen, J.F.; Lemkowitz, S.M.; Scarlett, B. Dust explosions in spherical vessels: The role of flame thickness in the validity of the ‘cube-root law’. J. Loss Prev. Process Ind. 1996, 9, 33–44. [Google Scholar] [CrossRef]
- Dahoe, A.E. Laminar burning velocities of hydrogen–air mixtures from closed vessel gas explosions. J. Loss Prev. Process Ind. 2005, 18, 152–166. [Google Scholar] [CrossRef]
- Ajrash, M.J.; Zanganeh, J.; Moghtaderi, B. Methane-coal dust hybrid fuel explosion properties in a large scale cylindrical explosion chamber. J. Loss Prev. Process Ind. 2016, 40, 317–328. [Google Scholar] [CrossRef]
- Saeed, K. Determination of the explosion characteristics of methanol—Air mixture in a constant volume vessel. Fuel 2017, 210, 729–737. [Google Scholar] [CrossRef]
- Sun, Z.-Y. Experimental studies on the explosion indices in turbulent stoichiometric H2/CH4/air mixtures. Int. J. Hydrogen Energy 2019, 44, 469–476. [Google Scholar] [CrossRef]
- Konnov, A.A. The effect of temperature on the adiabatic laminar burning velocities of CH4-air and H2-air flames. Fuel 2010, 89, 2211–2216. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Wang, S.; Whiddon, R.; He, Y.; Lv, Y.; Konnov, A.A. Parametrization of the temperature dependence of laminar burning velocity for methane and ethane flames. Fuel 2019, 239, 1028–1037. [Google Scholar] [CrossRef]
- Cashdollar, K.L.; Zlochower, I.A.; Green, G.M.; Thomas, R.A.; Hertzberg, M. Flammability of methane, propane, and hydrogen gases. J. Loss Prev. Process Ind. 2000, 13, 327–340. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Q.; Chen, J.; Huang, Y.; Shi, Y. Effects of hydrogen on combustion characteristics of methane in air. Int. J. Hydrogen Energy 2014, 39, 11291–11298. [Google Scholar] [CrossRef]
- Mittal, M. Explosion pressure measurement of methane-air mixtures in different sizes of confinement. J. Loss Prev. Process Ind. 2017, 46, 200–208. [Google Scholar] [CrossRef]
- Pasman, H.J.; Pekalski, A.; Braithwaite, M. To enable a better CAPE: The EU SAFEKINEX project. Comput. Aided Chem. Eng. 2005, 20, 355–360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).