Abstract
The biomass-to-liquid process is a promising alternative for sustainably meeting the growing demand for liquid fuels. This study focuses on the fabrication, characterization, and performance of a structured iron catalyst for producing hydrocarbons through Fischer–Tropsch synthesis (FTS). The catalyst was designed to address some drawbacks of conventional supported catalysts, such as low utilization, poor activity, and instability. The experimental investigation involved the manufacturing and characterization of both promoted and unpromoted iron-based catalysts. The performance of the structured iron catalyst was assessed in a fixed-bed reactor under relevant industrial conditions. Notably, the best results were achieved with a syngas ratio typical of the gasification of lignocellulosic biomass, where the catalyst exhibited superior catalytic activity and selectivity toward desired hydrocarbon products, including light olefins and long-chain paraffins. The resulting structured catalyst achieved up to 95% CO conversion in a single pass with 5% selectivity for CH4. The results indicate that the developed structured iron catalyst has considerable potential for efficient and sustainable hydrocarbon production via the Fischer–Tropsch synthesis. The catalyst’s performance, enhanced stability, and selectivity present promising opportunities for its application in large-scale hydrocarbon synthesis processes.
1. Introduction
Increased fuel prices, demand, and uncertainty regarding the secure supply of fuels have led to a review of various technologies related to converting coal, natural gas, and biomass into high-value fuels [1,2,3,4,5,6]. Fischer–Tropsch synthesis is one of the most well-known technologies for producing low-carbon-intensity fuels from synthesis gas, which can be produced from renewable energy sources such as biomass, thereby helping to satisfy the growing demand for fuel [7,8,9,10,11]. Additionally, the synthetic fuels produced by this technology have a low sulfur and aromatic content compared to gasoline and diesel obtained from petroleum [3]. The interest in this fuel type stems from the rise of a decentralized energy matrix and synthetic fuel production setups that do not rely on traditional fossil fuel networks [1,12,13]. Optimization is required to make this technology viable with biomass [11,13,14].
Depending on the catalyst and reaction conditions, Fischer–Tropsch synthesis can produce a wide range of hydrocarbons, such as light hydrocarbons, gasoline, diesel, and paraffins. The metals that exhibit the necessary activity for commercial Fischer–Tropsch applications include iron, nickel, cobalt, and ruthenium. For industrial applications, ruthenium and nickel are often discarded; the former is due to its high cost and low availability, and the latter is due to its production of a large amount of methane under operating conditions [7,15]. Although cobalt is considered the most effective catalyst for Fischer–Tropsch synthesis at an industrial level (due to its high activity and resistance to deactivation), iron should also be regarded as a viable alternative. It enables the conversion of synthesis gas with a low H2/CO ratio and can produce light hydrocarbons and olefins at moderate temperatures (250–270 °C). Furthermore, its reduction occurs at the same operating temperatures as the FTS (260–280 °C), while it is significantly more abundant and cheaper than cobalt. If an iron catalyst can be synthesized with a 5-fold higher activity per unit reactor volume, it would outperform the current cobalt-based catalysts [1,16]. Research indicates that impregnated iron catalysts demonstrate greater stability, a lower deactivation rate, and improved selectivity for long-chain hydrocarbons than precipitated iron catalysts [15,17]. However, precipitated iron-based catalysts are typically reported to have better activity [18,19,20,21], due to the higher level of reduction achieved with these catalysts and the higher Fe loading within the catalyst [22,23,24]. On the other hand, precipitated or impregnated catalysts also have disadvantages related to the dispersion of the metal on the support, the availability of active sites, the reducibility of the catalytic phase, and the stability of the catalyst [25,26,27]. Hence, numerous reports mention using different compounds during the precipitation, coprecipitation, or impregnation phase to control the deposition of the active phase, its dispersion, and the number of active sites. One of the trends to improve these drawbacks in Fischer–Tropsch processes is the use of meso-macroporous catalysts which show greater activity and lower selectivity to methane, possibly because the meso-macroporous structure reduces resistance to the diffusion of the species inside the catalyst, which not only leads to high catalytic activity, but also high stability as compared to mesoporous Fe2O3 catalysts that do not have macropores. Furthermore, the change in selectivity can be directly related to the size of the pores, since catalysts with small pores tend to produce lighter hydrocarbons. In contrast, macroporous ones usually favor the growth of the carbon chain [28]. This shows that diffusion limitations may play a crucial role in the activity and selectivity of the Fischer–Tropsch synthesis catalysts. Thus, considerable progress is required in developing new catalytic systems and promoters for enhanced activation and efficiency [29,30,31,32,33].
Some disadvantages of precipitated or impregnated catalysts could be addressed with a structured iron catalyst, which might provide a higher active site density along with a very low pressure drop [14,24,34,35,36,37,38,39]. The large surface area-to-volume ratio may reduce the effects of diffusion limitations while enhancing the heat transfer rate within the catalytic reactor [40,41,42,43].
Enhancing surface areas within compact reactor volumes increases void space while reducing pressure drop, a critical operational consideration in commercial settings [44]. A structured catalyst’s narrow residence time distribution may enable targeted product selectivity [45,46,47,48,49]. Hence, our work aims to study the catalytic performance of structured iron catalysts, examining the interplay between void fraction, promoter influence, and overall process efficiency through the analysis of CO and H2 conversion, selectivity to various hydrocarbons, and stability over prolonged operation times.
2. Materials and Methods
2.1. Catalyst Preparation and Characterization
2.1.1. Catalyst Manufacture
This study used iron structures as catalysts for the Fischer–Tropsch Synthesis (FTS). They were produced by replicating 3D-printed templates using a powder metallurgy technique known as sponge replication. The 3D-printed templates were cylinders (25 × 25 mm) generated using one of the predetermined infill pattern settings on the 3D printer (Flsun V400, Zhengzhou, China). The filament to produce the templates was Markforged 800cc Onyx Filament Spool purchased from A.W. Miller (East Aurora, NY, USA). A photograph of the templates is shown in Figure 1. The infill pattern used was Gyroid, and the infill density (the empty space between the material) was 28% and 35%.

Figure 1.
Notably, 3D printed templates for the iron structure catalyst used in the FTS. (a) gyroid infill 35 and (b) gyroid infill 28.
The replication method was performed as follows: A metallic slurry is produced using iron as metal precursor and a polymeric solution as solvent. The components of the polymeric solution were sodium carboxymethyl cellulose (1% wt. CMC, Mw 700,000), used as a viscosity agent; polyvinyl alcohol (6% wt. PVA, low Mw) that serves as a binder between the powder and template, and alginic acid sodium salt (0.5% SA, low viscosity), used as an electrostatic dispersant. All components were purchased from Alfa Aesar (Heysham, UK) and used as received. To prepare the polymeric solution, distilled water was heated to 80 °C, then CMC was subsequently added. Once the CMC was dissolved, the temperature was reduced to 50 °C, after which, polyvinyl alcohol was added, and once dissolved, sodium alginate was added. The polymeric solution was constantly stirred at 300 rpm using a magnetic stirrer until every component was dissolved. Once the solution was homogeneous, it was left to cool to room temperature. At this point, iron powder (60% wt.) was added to produce the slurry. According to the manufacturer, the iron used as the metallic precursor is 95% Fe, 2% Fe2O3, and could pass 200 mesh (Alfa Aesar). The metallic slurry was stirred manually. To coat the 3D-printed template, it was left soaking in the slurry for one hour under vacuum, rotating the structure every 15 min to prevent clogging on the base due to slurry accumulation. Afterward, the coated template was removed from the slurry and drained by applying a vacuum for 5 min or until the slurry stopped leaking. The structures were left to dry overnight at room temperature. Finally, the dry-coated foam was sintered to obtain the metallic structure. Sintering was conducted in four stages: first, a heating ramp of 5 °C/min to 375 °C, and an isotherm for 30 min to burn the 3D-printed template. Then, another heating ramp of 10 °C/min to 1100 °C and this temperature was held for 2 h.
Copper and potassium were introduced as promoters to the iron structure following sintering, utilizing a single incipient wetness impregnation step with an aqueous solution containing 5% wt. of KNO3, Cu(NO3)2, or both. After impregnation, the sample was dried at 105 °C for 2 h and calcinated at 325 °C for 4 h. The volume of the promoter solution (used for impregnation) was calculated based on the pore volume (pv) of each structure. The fluid displacement method was employed to determine this pore volume. Beginning with measuring the dry sample’s weight and subsequently the weight of the structure saturated with water, the difference between the dry and wet sample weights indicated the pore water weight. Dividing this weight by the density of water provides the occupied volume. To add the promoters, the Fe structure was washed with acetone in an ultrasonic bath for 10 min to remove any organics on the surface and then etched with 1M HCl solution for 10 min to increase the surface roughness and make it more chemically active. Subsequently, it was washed with deionized water repeatedly until the pH reached 7. Then, they were placed in a KNO3, Cu(NO3)2, or KNO3/Cu (NO3)2 solution, and once the structure was saturated, they were transferred into a 105 °C oven to dry overnight
2.1.2. Catalyst Characterization
The foam’s void fraction (α) was measured using Equation (1), where ρs [g/cm3] is the density of iron and ρb is the foam’s bulk density, as determined by the buoyancy method. This study examined two different void fractions to assess their impact on conversion and yield.
XRD analysis was performed to identify the bulk phase in the catalyst. The catalyst sample was manually ground in an agate mortar until a grain size of ≤45 µm was obtained. Then, the sample was placed in an XRD spectrophotometer (X-per Pro MPD, Panalytical, Malvern, UK), where the analysis was performed from 14° to 100°. Cu radiation was used as an X-ray source generated at 40 kV and 10 mA. Scans were taken with a 2θ step size of 0.04° and a counting time of 1.0 s to obtain the diffractogram. The JADE software package (version 7.5.0, 2019) was used to determine the phase composition in the samples using the Rietveld analysis.
X-ray fluorescence (XRF) was used to analyze the elemental composition and concentration of the catalysts used in this study. The apparatus was a Bruker S6 JAGUAR WD-XRF (Billerica, MA, USA).
The catalyst’s specific surface area was measured by nitrogen physisorption (ASAP 2020, Micromeritics, Norcross, GA, USA). To perform the measurement, pieces of the iron structure were placed on the sampling cell and outgassed overnight. Then, a 5-point BET isotherm was measured at −196 °C.
2.2. Catalysts Test
The catalyst structure was arranged within the reactor to form a catalytic bed with a height of 50 mm. Each cylinder had a diameter and height of 25 mm. The catalyst weight in each test was 25 g, unless specified otherwise. The reduction in the catalyst took place in situ using a mixture of hydrogen (70%, purity > 99) and nitrogen (30%, purity > 99) at atmospheric pressure. The reduction was conducted at 325 °C; these conditions were previously identified as optimal for Fe-based structured catalysts [50]. After the reduction, the reactor was cooled by passing the same gas mixture. Once the reaction temperature of 250 °C was attained, the gas mixture was switched to achieve the desired syngas composition (H2:CO of 1.2—indicating hydrogen deficiency), and the reactor was pressurized to 20 bars. A simplified diagram of the FTS reaction setup is illustrated in Figure 2.
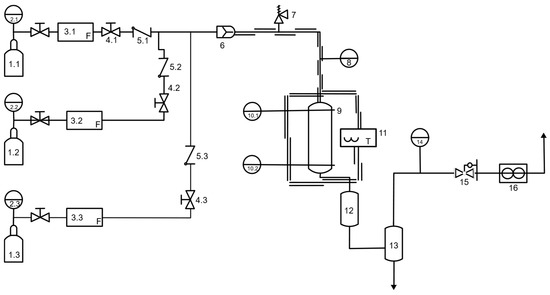
Figure 2.
Simplified Flow Diagram for Fisher–Tropsch reactor and peripheral components. 1. Gas cylinders (1.1 hydrogen, 1.2 nitrogen, 1.3 carbon monoxide); 2.1–2.3. Pressure regulators; 3.1–3.3. Mass flow controllers; 4.1–4.3. Valves; 5.1–5.3. Check valve; 6. Pressure gauge; 7. Relief valve; 8. Bypass valve; 9. Tube and shell catalytic reactor; 10.1–10.2. Temperature sensors; 11. Oven 12. Separation column (hot trap); 13. Separation column (cold trap); 14. Pressure gauge; 15. Backpressure regulator; 16. Gas flow counter.
The gases (carbon monoxide, hydrogen, and nitrogen) were taken from K-bottles and introduced into the reactor using electronic mass flow controllers, one for each gas, to ensure a consistent syngas composition for each experimental run. Before entering the reactor, the gases were mixed in a static mixer. The reactor outlet led to a gas-liquid separator, and a backpressure regulator was installed at the end of the piping system to maintain stable operational pressure during the experimental run. Non-condensable gases were vented or collected for GC analysis. The liquid product was collected in a pressurized trap and analyzed after each experimental run. The reactor, constructed from stainless steel 316, had an internal diameter of 26 mm and a height of 500 mm. A clamshell oven heated the reactor, and no cooling was used in it. Two thermocouples were installed in the reactor to monitor the temperature at the top and bottom of the catalytic bed. Stainless steel mesh was placed before and after the catalytic bed to maintain the catalyst position. The composition of the gas effluent was determined through GC analysis using a Bruker Scion 456-GC equipped with two columns: a Molsieve (13×, 80/100 mesh, 1.5 m × 1/8″ in IS) and a Hayesep (N 80/100 mesh, 0.5 m × 1/8″ IS), as well as a thermal conductivity detector and a flame ionization detector. The data were processed using Compass CDS software (version 4.0.0, 2016) with external standards. The performance of the catalyst was evaluated based on CO conversion (XCO) and CO2 and CH4 selectivity (SCH4, SCO2, respectively) using Equations (2)–(4). Conversion was calculated based on the product gas analysis for CO, H2, CO2, and CH4, where F denotes the molar flow rate [mol∙min−1].
2.3. Product Characterization
In this study, two types of liquid products were obtained from the FTS reaction: an aqueous phase and an organic phase. Density and pH measurements were taken to analyze these products. Furthermore, the organic samples underwent GC-MS analysis employing an Agilent (Santa Clara, CA, USA) 5975 series MSD connected to an Agilent 7820A GC. This GC was equipped with a J&W DB-5 ms column (non-polar phenyl arylene polymer) with dimensions of 15 m × 0.25 mm × 0.25 µm. The auto-injector (Agilent ALS) operated in a 10:1 split mode at 300 °C, with helium serving as the carrier gas at a flow rate of 1.2 mL·min−1. A 1 µL sample was injected, and the oven temperature was initially held at 50 °C for 1 min, then ramped up to 300 °C at a rate of 10 °C/min, after which it was maintained at 300 °C for 1 min.
The aqueous phase underwent centrifugation, followed by sterile filtration using a 0.22 µm membrane filter (Chromspec, Brockville, ON, Canada), and was subsequently analyzed using high-performance liquid chromatography (HPLC). The latter was an Agilent 1100 Series, equipped with a Rezex ROA-Organic Acid H+(8%) 300 × 7.8 mm column (Phenomenex, Torrance, CA, USA). The mobile phase consisted of 2.5 mM H2SO4 at a flow rate of 0.6 mL/min. The column temperature was maintained at 65 °C, and three calibration curves were generated for each compound of interest (ethanol, formic acid, and acetic acid).
3. Results and Discussion
3.1. Catalyst Characterization
Eight catalysts were tested, with the parameters investigated being the void fraction and the presence of promoters. In the catalyst nomenclature, the number refers to the void fraction, and the chemical symbols refer to the active catalytic phase (Fe) and the presence of promoters (K, Cu). For example, 72Fe refers to a structured iron catalyst with a void fraction of 0.72, and 77FeCu refers to a structured iron catalyst with a void fraction of 0.77 and copper as a promoter. The presence of promoters in the iron structures was achieved by direct impregnation with an aqueous solution of the Cu and K salts, resulting in a loading of approximately 3% wt.
Figure 3 shows a photograph of the structured iron catalyst obtained by applying the replication method of a 3D structure using a metallic slurry. By employing a precise infill pattern setting (Gyroid) with specific infill densities (28 or 35) for the 3D template, it was possible to achieve iron structures with varying void fractions. Panel (a) shows the structure with a void fraction of 0.77, and panel (b) shows a void fraction of 0.72. The objective of using this type of structure is to increase the void fraction without losing the mechanical resistance of the material (provide the maximum possible surface area while still being structurally resilient). The honeycomb-type monoliths that are the standard for this type of application (non-adiabatic catalytic process) have the disadvantage of restricting the free flow of reactant gases and liquid products, which leads to an increase in pressure (back pressure) and a decrease in performance [29,36,37,38,39,51]. Hence, a structure with a gyroid pattern, resembling the back mixing flow of a foam while maintaining the structural integrity of a honeycomb monolith, was tested [46,52,53].
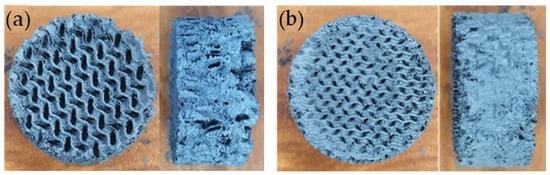
Figure 3.
Iron structures obtained from 3D-printed templates using the powder metallurgy replication technique. (a) void fraction 0.77 and (b) void fraction 0.72.
Different void fractions for structured catalysts aim to reach a residence time for iron catalysts similar to a cobalt catalyst without affecting the type of products obtained from the process [36,37,38,39,45]. Since the life span of the catalyst is influenced by the pressure drop in the catalytic bed, increasing the void fraction may reduce the pressure drop without negatively impacting catalytic activity, given the large geometric area that allows reactants to access the active sites [30,31,32,33,34,52,53,54,55,56]. In the literature, it is reported that the lifetime of Fe-based catalysts is short and that iron catalysts are replaced because of physical changes in the catalyst (attrition) and not due to the loss of catalyst sites due to poisoning and/or fouling [8,9]. For the catalysts tested in this work, it was expected that the high void fraction and the sintering process to which the catalysts were subjected would maintain the catalytic phase, preventing the catalyst from undergoing surface reconstruction and mechanical deactivation through attrition [19,20,23].
Table 1 presents the surface area and composition of the eight catalysts studied, as measured by nitrogen physisorption and X-ray fluorescence (XRF), respectively. The surface area was measured with the Brunauer-Emmett-Teller (BET) method. The results show a loss of surface area when promoters were added, with the copper-promoted catalysts having the lowest surface area, probably due to their crystal size.

Table 1.
BET surface area of the structured iron catalyst and promoter concentration of the structured iron catalysts used for FTS.
Given the size of the sample holder, the structured catalyst was broken down to carry out the surface area measurement, which implies losing the proportion between surface area and volume. Therefore, the measured surface area is consistent with expectations. For future work, we suggest measuring the textural properties of these materials by analyzing a precisely cut piece to maintain the pore structure and hierarchy. Additionally, Kr adsorption may be more suitable for calculating SBET than nitrogen adsorption. Additionally, the structure was subjected to a sintering process that, by its nature, causes the material to lose porosity. Consequently, the measured surface area is most likely not representative of the sample before crushing, meaning the external surface area created is negligible compared to the initial one. Nevertheless, the fact that the structures had a high void fraction (high area/volume ratio) implies little or no accumulation of liquid product within the catalyst’s structure, i.e., no filling of catalyst pores with hydrocarbons due to diffusion limitation and capillary condensation, which in theory could compensate for a low surface area in terms of mass transfer [36,37,38,57,58,59,60]. The effective catalyst composition, determined by XRF analysis and presented in Table 1, is consistent with the nominal K and Cu loading used during wet impregnation.
X-ray diffraction analysis in Figure 4 reveals that all catalysts contain the same iron oxide crystal (Fe2O3 hematite) as a bulk phase. Undoubtedly, the Fischer–Tropsch synthesis is not a fast reaction, so the reaction rate is significantly influenced by a reactor with a fast gas-liquid mass transfer, so the structure was made only using the catalytic phase without using a ceramic material as support, since the metal provides better radial heat transfer, but also because in this way, the highest possible catalyst inventory can be obtained, avoiding an inert volumetric fraction in the catalyst bed and eliminating complex interactions with the support. Furthermore, this approach eliminates the need for a wash coating on the structure to add the catalytic phase, which reduces the void fraction and increases the pressure drop. Ideally, the void fraction is such that Taylor Flow conditions can be achieved to ensure excellent gas-liquid-solid mass transfer [36,37,61].
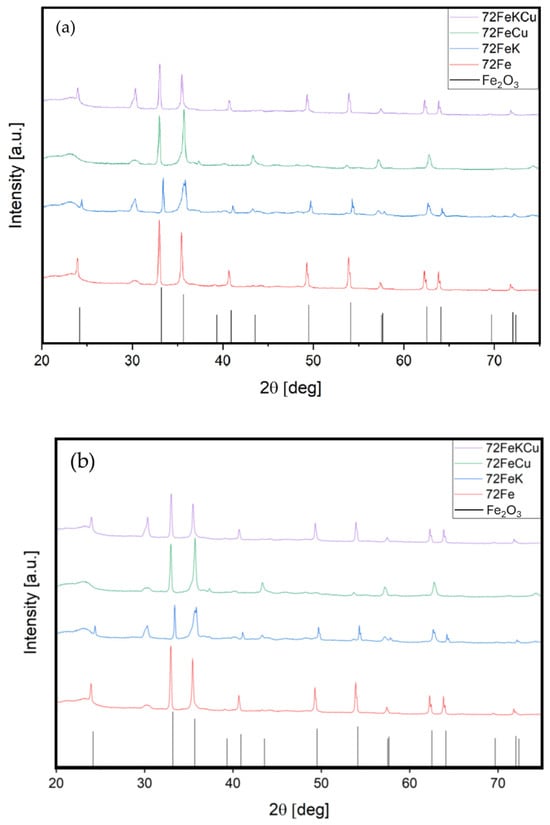
Figure 4.
XRD pattern for Fe-structure catalyst with and without copper and potassium as promoters, (a) void fraction 0.72 and (b) void fraction 0.77.
Figure 4 shows the XRD patterns of all eight catalysts. Panel (a) corresponds to a void fraction of 0.72, and panel (b) to a void fraction of 0.77. Compared to the hematite pattern, it is evident that the catalyst contains the iron oxide phase, leading to the conclusion that the promoter is the primary difference between the catalysts. Unfortunately, no copper or potassium oxide phase we examined accurately matches the promoters in question using the system database. Nevertheless, the patterns indicate that the catalysts had different compositions. This difference ultimately led to changes in catalytic performance, specifically in the conversion and selectivity of iron structures (see Section 3.2.2).
3.2. Catalytic Performance
The catalyst plays a crucial role in the Fischer–Tropsch synthesis (FTS), as the synthesis method directly influences activity, CO conversion, and product selectivity. Here, the catalytic performance of the Fe-structure catalyst was evaluated for FTS in a fixed-bed reactor, with the test conducted under conditions suitable for hydrogen-deficient syngas. The catalyst was reduced in situ under a 70% H2 flow at 325 °C for 4 h before the FTS; these conditions were previously identified as optimal for Fe-based structured catalysts [50]. The reaction was conducted at 250 °C, with a total pressure of 20 bar and an H2/CO ratio of 1.2, resulting in a hydrogen-deficient syngas feed. The weight hourly space velocity is calculated based on the CO mass feed rate to characterize the catalyst activity. The reaction was run uninterrupted for 48 h.
Literature shows that the operation conditions and promoters have an impact on the activity and selectivity of Fe-based catalysts. One condition that can be changed relatively easily in a structured catalyst is the void fraction, and simple wet impregnation is one of the techniques widely reported for catalyst promotion. Therefore, the effect of the void fraction of the catalytic bed and the addition of the most used promoters were evaluated. The aim is to compare the performance of a structured catalyst to that of a conventional powder catalyst.
3.2.1. Effect of Void Fraction
Figure 5 presents the performance of the structure catalyst in terms of CO and H2 conversion, as well as CO2 and CH4 selectivity, as a function of time on stream. The catalyst activity was investigated over several hours for two catalysts with different void fractions, while all other operating conditions remained constant for each experimental run. As shown in Figure 5, the catalyst exhibited very good activity from the onset of the experimental run, demonstrating the significant advantage of utilizing a catalytic bed entirely composed of catalytic material. Once a steady state was reached, the results indicate that a structured catalyst enables conversion levels that surpass those reported for the industrial iron-based catalyst, as well as the structured cobalt and iron-based catalyst [62]. Notably, greater porosity has a slight positive effect on conversion and selectivity, likely due to reactants having improved access to CO hydrogenation sites.
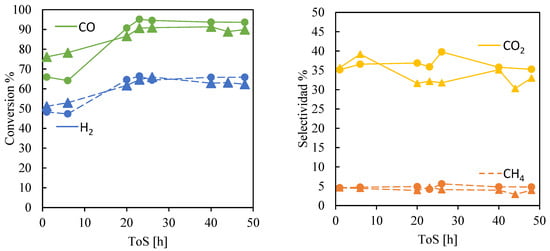
Figure 5.
Conversion and selectivity as a function of time on stream (ToS). Operation conditions: T = 250 °C, P = 20 bar, H2:CO = 1.2, WHSV = 600 L∙(gFe∙h)−1. The circles (●) denote a void fraction of 0.77 and the triangles (▲) of 0.72, respectively.
In a conventional catalyst (powder catalyst), the void fraction of the catalytic bed is determined by the size of the pellets and their packing capacity in the catalytic bed, which directly affects the pressure drop. In contrast, a structured catalyst (such as monoliths and foams) has a predetermined void fraction, which can be controlled during its fabrication by the manufacturer. In conventional catalysts, a high void fraction in the catalyst bed implies using larger pellets, which causes the reactants to face strong internal mass transfer restrictions due to non-uniform distribution on the pore site. Also, if the catalyst has eggshell-type pellets (to avoid non-uniform reactant distribution), there is low catalyst utilization since less active metal is available in the catalytic bed. Conversely, it has been reported that a low void fraction produces less selectivity towards high molecular weight hydrocarbons [14,34].
This work showed that a structured catalyst can be a solution to overcome pressure drop and diffusion limitations. In a structured catalyst, the void fraction becomes a controllable operational parameter, significantly affecting chain growth probability and hydrocarbon selectivity toward light or heavy products. Conversely, if the void fraction is high, a lower space velocity can be used without increasing the pressure drop. Lowering the space velocity would increase CO conversion, due to increased residence time of the reactants and products. Nevertheless, results obtained from this work show that increasing the void fraction decreased CO and CH4 selectivity while maintaining a high conversion level, meaning that the effect of the low H2/CO ratio was more pronounced and favored chain growth.
3.2.2. Effect of Promoters
The performance of both the promoted and unpromoted structure catalysts is presented in terms of CO and H2 conversions, as well as CO2 and CH4 selectivities, in Figure 6.
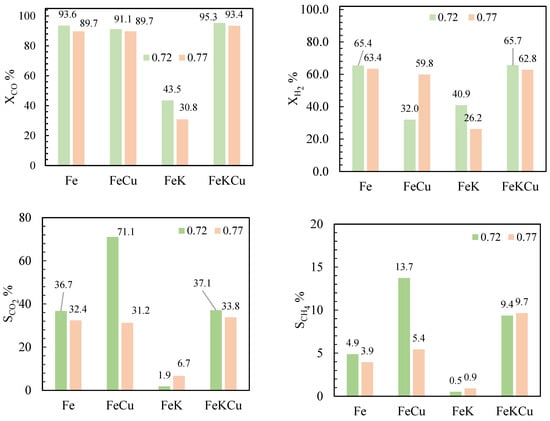
Figure 6.
Conversion and selectivity as a function of catalyst type. Operation conditions: T = 250 °C, P = 20 bar, H2:CO = 1.2, WHSV = 600 L∙(gFe∙h)−1.
The results are reported as a function of the void fraction and promoter use. The catalyst activity was investigated for 48 h, and the value of the parameter is the average of the sample points taken once the experiment reached a steady state. The operation conditions were the same for each experimental run. The promoters used were Cu and K. The former has been linked to the reduction in iron oxides and enhancement of the WGS reaction [19,20,24,63,64,65,66,67,68]. It was reported that K decreases CH4 selectivity, increases chain growth probability, and provides resistance to oxidation of the catalysts due to contact with the water produced in FT synthesis [1,69]. Both promoters were loaded at a 3% wt rate. The aim was to validate whether the promoters had the same behavior in structured catalysts as in powder catalysts.
As shown in Figure 6, the promoter behavior is the same regardless of the void fraction. Promotion with K at a 3 wt% loading diminishes catalytic activity, possibly due to catalytic site coverage and a lower extent of reduction, which is likely caused by the blockage of the active surface with potassium. Reports in the literature indicate that potassium promotion with K/Fe ratios higher than 0.03 hinders activity; our results support these findings [16,27,67,68,69,70,71,72]. However, since the selectivity toward heavier hydrocarbons increases, it is possible that the active sites to which the reactants have access possess better adsorption of CO and CO2, which could be related to the basicity of the catalyst surface.
Adding 3 wt% of copper as a promoter did not affect the CO conversion but decreased H2 conversion and selectivity to CO2, indicating that the WGS reaction activity is at its lowest. At the same time, selectivity towards CH4 increased, which suggests that this promoter is more active for methanation. This result is similar to previous reports in the literature, which indicate that copper can increase the carburization rate of the catalyst in a stable state; hence, methanation is favored over the WGS reaction [1,16,19,20,73]. The presence of Cu could accelerate the catalyst’s activation, probably because it facilitates the reduction in the catalyst. The activity of the structured catalyst promoted by K and Cu is higher than when it is not promoted or promoted by an individual promoter. The selectivity towards CO2 and CH4 also increases, and the molecular weight of the hydrocarbons is influenced by the presence of K [62,74].
3.2.3. Stability of Structured Catalysts
Figure 7 illustrates the performance of the structured iron catalyst with a void fraction of 0.72, promoted by K and Cu, in terms of conversion and selectivity. Conversion and selectivity are reported as a function of time on stream. For this test, catalytic activity was investigated for 100 h under the same operational conditions as the previous test.
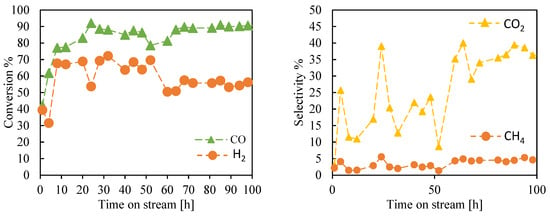
Figure 7.
Conversion and selectivity as a function of time on stream for the structure catalyst 72FeKCu in the FTS. Operation conditions: T = 250 °C, P = 20 bar, H2:CO = 1.2, WHSV = 600 L∙(gFe∙h)−1.
The objective was to verify catalyst stability, as it is well known that during reactions, catalyst composition changes, which may negatively influence catalyst activity. Small particles of iron carbide may sinter due to water formation and subsequent re-oxidation, leading to the formation of magnetite crystallites. Since sintering is a common mechanism of deactivation considered an irreversible phenomenon, it is crucial to understand the rate at which this phenomenon affects the structured catalyst. As seen in Figure 7, the structured catalyst undergoes a reduction-oxidation-reduction sequence around the 60 h mark, which appears to restore the initial activity. This may occur because the catalyst has a predefined shape that maintains the average crystallite size of the various phases present within the catalyst for an extended period.
3.2.4. Product Characterization
To characterize the liquid products of the FTS reactions, density and pH were measured, and HPLC identified the oxygenates present in water. In contrast, the hydrocarbons in the organic phase were identified by GC-MS analysis. Under the conditions presented, GC-MS analysis revealed a broad distribution of hydrocarbons from C8 to C27. These compounds correspond to boiling points spanning both the naphtha range (approximately 30–200 °C, typically C5–C12) and the diesel or middle range (200–350 °C, typically C13–C25) [75,76]. Figure 8 shows a photograph of the liquid products obtained from each run of the FTS reaction using a structured iron catalyst. Promoters influence product distribution and yield. The promoters alter the color of the samples, most likely due to the higher oxygen content in these samples. The samples with K as a promoter have a low yield of waxy hydrocarbon products.
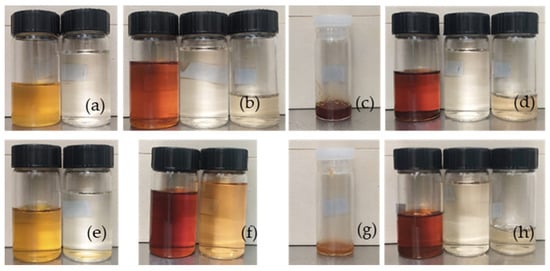
Figure 8.
Liquid products of FTS using a structured iron catalyst. Panel (a) 0.72 Fe, (b) 0.72FeCu, (c) 0.72FeK; (d) 0.72FeKCu; (e) 0.77Fe; (f) 0.77FeCu; (g) 0.77FeK; (h) 0.77FeKCu. Vials with black caps, 20 mL, and white caps, 10 mL.
Table 2 presents the pH, density, and viscosity values for the aqueous and organic phase products of the FTS reaction using a structured iron catalyst. The density and viscosity of the organic phase place these products within the gas oil range.

Table 2.
Viscosity, density, and pH of the liquid products of the FTS using structured iron catalysts.
The aqueous phase exhibits a very low pH, indicating the formation of oxygenates during the reaction. The HPLC analysis, shown in Table 3, confirms the production of some of these compounds, which could become value-added byproducts.

Table 3.
Oxygenate content in the aqueous phase produced by FTS using a structured iron catalyst.
The organic phase of the FTS contains an extensive array of products, making it challenging to identify them solely based on retention times from standards. Consequently, to pinpoint and characterize the compounds within these samples, gas chromatography-mass spectrometry (GC-MS) was employed. The GC-MS was operated in Total Ion Chromatogram (TIC) mode, allowing for the simultaneous collection of chromatograms and mass spectrometry (MS) data. The predominant branching in FTS involves single methyl-branched materials, with the diversity of these compounds reliant on the specific carbon number. As the carbon number increases, the range of methyl-branched paraffins becomes more diverse.
Figure 9 illustrates the chromatogram detailing the organic output obtained using an iron catalyst with a void fraction of 0.72. The panels display results for different catalyst compositions: (a) 0.72Fe, (b) 0.72FeCu, 0.72FeK, and 0.72FeKCu. The hydrocarbons observed in this sampling typically spanned from C8 to C27. The primary FTS products exhibit a distinct elution sequence: the initial peak corresponds to the 1-olefin, followed by paraffin, trans-2-olefin, and finally, the cis-2-olefin, ranging from C8 to greater than C27.
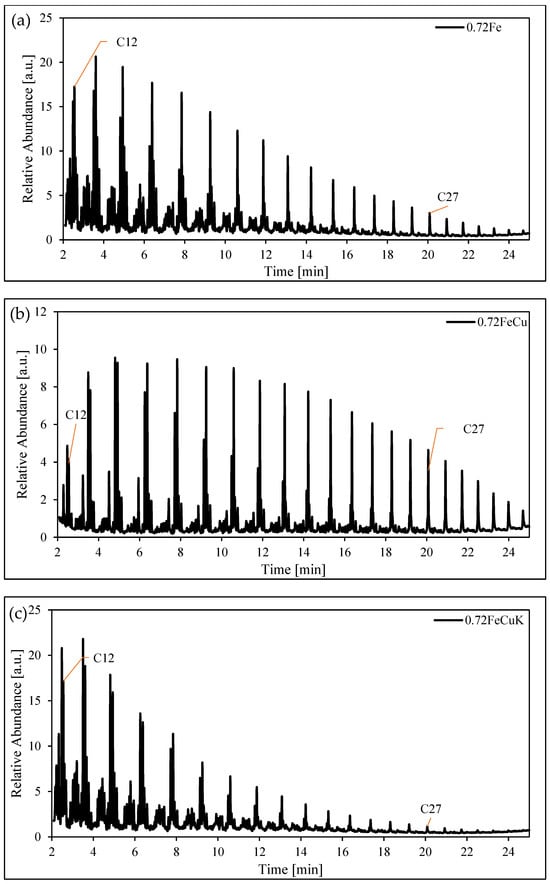
Figure 9.
Gas chromatograph of the organic phase produced with a structured iron catalyst. Panel (a) 0.72Fe, (b) 0.72FeCu and (c) 0.72FeKCu. Reaction conditions: 250C, 20 bar WHSV = 600, and H2/CO = 1.2.
The panels display results for different catalyst compositions: (a) 0.72Fe, (b) 0.72FeCu, 0.72FeK, and 0.72FeKCu. The hydrocarbons observed in this sampling typically spanned from C8 to C27. The primary FTS products exhibit a distinct elution sequence: the initial peak corresponds to the 1-olefin, followed by paraffin, trans-2-olefin, and finally, the cis-2-olefin, ranging from C8 to greater than C27. Before these major products, various hydrocarbon products with the same carbon number elute in clusters. Predominantly, these clusters comprise methyl-branched paraffins and olefins. Notably, the major oxygenate group detected in the samples consists of alcohols, eluting before the 1-olefin. The carbon number of the alcohols is two less than that of the corresponding hydrocarbon peak (for instance, when the C12 hydrocarbon elutes, the alcohol eluting is nonenol). It is noteworthy that the presence of potassium and copper alters the olefin-to-paraffin ratio, consequently impacting the abundance of hydrocarbons in the sample. The influence of potassium is more pronounced than that of copper.
Figure 10 shows the GC pattern of the organic sample produced using the structured iron catalyst with a void fraction of 0.77 (panel (a) 0.77Fe, (b) 0.77FeCu, 0.77FeK, and 0.77FeKCu).
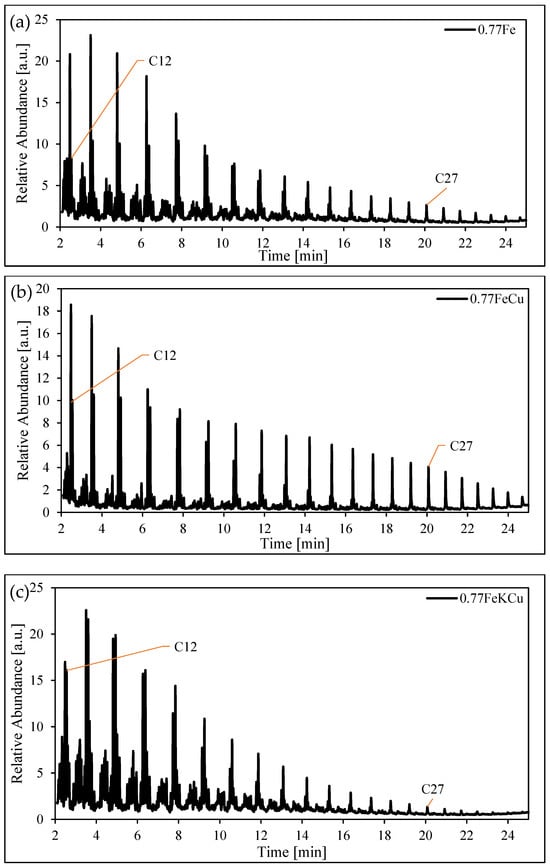
Figure 10.
Chromatographs of the organic phase produced with a structured iron catalyst. Panel (a) 0.77Fe, (b) 0.77FeCu and (c) 0.77FeKCu. Reaction conditions: 250C, 20 bar, WHSV = 600, and H2/CO = 1.2.
These samples exhibit the same trend as the catalyst with a void fraction of 0.72, suggesting that the promoter effect remains consistent regardless of the void fraction of the structured catalyst.
In general, the analysis of the physicochemical properties of the liquid, aqueous, and organic product (Table 2 and Table 3) indicates that the addition of Cu and the co-promotion of Cu and K promote the formation of oxygenated compounds in the aqueous phase, such as ethanol and acetic acid. On the other hand, promotion with K leads to a lower yield of wax-type hydrocarbons. All catalysts produced hydrocarbons in the range of a broad distribution of hydrocarbons from C8 to C27. Without promoters, the products suggest a typical chain growth route from CHx*. However, with K and Cu, it is observed that routes leading to partially oxygenated molecules such as ethanol and acetic acid can be favored [70,77]. Quantifying the molar fractions of the obtained products and calculating the chain growth probability (α) are suggested for future work to deepen the understanding of structured iron catalysts.
4. Conclusions
This study examined the effects of void fraction and Cu and K promoters on the performance of structured iron catalysts in Fischer–Tropsch synthesis. The results showed stable catalyst performance over time, with high activity and selectivity, indicating significant potential for the sustainable and efficient production of hydrocarbons at a large scale. Controlled void fraction in structured catalysts can positively impact CO conversion and selectivity in the FT synthesis. Additionally, all studied catalysts produced hydrocarbons in the C8 to C27 range, and the promotion of Cu and K enhanced the formation of oxygenated compounds in the aqueous phase. Furthermore, the presence of K significantly decreased CO conversion, leading to low production of wax-type hydrocarbons. These findings lay a solid foundation for future research to optimize the synthesis of structured catalysts for sustainable energy applications.
Author Contributions
Conceptualization, Y.H. and J.-M.L.; methodology, Y.H.; formal analysis, Y.H., I.D.M.-V. and J.-M.L.; investigation, Y.H.; writing—original draft preparation, Y.H. and I.D.M.-V.; writing—review and editing, Y.H., I.D.M.-V. and J.-M.L.; supervision, J.-M.L.; project administration, J.-M.L.; funding acquisition, J.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 101006717; the Canadian New Frontiers in Research Fund under grant number NFRFG-2020-00148; and the Canadian Fond de recherche Société et culture—Québec under grant number 308509.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the Biomass Technology Laboratory (BTL) of the Université de Sherbrooke and especially its sponsors: the Canadian New Frontiers in Research Fund under grant number NFRFG-2020-00148 and the Canadian Fond de recherche Société et culture—Québec under grant number 308509. Further acknowledgments go to the Biomass, Bioproducts, and Bioprocess Analysis Laboratory (L.A.B) for its support regarding HPLC analysis. Finally, the authors thank Henry Gauvin (Université de Sherbrooke) for his help in building the experimental setup of this work and Valérie Larouche for her assistance with the GC-MS analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ail, S.S.; Dasappa, S. Biomass to liquid transportation fuel via Fischer Tropsch synthesis—Technology review and current scenario. Renew. Sustain. Energy Rev. 2016, 58, 267–286. [Google Scholar] [CrossRef]
- Arutyunov, V.; Nikitin, A.; Strekova, L.; Savchenko, V.; Sedov, I. Utilization of renewable sources of biogas for small-scale production of liquid fuels. Catal. Today 2021, 379, 23–27. [Google Scholar] [CrossRef]
- Dry, M.E. High quality diesel via the Fischer–Tropsch process—A review. J. Chem. Technol. Biotechnol. 2002, 77, 43–50. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer–Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Baliban, R.C.; Elia, J.A.; Floudas, C.A. Biomass to liquid transportation fuels (BTL) systems: Process synthesis and global optimization framework. Energy Environ. Sci. 2013, 6, 267–287. [Google Scholar] [CrossRef]
- Van Steen, E.; Claeys, M. Fischer-Tropsch Catalysts for the Biomass-to-Liquid (BTL)-Process. Chem. Eng. Technol. 2008, 31, 655–666. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch synthesis: Current mechanism and futuristic needs. Fuel Process. Technol. 2001, 71, 157–166. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Steynberg, A.P.; Jager, B.; Vosloo, A.C. Low temperature Fischer–Tropsch synthesis from a Sasol perspective. Appl. Catal. A Gen. 1999, 186, 13–26. [Google Scholar] [CrossRef]
- Jager, B.; Espinoza, R. Advances in low temperature Fischer-Tropsch synthesis. Catal. Today 1995, 23, 17–28. [Google Scholar] [CrossRef]
- Khodakov, A.Y. Fischer-Tropsch synthesis: Relations between structure of cobalt catalysts and their catalytic performance. Catal. Today 2009, 144, 251–257. [Google Scholar] [CrossRef]
- Ram, V.; Salkuti, S.R. An Overview of Major Synthetic Fuels. Energies 2023, 16, 2834. [Google Scholar] [CrossRef]
- Demirbas, A. Converting Biomass Derived Synthetic Gas to Fuels via Fisher-Tropsch Synthesis. Energy Sources Part A Recover. Util. Environ. Eff. 2007, 29, 1507–1512. [Google Scholar] [CrossRef]
- Willauer, H.D.; Bradley, M.J.; Baldwin, J.W.; Hartvigsen, J.J.; Frost, L.; Morse, J.R.; DiMascio, F.; Hardy, D.R.; Hasler, D.J. Evaluation of CO2 Hydrogenation in a Modular Fixed-Bed Reactor Prototype. Catalysts 2020, 10, 970. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Wilcox, W.; Li, S. A compact and high throughput reactor of monolithic-structured catalyst bed for conversion of syngas to liquid fuels. AIChE J. 2012, 58, 2820–2829. [Google Scholar] [CrossRef]
- Davis, B.H.; Occelli, M.L. Fischer-Tropsch Synthesis, Catalysts, and Catalysis; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-429-09902-1. [Google Scholar]
- Van Steen, E.; Prinsloo, F.F. Comparison of preparation methods for carbon nanotubes supported iron Fischer–Tropsch catalysts. Catal. Today 2002, 71, 327–334. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch Synthesis: Reaction mechanisms for iron catalysts. Catal. Today 2009, 141, 25–33. [Google Scholar] [CrossRef]
- Eliseev, O.L.; Savost’yanov, A.P.; Sulima, S.I.; Lapidus, A.L. Recent development in heavy paraffin synthesis from CO and H2. Mendeleev Commun. 2018, 28, 345–351. [Google Scholar] [CrossRef]
- Li, S.; Krishnamoorthy, S.; Li, A.; Meitzner, G.D.; Iglesia, E. Promoted Iron-Based Catalysts for the Fischer–Tropsch Synthesis: Design, Synthesis, Site Densities, and Catalytic Properties. J. Catal. 2002, 206, 202–217. [Google Scholar] [CrossRef]
- Li, S.; Li, A.; Krishnamoorthy, S.; Iglesia, E. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer–Tropsch synthesis catalysts. Catal. Lett. 2001, 77, 197–205. [Google Scholar] [CrossRef]
- Daage, M.A.; Koveal, R.J.; Lapidus, A.L.; Krylova, A.J.; Brennan, S.P. Fischer-Tropsch Catalyst Enhancement. U.S. Patent 6355593B1, 1 September 2000. Available online: https://patents.google.com/patent/US6355593 (accessed on 26 February 2024).
- Lim, D.-H.; Jo, J.H.; Shin, D.Y.; Wilcox, J.; Ham, H.C.; Nam, S.W. Carbon dioxide conversion into hydrocarbon fuels on defective graphene-supported Cu nanoparticles from first principles. Nanoscale 2014, 6, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Wang, P.; Xu, W.; Hensen, E.J.M. Particle Size and Crystal Phase Effects in Fischer-Tropsch Catalysts. Engineering 2017, 3, 467–476. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, D.; Dai, Y.; Lin, J.; Li, Y.; Wen, C. Microstructure, mechanical properties, degradation behavior, and biocompatibility of porous Fe-Mn alloys fabricated by sponge impregnation and sintering techniques. Acta Biomater. 2020, 114, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-P.; Ding, Y.-J.; Lin, L.-W. Fischer−Tropsch Synthesis over Activated-Carbon-Supported Cobalt Catalysts: Effect of Co Loading and Promoters on Catalyst Performance. Ind. Eng. Chem. Res. 2004, 43, 2391–2398. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Mahmoudi, M.; Doustdar, O.; Jahangiri, H.; Tsolakis, A.; Gu, S.; LechWyszynski, M. A review of Fischer Tropsch synthesis process, mechanism, surface chemistry and catalyst formulation. Biofuels Eng. 2017, 2, 11–31. [Google Scholar] [CrossRef]
- Wang, J.; You, Z.; Zhang, Q.; Deng, W.; Wang, Y. Synthesis of lower olefins by hydrogenation of carbon dioxide over supported iron catalysts. Catal. Today 2013, 215, 186–193. [Google Scholar] [CrossRef]
- Otun, K.O.; Liu, X.; Hildebrandt, D. Metal-organic framework (MOF)-derived catalysts for Fischer-Tropsch synthesis: Recent progress and future perspectives. J. Energy Chem. 2020, 51, 230–245. [Google Scholar] [CrossRef]
- Pai, D.; Prabhu, M.K. Recent Advances in Substrate Materials and Thermal Analysis of Catalytic Converters. Mater. Today Proc. 2018, 5, 24221–24230. [Google Scholar] [CrossRef]
- Gascon, J.; Van Ommen, J.R.; Moulijn, J.A.; Kapteijn, F. Structuring catalyst and reactor—An inviting avenue to process intensification. Catal. Sci. Technol. 2015, 5, 807–817. [Google Scholar] [CrossRef]
- Kapteijn, F.; De Deugd, R.M.; Moulijn, J.A. Fischer–Tropsch synthesis using monolithic catalysts. Catal. Today 2005, 105, 350–356. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Beers, A.E.W.; Vergunst, T.; Hoek, I.; Kapteijn, F.; Moulijn, J.A. Preparation of monolithic catalysts. Catal. Rev. 2001, 43, 345–380. [Google Scholar] [CrossRef]
- Delparish, A.; Avci, A.K. Intensified catalytic reactors for Fischer-Tropsch synthesis and for reforming of renewable fuels to hydrogen and synthesis gas. Fuel Process. Technol. 2016, 151, 72–100. [Google Scholar] [CrossRef]
- Hilmen, A.-M.; Bergene, E.; Lindvåg, O.A.; Schanke, D.; Eri, S.; Holmen, A. Fischer–Tropsch synthesis on monolithic catalysts of different materials. Catal. Today 2001, 69, 227–232. [Google Scholar] [CrossRef]
- Park, J.C.; Roh, N.S.; Chun, D.H.; Jung, H.; Yang, J.-I. Cobalt catalyst coated metallic foam and heat-exchanger type reactor for Fischer–Tropsch synthesis. Fuel Process. Technol. 2014, 119, 60–66. [Google Scholar] [CrossRef]
- Tronconi, E.; Groppi, G.; Visconti, C.G. Structured catalysts for non-adiabatic applications. Curr. Opin. Chem. Eng. 2014, 5, 55–67. [Google Scholar] [CrossRef]
- Visconti, C.G.; Groppi, G.; Tronconi, E. Highly conductive “packed foams”: A new concept for the intensification of strongly endo- and exo-thermic catalytic processes in compact tubular reactors. Catal. Today 2016, 273, 178–186. [Google Scholar] [CrossRef]
- Visconti, C.G.; Tronconi, E.; Groppi, G.; Lietti, L.; Iovane, M.; Rossini, S.; Zennaro, R. Monolithic catalysts with high thermal conductivity for the Fischer–Tropsch synthesis in tubular reactors. Chem. Eng. J. 2011, 171, 1294–1307. [Google Scholar] [CrossRef]
- Visconti, C.G.; Tronconi, E.; Lietti, L.; Groppi, G.; Forzatti, P.; Cristiani, C.; Zennaro, R.; Rossini, S. An experimental investigation of Fischer–Tropsch synthesis over washcoated metallic structured supports. Appl. Catal. Gen. 2009, 370, 93–101. [Google Scholar] [CrossRef]
- Banville, M.; Labrecque, R.; Lavoie, J.-M. Dry reforming of methane under an electro-catalytic bed: Effect of electrical current and catalyst composition. In Proceedings of the ENERGY AND SUSTAINABILITY 2014, Lumpur, Malaysia, 23–24 October 2014; pp. 603–611. [Google Scholar]
- Kolaczkowski, S.T.; Awdry, S.; Smith, T.; Thomas, D.; Torkuhl, L.; Kolvenbach, R. Potential for metal foams to act as structured catalyst supports in fixed-bed reactors. Catal. Today 2016, 273, 221–233. [Google Scholar] [CrossRef]
- Yeetsorn, R.; Tungkamani, S.; Maiket, Y. Fabrication of a Ceramic Foam Catalyst Using Polymer Foam Scrap via the Replica Technique for Dry Reforming. ACS Omega 2022, 7, 4202–4213. [Google Scholar] [CrossRef] [PubMed]
- Cutler, W.A.; Lin, H.; Olszewski, A.R.; Sorensen, C., Jr. Thermally Conductive Honeycombs for Chemical Reactors. U.S. Patent 6881703B2, 8 August 2001. [Google Scholar]
- Hosseini, S.; Moghaddas, H.; Masoudi Soltani, S.; Kheawhom, S. Technological Applications of Honeycomb Monoliths in Environmental Processes: A review. Process Saf. Environ. Prot. 2020, 133, 286–300. [Google Scholar] [CrossRef]
- Cristiani, C.; Finocchio, E.; Latorrata, S.; Visconti, C.G.; Bianchi, E.; Tronconi, E.; Groppi, G.; Pollesel, P. Activation of metallic open-cell foams via washcoat deposition of Ni/MgAl2O4 catalysts for steam reforming reaction. Catal. Today 2012, 197, 256–264. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Monoliths in Heterogeneous Catalysis. Catal. Rev. 1994, 36, 179–270. [Google Scholar] [CrossRef]
- Danaci, S.; Protasova, L.; Snijkers, F.; Bouwen, W.; Bengaouer, A.; Marty, P. Innovative 3D-manufacture of structured copper supports post-coated with catalytic material for CO2 methanation. Chem. Eng. Process. Process. Intensif. 2018, 127, 168–177. [Google Scholar] [CrossRef]
- Giani, L.; Groppi, G.; Tronconi, E. Mass-Transfer Characterization of Metallic Foams as Supports for Structured Catalysts. Ind. Eng. Chem. Res. 2005, 44, 4993–5002. [Google Scholar] [CrossRef]
- Harmel, J.; Peres, L.; Estrader, M.; Berliet, A.; Maury, S.; Fécant, A.; Chaudret, B.; Serp, P.; Soulantica, K. hcp-Co Nanowires Grown on Metallic Foams as Catalysts for Fischer–Tropsch Synthesis. Angew. Chem. 2018, 130, 10739–10743. [Google Scholar] [CrossRef]
- Hurtado Castaño, Y.V. Development of a Structured Iron-Based Catalyst for Fischer–Tropsch Synthesis Using Bio-Syngas. Ph.D. Dissertation, University of Sherbrooke, Sherbrooke, QC, Canada.
- Giani, L.; Cristiani, C.; Groppi, G.; Tronconi, E. Washcoating method for Pd/γ-Al2O3 deposition on metallic foams. Appl. Catal. B Environ. 2006, 62, 121–131. [Google Scholar] [CrossRef]
- Konarova, M.; Aslam, W.; Ge, L.; Ma, Q.; Tang, F.; Rudolph, V.; Beltramini, J.N. Enabling Process Intensification by 3 D Printing of Catalytic Structures. ChemCatChem 2017, 9, 4132–4138. [Google Scholar] [CrossRef]
- William, C.; Lednor, P. Monolithic ceramics and heterogeneous catalysts: Honeycombs and foams. Curr. Opin. Solid State Mater. Sci. 1996, 1, 88–95. [Google Scholar] [CrossRef]
- Majidian, N.; Soltanali, S. Comparison of Fischer-Tropsch Fixed and Monolith Bed Reactors Using Pseudo-homogeneous 2D Model. J. Jpn. Pet. Inst. 2016, 59, 126–139. [Google Scholar] [CrossRef][Green Version]
- Guettel, R.; Kunz, U.; Turek, T. Reactors for Fischer-Tropsch Synthesis. Chem. Eng. Technol. 2008, 31, 746–754. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.A. Structured catalysts and reactors—Perspectives for demanding applications. Catal. Today 2022, 383, 5–14. [Google Scholar] [CrossRef]
- Tomašić, V.; Jović, F. State-of-the-art in the monolithic catalysts/reactors. Appl. Catal. Gen. 2006, 311, 112–121. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J. Automobile exhaust catalysts. Appl. Catal. Gen. 2001, 221, 443–457. [Google Scholar] [CrossRef]
- Heck, R.M.; Gulati, S.; Farrauto, R.J. The application of monoliths for gas phase catalytic reactions. Chem. Eng. J. 2001, 82, 149–156. [Google Scholar] [CrossRef]
- Li, C.; Xu, H.; Hou, S.; Sun, J.; Meng, F.; Ma, J.; Tsubaki, N. SiC foam monolith catalyst for pressurized adiabatic methane reforming. Appl. Energy 2013, 107, 297–303. [Google Scholar] [CrossRef]
- Pereira, V.G.F.; Rodrigues, C.P.; Toniolo, F.S. Ni/Al2O3 supported on cordierite monoliths for methane steam reforming: Influence of catalyst coating methodology. Catal. Commun. 2023, 183, 106759. [Google Scholar] [CrossRef]
- Todic, B.; Nowicki, L.; Nikacevic, N.; Bukur, D.B. Fischer–Tropsch synthesis product selectivity over an industrial iron-based catalyst: Effect of process conditions. Catal. Today 2016, 261, 28–39. [Google Scholar] [CrossRef]
- Kulikova, M.V. The new Fischer-Tropsch process over ultrafine catalysts. Catal. Today 2020, 348, 89–94. [Google Scholar] [CrossRef]
- Ding, M.; Yang, Y.; Li, Y.; Wang, T.; Ma, L.; Wu, C. Impact of H2/CO ratios on phase and performance of Mn-modified Fe-based Fischer Tropsch synthesis catalyst. Appl. Energy 2013, 112, 1241–1246. [Google Scholar] [CrossRef]
- Tu, J.; Ding, M.; Zhang, Y.; Li, Y.; Wang, T.; Ma, L.; Wang, C.; Li, X. Synthesis of Fe3O4-nanocatalysts with different morphologies and its promotion on shifting C5+ hydrocarbons for Fischer–Tropsch synthesis. Catal. Commun. 2015, 59, 211–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Wang, T.; Li, X. MnO2 coated Fe2O3 spindles designed for production of C5+ hydrocarbons in Fischer–Tropsch synthesis. Fuel 2016, 177, 197–205. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, C.; Qin, S.; Xiang, H.; Li, Y. Effect of interaction between potassium and structural promoters on Fischer–Tropsch performance in iron-based catalysts. J. Mol. Catal. Chem. 2008, 286, 137–142. [Google Scholar] [CrossRef]
- Pendyala, V.R.R.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Sparks, D.E.; Hopps, S.; Davis, B.H. Fischer–Tropsch Synthesis: Effect of Activation Gas After Varying Cu Promoter Loading Over K-Promoted Fe-Based Catalyst. Catal. Lett. 2014, 144, 1624–1635. [Google Scholar] [CrossRef]
- Luque, R.; De La Osa, A.R.; Campelo, J.M.; Romero, A.A.; Valverde, J.L.; Sanchez, P. Design and development of catalysts for Biomass-To-Liquid-Fischer–Tropsch (BTL-FT) processes for biofuels production. Energy Environ. Sci. 2012, 5, 5186–5202. [Google Scholar] [CrossRef]
- Shafer, W.; Gnanamani, M.; Graham, U.; Yang, J.; Masuku, C.; Jacobs, G.; Davis, B. Fischer–Tropsch: Product Selectivity—The Fingerprint of Synthetic Fuels. Catalysts 2019, 9, 259. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Su, X.; Fan, S.; Ma, Q.; Zhao, T. Selective formation of light olefins from CO2 hydrogenation over Fe–Zn–K catalysts. J. CO2 Util. 2015, 12, 95–100. [Google Scholar] [CrossRef]
- Zhang, J.; Abbas, M.; Chen, J. The evolution of Fe phases of a fused iron catalyst during reduction and Fischer–Tropsch synthesis. Catal. Sci. Technol. 2017, 7, 3626–3636. [Google Scholar] [CrossRef]
- Sonal; Ahmad, E.; Upadhyayula, S.; Pant, K.K. Biomass-derived CO2 rich syngas conversion to higher hydrocarbon via Fischer-Tropsch process over Fe–Co bimetallic catalyst. Int. J. Hydrogen Energy 2019, 44, 27741–27748. [Google Scholar] [CrossRef]
- Bukur, D.B.; Mukesh, D.; Patel, S.A. Promoter effects on precipitated iron catalysts for Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 1990, 29, 194–204. [Google Scholar] [CrossRef]
- Silva, A.P.; Bahú, J.O.; Soccol, R.; Rodríguez-Urrego, L.; Fajardo-Moreno, W.S.; Moya, H.; León-Pulido, J.; Cárdenas Concha, V.O. Naphtha Characterization (PIONA, Density, Distillation Curve and Sulfur Content): An Origin Comparison. Energies 2023, 16, 3568. [Google Scholar] [CrossRef]
- Borecki, M.; Geca, M.; Zan, L.; Prus, P.; Korwin-Pawlowski, M.L. Multiparametric Methods for Rapid Classification of Diesel Fuel Quality Used in Automotive Engine Systems. Energies 2024, 17, 4189. [Google Scholar] [CrossRef]
- Teimouri, Z.; Abatzoglou, N.; Dalai, A. Insights to the reaction kinetics of Fischer-Tropsch synthesis using an integral system over Cu-Mo promoted Fe catalyst. Fuel 2024, 360, 130512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).