Abstract
Biochar, as an emerging biotechnology, has been widely used in the remediation of soil organic pollution, mainly by promoting the abundance of related degrading bacteria in soil. In this study, we explored the influence of sewage sludge biochars pyrolyzed at different temperatures of 300–700 °C (SSB300-SSB700) and addition rates (1% and 5%) on the atrazine biodegradation in soils. After a 21-day incubation, the application of 5% SSB300 significantly increased soil catalase (CAT), urease activity, dissolved organic carbon (DOC), and electrical conductivity (EC). However, biochar amendment exhibited inhibitory effects on atrazine degradation in soils. The atrazine degradation ratio decreased with decreasing pyrolysis temperature and increasing addition rates. Further analysis found that there were two possible reasons for the significant decline of atrazine biodegradation in SSB300 groups: (1) SSB300 demonstrated higher adsorption capacity for atrazine compared to SSB500 and SSB700 and reduced atrazine bioavailability due to its stronger hydrophobic nature and more abundant surface functional groups; and (2) the SSB300 significantly decreased the abundances of dominant atrazine-degraders (Arthrobacter and Pseudomonas) and atrazine-degrading genes (atzA, atzB, and trzN).
1. Introduction
Pesticides, as synthetic chemicals of natural origin, have been extensively employed in agricultural landscapes for safeguarding crops against pests and diseases [1,2]. Global pesticide consumption amounts to approximately 5 million tons annually [3]. Notably, China and the United States have emerged as the largest pesticide consumers, with respective applications of 1.77 million tons and 407,779 tons in 2017 [4]. Only 1% of pesticides remain effective after application, while the remaining portion persists in the soil [5]. The extensive misuse of pesticides can have adverse effects on soil quality and crop production. This ultimately leads to negative impacts on soil microbial biomass and soil enzyme activities, resulting in soil degradation [6]. Additionally, the persistent, bioaccumulative, and toxic properties of pesticide residues pose a significant threat to both the environment and human health [7]. Among the pesticides, atrazine is frequently detected at levels exceeding permissible limits. In China’s Yangtze River Delta agricultural soils, atrazine ranges from <1.0 to 113 ng/g [8]. Dou et al. [9] found atrazine levels of 134 ng/g in open-field soils and 137 ng/g in greenhouse soils. Furthermore, atrazine’s carcinogenic and endocrine-disrupting effects may trigger potential ecotoxicological risks [10,11]. Hence, it is crucial to formulate strategies that expedite the removal and mitigate the toxic impacts of atrazine in agricultural soils, as this holds paramount significance for ecological integrity and human well-being.
Biochar is generated from biomass conversion via thermochemical processes in oxygen-limited environments [12,13]. Biochar exhibits potential for augmenting agriculture and fostering environmental well-being, particularly in soil organic contamination remediation. Biochar enhanced the biodegradation of soil organic contamination by promoting the proliferation of relevant degrading bacteria [14]. Bao et al. [15] investigated the effects of biochar on polycyclic aromatic hydrocarbon (PAH) removal in soil and observed that it facilitated PAH degradation through the increased abundance of PAH-degrading bacteria such as Bacillus, Lysobacter, Ohtaekwangia, Porphyrobacter, Rhizobium, and Sphingomonas. Furthermore, biochar promotes the degradation of atrazine, thiacloprid, polychlorinated biphenyls, and total petroleum hydrocarbons by providing a suitable habitat and nutrient source for microbes [16,17,18,19]. Numerous studies have consistently revealed that biochar has a positive impact on soil properties, including pH, electrical conductivity (EC), soil porosity, and water-holding capacity [20,21,22]. These improvements, in turn, influence the dynamics of soil microbial communities and facilitate the degradation of organic pollutants. However, previous studies have suggested that the strong adsorption capability of biochar for organic contaminants might impede microbial degradation processes [23,24], potentially resulting in higher residual levels of these contaminants in soils and posing ecological risks.
Furthermore, it is worth noting that biochar exhibits diverse properties due to different raw materials and pyrolysis conditions, which consequently influence the migration and transformation of soil organic pollutants. Sewage sludge (SS), a byproduct of wastewater treatment, was selected as the feedstock due to its high organic content and nutrient richness, making it a promising candidate for waste-to-resource conversion. Unlike crop-residue biochars, sewage sludge biochar (SSB) contains unique components, such as nitrogen-rich functional groups and mineral ash, which can alter soil physicochemistry (e.g., EC, dissolved organic carbon) and microbial habitats more profoundly [15,20,21]. Pyrolysis temperature was also a critical factor shaping biochar properties, directly influencing its carbon/nitrogen (C/N) content, surface functional groups, and porosity, which collectively affected pollutant transport in soils. Higher temperatures enhanced carbonization, increasing fixed carbon content while reducing volatile N-containing compounds and leading to lower N content and higher C/N ratios. Surface functional groups shifted from oxygen-rich moieties (e.g., hydroxyl, carboxyl) at low temperatures to aromatic and graphitic structures at high temperatures, altering surface polarity and adsorption capacity [15,21]. Low-temperature biochars (300–500 °C), with abundant polar groups, exhibited stronger interactions with polar organic pollutants (e.g., phenols) via hydrogen bonding, while high-temperature biochars (>600 °C), characterized by large pores and hydrophobic surfaces, excelled in adsorbing nonpolar contaminants (e.g., PAHs) through π-π interactions and pore-filling effects [16,20]. These properties modulated soil pollutant transport: biochar amendments either retarded (via enhanced adsorption) or facilitated (via pore-mediated pathways) contaminant migration, depending on temperature-derived characteristics [16,24]. Understanding this temperature–property–function relationship was essential for optimizing biochar production to address specific soil contamination challenges. Additionally, the application rates of biochar have shown an impact on pollutant biodegradation. For instance, Zhao et al. [25] found that a 4% addition of rape straw biochar resulted in greater removal efficiency for total polycyclic aromatic hydrocarbons compared to treatments with lower addition rates, such as 1% or 2%. Similarly, Sopeña et al. [26] found that adding wood charcoal biochar at a rate of 0.1% had a minimal effect on the isoproturon dissipation rate; however, higher addition rates, such as 1% or 2%, significantly reduced its dissipation rate. Therefore, there is an imperative need to investigate how both pyrolysis temperature and addition rate-dependent degradation mechanisms affect organic pollutant degradation in soils while exploring microbial degradation pathways.
Taking these concepts into consideration, atrazine was selected as a representative extensively utilized herbicide. Sewage sludge biochar (SSB) pyrolyzed at temperatures ranging from 300 °C to 700 °C was prepared for this study. The aim of this work was to explore the potential of SSB as a valuable resource for remediating atrazine-contaminated soil and achieving the goal of converting solid waste into a valuable asset. Following the addition of different types and dosages of SSB, the dissipation and bioavailability of atrazine in soils, enzyme activities, and soil properties, e.g., pH, EC, DOC, and dissolved nitrogen (DN), were evaluated. Additionally, the correlation between the atrazine degradation ratio and its bioavailability, environmental factors, and microbial community was also analyzed. Our research will contribute insights into understanding microbial responses towards atrazine degradation while guiding the further optimization of SSB characteristics.
2. Materials and Methods
2.1. Soil Samples
The soils utilized were obtained from agricultural soil (0–20 cm) located in northeast China. (126°49′40″E, 45°40′60” N) The soil atrazine was less than 0.02 mg/kg. Atrazine was purchased from Shanghai Macklin Biochemical Co., Ltd., Pudong, China. Subsequently, the soils were sieved (60-mesh) and spiked with atrazine dissolved in methanol to achieve 10.00 ± 0.14 mg/kg. Following this, all the soil was stored at 25 °C for 7 days for further investigation purposes. Detailed information on the soil samples is shown in Table S1. To ensure sterilization, the soil underwent four repetitions of the sterilization process at a temperature of 121 °C for a period lasting up to four hours each time.
2.2. Biochar Characteristics
The sewage sludge (SS) was obtained in a sewage plant in Beijing and then crushed and sieved (20-mesh) after drying. SSB was produced at 300, 500, and 700 °C under nitrogen conditions (2 h). The SSBs derived at 300, 500, and 700 °C were labeled as SSB300, SSB500, and SSB700, respectively. Detailed physicochemical properties of the SS and SSB are provided in Table S2. All elemental content determinations were performed on a dry weight basis. Fourier transform infrared (FT-IR) spectroscopy (Thermo Fisher Scientific Co., Ltd, Waltham, MA, USA) was applied to explore the biochar surface functional groups, while the hydrophilicity/hydrophobicity of the biochar was assessed using a Contact Angle measuring instrument (Zhongchen Digital Technology Equipment Co., Ltd., Shanghai, China) [27].
2.3. Pot Experiment
The experimental groups consisted of sterilized soil and non-sterilized soil. SSB300 was added at rates of 1% and 5%, denoted as 1% SSB300 and 5% SSB300, respectively. Similarly, the treatments with SSB500 and SSB700 were designated as follows: 1% SSB500, 5% SSB500, 1% SSB700, and 5% SSB700. The control group without any addition of SSB was labeled as CK. In summary, flowerpots (Φ10 cm) were filled with 200 g of soil along with the corresponding amounts of SSB for each experimental treatment described above. The soil moisture was maintained at 30% using deionized water, and incubation took place in darkness at a temperature of 25 °C. Each treatment was performed in triplicate, and samples were collected on days 3, 7, 14, and 21 for subsequent analysis.
2.4. Atrazine Analysis
The soil atrazine extraction was conducted as described by Luo et al. [28]. Briefly, 3 g of dry-weight soil, 10 mL of methanol, and 1.0 g of NaCl were added to a 50 mL centrifuge tube and subjected to ultrasound for 20 min. Subsequently, the supernatant was collected by centrifugation (3000 rpm, 5 min). The extraction process was repeated three times, with an additional addition of 10 mL of methanol each time. The combined supernatant was concentrated and dissolved in methanol and analyzed using UPLC [29].
The bioavailability of atrazine was obtained as described by Cao et al. [30]. Initially, a 0.01 M CaCl2 solution was supplemented with NaN3 to minimize microbial activity [31], resulting in a final concentration of 25 mg L−1 NaN3 (referred to as CaCl2-NaN3 solution). Subsequently, 3 g of soil and 40 mL of the CaCl2-NaN3 solution were combined in a 50 mL centrifuge tube and agitated at 60 rpm for 24 h. Finally, the supernatant was obtained through centrifugation and filtered for UPLC analysis.
2.5. Soil Enzyme Activity Analysis
The soil catalase (CAT) and urease were analyzed using the Soil CAT and Urease Assay Kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The detailed extraction procedures are described by Hou et al. [32]. A UV spectrophotometer (Puxi General Instrument Co., Ltd, Beijing, China) was used to measure the activities of soil enzymes.
2.6. Determination of Environmental Factors
Soils (3 g) were sampled and added to a 50 mL centrifuge tube with 15 mL H2O, then shaken at 180 rpm for 10 h. The supernatant was collected after centrifugation to analyze pH, EC, DOC, and DN. pH was determined by a pH meter (PHSJ-3F, Shanghai Precision Instruments Co.; Ltd., Shanghai, China), EC was analyzed by a digital conductivity meter (DDS-11A, Shanghai Precision Instruments Co., Ltd.), and DOC and DN were measured by multi N/C 2100 (Analytik Jena AG, Jena, Germany).
2.7. DNA Extraction and Sequencing
The 16s rDNA sequencing was carried out to analyze soil bacterial community structures, and details of the DNA extraction and determination referenced by Miao et al. [33] are presented in the Supplementary Materials.
2.8. Relative Quantification of Atrazine-Degrading Genes
The DNA extraction method was the same as in Section 2.7. Three atrazine degradation genes, atzA, atzB, and trzN, were selected for relative content determination. Primers refer to the reports of Mulbry et al. [34] and De Souza et al. [35], and detailed primers are provided in Table S3. Wcgene Biotechnology Co., Ltd., Shanghai, China, provided sequencing.
2.9. Statistical Analysis
One-way analysis of variance (ANOVA) was utilized to assess the variations among all groups (SPSS version 21.0). The bioinformatic analysis was conducted on the Majorbio Cloud Platform [36]. The remaining statistical analyses were carried out using Origin 2019 software.
3. Results and Discussion
3.1. Analysis of the Properties of Biochar and Soils Amended with Biochar
The characterization of SSB is shown in Table S2. In summary, biochar properties changed significantly with pyrolysis temperature. The increase in carbon content observed in sewage sludge biochar (SSB300, 48.40% wt.), compared to raw sewage sludge (RS, 41.31% wt.), was primarily attributed to the thermal decomposition and mass loss of non-carbonaceous components during low-temperature pyrolysis rather than actual carbon generation. During pyrolysis at 300 °C, volatile matter, such as low-molecular-weight organics (e.g., fatty acids, carbohydrates) and inorganic salts, is preferentially removed as gaseous byproducts (e.g., H2O, CO2, small hydrocarbons), concentrating the relatively stable carbonaceous structures in the residual biochar [20,37]. This process increases the proportion of fixed carbon in the biochar on a dry-weight basis, as the loss of non-carbon components exceeds the loss of carbon itself at low temperatures. With the higher pyrolysis temperature (from 300 °C to 700 °C), the content of C, N, and H decreased from 48.40% to 32.52% (C), 5.80 to 2.96% (N), and 2.02 to 0.74% (H). Zhang et al. [20] found that the content of C, N, and H in biochar decreased with an increase in pyrolysis temperature. As the temperature of pyrolysis increases, the majority of C and H are released as H2O, CO2, and CO [37]. Lang et al. [38] discovered that N primarily volatilizes during this process, with approximately 21–71% of N transforming into gaseous pyrolysis products, such as hydrogen cyanide, ammonia, and fulminic acid nitride [39,40]. In our work, SSB500 exhibited the highest BET surface area (47.53 m2/g), likely due to moderate pyrolysis promoting pore development without pore collapse. In contrast, SSB700 showed reduced porosity (7.16 m2/g), consistent with findings by Xing et al. [41], where high-temperature pyrolysis collapses micropores and increases ash content. The properties of soil amended with SSB are shown in Figure 1. HT-biochar contributed to increased soil pH compared to LT-biochar (Figure 1A). The increase in pH may be due to the residual inorganic minerals and alkaline components in HT-biochars [42]. Biochar significantly increased soil EC in the late incubation period (Figure 1B), which may be attributed to the high content of basic cations in biochar [43]. Biochar increased soil DOC concentration, especially at low temperatures (Figure 1C). Cui et al. [44] and Yang et al. [45] reported that biochar contributed to increasing soil DOC. Previous studies have also shown that LT-biochar had a higher concentration of DOC, and HT-biochar had lower DOC due to a higher degree of C aromatization [46]. The DN level showed an increase when LT-biochars were added, whereas the addition of HT-biochars resulted in a decrease in DN (Figure 1D). The inclusion of HT-biochars could potentially inhibit N availability by promoting its adsorption onto the biochar surface [47].
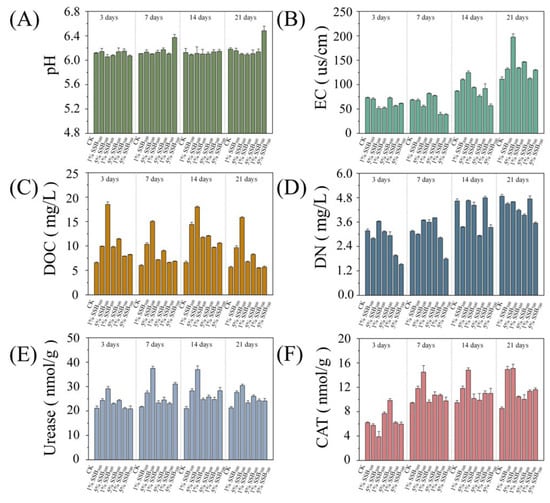
Figure 1.
The pH (A), EC (B), DOC (C), DN (D), Urease (E), and CAT (F) in biochar-amended soils.
Urease is an enzyme related to microbial N metabolism and a critical index of soil microbial activity [48]. LT-biochar application significantly increased soil urease (Figure 1E), and HT-biochar suppressed the urease, which might be due to the adsorption of N [49]. Catalase activity has been reported to be related to aerobic organisms’ metabolic activity and has been treated as an indicator of soil fertility [50]. In our study, LT-biochar significantly increased catalase activity, especially with 5% addition, while the change was not evident in the HT-biochar treatment (Figure 1F). Alef and Nannipieri [51] reported that catalase activity significantly correlated with organic carbon content. A positive correlation between DOC and catalase activity was found at 7 days in our study (R = 0.922**). Biochar is rich in many nutrient elements, such as C, N, P, and K, which can be used as a nutrient supplement for soil microorganisms, thus promoting the growth and vitality of microorganisms [13,20]. Our study suggested that DOC and DN might be the main factors affecting microbial communities. Because the DOC and DN of soil supplemented with biochar were significantly higher than those of CK, especially for LM-biochar, the increase in DOC and DN was more evident with biochar dosage.
3.2. Impacts of SSB on Atrazine Degradation and Bioavailability
The degradation of atrazine in the sterilized soil decreased in the order 5% SSB300 > 1% SSB300 > 5% SSB500 > 1% SSB500 > 1% SSB700 > 5% SSB700 > CK (Figure S1). After 21 days, atrazine degradation was at most 2.14 mg kg−1 in the 5% SSB300 treatment group, which was 1.59 mg kg−1 higher than that in the CK group. Biochar application generally increased atrazine chemical degradation in the sterilized soil, and this result could be confirmed by electron spin resonance analysis in Figure S2. On the contrary, biochar application inhibited atrazine degradation in unsterilized soil. The degradation of atrazine was in the order CK > 1% SSB700 > 1% SSB500 > 5%SSB700 1%SSB300 > 5%SSB500 > 5% SSB300 (Figure 2). After 21 days of incubation, the concentration of atrazine in the 5% SSB300 treatment group was 1.17 mg/kg, which was 1.00 mg/kg higher than that in the control group. The degradation ratio of atrazine in unsterilized conditions was a combination of both chemical degradation and biodegradation. At the end of 21 days of incubation, the degradation ratio of atrazine in the control in unsterilized and sterilized soils was 98.04% and 5.57%, respectively. In the 5% SSB300 treatment group, 86.63% and 24.62% atrazine degradation were found. Though a little arbitrary, the distinction between sterilized and unsterilized groups was acknowledged as the impact of atrazine biodegradation, a commonly accepted concept in the existing literature [18,52,53]. Consequently, based on the data provided, it can be inferred that biodegradation played a more significant role compared to chemical degradation. Additionally, the introduction of biochar hindered atrazine’s biodegradation within the soil.
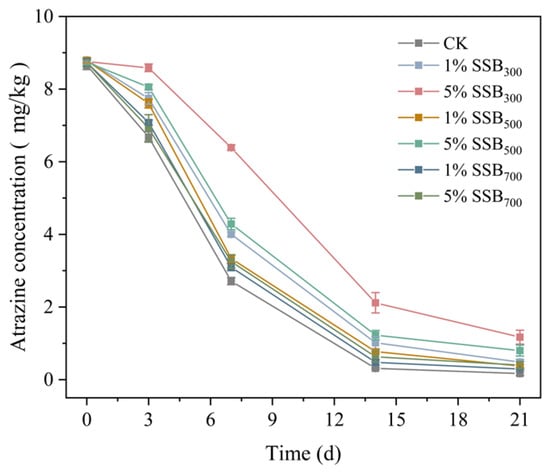
Figure 2.
Atrazine concentrations in unsterilized soils from different treatments.
The widespread observation of reduced pesticide biodegradation due to adsorption effects has been noted, and the addition of biochar in soil has been found to enhance the adsorption of pesticides. This is likely to result in a decrease in the availability of pesticides for biodegradation [54,55,56]. Hence, we also investigated the concentration of the bioavailability of atrazine (atrazine-CaCl2) in soils in different treatments. The bioavailability of atrazine in soils is shown in Figure 3. Compared to the CK, the SSB700 groups did not affect soil atrazine bioavailability. In SSB300 and SSB500, biochar application increased the adsorption of atrazine. The bioavailability of atrazine in 5% SSB300 decreased by 0.66 mg/kg on day 3 and 0.63 mg/kg on day 7 compared to the CK. Although SSB500 has a larger BET, SSB300 has a more substantial adsorption capacity for atrazine. Fruehwirth et al. [57] revealed that LT-biochar exhibited an increased abundance of organic functional groups, potentially impacting the adsorption behavior towards atrazine. This dissimilarity arises from inadequate combustion, as biochars generated under lower temperature conditions tend to retain their unique functional groups and structures, unlike those obtained through higher temperature processes [21]. In our study, we did not find any difference between the types of functional groups in LT-biochar and HT-biochar (Figure 4A), and we speculated that the difference in adsorption capacity may be caused by the number of functional groups. Zhao et al. [58] revealed that the hydrophobicity and low polarity of biochar facilitated atrazine adsorption. As shown in Figure 4B, SSB300 has lower hydrophilia, which can also explain the reason why LT-biochar has a stronger adsorption of atrazine. We did not detect the bioavailability of atrazine because the concentration of atrazine is below the quantitative detection limit (0.01 mg/L) on days 14 and 21 for all treatments.
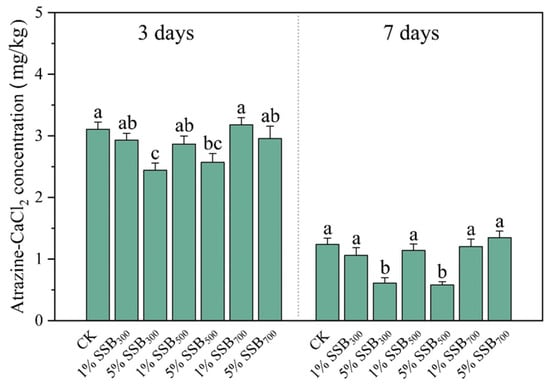
Figure 3.
The atrazine-CaCl2 concentration in biochar-amended soils.

Figure 4.
FTIR spectra (A) and contact angle (B) of different biochars.
3.3. Dynamics of Soil Microbial Community Structure with Biochar Application
According to the results of PCA (Figure 5), the first principal component (PC1) accounted for 49.04% of the total variance, and PC2 accounted for 88.02% of the total variance. Moreover, DOC, CAT, and urease were the top three parameters that affected the soil microbial community structure. The phylum-level classification of soil microbial composition is displayed in Figure 6. Biochar application increased the abundance of Actinobacteriota (4.09–21.72%) compared to CK, especially for 5% SSB300. HT-biochar (SSB500 and SSB700) contributed to increasing the abundance of Actinobacteriota (0.06–1.61%), Bacteroidota (0.07–0.23%), and Chloroflexi (0.70–2.63%) compared to CK, while LT-biochar (SSB300) caused opposite results.
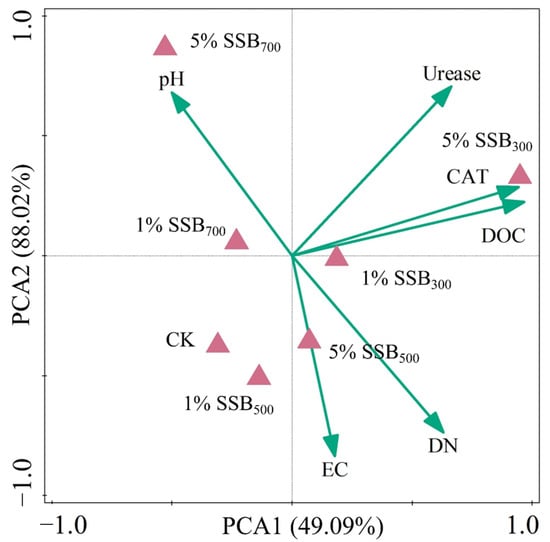
Figure 5.
The principal component analysis (PCA) in soils from different treatments. DOC: dissolved organic carbon; DN: dissolved nitrogen; CAT: catalase; EC: electrical conductance.
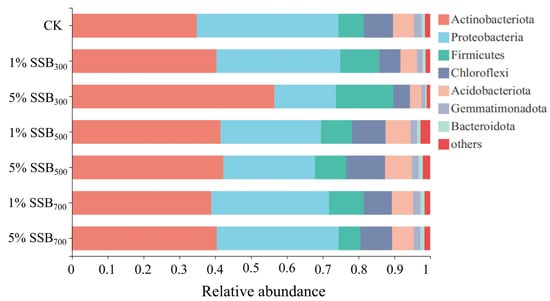
Figure 6.
Composition of the main bacterial phyla in soils from different treatments.
Additionally, Firmicutes, Proteobacteria, and Actinobacteria were the top three bacterial phyla in SSB-amended soils. Qiu et al. [59] found that Acidobacteria, Chloroflexi, Proteobacteria, and Actinobacteriota were the four most dominant phyla with peanut shell biochar amendment, accounting for approximately 77–78%. Yin et al. [60] also reported that Actinobacteriota (25.4–32.1%), Chloroflexi (7.6–9.6%), and Proteobacteria (35.5–38.4%) were the predominant phyla in cow manure biochar and reed straw biochar treatments. These abundant phyla have been reported to be involved in organic matter turnover in the C, N, and S cycle, restoring the nutrient levels in the soil [61].
Heatmap clustering based on the top 20 genera in all groups is shown in Figure 7. Among them, nine, six, and two genera belong to Actinobacteriota, Proteobacteria, and Chloroflexi, respectively. The other three genera (Subgroup, Bacillus, and Gemmatimonadaceae) belong to Acidobacteriota, Firmicutes, and Gemmatimonadota, respectively. LT-biochar amendment significantly increased the relative abundance of Bacillus, which was 1.87–2.95 times higher than CK (Figure 7). The higher addition rate of SSB300 led to the higher relative abundance of Bacillus. A similar finding was also reported in a previous study [62], in which LT-biochar increased the abundance of Bacillus more significantly than HT-biochar. Bacillus is an anaerobic bacterium that can degrade atrazine [63,64]. Ding et al. [62] revealed that the increased relative abundance of the Bacillus might be attributed to the higher content of DOM in LT-biochar. In contrast, the relative abundance of KD4-96, MB-AZ-108, Gemmatimonadaceae, and Massilia was enhanced by HT-biochar amendment. Among them, Massilia was aerobic, which prefers to live in larger pores (>5 µm) with more nutrients and air [45]. HT-biochar has a larger pore size, providing Massilia with a more comfortable habitat and the air, thus promoting its growth. Additionally, biochar decreased the relative abundance of Sphingomonas in all treatments. In a previous study, Sphingomonas was positively correlated with atrazine degradation [28]. In this work, the relative abundance of Streptomyces was induced to increase with LT-biochar application but not obviously in HT-biochar treatments. Saez et al. [65] found that the inoculation of bio-mixtures with Streptomyces induced atrazine removal. With biochar amendments, no regular changes were observed in Nocardia, which was reported as an atrazine-degrading bacterium in a previous study [66].
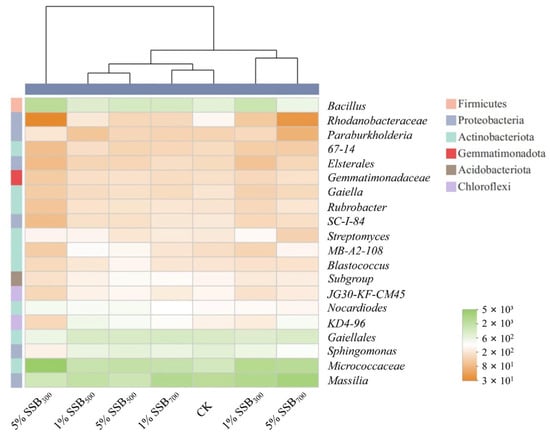
Figure 7.
Heatmap clustering based on the top 20 genera in soils from different treatments.
3.4. Biodegradation Mechanisms of Atrazine in SSB-Amended Soils
As stated in Section 3.2, the addition of LT- and HT-biochars had contrasting impacts on the atrazine biodegradation in SSB-amended soils. The addition of LT-biochar hindered the biodegradation ratio of atrazine, which decreased with higher application rates due to a decrease in atrazine’s bioavailability. Ding et al. [62] also observed that the amendment of LT-biochar inhibited the biodegradation ratio of phenanthrene, which declined as the pyrolysis temperature increased. Differently, in our study, the inhibition biodegradation of atrazine by HT-biochar was significantly lower than that by LT-biochar. The adsorption capacity of HT-biochar for atrazine becomes weaker, which may also be due to the smaller SSA; moreover, low-temperature biochar has lower hydrophilia and more functional groups, which led to a stronger adsorption of atrazine [57,67].
At present, atrazine degradation genes have been extensively explored. The gene atzA, encoding atrazine chlorohydrolase [EC:3.8.1.8], and trzN, encoding triazine hydrolase [EC:3.5.4.32], induces the dechlorination of atrazine to form hydroxyatrazine in the first step. Subsequently, atzB, encoding hydroxydechloroatrazine ethylaminohydrolase [EC:3.5.4.43], catalyzes hydroxyatrazine to form N-isopropylammelide [68]. Finally, other genes (atzC, atzD, trzN, atzE, and atzF) are further involved in N-isopropylammelide metabolism. To further explain the mechanisms for the changes in atrazine biodegradation with biochar amendment, the abundance of the atzA, atzB, and trzN genes was quantified. As shown in Figure 8, biochar addition significantly decreased the abundance of atzA in all treatments. SSB300 amendment led to a significant decline in the abundance of atzB (p < 0.01) and trzN, suggesting that SSB300 inhibited the atrazine-degrading bacteria. And the inhibition was more significant with the increase in addition rates. Through Pearson correlation analysis, we found that atzA was significantly correlated with the abundance of Pseudomonas (Figure 9A), which also indicated that in this experiment, Pseudomonas contained degradation genes, which played a role in degrading atrazine.
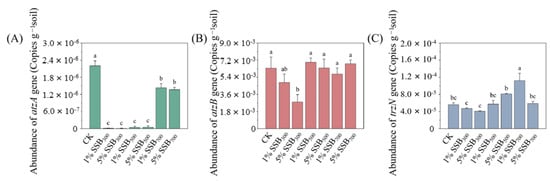
Figure 8.
Abundance of gene atzA (A), atzB (B), and traN (C) at day 7 in different treatments.
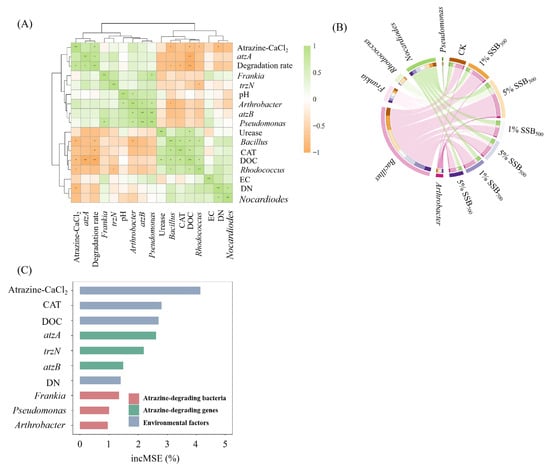
Figure 9.
Correlation analysis of atrazine degradation rate with environmental factors and atrazine degradation-related genera (one-way ANOVA, * p < 0.05, ** p < 0.01) (A); effect of biochar prepared at different temperatures on the relative abundance of genera related to atrazine degradation in soil on day 7 (B); determination of key factors affecting atrazine degradation rate based on random forest model (C).
Previous studies have shown that biochar application changes the soil microbial community by altering soil properties (e.g., pH, soil enzyme activities, and DOC), thus further affecting the biodegradation of pollutants [15,18]. As can be seen from Figure 9A, atzA and atrazine-CaCl2 significantly affected the degradation rate of atrazine (R = 0.89** with atzA, R = 0.86** with atrazine-CaCl2), suggesting that atrazine bioavailability and atrazine-degrading bacteria were the main factors of atrazine degradation in soil. Through correlation analysis, we found that atzB was significantly positively correlated with the atrazine-degrading bacteria Arthrobacter (R = 0.89**) and Pseudomonas (R = 0.96**). Previous studies also reported that Arthrobacter and Pseudomonas contained atzB genes [69,70], further confirming that Arthrobacter and Pseudomonas were involved in atrazine metabolism. We selected six genera—Arthrobacter [69,71], Bacillus [64], Frankia [72], Nocardioides [66], Pseudomonas [73], and Rhodococcus [74]—that have been reported to have the ability to degrade atrazine directly. These six genera also exhibited higher abundance in the CK treatment groups compared to the SSB treatment groups, which was an important contributing factor to the soil’s indigenous capacity for atrazine degradation [72,73]. As shown in Figure 9B, 5% SSB300 significantly decreased the relative abundance of the atrazine-degrading bacteria Arthrobacter and Pseudomonas. This may also explain why LT-biochar inhibits atrazine degradation.
In addition, DOC is an important factor affecting the degradation of atrazine. DOC was negatively correlated with atrazine degradation rate (R = −0.96**), atrazine-CaCl2 (R = 0.79*), and atzA (R = −0.96**). Previous studies have shown that LT-sludge biochar was more toxic than HT-biochar. Low-temperature biochar contains more aromatic CHON substances (e.g., amines, phenols, and heterocyclic-N) and shows stronger biotoxicity to pakchoi seeds, Vibrio qinghaiensis Q67, and zebrafish [75]. It can be inferred that the biotoxicity of low-temperature biochar to atrazine-degrading bacteria may also be the reason for its inhibition of atrazine degradation. In addition, the random forest model was used to further determine the effects of environmental factors, atrazine-degrading bacteria, and atrazine-degrading genes on the degradation rate of atrazine. It can be seen from Figure 9C that atrazine-CaCl2, CAT, DOC, and atzA are the most critical factors affecting the degradation of atrazine in soil.
In conclusion, the amendment of biochar with different temperatures and addition rates will not only affect the bioavailability of atrazine but also affect soil microbial activity and community structure. LT-biochar has a stronger adsorption capacity for atrazine, thus limiting its biodegradation. In addition, a large number of previous studies have found that LT-biochar has more potent toxic effects on soil microorganisms, plants, and invertebrates [75]. Ding et al. [62] also speculated that biochar might inhibit the abundance of specific PAH-degrading bacteria in the soil, resulting in a reduction in PAH degradation. In our study, we found that the decrease in the abundance of the important atrazine-degrading bacteria Arthrobacter and Pseudomonas may be the main factor affecting the biodegradation of atrazine. Moreover, we further amplified the atrazine degradation gene, which further proved that low-temperature biochar inhibited the abundance of atrazine-degrading bacteria, resulting in a decrease in atrazine degradation. This critical conclusion also gives us a warning that we should pay attention to the ecological risks caused by biochar land use.
4. Conclusions
This work explored the impact of pyrolysis temperature and addition rate on the biodegradation of atrazine in biochar-amended soils. Regardless of different pyrolysis temperatures and addition rates, biochar application was found to inhibit the degradation of atrazine. The soil microbial communities were primarily influenced by both the pyrolysis temperature and the addition rates of biochar, mainly due to changes in soil DOC content following biochar amendment. Furthermore, it was observed that soil DOC content played a crucial role in determining the total relative abundance of the dominant degradation compounds associated with atrazine. In soils amended with LT-biochar, decreased atrazine degradation occurred as a result of the reduced availability and abundance of atrazine-degrading bacteria, despite lower concentrations of bioavailable atrazine. Conversely, higher pyrolysis temperatures led to an increased availability and abundance of these bacteria, thereby promoting greater levels of atrazine degradation. Consequently, appropriate characteristics when amending biochar have the potential to mitigate high residual levels of atrazine in soils while simultaneously reducing ecological risks.
In addition, although SSB adsorbed atrazine and reduced the mobility of atrazine in the soil, it hindered the biodegradation rate of atrazine. In order to further achieve the bioremediation of organic polluted soil by SSB, future work will focus on the modification treatment of SSB, such as activation treatment (chemical/thermal activation), to reduce its adsorption of organic pollutants and realize its remediation effect on organic polluted soil in practical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en18123158/s1, Figure S1. Atrazine concentrations of atrazine in sterilized soils from different treatments. Figure S2. Electron spin resonance (ESR) signal analysis of different biochar. Table S1. The basic physical and chemical properties of soil samples. Table S2. The characteristics of biochar and raw sludge (RS). Table S3. Previously published primers (F for forward, R for reverse) used for PCR amplification of atrazine degradation genes, including individual nucleotide sequences and annealing temperatures.
Author Contributions
Methodology, Y.L.; Writing—original draft, S.L.; Writing—review & editing, X.L. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Science Foundation of National Engineering Research Center for Safe Disposal and Resources Recovery of Sludge (Harbin Institute of Technology, Grant No Z2024A006), National Key Research and Development Program of China (2019YFC1906501 and 2018YFC1901100).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, V.; Montanarella, L.; Fernández-Ugalde, O.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef] [PubMed]
- FAO. World Food and Agriculture—Statistical Pocketbook 2019; FAO: Rome, Italy, 2019. [Google Scholar]
- Roser, M. Pesticides. 2022. Available online: https://ourworldindata.org/pesticides (accessed on 29 September 2022).
- Sabzevari, S.; Hofman, J. A worldwide review of currently used pesticides’ monitoring in agricultural soils. Sci. Total Environ. 2022, 812, 152344. [Google Scholar] [CrossRef]
- Baxter, J.; Cummings, S.P. The degradation of the herbicide bromoxynil and its impact on bacterial diversity in a top soil. J. Appl. Microbiol. 2008, 104, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils–A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Sun, J.T.; Pan, L.L.; Tsang, D.C.W.; Zhan, L.Z.; Li, X.D. Atrazine contamination in agricultural soils from the Yangtze River Delta of China and associated health risks. Environ. Geochem. Health 2016, 39, 369–378. [Google Scholar] [CrossRef]
- Dou, R.; Sun, J.; Deng, F.; Wang, P.; Zhou, H.; Wei, Z.; Chen, M.; He, Z.; Lai, M.; Ye, T.; et al. Contamination of pyrethroids and atrazine in greenhouse and open-field agricultural soils in China. Sci. Total Environ. 2020, 701, 134916. [Google Scholar] [CrossRef]
- Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A.A.; Vonk, A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 2002, 99, 5476–5480. [Google Scholar] [CrossRef]
- Shenoy, V.S. Atrazine and its endocrine-disrupting effects: A review. Environ. Toxicol. Pharmacol. 2012, 33, 594–609. [Google Scholar]
- Manyà, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.M.; Xu, W.H.; Dong, J.; Yang, T.; Shangguan, Z.C.; Qu, J.; Li, X.; Tan, X. The effects of biochar and its applications in the microbial remediation of contaminated soil: A review. J. Hazard. Mater. 2022, 438, 129557. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.Y.; Wang, J.F.; Zhang, H.; Li, J.; Li, H.; Wu, F.Y. Effects of biochar and organic substrates on biodegradation of polycyclic aromatic hydrocarbons and microbial community structure in PAHs-contaminated soils. J. Hazard. Mater. 2020, 385, 121595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, P.; Xu, X.J.; Sun, H.W.; Jiang, B.; Liao, Y.H. Spectroscopic and molecular characterization of biochar-derived dissolved organic matter and the associations with soil microbial responses. Sci. Total Environ. 2020, 708, 134619. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, C.L.; Rong, Q.; Li, C.Z.; Mao, J.; Liu, Y.; Chen, J.; Liu, X. Effect of two organic amendments on atrazine degradation and microorganisms in soil. Appl. Soil Ecol. 2020, 152, 103564. [Google Scholar] [CrossRef]
- Zhang, P.; Ren, C.; Sun, H.W.; Min, L.J. Biochars change the sorption and degradation of thiacloprid in soil: Insights into chemical and biological mechanisms. Environ. Pollut. 2018, 236, 158–167. [Google Scholar] [CrossRef]
- Huang, S.Y.; Bao, J.P.; Shan, M.J.; Qin, H.; Wang, H.L.; Yu, X.J.; Chen, J.H.; Xu, Q.F. Dynamic changes of polychlorinated biphenyls (PCBs) degradation and adsorption to biochar as affected by soil organic carbon content. Chemosphere 2018, 211, 120–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.M.; Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Liu, N.; Charrua, B.A.; Weng, C.H.; Yuan, X.; Ding, F. Characterization of biochars derived from agricultural wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol. 2015, 198, 55–62. [Google Scholar] [CrossRef]
- Ji, M.Y.; Wang, X.X.; Usman, M.; Liu, F.H.; Dan, Y.T.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, S.; Xia, X.; Lu, X.; Zhang, X.; Xia, N.; Liu, T. Effects of carbonaceous materials on microbial bioavailability of 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) in sediments. J. Hazard. Mater. 2016, 312, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.H.; McAllister, L.E.; Chen, R.; Semple, K.T. Impact of activated charcoal on the mineralisation of 14C-phenanthrene in soils. Chemosphere 2010, 79, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Miao, R.H.; Guo, M.X.; Shang, X.T.; Zhou, Y.M.; Zhu, J.W. Biochar enhanced polycyclic aromatic hydrocarbons degradation in soil planted with ryegrass: Bacterial community and degradation gene expression mechanisms. Sci. Total Environ. 2022, 838, 156076. [Google Scholar] [CrossRef] [PubMed]
- Sopeña, F.; Semple, K.; Sohi, S.; Bending, G. Assessing the chemical and biological accessibility of the herbicide isoproturon in soil amended with biochar. Chemosphere 2012, 88, 77–83. [Google Scholar] [CrossRef]
- Fan, M.J.; Li, C.; Shao, Y.W.; Zhang, S.; Gholizadeh, M.; Hu, X. Pyrolysis of cellulose: Correlation of hydrophilicity with evolution of functionality of biochar. Sci. Total Environ. 2022, 825, 153959. [Google Scholar] [CrossRef]
- Luo, S.; Zhen, Z.; Zhu, X.; Ren, L.; Wu, W.; Zhang, W.; Chen, Y.; Zhang, D.; Song, Z.; Lin, Z.; et al. Accelerated atrazine degradation and altered metabolic pathways in goat manure assisted soil bioremediation. Ecotoxicol. Environ. Saf. 2021, 221, 112432. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Li, Y.Y.; Bao, H.Y.; Nan, J.; Xu, G.R. Rapid biodegradation of atrazine by a novel Paenarthrobacter ureafaciens ZY and its effects on soil native microbial community dynamic. Front. Microbiol. 2023, 13, 1103168. [Google Scholar] [CrossRef]
- Cao, X.D.; Ma, L.N.; Liang, Y.; Gao, B.; Harris, W. Simultaneous Immobilization of Lead and Atrazine in Contaminated Soils Using Dairy-Manure Biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef]
- Bergknut, M.; Sehlin, E.; Lundstedt, S.; Andersson, P.L.; Haglund, P.; Tysklind, M. Comparison of techniques for estimating PAH bioavailability: Uptake in Eisenia fetida, passive samplers and leaching using various solvents and additives. Environ. Pollut. 2007, 145, 154–160. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, W.X.; Yang, Y.; Hu, J.; Bian, C.S.; Jin, L.P.; Li, G.C.; Xiong, X.Y. Rhizosphere microbial diversity and community dynamics during potato cultivation. Eur. J. Soil Biol. 2020, 98, 103176. [Google Scholar] [CrossRef]
- Miao, S.J.; Tang, Y.J.; Xue, H.Q.; Qiao, Y.F. Soil bacterial community responses to land-use change in Mollisol of Northeast China. Ecol. Eng. 2022, 184, 106771. [Google Scholar] [CrossRef]
- Mulbry, W.W.; Zhu, H.; Nour, S.M. The triazine hydrolase gene trzN from Nocardioides sp. strain C190: Cloning and construction of gene-specific primers. FEMS Microbiol. Lett. 2002, 206, 75–79. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.L.; Seffernick, J.; Martinez, B.; Sadowsky, M.J.; Wackett, L.P. The atrazine catabolism genes atzABC are widespread and highly conserved. J. Bacteriol. 1998, 180, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Yu, G.; Shi, C.P.; Liu, L.M.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.Y.; Lei, H.W.; Zhang, X.S.; Wang, L.; Bu, Q.; Wei, Y. Production of hydrocarbons from biomass-derived biochar assisted microwave catalytic pyrolysis. Energy Fuels 2018, 2, 1781–1790. [Google Scholar] [CrossRef]
- Lang, T.; Jensen, A.D.; Jensen, P.A. Retention of organic elements during solid fuel pyrolysis with emphasis on the peculiar behavior of nitrogen. Energy Fuels 2005, 19, 1631–1643. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Tian, S.S.; Tan, Z.X.; Kasiulienė, A.; Ai, P. Transformation mechanism of nutrient elements in the process of biochar preparation for returning biochar to soil. J. Chem. Eng. 2017, 25, 477–486. [Google Scholar] [CrossRef]
- Xing, J.; Li, L.; Li, G.B.; Xu, G.R. Feasibility of sludge-based biochar for soil remediation: Characteristics and safety performance of heavy metals influenced by pyrolysis temperatures. Ecotoxicol. Environ. Saf. 2019, 180, 457–465. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Li, Y.Y.; Bao, H.Y.; Xing, J.; Zhu, Y.Z.; Nan, J.; Xu, G.R. Biochar acts as an emerging soil amendment and its potential ecological risks: A review. Energies 2023, 16, 410. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.D.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.I.; Bolan, N.S.; et al. Advances and future directions of biochar characterization methods and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- Cui, J.L.; Glatzel, S.; Bruckman, V.J.; Wang, B.; Lai, D.Y.F. Long-term effects of biochar application on greenhouse gas production and microbial community in temperate forest soils under increasing temperature. Sci. Total Environ. 2021, 767, 145021. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Chang, K.H.; Kim, Y.J.; Zhang, J.; Yoo, G. Effects of different biochar amendments on carbon loss and leachate characterization from an agricultural soil. Chemosphere 2019, 226, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Xu, G.R.; Li, G.B. Comparison of pyrolysis process, various fractions, and potential soil applications between sewage sludge-based biochars and lignocellulose-based biochars. Ecotoxicol. Environ. Saf. 2021, 208, 111756. [Google Scholar] [CrossRef]
- Cao, H.; Ning, L.; Xun, M.; Feng, F.; Yang, H. Biochar can increase nitrogen use efficiency of Malus hupehensis by modulating nitrate reduction of soil and root. Appl. Soil Ecol. 2019, 135, 25–32. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, H.J.; Cha, S.J.; Kwon, S.J.; Park, J.H. Effect of Pyroligneous Acid on Soil Urease, Amidase, and Nitrogen Use Efficiency by Chinese Cabbage (Brassica campestris var. pekinensis). Environ. Pollut. 2021, 291, 118132. [Google Scholar] [CrossRef]
- Feng, Y.F.; Sun, H.J.; Xue, H.J.; Wang, Y.M.; Yang, L.Z.; Shi, W.M.; Xing, B.S. Sawdust Biochar Application to Rice Paddy Field: Reduced Nitrogen Loss in Floodwater Accompanied with Increased NH3 Volatilization. Environ. Sci. Pollut. Res. 2018, 25, 8388–8395. [Google Scholar] [CrossRef]
- Trasar-Cepeda, B.; Gil-Sotres, F.; Leiros, M.C. Thermodynamic Parameters of Enzymes in Grassland Soils from Galicia, NW Spain. Soil Biol. Biochem. 2007, 39, 311–319. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995. [Google Scholar]
- Zhang, P.; Ren, C.; Sun, H.W.; Min, L.J. Sorption, Desorption and Degradation of Neonicotinoids in Four Agricultural Soils and Their Effects on Soil Microorganisms. Sci. Total Environ. 2018, 615, 59–69. [Google Scholar] [CrossRef]
- Ferrario, C.; Pittino, F.; Tagliaferri, I.; Gandolfi, I.; Bestetti, G.; Azzoni, R.S.; Diolaiuti, G.; Franzetti, A.; Ambrosini, R.; Villa, S. Bacteria Contribute to Pesticide Degradation in Cryoconite Holes in an Alpine Glacier. Environ. Pollut. 2017, 230, 919–926. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P. Bioavailability and Bioaccessibility of Polycyclic Aromatic Hydrocarbons (PAHs) in Historically Contaminated Soils after Lab Incubation with Sewage Sludge-Derived Biochars. Chemosphere 2016, 163, 480–489. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yao, T.; Tan, S.; Yu, B.Q.; Liu, K.L.; Hu, L.F.; Luo, K.; Liu, M.; Liu, X.Y.; Bai, L.Y. Effects of pH and Gallic Acid on the Adsorption of Two Ionizable Organic Contaminants to Rice Straw-Derived Biochar-Amended Soils. Ecotoxicol. Environ. Saf. 2019, 184, 109656. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Song, Y.; Shi, R.Y.; Liu, Z.T.; Bian, Y.R.; Wang, F.; Yang, X.L.; Gu, C.G.; Jiang, X. Biochar Reduces the Bioaccumulation of PAHs from Soil to Carrot (Daucus carota L.) in the Rhizosphere: A Mechanism Study. Sci. Total Environ. 2017, 601–602, 1015. [Google Scholar] [CrossRef] [PubMed]
- Fruehwirth, M.; Sbizzaro, M.; Rosa, D.M.; Sampaio, S.C.; dos Reis, R.R. Adsorption of Atrazine by Biochars Produced from Byproducts of the Wood Industry. Eng. Agric. 2020, 40, 769–776. [Google Scholar] [CrossRef]
- Zhao, Z.D.; Wu, Q.; Nie, T.T.; Zhou, W.J. Quantitative Evaluation of Relationships Between Adsorption and Partition of Atrazine in Biochar-Amended Soils with Biochar Characteristics. RSC Adv. 2019, 9, 4162–4171. [Google Scholar] [CrossRef]
- Qiu, H.S.; Liu, J.Y.; Boorboori, M.R.; Li, D.; Chen, S.; Ma, X.; Cheng, P.; Zhang, H. Effect of Biochar Application Rate on Changes in Soil Labile Organic Carbon Fractions and the Association between Bacterial Community Assembly and Carbon Metabolism with Time. Sci. Total Environ. 2023, 855, 158876. [Google Scholar] [CrossRef]
- Yin, S.J.; Zhang, X.; Suo, F.Y.; You, X.W.; Yuan, Y.; Cheng, Y.D.; Zhang, C.S.; Li, Y.Q. Effect of Biochar and Hydrochar from Cow Manure and Reed Straw on Lettuce Growth in an Acidified Soil. Chemosphere 2022, 298, 134191. [Google Scholar] [CrossRef]
- Close, D.C.; Sohi, S.P.; Bending, G.D. Microbial Diversity and the Carbon and Nitrogen Cycles in Soils Affected by Biochar. Soil Biol. Biochem. 2009, 41, 1016–1027. [Google Scholar]
- Ding, Z.; Zhang, F.; Gong, H.F.; Sun, N.; Huang, J.J.; Chi, J. Responses of Phenanthrene Degradation to the Changes in Bioavailability and Microbial Community Structure in Soils Amended with Biochars Pyrolyzed at Low and High Temperatures. J. Hazard. Mater. 2021, 410, 124584. [Google Scholar] [CrossRef]
- Khatoon, H.; Rai, J.P.N. Optimization Studies on Biodegradation of Atrazine by Bacillus badius abp6 Strain Using Response Surface Methodology. Biotechnol. Rep. 2020, 26, e00459. [Google Scholar] [CrossRef]
- Jakinala, P.; Lingampally, N.; Kyama, A.; and Hameeda, B. Enhancement of Atrazine Biodegradation by Marine Isolate Bacillus velezensis mhnk1 in Presence of Surfactin Lipopeptide. Ecotoxicol. Environ. Saf. 2019, 182, 109372. [Google Scholar] [CrossRef]
- Saez, J.M.; González, S.K.; Ocante, T.A.L.; Bigliardo, A.L.; Briceño, G.E.; and Benimeli, C.S. Actinobacteria Bioaugmentation and Substrate Evaluation for Biobeds Useful for the Treatment of Atrazine Residues in Agricultural Fields. J. Environ. Manag. 2022, 320, 115870. [Google Scholar] [CrossRef]
- Omotayo, A.E.; Ilori, M.O.; Radosevich, M.; Amund, O.O. Metabolism of Atrazine in Liquid Cultures and Soil Microcosms by Nocardioides Strains Isolated from a Contaminated Nigerian Agricultural Soil. Soil Sediment Contam. 2013, 22, 365–375. [Google Scholar] [CrossRef]
- Niu, L.; Liu, Y.; Zhang, W.; Chen, H.; Li, Z.; Zhao, Z. Adsorption of Atrazine on Biochar Derived from Rice Straw: Characterization and Effects of Solution pH. Chemosphere 2015, 138, 186–193. [Google Scholar]
- Zhang, Y.; Cao, B.; Jiang, Z.; Dong, X.N.; Hu, M.; Wang, Z.G. Metabolic Ability and Individual Characteristics of an Atrazine-Degrading Consortium DNC5. J. Hazard. Mater. 2012, 237–238, 376–381. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Wang, L.; Ma, F.; Yang, J.X. Characterisation of an Efficient Atrazine-Degrading Bacterium, Arthrobacter sp. ZXY-2: An Attempt to Lay the Foundation for Potential Bioaugmentation Applications. Biotechnol. Biofuels 2018, 11, 113. [Google Scholar] [CrossRef]
- Boundy-Mills, K.L.; Souza, M.L.D.; Mandelbaum, R.T.; Wackett, L.P.; Sadowsky, M.J. The atzB Gene of Pseudomonas sp. Strain ADP Encodes the Second Enzyme of a Novel Atrazine Degradation Pathway. Appl. Environ. Microbiol. 1997, 63, 916–923. [Google Scholar] [CrossRef]
- Bazhanov, D.P.; Yang, K.; Li, H.M.; Li, C.Y.; Li, J.S.; Chen, X.F.; Yang, H.T. Colonization of Plant Roots and Enhanced Atrazine Degradation by a Strain of Arthrobacter ureafaciens. Appl. Microbiol. Biotechnol. 2017, 101, 6809–6820. [Google Scholar] [CrossRef]
- Rehan, M.; Kluge, M.; Fränzle, S.; Kellner, H.; Ullrich, R.; Hofrichter, M. Degradation of Atrazine by Frankia alni ACN14a: Gene Regulation, Dealkylation, and Dechlorination. Appl. Microbiol. Biotechnol. 2014, 98, 6125–6135. [Google Scholar] [CrossRef]
- Tonelli Fernandes, A.F.; Braz, V.S.; Bauermeister, A.; Rizzato Paschoal, J.A.; Lopes, N.P.; Stehling, E.G. Degradation of Atrazine by Pseudomonas sp. and Achromobacter sp. Isolated from Brazilian Agricultural Soil. Int. Biodeterior. Biodegr. 2018, 130, 17–22. [Google Scholar] [CrossRef]
- Kolekar, P.D.; Phugare, S.S.; Jadhav, J.P. Biodegradation of Atrazine by Rhodococcus sp. BCH2 to N-Isopropylammelide with Subsequent Assessment of Toxicity of Biodegraded Metabolites. Environ. Sci. Pollut. Res. 2014, 21, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.L.; Hu, A.B.; Wang, Q.D.; Ai, J.; Zhang, W.J.; Liang, Y.; Cao, M.X.; Wu, H.J.; Wang, D.S. Molecular Composition and Biotoxicity Effects of Dissolved Organic Matters in Sludge-Based Carbon: Effects of Pyrolysis Temperature. J. Hazard. Mater. 2022, 424, 127346. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).