1. Introduction
The urgent necessity to mitigate climate change and reduce greenhouse gas (GHG) emissions has propelled the investigation of alternative energy carriers that are both sustainable and scalable. As global temperatures continue to rise and the impacts of climate change become increasingly evident, the need for innovative solutions in the energy sector has never been more critical [
1]. Among the various options being explored, ammonia has emerged as a promising candidate due to its unique characteristics and advantages [
2]. Its carbon-free nature ensures that combustion does not produce carbon dioxide, the primary greenhouse gas driving global warming. Furthermore, ammonia’s relatively high energy density enables efficient storage and transportation, making it particularly suitable for long-distance energy distribution. The well-established infrastructure for ammonia production and handling, originally developed for the fertilizer industry, provides a robust foundation for its integration as an energy carrier. Notably, ammonia can be synthesized using renewable electricity through the Haber–Bosch process, positioning it as a potential transitional fuel on the path toward a fully decarbonized energy future [
3,
4]. However, ammonia faces challenges as a standalone fuel in internal combustion engines, including low reactivity and narrow flammability limits [
5]. These limitations can lead to poor combustion efficiency, possible emission of unburned ammonia (ammonia slip) [
6], and difficulties in engine starting and operation. The low flame speed and high ignition energy requirements of ammonia pose significant hurdles for its direct use in conventional engine designs.
To address these issues, researchers are exploring the use of hydrogen in dual-fuel configurations with ammonia [
7,
8,
9,
10,
11,
12]. Hydrogen, the lightest and most abundant element in the universe, offers complementary properties that can enhance ammonia’s performance as a fuel. Hydrogen’s high reactivity and clean-burning properties complement ammonia’s limitations, enhancing flame stability and ignition characteristics. The addition of hydrogen to ammonia fuel mixtures can significantly improve combustion efficiency, reduce ignition delay, and expand the operating range of engines. This dual-fuel approach improves combustion efficiency and reduces emissions while minimizing the need for extensive engine modifications [
13]. By carefully balancing the ratio of ammonia to hydrogen, engine performance can be optimized across various operating conditions. The presence of hydrogen helps initiate and sustain combustion, allowing for more complete burning of the ammonia fuel. This results in lower emissions of unburned ammonia, addressing one of the environmental concerns associated with ammonia combustion. Furthermore, the dual-fuel strategy allows for greater flexibility in fuel composition, enabling engines to adapt to varying availability of ammonia and hydrogen. This adaptability is particularly valuable during the transition period as renewable hydrogen production and ammonia infrastructure are being developed and scaled up. On the other hand, the addition of ammonia to hydrogen can play a crucial role in moderating peak flame temperatures during the combustion processes. This reduction in temperature is particularly advantageous, as it directly contributes to the mitigation of thermal nitrogen oxides (NO
x) emissions [
14].
As the global energy sector moves toward integrating renewable hydrogen production and ammonia transport infrastructure, the study of ammonia–hydrogen dual-fuel systems becomes increasingly relevant [
15]. Developing optimized strategies for their combustion is critical to overcoming technical barriers, minimizing emissions, and maximizing energy efficiency.
This review addresses these challenges by examining the current state of knowledge on ammonia–hydrogen “dual-fuel” combustion in internal combustion engines. It aims to consolidate experimental insights and modeling advancements, identify knowledge gaps, and propose future research directions to enable the widespread adoption of this sustainable dual-fuel technology. Compared to the existing literature, this review provides a focused analysis of dual-fuel ammonia–hydrogen applications, offering a high level of technical detail on both injection and ignition strategies, and integrating recent advances in optical diagnostics and computational modeling.
2. Properties of Ammonia and Hydrogen
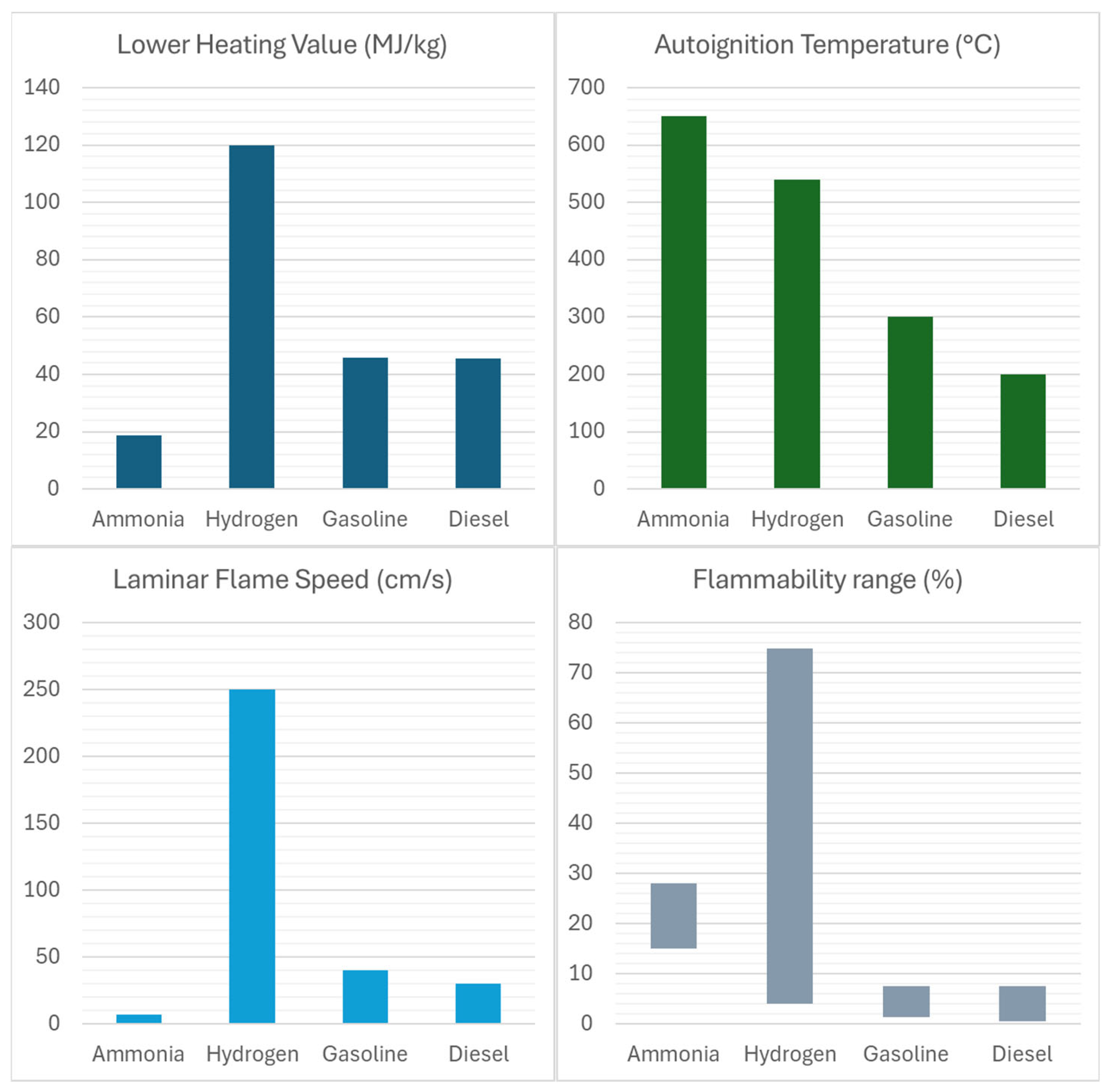
Table 1 summarizes the general physicochemical properties of ammonia and hydrogen fuels, while
Figure 1 provides a comparative overview of their key characteristics relative to conventional fuels.
Ammonia is a colorless, pungent-smelling gas at standard temperature and pressure, composed of one nitrogen atom and three hydrogen atoms. It has several unique chemical and thermodynamic properties that are relevant for its consideration as a fuel:
Low flammability: Ammonia has a narrow flammability range, requiring high temperatures and ignition energy to sustain combustion. The flammability limits for ammonia in air at standard atmospheric conditions are 15% by volume (lower flammability limit, LFL) and 28% by volume (upper flammability limit, UFL). This limited flammability range poses significant challenges for the use of ammonia as a fuel in internal combustion engines, as it can lead to poor combustion efficiency, unstable engine operation, and increased emissions of unburned ammonia and other pollutants [
12].
High autoignition temperature: Ammonia has a high autoignition temperature (around 650 °C) [
18], which is significantly higher than that of conventional hydrocarbon fuels (gasoline 300 °C and diesel 230 °C). This characteristic makes it difficult to initiate combustion in engines without the use of an additional ignition source, such as a spark plug or diesel pilot injection.
Low flame speed: Ammonia has a relatively low laminar flame speed compared to other hydrocarbon fuels. This property can result in incomplete combustion and increased emissions, particularly at high engine loads and speeds [
19].
High heat of vaporization: Ammonia has a high heat of vaporization (1.371 MJ/kg), which can lead to extreme charge cooling that lowers in-cylinder temperatures, potentially resulting in incomplete combustion and reduced thermal efficiency. On the other side, the charge cooling, depending on the injection configuration and strategies, could improve volumetric efficiency and extend knock limits, with potential benefits for efficiency.
Corrosiveness and toxicity: Ammonia is a corrosive substance, which can cause compatibility issues with engine materials and require specialized fuel system components. Moreover, it is toxic to humans and ecosystems [
20].
NO
x potential: The combustion of ammonia can lead to the formation of nitrogen oxides due to the presence of N in the fuel molecule [
21]. Careful combustion management and optimization of the engine operating parameters are required to mitigate NO
x emissions and ensure the environmental benefits of ammonia as a fuel. This may involve adjusting the air–fuel ratio, combustion chamber design, and other engine parameters to promote more complete and cleaner combustion of the ammonia fuel.
Despite these challenges, ammonia offers practical benefits that make it an attractive alternative fuel:
CO2-less combustion: The primary and most significant advantage of using ammonia as a fuel is its zero CO2 emissions during combustion.
Energy Density: Ammonia has a moderate energy density, 15.6 MJ/L, allowing it to store and transport large amounts of energy in a relatively compact form.
Storability: Ammonia can be liquefied under moderate pressure, allowing for efficient storage and transportation. This is advantageous compared to other gaseous fuels.
Transportability: The existing infrastructure for ammonia production and distribution, primarily developed for the fertilizer industry, provides a foundation for its adoption as an energy carrier.
Hydrogen is a highly reactive and clean-burning fuel, making it an ideal complement to ammonia in dual-fuel internal combustion engines. More in detail:
An ammonia–hydrogen dual-fuel approach can leverage the complementary properties of these two fuels, leading to improved engine performance, lower emissions, and increased overall energy efficiency.
Synergies in Dual-Fuel Mode
The combination of ammonia and hydrogen in a dual-fuel system effectively addresses many of the challenges associated with their individual use. By leveraging their complementary chemical and physical properties, this approach improves ignition stability, enhances combustion efficiency, and mitigates emissions, making it a promising solution for decarbonized internal combustion engines.
Ammonia and hydrogen have complementary chemical properties. The high flame speed and wide flammability range of hydrogen play a crucial role in compensating for the slow combustion and narrow flammability range of ammonia. These properties enable stable ignition and complete combustion, even under lean conditions where ammonia alone would struggle. From an emissions perspective, the presence of hydrogen helps minimize ammonia slip (unburned ammonia), allowing for more complete combustion, addressing a key drawback of ammonia-fueled engines. Moreover, the combustion of ammonia mainly produces nitrogen and water vapor, with nitrogen oxides (NOx) being the main concern. Hydrogen addition allows also leaner combustion, lowering peak flame temperatures and thus reducing NOx formation. Finally, its faster combustion shortens the residence time at high temperatures, further limiting NOx production.
In addition to their combustion properties, ammonia and hydrogen also complement each other in terms of storage and handling. Ammonia has a much higher volumetric energy density and is easier to store and transport than hydrogen, as it remains liquid under moderate pressure and temperature. This makes it an efficient hydrogen carrier and enables a flexible fuel supply, also facilitating high-pressure injection by means of more efficient pumping systems. Controlled decomposition of ammonia, by catalytic cracking, can further optimize dual-fuel operation by providing hydrogen on demand.
The dual-fuel approach offers a well-balanced solution that utilizes the strengths of both fuels while mitigating their individual limitations. Ammonia alone struggles with ignition stability and slow flame propagation, which are effectively improved by hydrogen. Hydrogen alone struggles with high combustion temperature, storage, and distribution issues, which ammonia can overcome due to its cooling effect, its liquid state, and existing infrastructure. Together, they form a practical and efficient fuel system that combines the superior combustion properties of hydrogen with the storage and transportation benefits of ammonia to create a viable, decarbonized internal combustion engine.
3. Fuel Injection Strategies for Ammonia–Hydrogen Dual Fuel
Fuel injection plays a pivotal role in the performance and emissions characteristics of dual-fuel ammonia–hydrogen engines. The choice of injection strategy, whether direct, indirect, or a combination of both, has a significant impact on combustion stability, thermal efficiency, and pollutant formation. Given the contrasting properties of ammonia and hydrogen, these strategies need to be carefully designed to capitalize on their respective strengths while mitigating their limitations.
3.1. Direct Injection (DI)
In direct injection (DI) systems, the fuel is introduced directly into the combustion chamber: this allows precise control over the fuel–air mixture and combustion timing [
31]. This approach is particularly advantageous for pure ammonia, as it ensures optimal mixing and minimizes unburned ammonia emissions (ammonia slip). Additionally, direct injection supports higher compression ratios, leading to improved thermal efficiency.
3.1.1. Ammonia DI
Ammonia can be directly injected into the combustion chamber in liquid or gaseous form. Due to ammonia’s high latent heat of vaporization, injecting it as a liquid helps with in-cylinder cooling, which can be beneficial in mitigating knock (SI case) and controlling the combustion temperature. However, the phase transition from liquid to gas must be carefully managed to ensure proper mixing with air and to prevent excessive cooling that could hinder ignition. Direct injection of gaseous ammonia can also be considered [
32], but gas injection presents greater difficulties due to the inherent physical properties of ammonia: it condenses at a pressure of more than about 6 bar. This imposes limitations on engine operation, as a low compression ratio must be maintained, and the time available for injection is extremely short—essentially limited to the interval between the exhaust valves fully closing and the moment when the pressure in the cylinder exceeds 6 bar. If this timing is not adhered to, the injected gas can escape through the exhaust system. Such an approach may be more practical for low-speed marine engines, where the use of low-pressure ammonia injection lines could be considered. Therefore, direct liquid injection remains the preferred method to achieve better fuel control, optimize spray formation, and improve overall combustion efficiency in DI ammonia-powered internal combustion engines.
As a drawback, direct injection of ammonia requires injectors designed to handle its physical properties, such as the low vapor pressure. Despite the complexities, DI remains a compelling choice for applications where maximizing efficiency and minimizing slip emissions are priorities.
In [
33], the authors investigated direct injection of ammonia sprays, comparing their behavior to that of Diesel fuel. The findings revealed that ammonia exhibits slower penetration and a mixing-controlled evolution under non-reacting conditions, offering insights relevant to combustion strategy development. In [
34], the study focused on ammonia direct injection in spark-ignition (SI) engines, analyzing combustion characteristics with increased compression ratios and high-flow rate injectors. The results showed that higher compression ratios led to elevated unburned NH
3 emissions, while the use of high-flow rate injectors improved network output by 4.7%.
3.1.2. Hydrogen DI
For hydrogen-only engines, introducing hydrogen directly into the combustion chamber (DI) instead of premixing it with air in the intake manifold (PFI) offers numerous advantages in terms of combustion control, thermal efficiency, and emission reduction.
One of the principal advantages of hydrogen DI lies in the enhancement of combustion efficiency [
35,
36]. Direct injection allows precise modulation of the air–fuel ratio and supports the formation of locally rich or lean mixtures as required, which in turn promotes more complete combustion and improves engine thermal efficiency. The ability to meter hydrogen directly into the cylinder allows more effective utilization of hydrogen’s high diffusivity and reactivity, resulting in higher output energy and lower unburned fuel losses. Hydrogen DI also plays an important role in controlling pollutant emissions. In hydrogen combustion, NO
x formation remains a critical challenge, especially under stoichiometric and lean conditions. Advanced injection strategies, including retarded injection timing and multi-pulse injections, have shown potential for NO
x reduction.
From the point of view of combustion stability, direct injection during the compression stroke significantly reduces the risk of pre-ignition, which is usually associated with port fuel injection of hydrogen. In PFI systems, the hydrogen–air mixture can linger in the intake manifold, where it can encounter high temperatures that promote backfire. By limiting the residence time of the hydrogen in the hot intake environment and ensuring a stratified mixture at ignition, DI contributes to safer and more controllable engine operation [
37]. In addition, DI contributes to the suppression of pre-ignition phenomena: early, uncontrolled ignitions that can precede a backfire and severely disrupt the combustion process. These phenomena are often triggered by residual hot spots or sub-optimal valve timing, conditions that are more common in PFI systems. Injecting hydrogen during the compression stroke in DI systems not only circumvents these triggers but also reduces the time available for reaching the thermodynamics favorable for auto-ignition.
Nevertheless, the implementation of hydrogen DI is associated with a number of technical challenges. The design and positioning of the injector are critical factors influencing mixture formation and combustion characteristics. Empirical and simulation-based studies consistently indicate that a centrally mounted injector, especially when placed close to the spark plug, enables better stratification and increases ignition reliability. Variations in nozzle geometry also affect the spray pattern, penetration, and interaction with in-cylinder turbulence, all of which influence combustion efficiency and emissions [
35,
38]. Due to the very short injection windows imposed by the engine cycle, high-pressure hydrogen injection systems are essential. These systems must deliver precise fuel quantities at high speed, which requires the development of robust and fast-acting injectors that can operate under demanding conditions.
Both experimental investigations and numerical modeling play a vital role in advancing the understanding of hydrogen DI processes. Computational fluid dynamics (CFD) simulations are widely used to optimize injector design and injection strategies by providing detailed insight into mixture formation, in-cylinder flow behavior, and flame development. These simulations are often supported by experimental campaigns using optically accessible engines and endoscopic diagnostics, which allow direct visualization of spray dynamics and combustion phenomena [
25].
3.2. Indirect Injection (PFI)
Indirect injection systems introduce the fuel into the intake manifold or port, allowing it to mix with air before entering the combustion chamber.
Indirect injection offers several advantages for both hydrogen and ammonia, mainly due to its simplicity. The injection system operates at lower pressures compared to DI, reducing the complexity and cost of the injection system. This feature makes PFI particularly attractive for retrofitting existing combustion engines, as it requires fewer modifications to fuel injectors and fuel lines compared to high-pressure DI systems [
39]. In addition, the injection of fuel into the intake manifold allows a longer residence time for the fuel–air mixture, which promotes homogeneous charge formation and can improve combustion stability under certain operating conditions. This aspect is particularly beneficial for spark-ignition engines, where a well-mixed charge can improve flame propagation.
In addition, the risk of injector clogging and icing—potential problems in high-pressure DI systems due to ammonia freezing and cavitation—is reduced in PFI configurations, as the injection process takes place at lower injection pressures [
40].
Despite these advantages, PFI presents several inherent drawbacks.
A general disadvantage of indirect injection systems is that the thermal efficiency is generally lower compared to DI. In PFI configurations, one of the most critical limitations is the reduction in volumetric efficiency. Since the fuel is injected into the intake manifold in gaseous or vaporizing liquid form, it occupies space that would otherwise be available for fresh air intake, limiting the amount of oxygen available for combustion, which reduces the power density and volumetric efficiency of the engine. This phenomenon is particularly detrimental in high-load conditions, where maximum air intake is essential for power output [
41].
Moreover, premixing hydrogen with air in the intake manifold can introduce significant risks and challenges. Hydrogen has a wide flammability range, and when mixed with air, it can lead to unpredictable combustion characteristics. This variability may increase the likelihood of backfire.
Finally, incomplete mixing and the potential for early combustion can negatively impact overall engine efficiency.
In the case of ammonia, an important drawback of PFI is the increased risk of ammonia slip, i.e., the presence of unburned ammonia in the exhaust gases. Because PFI relies on a premixed charge, it lacks the benefits of mixture stratification offered by DI, where localized fuel-rich zones can improve ignition. Therefore, PFI systems can result in incomplete combustion of ammonia, misfiring, and increased unburned ammonia emissions. In addition, less precise dosing can lead to ammonia slip, especially in configurations with valve overlapping and scavenging. Consequently, higher ammonia emissions may require advanced aftertreatment systems such as selective catalytic reduction (SCR) to prevent environmental impacts [
42].
3.3. Dual-Fuel Injection Strategies
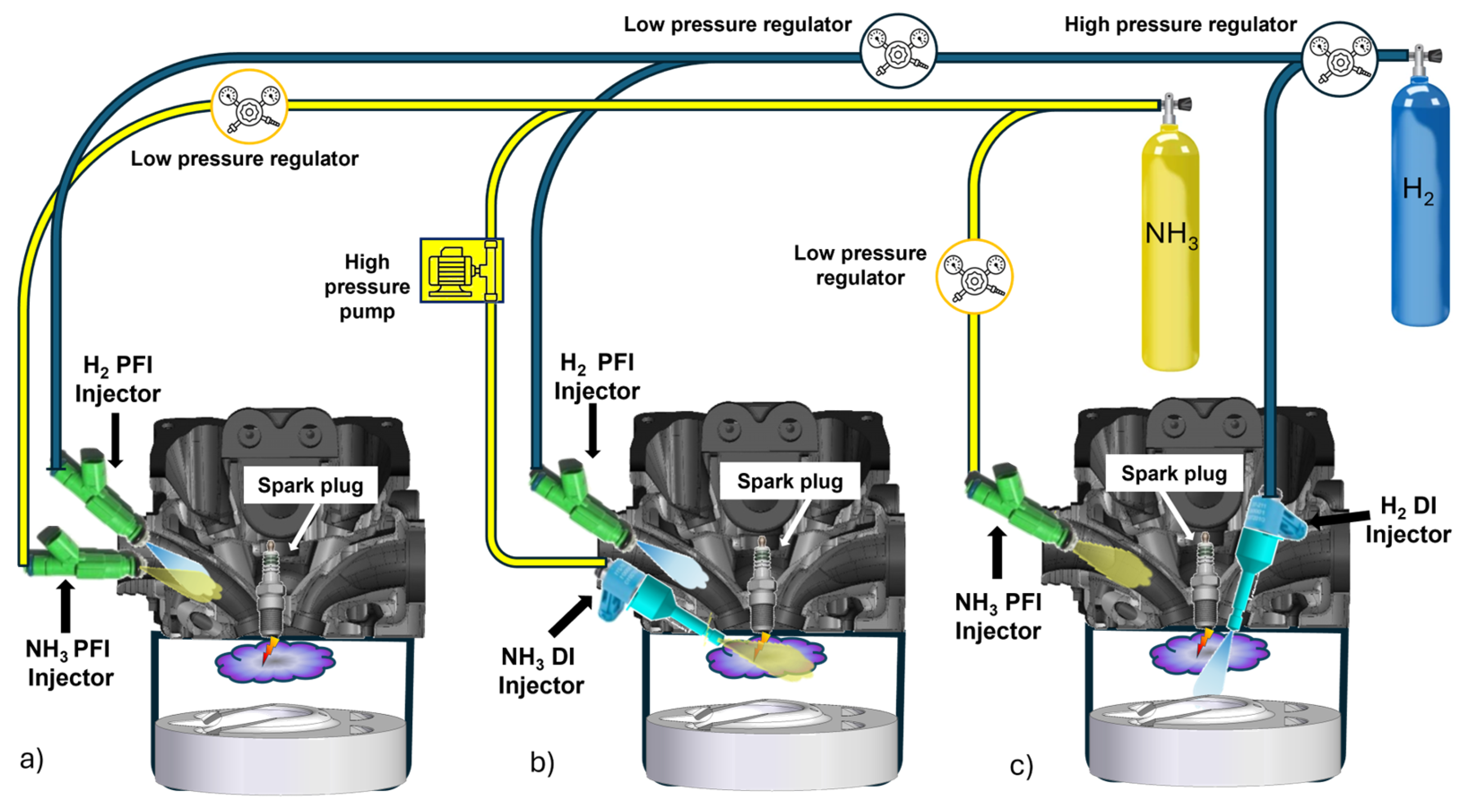
In dual-fuel hydrogen–ammonia engines, see
Figure 2, it is essential to understand the most effective injection configuration for each fuel, as the choice between direct and indirect injection—whether applied to hydrogen, ammonia, or both—plays a critical role in shaping combustion stability, thermal efficiency, and pollutant formation.
Frigo et al. investigated the use of hydrogen and ammonia as dual fuels in a spark-ignition engine equipped with two port fuel injection systems [
7],
Figure 2a. Hydrogen was the primary fuel, while ammonia contributed 25% of the total energy input. Due to concerns about backfiring associated with hydrogen PFI, the experiments were conducted under low-load conditions. Morch et al. explored a similar approach, supplying a mixture of hydrogen and ammonia through a single PFI injector in a spark-ignition engine. Their findings indicated that the maximum achievable load was 0.6 MPa, but the resulting indicated thermal efficiency was below 33% [
43]. Indirect injection is still a simpler alternative, especially for smaller engines or applications where cost is an important consideration. However, current research is mainly focused on direct injection of hydrogen and ammonia, as it allows for higher performance, better efficiency, and better control over combustion.
To balance the benefits of the DI and PFI approaches, hybrid injection strategies are increasingly being explored in dual-fuel engines. In these systems, hydrogen is typically injected indirectly into the intake manifold to stabilize the ignition and support combustion, while ammonia is injected directly into the combustion chamber to ensure precise control and minimize unburned emissions. This combination allows for optimized combustion dynamics: the premixed hydrogen–air mixture provides reliable ignition and rapid flame propagation, while the direct injection of ammonia enhances thermal efficiency and reduces emissions. As an example, in [
44], ammonia was directly injected into the cylinder of a spark-ignition engine, while hydrogen was introduced via the intake port,
Figure 2b. This dual-fuel strategy was designed to enhance combustion stability and expand the operating range of ammonia-fueled engines. The results demonstrated significant improvement: the operable range increased from 0.2 to 1.4 MPa, and the brake thermal efficiency was enhanced by 4.7%.
A recent paper [
45] demonstrates the feasibility of a direct-injection ammonia engine enhanced with hydrogen indirect injection for stable and efficient combustion. By adapting Hyundai’s 2.5 L LPG turbocharged direct injection (T-DI) engine, the authors successfully designed a system capable of overcoming ammonia’s inherent challenges, such as its high ignition energy requirement, slow flame speed, and emission concerns. To achieve this, significant modifications were introduced. The ignition system was enhanced to deliver higher energy, while the fuel system was upgraded with larger injectors and pumps to handle the greater fuel volume needed for ammonia combustion. The integration of a direct injection system for liquefied ammonia allowed precise control, essential for ensuring stable and efficient combustion. The study demonstrates that the optimized ammonia engine achieved performance levels comparable to the original LPG engine, with even better knock resistance at lower speeds and a peak brake thermal efficiency (BTE) of 40%. Hydrogen was introduced as a secondary fuel to support stable combustion, and the research provided valuable insights into the specific hydrogen requirements across various operating conditions. To make this approach more practical, the authors underscored the need for an onboard ammonia cracking system to supply hydrogen efficiently during operation. However, ammonia’s use as a primary fuel introduces challenges with raw emissions, particularly NH
3 and NO
x, which were significantly higher than those of conventional engines. The authors propose that a 30% reduction in these emissions, combined with optimized aftertreatment systems, could meet stringent EU7 regulations, paving the way for commercial viability.
On the other hand, the hybrid strategy of hydrogen direct injection and ammonia port injection can improve combustion stability compared to hydrogen port injection, which can lead to issues like premature ignition and backfiring. The direct injection of hydrogen improves the ignition characteristics, while the combustion stability of ammonia can be increased by the intake manifold injection, resulting in a more controlled combustion process,
Figure 2c. This dual-injection strategy was proposed in the study [
46] and offers a novel approach to improve combustion efficiency and reduce emissions in engines designed to meet environmental targets in the automotive sector. The study investigates the effects of different hydrogen blending ratios (HBRs), ranging from 6.1% to 57.7%, on combustion and emission characteristics. The results show that increasing the HBR improves engine performance, as indicated by higher mean effective pressures (IMEPs), but diminishing returns are observed beyond a 27.3% HBR. Furthermore, while higher hydrogen fractions lead to a reduction in ammonia emissions, they also lead to an increase in NO
x emissions, highlighting the trade-offs in emission profiles. The study identifies optimal operating conditions with an excess air coefficient (λ) of 1.2 and a hydrogen blending ratio of 27.3%, achieving a balance between performance and emissions.
Hybrid systems offer enhanced flexibility, as they enable real-time adjustments to fuel delivery based on engine load and operating conditions. However, these systems are more complex, requiring sophisticated control mechanisms and injector designs to manage the differing properties of the two fuels effectively.
Current Practices and Trends
In practice, the choice of injection strategy depends on the specific engine design and application. For stationary power generation, where efficiency and emissions control are critical, direct injection of ammonia combined with indirect injection of hydrogen is a popular choice. In mobile applications, such as marine or heavy-duty vehicles, hybrid systems are gaining traction due to their ability to adapt to varying load conditions while maintaining performance and emissions standards.
In some configurations, particularly those that include a third fuel like diesel, ammonia is injected directly, hydrogen is supplied indirectly, and diesel is used as a pilot fuel to assist ignition. These multi-fuel strategies highlight the versatility of injection systems in accommodating the diverse demands of dual-fuel ammonia–hydrogen engines.
4. Ignition Strategies for Ammonia–Hydrogen Dual Fuel
The ignition process in dual-fuel engines using ammonia and hydrogen plays a pivotal role in ensuring stable and efficient combustion.
As already mentioned, ammonia poses a major challenge for ignition due to its properties, in particular, its high minimum ignition energy and relatively low flame propagation speed. When hydrogen is mixed with ammonia, the overall reactivity of the fuel mixture improves considerably [
47]. This synergistic effect is particularly important in low engine load and cold start scenarios, where the ignition properties of ammonia may not be sufficient to ensure reliable combustion. In addition, the integration of hydrogen in the ammonia–fuel mixture plays a critical role in initiating and sustaining combustion, especially under lean operating conditions. To further enhance ignition reliability and efficiency, advanced ignition systems are often used in engines with ammonia–hydrogen mixtures.
4.1. Spark Ignition (SI) with Hydrogen Enhancement
Conventional spark-ignition systems can be adapted to meet the demanding ignition requirements of ammonia. By increasing the energy output of the ignition coil or using multi-spark systems, the ignition process can be made more robust. Studies show that optimizing the spark energy and discharge characteristics can provide stable ignition with 100% ammonia. In addition, advancing the ignition timing has been shown to be effective in balancing emissions, particularly NO
x and unburned ammonia, while minimizing N
2O formation [
48]. The presence of hydrogen in the fuel mixture plays a crucial role in ensuring that the spark is sufficient to reliably ignite the mixture, even when ammonia constitutes the majority of the fuel. The synergistic effect of the high reactivity of hydrogen combined with the improved ignition system enables stable and efficient combustion, even under lean air–fuel conditions or in cold start situations where ammonia alone is difficult to ignite.
4.2. Compression Ignition (CI) with Ammonia–Hydrogen Mixtures
Ammonia’s high autoignition temperature poses significant challenges for its use as pure fuel in compression-ignition (CI) engines. This characteristic makes it impractical for straightforward CI operation without modifications or additional fuels [
49]. As a result, the scientific community shifted focus towards using ammonia in dual-fuel configurations, where it can be combined with a secondary fuel that has a lower autoignition temperature, such as diesel or biodiesel. This approach allows for more feasible combustion and better performance [
50].
Hydrogen, on the other hand, offers high reactivity and flame speed, and when used as a blend with ammonia, it significantly improves ignition behavior and combustion phasing. However, blending these two fuels also raises concerns. While hydrogen enhances ammonia’s reactivity, excessive hydrogen content can lead to advanced combustion timing, which may compromise thermal efficiency [
51]. Moreover, ammonia combustion tends to produce substantial NO
x and N
2O emissions due to its nitrogen content, posing a challenge from both environmental and regulatory perspectives [
52,
53].
Dual-fuel systems, where diesel or hydrogen is used as a pilot or co-fuel, offer a compromise between efficiency and stability. Diesel pilot injection is especially effective in ensuring consistent ignition, while hydrogen supports flame development, resulting in better efficiency and lower ammonia slip [
54,
55]. More recently, tri-fuel strategies combining ammonia, hydrogen, and diesel have been tested to maximize the advantages of each fuel [
53].
Emission control remains a key concern. Exhaust gas recirculation (EGR) is commonly applied to reduce NO
x by lowering peak temperatures, though it can also raise unburned ammonia levels. Injection timing optimization and hydrogen enrichment have also been used to reduce N
2O and NH
3 emissions, though these strategies must be carefully balanced to avoid increasing thermal NO
x [
56].
Numerical modeling has proven essential in guiding these developments. RANS-based CFD simulations and thermodynamic studies have helped identify optimal ranges for air-to-fuel ratios, ignition phasing, and injection strategies [
57,
58]. These tools are crucial in designing engines that can operate reliably and cleanly on ammonia–hydrogen blends.
In conclusion, while ammonia–hydrogen combustion in CI engines poses several fundamental challenges, especially in terms of ignition and emissions, significant progress has been made. Through intelligent fuel blending, advanced combustion modes, and robust control strategies, these zero-carbon fuels have the potential to play a central role in the decarbonization of heavy-duty transport and power generation.
4.3. Pre-Chamber Combustion (PCC) with Turbulent Jet Ignition
For even greater efficiency and stability, pre-chamber ignition systems have been explored. In this setup, a small amount of hydrogen or a hydrogen-rich mixture is injected into a pre-chamber, where it is ignited to create high-energy jets of reactive species. These jets penetrate the main combustion chamber, ensuring rapid and uniform ignition of the ammonia-rich air–fuel mixture. Pre-chamber systems not only enhance ignition reliability but also improve combustion efficiency by promoting turbulence and better air–fuel mixing. This approach has shown impressive outcomes, achieving an indicated thermal efficiency (ITE) of 50.4% while maintaining low ammonia emissions [
59]. The design of the pre-chamber, especially parameters such as throat diameter, plays a crucial role in its performance. For example, narrower throats improve efficiency by increasing the reactivity of the jet, although they can lead to increased heat loss at higher hydrogen contents [
59]. A study by Wang et al. (2024) [
60] explores hydrogen multiple-injection jet-ignition (MIJI) as an innovative ignition system for ammonia–hydrogen dual-fuel combustion. The active jet system consisted of an H
2 injector, a spark plug, and a jet chamber. The H
2 injector and spark plug were positioned atop the jet chamber, which features seven orifices, including one bottom orifice and six peripheral ones. The methodology applied in this research utilizes a strategic triple-injection technique, which has proven to significantly enhance both fuel reactivity and flame front propagation, achieving an ITE of 42.5%. A particularly compelling feature of this method is its capability to function with a hydrogen energy ratio of less than 3%.
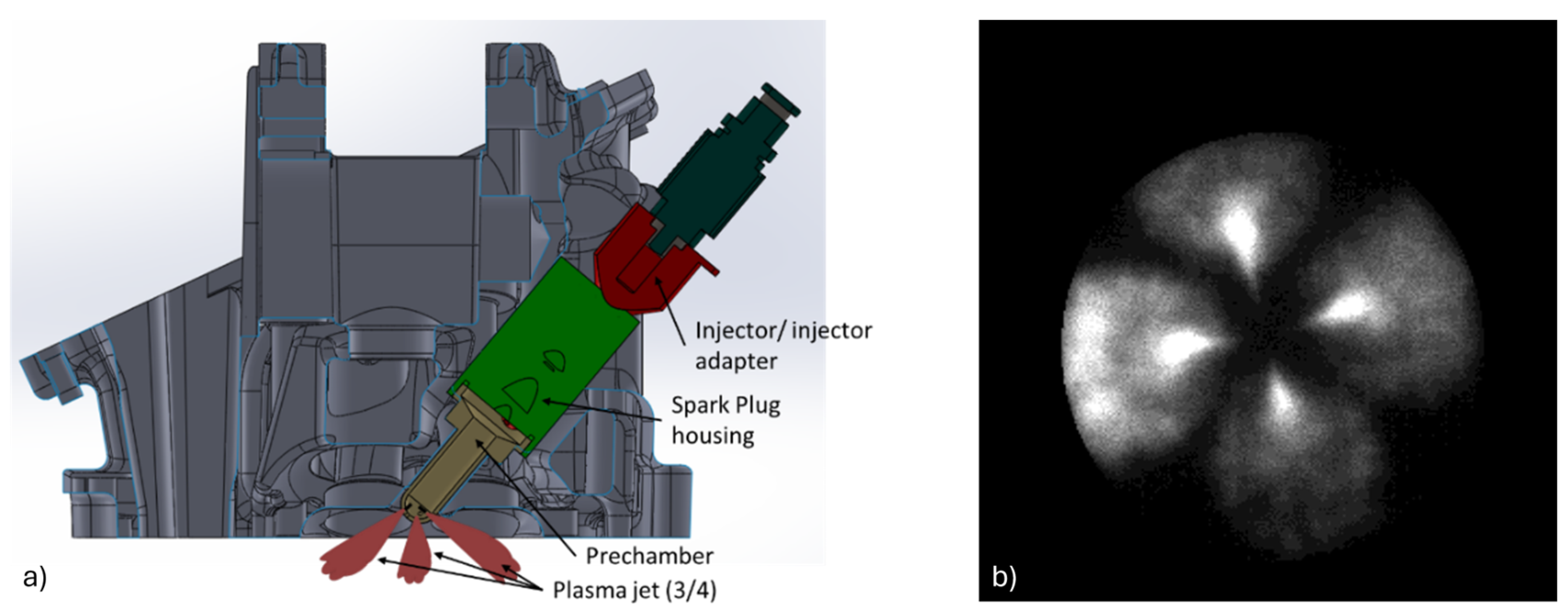
Figure 3, adapted from the authors’ previous work [
61], shows (
a) a section of a spark-ignition (SI) engine head equipped with an active pre-chamber and (
b) the ignition process inside the combustion chamber, triggered by the reactive jets emerging from the pre-chamber nozzles.
4.4. Plasma-Assisted Ignition
To improve ignition performance in ammonia–hydrogen dual-fuel engines, researchers have explored the innovative potential of plasma-assisted ignition systems. This cutting-edge approach uses the generation of high-energy plasma to generate reactive radicals, boosting local reactivity and promoting stable ammonia combustion [
62]. These plasma discharges create chemically active species, fuel fragments, and localized heating, which modify conventional combustion pathways and accelerate fuel oxidation [
63,
64].
Figure 4 shows a gliding arc; the arc forms between diverging electrodes and is carried upward by the gas flow, producing an elongated, dynamic plasma region. Experimental and numerical studies have demonstrated that plasma can extend the lean blow-off limits of NH
3 flames while reducing NO
x emissions [
65,
66]. Furthermore, non-equilibrium plasma has shown significant advantages in enhancing ignition, extending flammability, accelerating low-temperature oxidation, and reducing emissions in ammonia combustion.
Numerical modeling has been used to investigate the kinetic effects of plasma on NH3 ignition, revealing that excited species and radicals significantly improve ignition characteristics. However, discrepancies remain in understanding plasma-assisted ignition mechanisms, particularly regarding the role of vibrationally and electronically excited species and their influence on NOx formation. Recent advancements, such as the development of a validated NH3/O2/N2 plasma-combustion kinetic model, have enabled more accurate numerical studies.
The introduction of hydrogen in addition to ammonia further enhances combustion performance by increasing the laminar flame speed, reducing the auto-ignition temperature, and broadening the flammability limits.
4.5. On-Site Partial Ammonia Dissociation
An innovative approach that is gaining acceptance is the partial dissociation of ammonia; that means a cracking of a portion of ammonia into hydrogen and nitrogen before it enters the combustion chamber.
Thermo-catalytic cracking is the most studied ammonia decomposition option, but to be thermodynamically favored, it requires high temperatures (>300 °C), with 400–600 °C needed to achieve NH
3 conversion above 99.5% [
68,
69]. The process can be enhanced by increasing the temperature and using catalysts [
70]. In internal combustion engines, the heat required for ammonia dissociation can be efficiently supplied by exhaust gases [
71]. A catalytic dissociation reactor can be integrated into the system to provide partially dissociated ammonia, improving combustion characteristics. Additionally, the installation of heat exchangers and heaters facilitates the interaction between the ICE and the reactor, optimizing thermal efficiency. The use of a catalytic dissociation reactor reduces the need for auxiliary gases, further enhancing the feasibility and performance of ammonia-fueled ICEs. An example of an ammonia dissociation catalyst assembly is illustrated in
Figure 5. The setup includes catalyst pellets housed in a cylindrical tube, preceded by another tube containing heat-exchange wiring. The entire system was positioned in the engine exhaust line, where temperatures exceeded 800 °C, suitable for ammonia cracking. This configuration not only exploited the exhaust heat to drive the dissociation reaction but also showcased energy recovery from engine operation.
Ganley et al. [
72] conducted a foundational study on the catalytic decomposition of ammonia, ranking the effectiveness of various metals in promoting hydrogen release. Their results indicated that ruthenium exhibited the highest catalytic activity, followed by nickel, rhodium, cobalt, and other metals in decreasing order, with elements such as tellurium, selenium, and lead showing minimal activity. Building on these insights, Comotti et al. [
10] designed a hydrogen generation system (HGS) tailored for ammonia–hydrogen dual-fuel engines, employing a commercially available ruthenium-based catalyst (ACTA 10010). The system, which utilized engine exhaust heat to drive the decomposition process, demonstrated promising results in terms of hydrogen yield, brake thermal efficiency, and overall heat release. However, it also led to elevated NO
x emissions. Comparable findings were reported by Ryu et al. [
71], who noted that improvements in combustion performance were only achieved when the ammonia feed rate was kept relatively low [
73].
Another approach involves the decomposition of ammonia into hydrogen using plasma catalysis. Plasma catalysis provides the benefit of functioning at lower temperatures while achieving a high conversion rate. The combined effect of plasma and catalysts significantly boosts ammonia cracking beyond the capabilities of each process on its own. As reported in [
74], recent studies have demonstrated that plasma–catalytic processes significantly enhance ammonia decomposition, both in diluted and pure NH
3 feeds. Research has shown that dielectric barrier discharge (DBD) reactors combined with various catalysts can achieve high NH
3 conversion rates without external heating. Notably, Wang et al. achieved 99.9% NH
3 conversion using a Fe-based catalyst at 410 °C, far surpassing plasma or catalyst alone. Similarly, Co/SiO
2 catalysts reached 99.2% conversion when combined with plasma. Additionally, membrane DBD reactors have been shown to boost H
2 production by 87%, indicating that selective H
2 removal can further enhance NH
3 decomposition efficiency.
By utilizing thermal or plasma-assisted methods to partially dissociate ammonia into hydrogen and nitrogen, combustion can be enhanced through the presence of hydrogen, improving ignition and flame stability. The integration of such technologies with existing pre-chamber systems could yield synergistic effects, amplifying the benefits of turbulence and reactive jet formation while maintaining operational stability. As studies continue to explore optimal conditions for dissociation, including temperature thresholds and energy input, the potential for achieving higher indicated thermal efficiency alongside lower environmental impact becomes increasingly viable.
5. Experimental Insights into Ammonia–Hydrogen Combustion
5.1. Optical Engine Studies
The combustion of ammonia, particularly when blended with hydrogen, poses several fundamental challenges. A key question concerns the interaction between turbulence and ammonia chemistry, given ammonia’s low reactivity and slow burning velocity. Understanding how turbulence influences ammonia’s ignition, flame propagation, and pollutant formation is critical for optimizing its use as a fuel. Another major issue is the role of differential diffusion in turbulent ammonia/hydrogen flames, as the significant differences in molecular diffusivities among the reactants can affect local burning rates and overall flame behavior. Additionally, the impact of hydrogen enrichment remains insufficiently understood, especially in engine-relevant conditions. Finally, developing accurate predictive models for turbulent ammonia combustion is essential, as existing correlations may not fully capture the unique characteristics of ammonia/hydrogen mixtures.
In recent years, several research groups have made significant progress in analyzing the fundamental mechanisms governing the turbulent combustion of ammonia and ammonia/hydrogen blends. These studies have been primarily conducted in controlled environments, such as burners or fan-stirred constant-volume combustion chambers [
75].
Fan et al. [
76] focused on improving the fundamental understanding of turbulence–chemistry interactions in ammonia combustion under highly turbulent conditions. They employed laser-based diagnostics, including Rayleigh scattering for temperature measurements and planar laser-induced fluorescence (PLIF) to determine the spatial distributions of NH and NO in highly turbulent premixed ammonia/air flames. High-resolution imaging was used to analyze the preheating zone, characterized by the temperature field, and the fuel consumption layer, marked by NH radicals. These features were further examined using statistical methods to establish correlations between the NH/NO concentrations and temperature, providing insights into the relationships between these key variables. The study revealed a linear increase in the ratio of turbulent to laminar flame speed for ammonia/air flames with increasing turbulence intensity, particularly under high Karlovitz number conditions (high turbulent conditions).
Cai et al. [
77] investigated the impact of differential diffusion on the turbulent burning velocity of ammonia/hydrogen flames under highly turbulent conditions, also at high Karlovitz numbers, using NH-PLIF diagnostics. The Lewis number (
Le) is used to evaluate the thermal-diffusion instability of a premixed flame. The premixed flame is stable when the Lewis number is greater than unity (
Le > 1) and unstable for a Lewis number less than unity (
Le < 1). The results of this work demonstrated that flames with a lower Le burn significantly faster—up to three times more—than those with a higher Le. This finding highlights the crucial role of differential diffusion in turbulent flame propagation. Despite differences in burning velocity, the total flame surface area was found to be similar across different Lewis numbers. However, flames with lower Lewis numbers exhibited greater flame stretch, which enhanced local burning rates rather than increasing the flame’s overall surface area. To better describe these effects, a new correlation was developed and validated across various flame conditions, underscoring the need to incorporate differential diffusion effects into turbulent combustion models.
Lhuillier et al. [
78] explored the combustion characteristics of ammonia-based mixtures under engine-relevant turbulent conditions. Laminar and turbulent flame experiments were conducted in a constant-volume vessel to study ammonia/hydrogen/air, ammonia/methane/air, and methane/hydrogen/air mixtures as observed in a single-cylinder all-metal SI engine. The results indicated that the effects of hydrogen or methane enrichment on combustion characteristics in the SI engine could not be fully explained by the low measured laminar burning velocities of the mixtures. The combustion regimes were found to be near the boundary between the thin and broken reaction zones, implying that turbulence-chemistry interactions play a dominant role. Ammonia-based blends exhibited a stronger response to turbulence compared to methane-based fuels. Furthermore, the study revealed opposing effects of ammonia enrichment with hydrogen or methane on the turbulent burning velocity, linked to differences in thermochemical properties and flame stretch sensitivity. An unexpected bending effect was observed in the ratio of turbulent-to-laminar velocity when the hydrogen fraction in the ammonia/hydrogen blend increased. Nevertheless, a strong correlation was established between the turbulent burning velocity and the Karlovitz and Damköhler numbers, suggesting that ammonia combustion is heavily influenced by turbulence and reaction kinetics.
These studies contribute to a deeper understanding of the fundamental aspects of ammonia and ammonia/hydrogen turbulent combustion. However, further research is needed to refine predictive models and improve the practical application of ammonia as a viable fuel in turbulent combustion systems.
Moreover, the first experimental studies exploring flame propagation characteristics of ammonia and ammonia-based fuel blends in optically accessible engines have recently begun to emerge in the literature [
79,
80,
81].
The study by Zhang et al. [
79] compares ammonia (NH
3) and methane (CH
4) combustion in a high-compression optical SI engine, highlighting ammonia’s challenges due to low burning velocity and poor early flame development. Optical diagnostics reveal that ammonia’s flame propagation is more turbulence-dependent, leading to higher cyclic variations and lower efficiency (~23% vs. ~32% for CH
4). Ammonia’s inefficient combustion behavior is predominantly governed by the early-stage flame evolution rather than bulk combustion duration. Statistical analysis based on early flame area growth (ST-FA = 300 mm
2) confirms that NH
3 combustion is highly sensitive to turbulence dissipation near top dead center (TDC), resulting in poor initial flame kernel development. Additionally, turbulence interactions play a more dominant role in ammonia’s propagation process compared to methane, which relies more heavily on its intrinsic laminar burning velocity.
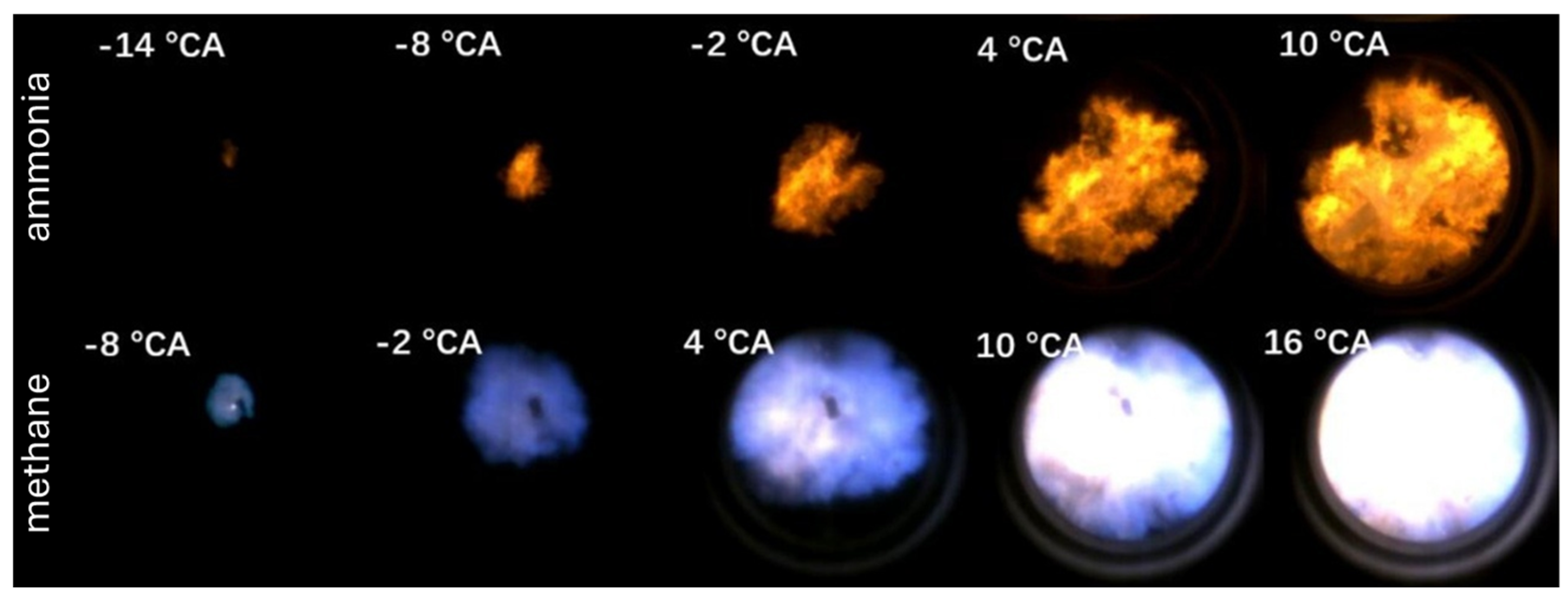
Figure 6 shows the evolution of the flame front in the combustion chamber for ammonia (upper case) and methane (lower case). Ammonia and methane flames exhibit distinct characteristics: ammonia appears bright orange due to NO
2 and NH
2 radiation (550–650 nm), while methane appears blue due to CH*, C
2*, and CO
2 fluorescence (340–650 nm).
Extending the investigations to hydrogen blends, Li et al. [
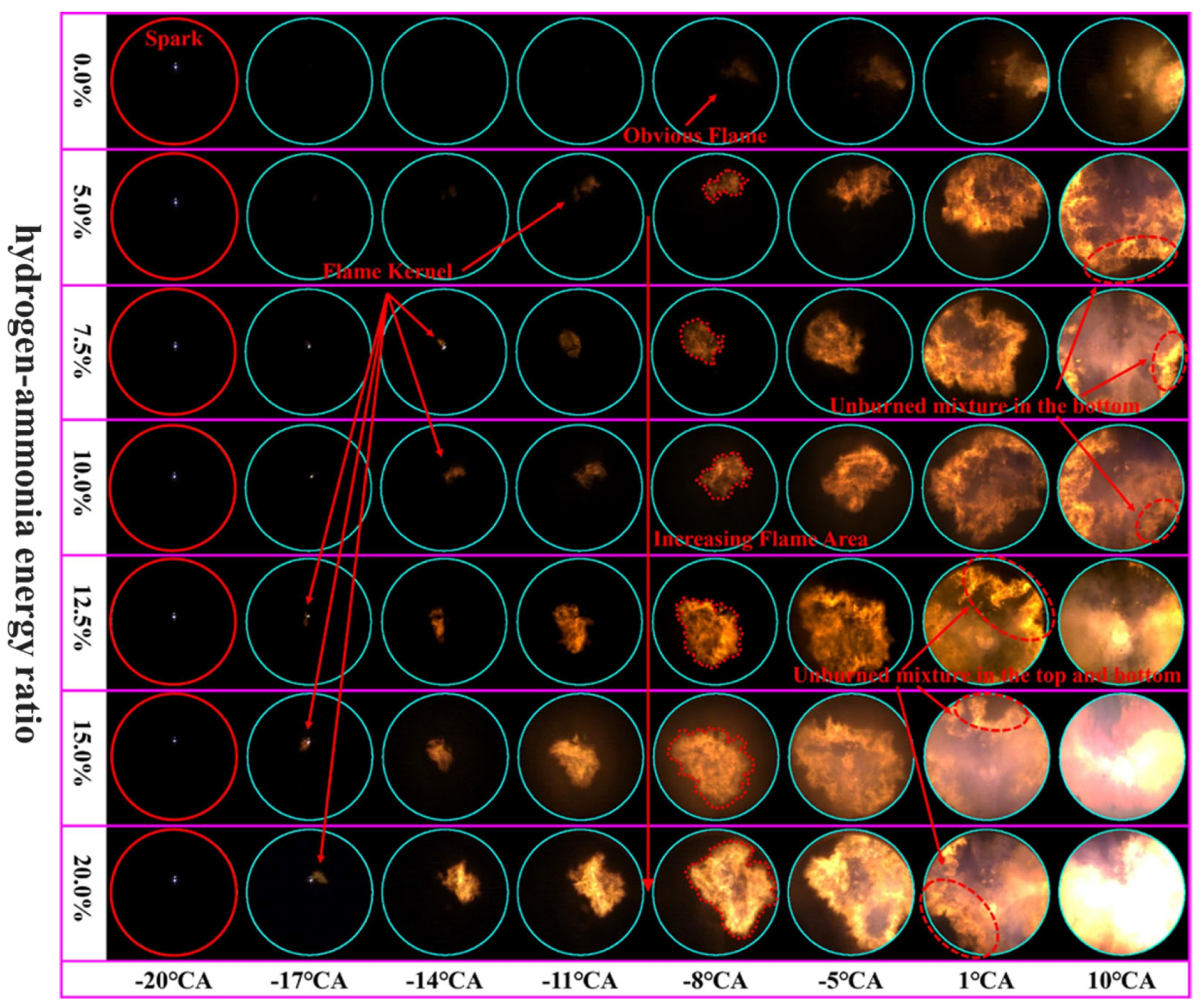
79] investigated the effects of hydrogen addition on the combustion of ammonia in an optical spark-ignition engine with high compression ratios. The study shows that at a hydrogen-to-ammonia energy ratio of less than 10%, the addition of hydrogen significantly improves the flame speed of ammonia and increases the stability of combustion in the early stage. This leads to more even ignition and combustion behavior and thus to improved overall engine performance. However, when the energy ratio exceeds 12.5%, hydrogen begins to affect the initial flame formation differently. At these higher ratios, the flame stretch-sensitivity decreases, altering the spread of the flame in the early stages of combustion. This change in flame dynamics plays a critical role in shaping the overall combustion process. The optimum hydrogen-to-ammonia energy ratio to achieve stable combustion and high thermal efficiency is around 7.5%, beyond which the benefits diminish due to increased heat loss.
Figure 7 shows flame images of different hydrogen–ammonia energy ratios, which illustrate the process of flame propagation in the cylinder. These images show that when pure ammonia is burned, a visible flame does not appear until −8 °CA, which is like a spontaneous combustion phenomenon. This delayed appearance is due to the long ignition delay time of ammonia, the low initial heat release, and the low flame brightness, making the early flame propagation difficult to observe. The flame appears as an orange glow starting at −8 °CA. As the hydrogen-to-ammonia energy ratio increases, the flame kernel forms earlier, indicating a faster ignition process. The flame front moves towards the cylinder wall with noticeable turbulence and appears more stretched and wrinkled. In addition, at −8 °CA, the flame area increases with increasing hydrogen content, which confirms an acceleration of flame propagation.
5.2. Performance Testing and Emission Control
The performance and emissions of ammonia–hydrogen engines have been the subject of increasing research interest in recent years.
The study presented in [
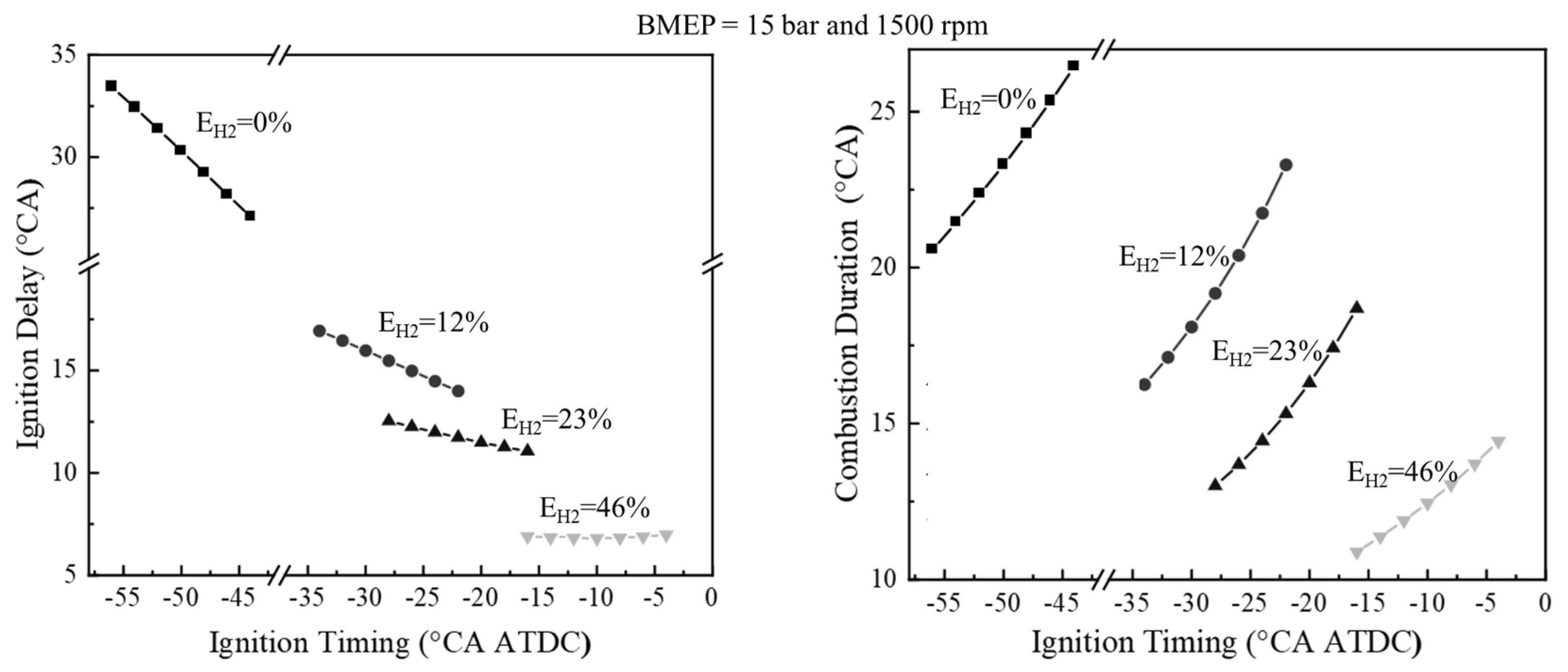
82] provides a detailed numerical analysis of the combustion behavior of ammonia–hydrogen fuel blends in internal combustion engines and highlights several key effects of hydrogen enrichment on performance parameters. One of the most important findings concerns the improvement of ignition and combustion characteristics: it has been shown that increasing the hydrogen content in the fuel mixture significantly shortens both the ignition delay and the overall duration of combustion. This improvement enables faster and more stable combustion, so that the engine can ignite and burn the fuel more effectively.
Figure 8 shows that increasing the hydrogen blending ratio significantly reduces both the ignition delay and combustion duration at BMEP = 15 bar and 1500 r/min. The ignition delay drops from 30.3 to 6.8 °CA and the combustion duration from 23.3 to 12.4 °CA as hydrogen content increases from 0% to 46%, due to hydrogen’s lower ignition energy and faster flame propagation.
Another important result relates to thermal efficiency. The study found that a suitable mixture of hydrogen with ammonia improves the engine’s brake thermal efficiency, meaning that a greater portion of the fuel’s chemical energy is converted into useful mechanical work. This improvement is particularly valuable in the context of decarbonizing transport while maintaining high engine performance. The role of engine speed was also investigated. As engine speed increases, it was found that a higher hydrogen fraction becomes necessary to maintain reliable ignition. This finding suggests that engine operating conditions, such as load and speed, need to be considered when determining the optimal fuel blend, as combustion requirements vary across the engine map. In addition, the study highlights the importance of determining the most appropriate blend ratio of hydrogen and ammonia based on specific operating conditions. Adjusting the mixture allows for an effective compromise between performance, efficiency, and emissions.
In [
54], the authors experimentally investigated the performance of internal combustion engines fueled with an ammonia–hydrogen blend, using a small diesel pilot injection to initiate ignition. The results demonstrated that increasing the hydrogen fraction from 20% to 40% by volume significantly improved key combustion parameters such as the in-cylinder pressure and heat release rate. Overall, the study confirms that hydrogen blending within the 20–40% range is particularly effective in promoting faster and more complete combustion, thereby contributing to improved thermal efficiency and more stable engine operation.
Moreover, the study [
83] investigates the impact of ammonia addition on combustion and emissions in a spark-ignition engine fueled with hydrogen, highlighting several key findings. As ammonia content increases, both flame development and combustion duration are prolonged, causing a delay in combustion phasing and reducing engine efficiency. Higher ammonia mass ratios also lead to increased combustion instability under stoichiometric conditions, along with a noticeable drop in the peak cylinder pressure and heat release rate, which may hinder power output. Exhaust temperatures rise with more ammonia, posing potential challenges for thermal management. Regarding fuel efficiency, brake-specific fuel consumption (BSFC) reaches its minimum at an ammonia mass ratio of 0.4–0.6, beyond which it begins to increase. Emission trends show that unburned ammonia and N
2O emissions rise steadily with more ammonia, while NO
x emissions peak at low ammonia ratios and then decline. Overall, a 0.6 ammonia mass ratio under stoichiometric conditions is recommended for optimal performance, while lean-burn operation may benefit from higher ammonia content.
Lhuillier et al. [
11] conducted a comprehensive study of the combustion characteristics, engine performance, and emission behavior of a spark-ignition engine operating on ammonia–hydrogen–air mixtures. In the experiments, the volumetric content of hydrogen in the fuel mixture was increased from 0% up to 60%. The results demonstrate that introducing hydrogen into the fuel blend, up to 20% by volume, significantly enhances combustion stability, improves thermal efficiency, and increases the engine’s work output. However, when the hydrogen content exceeds this threshold, the benefits are offset by greater heat losses, which can negatively impact overall performance. Combustion analysis reveals that hydrogen addition accelerates flame propagation and shortens ignition delays, promoting more reliable and complete combustion. Engine performance data further show that stable operation is particularly evident under lean-burn conditions in which ammonia alone would typically result in misfiring or incomplete combustion. As the ammonia content increases, advancing the spark timing becomes necessary to achieve optimal combustion phasing. Maximum efficiency is observed near an equivalence ratio of 1.1, where the balance between reactivity and combustion completeness is most favorable. In terms of emissions, the findings revealed a clear and consistent trend: as the hydrogen fraction increased, the amount of unburned ammonia in the exhaust gases decreased steadily, as shown in
Figure 9.
The addition of H2 in ammonia induces an NH3 emission decrease at the exhaust due to less NH3 as fuel at the intake, but the behavior is also attributable to the enhanced reactivity and improved combustion efficiency brought about by the presence of hydrogen, which promotes more complete oxidation of ammonia. This benefit came with a trade-off, as the higher hydrogen content also led to a monotonic rise in nitrogen oxides (NOx) emissions. This increase can be explained by the elevated flame temperatures and higher combustion rates associated with hydrogen-enriched mixtures, which are known to favor NOx formation through thermal mechanisms. Operating the engine with 20% hydrogen at an equivalence ratio of 0.7 emerges as a promising compromise, offering a satisfactory balance between performance and emissions. Overall, the study highlights the dual effect of hydrogen addition: while it contributes to cleaner combustion in terms of unburned fuel, it also exacerbates NOx emissions, underscoring the importance of carefully balancing fuel composition and engine operating strategies.
In [
50], the authors report on the emissions associated with ammonia and hydrogen engines. It is shown that the combustion of both ammonia and hydrogen can lead to increased emissions of nitrogen oxides. In particular, the combustion of ammonia was found to produce increased amounts of nitrous oxide (N
2O), a potent greenhouse gas that is about 300 times more harmful than CO
2 over a century. The addition of hydrogen to ammonia combustion can exacerbate NO
x emissions due to the higher combustion temperatures associated with hydrogen, which can lead to greater NO
x formation.
The reviewed studies collectively underscore the complex interaction between ammonia and hydrogen in combustion engines. The addition of hydrogen to ammonia-rich mixtures consistently improves the ignition properties, accelerates flame propagation, and increases thermal efficiency, particularly when blended in the 20–40% volume range. This blending range proves to be optimal for promoting stable, efficient combustion in both spark-ignition and dual-fuel compression-ignition systems. However, the benefits of hydrogen enrichment are not without trade-offs. A higher hydrogen content leads to higher pressures in the cylinder and higher heat release rates, but also increases combustion temperatures, which in turn exacerbate NOx emissions. Similarly, unburned ammonia emissions tend to decrease with the addition of hydrogen, while nitrous oxide (N2O) emissions increase with a higher ammonia content, posing a major challenge for climate impact mitigation. The studies also highlight the importance of engine operating conditions: factors such as the equivalence ratio, engine speed, and spark timing must be carefully calibrated to take advantage of the benefits of ammonia–hydrogen blends while minimizing their drawbacks. An optimal strategy appears to be moderate hydrogen enrichment (around 20%) combined with operation at slightly rich or lean equivalence ratios, depending on the specific application goal, be it maximizing efficiency or minimizing emissions. The collective results emphasize the need for a balanced and application-specific approach to ammonia–hydrogen combustion that combines fuel composition with engine design and control strategies to achieve viable, low-carbon propulsion solutions.
6. Conclusions
Ammonia and hydrogen represent promising fuel alternatives for decarbonization and the energy transition due to their potential for near-zero emissions and integration with renewable energy systems. In particular, their combined use in dual-fuel configurations has attracted significant attention in recent years, prompting numerous studies aimed at understanding their combustion characteristics, enhancing performance, and addressing the technical challenges associated with their integration into existing engine systems.
Given the different properties of ammonia and hydrogen, the fuel injection strategy is critical, as the method chosen has a direct impact on combustion stability, thermal efficiency, and the formation of pollutants. Many configurations have been investigated, and hybrid strategies combining the advantages of DI and PFI strategies have proven to be more suitable for tailoring combustion dynamics—improving flame propagation, reducing pollutant formation, and achieving greater flexibility and adaptability to different systems and applications.
Moreover, reliable ignition of ammonia–hydrogen mixtures requires overcoming the inherent challenges of ammonia—high minimum ignition energy and slow flame propagation—through synergistic blending with hydrogen. Advanced techniques such as optimized spark ignition with hydrogen enhancement, dual-fuel compression ignition (with co-fuels or alternative strategies such as SACI), pre-chamber combustion, and plasma-assisted ignition have each shown the potential to stabilize the combustion process, improve efficiency, and reduce emissions. The integration of numerical models and advanced control systems is essential for fine-tuning the ignition phases and optimizing the air–fuel ratio.
Emerging technologies such as on-site partial dissociation of ammonia, achieved by thermo-catalytic cracking or plasma-assisted methods, offer promising strategies to improve the performance of ammonia-fueled engines. By converting a portion of the ammonia into hydrogen and nitrogen using exhaust heat and optimized catalyst systems, ignition characteristics and flame stability can be significantly improved. The synergistic integration of these dissociation technologies with pre-chamber combustion systems further increases efficiency and leads to high conversion rates and reduced emissions.
Many studies on optical and research engines have also underlined the importance of both in-cylinder turbulence and the ammonia–hydrogen ratio.
Turbulent combustion plays a pivotal role in optimizing the performance of ammonia and ammonia/hydrogen engines. Key studies consistently demonstrate that ammonia’s inherently low reactivity and slow burning velocity render its combustion highly sensitive to turbulence. Enhanced turbulence increases flame stretch and accelerates flame propagation—which improves the ratio of turbulent to laminar flame speed—but also poses a challenge for early flame kernel development and cyclic stability. Different diffusion effects and enrichment with hydrogen further change these interactions. While hydrogen improves ignition and combustion rates, its effects must be carefully balanced to maintain overall stability and efficiency. Consequently, optimizing turbulence characteristics and incorporating accurate models of turbulence chemistry are critical to exploiting the potential of ammonia as a low-carbon fuel and achieving superior engine performance under practical, engine-relevant conditions.
The collective findings underscore that optimizing the balance between ammonia and hydrogen in fuel blends is critical. Enrichment with hydrogen, typically in the range of 20–40% by volume, significantly improves ignition characteristics, accelerates flame propagation, and increases thermal efficiency; however, excessive hydrogen can increase combustion temperatures and NOx emissions. Conversely, while higher ammonia content can reduce unburned fuel emissions, it tends to increase combustion duration and increase N2O formation. Therefore, careful calibration of the fuel mixture, which also depends on the operating conditions of the engine, is essential to balance performance, efficiency, and environmental impact.
In summary, numerous and accurate works dealing with ammonia–hydrogen engines reveal the complexity of the phenomena involved in optimizing the design and calibration of ammonia–hydrogen engines. Further studies could be useful to improve the numerical models. Ammonia–hydrogen engines represent a promising route to low-carbon propulsion and offer significant potential for efficiency and reduced emissions. Although extensive research has already led to a better understanding of these systems, further scientific and technological advances are essential to fully elucidate the complex phenomena that govern their combustion behavior. The continued development of advanced numerical models, as well as flexible and optimized injection and combustion strategies, will be critical for the development of next-generation engines that are both more efficient and more environmentally friendly.
Looking ahead, several key research directions are essential to advance and implement ammonia–hydrogen dual-fuel technologies in internal combustion engines. A top priority is the development of fuel injection and ignition systems specifically engineered for ammonia-based dual-fuel operations. Tailoring these components to the unique properties of ammonia is critical for mitigating challenges such as ignition delay and combustion instability, which directly affect efficiency and engine performance.
Equally important is the integration of these combustion technologies with the upstream production of green hydrogen and green ammonia. Leveraging excess renewable energy, particularly from solar and wind, to produce hydrogen via electrolysis and ammonia through sustainable nitrogen fixation enables a truly decarbonized fuel cycle. This closed-loop system minimizes fossil fuel dependence and aligns with broader climate and sustainability targets.
On the modeling and control front, the adoption of advanced CFD simulations, augmented by real-time optimization through machine learning, offers a powerful toolset for enhancing combustion control and minimizing emissions under varying engine loads. These computational methods provide critical insight into the intricate in-cylinder processes, allowing for more accurate and responsive engine tuning.
Finally, demonstration-scale testing under realistic operating conditions, supported by comprehensive techno-economic evaluations, is vital to translate lab-scale advances into viable commercial solutions. These efforts will ensure that ammonia–hydrogen dual-fuel systems can contribute meaningfully to future low-carbon transportation and energy scenarios.
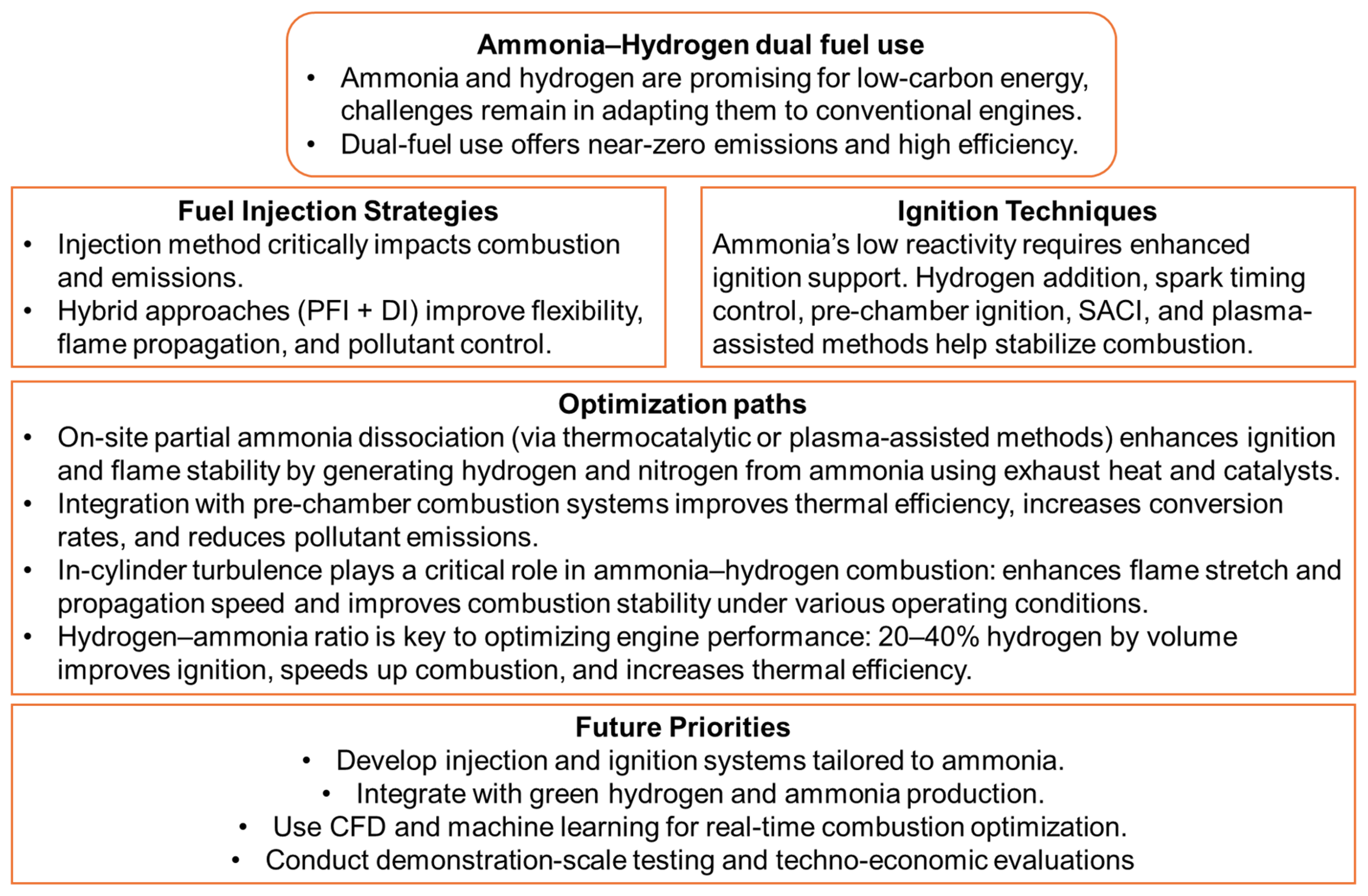
Figure 10 shows a graphical summary that clearly synthesizes the key conclusions.