Abstract
The production of microalgal biomass on a commercial scale remains a significant challenge. Despite the positive results obtained in the laboratory, there are difficulties in obtaining similar results in industrial photobioreactors. Changing the cultivation conditions can affect not only the growth of microalgae but also their metabolism. This is of particular importance for the use of biomass for bioenergy production, including biofuel production. The aim of this study was to determine the biomass production efficiency of selected microalgal strains, depending on the capacity of the photobioreactor. The lipid and ash content of the biomass were also taken into account. It was found that as the scale of production increased, the amount of biomass decreased, irrespective of the type of strain. The change in scale also affected the lipid content of the biomass. The highest values were found in 2.5 L photobioreactors (ranging from 26.3 ± 2.2% for Monoraphidium to 13.9 ± 0.3% for Chlorella vulgaris). The least favourable conditions were found with industrial photobioreactors, where the lipid content of the microalgal biomass ranged from 7.1 ± 0.6% for Oocycstis submarina to 10.2 ± 1.2% for Chlorella fusca. The increase in photobioreactor capacity had a negative effect on the ash content.
1. Introduction
The use of microalgae for energy purposes depends on the possibility of obtaining sufficient amounts of biomass. An appropriate scale-up strategy is therefore essential for the gradual multiplication of microalgae from the laboratory stage to the industrial production stage. The first step is to create a culture in small-volume shake flasks. Typically, ten percent of the inoculate that is prepared in this way constitutes the input in the next step. In the intermediate stages, photobioreactors with volumes of a few litres and several litres, respectively, are used. Such prepared biological material can be used to inoculate industrial photobioreactors.
The efficiency of biomass production under laboratory conditions is generally high, which is related to the ease of providing suitable conditions for microalgal growth. In large-scale photobioreactors, liquid flow processes and mass exchange may be slower, potentially affecting biomass production efficiency. The average productivity of industrial strains has been found to be lower compared to maximum theoretical estimates [1].
Optimising the culture medium composition prevents the reduction in microalgae growth associated with mineral deprivation [2]. A more significant problem is the abiotic factor. One of the most important cultivation parameters is light, which affects the rate of photosynthesis [3] and biomass production. The optimum irradiance for different algal strains is approximately 26–400 μmol photons m−2 s−1 [4]. To maximise production efficiency, a single microalgae cell should be exposed to a light intensity of approximately 0.2–0.4 µmol·m−2·s−1. At this value, the photosynthetic rate in relation to light intensity starts to equalise. Due to the self-darkening of the cells, this is difficult, especially at high culture densities [5]. The cells inside the culture receive only part of the radiation, which reduces the light efficiency compared to cells in the outer, light-exposed zone [6]. When cells stay in the illuminated zone for a short time, they are unable to absorb enough photons for photosynthesis, and the rate of volumetric biomass production decreases [7]. Due to the geometry of the different types of photobioreactors, the light pathway and, therefore, the light intensity in the different zones of the photobioreactor will be different. At increasing distances from the exposed side, the light intensity decreases almost exponentially. Both an excess of light, causing photoinhibition, and a deficiency of light, i.e., photolimitation, are disadvantageous for the culture [8]. Light should be distributed throughout the photobioreactor to allow the photons to penetrate the cells [9]. Reducing the light pathway can significantly increase the biomass yield [10]. Microalgae require light for the production of ATP and NADPH and for the synthesis of molecules essential for their growth [11]. Industrial cultures should have a high biomass concentration and the ability to absorb high levels of light, thus limiting losses due to photoinhibition [12]. The efficiency of microalgal biomass production is also determined by the time of illumination. Increasing the light/dark ratio can increase productivity [13,14].
The growth of microalgae depends on the nutrient supply. Elements such as nitrogen and phosphorus are responsible for the metabolic regulation processes. The availability of nutrients is determined by adjusting the pH of a culture medium [15]. The optimum pH range for most microalgae is between six and ten. At acidic conditions, a decrease in nutrient uptake is observed, while high values reduce the ability to assimilate carbon dioxide [16].
It is also possible that, due to modifications in the design of photobioreactors, gas transport may become inefficient [17], so another important parameter in culture is stirring. This process prevents cell sedimentation [18]. Stirring is also important for carbon dioxide transport to photosynthesis [2] and oxygen removal, limiting photorespiration [19]. Culture stirring allows for the efficient use of available light by frequently changing the cell position between zones with appropriate and limited lighting [12]. In photobioreactors, there is a three-phase system: liquid (medium)–gas–solid phase, i.e., microalgae cells [20]. Changing the rate of liquid flow inside the photobioreactor can have a negative effect on the cells related to shear stress. Excessive shear stress can result in reduced cell growth rates and cell viability [21]. The typical linear flow rate in tubular bioreactors is 0.3–0.5 m·s−1 [22]. Increased cell density in culture can affect the culture medium’s properties, including gas retention, but the nature of these changes is not clear [23]. In vertical tubular photobioreactors, large gas bubbles rising rapidly can direct smaller bubbles towards the walls. Due to the high shear rate, they can break up [24], which can affect the stability of the flow throughout the volume. As a result of the stirring process in photobioreactors, microalgal cells are also subject to rapid changes in the light–dark cycle [2].
The aim of this study was to determine the biomass yield of selected microalgal strains in relation to the scale of production. Vertical tubular laboratory photobioreactors and those used on an industrial scale were used in the experiments. The capacity of the photobioreactors was selected according to the successive stages of culture on an enlarged scale, ranging from a few to several tens of litres. This study assessed the efficiency of the biomass production at each stage, as well as the content of lipids and ash, the amount of which is important in the energetic use of biomass.
2. Materials and Methods
2.1. Microalgae Strains
A total of six strains of microalgae were used in the study. The genus Scenedesmus was from the Department of Renewable Energy Engineering’s own collection; the other five strains (Chlorella vulgaris, Chlorella fusca, Oocystis submarina, Chlorella minutissima, and Monoraphidium) were obtained from the Culture Collection of Baltic Algae (CCBA).
2.2. Preincubation
Microalgae cultures were stored in F/2 liquid medium at 4 °C under LED illumination for a 12/12 h cycle (light/dark). The inoculum culture was prepared in 1000 mL culture bottles containing 500 mL of F/2 medium, pH 7, which was inoculated with 50 mL of individual microalgae suspension. Preculture conditions were 23 ± 1 °C, with a light intensity of 125 µmol m−2 s−1 and illumination for 12/12 h cycle (light/dark). SonT Agro sodium lamps (High Pressure Sodium, HPS; PHILIPS, Amsterdam, The Netherlands) were used to illuminate the culture. Carbon dioxide for photosynthesis was supplied with atmospheric air using a 7.2 L·min−1 membrane pump (HAILEA ACO-9602, Guangdong, China). The cultures were continued for 7 consecutive days.
2.3. Experimental Setup
During the experiment, vertical tubular photobioreactors of various total volumes were used: 2.5 L, 14 L, and 100 L with diameters of 80, 200, and 242 mm. Depending on the total volume, the photobioreactors were filled with 2 L, 12 L, or 80 L of F/2 nutrient solution at pH 7. Next, a 7-day inoculum was introduced in volumes of 0.2 L, 1.2 L, and 8.0 L, respectively. The changes in the culture medium pH were not analysed during the experiment. Mixing in the photobioreactors was provided by 2 L·min−1 membrane pumps (Aqua Medic Mistral 2000, Bissendorf, Germany) for the 2.5 L photobioreactor, 14 L·min−1 (HAILEA ACO-9620, Guangdong, China) for the 14 L photobioreactor, and 240 L·min−1 (HAILEA ACO-300A, Guangdong, China) for the 100 L photobioreactor. All cultures were carried out at 23 ± 1 °C. An LED light at 285 µmol m−2 s−1 was used for illumination, with an 18/6 h light/dark cycle. The light sources were 1200 × 50 mm light bars placed above the photobioreactors at a height that provided appropriate illumination. The experiment was carried out as a batch culture for 15 days. The study determined biomass growth dynamics, average biomass content, biomass productivity, and lipid and ash content.
2.4. Analytical Methods
The biomass content of the culture was assessed by the gravimetric method using a moisture analyser (AXIS ATS60, Gdansk, Poland). The methodology was described in an earlier paper [14]. The results were converted and reported in mg·L−1. The measurement was carried out at the beginning of the experiment and then after 5, 10, and 15 days of culture.
Lipid content was determined by gravimetric analysis after extraction of the biomass with a solvent mixture (chloroform–methanol) using the method according to Bligh and Dyer [25]. The analysis was carried out after the evaporation of the extracting solvents, based on the equation given below:
where LC = lipid content; mL = lipid mass (g); mDAB = dry biomass of microalgae (g).
The ash content of the biomass was determined gravimetrically according to PN-EN ISO 18122:2016-01 [26]. A total of 0.2 grammes of microalgal biomass, dried at 105 °C, was weighed into porcelain crucibles and placed at 550 °C for 6 h. The results were recalculated and given as a percentage.
2.5. Statistical Analysis
The data were presented as mean values ± standard deviation of the mean. The results were evaluated using an analysis of variance (ANOVA) with Statistica ver. 13.1 by StatSoft. A post hoc Tukey’s tests was performed at a statistical significance of p ≤ 0.05.
3. Results and Discussion
3.1. Algal Biomass Concentration
The growth of microalgal biomass in the 2.5 L photobioreactors is shown in Figure 1A. At the beginning of the experiment, the biomass content in particular photobioreactors ranged from 135 ± 0.8 mg·L−1 for C. minutissima to 212 ± 1.0 mg·L−1 for C. vulgaris and C. fusca. On the first measurement date, after 5 days of cultivation, the highest biomass increase was observed for Scenedesmus (770 ± 20.4 mg·L−1). A slightly lower value was obtained for C. fusca (716 ± 11.1 mg·L−1). During the following days, the biomass gradually increased in all photobioreactors. After 15 days of culture, the highest values were determined for C. fusca (1072 ± 7.0 mg·L−1) and Scenedesmus (1063 ± 7.0 mg·L−1). No lag phase was observed in the 2.5 L photobioreactors, indicating favourable environmental conditions after the inoculation of the culture medium. This could have been the result of efficient stirring of the culture medium [27] and appropriate light transmission [28], parameters that characterise cultures cultivated in smaller capacity photobioreactors [29]. Nguyen et al. [30] achieved a stationary growth phase for C. vulgaris as late as day 18 of culture. In contrast, Han et al. [31], in a 3 dm3 photobioreactor, observed biomass growth for S. quadricauda up to day 13 of the experiment. In an earlier study [14] in a C. vulgaris culture, the stationary phase was determined as early as day 10 of culture, with cultures illuminated with a SON-T Agro sodium light.
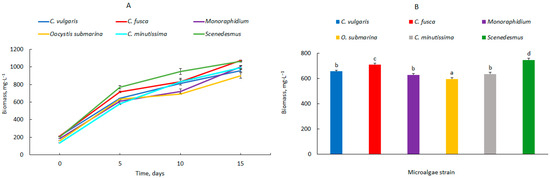
Figure 1.
Dynamics of changes in biomass content (A) and average biomass content in the culture, (B) fotobioreaktor 2.5 L; mean over each column not marked with the same letter is significantly different at p ≤ 0.05).
The mean microalgal biomass content, shown in Figure 1B, ranged from 596 ± 10.6 mg·L−1 (O. submarina) to 747 ± 15.4 mg·L−1 (Scenedesmus).
The dynamics of microalgal biomass changes in 14 L photobioreactors are shown in Figure 2A. Although the parameters of the culture medium pH, temperature, and light intensity were the same, differences in the growth of the individual microalgal strains were observed. The highest biomass was found after 10 days for O. submarina (1039 ± 12.2 mg·L−1). In the other photobioreactors, the values were lower and ranged from 558 ± 9.2 mg·L−1 for C. fusca to 749 ± 9.2 mg·L−1 for Scenedesmus. After 15 days, biomass growth was observed for C. vulgaris, C. fusca, C. minutissima, and Monoraphidium. For O. submarina and Scenedesmus strains, the amount of biomass decreased from the 10th day of incubation onwards. These differences are a result of the nutritional requirements of the individual algal species [32]. The main variable parameters in this stage were the volume of the photobioreactor and the related requirements for a higher flow rate pump. The biomass production can therefore be increased by selecting a suitable culture medium composition. Another way to achieve high growth rates is to optimise the environmental conditions [33]. In the present study, culture-relevant parameters such as pH and temperature were in ranges that are considered suitable for most microalgae.
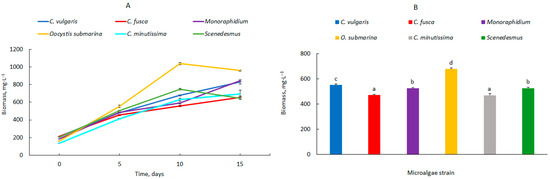
Figure 2.
Dynamics of changes in biomass content (A) and average biomass content in the culture, (B) fotobioreaktor 14 L; mean over each column not marked with the same letter is significantly different at p ≤ 0.05).
The average amount of microalgal biomass in the 14-litre photobioreactors is shown in Figure 2B. It ranges from 469 ± 16.3 mg·L−1 for C. minutissima to 677 ± 8.0 mg·L−1 for O. submarina.
In the 100 L photobioreactors, the growth rate was lower compared to the smaller photobioreactor volumes presented in this study (Figure 3A). The highest growth was observed for the genus Scenedesmus, from 203 ± 0.8 mg·L−1 (day 0) to 524 ± 13.3 mg·L−1 (day 15). Significantly lower values were recorded for C. vulgaris, Monoraphidium, and O. submarina. The biomass content on the last day of culture was 305 ± 14.0, 396 ± 8.1, and 343 ± 7.0 mg·L−1, respectively. For C. fusca and C. minutissima, the stationary phase of growth started between days 5 and 10 of cultivation. A similar relation was also presented by Kim et al. [34], who analysed the optimal conditions for Chlorella sp., Dunaliella salina, and Dunaliella sp. and observed growth up to day 8 of culture. According to Min et al. [35], a much larger culture volume complicates light availability, but theoretically, in barbotage-based columns, as in the study presented here, the cross-shading of cells with constant stirring of the photobioreactor should not affect the results so much. Gas exchange, including carbon dioxide fixation in the culture medium, may be more important. Oxygen accumulation in photobioreactors also has a negative impact on microalgal growth [36,37]. In industrial photobioreactors, higher efficiency pumps are used to prevent cell sedimentation and ensure proper CO2 and O2 transfer. The hydrodynamic stresses under such conditions may also be responsible for the reduced growth rate of microalgae. The cell damage due to intensive stirring is confirmed by Camacho et al. [38]. In the case of animal cells, damage occurred during the bursting of gas bubbles [39]. A similar mechanism cannot be excluded for microalgae grown in photobioreactors.
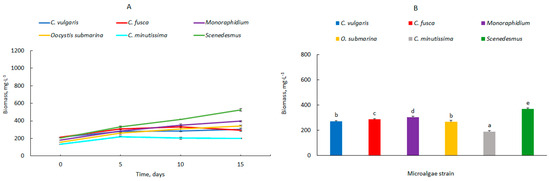
Figure 3.
Dynamics of changes in biomass content (A) and average biomass content in the culture (B) 100 L; mean over each column not marked with the same letter is significantly different at p ≤ 0.05).
The average biomass content in the individual photobioreactors over the entire experimental period is shown in Figure 3B. Biomass amounts ranged from 189 ± 5.9 mg·L−1 (C. minutissima) to 369 ± 7.3 mg·L−1 (Scenedesmus). There are many possibilities to increase the production of microalgal biomass, including by increasing the amount of nutrients in the culture medium or increasing the light intensity of the culture [32,40]. In the present study, the composition of the culture medium and the same environmental parameters for cultivation were maintained at each stage, but the microalgal biomass decreased.
The highest productivity of microalgal biomass (Table 1), irrespective of the scale of culture, was observed during the first 5 days of the experiment. The values range from 85.6 ± 0.7 mg·L−1·d−1 (Monoraphidium) to 113.5 ± 4.1 mg·L−1·d−1 (Scenedesmus). These values decreased on subsequent days, and after 15 days, a maximum value of 57.4 ± 0.5 mg·L−1·d−1 was determined for Scenedesmus and C. fusca. An increase in photobioreactor volume up to 14 L generally reduced productivity. A similar situation was observed in 100 L photobioreactors. The results obtained were below the data available in the literature [41].

Table 1.
Comparison of biomass productivity obtained with different microalgal strains.
3.2. Lipid Content
The lipid content of the biomass is shown in Figure 4. The high lipid content of the microalgal biomass determines its potential utilisation in biodiesel production [42]. The highest values were found for cultures cultivated in photobioreactors of 2.5 L capacity. In general, increasing the volume of the photobioreactor decreased the lipid content of the biomass. There was one exception for the C. fusca strain, where the lipid content in the 100 L volume photobioreactor was higher compared to the 14 L capacity, 10.2 ± 1.2% versus 6.4 ± 1.1%, respectively. The decrease in lipids in the biomass of microalgae grown in the 14 L photobioreactor ranged from 25% (Scenedesmus) to 57% (C. fusca). In 100 L photobioreactors, lipid reduction ranged from 32% (C. fusca) to 64% (Monoraphidium). The lower lipid content in higher capacity photobioreactors may be related to the differential transmission of light in the culture medium. According to Amaro et al. [43], the appropriate kind of lighting and light intensity can potentially increase lipid content between 25 and 42%. Also, the shape of the photobioreactor and the method of culture stirring could have a strong influence on the lipid accumulation potential [44,45] In the present study, the highest lipid accumulation rate for the highest photobioreactor capacity was observed for Monoraphidium (10 ± 1.2%). The average amount of lipids for this strain ranged from 5 to 58% [46].
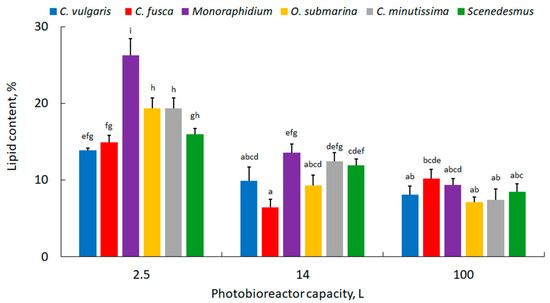
Figure 4.
Lipid content in microalgal biomass; mean over each column not marked with the same letter is significantly different at p ≤ 0.05.
In different capacity photobioreactors, the optimal conditions for the growth of the same microalgal strains may be significantly different (Table 2); thus, Rodolfi et al. [47] indicate that a more important property than the high lipid content of the cells may be the lipid yield per unit area.

Table 2.
Comparison of microalgal biomass production and lipid yield during different cultivation conditions.
3.3. Ash Content
The microalgal biomass ash content is shown in Figure 5. This parameter is important when the biomass is intended as feedstock for biofuel production. An excessive ash content has a negative impact on biofuel properties [67]. In the presented study, the lowest values were recorded for strains grown in 2.5 L photobioreactors and ranged from 7.8 ± 0.2% for C. fusca to 11.3 ± 0.5% for C. vulgaris. When the culture scale was increased to 14 L, there was an increase in ash biomass, with values ranging from 17.3 ± 1.1% for C. minutissima to 21.6 ± 0.3% for Monoraphidium. Metsoviti et al. [68] cultivated C. vulgaris in a 25 L photobioreactor and determined an ash content of approximately 12%. In this study, compared to the 14 litre capacity, the ash content for C. vulgaris, Monoraphidium, C. minutissima, and Scenedesmus increased by 11, 1, 45, and 33%. In the biomass of C. fusca and O. submarina, the ash content decreased slightly to 11.1 ± 0.3 and 13.4 ± 0.4%, respectively. The ash content of the biomass varied depending on the microalgae species and the culture methods and growing conditions [69,70,71]. In our previous studies, the decrease in ash content was associated with a limited nutrient availability [56]. In the present study, the culture medium was used in complete composition at all steps, and a reduction in ash content was noted at the industrial stage for only two microalgae species. However, at the same time, the increase in lipid content that is reported under stress conditions was not observed.
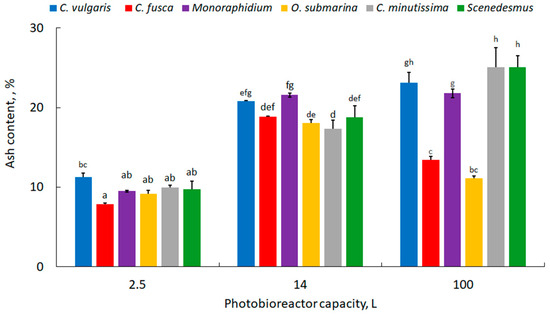
Figure 5.
Ash content in microalgal biomass; mean over each column not marked with the same letter is significantly different at p ≤ 0.05.
4. Conclusions
In this study, the relation between the photobioreactor capacity and microalgae growth was analysed according to the amount of biomass. As the scale of cultivation increased, there was a decrease in biomass production and cellular lipid content with an increase in ash content. In the case of the Scenedesmus strain, the large increase in biomass did not result in significant differences in lipid and ash content. The highest lipid content and lower ash content of the biomass in the highest capacity 100 L photobioreactor were recorded for C. fusca. The data presented here represent the real amounts of biomass that can be obtained during biomass cultivation on an industrial scale compared to yields obtained under laboratory conditions. According to the authors, they can be useful in assessing the potential for commercial-scale biomass production.
Author Contributions
Conceptualisation, M.H.-P., methodology, M.H.-P.; validation, M.H.-P. and P.R.; formal analysis, P.R.; investigation, M.H.-P. and P.R.; writing—original draft preparation, M.H.-P. and P.R.; writing—review and editing, M.H.-P. and P.R.; visualisation, M.H.-P. and P.R.; supervision, M.H.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef]
- Pruvost, J.; Le Borgne, F.; Artu, A.; Cornet, J.F.; Legrand, J. Chapter Five—Industrial Photobioreactors and Scale-Up Concepts. In Advances in Chemical Engineering; Legrand, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 257–310. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Masojídek, J.; Kopecký, J.; Giannelli, L.; Torzillo, G. Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J. Ind. Microbiol. Biotechnol. 2011, 38, 307–317. [Google Scholar] [CrossRef]
- Sforza, E.; Simionato, D.; Giacometti, G.M.; Bertucco, A.; Morosinotto, T. Adjusted Light and Dark Cycles Can Optimize Photosynthetic Efficiency in Algae Growing in Photobioreactors. PLoS ONE 2012, 7, e38975. [Google Scholar] [CrossRef] [PubMed]
- Zarmi, Y.; Bel, G.; Aflalo, C. Theoretical Analysis of Culture Growth in Flat-Plate Bioreactors: The Essential Role of Timescales. In Handbook of Microalgal Culture; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Loera-Quezada, M.M.; Angeles, G.; Olguín, E.J. Effect of irradiance on the cell density, size and lipid accumulation of Neochloris oleoabundans. Rev. Latinoam. Biotechnol. Ambient. Algal 2011, 2, 81–92. [Google Scholar]
- González-Camejo, J.; Aparicio, S.; Jiménez-Benítez, A.; Pachés, M.; Ruano, M.V.; Borrás, L.; Barat, R.; Seco, A. Improving membrane photobioreactor performance by reducing light path: Operating conditions and key performance indicators. Water Res. 2020, 172, 115518. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Hu, B.; Min, M.; Chen, P.; Ruan, R.R. Effect of light intensity on algal biomass accumulation and biodiesel production for mixotrophic strains Chlorella kessleri and Chlorella protothecoide cultivated in highly concentrated municipal wastewater. Biotechnol. Bioeng. 2012, 109, 2222–2229. [Google Scholar] [CrossRef]
- Fernández del Olmo, P.; Acién, F.G.; Fernández-Sevilla, J.M. Analysis of productivity in raceway photobioreactor using computational fluid dynamics particle tracking coupled to a dynamic photosynthesis model. Bioresour. Technol. 2021, 334, 125226. [Google Scholar] [CrossRef]
- Rai, M.P.; Gautom, T.; Sharma, N. Effect of salinity, pH, light intensity on growth and lipid production of microalgae for bioenergy application. Online J. Biol. Sci. 2015, 15, 260–267. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M. Production of Chlorella vulgaris biomass in tubular photobioreactors during different culture conditions. Appl. Sci. 2021, 11, 3106. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Rai, S.V.; Rajashekhar, M. Effect of pH, salinity and temperature on the growth of six species of marine phytoplankton. J. Algal Biomass Util. 2014, 5, 55–59. [Google Scholar]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Legrand, J.; Artu, A.; Pruvost, J. A review on photobioreactor design and modelling for microalgae production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- Schediwy, K.; Trautmann, A.; Steinweg, C.; Posten, C. Microalgal kinetics—A guideline for photobioreactor design and process development. Eng. Life Sci. 2019, 19, 830–843. [Google Scholar] [CrossRef]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Chisti, Y. Fermentation (industrial) Basic Considerations. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 751–761. [Google Scholar]
- Luzi, G.; McHardy, C. Modeling and Simulation of Photobioreactors with Computational Fluid Dynamics—A Comprehensive Review. Energies 2022, 15, 3966. [Google Scholar] [CrossRef]
- Lucas, D.; Tomiyama, A. On the role of the lateral lift force in poly-dispersed bubbly flows. Int. J. Multiph. Flow. 2011, 37, 1178–1190. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 18122:2016-01; Biopaliwa Stałe—Oznaczanie zawartości Popiołu. Polish Committee for Standardisation: Warszawa, Poland, 2016. Available online: https://sklep.pkn.pl/pn-en-iso-18122-2016-01p.html (accessed on 26 April 2021). (In Polish)
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, J.M. Attenuation of monochromatic and polychromatic lights in Chlorella vulgaris suspensions. Appl. Microbiol. Biotechnol. 2001, 55, 765–770. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Elmore, S.; Van Gerven, T.; Stankiewicz, A. Hydrodynamic evaluations in high rate algae pond (HRAP) design. Chem. Eng. J. 2013, 217, 231–239. [Google Scholar] [CrossRef]
- Nguyen, L.; Nguyen, D.K.; Nguyen, T.; Nguyen, B.; Nghiem, T.X. Analysis of Microalgal Density Estimation by Using LASSO and Image Texture Features. Sensors 2023, 23, 2543. [Google Scholar] [CrossRef]
- Han, F.; Pei, H.; Hu, W.; Song, M.; Ma, G.; Pei, R. Optimization and lipid production enhancement of microalgae culture by efficiently changing the conditions along with the growth-state. Energy Convers. Manag. 2015, 90, 315–322. [Google Scholar] [CrossRef]
- de Alva, M.S.; Luna-Pabello, V.M.; Cadena, E.; Ortiz, E. Green microalga Scenedesmus acutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour. Technol. 2013, 146, 744–748. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Sąsiadek, M. Optimization of Microalgal Biomass Production in Vertical Tubular Photobioreactors. Energies 2023, 16, 2429. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.M.; Gim, G.H.; Jeong, S.-H.; Kang, C.M.; Kim, D.-J.; Kim, S.W. Optimization of culture conditions and comparison of biomass productivity of three green algae. Bioprocess Biosyst. Eng. 2012, 35, 19–27. [Google Scholar] [CrossRef]
- Min, M.; Wang, L.; Li, Y.; Mohr, M.J.; Hu, B.; Zhou, W.; Chen, P.; Ruan, R. Cultivating Chlorella sp. in a Pilot-Scale Photobioreactor Using Centrate Wastewater for Microalgae Biomass Production and Wastewater Nutrient Removal. Appl. Biochem. Biotechnol. 2011, 165, 123–137. [Google Scholar] [CrossRef]
- Kazbar, A.; Cogne, G.; Urbain, B.; Marec, H.; Le-Gouic, B.; Tallec, J.; Takache, H.; Ismail, A.; Pruvost, J. Effect of dissolved oxygen concentration on microalgal culture in photobioreactors. Algal Res. 2019, 39, 101432. [Google Scholar] [CrossRef]
- Kishi, M.; Tanaka, K.; Tagawa, M.; Akizuki, S.; Toda, T. Energy-efficient algal culture through aeration-less oxygen removal in a gas-permeable bag photobioreactor. Algal Res. 2023, 69, 102959. [Google Scholar] [CrossRef]
- Camacho, F.G.; Gómez, A.C.; Sobczuk, T.M.; Molina-Grima, E. Effects of mechanical and hydrodynamic stress in agitated, sparged cultures of Porphyridium cruentum. Process Biochem. 2000, 35, 1045–1050. [Google Scholar] [CrossRef]
- Wu, J. Mechanisms of animal cell damage associated with gas bubbles and cell protection by medium additives. J. Biotechnol. 1995, 43, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Dhup, S.; Dhawan, V. Effect of nitrogen concentration on lipid productivity and fatty acid composition of Monoraphidium sp. Bioresour. Technol. 2014, 152, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.-M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022, 12, 5877. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Advances and perspectives in using microalgae to produce biodiesel. Appl. Energy 2011, 88, 3402–3410. [Google Scholar] [CrossRef]
- Xu, L.; Weathers, P.J.; Xiong, X.-R.; Liu, C.-Z. Microalgal bioreactors: Challenges and opportunities. Eng. Life Sci. 2009, 9, 178–189. [Google Scholar] [CrossRef]
- Pham, H.-M.; Kwak, H.S.; Hong, M.-E.; Lee, J.; Chang, W.S.; Sim, S.J. Development of an X-Shape airlift photobioreactor for increasing algal biomass and biodiesel production. Bioresour. Technol. 2017, 239, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, G.C.; Zhou, B.C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef]
- Feng, Y.; Li, C.; Zhang, D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Ho, K.C.; Leung, H.M.; Yung, K.K.L. Growth medium screening for Chlorella vulgaris growth and lipid production. J. Aquac. Mar. Biol. 2017, 6, 00143. [Google Scholar] [CrossRef]
- Papapolymerou, G.; Karayannis, V.; Besios, A.; Riga, A.; Gougoulias, N.; Spiliotis, X. Scaling-up sustainable Chlorella vulgaris microalgal biomass cultivation from laboratory to pilot-plant photobioreactor, towards biofuel. Glob. Nest J. 2018, 21, 37–42. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M.; Koniuszy, A. Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes. Sustainability 2021, 13, 9118. [Google Scholar] [CrossRef]
- Jerez, C.G.; Malapascua, J.R.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Effect of Nutrient Starvation under High Irradiance on Lipid and Starch Accumulation in Chlorella fusca (Chlorophyta). Mar. Biotechnol. 2016, 18, 24–36. [Google Scholar] [CrossRef]
- Arrojo, M.Á.; Regaldo, L.; Orquín, J.C.; Figueroa, F.L.; Díaz, R.T.A. Potential of the microalgae Chlorella fusca (Trebouxiophyceae, Chlorophyta) for biomass production and urban wastewater phycoremediation. AMB Express 2022, 12, 43. [Google Scholar] [CrossRef]
- Deamici, K.M.; Cardias, B.B.; Costa, J.A.V.; Santos, L.O. Static magnetic fields in culture of Chlorella fusca: Bioeffects on growth and biomass composition. Process Biochem. 2016, 51, 912–916. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Ratomski, P.; Koniuszy, A.; Golimowski, W.; Teleszko, M.; Grygier, A. Fatty Acid Profile of Microalgal Oils as a Criterion for Selection of the Best Feedstock for Biodiesel Production. Energies 2021, 14, 7334. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Ding, K.; Che, R.; Xu, J.-W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Production of biomass and lipids by the oleaginous microalgae Monoraphidium sp. QLY-1 through heterotrophic cultivation and photo-chemical modulator induction. Bioresour. Technol. 2016, 211, 669–676. [Google Scholar] [CrossRef]
- Guerrero-Cabrera, L.; Rueda, J.A.; García-Lozano, H.; Navarro, A.K. Cultivation of Monoraphidium sp., Chlorella sp. and Scenedesmus sp. algae in batch culture using Nile tilapia effluent. Bioresour. Technol. 2014, 161, 455–460. [Google Scholar] [CrossRef]
- Bogen, C.; Klassen, V.; Wichmann, J.; La Russa, M.; Doebbe, A.; Grundmann, M.; Uronen, P.; Kruse, O.; Mussgnug, J.H. Identification of Monoraphidium contortum as a promising species for liquid biofuel production. Bioresour. Technol. 2013, 133, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M. Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy. Energies 2020, 13, 5975. [Google Scholar] [CrossRef]
- Klin, M.; Pniewski, F.; Latała, A. Characteristics of the growth rate and lipid production in fourteen strains of Baltic green microalgae. Oceanol. Hydrobiol. Stud. 2018, 47, 10–18. [Google Scholar] [CrossRef]
- Tang, H.; Chen, M.; Garcia, M.E.D.; Abunasser, N.; Ng, K.Y.S.; Salley, S.O. Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol. Bioeng. 2011, 108, 2280–2287. [Google Scholar] [CrossRef]
- Freitas, B.C.B.; Cassuriaga, A.P.A.; Morais, M.G.; Costa, J.A.V. Pentoses and light intensity increase the growth and carbohydrate production and alter the protein profile of Chlorella minutissima. Bioresour. Technol. 2017, 238, 248–253. [Google Scholar] [CrossRef]
- Amaral, M.S.; Loures, C.C.A.; Naves, F.L.; Baeta, B.E.L.; Silva, M.B.; Prata, A.M.R. Evaluation of cell growth performance of microalgae Chlorella minutissima using an internal light integrated photobioreactor. J. Environ. Chem. Eng. 2020, 8, 104200. [Google Scholar] [CrossRef]
- Di Caprio, F.; Altimari, P.; Toro, L.; Pagnanelli, F. Effect of Lipids and Carbohydrates Extraction on Astaxanthin Stability in Scenedesmus sp. Chem. Eng. Trans. 2015, 43, 205–210. [Google Scholar] [CrossRef]
- Wu, Y.H.; Yu, Y.; Li, X.; Hu, H.Y.; Su, Z.F. Biomass production of a Scenedesmus sp. under phosphorous-starvation cultivation condition. Bioresour. Technol. 2012, 112, 193–198. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.M.; Ross, A.B.; Anastasakis, K.; Hodgson, E.M.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Laurens, L.M.L.; Dempster, T.A.; Jones, H.D.T.; Wolfrum, E.J.; Van Wychen, S.; McAllister, J.S.P.; Rencenberger, M.; Parchert, K.J.; Gloe, L.M. Algal biomass constituent analysis: Method uncertainties and investigation of the underlying measuring chemistries. Anal. Chem. 2012, 84, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. Characterization of ash in algae and other materials by determination of wet acid indigestible ash and microscopic examination. Algal Res. 2017, 25, 307–321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).