Abstract
This study focuses on the semiconductive silicone rubber of 10 kV cold-shrink accessories. Accelerated thermal aging tests were conducted on the semiconductive silicone rubber, obtaining tensile stress–strain curves at various time points after thermal aging. The corresponding parameters of the Yeoh hyperelastic model were calculated. The results indicate that the initial shear modulus of the samples decreases with the increase in the aging temperature and time. Microscopic morphology, changes in cross-sectional content, thermal residual values, and chemical structure changes of the samples after aging were studied using electron microscopy, EDS testing, TG curves, and Fourier spectra. The results show that the surface roughness of the aged semiconductive silicon rubber increases, the residual values decrease, the thermal stability decreases, the main chain absorbance decreases, the main chain integrity decreases, and the organic functional groups Si-CH3 and Si(CH3)2 decrease, leading to a reduction in organic content.
1. Introduction
With the vigorous development of modern industrialization, the demand for power cables has grown rapidly. Transmission cables are referred to as the ‘arteries and nerves’ of the national economy. The urban cable rate has become an important indicator for measuring urban modernization and living environment [1,2]. To meet the power demand and ensure stability and cost-effectiveness in power transmission, the power grid is continuously evolving towards higher capacities and voltage levels. This poses higher requirements for the conduciveness and reliability of power cables, consequently accelerating the aging process of power cables [3,4].
The intermediate joints of cables are limited by the manufacturing process, resulting in significant variations in quality and higher failure rates. Wang et al. [5] pointed out that compared to the main body of the cable, the diameter of the cable intermediate joint is larger, leading to inadequate heat dissipation capacity. Additionally, the contact resistance at the crimping tube increases the heat generation of the cable joint, causing the conductor temperature at the cable intermediate joint to be higher than that of the cable body. This is more likely to result in a decline in the insulation performance of the cable joint. Gao et al. [6] pointed out that during the installation process of cable joints, it is necessary to strip the semiconductive shielding layer of the cable body. If the construction is not carried out properly, serious electric field distortion may occur at the breakage points. Yang et al. [7] pointed out that the electric field strength and temperature at the cable joint are higher, making it more prone to thermal stress and electrical stress distortion, thereby affecting the safe operation of the cable joint. Other studies [8,9] also indicate the cable joints are the weak links in cable circuits and are most susceptible to failures.
In the faults occurring at cable joints, a relatively high proportion is attributed to the degradation of the insulation performance in cable accessories. Silicone rubber and semiconductive silicone rubber are widely used as insulation materials in cross-linked polyethylene cable (XLPE) cable joints due to their high elasticity, resistance to high and low temperatures, and excellent electrical properties. However, with the increase in operational time, the insulation of silicone rubber cable accessories undergoes a gradual decline in electrical performance under the influence of electrical and thermal stresses. Zhou et al. [10] found that prolonged exposure of silicone rubber to normal operating temperatures can lead to damage to its insulation performance, thereby affecting the reliability of cable accessory operation. Lv et al. [11] found that during the thermal aging process, the relative dielectric constant of silicone rubber specimens gradually increased, and the electrical strength decreased with the increase in aging time. Chen et al. [12] revealed that the volume resistivity of silicone rubber increases after thermal aging, and the breakdown field strength shows a trend of initially increasing and then decreasing with the progression of aging. S. Kashi et at. [13] found that the tensile strength and elongation at break of silicone rubber gradually decrease with the increase in the aging time before and after accelerated thermal aging. Tear strength and hardness, in the early stages of aging, initially increase and then decrease. Fourier transform infrared spectroscopy and thermal stability, however, show no significant changes.
Silicone rubber, as a polymer material, undergoes processes such as crosslinking or breakage between polymer molecular chains, exhibiting different mechanical properties [14]. Analyzing the changes in these mechanical properties through testing can to some extent reflect the molecular structure and aging status of the material [15]. S. Ito et al. [16] revealed that after aging, silicone rubber develops a cross-linked structure through the formation of siloxane bonds internally. This results in silicone rubber becoming harder and more brittle, causing a decline in mechanical properties. However, it leads to improved dielectric characteristics. Zhou et al. [17] pointed out the presence of the interface effect within the insulation of decommissioned cables. The inner side of the insulation, experiencing higher electric field intensity, exhibits more severe molecular breakage and oxidation, resulting in higher conductivity and dielectric constant. As a result, the degree of aging is deeper on the inner side compared to the outer side of the insulation. Chen et al. [18] conducted a study on the physicochemical properties of decommissioned cable accessories and found that the reduction of C-O-C and organic groups on the molecular chains of ethylene propylene diene monomer (EPDM) is a sign of insulation aging in the accessories. Furthermore, the insulation’s dielectric performance deteriorates significantly in severely aged accessories. Shao et al. [19] found that the electrical and thermal interactions result in a weakening of the interaction forces between SiO2 fillers and SIR polymer chains, leading to charge accumulation and a decrease in the insulation breakdown strength.
Therefore, to ensure the safe and reliable operation of the insulation interface between cables and accessories, it is necessary to pay attention to the insulation condition and physicochemical properties of cable accessories during long-term operation [20,21,22]. Currently, there have been numerous studies on the aggregation state and mechanical properties of insulation materials for cable accessories both domestically and internationally. However, most of these studies have focused on the primary insulation material for cable accessories, namely silicone rubber. There is a lack of reported research on the insulation and physicochemical characteristics of semiconductive silicone rubber.
This article investigates the macroscopic properties and microscopic characteristics of semiconductive silicone rubber used in 10 kV cold-shrink cable accessories through methods such as mechanical tensile testing, Fourier transform infrared spectroscopy analysis (FTIR), scanning electron microscopy (SEM), energy dispersive spectrometry (EDS), and thermogravimetric analysis (TGA). The study analyzed the insulation condition and physicochemical properties of semiconductive silicone rubber in cable accessories after accelerated thermal aging. It investigated the aging mechanism of semiconductive silicone rubber, providing experimental evidence for the aging state monitoring and lifespan assessment of medium-voltage cable accessories.
2. Materials and Methods
2.1. Specimen
Semiconductive silicone rubber is an important component of cold-shrink cable accessories, addressing the issue of electric field distortion at the joints and pressure tubing ends. In this study, semiconductive silicone rubber specimens measuring 10 × 10 cm with a thickness of 2 mm were prepared using a high temperature vulcanization process. Subsequently, according to the national standard HG/T 2645-2011 [23] for rubber specific cutting knives, the semiconductive silicone rubber formed by high temperature vulcanization was cut into dumbbell Type II shapes, as shown in Figure 1. The dimensions of the semiconductive silicone rubber specimens were as follows: an average width of 4 mm, an average thickness of 2 mm, a gauge length of 25 mm, and an average length of 75 mm.

Figure 1.
Dumbbell type semiconductive silicone rubber sample.
Finally, in accordance with the national standard GB/T 3512-2001 [24] for rubber aging, accelerated thermal aging tests were conducted on the specimens using an electric constant temperature drying oven. Generally, the rated temperature for the normal operation of cables does not exceed 90 °C. The temperature does not exceed 130 °C during overload. However, in the event of abnormal conditions such as a short circuit, the cable’s maximum temperature can reach 200 °C to 250 °C. Here, the aging temperatures were set at 130 °C, 150 °C, 170 °C, and 200 °C, with aging times of 2 days, 4 days, 8 days, 15 days, 22 days, and 30 days. After the aging specimens were prepared, the samples were removed and allowed to stand at room temperature for 30 min.
2.2. Test Method
2.2.1. Mechanical Performance Testing
Following the testing standard GB/T 528-2009 [25], a universal tensile testing machine was utilized for the uniaxial tensile testing of semiconductive rubber. Each set of tensile tests was repeated three times, and the pulling rate was set at 500 mm/min.
2.2.2. Microstructure Analysis
We used a scanning electron microscope to observe the microstructure of the surface and cross-section of the semiconductive silicon rubber material and employed energy dispersive X-ray spectroscopy configured on the SEM for the qualitative and quantitative analysis of specific areas.
2.2.3. Thermogravimetric Analysis
We utilized a thermogravimetric analyzer to investigate the mass changes in the semiconductive silicon rubber specimens during the temperature elevation process, with a temperature ramp rate of 20 °C/min.
2.2.4. Material Characterization Analysis
We analyzed the chemical composition and structure of aged semiconductor silicon rubber specimens using Fourier transform infrared spectroscopy. The FTIR spectrometer used was model THERMO IS5, with an instrumental resolution of 0.8 cm−1 and a spectral range from 7800 to 350 cm−1.
3. Results
3.1. Mechanical Performance Testing
The stress–strain curve of the semiconductor silicone rubber aged at 130 °C is shown in Figure 2. The stress–strain data for the semiconductor silicone rubber at 150 °C, 170 °C, and 200 °C are shown in Figure 3, Figure 4 and Figure 5, respectively. In order to make the figures clearer and more understandable, only the stress–strain curves for aging periods of 96 h, 360 h, and 720 h are presented in the figures [26].
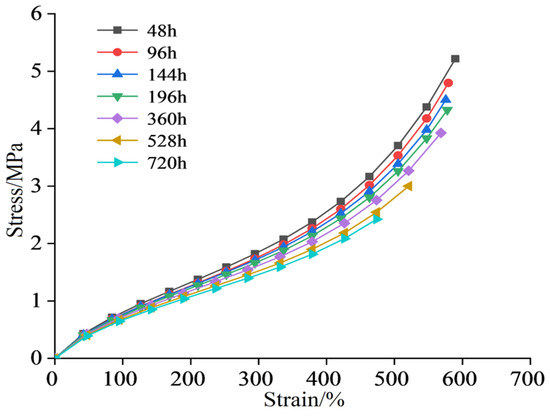
Figure 2.
Stress and strain of semiconductive silicone rubber after aging at 130 °C.
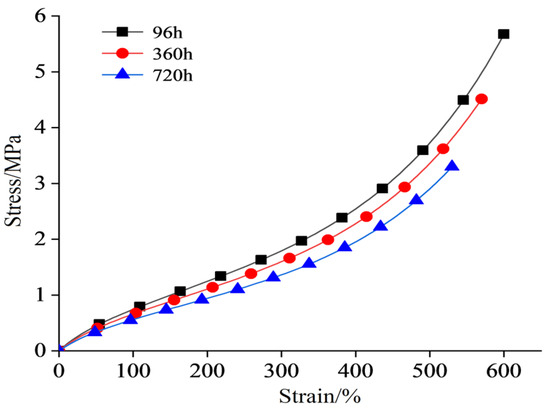
Figure 3.
Stress–strain curve after aging at 150 °C.
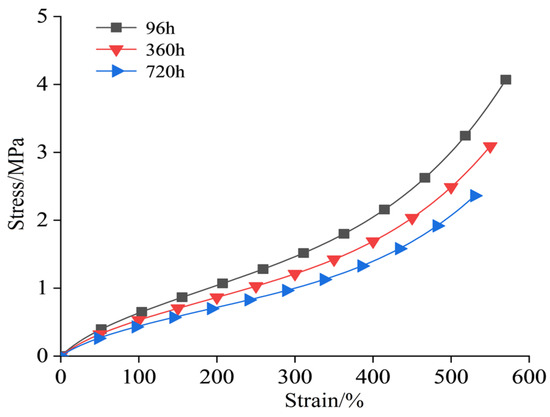
Figure 4.
Stress–strain curve after aging at 170 °C.
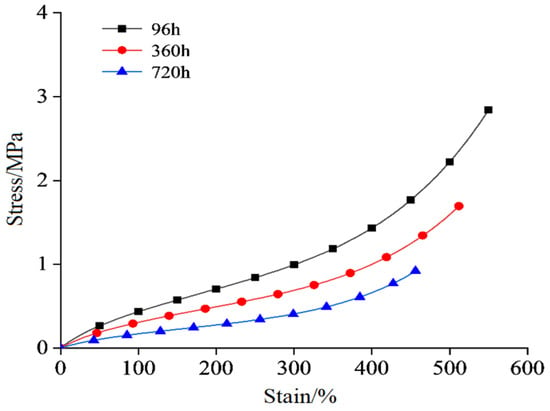
Figure 5.
Stress–strain curve after aging at 200 °C.
We utilized the experimental data of stress–strain obtained from the tests, imported the data into Origin 2022, and then fit the parameters of the Yeoh constitutive model [27] for thermally aged semiconductor silicon rubber, which are shown in Table 1, Table 2, Table 3 and Table 4. In the Yeoh constitutive model, C10 represents the initial shear modulus at small strains, the negative value of C20 reflects the material softening process during moderate deformations, and C30 indicates an upward curve at large deformations, representing the hardening phenomenon during significant strains.

Table 1.
Yeoh model parameters of semiconductive silicone rubber after aging at 130 °C.

Table 2.
Yeoh model parameters of semiconductive silicone rubber after aging at 150 °C.

Table 3.
Yeoh model parameters of semiconductive silicone rubber after aging at 170 °C.

Table 4.
Yeoh model parameters of semiconductive silicone rubber after aging at 200 °C.
As shown in Figure 6, Figure 7, Figure 8 and Figure 9, with the increasing aging time, at the same elongation rate, the tensile stress continuously decreases, indicating a gradual reduction in the mechanical properties of the semiconductive silicone rubber due to aging. Similarly, for semiconductive silicone rubber at different aging temperatures, at the same tensile stress, samples with higher thermal aging temperatures require higher elongation rates, suggesting that higher temperatures lead to more severe aging in semiconductive silicone rubber. As seen in Table 1, Table 2, Table 3 and Table 4, at the same temperature, with the increasing aging time, the value of C10 gradually decreases. Similarly, at the same aging time, with the temperature rising, the value of C10 also exhibits a decreasing trend, indicating that the initial shear modulus of the semiconductive silicone rubber gradually decreases with the increase in the aging time and aging temperature.

Figure 6.
Surface morphology of the unaged samples (SEM). (a) 200 μm; (b) 50 μm.
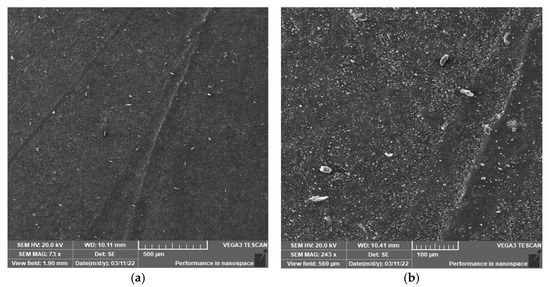
Figure 7.
Surface morphology of the sample aged at 170 °C for 30 days (SEM). (a) 500 μm; (b) 100 μm.
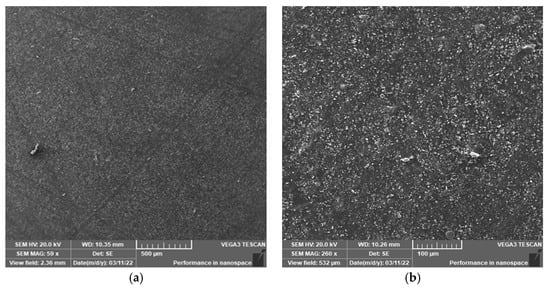
Figure 8.
Surface morphology of the sample aged at 200 °C for 30 days (SEM). (a) 500 μm; (b) 100 μm.
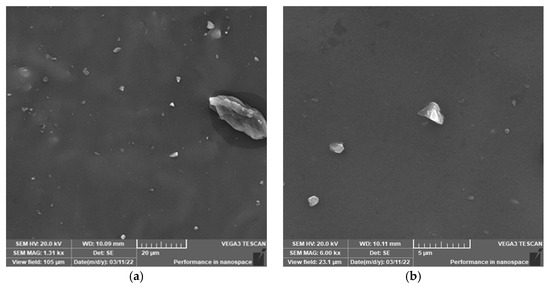
Figure 9.
Cross section morphology of unaged samples. (a) 20 μm; (b) 5 μm.
3.2. Microstructure Analysis
3.2.1. Surface Topography Analysis
Figure 6, Figure 7 and Figure 8 show the surface SEM images of the samples before aging, aged at 170 °C, and aged at 200 °C for 30 days, respectively. It can be observed that thermal aging has a certain influence on the surface morphology of the semiconductor silicon rubber. Moreover, at the same aging time, the higher the thermal aging temperature, the higher the surface roughness of the semiconductor silicon rubber, indicating a more severe aging process.
Figure 9 shows the cross-sectional morphology of the semiconductor silicon rubber before aging, Figure 10 depicts the cross-sectional morphology after aging for 30 days at 170 °C, and Figure 11 illustrates the cross-sectional morphology after aging for 30 days at 200 °C. It can be observed that before aging, there are few particle distributions on the surface of the semiconductor silicon rubber. After aging, a large number of microparticles are distributed on the surface of the semiconductor silicon rubber, and the particle sizes are uneven, with some particles floating on the surface. Additionally, when the aging time is the same, a higher thermal aging temperature results in a denser distribution of microparticles on the surface of the semiconductor silicon rubber, and the particle size of the microparticles is larger. With the increase in the aging temperature, the white powder particles attached to the surface also gradually increase, and the white particles mainly consist of some small molecular siloxanes and inorganic particles. With the increase in the temperature, more and more white particles are precipitated from the surface of the material, and an obvious powdering phenomenon appears on the surface of the aging sample, indicating that under the condition of high aging temperature, the surface of the aging sample is gradually increased. Powder easily accumulates on the surface of the material. The analysis shows that when the ambient temperature becomes higher, the movement of the molecules becomes intensified after being heated, and the high temperature will accelerate the migration rate of small molecular substances to the surface and accelerate the degumming of the filler to the surface of the material.

Figure 10.
Cross-section morphology of the sample aged at 170 °C for 30 days (SEM). (a) 20 μm; (b) 5 μm.
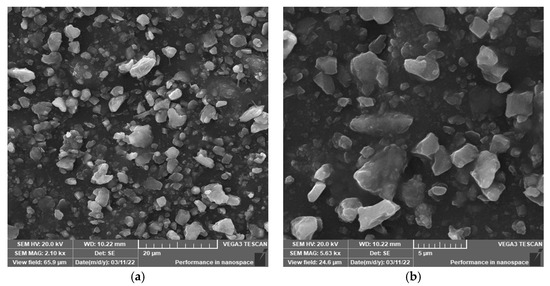
Figure 11.
Cross-section morphology of the sample aged at 200 °C for 30 days (SEM). (a) 20 μm; (b) 5 μm.
3.2.2. Cross-Sectional EDS Analysis
The cross-sectional EDS analysis was performed on localized positions of the cable accessory semiconductive silicone rubber samples before aging, aged at 170 °C, and aged at 200 °C for 30 days, respectively. The results are shown in Figure 12, Figure 13 and Figure 14. The elemental composition of the semiconductive silicone rubber for the unaged, aged at 170 °C for 30 days, and aged at 200 °C for 30 days are shown in Table 5, Table 6, and Table 7, respectively.
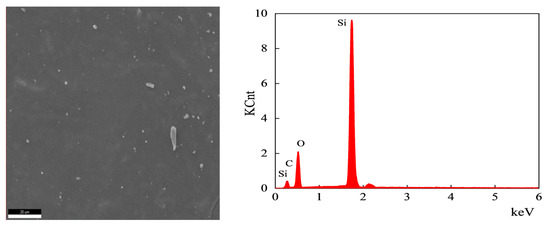
Figure 12.
EDS analysis of the cross-sectional area of the unaged sample.
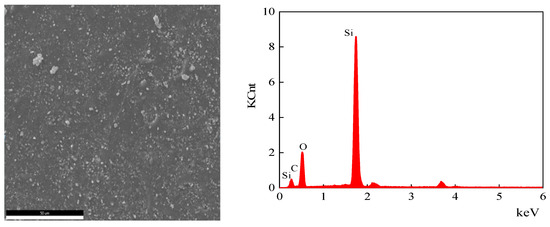
Figure 13.
EDS analysis of the cross-section area of the sample aged at 170 °C for 30 days.
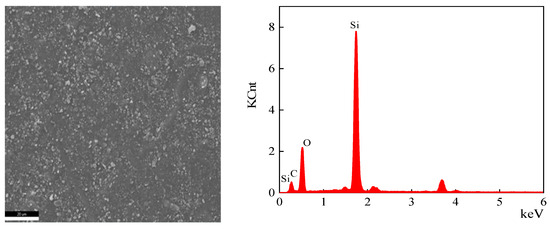
Figure 14.
EDS analysis of the cross-section area of the sample aged at 200 °C for 30 days.

Table 5.
Element content of the section of the unaged sample.

Table 6.
Element content after aging at 170 °C for 30 days.

Table 7.
Element content after aging at 200 °C for 30 days.
According to the test results, the main elements of the semiconductor silicon rubber are silicon, oxygen, and carbon. The types of elements remain unchanged before and after thermal aging, but their elemental content changes. After aging at 170 °C for 30 days, the atomic percentage of carbon elements decreases by 3.62%, the atomic percentage of oxygen elements increases by 4.68%, and the atomic content of silicon elements decreases by 1.06%. After aging at 200 °C for 30 days, the atomic percentage of carbon elements decreases by 6.75%, the atomic percentage of oxygen elements increases by 8.82%, and the atomic content of silicon elements decreases by 2.07%. When the aging time is the same, a higher thermal aging temperature leads to a greater decrease in the atomic percentage content of carbon elements, a greater increase in the atomic percentage content of oxygen elements, and a greater decrease in the atomic percentage content of silicon elements. The reason for the decrease in the atomic percentage content of silicon elements due to thermal aging may be the depolymerization of Si-O structure rings, leading to the degradation of semiconductive silicon rubber. The cyclic oligomers diffuse from the interior to the surface, resulting in a reduced Si element atomic percentage content measured on the internal cross section.
EDS uses the characteristic X-rays of the sample for elemental analysis and stoichiometric studies. K usually refers to the characteristic X-ray series. C K, O K, and Si K are the K series of C, the K series of O, and the K series of Si, respectively. To compare the variations in the C and O elements on the cross section of the semiconductive silicone rubber samples, the atomic percentage of the C and O elements relative to the atomic percentage of Si element was calculated. For the unaged sample, the C/Si atomic percentage ratio was 1.41, and the O/Si atomic percentage ratio was 1.303. After aging at 170 °C for 30 days, the C/Si atomic percentage ratio decreased to 1.33, while the O/Si atomic percentage ratio increased to 1.53. After aging at 200 °C for 30 days, the C/Si atomic percentage ratio further decreased to 1.26, and the O/Si atomic percentage ratio increased to 1.76. With the increase in the aging temperature, the C/Si atomic percentage ratio on the cross section of the semiconductive silicone rubber decreased, while the O/Si atomic percentage ratio generally increased. This can be mainly attributed to the cyclization of the main chain and oxidation of the side chains, resulting in an increase in the O element and a decrease in the C element on the cross section of the semiconductive silicone rubber.
3.3. Thermogravimetric Analysis
The TG and DTG curves of samples subjected to no aging, aging at 170 °C, and aging at 200 °C in an atmospheric environment with a maximum temperature ramp of 800 °C are shown in Figure 15, Figure 16 and Figure 17.
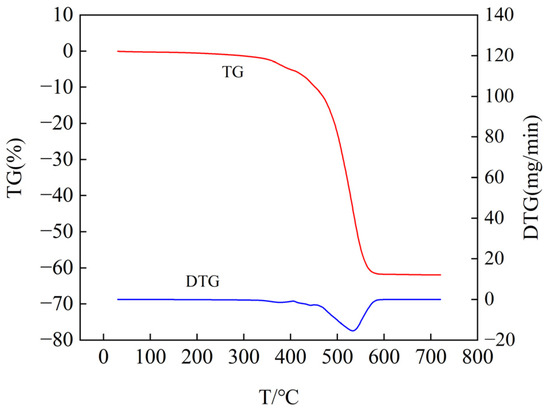
Figure 15.
Thermogravimetric analysis curve of unaged samples (air atmosphere).
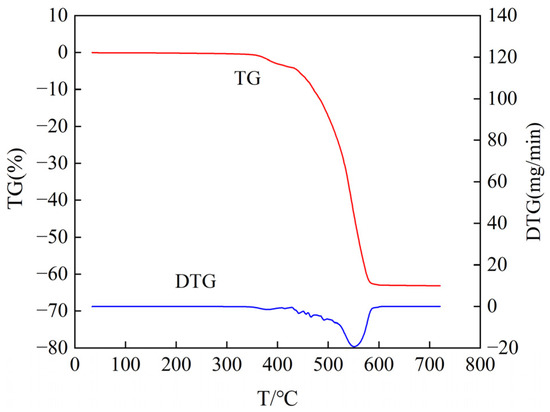
Figure 16.
Thermogravimetric analysis curve of samples aged at 170 °C for 30 days (air atmosphere).
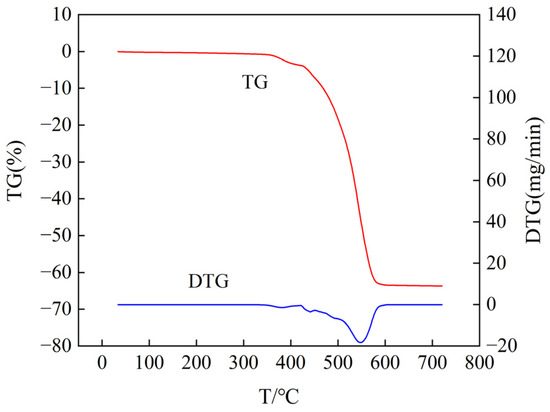
Figure 17.
Thermogravimetric analysis curve of samples aged at 200 °C for 30 days (air atmosphere).
In Figure 15, the point where the TG curve starts to decline represents the onset of decomposition for the semiconductive silicone rubber, occurring at a temperature of 380.48 °C. At 520.55 °C, the TG curve exhibits the highest slope, indicating that the sample experiences the maximum rate of mass change and decomposition at this temperature. Beyond 573.56 °C, the TG curve remains unchanged, indicating that the decomposition of the semiconductive silicone rubber sample ceases, marking the termination temperature of decomposition. By differentiating the TG curve with respect to time, we obtain the DTG curve, which accurately and visually illustrates the relationship between the mass and temperature changes. The peak of the DTG curve directly represents the maximum rate of sample decomposition. From the DTG curve, we find that the onset temperature of weight loss for the nonaged sample is 469.1 °C, the temperature of maximum weight loss rate is 533.3 °C, the maximum weight loss rate is 15.39 mg/min, and the weight loss ends at 568.28 °C.
From Figure 16 and Figure 17, it can be observed that the semiconductive silicone rubber samples undergo oxidative degradation reactions and decrease in mass with increasing temperature. The DTG curves exhibit degradation peaks. Analyzing the TG curve of the semiconductive silicone rubber sample aged at 170 °C for 30 days in Figure 16, we can see that the mass begins to change at 365.25 °C, indicating the onset temperature of decomposition. At 557.9 °C, the mass no longer changes, indicating the end of decomposition. From the DTG curve, we find that the onset temperature of weight loss for the sample is 431.67 °C, the temperature of maximum weight loss rate is 548.37 °C, the maximum weight loss rate is 19.37 mg/min, and the weight loss ends at 578.18 °C. Analyzing the TG curve of the semiconductive silicone rubber sample aged at 200 °C for 30 days in Figure 17, we can observe that the mass begins to change at 358.65 °C, indicating the onset temperature of decomposition. At 585.60 °C, the mass no longer changes, indicating the end of decomposition. From the DTG curve, we find that the onset temperature of the weight loss for the sample is 425 °C, the temperature of the maximum weight loss rate is 551.26 °C, and the weight loss ends at 586.40 °C.
From the TG curve results, it can be observed that, compared to the non-aged semiconductive silicone rubber specimen, as the aging temperature increases, the temperature at which the mass of the semiconductive silicone rubber begins to decline gradually decreases. This indicates that with the increasing degree of aging, the thermal stability of the semiconductive silicone rubber gradually decreases. The analysis suggests that the reason for this phenomenon may be the disruption of the molecular chain structure inside the aged semiconductive silicone rubber, leading to a decrease in the cross-linking degree, a reduction in the number of cross-linking points, a decrease in the cross-linking density, and a loosening of the dense cross-linking structure between the semiconductive silicone rubber molecules. This results in an accelerated rate of molecular degradation and weight loss in the semiconductive silicone rubber, leading to a lower initial thermal degradation temperature after thermal aging. By calculating the weight loss rates from TG curves for non-aged, aged at 170 °C for 30 days, and aged at 200 °C for 30 days semiconductive silicone rubber samples, it is observed that the final residual mass of the aged samples slightly decreases. The weight loss rate of the non-aged sample is 61.89% of the aged at 170 °C for 30 days sample is 63.13%, and that of the aged at 200 °C for 30 days sample is 63.62%. The analysis suggests that with the gradual increase in the thermal aging time, the degradation reaction in the semiconductive silicone rubber material intensifies, leading to a decrease in the final residual mass.
In conclusion, thermal aging has an impact on the thermal stability of semiconductive silicone rubber. The longer the thermal aging time and the higher the thermal aging temperature, the greater the decrease in the thermal stability of semiconductive silicone rubber, indicating an intensified effect of thermal aging.
3.4. Material Characterization Analysis
The infrared absorption peaks of the main functional groups in semiconductor silicone rubber, as shown in Figure 18 and Table 8, undergo changes in absorbance with accelerated aging of the sample, while the wavelengths remain approximately constant.
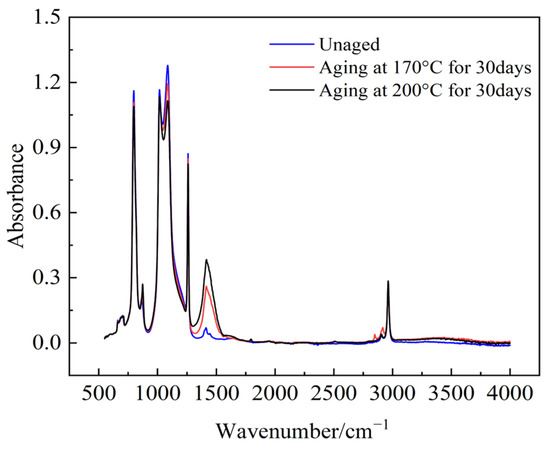
Figure 18.
Changes in the FTIR spectra of the samples after thermal aging.

Table 8.
Infrared absorption peaks of the main characteristic functional groups of semiconductive silicone rubber.
The magnitude of absorption peaks for various functional groups represents the content of the functional groups in semiconductive silicone rubber samples. Research [28,29] indicates that using the absorbance of three functional groups, Si-O-Si, Si-CH3, and Si(CH3)2, provides a relatively high accuracy in characterizing semiconductive silicone rubber. In this study, Si-O-Si, Si-CH3, and Si(CH3)2, three of the most significant functional groups, were selected to analyze the changes in the peak values of different functional groups after the aging of semiconductive silicone rubber, as shown in Figure 19, Figure 20 and Figure 21.
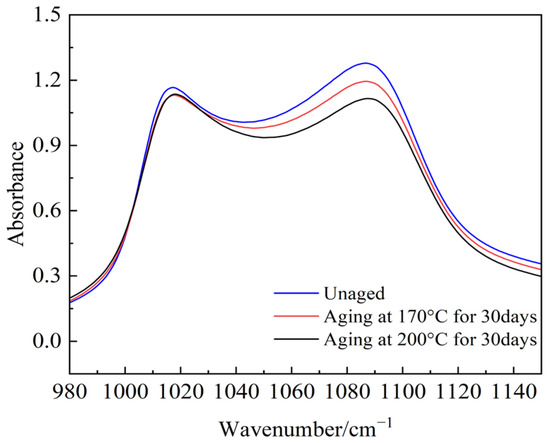
Figure 19.
The absorbance of Si-O-Si.
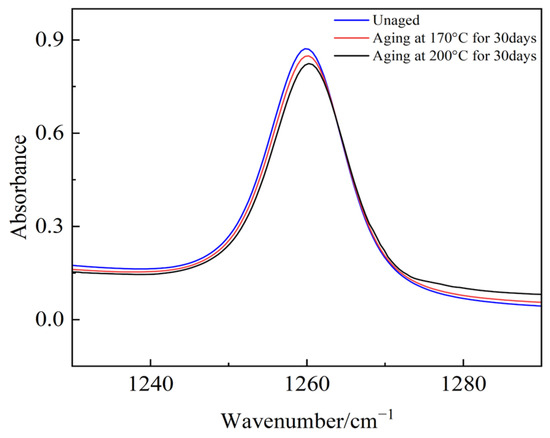
Figure 20.
The absorbance of Si-Ch3.
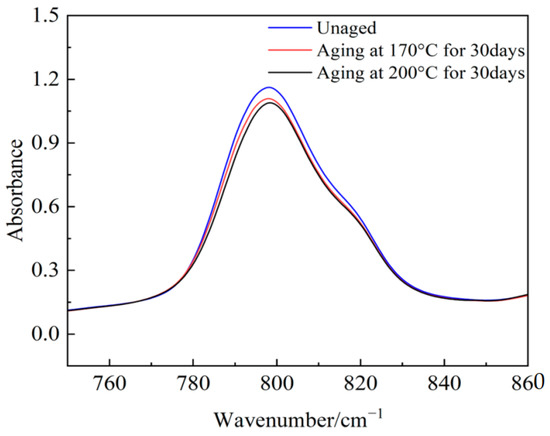
Figure 21.
The absorbance of Si(CH3)2.
The absorption peak values of the main chain Si-O-Si, Si(CH3)2, and the organic group Si-CH3 in semiconductive silicone rubber change with an increase in the aging days. Compared to the non-aged semiconductive silicone rubber sample, the absorption peak value of Si-O-Si decreases after 30 days of aging, indicating that the main chain of semiconductive silicone rubber has undergone degradation. The absorbance peak values of various side chain groups decrease, and as the aging temperature increases, the absorbance peak values decrease further. This suggests that the absorbance of side-chain organic functional groups decreases with thermal aging, leading to a reduction in organic components in the semiconductive silicone rubber system. This reduction manifests macroscopically as a decrease in the mechanical properties, namely a reduction in the interface pressure in cold-shrink accessories. High temperatures intensify molecular chain movement, and when it exceeds the dissociation energy of chemical bonds, it can cause the thermal decomposition of polymer chains or detachment of the functional groups. Oxygen, under high temperature conditions, easily attacks the organic functional groups on the semiconductive silicone rubber side chains, forming highly reactive oxygen free radicals or peroxides, leading to the detachment of side-chain groups.
4. Discussion
The semiconductive silicone rubber in cable accessories undergoes aging due to the influence of electrical, thermal, and mechanical factors. As the operating years increase, the aging level of cable accessories gradually deepens. At this point, changes occur in the surface microscopic morphology and elemental content of the insulation in cable accessories. Macroscopically, this is manifested as a decline in the electrical and mechanical properties.
The microscopic morphology of a material is correlated with its macroscopic mechanical properties. After undergoing accelerated thermal aging, the surface of semiconductive silicone rubber exhibits a dense distribution of microparticles, with an increase in the particle diameter. This indicates that with the progression of thermal aging, cross-linking reactions occur between molecular chains in the semiconductive silicone rubber. Some molecules entangle, forming a new dense cross-linked structure. The Fourier transform infrared spectroscopy test results reveal that the thermally aged semiconductive silicone rubber experiences a decrease in main chain absorbance, leading to a reduction in main chain integrity. The organic functional groups Si-CH3 and Si(CH3)2 also decrease, resulting in a decrease in the organic content, reduced elasticity, and a decline in the mechanical properties. Under the influence of high temperature, the internal molecular structure undergoes severe damage, causing degradation of the main chain and oxidation of side groups. As the aging temperature increases and the duration extends, the degradation reaction of the main chain inside the specimens accelerates, leading to a deterioration in the mechanical properties and a decrease in the interface pressure in cold-shrink accessories. The thermogravimetric analysis indicates a reduction in the residue value for the thermally aged semiconductive silicone rubber, indicating a decrease in thermal stability.
Changes in the chemical element content of the cross section of semiconductive silicone rubber after aging were studied through micro-area EDS tests. After aging at 170 °C and 200 °C for 30 days, the C/Si ratio decreased, and the O/Si element content increased on the cross section of semiconductive silicone rubber. This is attributed to the degradation of the main chain and oxidation of the side chains in the specimen.
5. Conclusions
The study investigated the changes in the mechanical properties of semiconductive silicone rubber after accelerated thermal aging. It was observed that the initial shear modulus of the semiconductive silicone rubber exhibited a decreasing trend with an increase in the aging temperature and aging time, leading to a reduction in tensile performance. Scanning electron microscopy was employed to study the morphological changes on the surface and cross section of the aged semiconductive silicone rubber. It was found that the roughness increased during the aging process.
Chemical analysis methods were employed to investigate the aging mechanism of the semiconductive silicone rubber. The thermal stability was analyzed through thermogravimetric experiments, revealing a decrease in the residual values of the specimens after aging. This indicates a reduction in stability after thermal aging. The changes in chemical element content on the cross section of the aged semiconductive silicone rubber were studied using energy dispersive X-ray spectroscopy on microregions. After aging at 170 °C and 200 °C for 30 days, the C/Si ratio on the cross section of the semiconductive silicone rubber showed a decreasing trend, while the O/Si element content exhibited a general increasing trend. This is primarily due to the cyclization degradation of the main chain and oxidation of the side chains, leading to an increase in internal O elements and a decrease in C elements.
The chemical structure changes during the aging process were studied using Fourier transform infrared spectroscopy. After aging at 170 °C and 200 °C for 30 days, the absorbance of the main chain decreased, indicating a decline in the main chain integrity. The organic functional groups Si-CH3 and Si(CH3)2 also decreased, leading to a reduction in the organic content and elasticity, manifesting as a decrease in the mechanical properties at the macroscopic level. Under the influence of high temperatures, the internal molecular structure of semiconductive silicone rubber undergoes severe damage, leading to degradation of the main chain and oxidation of side groups. As the aging temperature increases and the duration of aging extends, the degradation reaction of the main chain inside the specimen accelerates. This results in a decline in the mechanical properties and a decrease in the interface pressure in cold-shrink accessories.
Author Contributions
Conceptualization, J.Y. and Z.Z.; methodology, J.Y., W.R. and D.Y.; validation, Z.Z. and D.W.; formal analysis, Z.N.; investigation, J.Y., C.F. and J.W.; data curation, J.Y., Z.Z. and C.F.; writing—original draft preparation, C.F. and J.W.; writing—review and editing, J.Y., C.F. and J.W.; visualization, Z.Z. and W.R.; supervision, D.Y., D.W. and Z.N.; project administration, C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The industrial partner’s policy prevents public access to the data.
Conflicts of Interest
Authors Jun Yu, Weifeng Ren, Dongxing Yang, Dian Wu, Zhiqiang Ning were employed by the company Wuxi Guangying Group Co., Ltd. Author Zhijian Zhang was employed by the State Grid Wuxi Power Supply Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lee, H.; Lee, B.; Han, G.; Kim, Y.; Kim, Y. Development of Methods for an Overhead Cable Health Index Evaluation That Considers Economic Feasibility. Energies 2023, 16, 7122. [Google Scholar] [CrossRef]
- Kwon, D.-J. Technology Trends for Asset Management System of Power Facilities. Korean Inst. Electr. Eng. 2018, 67, 30–33. [Google Scholar]
- Shaalan, E.M.; Ward, S.A.; Youssef, A. Analysis of a Practical Study for Under-Ground Cable Faults Causes. In Proceedings of the 2021 22nd International Middle East Power Systems Conference (MEPCON), Assiut, Egypt, 14–16 December 2021. [Google Scholar]
- Wang, C.; Zhao, X.; Qiao, J.; Xiao, Y.; Zhang, J.; Li, Y.; Cao, H.; Yang, L.; Liao, R. Structural Changes and Very-Low-Frequency Nonlinear Dielectric Response of XLPE Cable Insulation under Thermal Aging. Materials 2023, 16, 4388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mu, Y.; Wang, B.; Liu, C. Research on Design of New 10 kV Intelligent Explosion-proof Cable Joint. In Proceedings of the 2023 6th International Conference on Energy, Electrical and Power Engineering (CEEPE), Guangzhou, China, 21–23 April 2023; pp. 251–255. [Google Scholar]
- Gao, Y.; Zhao, J.; Li, X.; Wang, Q.; Zhang, W.; Liu, Z. Effect of Expansion rate in Cable Joint on Stress and Electric Field Distribution of Stress Cone. In Proceedings of the 2022 IEEE International Conference on High Voltage Engineering and Applications, ICHVE 2022, Chongqing, China, 25–29 September 2022. [Google Scholar]
- Yang, X.; Liu, Z.; Liang, Z.; Qiu, W.; Cui, J.; Zhong, Q. Simulation calculation of thermal stress distribution of composite interface in operation of high voltage cable joint under electrothermal coupling. Electr. Mach. Control. 2020, 24, 100–108. [Google Scholar]
- Li, X.; Liu, T.; Sun, W.; Liang, X.; Wei, Y.; Hao, C.; Li, S.; Li, G. Influence and mechanism analysis of acid or alkali damp environment on insulation performance of distribution cable accessories. Eng. Fail. Anal. 2023, 152, 107469. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, R.; Wu, X.; Xu, C.; Suo, C. Study on the Application of Modified Sn-Based Solder in Cable Intermediate Joints. Materials 2022, 15, 8385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Zhang, X.; Liu, R.; Wang, M.; Gao, S. Influence of Thermal Aging Time on Electrical Tree Initiation of Silicone Rubber. High Volt. Eng. 2014, 40, 979–986. [Google Scholar]
- Lv, H.; Ma, G.W.; Yang, X.; Zeng, Y.; Yu, X.; Jin, H. Influence of Thermal Aging on Dielectric Properties of Insulating Materials in 220 kV Silicone Rubber Cable Joint. Insul. Mater. 2019, 52, 47–51. [Google Scholar]
- Chen, Q.; Shang, N.; Wei, X. Influence of Thermal Oxygen Aging on Dielectric and Mechanical Properties of Liquid Silicone Rubber. Electr. Mach. Control. 2020, 24, 141–148. [Google Scholar]
- Kashi, S.; Varley, R.; De Souza, M.; Al-Assafi, S.; Di Pietro, A.; de Lavigne, C.; Fox, B. Mechanical, thermal, and morphological behavior of silicone rubber during accelerated aging. Polym.-Plast. Technol. Eng. 2018, 57, 1687–1696. [Google Scholar] [CrossRef]
- Jiang, Q.; Ouyang, X.; Zhou, Q.; Li, Y.; Luo, Z.H.; Chen, M.H. Analysis of Joint Deterioration and Interaction Mechanism of Multiple Dendritic Defects in XLPE Insulated Cables. Proc. CSEE 2021, 41, 6806–6815. [Google Scholar]
- Hu, H.; Jia, Z.; Wang, X. Aging Mechanism of Silicone Rubber Under Thermal–Tensile Coupling Effect. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 185–192. [Google Scholar] [CrossRef]
- Ito, S.; Hirai, N.; Ohki, Y. Changes in mechanical and dielectric properties of silicone rubber induced by severe aging. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 722–730. [Google Scholar] [CrossRef]
- Kai, Z.; Shiyu, L.; You, Y.; Lin, S.; Yun, H. Analysis of Aging Characteristics of Medium Voltage XLPE and EPR Retired Cables. Trans. China Electrotech. Soc. 2020, 35, 5197–5206. [Google Scholar]
- Chen, J.; Wu, S.; Hu, L.; Ren, C.; Shao, T. Analysis of Insulation State and Physicochemical Property of Retired High-Voltage Cable Accessories. Trans. China Electrotech. Soc. 2021, 36, 2650–2658. [Google Scholar]
- Shao, G.; Qin, F.; Zhao, J.; Li, Z.; Zhen, Z.; Li, X. Study on electrical characteristics of Silicone rubber insulation for cable joints. Insul. Mater. 2020, 53, 38–43. [Google Scholar]
- Jing, W.; Xie, K.; Li, H.; Chen, J.; Zhang, C.; Wu, S.; Ren, C.; Shao, T. Research on Aging Characteristics of Silicone Rubber Insulated High Voltage Cable Accessories. High Volt. Appar. 2023, 59, 201–210, 223. [Google Scholar]
- Wang, H.; Sun, M.; Zhao, K.; Wang, X.; Xu, Q.; Wang, W.; Li, C. High-Voltage FDS of Thermally Aged XLPE Cable and Its Correlation with Physicochemical Properties. Polymers 2022, 14, 3519. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhang, T.; Ma, M.; Gong, W.; Wang, W.; Li, Q. Research on Mechanical, Physicochemical and Electrical Properties of XLPE-Insulated Cables under Electrical-Thermal Aging. J. Nanomater. 2020, 2020, 3968737. [Google Scholar] [CrossRef]
- HG/T 2645-2011; Technical Specifications for Rubber Dies. Chemical Industry Press: Beijing, China, 2011.
- GB/T 3512-2001; Rubber, Vulcanized or Thermoplastic—Accelerated Aging and Heat Resistance Tests—Air-Oven Method. Standards Press of China: Beijing, China, 2001.
- GB/T 528-2009; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. Standards Press of China: Beijing, China, 2009.
- Xia, R.; Ouyang, B.; Wang, Y.; Yuan, J.; Huang, K.; Fang, C.; Wang, Y. Effect of Temperature and Thermal Ageing of Cable Silicone Rubber Accessories on Interface Pressure of Cable Joints. Appl. Sci. 2023, 13, 10406. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, G.; Geng, H.; Suo, S.; Zhang, J. Finite element analysis of silicone rubber based on Yeoh constitutive model and Ogden constitutive model. IOP Conf. Ser. Earth Environ. Sci. 2021, 714, 032078. [Google Scholar] [CrossRef]
- Li, Y.; Yu, D.; Cui, J.; Sun, T.; Qiu, W. Effect of Different Ageing Treatments on Breakdown Characteristics of XLPE/SIR Interface After Coated with Silicone Grease. Insul. Mater. 2020, 53, 41–49. [Google Scholar]
- Wang, J.; Zhang, F.; Cheng, Z.; Wang, J.; Zhou, X.; Qi, P.; Wei, Y.; Li, G. Effect and Mechanism of Salt Spray on Electrical Insulation Properties of Silicone Rubber for Cable Accessories. Electr. Power 2023, 56, 82–89, 100. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).