Abstract
The subject of the article was the chemical analysis of gasoline and exhaust gas samples taken from an urban two-wheeled vehicle. The main aim of the work was to identify chemical compounds emitted by a group of urban two-wheeled vehicles depending on the engine’s operating parameters. First, engine operating parameters and driving parameters of three urban two-wheeled vehicles were measured in real operating conditions. Based on the averaged results, engine operating points were determined for exhaust gas samples that were collected into Tedlar bags. The exhaust gas composition of individual chemical substances obtained in the chromatographic separation process were subjected to a detailed analysis relating the engine operating point with their emission rate, with each individual component being assessed in terms of its impact on human health. The obtained qualitative analysis results indicated the presence of alkenes, alkanes, aliphatic aldehydes, and aromatic and cyclic hydrocarbons (cycloalkanes) in the tested samples. The experiments provided a variety of conclusions relating to the operating parameters of a two-wheeler engine. Qualitative assessment of exhaust samples showed that a two-wheeled vehicle was characterized by the most varying composition of BTX aromatic hydrocarbons derivatives, which are particularly dangerous to human health and life. Therefore, the authors suggest that in the future, approval procedures regarding toxic emissions should be extended to include chromatographic tests. The presented results are an extension of previous studies on toxic emissions from urban two-wheeled vehicles in real operating conditions that were published in other journals.
1. Introduction
Combustion engines emit complex mixtures of volatile organic compounds (VOCs) and polycyclic aromatic hydrocarbons (PAHs). The European Commission Directive [] states that a volatile organic compound is any organic compound with an initial boiling point less than or equal to 250 °C measured at a standard pressure of 1010.3 kPa. The most numerous groups of VOCs include aliphatic hydrocarbons, cyclic hydrocarbons, aromatic hydrocarbons, halogenated hydrocarbons, nitro hydrocarbons, alcohols, and phenols. It is estimated that VOCs constitute 60% of all substances polluting the atmosphere, while as many as 73% of them are on the list of carcinogenic compounds. They can therefore cause a number of short- and long-term effects, ranging from the irritation of the mucous membranes of the nose, eyes, and throat, headache, nausea, and dizziness to the development of cancer and damage to the nervous system. Emissions of these compounds in the range of 200–300 µg/m3 are considered potentially harmful, while the range of 300–500 µg/m3 is deemed harmful to human health. According to reports [,] by the National (Poland) Center for Emission Balancing and Management, the main anthropogenic source of VOCs with the second largest share (after the use of solvents and other products of this type) includes the transport sector. Polycyclic aromatic hydrocarbons, on the other hand, are compounds consisting of two or more aromatic rings. They are characterized by various structural forms with different relative positions of benzene rings in the molecule. These compounds have strong genotoxic, mutagenic, and carcinogenic properties [] and are always present in a mixture, never individually. It is estimated that in India, 37% of CO (carbon oxide) emissions and 17.5% of volatile organic compound (VOC) emissions from transport are generated by two-wheelers. The cited data demonstrate the scale of the problem of emissions from two-wheeled vehicles, although the scale varies depending on the location.
Currently, the interdisciplinarity of scientific research is important, which enables a multi-aspect approach to a selected issue. Therefore, the identification of toxic compounds from motor vehicles in real operating conditions, in addition to tests using PEMS (Portable Emission Measurement System) equipment, can be additionally undertaken with a qualitative chemical analysis []. Due to the fact that some semi-volatile organic compounds emitted from motor vehicles (i.e., PAHs—polycyclic aromatic hydrocarbons) play a significant role in the formation of photochemical smog and secondary organic aerosol, there is a need to understand the sources and fate of these compounds in the atmosphere. Analyses of this type require researchers to optimize sample collection and determine an appropriate analytical method. A literature review showed that scientists use a number of available methods of collecting research material and chromatographic methods, i.e., GC–MS [,,,,,,,,], LC–MS [], TD–GC–MS [] with sampling into sorbent tubes [], feeding whole air or raw exhaust gases into Tedlar bags [,,,,,,,], microextraction into the solid phase [], or the flame ionization method [,,]. In the work of other research centers [,], volatile organic compounds were collected from two-wheeled vehicles into a set of Tedlar bags. Taking into account the current state of knowledge presented in the following chapters, theoretical and laboratory work should be developed in the field to generate a more detailed assessment of exhaust emissions. Therefore, the article presents a test procedure for a two-wheeled vehicle, based on GC–MS analysis, in traffic conditions representative of the tested vehicle. The methodology used, including the selection of specific operating conditions, was a novelty in the field of this type of work.
2. Materials and Methods
2.1. Collection and Preparation of Measurement Samples
Research carried out in real operating conditions of three urban two-wheeled vehicles using equipment from the PEMS group made it possible to determine the most frequently used (in terms of overall duration) engine operating points [,,,] (Figure 1).
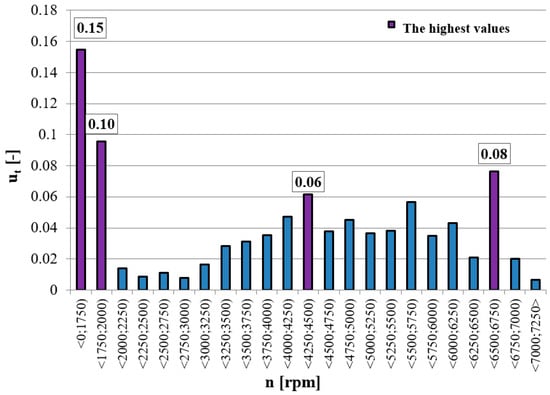
Figure 1.
Characteristics of the operating time density of three urban two-wheeled vehicles in real operating conditions.
Exhaust gas samples were collected at these operating points for further chromatographic analysis. Based on the characteristics of the averaged results for all test vehicles, it could be seen that the vehicles most often operated around idle speed. The share of operating time in this parameter range amounted to 15% on average. The next two engine operating points most frequently used by vehicles during research tests were the speed ranges of 4250–4500 rpm and 6500–6750 rpm. Vehicle engines worked at these points for an average of 6 and 8% of the entire test duration respectively.
Tests performed on a chassis dynamometer constitute simplified operating parameters of the facility in real operating conditions. Exhaust gas samples were collected at a constant vehicle speed—the area of variability in the object’s parameters (acceleration, deceleration, and change of the road slope angle) was not taken into account. During the study, the vehicle was simulated driving at a constant speed on flat terrain, taking into account the driver’s load and vehicle resistance. Based on current readings from the dynamometer and operational parameters from actual operation, it was assumed that the following loads occurred at the measurement points: idle speed—(load 0%); 4250–4500 rpm—(load 45–55%); 6500–6750 rpm—(load 85–95%).
Significant ranges obtained during vehicle tests in real operating conditions made it possible to determine the operating points of the test vehicle engine, which were recreated under laboratory conditions in order to collect a sample of exhaust gases. Samples of volatile organic compounds were taken from an urban two-wheeled vehicle belonging to the L3e category. The moped was equipped with a four-stroke engine with a displacement of 49 cm3 and a power of 2.5 kW. The vehicle was manufactured in 2013, so it complied with the Euro 3 standard [] (Table 1).

Table 1.
Technical data of the tested vehicle.
During the research process, samples of exhaust gases were collected into Tedlar testing bags. The moped tests were carried out on a chassis test bench designed for two-wheeled vehicles testing. The DYNOmite motorcycle test stand was manufactured by LAND&Sea (Concord, MA, USA). The device can record vehicle operating parameters (instantaneous power, torque, speed, acceleration) and the distance traveled. The technical data of the chassis dynamometer are presented in Table 2.

Table 2.
Technical specifications of the dynamometer test bench.
Samples of volatile organic compounds were collected into special sampling bags, two for each engine operating point, in order to average the results of subsequent chromatographic analysis. First, samples were taken from an urban two-wheeled vehicle while running at idle speed for different engine thermal states: cold (Sample 1) and hot (Sample 2). Another sample was obtained at a crankshaft speed of 4250 rpm–4500 rpm (Sample 3), and the last one was taken at an engine speed in the range of 6500 rpm–6750 rpm (Sample 4). The measurement station also included a probe and a pump (Figure 2).
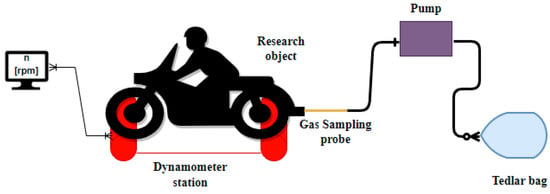
Figure 2.
Measurement station for collecting exhaust gas samples.
The next stage was the preparation of the collected gas samples for chemical analysis and recovery of research material, which involved collecting exhaust gases from test bags onto a sorbent in the form of Tenax. An aspirator was used for this purpose, and the exhaust gas flow was set at 20 mL/min. The aspirator technical data are provided (Table 3). Due to the fact that volatile organic compounds contained in exhaust gases are photosensitive, the samples were stored in brown bottles to minimize photolytic decomposition. The sorbent fill—Tenax—was therefore contained in a copper sorbent tube. Tenax porous polymers, based on 2,6 diphenyl-p-phenylene oxide, are widely used as an adsorbent in both air collection and gas release applications. Its unique structure provides alternative and desirable adsorption/desorption properties compared to other porous polymers []. Poly(2,6-diphenylphenylene oxide) is characterized by low leakage, low level of contamination, and high thermal stability up to 350 °C. Figure 3 shows a diagram of the exhaust gas collection process on the Tenax surface.

Table 3.
Aspirator technical data.
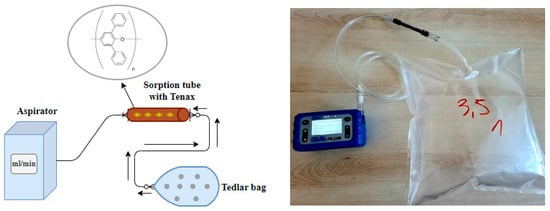
Figure 3.
Scheme of sample collection on the Tenax surface.
2.2. Measuring Equipment
The qualitative analysis of the components isolated using a capillary column with a diameter of 0.25 mm was carried out and identified by gas chromatography (GC—gas chromatography) with thermal desorption (TD—thermal desorption) and mass spectrometry (MS—mass spectrometry) from Perkin Elmer (Kraków, Poland). The TD–GC–MS technique makes it possible to determine the composition of mixtures containing up to several hundred chemical compounds. Due to its universality, it is widely used, among others, in agriculture and pharmaceuticals. The measurement process using the TD–GC–MS technique is shown step by step in Figure 4.
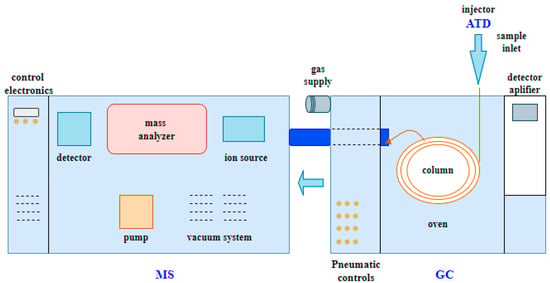
Figure 4.
Schematic of the ATD–GC–MS measurement technique.
The Clarus 690 gas chromatograph (Perkin Elmer, Kraków, Poland) used in this research allows for a relatively quick and accurate analysis of complex substances in the form of gases or vapors. The method of chromatographic analysis of a multi-component gas involves separating the mixture into individual chemical components by migrating them between the mobile phase and the stationary phase in the separation column and then measuring the content of each of these components in the carrier gas at the column outlet. The separation process in the chromatographic system was divided into two phases—the mobile phase (carrier gas) and the stationary phase (column packed with a liquid coating or appropriate material). The components were separated in a thermal process based on each component’s differences in affinity for either phase. The gas chromatography technique identifies individual components based on the location of chromatographic peaks corresponding to specific substances and by determining their retention times. The device used had a heating rate of 160 °C/min, while cooling from 450 °C to 50 °C took less than 2 min. The temperature range of the chromatograph oven was 0–450 °C. All time and temperature functions were controlled by a microprocessor.
The final qualitative analysis of the exhaust gas samples was performed by unambiguous identification of individual components using mass spectra of the analyzed sample. For this purpose, a Clarus SQ8 mass spectrometer (Perkin Elmer, Kraków, Poland) was used as a detector. The main advantage of this detector is that it allows the identification of compounds based on mass-to-charge ratios (m/z) and the relative amounts of molecular and fragment ions resulting from electronic ionization. Therefore, the identification of a compound can be concluded by analyzing the MS spectrum and comparing it with a commercial MS library. The device uses advanced technologies, thanks to which it is characterized by high sensitivity and stability both in the case of analyses requiring only identification, as well as in quantitative assessment. The speed of data collection enables the registration of a large number of spectra in the mass range of 1–1200 amu, which allowed for unambiguous identification and determination of the content of components based on very narrow chromatographic peaks. Samples were dosed using a TurboMatrix 350 ATD thermal desorber (Perkin Elmer, Kraków, Poland) with automatic pneumatics and a feeder for 50 sorption tubes (Figure 5). In the method used, the high frequency of measurements was extremely important.

Figure 5.
View of the research equipment.
2.3. Conditions of Chromatographic Analysis
Qualitative analysis of samples taken from an urban two-wheeled vehicle was performed at a gas flow of 30 mL/min and a dispenser temperature of 270 °C. The experiment time for each sample was set at 34 min, and the temperature program of the oven and column was defined as follows:
- initial temperature: 50 °C, maintained for 2 min;
- temperature level 1: 7 °C/min to reach a temperature of 160 °C;
- temperature level 2: 10 °C/min to reach a temperature of 280 °C, maintained for 4.29 min (Figure 6).
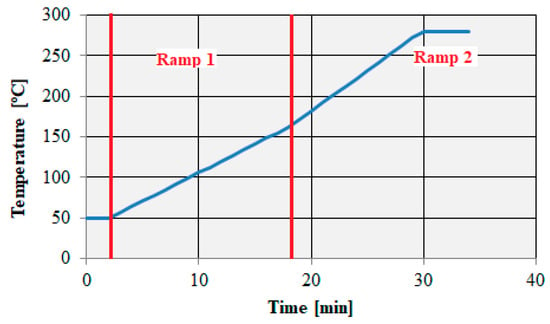 Figure 6. Temperature program of the oven and column.
Figure 6. Temperature program of the oven and column.
3. Chromatographic Analysis of the Chemical Composition of Exhaust Gas Samples
The use of the gas chromatography–thermal desorption–mass spectrometry method provides valuable information, primarily about volatile substances produced at particular operating points of a gasoline-powered engine. The identification of exhaust compounds (qualitative analysis) was carried out by locating chromatographic peaks of individual substances and determining their retention time (Rt). A chromatogram was obtained for each analyzed sample (Figure 7). Additional confirmation was provided by the analysis of mass spectra (MS), which were compared with the commercial library of NIST (National Institute of Standards and Technology). This enabled the final identification of compounds based on mass-to-charge ratios (m/z) and the relative amounts of molecular and fragment ions resulting from electronic ionization. Mass spectrometry is a method that allows the determination of the molecular weight of a tested compound and the mass of fragments resulting from its decay during ionization in a mass spectrometer. Thus, mass spectra were compiled for the detected compounds with approximate retention times of Rt = 1.66 and 1.67.
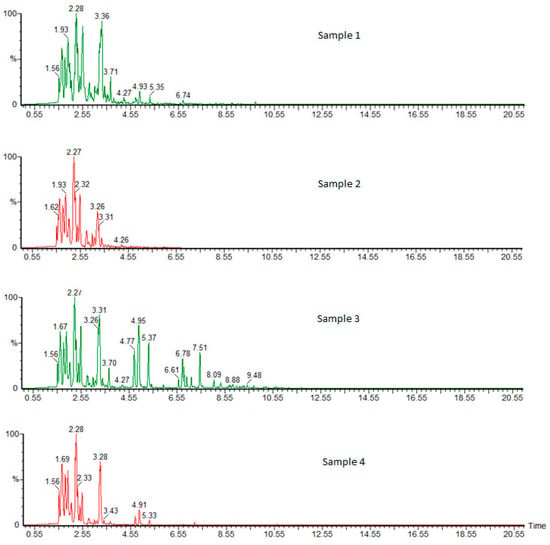
Figure 7.
Chromatograms of exhaust gas samples from an urban two-wheeled vehicle at various operating points of the combustion engine.
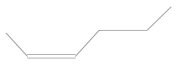
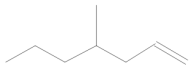
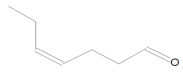
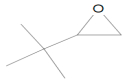
In the mass spectrum of 3,3-dimethyl-1,2-epoxybutane, the most visible molecular ion had a mass-to-charge ratio m/z = 83, while for 4-methyl-1-heptene, the ion was measured to have a ratio m/z = 42 (Figure 8a,b). The relative intensity was calculated in relation to the ion with the highest measured intensity, which was taken as the main ion and assigned an intensity of 100%. The intensities of the remaining peaks were then presented as percentages relative to the main peak.
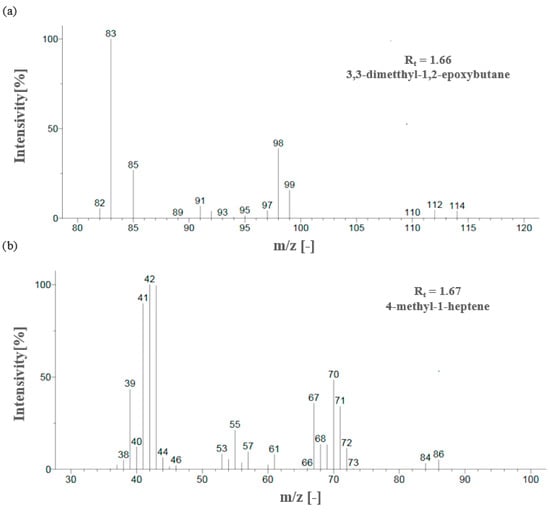
Figure 8.
Mass spectra of eluted substances for retention times (a) 1.66 min (b) 1.67 min.
Table 4 shows the chemicals identified for all samples that were detected using the measurement method described in Section 2. The tabular list of chemical components contained in the gas samples obtained from an urban two-wheeled vehicle was additionally supplemented with a literature review. Studies and scientific articles that discussed each specific substance were analyzed. Their presence was recorded in chemical tests of samples of pollutants from motor vehicles, which were described in the cited works.

Table 4.
Results of chromatographic analysis of exhaust gas samples.
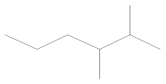
The obtained results indicated the presence of volatile organic compounds in the tested samples, including many aromatic and cyclic hydrocarbons (cycloalkanes). Qualitative analysis did not reveal significant differences in the four samples obtained (Table 5). Gasoline is a fuel that does not occur in the natural environment but is produced in primary or destructive refining processes, which means that it consists of up to several hundred substances with various physical and chemical properties. However, the main components of gasoline include aliphatic hydrocarbons with carbon atoms ranging from 5 to 12 and trace amounts of unsaturated and aromatic hydrocarbons. Therefore, alkenes and alkanes were observed in all samples, regardless of the engine operating point, as they are the main components of petroleum gases, i.e., butene, octane, heptane, hexene, and 3,3-dimethyl-1,2-epoxybutane, formed as a result of the oxidation of 4-heptanal and 2,3-dimethylhexane. These are primarily branched alkanes with long carbon chains and retention times of 1.66–1.95.

Table 5.
Results of chromatographic analysis depending on the operating point of the engine of an urban two-wheeler.
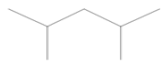
Gasoline components that were a product of crude oil distillation—4-methyl-1-heptene, 2,4-dimethylpentane, and 1,4-epoxycyclohexane—were also found in each of the analyzed samples. Moreover, toxicologically important compounds in the composition of gasoline were noted, defined as aromatic hydrocarbons from the BTX group (benzene, toluene, and xylenes),their derivatives (cumene, phenylethane, and dimethylbenzene), and benzoic acid, resulting from the oxidation of toluene.
The presence of these components in the exhaust gases may indicate incomplete combustion and complex reactions in the engine combustion chamber. The qualitative assessment of exhaust gas samples showed that the two-wheeled vehicle was characterized by greater qualitative diversity depending on the engine operating point in the case of aromatic hydrocarbon derivatives of the BTX group.
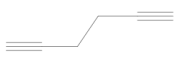
Ethylbenzene (phenylethane) with a retention time of 4.77 was not recorded in sample no. 4 (crankshaft rotational speed in the range of 6500–6750 rpm). The high temperature reached at this operating point resulted in a complete combustion of this compound. Increasing the speed and, consequently, a more effective combustion process also resulted in the lack of the presence of cumene (belonging to the group of aromatic hydrocarbons) in the last two analyzed samples. A more efficient combustion process resulted in the oxidation of aromatic hydrocarbons or their complete combustion, thus removing them from the emissions. The compound 1,5-Heptadien-3-in also appeared only in sample no. 1. Too low a temperature in the combustion chamber and cold walls and piston head were the causes of the formation of this compound. This is referred to as the wall effect—where the flame is dimmed near the cold walls of the combustion chamber.
In order to confirm the validity of the above considerations regarding the chemical composition of a gaseous sample from an urban two-wheeled vehicle, a gasoline sample was also analyzed to determine its detailed composition. The gas chromatography–mass spectrometry method was also used in this case. The technique utilizes three primary components: the separation power of high-resolution capillary gas chromatography, a mass spectrometer with controlled ion source and ion fragmentation coefficients, and unique data processing and reporting software. The method covers the range of hydrocarbons from C4 to C12 in gasoline.
There are a number of items in the literature regarding the gas chromatographic analysis of gasoline and related petroleum products. Retention index databases and computer software for detailed gasoline analysis are available from references [,,,,,,]. Gas chromatographic classification of liquid petroleum products is a well-established technique. The primary parameters used to distinguish different classes of products include boiling point range, aliphatic and aromatic hydrocarbon content, and the relative concentration of major and minor components.
The analysis showed that the composition of gasoline is mainly light aliphatic hydrocarbons (butane, pentane, methylpentane, hexane, methylhexane, and heptane) and aromatic compounds (toluene, xylenes, and trimethylbenzenes), with smaller amounts of olefinic hydrocarbons (pentanes, hexenes, heptenes, octenes, and nonenes; Table 6). It is worth noting that conventional combustion engine solutions use gasoline and diesel oil, which are derivatives of crude oil. The approximate elemental structure of an average crude oil sample is assumed to be 84% carbon, 14% hydrogen, 1–3% sulfur, and less than 1% nitrogen, oxygen atoms, metals, and salts. There is a wide range of hydrocarbon compounds in crude oil consisting of alkanes, alkenes, naphthenes, and aromatics. These are very small molecular structures, such as propane (C3H8) and butane (C4H10), but can also consist of mixtures of different structures with very large molecules, such as heavy oils and asphalt. Therefore, crude oil must be distilled to be used for automotive applications.

Table 6.
Results of chromatographic analysis of a liquid gasoline sample.
As a result of the thermal distillation of crude oil, petroleum products are obtained, such as petroleum gases, aviation fuel, kerosene, gasoline, diesel oil, heavy fuels, machine oils, and asphalt. Generally, crude oil distillation produces on average 30% gasoline, 20–40% diesel oil, 20% heavy fuel oil, and heavy oils from 10 to 20% []. The lack of derivatives of many compounds in the tested samples from the urban two-wheeler was due to the combustion reaction taking place in the engine and the detection limit of the concentration of some emitted substances. Additionally, the analysis time, which differed between the analysis of a liquid substance (gasoline) and a gaseous substance, could also have played a significant role.
Of the identified volatile toxic compounds found in a sample from an urban two-wheeler, almost half were aromatic compounds that are hazardous to health, including benzene, toluene, xylenes, and ethylbenzene. Moreover, most of them take part in the process of creating photochemical smog. VOCs can cause serious health effects such as drowsiness, headaches and dizziness, irritation of mucous membranes, and permanent damage to the liver or nervous system. Some of them are characterized by highly toxic, carcinogenic, neurotoxic, or mutagenic properties. They enter the body through the skin, respiratory, and digestive systems. Benzene and its derivatives are considered to be the most toxic compounds detected in exhaust gas samples from urban two-wheeled vehicles. Benzene is a simple aromatic hydrocarbon that causes both acute and chronic poisoning. It usually occurs through the inhalation of vapors through the respiratory system, but absorption through the skin and food intake are also possible. It may also lead to genetic defects [].
Toluene, although much less toxic than benzene, is considered a compound harmful to the respiratory, circulatory, reproductive, nervous, and immune systems, as well as the kidneys and the liver. In the case of toluene, the nervous system is particularly vulnerable, as toluene has a high affinity for tissues rich in lipids, in which it is soluble. Xylenes are compounds from the BTX group, which, due to the lowest volatility and higher boiling point, are considered safer than benzene and toluene. However, its unpleasant-smelling vapors are extremely easily absorbed by the respiratory tract, mucous membranes, and skin. Table 7 presents the types of hazards and the chemical compounds assigned to them that were recorded in exhaust gas samples from two-wheeled vehicles [].

Table 7.
Types of threats to compounds detected in exhaust gases [,].
Most of the substances detected in the tested exhaust gases, depending on the method of exposure, may cause more or less serious health effects. For example, contact with liquid heptane causes redness, rash, and dry skin and is irritating to the eyes, but short-term exposure to heptane vapors may cause dizziness, headache, vomiting, a feeling of intoxication, problems with motor coordination, and loss of consciousness. Moreover, after entering water, heptane settles on solids in the water, including animals. For this reason, it is defined as a compound that is highly toxic to aquatic organisms and causes negative and long-lasting effects in the aquatic environment. Hexene, heptanal, 1,5-Hexadiene, and octane found in exhaust gases also have similar properties. The main way octane enters organisms is through the inhalation of its vapors []. This aliphatic hydrocarbon mainly affects the central nervous system; however, in case of aspiration, there are also symptoms related to the respiratory system, i.e., cough, irritation of the upper respiratory tract, and in extreme cases it may lead to hemorrhagic pneumonia and pleurisy [,].
4. Conclusions
- The results of the qualitative analysis of chemical components indicate the presence of alkenes, alkanes, aliphatic aldehydes, aromatic and cyclic hydrocarbons (cycloalkanes) in the tested samples. Most of them have a negative impact on the environment as well as on human health. However, it is not known whether the samples contained significant or trace amounts. Therefore, further research is necessary, i.e., quantitative analysis of exhaust gases from the discussed group of vehicles, taking into account the specificity of the operation of urban two-wheeled vehicles. The literature review showed a lack of this type of research for vehicles in this category.
- The qualitative assessment of exhaust gas samples showed that the two-wheeled vehicle had a greater qualitative diversity depending on the engine operating point (crankshaft rotation speed) in the case of derivatives of aromatic hydrocarbons from the BTX group, which are particularly important in terms of toxicology.
- The presented research and the obtained results showed that there is a need to extend the approval tests of two-wheeled vehicles to include chromatographic analysis. The authors suggest collecting exhaust gas samples while the engine is idling in cold and hot thermal states for further chromatographic analysis.
- The need to perform chromatographic analyzes in this type of approval process would force manufacturers of two-wheeled vehicles to use design solutions aimed at meeting specific permissible values of chemical compounds. As a consequence, the air quality in many crowded urban centers would improve.
- Due to the quality of their results, chromatographic tests should be used in other scientific works. This may be particularly useful in assessing the use of solutions aimed at reducing emissions, e.g., through the use of fuel mixtures or dual-fuel systems [,,].
Author Contributions
Conceptualization, N.S.; methodology, N.S. and B.K.; formal analysis, N.S., Ł.R. and B.K.; investigation, N.S. and B.K.; data curation, N.S. and B.K.; writing—original draft preparation, N.S.; writing—review and editing, N.S., Ł.R. and B.K.; supervision, Ł.R. and B.K.; funding acquisition, Ł.R. All authors have read and agreed to the published version of the manuscript.
Funding
The study presented in this article was performed within the statutory research (Contract No. 0415/SBAD/0342).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Union. Directive 2004/42/CE of the European Parliament and of the Council of 21 April 2004 on the Limitation of Emissions of Volatile Organic Compounds Due to the Use of Organic Solvents in Certain Paints and Varnishes and Vehicle Refinishing Products and Amending Directive 1999/13/EC 2004; European Union: Brussels, Belgium, 2004. [Google Scholar]
- Bebkiewicz, K.; Boryń, E.; Chłopek, Z.; Chojnacka, K.; Doberska, A.; Kanafa, M.; Kargulewicz, I.; Olecka, A.; Rutkowski, J.; Skośkiewicz, J.; et al. Krajowy Bilans Emisji SO2, NOx, CO, NH3, NMLZO, Pyłów, Metali Ciężkich i TZO Za Lata 1990–2020. 2022. Available online: https://www.kobize.pl/uploads/materialy/materialy_do_pobrania/krajowa_inwentaryzacja_emisji/Bilans_emisji_za_2020.pdf (accessed on 30 November 2023).
- Materiał Dotyczący Regulacji Oraz Wymagań w Zakresie Bilansowania Emisji Niemetanowych Lotnych Związków Organicznych (NMLZO) 2015. Available online: https://krajowabaza.kobize.pl/docs/bilansowanie_LZO_aktualizacja-final-15-12-2015.pdf (accessed on 4 December 2023).
- Manahan, S.; Środowiska, T. Aspekty Chemiczne i Biochemiczne; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006. [Google Scholar]
- Kęska, A. Metoda Oceny Toksyczności Spalin Silnikowych w Aspekcie Analizy Rozwoju Standardów Emisyjnych. Rap. Wydziału Mech. Politech. Wrocławskiej 2020, 26, 155. [Google Scholar]
- Di Francesco, F.; Loccioni, C.; Fioravanti, M.; Russo, A.; Pioggia, G.; Ferro, M.; Roehrer, I.; Tabucchi, S.; Onor, M. Implementation of Fowler’s Method for End-Tidal Air Sampling. J. Breath Res. 2008, 2, 037009. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Reducing Volatile Organic Compound Emissions from Diesel Engines Using Canola Oil Biodiesel Fuel and Blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Hu, N.; Tan, J.; Wang, X.; Zhang, X.; Yu, P. Volatile Organic Compound Emissions from an Engine Fueled with an Ethanol-Biodiesel-Diesel Blend. J. Energy Inst. 2017, 90, 101–109. [Google Scholar] [CrossRef]
- Jin, D.; Choi, K.; Myung, C.-L.; Lim, Y.; Lee, J.; Park, S. The Impact of Various Ethanol-Gasoline Blends on Particulates and Unregulated Gaseous Emissions Characteristics from a Spark Ignition Direct Injection (SIDI) Passenger Vehicle. Fuel 2017, 209, 702–712. [Google Scholar] [CrossRef]
- Salvo, P.; Ferrari, C.; Persia, R.; Ghimenti, S.; Lomonaco, T.; Bellagambi, F.; Di Francesco, F. A Dual Mode Breath Sampler for the Collection of the End-Tidal and Dead Space Fractions. Med. Eng. Phys. 2015, 37, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Volckens, J.; Olson, D.A.; Hays, M.D. Carbonaceous Species Emitted from Handheld Two-Stroke Engines. Atmos. Environ. 2008, 42, 1239–1248. [Google Scholar] [CrossRef]
- Wang, X.; Ge, Y.; Zhang, C.; Tan, J.; Hao, L.; Liu, J.; Gong, H. Effects of Engine Misfire on Regulated, Unregulated Emissions from a Methanol-Fueled Vehicle and Its Ozone Forming Potential. Appl. Energy 2016, 177, 187–195. [Google Scholar] [CrossRef]
- Xiao, Q.; Li, M.; Liu, H.; Fu, M.; Deng, F.; Lv, Z.; Man, H.; Jin, X.; Liu, S.; He, K. Characteristics of Marine Shipping Emissions at Berth: Profiles for Particulate Matter and Volatile Organic Compounds. Atmos. Chem. Phys. 2018, 18, 9527–9545. [Google Scholar] [CrossRef]
- Zhao, H.; Ge, Y.; Zhang, T.; Zhang, J.; Tan, J.; Zhang, H. Unregulated Emissions from Diesel Engine with Particulate Filter Using Fe-Based Fuel Borne Catalyst. J. Environ. Sci. 2014, 26, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Hata, H.; Okada, M.; Funakubo, C.; Hoshi, J. Tailpipe VOC Emissions from Late Model Gasoline Passenger Vehicles in the Japanese Market. Atmosphere 2019, 10, 621. [Google Scholar] [CrossRef]
- Chin, J.-Y.; Batterman, S.A.; Northrop, W.F.; Bohac, S.V.; Assanis, D.N. Gaseous and Particulate Emissions from Diesel Engines at Idle and under Load: Comparison of Biodiesel Blend and Ultralow Sulfur Diesel Fuels. Energy Fuels 2012, 26, 6737–6748. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.A.; Lopes, D.J.; Calvo, A.I.; Evtyugina, M.; Rocha, S.; Nunes, T. Emissions from Light-Duty Diesel and Gasoline in-Use Vehicles Measured on Chassis Dynamometer Test Cycles. Aerosol Air Qual. Res. 2015, 15, 99–116. [Google Scholar] [CrossRef]
- Gawron, B.; Górniak, A.; Białecki, T.; Janicka, A.; Włostowski, R.; Włóka, A.; Molska, J.; Zawiślak, M. Impact of a Synthetic Component on the Emission of Volatile Organic Compounds during the Combustion Process in a Miniature Turbine Engine. Energies 2021, 14, 8462. [Google Scholar] [CrossRef]
- Janicka, A.B.; Zawiślak, M.; Gawron, B.; Górniak, A.; Białecki, T. Emission of Volatile Organic Compounds during Combustion Process in a Miniature Turbojet Engine. Environ. Prot. Eng. 2018, 44, 57–67. [Google Scholar] [CrossRef]
- Kim, H.Y.; Choi, N.J. Study on Volatile Organic Compounds from Diesel Engine Fueled with Palm Oil Biodiesel Blends at Low Idle Speed. Appl. Sci. 2020, 10, 4969. [Google Scholar] [CrossRef]
- Nakashima, Y.; Kamei, N.; Kobayashi, S.; Kajii, Y. Total OH Reactivity and VOC Analyses for Gasoline Vehicular Exhaust with a Chassis Dynamometer. Atmos. Environ. 2010, 44, 468–475. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Huang, P.-H.; Chiang, H.-L. Characteristics of Volatile Organic Compounds from Motorcycle Exhaust Emission during Real-World Driving. Atmos. Environ. 2014, 99, 215–226. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Yao, Y.-C.; Huang, P.-H.; Chiang, H.-L. Criteria Pollutants and Volatile Organic Compounds Emitted from Motorcycle Exhaust under Various Regulation Phases. Aerosol Air Qual. Res. 2017, 17, 1214–1223. [Google Scholar] [CrossRef]
- Grote, C.; Pawliszyn, J. Solid-Phase Microextraction for the Analysis of Human Breath. Anal. Chem. 1997, 69, 587–596. [Google Scholar] [CrossRef]
- Costagliola, M.; De Simio, L.; Iannaccone, S.; Prati, M. Combustion Efficiency and Engine out Emissions of a SI Engine Fueled with Alcohol/Gasoline Blends. Appl. Energy 2013, 111, 1162–1171. [Google Scholar] [CrossRef]
- Costagliola, M.A.; Murena, F.; Prati, M.V. Exhaust Emissions of Volatile Organic Compounds of Powered Two-Wheelers: Effect of Cold Start and Vehicle Speed. Contribution to Greenhouse Effect and Tropospheric Ozone Formation. Sci. Total Environ. 2014, 468, 1043–1049. [Google Scholar]
- Mattrel, P.; Vasic, A.-M.; Gujer, E.; Haag, R.; Weilenmann, M. VOC Composition and Ozone-Forming Potential of the Exhaust Gas of in-Use Motorcycles. Int. J. Environ. Pollut. 2004, 22, 301–311. [Google Scholar] [CrossRef]
- Merkisz, J.; Lijewski, P.; Fuc, P.; Siedlecki, M.; Ziolkowski, A. Development of the Methodology of Exhaust Emissions Measurement under RDE (Real Driving Emissions) Conditions for Non-Road Mobile Machinery (NRMM) Vehicles; IOP Publishing: Bristol, UK, 2016; Volume 148, p. 012077. [Google Scholar]
- Bielaczyc, P.; Merkisz, J.; Pielecha, J. A Method of Reducing the Exhaust Emissions from DI Diesel Engines by the Introduction of a Fuel Cut off System during Cold Start; SAE: Warrendale, PA, USA, 2001. [Google Scholar]
- Rymaniak, Ł.; Merkisz, J.; Szymlet, N.; Kamińska, M.; Weymann, S. Use of Emission Indicators Related to CO2 Emissions in the Ecological Assessment of an Agricultural Tractor. Maint. Reliab. 2021, 23, 605–611. [Google Scholar] [CrossRef]
- Ziolkowski, A. Automotive Thermoelectric Generator Impact on the Efficiency of a Drive System with a Combustion Engine; EDP Sciences: Les Ulis, France, 2017; Volume 118. [Google Scholar]
- European Commission Regulation (EU) No 168/2013 of the European Parliament and of the Council of 16 December 2013 on supplementing and amending Regulation (EU) No 168/2013 of the European Parliament and of the Council as regards environmental performance and power unit performance requirements annex V. Off. J. Eur. Union 2014, 60, 52–128.
- Available online: https://www.sigmaaldrich.com (accessed on 19 December 2023).
- Dhital, N.B.; Yang, H.-H.; Wang, L.-C.; Hsu, Y.-T.; Zhang, H.-Y.; Young, L.-H.; Lu, J.-H. VOCs Emission Characteristics in Motorcycle Exhaust with Different Emission Control Devices. Atmos. Pollut. Res. 2019, 10, 1498–1506. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R. Measurement of Emissions from Air Pollution Sources. 5. C1−C32 Organic Compounds from Gasoline-Powered Motor Vehicles. Environ. Sci. Technol. 2002, 36, 1169–1180. [Google Scholar]
- Tsai, J.-H.; Chiang, H.-L.; Hsu, Y.-C.; Weng, H.-C.; Yang, C.-Y. The Speciation of Volatile Organic Compounds (VOCs) from Motorcycle Engine Exhaust at Different Driving Modes. Atmos. Environ. 2003, 37, 2485–2496. [Google Scholar] [CrossRef]
- He, Z.; Zhang, L.; Liu, G.; Qian, Y.; Lu, X. Evaluating the Effects of Olefin Components in Gasoline on GDI Engine Combustion and Emissions. Fuel 2021, 291, 120131. [Google Scholar] [CrossRef]
- Ho, K.; Lee, S.; Ho, W.K.; Blake, D.; Cheng, Y.; Li, Y.S.; Ho, S.S.H.; Fung, K.; Louie, P.; Park, D. Vehicular Emission of Volatile Organic Compounds (VOCs) from a Tunnel Study in Hong Kong. Atmos. Chem. Phys. 2009, 9, 7491–7504. [Google Scholar] [CrossRef]
- Lough, G.C.; Schauer, J.J.; Lonneman, W.A.; Allen, M.K. Summer and Winter Nonmethane Hydrocarbon Emissions from On-Road Motor Vehicles in the Midwestern United States. J. Air Waste Manag. Assoc. 2005, 55, 629–646. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Lan, C.-H.; Yang, C.-Y. Effects of Biodiesel Blend Fuel on Volatile Organic Compound (VOC) Emissions from Diesel Engine Exhaust. Biomass Bioenergy 2012, 36, 96–106. [Google Scholar] [CrossRef]
- Petrovic, V.S.; Janković, S.P.; Tomić, M.V.; Jovanović, Z.S.; Knežević, D.M. The Possibilities for Measurement and Characterization of Diesel Engine Fine Particles-A Review. Therm. Sci. 2011, 15, 915–938. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Xu, C.; Tu, X. Direct Conversion of Methanol to N-C4H10 and H2 in a Dielectric Barrier Discharge Reactor. Green Chem. 2016, 18, 5658–5666. [Google Scholar] [CrossRef]
- Awad, O.I.; Ali, O.M.; Hammid, A.T.; Mamat, R. Impact of Fusel Oil Moisture Reduction on the Fuel Properties and Combustion Characteristics of SI Engine Fueled with Gasoline-Fusel Oil Blends. Renew. Energy 2018, 123, 79–91. [Google Scholar] [CrossRef]
- Lai, C.H.; Wu, Y.-L.; Lin, C.H.; Yeh, S.H. Measurements of the Speciation of Ozone Precursor from Kaoping Petrochemical/Industrial Area in Taiwan. In Proceedings of the 103rd Air and Waste Management Association Annual Conference and Exhibition 2010, Calgary, AB, Canada, 22–25 June 2010; pp. 6290–6295. [Google Scholar]
- Stupp, D.; Gass, M.; Leiteritz, H.; Pijls, C.; Thornton, S.; Smith, J.; Dunk, M.; Grosjean, T.; Den Haan, K. Gasoline Ether Oxygenate Occurrence in Europe, and a Review of Their Fate and Transport Characteristics in the Environment. 2012. Available online: https://www.concawe.eu/wp-content/uploads/2017/01/report-no-4_12.pdf (accessed on 14 November 2023).
- Eluri, S.; Cappa, C.D.; Friedman, B.; Farmer, D.K.; Jathar, S.H. Modeling the Formation and Composition of Secondary Organic Aerosol from Diesel Exhaust Using Parameterized and Semi-Explicit Chemistry and Thermodynamic Models. Atmos. Chem. Phys. 2018, 18, 13813–13838. [Google Scholar] [CrossRef]
- Koul, M.; Shadangi, K.P.; Mohanty, K. Thermo-Chemical Conversion of Kusum Seed: A Possible Route to Produce Alternate Fuel and Chemicals. J. Anal. Appl. Pyrolysis 2014, 110, 291–296. [Google Scholar] [CrossRef]
- Cain, P. Comparison of Kerosenes Using Capillary Column Gas Liquid Chromatography. J. Forensic Sci. Soc. 1975, 15, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.; Boscher, Y.; Petroff, N.; Berthelin, M. Automatic Gas Chromatographic Determination of Gasoline Components: Application to Octane Number Determination. J. Chromatogr. A 1987, 395, 229–240. [Google Scholar] [CrossRef]
- Hayes, P.C., Jr.; Anderson, S.D. High Resolution Multidimensional Chromatographic Analysis of Hydrocarbon Distillate Fuels: Matrix Simplification Using off-Line Preparative HPLC/DCD with on-Column GC/MSD and GC/FID. J. Chromatogr. Sci. 1988, 26, 250–257. [Google Scholar] [CrossRef]
- Wiedemann, L.S.; d’Avila, L.A.; Azevedo, D.D.A. Brazilian Gasoline Quality: Study of Adulteration by Statistical Analysis and Gas Chromatography. J. Braz. Chem. Soc. 2005, 16, 139–146. [Google Scholar] [CrossRef]
- Miki, Y.; Sugimoto, Y. GC/FID and GCIMS Analysis on the Compositions of Coal-Liquid Naphtha and Petroleum Gasoline. Fuel Energy Abstr. 1998, 1, 13. [Google Scholar]
- Olson, K.L.; Sinkevitch, R.M.; Sloane, T.M. Speciation and Quantitation of Hydrocarbons in Gasoline Engine Exhaust. J. Chromatogr. Sci. 1992, 30, 500–508. [Google Scholar] [CrossRef]
- Toth, T. Identification of C2 & C4 Alkylated Benzenes in Flash Pyrolysates of Kerogens, Coals and Asphaltenes. J. Chromatogr. Sci. 1987, 279, 156–157. [Google Scholar]
- Mueller, C.J.; Cannella, W.J.; Kalghatgi, G.T. Fuels for Engines and the Impact of Fuel Composition on Engine Performance. Encycl. Automot. Eng. 2014, 1–27. [Google Scholar] [CrossRef]
- Powell, C.H.; Bingham, E.; Cohrssen, B. Patty’s Toxicology; John Wiley: Hoboken, NJ, USA, 2001; ISBN 1-59124-485-4. [Google Scholar]
- U.S. Coast Guard. Chemical Hazard Response Information System (CHRIS)-Hazardous Chemical Data; U.S. Coast Guard: Washington, DC, USA, 1999; p. 16465. [Google Scholar]
- Mutschler, E.; Malinowska, B.; Droździk, M.; Kocić, I.; Pawlak, D.; Geisslinger, G.; Kroemer, H.K.; Menzel, S.; Ruth, P.; Grotthus, B. Mutschler Pharmacology and Toxicology; MedPharm: Wrocław, Poland, 2016; ISBN 83-7846-037-1. [Google Scholar]
- Chen, Z.; Li, K.; Liu, J.; Wang, X.; Jiang, S.; Zhang, C. Optimal Design of Glucose Solution Emulsified Diesel and Its Effects on the Performance and Emissions of a Diesel Engine. Fuel 2015, 157, 9–15. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Wei, Z.; Wang, Y.; Deng, J. Effect of Components on the Emulsification Characteristic of Glucose Solution Emulsified Heavy Fuel Oil. Energy 2022, 244, 123147. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.; Chen, H.; Chen, Z.; Zhang, P.; Chen, Z. Diesel/Methanol Dual-Fuel Combustion: An Assessment of Soot Nanostructure and Oxidation Reactivity. Fuel Process. Technol. 2022, 237, 107464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).