Abstract
Microalgae and their bioproducts have diverse applications, including wastewater remediation, CO2 fixation, and the synthesis of nutraceuticals, pharmaceuticals, and biofuels. However, the production of these organisms heavily relies upon environmental conditions, which can significantly impact growth. Furthermore, microalgae cultivation itself can be a source of economic and environmental concerns. Thus, microalgae growth systems have become a critical consideration for both research and industry, to bolster microalgae cultivation and address its accompanying issues. Both open and closed systems, such as raceway ponds and photobioreactors, respectively, are commonly used during the growth process but have their own advantages and drawbacks. However, for microalgae growth, photobioreactors may address most concerns as the system’s design lowers the risk of contamination and provides the ability to control the delivery of desired growth factors. To determine the appropriate system for targeted microalgae cultivation, it is crucial to determine factors such as the scale of cultivation and growth and productivity targets. Additionally, efficient usage of these growth systems and carefully selected incubation factors can aid in addressing some of the economic and environmental issues associated with microalgae production. This review will summarize the current applications of bioreactors in both research and industrial capacities and summarize growth and incubation factors for microalgae.
1. Introduction
Microalgae are unicellular, photosynthetic organisms with a variety of potential applications. As they can be involved in the production of renewable energy sources and are efficient producers of compounds commonly utilized in a variety of fields, such as pharmaceuticals and nutraceuticals, as depicted in Figure 1 [1,2,3,4]. This productivity has made microalgae highly sought after in research and industrial capacities, including efforts to improve sustainability.
One such function of microalgae is environmental betterment. Although microalgae cultivation can propose some environmental concerns such as climate change, ecotoxicity, space requirements, and nutrient depletion, to curb these issues, several pathways have been explored [5]. One such pathway involves life cycle assessments of varying microalgae cultivation methods, such as mixotrophic cultivation and the introduction of a magnetic field, to determine the economic and environmental impacts of the process [5,6]. Thus, aid in identifying areas of improvement to offset the concerns related to traditional microalgae cultivation.
Microalgae can be utilized to remediate wastewater while recovering useful products such as pigments and biogas [7]. This methodology, which utilizes wastewater in place of a traditional growth medium for the recovery process, encourages a circular bioeconomy by taking urban and industrial food wastewater and cultivating microalgae with the ability to reclaim useful products [7]. Additionally, the usage of wastewater addresses some environmental concerns involved with the production of microalgae-based biofuel by decreasing the synthesis of undesirable byproducts [8]. This process also improves the quality of resulting biofuel, of which cannot be ascertained through productivity increases in microalgae cultivation alone [8]. Additionally, the usage of biochar in microalgae culture has been observed to be an effective combination for wastewater remediation through the removal of tetracycline [9]. Tetracycline is a popular antibiotic for human and animal treatment; however, the compound does not break down easily and has been located in the soil and both surface and ground water [10,11]. While in these environments, tetracycline causes toxicity in microbial communities and, if ingested by humans, can disrupt the gut’s microbial community [10,11]. Thus, pursuing its removal from the environment is highly preferable. With the addition of biochar and Chlorella protothecoides, tetracycline was completely removed within the culture, and biomass increased by 13.26% [9]. Furthermore, due to the organisms’ photosynthetic status, their consumption of carbon dioxide can be utilized for CO2 sequestering and simultaneous production of biomass [12], which can assist in decreasing the presence of CO2 in the atmosphere and alleviate the impact of greenhouse gas emissions. Currently, CO2 in the atmosphere is trending upward [13,14], wherein the current preliminary global monthly CO2 emissions, reported by the National Oceanic and Atmospheric Administration (NOAA), were 421.20 ppm in July 2024 [13]. These staggering statistics have emphasized the importance of addressing carbon dioxide emissions, which can be done through the cultivation of microalgae and the utilization of microalgae-based products produced in a sustainable manner.
Microalgae productivity encompasses the formation of lipids like polyunsaturated fatty acids (PUFAs) that can be used in the synthesis of biofuel or nutraceuticals [2,15]. For instance, Chlorella vulgaris can be the source of monounsaturated and saturated fatty acids that contribute to its potential in nutraceutical production [16]. Microalgae can also produce pigments like carotenoids, including compounds such as β-carotene, zeaxanthin, and astaxanthin, with high antioxidant capabilities [17,18,19]. As such, microalgae-based nutraceuticals have been synthesized to make use of microalgae extract’s healthy properties. For instance, compounds extracted from C. vulgaris exhibit a variety of health benefits, namely anti-obesity activity [2]. Furthermore, these bioproducts can be included in animal feed to improve the health of livestock. For instance, it is possible to enhance the growth of domesticated chicken flocks under heat stress through the introduction of antioxidant-rich microalgae in their diet [20]. The cultivation of these photosynthetic organisms, thus, becomes a point of focus in obtaining these desirable microalgae bioproducts.
There are a variety of factors that impact microalgae growth and, occasionally, productivity. These include conditions such as light availability, temperature, pH, the rates of CO2 in airflow, nitrogen supply, and carbon source [21,22,23,24]. Under a 12 h dark and 12 h light period, the biomass and growth rate of Nannochloropsis salina is less compared to cultures grown under constant light [21]. However, under the former conditions, it was noted that cells were more efficient during the 12 h light period [21]. When grown under constant light, a larger productivity rate was also observed in Nannochloropsis QU130, a strain that has adapted to the harsh climate of the Qatar desert, compared to cultures grown under light cycles [23]. Additionally, in the same strain, fluctuating temperatures increased cell size compared to constant temperatures [23], wherein observing cells cultivated under varied temperatures and continuous light resulted in the highest productivity [23].
pH is also a consideration when cultivating microalgae. As more CO2 is taken up by an increasing microalgae population, pH fluctuations occur, thus rendering it necessary to balance pH to keep the microalgae within a tolerable range [21]. The preferred pH for growth can vary depending on strain [25,26]. However, it has been observed that lipid productivity and accumulation are not significantly influenced by unideal pH environments in different microalgae strains [25,26].
In terms of airflow rates, 1 L/h of 5% CO2 supplemented air facilitated faster growth for N. salina, as cultures reached a plateau or the stationary phase at about 10 days [21]. Whereas, at the lower rate of 0.25 L/h, the stationary phase occurred at approximately 17 days [21]. By increasing both the nitrogen and CO2 sources, an approximately four-fold increase in biomass was observed [21]. Furthermore, in nitrogen-deficient medium, 63% of N. salina dry weight was comprised of lipids [21]. Beyond air supply and medium type, the carbon source provided for microalgae cultures can also impact growth rates.
Some microalgae are mixotrophic and thus can utilize inorganic and organic forms of carbon and light for growth. An example is C. vulgaris [22]. In comparison to photoautotrophic growth, mixotrophy can increase productivity, which is dependent on microalgae species and other growth factors [22,27]. To elucidate the impact of mixotrophy in microalgae, a mathematical model was designed to observe the photoautotrophic, heterotrophic, and mixotrophic growth of C. vulgaris [22]. This revealed the organism prefers photoautotrophic methods in a mixotrophic environment [22]. Therefore, to cater to specific growth and productivity rates, these growth factors must be adjusted accordingly.
Productivity can also be altered by culturing microalgae under stress conditions or extreme growth conditions. For instance, adjusting factors, like temperature, can increase the production of PUFAs [28], wherein Nannochloropsis oculata and Isochrysis galbana were identified as microalgae species with high eicosapentaenoic acid (EPA) productivity at 20 °C and docosahexaenoic acid (DHA) productivity at 14 °C, respectively [28].
Light intensity can also be adjusted to increase growth rates. For instance, high light (360 µmol photons/(m2s)) increased growth approximately three-fold compared to low light conditions (6 µmol photons/(m2s)) for Nannochloropsis gaditana [29]. A similar result was observed in other microalgae species, where increases in light intensity invoked an approximate three-fold increase in fatty acid content in both Desmodesmus sp. and Scenedesmus obliquus after 15 days of cultivation [30]. Furthermore, under higher light intensity conditions, or 300 µE/m2s, the percentage of lipids within their biomass increased compared to the lower light intensity environment of 50 µE/m2s [30].
Nutritional deficiencies and unideal salt concentrations are also stress factors related to growth in microalgae [31], wherein under salinity stress, three strains of freshwater microalgae, Ankistrodesmus braunii, Ankistrodesmus falcatus, and Scenedesmus incrassatulus, have heightened lipid content present in their dry biomass [31]. With the addition of varying temperatures and nitrogen supply, the accumulation of lipids increased in Xanthonema hormidioides [32], where with a nitrogen concentration of 3 mM and a temperature of 25 °C, lipid content within microalgal dry weight increased to its highest at 57.49% dry weight [32]. However, achieving stress conditions is not a requirement to ensure high biomass and productivity.
Microalgae growth can also be assisted by providing optimal growth conditions, which can result in an increase in growth and biomass productivity. This was described by Josephine et al. for C. vulgaris, which revealed that specific temperature, pH, salinity, and light provide the ideal conditions for microalgae growth [33]. Thus, to provide optimal growth conditions, cultivation systems or devices were introduced to provide these growth factors for both research and industrial purposes alike.
The most common microalgae cultivation systems can be divided into two categories: open systems, such as ponds, and closed systems, like bioreactors [34,35]. Open systems are generally susceptible to contamination, whether it is bacterial or cross-contamination [36]. There are systems that prevent the latter but still detect concentrations of the former, such as the twin-layer solid-state photobioreactor [36]. Photobioreactors (PBRs) are an example of a closed cultivation system with an additional light module and thus tend to be preferable for microalgae cultivation. They do not require as much space as open raceway ponds and have a low possibility of contamination [34]. Whereas, for an open system, like a raceway pond, space requirements and the possibility of contamination are higher [34]. However, it is important to note that, generally, other factors, such as maintenance and setup costs, can be more reasonable [34]. One of the limiting factors for microalgae growth in open raceway ponds is light, as natural light tends to be the only source of this key cultivation factor [24]. Thus, it can only be distributed from the sun, which requires harvesting to occur more often to inhibit negative responses to light attenuation [24]. Furthermore, closed systems, like bubble column photobioreactors, can cultivate higher biomass yield and nitrogen intake in microalgae compared to an open high-rate pond (HRP) system [37]. However, in an HRP, the net energy ratio or energy efficiency is higher, rendering it more favorable in that aspect [37]. Therefore, dependent on the purpose of the microalgae culture, there are many growth systems that can be tailored for a desirable result.
However, for research purposes, it may be preferable to utilize PBRs, as they can cover a variety of roles. The first role largely involves microalgae cultivation. PBRs allow the controlled delivery of a variety of growth factors, including mass transfer and agitation of the culture, which allows for the ability to adjust these factors for or to observe a certain outcome [38]. For instance, by taking advantage of the device’s ability to control growth conditions, PBRs have been used to observe the impact of various cultivation conditions on productivity in various microalgal species, such as C. vulgaris and Nannochloropsis oceanica [39,40,41,42]. They can also play an indirect role by cultivating microalgae for the design of a model to optimize the organism for biofuel production [40]. This paper will, therefore, delve into the importance of PBRs and their design in microalgae research and industry.
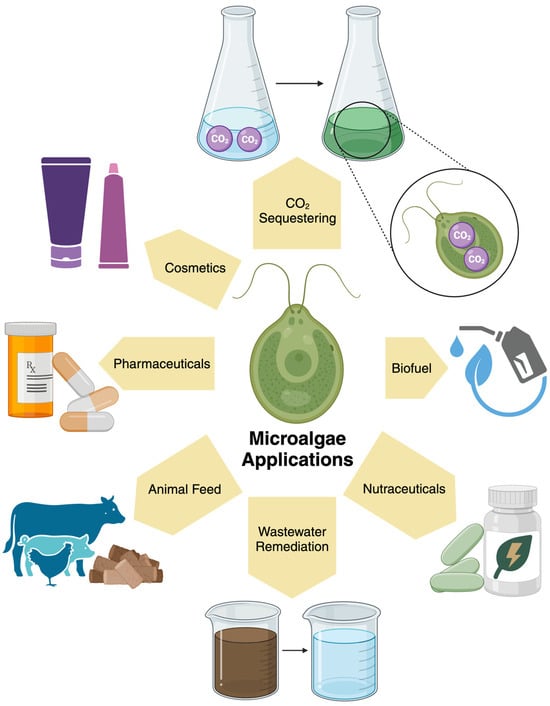
Figure 1.
The varied applications of microalgae [1,2,3,4,7,12,15,16,17,18,19,20,43,44,45,46].
2. Types and Uses of Bioreactors
PBRs come in various designs and capacities (Table 1). For instance, a Fibonacci-type PBR was designed and scaled up to cultivate Dunaliella salina in an extreme solar environment where growth factors like temperature and pH were controlled within optimal values for the microalgae [47]. This design scaled the culture to 1250 L and increased biomass concentrations three-fold compared to the culture grown in the same conditions in a raceway pond [47]. There are also tubular and panel PBRs, which can increase the efficiency of microalgae biomass productivity in the aerobic phase and biohydrogen production phase, respectively, for Chlamydomonas reinhardtii [48].

Table 1.
The diverse uses of bioreactors.
In an outdoor photobioreactor with a capacity of 50 L, C. vulgaris FSP-E was grown to optimize the production of protein in the microbial species to lower protein production costs for a fishmeal alternative [39]. A biomass productivity level of 268.1 mg/L/d and a protein productivity level of 155.4 mg/L/d were achieved with this cultivation system [39]. Analysis of the synthesized proteins validated the potential of utilizing the species as a feedstock for the production of a fishmeal alternative [39]. Beyond research applications, PBRs can be used for larger-scale functions.
PBRs have diverse uses for industrial purposes. For large-scale wastewater treatment and biofuel production, a floating offshore PBR was designed to address a couple of identifiable issues with biofuel feedstock cultivation [49]. This included scaling up the culture and its longevity [49]. One key element of this PBR’s wastewater remediation function is the cultivation of polycultures, which has aided in the stability, efficiency, and consistency of the culture and its products [49]. Beyond microalgae, wastewater can also be utilized for the cultivation of other microbes, including Zoogloea [50]. These microbes can produce polyhydroxyalkanoates (PHA), a biopolymer studied as an alternative for common plastics but have not yet been widely adopted due to high production costs [50,60]. As such, a model was designed to predict microbe growth and PHA production in rice winery wastewater with varying organic loading rates (OLRs), which is related to microbe metabolism and productivity [50]. As an average, OLR improved PHA productivity [50].
Wastewater as growth media is a method of addressing environmental and economic concerns regarding microalgae cultivation. There are several versions of membrane PBRs (MPBRs) designed for wastewater remediation and microbe cultivation. For instance, mixotrophic growth of Chlorella pyrenoidosa in an MPBR was observed when acetate was added to wastewater [45]. The addition of acetate exacerbated the membrane fouling of the MPBR, an occurrence that was attributed to the success of the culture [45]. Thus, it was concluded that the MPBR system is a good candidate for the simultaneous production of microalgae and wastewater remediation [45]. Other versions of MPBRs have also been utilized for this purpose, as the combination of C. pyrenoidosa within both an anaerobic membrane bioreactor (AnMBR) and MPBR for mixotrophic cultivation, also referred to as an AnMBR-MPBR system, was recognized to participate in carbon mitigation, wastewater remediation, and desirable microalgae productivity [44]. Additionally, novel MPBR designs have also been identified as preferable candidates. One such model, the biofilm MPBR (BF-MPBR), was described as an effective vessel for continuous microalgae productivity, thus optimal for the removal of nutrients and sulfonamides from aquaculture wastewater [43]. As a result of batch cultivation with a duration of ten days, the biomass productivity rate reached 6.64 mg/L·d, whereas cultivation in a BF-MPBR achieved 22.03 mg/L·d, an approximate three-fold increase [43]. Beyond wastewater treatment, PBRs can also be involved in the mitigation of CO2.
When scaling up Tetraselmis sp. CTP4, tubular PBRs were tested for industrial-scale cultivation [51]. The productivity of photosynthetic microalgae is tied to CO2. As such, CO2 mitigation is a factor observed when considering the success of cultures. Within a tubular PBR, CO2 mitigation efficiency reached 65%, contributing to the potential of the species for industrial-sized production [51]. Additionally, to improve biomass, this system can be used during the spring and summer months or modified to provide mixotrophic growth conditions [51]. In addition to industrial-scale PBR usage, microalgae applications in industrial capacities have been explored.
Prospective industrial applications of microalgae have been identified with the use of PBRs. This has been achieved through the production of PBR models to simulate microalgae growth and productivity in various climates [52]. Through studying Microchloropsis salina, such a model was created for open thin-layer cascade (TLC) PBRs by observing the species’ lipid productivity while utilizing two scalable TLC PBRs [52]. Through microalgae cultivation in the TLC PBRs and an accompanying model, the potential of M. salina for full-scale productivity of lipids on an industrial scale was revealed and confirmed [52]. However, these examples describe a fraction of bioreactors’ contribution to microbe research.
Bioreactors have diverse functions in the research of microbes, as they can also play an additional role in research by cultivating transgenic organisms. A genetically engineered strain of Streptococcus equi subsp. zooepidemicus was cultivated in a 3 L bioreactor to observe the strain’s ability to produce chondroitin, a precursor of chondroitin sulfate, and hyaluronic acid [56]. The latter two compounds are glycosaminoglycans, which are used in cosmetic and pharmaceutical industries [56]. The inclusion of genes from Escherichia coli known to be associated with the synthesis of a chondroitin-like sugar, kfoC and kfoA, gave the recombinant S. equi subsp. zooepidemicus the ability to produce both chondroitin and hyaluronic acid [56]. The 3 L bioreactors were utilized in the cultivation of the recombinant bacteria and, therefore, the production of desired compounds [56]. Bioreactors have also been involved in the larger-scale production of transgenic organisms. Saccharomyces cerevisiae was genetically engineered to produce high amounts of tyrosol and salidroside, used in the production of cosmetics, nutraceuticals, and pharmaceuticals [53]. In terms of bioreactor usage, a 5 L bioreactor was utilized to scale up the productivity of S. cerevisiae from shake flasks [53]. With this method, the yeast species was able to produce a high amount of the desired products [53].
A bioreactor was also utilized to increase lipid productivity, squalene, and DHA in Aurantiochytrium sp. T66 [46], the latter of which has nutritional benefits for humans when incorporated into the diet [46,61,62]. Moreover, squalene is the precursor to human steroids, the production of which makes Aurantiochytrium sp. a good candidate for the industrial production of nutraceuticals, cosmetics, and pharmaceuticals [46]. After cultivation in a bioreactor, total lipid concentration was 5.90 g/L, where DHA made up 35.76%, and squalene yield increased by approximately 0.28 g/L in comparison to flask-cultivated microbes [46].
Bioreactors can also be used to cultivate plant cell lines with the ability to produce compounds with various applications in cosmetics, nutraceuticals, and food coloring [57]. This plant biomass, from the red carrot R4G cell line, was observed to contain high concentrations of anthocyanins, which may point to its use as a food colorant [57]. Furthermore, the carrot cells’ biomass had a variety of health benefits in mouse cells, including anti-aging, anti-inflammatory, and antioxidant activity [57]. These results further identify the impact of bioreactors on research to produce a variety of useful products, the diverse applications of which are noted in Figure 2.

Figure 2.
Diverse applications of bioreactors [39,41,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59].
When scaling up the production of microalgae with photobioreactors, several issues may arise (Table 2). Notably, keeping the process low-cost, culture collapse, and contamination [41,55]. However, to address the latter two, a two-stage process was created with C. vulgaris culture [41]. The first stage of this methodology requires the cultivation of the microalgae species in fermenters [41]. Stage two is inoculating the culture in a larger capacity flat-panel PBR with the ability to culture 1000 L of microalgae [41]. These steps describe a heterotrophic method of scaling up productivity [41]. By utilizing the autotrophic growth method, growing 1000 L of C. vulgaris would take approximately 35 days, whereas the previously mentioned method requires approximately five days [41]. Furthermore, the heterotrophic method essentially decreases the time and space required for scaled-up microalgae production in PBRs [41]. Therefore, this emphasizes the importance of identifying the ideal growth system for an intended purpose, whether that be for research or industry.

Table 2.
Summarization of challenges faced by current photobioreactors.
3. Photobioreactors for Energy
Currently, there is interest in increasing sustainability through the production of biofuels sourced from microbes like microalgae, illustrated in Figure 3. However, there are several challenges that have inhibited the usage of the product worldwide [54]. For instance, identifying the microbe species to be used as feedstock and determining its success in scaled-up systems can pose difficulties [54].
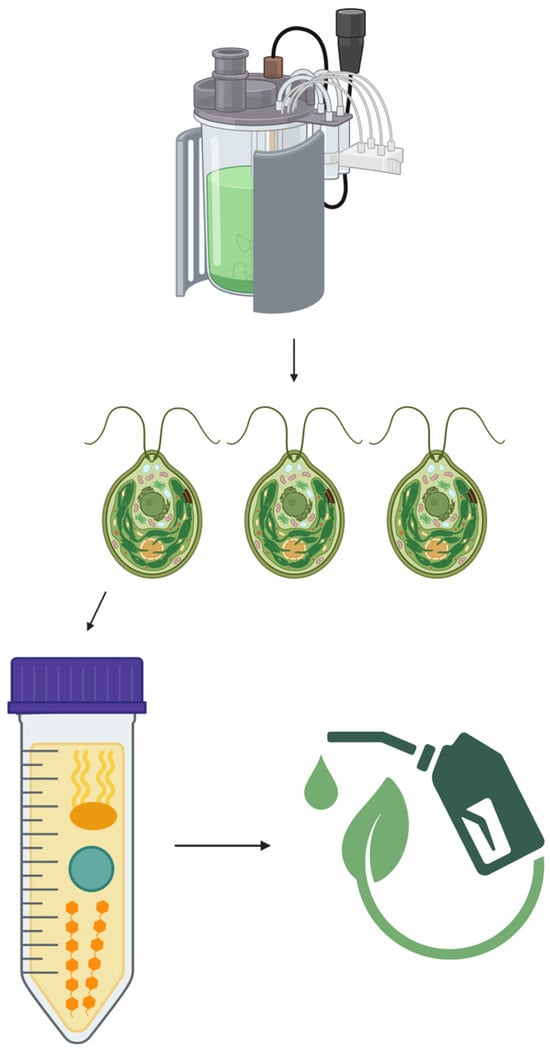
Figure 3.
Synthesis of biofuels [1,54,58,59].
Biodiesel quality is correlated with the concentration of fatty acid type, which plays a role in the biofuel’s characteristics, such as cold-flow properties [59]. This makes it necessary to design an efficient method for microalgae cultivation to maximize ideal lipid concentrations for biodiesel production [59], which was performed through validation of Coelastrum sp. SM cultivated in an air-lift PBR as a feedstock for biofuel and fixation of CO2 [59]. Analysis revealed that the highest lipid content reached 37.91% of dry weight and the highest carbohydrate content was 58.45% of dry weight. Furthermore, the culture had a CO2 fixation rate of 0.302 g/L·h, confirming the utility of the species in biodiesel production and CO2 fixation simultaneously [59].
CO2 fixation has also been recorded in C. vulgaris cultivated in tubular PBRs [42]. The system was tested for its ability to scale up the microalgae culture to 100 L while utilizing sodium bicarbonate as a CO2 source [42]. As a result, lipid concentrations increased to about 26%, and CO2 fixation was approximately 0.925 g/L·d [42]. As the maximum concentration of sodium bicarbonate to completely increase lipid productivity has not yet been elucidated, we can only conclude that lipid productivity and sodium bicarbonate concentration are directly related, wherein an increase in the latter results in the increase in the former [42].
To produce biodiesel or biofuel, microalgae’s lipid productivity is a point of interest. In an open system, a raceway pond, Nannochloropsis sp. KMMCC 290, was cultivated to produce biodiesel [1]. In a flat-plate photobioreactor (FPP) under control conditions, or with a light intensity of 5800 lux and continuous air supply with no carbon dioxide, lipid content was higher than cultures grown in the raceway pond [1]. Whereas, under increased light intensity, gas exchange rates, and CO2 supply, lipid productivity was about 16.6-fold higher than in the raceway pond [1]. At the conclusion of this research, the productivity in different systems, such as a bubble column PBR and air-lift PBR, provided varying productivity rates, thus further pointing to the importance of identifying the optimal growth system for a desired result [1]. Another species, S. obliquus, was cultivated in a plastic-type flat-panel PBR with a volume of 5 L [58]. When the nitrogen source for the culture was urea and light intensity reached 3000 lux with white-colored, fluorescent bulbs, the resulting culture’s dry biomass contained 40% lipids [58]. Furthermore, 66.6% of lipids were unsaturated fatty acids, which is ideal for biofuel production [58]. The potential of microalgae species for alternative energy sources has also been realized through the production of models.
A computational fluid dynamic model was utilized to experimentally scale up a mutant cell line of C. reinhardtii by simulating varying sparger designs in a 120 L flat-plate photobioreactor [54]. The model predicted a biomass productivity increase of 18% in C. reinhardtii grown within the optimized closed PBR [54]. These studies have revealed the importance of PBRs in the synthesis of renewable energy sources utilizing the productivity of microalgae.
4. Incubation Factors for Photobioreactors
An effective photobioreactor design must take into consideration a variety of growth factors, such as hydrodynamics, light, growth kinetics, agitation, nutrient supply, and gas exchange (Table 3 and Figure 4) [64].

Table 3.
The incubation and growth factors for microalgae cultivation.

Figure 4.
Photobioreactor (PBR) incubation factors [1,4,21,23,25,26,28,29,30,31,32,33,39,42,47,48,54,58,59,65,66,67,68,69,70,71,72,73].
Light is an important factor for microalgae growth. For example, a light intensity of 350 μmol/m2s, results in the highest productivity of carbohydrates at 48.11 gC/m3d for I. galbana [68].
Light intensity, gas exchange, and CO2 supply impact cell count in Nannochloropsis sp. KMMCC 290 cultures [1]. Increasing the growth factors previously listed can improve lipid content in cells [1]. Furthermore, light cycles can inhibit cell replication and stress cells, which induces an increase in lipid production [1]. Light as a growth factor can be controlled with cultivation systems.
The thickness of a flat-plate photobioreactor (FPP) impacts the light intensity received by the algae culture in the device [66]. The thinner the FPP, or the smaller the light path, the higher the lipid productivity and content in cells [66]. However, for a light path of 5 cm compared with the 10 cm light path, values such as growth rate, cell dry weight, and biomass productivity were lower [66]. Thus, it is important to consider light intensity for the purpose of providing ideal growth conditions.
In addition to light intensity, cell density, growth medium, and light cycles also influence microalgae growth [67]. For Chlorococcum sp., saline water, 2500–3500 lux, and growth under 24 h of light resulted in optimal growth of the species on the fifth day [67]. However, this growth rate additionally relies on cell density, wherein low initial cell densities have a longer death phase after the 11th day of growth [67].
Temperature conditions are also an important consideration for microalgae cultivation. Under high-temperature stress, at 30 °C, lipid productivity improved in a mixed microalgae culture collected from the Nacharam Cheruvu in India [73]. Furthermore, analysis of lipid content noted a high concentration of saturated fatty acids due to stress phase growth under the mentioned temperature conditions [73]. At 25 °C, the growth rate was higher than at hotter temperatures for Chaetoceros sp. FIKU035 and Nannochloropsis sp. FIKU036 [65], whereas Tetraselmis suecica FIKU032 had a slightly higher growth rate at 30 °C and could not be cultivated at higher temperatures [65]. Variability was also observed in biomass productivity and concentration [65], where these values were the highest for Nannochloropsis sp. FIKU036 cultivated at 25 °C and the highest for Chaetoceros sp. FIKU035 and T. suecica FIKU032 at 30 °C [65].
Nutrient supply is highly involved with productivity in microalgae [71]. In freshwater with fertilizer, Tetradesmus almeriensis cultivated in a pilot-scale thin-layer cascade PBR had the most biomass productivity of 30.3 g/m2·day [71], whereas when the microalgae were grown in wastewater, protein and lipid productivity increased [71]. When Chlorella sp. GN1 was grown in nitrogen deprivation conditions, the lipid content increased in dry cell weight compared to phosphorous limitation or nitrogen and phosphorous sufficient conditions [66]. Under nitrogen-deprived conditions, lipid productivity reached 63.5 mg/L·day [66]. However, lipid concentration under nutrient-limited conditions was lower than Chlorella sp. GN1 grown in nitrogen and phosphorous-sufficient conditions after eight days of growth [66]. Nutrient availability also impacts the productivity of other beneficial compounds in microalgae.
Fucoxanthin is a pigment with a variety of health benefits, including as an antioxidant, which has made the compound highly sought after to produce nutraceuticals and pharmaceuticals [3,4]. As such, methods to optimize the productivity of fucoxanthin have been pursued [4]. This has revealed that providing high nitrogen levels in the initial f/2 growth medium for Phaeodactylum tricornutum can result in high productivity, thus increasing biomass and concentrations of fucoxanthin [4]. Furthermore, high nitrogen media and low light conditions can increase fucoxanthin production [4]. This points to both growth factors attributing to the fucoxanthin production process in P. tricornutum [4].
Higher airflow is correlated with higher cell density in Tisochrysis lutea cultivated in a bench-scale air-lift PBR [72]. Generally, for column-style photobioreactors, air supply has a positive correlation with gas exchange as a result of each microalgae cell within the culture receiving more light exposure [72]. Furthermore, reducing hydrodynamic stress, caused by factors like shear stress, can further increase cell growth [72].
Different microalgae species may react to hydrodynamic stress differently [74]. Both shear rate and shear stress are values associated with calculating shear forces [75]. High shear stress and shear rate can damage cells, and microalgae’s reaction and resilience to such stress vary by strain [75]. However, shear stress near the walls of closed growth systems is necessary to inhibit the growth of a biofilm [65]. Biofilm formation can prevent the microalgae culture from receiving light [76]. Thus, C. vulgaris biofilm prevention and removal through wall shear forces was pursued through cultivation in a flat-panel PBR [76]. It was revealed that 0.2 Pa of wall shear stress prevents biofilm formation, whereas 6 Pa can disrupt an established biofilm, and 53 Pa is necessary to remove it [76].
Shear force can also be utilized to increase mass transfer, thus improving microalgae’s CO2 fixation [70]. This was achieved through applying shear force through water centrifugation to decrease the overall size of bubbles or increase the bubbles’ surface area within the microalgae culture [70]. This method increased biomass productivity by 50.7% [70]. However, high aeration creates high levels of shear stress, preventing biomass productivity of Arthrospira platensis in 2 L photobioreactors [77]. Thus, it is important to develop optimal growth settings to introduce reasonable levels of shear stress for growing microalgae cultures.
Simulations of an air-lift photobioreactor predict that higher gas flow rates cause increases in photosynthetic efficiency [69]. This is due to the cells’ light exposure increasing as a result of introducing agitation [69]. However, this comes with a disclaimer, as high gas flow rates also cause high levels of shear stress, which decrease biomass [69]. Thus, for Porphyridium sp., the increased fluid mixing, a result of higher gas flow rates, also increases shear stress, causing cell damage, thus leading to decreased biomass productivity [69]. Additionally, challenges in microalgae cultivation come in the form of contamination.
Rotifers are predators of microalgae [78,79,80]. These organisms can decimate microalgae cultures in both open ponds and closed photobioreactors when introduced [79]. However, rotifer contamination is restricted in thin-layer cascade reactors, as their population density is limited in the system [79]. This indicates the impacts of rotifer contamination vary in different growth systems [79].
The role of growth kinetic models is to convey how microalgae grow in specific environments within a time period [24,81]. As such, there are currently a variety of growth kinetic models available for microalgae species [24]. These models can point to the downsides of different growth methods; for instance, in open raceway ponds, growth kinetics have pointed toward the main issue of light attenuation [24]. One such model for an open raceway pond notes that systems around 30–35 cm are advantageous for outdoor growth, whereas values above this range are not [24].
One method to analyze cell growth kinetics involves the maximum growth rate, a value calculated with the biomass concentration of the desired microalgae strain at a specific time period [77]. Additionally, cell productivity must also be calculated through the varied cell density over the cultivation period [77]. This method was utilized to calculate the growth kinetics of A. platensis in PBRs [77]. Growth rate kinetics can also be calculated with the Contois equation, the differential of substrate concentration with consideration to time, and the differential of carbohydrate concentration with consideration to time [82]. This method can reveal the growth rate of microalgae cultivated using thin-layer photobioreactors in different mediums [82].
Furthermore, different growth kinetic models fit with experimental data [81]. For instance, the Gompertz model is more accurate than the logistic model in determining the cultivation of Scenedesmus parvus in a PBR [81]. Both of these models utilize the same variables to calculate growth kinetics, such as initial and largest biomass, growth rate, and time; however, they are arranged differently to gain varying results [81]. As a result, the more accurate model can be utilized to scale up cultures for industrial purposes in the future [81].
Growth kinetics can also model growth limitations reliant on dissolved inorganic carbon (DIC) concentration in the microalgae culture [83], wherein low levels of DIC, or limited carbon, can result in a decrease in growth kinetics [83]. However, this model has not been developed to consider the impact of mixotrophic growth on CO2 remediation by microalgae cultures [83]. Thus, growth kinetics can assist with determining the ideal growth factors and system for microalgae cultivation.
5. Challenges and Perspectives
The cultivation of microalgae species currently has identifiable economic and environmental impacts; however, it is possible to discover pathways to sustainable and cost-efficient methods for microalgae production [84,85]. These environmental challenges include the production of fertilizers and the utilization of certain types of growth media [84]. The former are used in microalgae cultivation but pose environmental concerns such as ecotoxicity and ozone layer damage [6]. The latter refers to growth media that utilize compounds like ammonia, which, when produced, have high greenhouse gas emissions [86]. Although, to combat this concern, several pathways have been revealed, such as the usage of modified wastewater as growth media and adapting more sustainable ammonia production methods [8,87]. However, for the former solution, the consistency of the product cannot be guaranteed, as wastewater itself is not consistent [8], which emphasizes the importance of analyzing wastewater for specific nutrient requirements needed for successful microalgae growth.
For biofuel production, economic challenges are prevalent, for instance, identifying suitable feedstock species and decreasing production costs [54]. However, through integrated production of various fuel types, microalgae-based biofuel production can increase in economic value [84]. Additional efforts in addressing these concerns include implementing optimal growth conditions, decreasing space requirements, and utilizing greener energy during the production process [84]. Generally, microalgae cultivation and its accompanying economic and environmental concerns vary when considering laboratory or industrial scale production, microalgae species, and cultivation conditions [6]. Thus, the accompanying impacts of microalgae production vary for specific targeted growth and must be considered on a case-by-case basis.
The cultivation of microalgae heavily relies upon growth conditions, wherein the delivery of such factors can be dictated through the usage of cultivation systems [34,38]. These systems, categorized as either open or closed, differ greatly in their characteristics [34,35]. Generally, open systems have a higher risk of contamination from either other eukaryotic species or predators of microalgae but are more suitable for larger volume cultivation [34,36]. As such, for monoculture of smaller volumes, a PBR may be preferable, as the system provides the ability to deliver controlled growth conditions such as light and temperature, of which these qualities in an open system, like a raceway pond, are more difficult to control [24,51]. Additionally, the usage of closed systems has a lower risk of contamination [34]. However, there are some recognizable challenges that PBRs face, which have been mainly tied to scaling up cultures [41,55], where production costs and limited cultivation volumes of PBRs pose issues [55]. Furthermore, large-volume outdoor PBRs are exposed to seasonal changes and natural light, which can vary drastically as time passes [47,51]. Thus, these challenges emphasize a need for a variety of PBRs that can address the downsides of current models available. Several PBR designs have been designed to address this and are slightly more favorable for larger-scale microalgae production [41,47]. However, these models further confirm that there is a wide range of considerations when identifying or designing an ideal microalgae growth system. One must consider the purpose of the final microalgae culture, whether that be for research or industrial purposes. The scale of the product and, if applicable, desired enhancements in productivity. This will assist in determining the type of microalgae growth system and the conditions required to induce desired biomass accumulation and productivity rates. For microalgae research, many of these considerations are addressed through the usage of PBRs that have allowed finer control of various growth factors.
Author Contributions
Z.-Y.D. developed the idea and outline of the article; S.D. made the figures and tables and wrote the manuscript; Z.-Y.D. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by UHM UROP-Undergraduate Research Opportunities Program, USDA-NIFA HATCH project HAW05047-H, State of Hawaii Department of Agriculture, Center for Tropical and Subtropical Aquaculture through Grant No. 2020-38500-32559 and 2022-38500-38099 from the U.S. Department of Agriculture National Institute of Food and Agriculture.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Ju-Ling Chen (UROP, UHM), Kanak Pal, Ty Shitanaka, Cade Kane, and Rumesh Senthilnathan, for their help with this paper.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kwon, M.H.; Yeom, S.H. Evaluation of closed photobioreactor types and operation variables for enhancing lipid productivity of Nannochloropsis sp. KMMCC 290 for biodiesel production. Biotechnol. Bioprocess Eng. 2017, 22, 604–611. [Google Scholar] [CrossRef]
- Regueiras, A.; Huguet, Á.; Conde, T.; Couto, D.; Domingues, P.; Domingues, M.R.; Costa, A.M.; da Silva, J.L.; Vasconcelos, V.; Urbatzka, R. Potential Anti-Obesity, Anti-Steatosis, and Anti-Inflammatory Properties of Extracts from the Microalgae Chlorella vulgaris and Chlorococcum amblystomatis under Different Growth Conditions. Mar. Drugs 2022, 20, 9. [Google Scholar] [CrossRef]
- Arunkumar, K.; Nalluri, M.; Anjana, K.; Mohan, G.; Raja, R. Fucoxanthin as antioxidant, anti-hyaluronidase and cytotoxic agent: Potential of brown seaweeds decoction for tea supplement. J. Food Meas. Charact. 2023, 17, 3980–3989. [Google Scholar] [CrossRef]
- Truong, T.Q.; Park, Y.J.; Winarto, J.; Huynh, P.K.; Moon, J.; Choi, Y.B.; Song, D.-G.; Koo, S.Y.; Kim, S.M. Understanding the Impact of Nitrogen Availability: A Limiting Factor for Enhancing Fucoxanthin Productivity in Microalgae Cultivation. Mar. Drugs 2024, 22, 93. [Google Scholar] [CrossRef]
- D’Imporzano, G.; Veronesi, D.; Salati, S.; Adani, F. Carbon and nutrient recovery in the cultivation of Chlorella vulgaris: A life cycle assessment approach to comparing environmental performance. J. Clean. Prod. 2018, 194, 685–694. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, Y.; Liu, Y.; Esakkimuthu, S.; Chen, H.; Wang, S. Impact of constant magnetic field on enhancing the microalgal biomass and biomolecules accumulations and life cycle assessment of the approach. Algal Res. 2024, 80, 103563. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Josa, I.; Ferrer, I.; Van Hulle, S.W.H.; Rousseau, D.P.L.; Garfí, M. Life cycle assessment of microalgae systems for wastewater treatment and bioproducts recovery: Natural pigments, biofertilizer and biogas. Sci. Total Environ. 2022, 847, 157615. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, J.; Zhang, Y.; He, S.; Esakkimuthu, S.; Zhu, K.; Kumar, S.; Lv, G.; Hu, X. Biochar assisted cultivation of Chlorella protothecoides for adsorption of tetracycline and electrochemical study on self-cultured Chlorella protothecoides. Bioresour. Technol. 2023, 389, 129810. [Google Scholar] [CrossRef]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the environment: An overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Prigiobbe, V.; Abimbola, T.; Christodoulatos, C. Integrating biological and chemical CO2 sequestration using green microalgae for bioproducts generation. Front. Clim. 2023, 4, 949411. [Google Scholar] [CrossRef]
- Lan, X.; Tans, P.; Thoning, K.W. Trends in Globally-Averaged CO2 Determined from NOAA Global Monitoring Laboratory Measurements, Version 2024-10; NOAA Global Monitoring Laboratory: Boulder, CO, USA, 2024. [CrossRef]
- Wu, Z.; Vermeulen, A.; Sawa, Y.; Karstens, U.; Peters, W.; de Kok, R.; Lan, X.; Nagai, Y.; Ogi, A.; Tarasova, O. Investigating the differences in calculating global mean surface CO2 abundance: The impact of analysis methodologies and site selection. Atmos. Chem. Phys. 2024, 24, 1249–1264. [Google Scholar] [CrossRef]
- Xin, Y.; Shen, C.; She, Y.; Chen, H.; Wang, C.; Wei, L.; Yoon, K.; Han, D.; Hu, Q.; Xu, J. Biosynthesis of Triacylglycerol Molecules with a Tailored PUFA Profile in Industrial Microalgae. Mol. Plant 2019, 12, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.L.; Falé, P.; Afonso, C.M.; Bandarra, N.M. Investigation of nutraceutical potential of the microalgae Chlorella vulgaris and Arthrospira platensis. Int. J. Food Sci. Technol. 2020, 55, 303–312. [Google Scholar] [CrossRef]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as Singlet Oxygen Quenchers in Marine Organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 2014, 165, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, B.; Hu, Q.; Sommerfeld, M.; Li, Y.; Han, D. A New Paradigm for Producing Astaxanthin from the Unicellular Green Alga Haematococcus pluvialis. Biotechnol. Bioeng. 2016, 113, 2088–2099. [Google Scholar] [CrossRef]
- Chaudhary, A.; Mishra, P.; Amaz, S.A.; Mahato, P.L.; Das, R.; Jha, R.; Mishra, B. Dietary supplementation of microalgae mitigates the negative effects of heat stress in broilers. Poult. Sci. 2023, 102, 102958. [Google Scholar] [CrossRef]
- Sforza, E.; Bertucco, A.; Morosinotto, T.; Giacometti, G.M. Photobioreactors for microalgal growth and oil production with Nannochloropsis salina: From lab-scale experiments to large-scale design. Chem. Eng. Res. Des. 2012, 90, 1151–1158. [Google Scholar] [CrossRef]
- Manhaeghe, D.; Blomme, T.; Van Hulle, S.W.H.; Rousseau, D.P.L. Experimental assessment and mathematical modelling of the growth of Chlorella vulgaris under photoautotrophic, heterotrophic and mixotrophic conditions. Water Res. 2020, 184, 116152. [Google Scholar] [CrossRef] [PubMed]
- Al Jabri, H.; Taleb, A.; Touchard, R.; Saadaoui, I.; Goetz, V.; Pruvost, J. Cultivating Microalgae in Desert Conditions: Evaluation of the Effect of Light-Temperature Summer Conditions on the Growth and Metabolism of Nannochloropsis QU130. Appl. Sci. 2021, 11, 3799. [Google Scholar] [CrossRef]
- Romagnoli, F.; Weerasuriya-Arachchige, A.R.P.P.; Paoli, R.; Feofilovs, M.; Ievina, B. Growth Kinetic Model for Microalgae Cultivation in Open Raceway Ponds: A System Dynamics Tool. Environ. Clim. Technol. 2021, 25, 1317–1336. [Google Scholar] [CrossRef]
- Bartley, M.L.; Boeing, W.J.; Dungan, B.N.; Holguin, F.O.; Schaub, T. pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. J. Appl. Phycol. 2014, 26, 1431–1437. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Hrůzová, K.; Rova, U.; Christakopoulos, P. Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass. Mar. Drugs 2019, 17, 119. [Google Scholar] [CrossRef]
- Aussant, J.; Guihéneuf, F.; Stengel, D.B. Impact of temperature on fatty acid composition and nutritional value in eight species of microalgae. Appl. Microbiol. Biotechnol. 2018, 102, 5279–5297. [Google Scholar] [CrossRef]
- Perin, G.; Cimetta, E.; Monetti, F.; Morosinotto, T.; Bezzo, F. Novel micro-photobioreactor design and monitoring method for assessing microalgae response to light intensity. Algal Res. 2016, 19, 69–76. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Fawzy, M.A.; El-Otify, A.M.; Adam, M.S.; Moustafa, S.S.A. The impact of abiotic factors on the growth and lipid accumulation of some green microalgae for sustainable biodiesel production. Environ. Sci. Pollut. Res. 2021, 28, 42547–42561. [Google Scholar] [CrossRef]
- Gao, B.; Hong, J.; Chen, J.; Zhang, H.; Hu, R.; Zhang, C. The growth, lipid accumulation and adaptation mechanism in response to variation of temperature and nitrogen supply in psychrotrophic filamentous microalga Xanthonema hormidioides (Xanthophyceae). Biotechnol. Biofuels Bioprod. 2023, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Josephine, A.; Kumar, T.S.; Surendran, B.; Rajakumar, S.; Kirubagaran, R.; Dharani, G. Evaluating the effect of various environmental factors on the growth of the marine microalgae, Chlorella vulgaris. Front. Mar. Sci. 2022, 9, 954622. [Google Scholar] [CrossRef]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of Microalgae Cultivation in Photobioreactor, Open Raceway Pond, and a Two-Stage Hybrid System. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Prado, L.O.; Bolzani, H.R.; Souza, H.H.S.; Ruas, G.; Silva, G.H.R. Microalgal cultivation in open and closed systems under a tropical climate: A life cycle comparison. J. Clean. Prod. 2023, 422, 138631. [Google Scholar] [CrossRef]
- Naumann, T.; Çebi, Z.; Podola, B.; Melkonian, M. Growing microalgae as aquaculture feeds on twin-layers: A novel solid-state photobioreactor. J. Appl. Phycol. 2013, 25, 1413–1420. [Google Scholar] [CrossRef]
- Magalhães, I.B.; Ferreira, J.; Castro, J.d.S.; de Assis, L.R.; Calijuri, M.L. Agro-industrial wastewater-grown microalgae: A techno-environmental assessment of open and closed systems. Sci. Total Environ. 2022, 834, 155282. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.D.; Mota, A.; Ferreira, A.; Dragone, G.; Teixeira, J.A.; Vicente, A.A. Characterization of split cylinder air-lift photobioreactors for efficient microalgae cultivation. Chem. Eng. Sci. 2014, 117, 445–454. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, Y.-H.; Chang, H.-Y. Outdoor cultivation of Chlorella vulgaris FSP-E in vertical tubular-type photobioreactors for microalgal protein production. Algal Res. 2016, 13, 264–270. [Google Scholar] [CrossRef]
- del Rio-Chanona, E.A.; Liu, J.; Wagner, J.L.; Zhang, D.; Meng, Y.; Xue, S.; Shah, N. Dynamic modeling of green algae cultivation in a photobioreactor for sustainable biodiesel production. Biotechnol. Bioeng. 2018, 115, 359–370. [Google Scholar] [CrossRef]
- Barros, A.; Pereira, H.; Campos, J.; Marques, A.; Varela, J.; Silva, J. Heterotrophy as a tool to overcome the long and costly autotrophic scale-up process for large scale production of microalgae. Sci. Rep. 2019, 9, 13935. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M.; Koniuszy, A. Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes. Sustainability 2021, 13, 9118. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Gao, F.; Yang, H.-L.; Wu, H.-W.-J.; Li, C.; Lu, M.-M.; Yang, Z.-Y. Simultaneous removal of nutrient and sulfonamides from marine aquaculture wastewater by concentrated and attached cultivation of Chlorella vulgaris in an algal biofilm membrane photobioreactor (BF-MPBR). Sci. Total Environ. 2020, 725, 138524. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, Z.-Y.; Zhao, Q.-L.; Chen, D.-Z.; Li, C.; Liu, M.; Yang, J.-S.; Liu, J.-Z.; Ge, Y.-M.; Chen, J.-M. Mixotrophic cultivation of microalgae coupled with anaerobic hydrolysis for sustainable treatment of municipal wastewater in a hybrid system of anaerobic membrane bioreactor and membrane photobioreactor. Bioresour. Technol. 2021, 337, 125457. [Google Scholar] [CrossRef]
- Huang, K.-X.; Mao, B.-D.; Lu, M.-M.; Chen, D.-Z.; Qiu, J.; Gao, F. Effect of external acetate added in aquaculture wastewater on mixotrophic cultivation of microalgae, nutrient removal, and membrane contamination in a membrane photobioreactor. J. Environ. Manag. 2024, 349, 119391. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Simultaneous production of DHA and squalene from Aurantiochytrium sp. grown on forest biomass hydrolysates. Biotechnol. Biofuels 2019, 12, 255. [Google Scholar] [CrossRef]
- Díaz, J.P.; Inostroza, C.; Acién, F.G. Scale-up of a Fibonacci-Type Photobioreactor for the Production of Dunaliella salina. Appl. Biochem. Biotechnol. 2021, 193, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Oncel, S.; Kose, A. Comparison of tubular and panel type photobioreactors for biohydrogen production utilizing Chlamydomonas reinhardtii considering mixing time and light intensity. Bioresour. Technol. 2014, 151, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Novoveská, L.; Zapata, A.K.M.; Zabolotney, J.B.; Atwood, M.C.; Sundstrom, E.R. Optimizing microalgae cultivation and wastewater treatment in large-scale offshore photobioreactors. Algal Res. 2016, 18, 86–94. [Google Scholar] [CrossRef]
- Fang, F.; Xu, R.-Z.; Huang, Y.-Q.; Wang, S.-N.; Zhang, L.-L.; Dong, J.-Y.; Xie, W.-M.; Chen, X.; Cao, J.-S. Production of polyhydroxyalkanoates and enrichment of associated microbes in bioreactors fed with rice winery wastewater at various organic loading rates. Bioresour. Technol. 2019, 292, 121978. [Google Scholar] [CrossRef]
- Pereira, H.; Páramo, J.; Silva, J.; Marques, A.; Barros, A.; Maurício, D.; Santos, T.; Schulze, P.; Barros, R.; Gouveia, L.; et al. Scale-up and large-scale production of Tetraselmis sp. CTP4 (Chlorophyta) for CO2 mitigation: From an agar plate to 100-m3 industrial photobioreactors. Sci. Rep. 2018, 8, 5112. [Google Scholar] [CrossRef]
- Schädler, T.; Thurn, A.-L.; Brück, T.; Weuster-Botz, D. Continuous Production of Lipids with Microchloropsis salina in Open Thin-Layer Cascade Photobioreactors on a Pilot Scale. Energies 2021, 14, 500. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Zhou, Y.; Kan, Y.; Wu, T.; Xiao, W.; Luo, Y. Multi-modular engineering of Saccharomyces cerevisiae for high-titre production of tyrosol and salidroside. Microb. Biotechnol. 2021, 14, 2605–2616. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Solsvik, J.; Wagner, J.L.; Zhang, D.; Hellgardt, K.; Park, C.W. CFD and kinetic-based modeling to optimize the sparger design of a large-scale photobioreactor for scaling up of biofuel production. Biotechnol. Bioeng. 2019, 116, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Bani, A.; Fernandez, F.G.A.; D’Imporzano, G.; Parati, K.; Adani, F. Influence of photobioreactor set-up on the survival of microalgae inoculum. Bioresour. Technol. 2021, 320, 124408. [Google Scholar] [CrossRef]
- Cimini, D.; Iacono, I.D.; Carlino, E.; Finamore, R.; Restaino, O.F.; Diana, P.; Bedini, E.; Schiraldi, C. Engineering S. equi subsp. Zooepidemicus towards concurrent production of hyaluronic acid and chondroitin biopolymers of biomedical interest. AMB Express 2017, 7, 61. [Google Scholar] [CrossRef]
- Bianconi, M.; Ceriotti, L.; Cuzzocrea, S.; Esposito, E.; Pressi, G.; Sgaravatti, E.; Bertaiola, O.; Guarnerio, C.; Barbieri, E.; Semenzato, A.; et al. Red Carrot Cells Cultured in vitro Are Effective, Stable, and Safe Ingredients for Skin Care, Nutraceutical, and Food Applications. Front. Bioeng. Biotechnol. 2020, 8, 575079. [Google Scholar] [CrossRef]
- Abdel-Baset, A.; Matter, I.A.; Ali, M.A. Enhanced Scenedesmus obliquus Cultivation in Plastic-Type Flat Panel Photobioreactor for Biodiesel Production. Sustainability 2024, 16, 3148. [Google Scholar] [CrossRef]
- Mousavi, S.; Najafpour, G.D.; Mohammadi, M. CO2 bio-fixation and biofuel production in an air-lift photobioreactor by an isolated strain of microalgae Coelastrum sp. SM under high CO2 concentrations. Environ. Sci. Pollut. Res. 2018, 25, 30139–30150. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Show, P.L.; Lin, H.C.; Chang, C.K.; Loh, H.-S.; Lan, J.C.-W.; Ling, T.C. Preliminary integrated economic and environmental analysis of polyhydroxyalkanoates (PHAs) biosynthesis. Bioresour. Bioprocess. 2016, 3, 41. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clin. Proc. 2021, 96, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.L.; Lee, S.-M.; Choi, H.-J. A mini review: Photobioreactors for large scale algal cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-J.; Tong, Z.-X.; Zhou, Z.-J.; Huang, D.; Wang, R.-L. A numerical model coupling bubble flow, light transfer, cell motion and growth kinetics for real timescale microalgae cultivation and its applications in flat plate photobioreactors. Algal Res. 2019, 44, 101727. [Google Scholar] [CrossRef]
- Chaisutyakorn, P.; Praiboon, J.; Kaewsuralikhit, C. The effect of temperature on growth and lipid and fatty acid composition on marine microalgae used for biodiesel production. J. Appl. Phycol. 2018, 30, 37–45. [Google Scholar] [CrossRef]
- Feng, P.; Xu, Z.; Qin, L.; Alam, M.A.; Wang, Z.; Zhu, S. Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresour. Technol. 2020, 301, 122762. [Google Scholar] [CrossRef]
- Putri, D.S.; Sari, D.A.; Marianah; Astuti, S.P.; Wangiyana, I.G.A.S. Effect of medium type, light intensity, and photoperiod on the growth rate of microalgae Chlorococcum sp. local isolate. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012071. [Google Scholar] [CrossRef]
- Guzmán-Palomino, A.; Aguilera-Vázquez, L.; Hernández-Escoto, H.; García-Vite, P.M.; Martínez-Salazar, A.L. Dynamical Simulation, Sensitivity, and Productivity Analysis of a Light-Photoacclimation Model for Microalgae-Based Carbohydrate Production in Continuous Photobioreactors. Processes 2023, 11, 1866. [Google Scholar] [CrossRef]
- Gao, X.; Kong, B.; Vigil, R.D. Multiphysics simulation of algal growth in an air-lift photobioreactor: Effects of fluid mixing and shear stress. Bioresour. Technol. 2018, 251, 75–83. [Google Scholar] [CrossRef]
- Cheng, J.; Miao, Y.; Guo, W.; Song, Y.; Tian, J.; Zhou, J. Reduced generation time and size of carbon dioxide bubbles in a volute aerator for improving Spirulina sp. growth. Bioresour. Technol. 2018, 270, 352–358. [Google Scholar] [CrossRef]
- Villaró, S.; Sánchez-Zurano, A.; Ciardi, M.; Alarcón, F.J.; Clagnan, E.; Adani, F.; Morillas-España, A.; Álvarez, C.; Lafarga, T. Production of microalgae using pilot-scale thin-layer cascade photobioreactors: Effect of water type on biomass composition. Biomass Bioenergy 2022, 163, 106534. [Google Scholar] [CrossRef]
- Falinski, K.A.; Timmons, M.B.; Callan, C.; Laidley, C. Response of Tisochrysis lutea [Prymnesiophycidae] to aeration conditions in a bench-scale photobioreactor. J. Appl. Phycol. 2018, 30, 2203–2214. [Google Scholar] [CrossRef]
- Venkata Subhash, G.; Rohit, M.V.; Devi, M.P.; Swamy, Y.V.; Venkata Mohan, S. Temperature induced stress influence on biodiesel productivity during mixotrophic microalgae cultivation with wastewater. Bioresour. Technol. 2014, 169, 789–793. [Google Scholar] [CrossRef]
- Leupold, M.; Hindersin, S.; Gust, G.; Kerner, M.; Hanelt, D. Influence of mixing and shear stress on Chlorella vulgaris, Scenedesmus obliquus, and Chlamydomonas reinhardtii. J. Appl. Phycol. 2013, 25, 485–495. [Google Scholar] [CrossRef]
- Guler, B.A.; Deniz, I.; Demirel, Z.; Oncel, S.S.; Imamoglu, E. Computational fluid dynamics modelling of stirred tank photobioreactor for Haematococcus pluvialis production: Hydrodynamics and mixing conditions. Algal Res. 2020, 47, 101854. [Google Scholar] [CrossRef]
- Belohlav, V.; Zakova, T.; Jirout, T.; Kratky, L. Effect of hydrodynamics on the formation and removal of microalgal biofilm in photobioreactors. Biosyst. Eng. 2020, 200, 315–327. [Google Scholar] [CrossRef]
- Hussin, A.A.; To, S.W.; Sani, M.H.; Amin, M.F.M.; Kamaroddin, M.F. Optimisation and growth kinetic analysis of Microalgae, Arthrospira platensis in 2-L Photobioreactors. IOP Conf. Ser. Earth Environ. Sci. 2021, 842, 012036. [Google Scholar] [CrossRef]
- Wang, Y.; Castillo-Keller, M.; Eustance, E.; Sommerfeld, M. Early detection and quantification of zooplankton grazers in algal cultures by FlowCAM. Algal Res. 2017, 21, 98–102. [Google Scholar] [CrossRef]
- Deruyck, B.; Nguyen, K.H.T.; Decaestecker, E.; Muylaert, K. Modeling the impact of rotifer contamination on microalgal production in open pond, photobioreactor and thin layer cultivation systems. Algal Res. 2019, 38, 101398. [Google Scholar] [CrossRef]
- Martínez, C.; Pessi, B.A.; Bernard, O. Dynamics and productivity of microalgae in presence of predators. IFAC-PapersOnLine 2021, 54, 673–678. [Google Scholar] [CrossRef]
- Tan, K.M.; Kassim, M.A.; Ng, Z.J.; Lalung, J. Isolation and characterization of novel acidophilic microalgae from abandoned mining site area for carbohydrate biosynthesis and its kinetic growth study in photobioreactor. IOP Conf. Ser. Mater. Sci. Eng. 2020, 716, 012011. [Google Scholar] [CrossRef]
- Yuarrina, W.P.; Pradana, Y.S.; Budiman, A.; Majid, A.I.; Indarto; Suyono, E.A. Study of cultivation and growth rate kinetic for mixed cultures of local microalgae as third generation (G-3) bioethanol feedstock in thin layer photobioreactor. J. Phys. Conf. Ser. 2018, 1022, 012051. [Google Scholar] [CrossRef]
- Pruvost, J.; Le Gouic, B.; Cornet, J.-F. Kinetic Modeling of CO2 Biofixation by Microalgae and Optimization of Carbon Supply in Various Photobioreactor Technologies. ACS Sustain. Chem. Eng. 2022, 10, 12826–12842. [Google Scholar] [CrossRef]
- Wang, S.; Mukhambet, Y.; Esakkimuthu, S.; Abomohra, A.E.-F. Integrated microalgal biorefinery—Routes, energy, economic and environmental perspectives. J. Clean. Prod. 2022, 348, 131245. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I.; Vezina, G.; Raso, F. Impact Assessment and Environmental Evaluation of Various Ammonia Production Processes. Environ. Manag. 2017, 59, 842–855. [Google Scholar] [CrossRef]
- Ye, L.; Li, H.; Xie, K. Sustainable ammonia production enabled by membrane reactor. Nat. Sustain. 2022, 5, 787–794. [Google Scholar] [CrossRef]
- Deprá, M.C.; Severo, I.A.; dos Santos, A.M.; Zepka, L.Q.; Jacob-Lopes, E. Environmental impacts on commercial microalgae-based products: Sustainability metrics and indicators. Algal Res. 2020, 51, 102056. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).