Abstract
In this study, the combined combustion characteristics and gaseous product emissions of coal slime and corn stover were compared at different blending ratios. The TG-DTG curves indicate that the optimal performance is achieved when the corn straw blending ratio is 20%. Furthermore, the TG-FTIR coupling results demonstrated an increase in gas species as the blending ratio increased. The composition analysis of ash samples formed at various combustion temperatures using XRD and XRF indicated that a portion of KCl in the fuel was released as volatile matter, while another part reacted with Al2O3 and SiO2 components in the slime to form silica–aluminate compounds and other substances. Notably, interactions between the components of slime and potassium elements in corn stover primarily occurred within the temperature range of 800–1000 °C. These findings contribute to a comprehensive understanding of biomass and coal co-firing combustion chemistry, offering potential applications for enhancing energy efficiency and reducing emissions in industrial processes.
1. Introduction
Carbon peaking is the point at which a region or country’s CO2 emissions reach their highest level before starting to decline. In the context of China’s “Peak Carbon”, the rational and clean utilization of coal resources is of paramount importance [1]. Slime, a by-product of coal production and washing process [2], possesses characteristics such as fine particle size, high water content, high viscosity, high water holding capacity, high ash content, low calorific value, and difficulties in transportation and usage [2,3]. The accumulation of slime not only leads to environmental pollution but also results in resource wastage [4]. In a carbon-constrained world, the heavy reliance on coal has necessitated the exploration of alternative, carbon-neutral energy sources. Biomass waste currently constitutes 14% of the global energy production [5]. Co-combustion of biomass with slime has proven to be a promising way to support the transition from fossil to renewable energy carriers. Straw-based biomass contains significant amounts of K (potassium), Na (sodium), and Cl (chlorine) compared with slime. Burning large quantities of biomass leads to the formation of alkali chlorides, which condense into fly ash or aerosols. These particles are subsequently deposited on heat transfer surfaces through chemical reactions or physical transformations [6]. Chlorine and alkali metals in ash and gas can cause complex metal reactions, leading to severe corrosion of heat transfer surfaces [7,8]. One of the key factors affecting the feasibility of this technology is the high alkali metal content of biomass.
In recent years, the combustion characteristics, kinetic analysis, and pollutant emissions of slime mixed with biomass have been widely studied, for example, Wang [9] found that increasing the furnace temperature or increasing the ratio of sawdust to Shanxi slime could accelerate the combustion of the mixed samples to shorten the ignition delay and burnout time; Yang [10] discovered the combustion reactivity of anthracite was enhanced by mixing acacia charcoal with a lower degree of order, a higher degree of defects and a more developed porous structure; and Ni [11,12] reported that combustion of rice husk mixed with slime could change the mechanism of the combustion process, using cellulose and lignin mixed with slime found that the overall combustion performance increased exponentially with the addition of cellulose and lignin to the slime. Mixed combustion of biomass and slime solved the deficiencies of slime in combustion characteristics and pollutant gas emissions to a certain extent, but further research on the mechanism is needed. Liu [13] found that the higher the proportion of corn stover in the mixing process of quandong coal and corn stover, the earlier the peak concentration of alkali appears, and the peak concentration of sodium decreases the increase in the peak concentration of potassium; Zhang [14] proposed a migration mechanism for depolymerized silica aluminates, where alumina replaces silicon to form aluminum tetrahedra while promoting combustion by bringing K+ to the interface. This is because the combustion of carbon particles on the slag surface leads to the phenomenon of temperature increase; Zhou [15] suggested that elements such as Al and Si in coal may react with K in biomass to form basic alumino-silicates, resulting in more K remaining in the ash; Song [16] demonstrated that the reaction temperature and the ratio of Al to Si from coal minerals are the key issues for potassium redistribution in biomass; Wang [17] reviewed the occurrence of synergistic effects in the pyrolysis of coal and biomass as a result of the catalytic effect of potassium; Xue [18] investigated the reaction mechanism involving coal, straw, and kaolin. The results indicated that kaolin transfers potassium to fly ash and bottom solids, negatively impacting potassium release, with this effect increasing at higher temperatures; He [19] investigated the transformation morphology of potassium under steam and CO2 atmospheres. The results showed that the transformation morphology of potassium was affected under different atmospheres. Recent studies have focused on the interactions of elements during mixed combustion, particularly the roles of alkali and alkaline earth metals in the combustion of biomass and slime. This paper specifically examines the morphology and migration process of potassium in slime and corn stover.
The present study investigates the combustion characteristics of slime and biomass in an air atmosphere, examining the correlation between combustion variations and potassium content in biomass, as well as elucidating the specific migration process of potassium during combustion. The primary objective of this research is to elucidate the role played by potassium in the co-combustion of slime and biomass, thereby providing theoretical guidance for enhancing slime utilization and addressing low-carbon environmental protection requirements.
2. Materials and Methods
2.1. Raw Materials
Corn stover was purchased from Huifeng Straw Agricultural Products Deep Processing Plant and the particle size was less than 74 um. The slime samples were selected from the flotation tailings of Yulin Hebi Coal Selection Plant, and the particle size was less than 149 um. Table 1 presents the results of the elemental and industrial analysis of these two raw materials by elemental analyzers (the Vario MACRO cub, Germany) and industrial analyzers (SX2-10-12N, Shanghai Yiheng, Shanghai, China). The % in Table 1 is the dry basis of the fuels.

Table 1.
Elemental and industrial analysis of fuels.
As shown in Table 1, slime had a dry basis ash content of 37.15% and corn stover 6.42%; the ash content and fixed carbon content of slime were larger than that of corn stover; and the volatile fraction of corn stover was 60.58%, while the volatile fraction of slime was 22.48%, and the volatile fraction content of corn stover was nearly three times that of slime.
Table 2 shows the comparative elemental content analysis of the two raw materials by X-ray fluorescence spectroscopy (Panalytical Zetium, The Netherlands).

Table 2.
Comparative analysis of elemental content of fuels.
From Table 2, it can be seen that slime and corn stover also have a great difference in elemental content. The main element of the two fuels is Si, but the K content of corn stover is second, and the Al content of slime is second.
2.2. Preparation of Raw Materials
Corn stover and slime were mixed according to the mixing ratios of 20%, 40%, and 60% of corn stover, with a total of 10 g. In the experiment, 2 g, 4 g, and 6 g of corn stover were weighed and mixed with 8 g, 6 g, and 4 g of slime, respectively. The samples were put into an onyx mortar and ground for not less than 15 min to ensure that the samples were well mixed. The samples with a blending ratio of 20% and the raw materials slime and corn stover were subjected to ash sampling. The samples were burned in a muffle furnace at 300 °C, 500 °C, 800 °C, and 1000 °C to produce ash samples with the following firing process: the samples were raised to the target temperature at room temperature with a heating rate of 10 °C/min, and the samples were kept warm for 3 h at the target temperature so that the organic matter was fully combusted.
2.3. Methods of Analysis
2.3.1. Thermogravimetric Analysis
To investigate the combustion characteristics of two raw materials and blended fuels obtained at the same rate of heating. Thermal analysis experiments were carried out on coal using a comprehensive thermal analyzer (TGA 550, USA). Five fuels at different blending ratios were prepared, weighed (10 ± 0.5 mg), and placed in a crucible to prepare for the test. The testing conditions of the instrument were starting temperature of 30 °C, termination temperature of 1000 °C, heating rate of 20 °C/min, and air was used as a gas in the test chamber. The temperature accuracy and sensitivity of the thermogravimetric analyzer were 0.1 °C and 0.1 μg, respectively.
2.3.2. XRF Analysis of Fuel Ash Samples under Different Conditions
In order to investigate the changes in the elemental content of the ash samples at different temperatures for each blending ratio, the ash samples were tested and analyzed by X-ray fluorescence spectrometer (Panalytical Zetium, The Netherlands) under each condition.
2.3.3. XRD Analysis of Fuel Ash Samples
To investigate the physical phase changes in the ash samples under various conditions, the ash samples were analyzed by XRD (XRD-6000, Japan), with the XRD test conditions: the test angle of 5–90° and the scanning speed of 5°/min. Then, based on the analysis results and related literature, a series of evolution mechanisms of the blended materials at different temperatures were proposed.
2.3.4. Thermogravimetric and Infrared Coupling
To investigate the gas composition of the fuel co-combustion in the combustion process, it was tested through TG-FTIR (Netzsch STA-2500, Germany/Thermo Fisher IS-50, USA) coupled with the following test conditions: thermogravimetric test conditions were the same as those in Section 2.3.1, and the wavelength range of infrared test was 400–4000 cm−1, with a resolution of 2 cm−1. The scanning time interval was 11 s.
2.4. Combustion Characterization Parameters
The ignition temperature (Ts, °C) and the burnout temperature (Tf, °C) are important parameters used to evaluate the combustion characteristics of the fuel and are obtained from the TG and DTG curves [20,21]. The combustion performance of the fuel was evaluated by the combined combustion index (S, %2/(°C3 min2)), and Equation (1) was used for the calculation.
where Ts is the ignition temperature; Tf is the burnout temperature; (dω/dt)mean is the average mass loss rate (%/min); (dω/dt)max is the maximum mass loss rate (%/min).
S = ((dω/dt)max(dω/dt)mean)/(TS2Tf)
The TG-DTG tangent method is used to determine Ts and Tf, and the specific steps are shown in Figure 1a: firstly, the TG curve and the DTG curve of the sample are made in the same coordinate axis, and a straight line perpendicular to the x-axis is made through the point A, intersecting with the TG curve at a point B. The temperature corresponding to point B represents the temperature of maximum combustion rate. Extension of the curve and the initial mass of the TG curve intersect at a point C, the point corresponding to the temperature that is Ts. Determination of Tf follows a similar procedure as Ts: a straight line passing through point B intersects with the graph at point D, and extending this line downwards to intersect with x-axis gives Tf. If multiple peaks exist in the weight loss graph, ignition temperature is determined based on the first peak, while burning-out temperature is considered beyond the last peak.
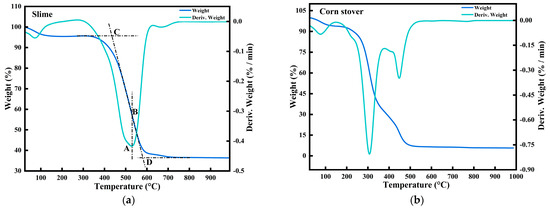
Figure 1.
Thermogravimetric profile of slime and corn stover combustion alone: (a) slime and (b) corn stover.
3. Results and Discussion
3.1. Analysis of Combustion Results
3.1.1. Separate Combustion of Slime and Corn Stover
The combustion reaction is divided into four stages. The first stage is the dehydration stage; this stage generally occurs at about 100 °C; the second stage is the precipitation and combustion of volatile substances, which to some extent serves to ignite the fixed carbon, and this stage accounts for about 1/10th of the total combustion time; the third stage is mainly the combustion of the fixed carbon, which is slower and lasts for a longer period of time; the fourth stage is the decomposition of the minerals and the combustion of the residual fixed carbon, the strength of the reaction mainly depends on the nature of the combustion material, such as the ash composition of the coal. Next, the combustion performance will be examined from the second stage.
Figure 1a shows the thermogravimetric curve of the mucilage. The combustion of the mucilage shows a clear combustion peak of fixed carbon, and due to the low volatile content of the mucilage of about 20%, there is no obvious combustion peak during combustion. From the TG curve, it can be seen that the slime starts to lose weight at about 300 °C and, with the increase in temperature, reaches the maximum weight loss corresponding to the peak of the DTG curve at about 520 °C. Figure 1b shows the thermogravimetric curve of corn stover. It can be seen that corn stover shows two peaks during combustion from the DTG curve, which is due to the fact that biomass consists mainly of cellulose, hemicellulose, and lignin. Biomass combustion first undergoes pyrolysis of the biomass. Hemicellulose and cellulose burn relatively easily, lignin is the most difficult to burn and has the longest reaction time. Hemicellulose burns mainly as volatiles, and lignin burns mainly as coke. Table 1 shows that the volatile content of slime is much smaller than that of corn stover. The combustion process of volatile analysis is almost simultaneous with the combustion of coke, so the curve change is not obvious in the combustion stage of volatile analysis.
3.1.2. Combustion of Slime Mixed with Corn Stover
Figure 2 is the thermogravimetric profiles of slime and corn stover according to the blending ratios of 20%, 40%, and 60% of corn stover.
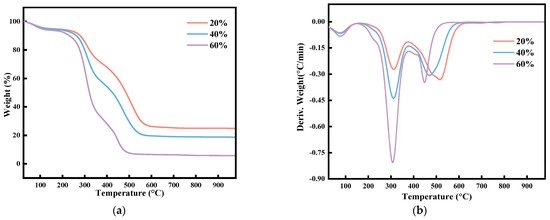
Figure 2.
Thermogravimetric profile of mixed combustion of slime and corn stover: (a) TG curve and (b) DTG curve.
Figure 2 shows that the thermogravimetric profile after blending corn stover is significantly different from burning coal slime alone (Figure 1a). This is because the ignition point of corn stover is lower than the slime, and the early combustion of corn stover provides thermal energy for the subsequent combustion of the slime. Thus, the ignition point of slime is advanced. The TG and DTG curves of the combustion of corn stover mixed with slime in different proportions have similar trends, but the magnitude of the values is different. From the TG curve, at the same temperature, the larger the mixing proportion corresponds to the smaller weight percentage, indicating that the weight loss is becoming more and more obvious. At the same time, as the corn stover ratio increases, the TG curve shifts to the low-temperature region. The blended fuel is easier to ignite, burns to a high degree, and leaves less final residue. From the DTG curve, as the blending ratio increases, the peak of volatile matter becomes larger and wider, the peak of fixed carbon becomes smaller and narrower, and the temperature of the maximum rate of combustion of fixed carbon decreases with the increase in the blending ratio. Table 3 is obtained after analyzing the aspects of ignition temperature and combustion exhaustion temperature in these three cases.

Table 3.
Combustion characteristics of slime and corn stover at different blending ratios.
Table 3 shows that the ignition temperature decreases as the mix ratio increases. The mixing ratio is 0, which means that the ignition temperature of coal slurry burned alone is 439.52 °C. The addition of corn stover reduces the ignition temperature by about 50 °C. The ignition temperature decreases with the addition of corn stover. There is no significant change in the ignition temperature when the mix ratio is 60 percent compared to 40 percent. At this time, corn stover accounted for a larger proportion of the corn stover, corn stover characteristics are reflected, combustion volatile fraction is in the dominant position because corn stover contains potassium, and alkali metals can catalyze the combustion of coal. The silica–alumina oxides in the coal slime interact with potassium in favor of reducing ash deposition during co-firing [22], resulting in a decrease in the final residual mass percentage with an increase in the blending ratio.
3.2. Fuel Gas Products
Figure 3 illustrates the evolution of gaseous products during the co-combustion of slime and corn stover by using thermogravimetric and infrared measurements of gas functional groups at different blending ratios.
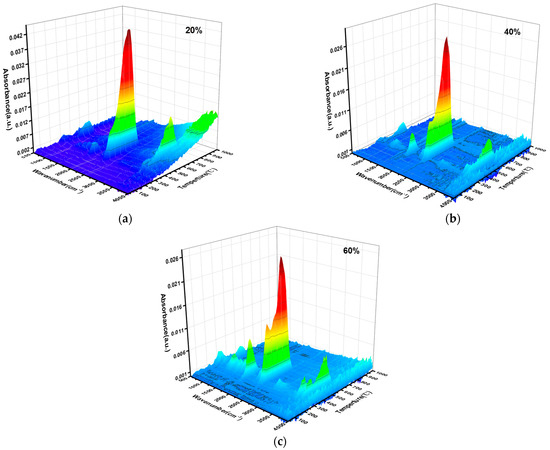
Figure 3.
TG-FTIR profiles of gases released during combustion at different blending ratios: (a) blend ratio 20%, (b) blend ratio 40%, and (c) blend ratio 60%.
In Figure 3, the gaseous products and major weight loss peaks mainly occur in the temperature interval of 300~700 °C. Thus, the main combustion processes of the blended fuels occur in this interval. According to the related FTIR literature [23,24], Figure 3 indicates the presence of distinct absorption peaks in the wave number ranges of 1673~1850 cm−1 and 2259~2420 cm−1, which are caused by the contraction vibrations of the carbonyl group, and the product is CO2, the first of peak is from the decomposition of the organic matter, and the second peak is produced due to the combustion of the coke or the decomposition of CaCO3, and the outputs of the two gaseous products are in agreement with the weight loss process in Figure 2a. The peaks at 3500~4000 cm−1 are -O-H absorption peaks, indicating that water, phenols, carboxylic acids, and alcohols were produced during the combustion process. The H2O in corn stover combustion comes from two main sources: the release of free and bound water and the reaction of volatile compounds produced by thermal decomposition with oxygen. The absorption peaks of oxidizable compounds were not obvious, such as C-O, C=O, and CO, which was because of the fact that a large number of volatile compounds were oxidized to carbon dioxide at high temperatures, and only a small portion of the large molecule volatile compounds were not burned. The first peak of CO2 was enhanced with the increase in the mixing ratio of corn stover in the slime, which was due to the increase in the content of readily decomposable compounds and the increase in CO2 released during the heating process. In addition, the peaks of other organic compounds appeared gradually. The higher the biomass content, the more types of gaseous products were detected by FTIR [25]. This is due to the fact that a large number of volatile compounds are separated and combusted, creating an oxygen-deficient environment in which a large number of volatile compounds cannot be oxidized. The higher the slurry content, the faster the volatile compounds are released, and the more unoxidized organic compounds are detected by FTIR.
3.3. Compositional Analysis of Fuel Ash Samples
X-ray fluorescence spectroscopy analysis of the ash samples was prepared at 300 °C, 500 °C, 800 °C, and 1000 °C at a blending ratio of 20%.
Figure 4 presents that the main oxides in slime are SiO2, Al2O3, and Fe2O3. while the main oxides in corn stover are SiO2 and K2O in addition to Na2O, CaO, MgO, SO3 and P2O5. Figure 4a illustrates that with the increase in temperature, the content of SiO2 and Al2O3 increases at 300~800 °C because the content of Fe2O3 and CaO decreases, making its proportion relatively increase at 800~1000 °C but shows the opposite trend. The reason for this phenomenon is that Fe2O3 and CaO react with SO3 and Cl in the material during combustion to form substances such as CaSO4 and FeCl2. In Figure 4b, with the increase in the ashing temperature, the content of SiO2 and Al2O3 basically increases, and the content of K2O gradually decreases, in combination with the thermogravimetric curves of corn stover during combustion, it can be guessed that potassium is expected to be released during combustion in two ways [26]: (1) The sample decomposes as water and vaporized material are expelled from the pores. (2) Elevated particle temperatures increase the vapor pressure of low melting point potassium compounds. Reacting with other components during the volatile fraction and fixed carbon combustion phase in corn stover, K2SO4 appears through sulfation by KCl [27]. Compared with Figure 4b,c, the content of K2O in the ash sample after combustion of corn stover blended with slime increased at 1000 °C, which may be due to the fact that some of the elemental K enters into the crystals of Al2O3 and SiO2 to form KAlSi3O8 when the combustion temperature of corn stover reaches more than 800 °C. Combined with the XRD profiles of the combusted ash samples to analyze the elemental migration during combustion, Figure 5 shows the XRD patterns of the ash samples at different temperatures.
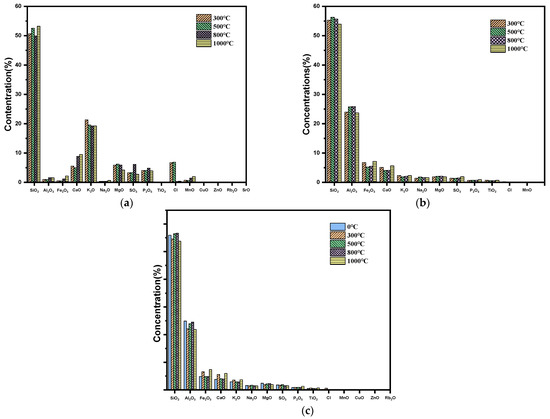
Figure 4.
XRF analysis pattern of gray sample: (a) corn stover, (b) coal slime, and (c) mixture of corn stover and coal slime.
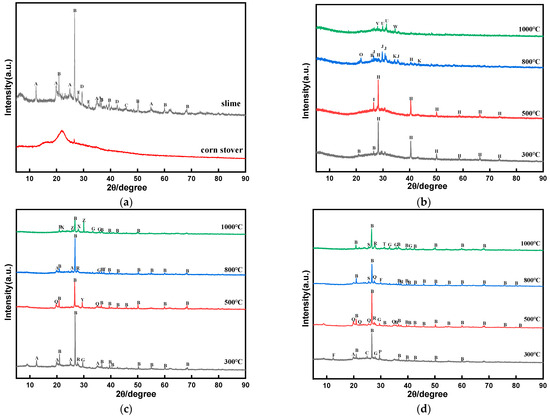
Figure 5.
XRD patterns of ash samples after different combustion temperatures: (a) raw materials, (b) corn stover, (c) coal slime, and (d) mixture of corn stover and coal slime. A—Al4(OH)8(Si4O10); B—SiO2; C—MgFeSiAlOOH; D—Mg7Si8O22(OH)2; E—Pb4(S2O3)O2(OH)2; F—Al2Si2O5(OH)4; G—Al2Si2O5; H—KCl; I—SnO2; J—CaMgSi2O6; K—Al2O3; O—KAlSiO4; P—CaCO3; Q—KAl2(AlSi3O10)(OH)2; R—KAlSi3O8; S—TiO2; T—Pb2O(SO4); U—CaSiBO5H; V—K2Ca2Mg(SO4)4; W—Si3N4; X—Na(AlSi3O8); Y—Al2(SiO4)(OH)2; Z—KAl3(SiO4)(OH)6.
Figure 5a illustrates that no distinct peaks appear for corn stover, indicating that no crystals are produced. It was shown that element K is mainly bound to organic matter in biomass and exists as potassium in an amorphous phase [28]. The main components of slime are quartz (SiO2), kairomite (Al4(OH)8(Si4O10)) and some other impurities. Figure 5b illustrates that the main components of slime after combustion at 300 °C are quartz (SiO2), kaolinite (Al2Si2O5), and diorite (Al4(OH)8(Si4O10)), while potassium feldspar (KAlSi3O8) appeared in the ash of slime at 800 °C, which indicates that in the process of ashing, the minerals in the slurry will change accordingly, and at 1000 °C, potassium feldspar (KAlSi3O8) and alunite (KAl3(SiO4)(OH)6) and other substances, so coal slime may undergo the following transformation of substances.
CaCO3 → CaO + CO2
2CaCO3 + 2SO2 + O2 → 2CaSO4 + 2CO2
Al2Si2O5(OH)4 → Al2O3 + 2SiO2 + 2H2O
Figure 5c presents that corn stover at 300 °C its main components are KCl and SiO2 composition. As the temperature increases, the intensity of the KCl peak becomes smaller and smaller, which indicates that the water-soluble KCl will be gradually released into the gas phase or converted into silica–aluminate of K with the increase in temperature. The KCl release process will be influenced by other elements in the biomass, which will promote the release of K in the form of KCl. During combustion, the process of potassium release is affected by other elements in the biomass, such as Cl in the form of KCl, which facilitates K release. At 800 °C, its main component is CaMgSi2O6, and the peak of diffraction peak of KCl is almost invisible because KCl is almost completely evaporated at 700–800 °C, and when it reaches 1000 °C its main component no longer has KCl.
KCl → Volatilization
CaO + MgO+ 2SiO2 → CaMgSi2O6
Figure 5d shows when the slime is mixed with corn stover at 300 °C, the main component is SiO2, accompanied by MgFeSiAlOOH, Al2Si2O5, Al4(OH)8(Si4O10), and Al2Si2O5(OH)4. When the temperature reaches 500~800 °C, there are substances such as mica (KAl2(AlSi3O10)(OH)2) and so on. When the temperature reached 1000 °C, the main substances such as Pb2O(SO4) and Al2Si2O5 appeared the same as in the coal slurry ash samples alone, which may be due to the fact that the coal slurry plays a dominant role when the mixture of corn stover is relatively small.
Al4(OH)8(Si4O10) → 2Al2O3 + 4SiO2 + 8H2O
KAl2(AlSi3O10)(OH)2 + SiO2 → KAlSi3O8 + Al2O3•SiO2 + H2O
2KCl + Al2O3 +3SiO2 + H2O → 2KAlSi3O8 + 2HCl
6KCl + 3Al2Si2O5 + 6SiO2 + 3H2O → 6KAlSi3O8 + 6HCl
It can be inferred that a series of chemical reactions occurred between the minerals during the combustion of coal slime and corn stover into ash; the possible reaction mechanisms are shown in Figure 6. The numbers in the graph are combustion temperatures in °C.
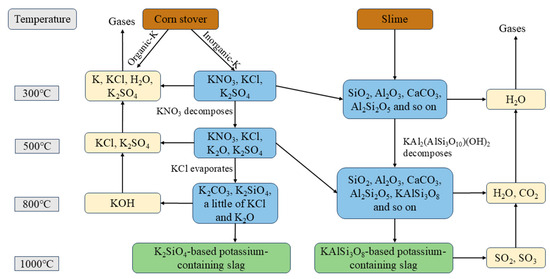
Figure 6.
Possible transformation pathways of elemental K during combustion of slime and corn stover.
In Figure 6, inorganic potassium in corn stover mainly exists in the form of KNO3, KCl, K2SO4, and during combustion KNO3 decomposes above 450 °C, and KCl is almost completely evaporated at 700~800 °C, during which it reacts with Al2O3 and SiO2 in the slime to form KAl2(AlSi3O10)(OH)2 and a potassium-containing ash slag dominated by KAlSi3O8.
4. Conclusions
- (1)
- The addition of corn stover reduced the ignition temperature of the coal slurry by about 50 °C. The ignition temperature increased continuously with the increase in the blending ratio of corn stover. After the corn stover blending ratio was 40%, the ignition temperature was basically unchanged. By examining the comprehensive combustion characteristic curve under the corn stover blending ratio of more than 40%, the best combustion effect was obtained when the blending ratio was 20%.
- (2)
- The gaseous products during the combustion process were monitored in real time by TG-FTIR coupling. The formation of these gaseous products primarily occurred within the temperature range of 300–700 °C, which coincided with the main weight loss peaks observed during combustion. CO2 was identified as the predominant gaseous product. As the mixing ratio increased, the combustion process produced a greater variety of gaseous products. This can be attributed to the higher mixing ratio of corn stover, which leads to the release and combustion of a large amount of volatile compounds, creating an oxygen-deficient environment. As a result, many volatile compounds are not fully oxidized. Additionally, with an increase in corn stover content, the rate of volatile compound release accelerated, resulting in more unoxidized organic compounds being detected by FTIR.
- (3)
- The influence of combustion temperature on the release and transformation of K in the process of mixed combustion is significant, as evidenced by XRD and XRF analysis of the composition of the ash samples after combustion at different temperatures. The K element primarily combines with the organic matter in the corn stover to form the amorphous form of potassium. With the increase in combustion temperature, a fraction of the KCl is released in the form of volatile matter, and another fraction is combined with the components of coal slime, such as Al2O3 and SiO2, to generate silica–aluminate. On one hand, the substances in the coal slime play the role of fixing potassium, on the other hand, the release of volatile matter promotes the coal slime combustion. The formation of potassium-containing complexes between potassium in corn stover and coal slime mainly occurs in the temperature range of 800~1000 °C.
Author Contributions
Conceptualization, J.Z.; methodology, C.C.; software, J.Z.; validation, J.Z.; formal analysis, J.Z.; investigation, J.Z.; resources, C.C.; data curation, J.Z.; writing—original draft preparation, J.Z.; writing—review and editing, C.C.; visualization, T.G. and M.Z.; supervision, Tao Ge and M.Z.; project administration, C.C.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Research Project of Anhui Educational Committee (Grant No. 2023AH051191), National Natural Science Foundation of China (Grant No. 52174231), Anhui Province Coal Clean Processing and Carbon Reduction Engineering Research Center Foundation (Grant No. CCCE-2023007).
Data Availability Statement
The data that were used are confidential.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xing, Z.; Zhao, S.; Guo, W.; Meng, F.; Guo, X.; Wang, S.; He, H. Coal resources under carbon peak: Segmentation of massive laser point clouds for coal mining in underground dusty environments using integrated graph deep learning model. Energy 2023, 285, 128771. [Google Scholar] [CrossRef]
- Dong, C.; Qi, Y.; Nemet, G. A government approach to address coal overcapacity in China. J. Clean. Prod. 2021, 278, 123417. [Google Scholar] [CrossRef]
- Du, M.; Wang, S. Investigation of the segregation of a binary particle mixture in a square circulating fluidized bed with air staging. Particuology 2019, 47, 70–76. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, S.; Xia, D.; Wang, L.; Lv, J.; Yu, H.; Jiao, X. Efficient utilization of coal slime using anaerobic fermentation technology. Bioresour. Technol. 2021, 332, 125072. [Google Scholar] [CrossRef]
- Balraj, A.; Krishnan, J.; Selvarajan, K.; Sukumar, K. Potential use of biomass and coal-fine waste for making briquette for sustainable energy and environment. Environ. Sci. Pollut. Res. 2021, 28, 63516–63522. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, W.; Zhou, J.; Yu, Z. Effects of S and Al on K Migration and Transformation during Coal and Biomass Co-combustion. ACS Omega 2022, 7, 15880–15891. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Wang, Y.; Li, S.; Wei, X. Release of alkali metals during co-firing biomass and coal. Renew. Energy 2016, 96, 91–97. [Google Scholar] [CrossRef]
- Zhihua, W.; Siyu, L.; Wubin, W.; Yong, H.; Marcus, A.; Zhongshan, L. Alkali metal release in thermochemical conversion of biomass and coal: Optical measurements and modeling. Prog. Energy Combust. Sci. 2023, 100, 101131. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Zhao, K.; Yang, H.; Chen, J. Combustion characteristics of spherical particles mixed with coal slime and sawdust. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 535–549. [Google Scholar] [CrossRef]
- Guang, Y.; Yiran, L.; Lijuan, G.; Yingjie, S. Investigation of the synergistic effect and kinetic behavior of anthracite and biochar during co-combustion process in pure oxygen atmosphere. J. Energy Inst. 2021, 101, 1–18. [Google Scholar] [CrossRef]
- Zhanshi, N.; Haobo, B.; Hao, S.; Xiang, L.; Junjian, T.; Yurou, Y.; Liqun, H.; Kesheng, M.; Qizhao, L. Effect of lignin on coal slime combustion characteristics and carbon dioxide emission. J. Clean. Prod. 2024, 441, 140884. [Google Scholar] [CrossRef]
- Zhanshi, N.; Zhihui, S.; Haobo, B.; Chunlong, J.; Hao, S.; Zhicong, Q.; Liqun, H.; Qizhao, L. The effect of cellulose on the combustion characteristics of coal slime: TG-FTIR, principal component analysis, and 2D-COS. Fuel 2022, 333, 126310. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Wang, Z.; Xia, J.; Wan, K.; Whiddon, R.; Cen, K. Characteristics of alkali species release from a burning coal/biomass blend. Appl. Energy 2018, 215, 523–531. [Google Scholar] [CrossRef]
- Haigang, Z.; Zhongjie, S.; Jianliang, X.; Qinfeng, L.; Zhenghua, D.; Haifeng, L. New insight to migration and influence of potassium element on combustion of coal/biomass char-slag interface. Combust. Flame 2023, 257, 112969. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Zhong, W.-q.; Yu, Z.-w. Migration and transformation law of potassium in the combustion of biomass blended coal. J. Fuel Chem. Technol. 2020, 48, 929–936. [Google Scholar] [CrossRef]
- Song, Y.C.; Li, Q.T.; Li, F.Z.; Wang, L.S.; Hu, C.C.; Feng, J.; Li, W.Y. Pathway of biomass-potassium migration in co-gasification of coal and biomass. Fuel 2019, 239, 365–372. [Google Scholar] [CrossRef]
- Wei, W.; Romain, L.; Ammar, B.; Denis, L. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Zeyu, X.; Zhaoping, Z.; Ping, L.; Feihong, G. Capture effect of K and Pb by kaolin during co-firing of coal and wheat straw: Experimental and theoretical methods. Fuel 2023, 360, 105479. [Google Scholar] [CrossRef]
- Zi-Meng, H.; Yu-Jie, D.; Jing-Pei, C.; Xiao-Yan, Z. Agglomeration and transformation of different types of inorganic potassium in biomass during co-gasification with coal. Fuel 2023, 357, 129728. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, H.; Yang, T.; Zhu, Y.; Li, R. Combustion Characterization and Kinetic Analysis of Mixed Sludge and Lignite Combustion. ACS Omega 2024, 9, 6912–6923. [Google Scholar] [CrossRef]
- Zhuikov, A.V.; Glushkov, D.O. Characteristics of the Joint Combustion of Brown Coal and Sewage Sludge under Nonisothermal Heating Conditions. Solid Fuel Chem. 2022, 56, 353–359. [Google Scholar] [CrossRef]
- Dayton, D.C.; Belle-Oudry, D.; Nordin, A. Effect of Coal Minerals on Chlorine and Alkali Metals Released during Biomass/Coal Cofiring. Energy Fuels 1999, 13, 1203–1211. [Google Scholar] [CrossRef]
- Tao, J.; Liang, R.; Li, J.; Yan, B.; Chen, G.; Cheng, Z.; Li, W.; Lin, F.; Hou, L. Fast characterization of biomass and waste by infrared spectra and machine learning models. J. Hazard. Mater. 2019, 387, 121723. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.; Yoo, C. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Xinjie, L.; Shihong, Z.; Xincheng, W.; Jinai, S.; Xiong, Z.; Xianhua, W.; Haiping, Y.; Hanping, C. Co-combustion of wheat straw and camphor wood with coal slime: Thermal behaviour, kinetics, and gaseous pollutant emission characteristics. Energy 2021, 234, 121292. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Xia, J.; Vervisch, L.; Wan, K.; He, Y.; Whiddon, R.; Bahai, H.; Cen, K. Measurement and kinetics of elemental and atomic potassium release from a burning biomass pellet. Proc. Combust. Inst. 2018, 37, 2681–2688. [Google Scholar] [CrossRef]
- Deng, L.; Jiang, J.; Tie, Y.; Ma, S.; Fan, G.; Zhu, T.; Che, D. Potassium transformation and release during biomass combustion. Can. J. Chem. Eng. 2022, 101, 337–346. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Yang, X.; Guo, S.; Zhan, H.; Zhang, Y.; Fang, Y. Influence of coal ash on potassium retention and ash melting characteristics during gasification of corn stalk coke. Bioresour. Technol. 2018, 270, 416–421. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).