Abstract
Lithium, a silver-white alkali metal, with significantly high energy density, has been exploited for making rechargeable lithium-ion batteries (LiBs). They have become one of the main energy storage solutions in modern electric cars (EVs). Cobalt, nickel, and manganese are three other key components of LiBs that power electric vehicles (EVs). Neodymium and dysprosium, two rare earth metals, are used in the permanent magnet-based motors of EVs. The operation of EVs also requires a high amount of electricity for recharging their LiBs. Thus, the CO2 emission is reduced during the operation of an EV if the recharged electricity is generated from non-carbon sources such as hydroelectricity, solar energy, and nuclear energy. LiBs in EVs have been pushed to the limit because of their limited storage capacity and charge/discharge cycles. Batteries account for a substantial portion of the size and weight of an EV and occupy the entire chassis. Thus, future LiBs must be smaller and more powerful with extended driving ranges and short charging times. The extended range and longevity of LiBs are feasible with advances in solid-state electrolytes and robust electrode materials. Attention must also be focused on the high-cost, energy, and time-demand steps of LiB manufacturing to reduce cost and turnover time. Solid strategies are required to promote the deployment of spent LiBs for power storage, solar energy, power grids, and other stationary usages. Recycling spent LiBs will alleviate the demand for virgin lithium and 2.6 × 1011 tons of lithium in seawater is a definite asset. Nonetheless, it remains unknown whether advances in battery production technology and recycling will substantially reduce the demand for lithium and other metals beyond 2050. Technical challenges in LiB manufacturing and lithium recycling must be overcome to sustain the deployment of EVs for reducing CO2 emissions. However, potential environmental problems associated with the production and operation of EVs deserve further studies while promoting their global deployment. Moreover, the combined repurposing and remanufacturing of spent LiBs also increases the environmental benefits of EVs. EVs will be equipped with more powerful computers and reliable software to monitor and optimize the operation of LiBs.
1. Introduction
Electric vehicles (EVs) have been advocated as game changers to tackle climate change and greenhouse gas CO2 emissions. EVs do not emit tailpipe CO2, nitrogen oxides (NOx), CO, SO2, etc. and the elimination of such gases is critical in the urban environment. EVs only need smaller engines without the cooling unit, gear shift, clutch, etc. compared to internal combustion engine vehicles (ICEVs). So, EVs produce minimal noise and require low maintenance. Some pros and cons of EVs are summarized in Table 1. General information on lithium battery features, system design, and safety, is described in Ref. [1].

Table 1.
Key advantages and disadvantages of EVs.
The deployment of EVs is anticipated to limit global warming to below 1.5–2 °C, in agreement with the Paris Agreement’s targets to abandon the current fuel-intensive energy system. With this ambitious material-intensive plan, only EVs equipped with massive batteries are allowed to hit the roads. Such batteries are fabricated from lithium and other expensive materials metals. The design of LiBs should address two important safety points: short circuitry and potential battery explosion. Petrol-powered and diesel cars might face extinction as General Motors and Audi of Germany aim to stop selling such cars by 2033–2035. The production of diesel/petrol cars will cease in the UK after 2030 and other automotive multinationals might follow this path. Multinationals are racing to secure the purchase of lithium, a principal “white oil” component in rechargeable batteries.
Like fossil oil, lithium is not renewable, with about 14 million tons on land, compared to 230 million tons in seawater. However, there are some conflicting reports about lithium reserves, which could be up to 22 or even 80 million tons. Lithium is dissolved in brine, whereas rock and clay also have it as solid minerals. Lithium is the least dense metal, which can store voluminous energy to its weight, a critical parameter for developing powerful batteries. The rush for lithium might trigger some environmental issues in industrial production and the operation of massive EVs. The lithium content in seawater is fairly dilute, 0.1–0.2 ppm (mg/L), i.e., its isolation from seawater is not economically viable with current technology. Based on the increasing manufacturing of EVs, lithium from proven reserves will run out in 2080, a scenario similar to crude oil [5]. Lithium must be mined, isolated, and purified for making car batteries, and this is an energy-consuming step toward the fabrication of EVs.
For the global deployment of EVs, three important points must be addressed: (i) the manufacturing process with carbon footprint; (ii) the operation of EVs with electricity charge; and (iii) the reuse, recycling, or disposal of batteries. Such information is critical to assess the sustainability and environmental impact of EVs. This paper will address the technical challenges and performance of EVs concerning global warming and CO2 reduction. The paper also discusses the feasible recycling of spent LiBs and the extraction of lithium from seawater. There is a paradox between “clean” EVs versus “dirty” mining of lithium and other metals with CO2 footprints. Another important factor is whether the electricity required for the operation of EVs can be generated from carbon-free sources. This paper focuses on EVs, which are propelled by only electric power; therefore, other types of EVs such as plug-in hybrid EVs (PHEVs) [6,7], hybrid EVs [8], fuel cell EVs [9], and extended-range EVs [10] are excluded in this review.
2. Scope of the Review
This review focuses on the operation of EVs concerning the reduction of CO2 emissions as it is highly dependent on the electricity generated during their production and use stage. The review also highlights some technical challenges and social concerns regarding the extraction of lithium and other metals. The recycling aspect of lithium batteries is also discussed together with the development of solid-state electrolytes and batteries without lithium. Scopus, Google Scholar, Research Gate, and Web of Science were used to search for pertinent papers related to the fabrication of lithium batteries and their limitation. The filter for document type was set to “Article” and “Review”. Reference lists were then examined to broaden the selection of highly cited older literature and include them in the reference list. A similar methodology was adopted to look at the mining of lithium, cobalt, other metals, etc. Some features of spent batteries, natural resources for lithium, other metals, and rare earth metals were searched from “open-access” sources and different websites. The search was conducted with the following keywords: electric vehicles; lithium battery; lithium mining/extraction; recovery of spent lithium batteries; batteries with cobalt; nickel, and manganese; rare earth metals, life cycle analysis; lithium-cobalt, nickel reserves, etc. Information on a specific EV is cited from its official website.
This review updates the global perspectives on EVs powered by LiBs, lithium mining, reuse and recycling of spent batteries, repurposing of batteries, and future advances in battery manufacturing. The content of this paper will be useful to researchers and scholars in various disciplines in comprehending the current status of the manufacturing and operation of EVs. The review also helps policymakers familiarize themselves with technical challenges and social concerns in planning their policies during the transition period of decarbonization.
3. General Aspects of EVs
There are some pertinent surveys related to the production of EVs and their sales, efficiency/capacity of EVs, charging methods, battery diagnosis, environmental impacts, etc. Detailed information on these subjects is available in the literature and will not be repeated here due to space limitations (Table 2).

Table 2.
Literature surveys on the production, performance, and other features of EVs.
At first glance, EVs equipped with batteries run on electricity; therefore, they produce no CO2 emissions as encountered with internal combustion engine vehicles (ICEVs). As cars often last over 5 years, LiBs must be designed to function for several years. Ideally, an EV must have a minimum range of over 300 miles with a minimal charging time, of 30 min or less. Among various types of batteries, lithium-ion batteries (LiBs) appear to meet such requirements, albeit further improvements to reduce the production cost and extend their lifetime are urgently needed. In brief, car batteries are fabricated from specialty materials such as lithium and metals and must be recharged with electricity. Thus, energy is needed to generate electricity for recharging batteries during their lifetime operation. For CO2 reduction, the benefits of EVs are timid if electricity is generated from coal or other carbon-based materials. Conversely, the benefits of EVs are more substantial as several countries decarbonize electricity generation to meet their climate targets. As an example, the electricity sector in Norway relies predominantly on hydroelectricity, wind, and thermal energy. It only has one coal-fired power station, known as Longyearbyen Heat and Power Plant. The Norwegian government is now working on a new energy plan for Longyearbyen with the usage of natural gas, bio pellets, or other renewable resources [25]. In contrast, coal, oil, and solid biomass are the main sources of energy needed, over 80% in India [26]. The electricity sector in China is more diversified; however, about 52% of electricity is still generated by coal and 4.5% from natural gas [27]. In the USA, 80% of the nation’s electricity generated in 2020 stemmed from natural gas, nuclear energy, and coal versus 20% from renewable sources and geothermal [28]. In several countries, the electricity sector advocates renewable energy resources such as solar, wind, and hydropower to support the emerging electricity demands from EVs. The life cycle assessment (TCA) has been often conducted for internal combustion vehicles fueled by diesel and petrol, plug-in hybrid vehicles, and EVs with future electricity energy. As an example, EVs are an optimal choice with the least environmental impact in Hong Kong [29]. The use of clean energy electricity decreases the environmental impact and mitigates climate change globally [30]. In Norway with an electricity mix in 2030, the choice of EVs is optimal with the least environmental impact among 10 selected countries including the USA, the UK, Germany, China, and Canada.
Lithium, cobalt, and nickel are three important metals for the fabrication of advanced LiBs, whereas neodymium, samarium, and dysprosium, three rare-earth” metals are needed in electric drive motors. Carbon fiber and other lightweight materials are often used in the structure of EVs. Like fossil oil, lithium is not renewable, and its extraction or mining is also harmful to the planet. Its removal can result in soil degradation, water shortages, impair ecosystem functions, and might cause global warming. The mining of cobalt in DR Congo is widespread with human rights abuses, including the use of labor from 40,000 children. The operation also lacks proper safety precautions, resulting in frequent accidents [31]. In this context, the reuse of LiBs from EVs is advocated for low-performance operations such as energy storage for solar energy and power grids. As spent LiBs are utilized for a second life, this practice will give time for the development of LiBs recycling technology, batteries without lithium, and the set-up of improved power grids with increasing capacities to accommodate the electricity demand from the operation of EVs. In particular, material flow analysis for spent LiBs in the USA and China is discussed by Shafique et al. [32].
4. Lithium Sources, Other Metals, and Production
4.1. Lithium Reserves
Among three popular rechargeable batteries, lead acid batteries (LABs) are the cheapest and fully developed with 97% recyclability [33]. Nickel metal hydride batteries are more expensive than LABs but provide higher output and better performance [34]. LiBs are lighter than lead acid and nickel batteries and their most expensive cost can be compensated by extra performance and running range. LiBs are more power denser than LABs, to fulfill a vehicle’s performance with considerably less weight and last longer; 10 years vs. 4 years. A review of working principles, cell structures, and safety issues of LiBs is available elsewhere with 182 relevant references [35].
There are three main sources of lithium: salt-flat brines, geothermal brines, and mineral ores, mainly spodumene (Table 3).

Table 3.
General characteristics, resources, quantity, and commercial production of lithium.
Salt-flat brines are up to 75% of reserves, located in South America, (Chile, Argentina, and Bolivia—together referred to as the “Lithium Triangle) [43], whereas 17% are in hard rock as mineral deposits, mainly in Australia [44]. Most energy used for the extraction or mining of lithium is based on CO2-emitting fossil fuels. The mining operation of lithium, metals (cobalt and nickel), and rare earth metals is labor-intensive and requires chemicals and an enormous amount of water. Lithium brine recovery is very time-consuming, several months to a few years and only a few companies can produce high-quality, high-purity lithium [45]. Salar brines, up to 2000–6000 ppm (mg/L) (0.2–0.6%) of lithium, are subject to solar evaporation until significant water has been removed, corresponding to about 6% of lithium [46]. Besides Li, brines also consist of boron, sodium, magnesium, and potassium. In some cases, water is removed by reverse osmosis (RO) to accelerate the evaporation process. The brine is then subject to selective precipitation steps to remove contaminants or unwanted materials including boron, depending on the purity required for lithium products [47,48,49]. The collected brine is then treated with various reagents to form Li2CO3, LiOH, LiCl, LiBr, or (CH3)3CLi.
The evaporative method is only economical by solar energy; thus, it depends on geographical locations where the brine composition can be drastically different. Endogenous Mg2+ ions often co-precipitate as Mg carbonate along with Li carbonate. Evaporative technology also causes severe water loss, which is more problematic for locations with scarce water. The recovery of lithium from lithium-bearing ores, e.g., spodumene, involves crushing, roasting, and acid leaching. Similar to any mining or extraction operation, the affected sites would suffer from soil damage, air contamination, water pollution, and water depletion [50]. The production of lithium through evaporation ponds needs 500,000 gallons of water for every metric ton of lithium [51]. Undoubtedly, the extraction of lithium might impair biodiversity, ecosystem functions, and soil properties, resulting in global warming and water shortages. In this context, the Jadar lithium mine in Serbia, perhaps one of the biggest in the world, is always subject to environmental and social concerns.
4.2. Other Precious Metals
Besides lithium, other metals are also used for the production of EV batteries and their amounts might differ on the battery type and vehicle. There are some estimated amounts of lithium and other materials to build 20 million cars a year: 127, 302 tons of lithium (6.35 kg/car), 68, 315 tons of cobalt (3.41 kg/car), and 750, 410 tons of nickel (37.5 kg/car) [52]. Cobalt is now USD 56,000-per-ton level [53], well below its peak of USD 82,000 and it is mainly mined in DRC (the Democratic Republic of the Congo) to supply 70% of the global production, followed by Russia. The mining of this expensive metal has been linked to birth defects in DRC [54]. Nickel is much cheaper, USD 24,000 per ton, compared to cobalt. Manganese only trades around 4.5 U.S. dollars per ton. According to the IEA’s (International Energy Programme) Net Zero by 2050 roadmap, 2 billion EVs and battery-related vehicles are needed globally to meet the target. The total cobalt reserve in China is only 80,000 tons; therefore, China has a strong motivation to avoid the use of this metal in LiB (lithium batteries). In principle, cobalt can be replaced by other metals from cathodes without compromising the performance of lithium batteries [55]. Indeed, half of Tesla’s new cars use cobalt-free ion phosphate (LFP) batteries. Without both cobalt and nickel, LFP batteries are cheaper and safer, but have less energy density, resulting in less efficient and shorter range. Nonetheless, the batteries are improved and used in lower-end and shorter-range vehicles [56]. Note that the reserves for lithium, cobalt, nickel, and even manganese in the USA are negligible. Therefore, the manufacture of EVs relies on such imported metals, a similar scenario in Europe. Australia might be a friendly supplier of such metals to both the USA and Europe in the short term. Perhaps this is the reason why Tesla, an American company, is ramping up production at its factories in Texas (USA) and Shanghai (China). Considering that 3.42 kg cobalt is required for making an EV [52], the global reserve of this metal is 7.1 m tons (Table 4), which is sufficient for making 2 billion EVs.

Table 4.
Global reserves of lithium, cobalt, nickel, and manganese (million tons).
4.3. Suppliers of Lithium and Lithium Reserves
Proven land reserves of lithium with 14 million tons are located in four countries: Chile, Australia, Argentina, and China [61]. The global lithium reserve might be higher, 80 million tons (80 billion kg), according to the US Geological Survey in 2019 [62]; however, only a few locations are considered economically viable (Table 5). For the cost-effective recovery of lithium, the level of lithium must be above some threshold concentrations. Another important factor is the presence of magnesium (Mg) together with lithium in salar (a salt-encrusted depression) or salt lake brines because the removal of Mg is necessary for battery-grade lithium [63]. In this context, lithium from the Dead Sea in Israel is not a viable source. In contrast, the lithium level in Zabuye, China is very high together with an extremely low Mg/Li ratio, which favors its profitable recovery. The Zabuye Salt Lake has lithium carbonate reserves of about 1.84 M tons [64]. The lake got its name from a natural mineral form of zabuyelite, lithium carbonate (Li2CO3). The level of magnesium is negligible in comparison to that of lithium, a unique case. Of note is the Salton Sea’s lithium capacity, which is about 32 million metric tons, almost the reserves of the world’s largest combined deposits in Bolivia and Chile [65].

Table 5.
Lithium and magnesium concentrations in some known reserves.
In terms of production, 55% of global lithium production comes from Australia, followed by Chile (23%), China (10%), and Argentina (8%). In 2021, Australia has been the world leader in lithium production, with an estimate of 55 × 103 tons, followed by Chile (26 × 103 tons), and China with 14 × 103 tons. Therefore, the production of EVs in Europe is heavily dependent on imported lithium. Undoubtedly, the European Commission (EC) wants to create a lithium industry and significantly reduce its dependence on third-party countries. Europe in the near term does not have sufficient access to sources of lithium to make the batteries and factories to make them. In 2021, China produced 79% of all lithium-ion batteries for the global market and its price is extremely volatile, 6000 USD/ton in 2000 and 78,032 USD/ton in 2022 [66]. A significant amount of lithium is likely produced by other countries over the next decade to reach over 2.7 M tons of LCE (lithium carbonate equivalent) by 2030. After South America (mainly Bolivia, Chile, and Argentina), the most lithium producer is Australia, followed closely by China. If each EV requires only 6.35 kg [52], the total estimated reserve of 14 million tons is sufficient for making 1.47 billion EVs. Eventually, the recycling of spent lithium batteries will play an important role as addressed later.
5. Types of Lithium Batteries and Their Characteristics
5.1. Types of Batteries
As rechargeable batteries, LiBs consist of a cathode (layered lithium-rich material), an anode (usually graphite), and an electrolyte. Different types of LiBs have been developed for deployment in EVs and portable electronic devices [67], as summarized in Table 6.

Table 6.
Type of electrodes (cathodes) and their characteristics.
The cathode is most commonly a lithiated metal oxide, consisting of three types: LixCoO2, LiMn2O4, LiFePO4, and Li2FeSiO4. One of the most common anodes is lithiated graphite LixC6, comprising graphene sheets intercalated with lithium. New materials, including silicon and other elemental blends, are under investigation. All electrolytes are based on lithium-containing materials that allow for the easy diffusion of lithium with LiPF6 or similar lithium salt and ethylene carbonate as a solvent. Solid states and polymers are under investigation, but they are rarely used due to the low diffusivity of lithium. The electrochemical cell of lithium batteries is described as follows:
At anode: LixC6 ⇋ xLi+ + C6 +xe−
At cathode: Li1−x XXO2 + xLi+ + xe− ⇋ Li XXO2
Overall equation: LixC6 + Li1−x XXO2 ⇋ Li XXO2 + C6
During discharge, lithium is oxidized by the lithium-graphite anode; C6Li → 6C (graphite) + Li+ + e−. Released lithium ions flow to and are incorporated in the cobalt cathode, resulting in the reduction of Co4+ to Co3+. During recharging, the lithium ions leave the lithium cobalt oxide cathode and migrate back to the anode. They are then reduced back to neutral lithium and reincorporated into the graphite network to complete a cycle (Figure 1). At the time of this writing, a new 90 kWh unit costs over USD 20,000. The capacity of batteries increases continuously and EVs equipped with 100 kWh batteries are available with Tesla Model S [68]. A typical Li-ion battery consists of a cathode, an anode, a separator, a copper current collector, an aluminum current collector, an electrolyte solution, and other binders.
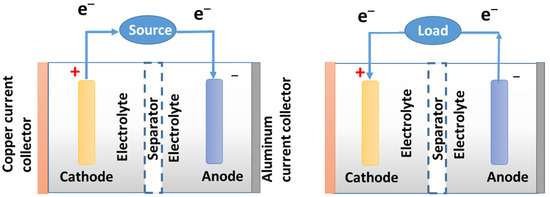
Figure 1.
Charge (left) and discharge (right) of a conventional rechargeable battery. Different Li-ion cathodes have been developed (Table 6) but the anode is often based on carbon (graphite or microspheres) because carbon can reversibly absorb and release a large quantity of Li (Li:C) = 1:6 as discussed earlier.
5.2. Battery Life
An EV battery will lose about 2.3 percent of its initial range per year, according to Geotab [69]. In terms of mileage, the battery pack in the Tesla Model S loses about 5% of its capacity over the first 50,000 miles on the road. Manufacturers are mandated to cover their batteries with at least 8 years or 100,000 miles of warranty, whichever comes first [70]. Generally, the average electric car battery life is approximately 10 years before a replacement is required. For the best performance of LIBs, they should not be discharged fully or recharged more often. The capacity of Li- and Ni-based batteries loses about 20% after 300 and 500 full discharge/charge cycles [71]. A battery cycle refers to a single full recharge of a completely exhausted battery or two full recharges of a battery with 50% capacity. After each cycle, mobile Li-ions are reduced as they are trapped by newly formed compounds in the electrolyte. The battery’s lifetime is also affected by high temperatures due to the electrolyte breakdown. The electrode structure is also damaged through structural disordering during the flow of Li-ions in and out of the electrodes. Consequently, the electrode accepts fewer Li-ions into its structure, resulting in low capacity. The additive introduction into the electrolyte is one remedy, whereas the structural reinforcement of electrode materials can be achieved by cationic doping. Ongoing LiB research is essential to equip EVs with longer battery lifetimes, comparable to the longevity of their fossil-fueled competitors. Detailed information on electrode degradation in lithium batteries is discussed elsewhere [72].
5.3. Extra Energy and CO2 Released for Making EVs
EVs are equipped with a pack of LiBs, consisting of a mixture of metals that needs to be extracted and refined. Such operations heavily depend on carbon-based energy, which emits greenhouse gases, mainly CO2. The amount of CO2 emitted in production, known as CO2 footprint, is associated with electricity use. Producing a 75-kW.h battery at Tesla’s battery factory in Nevada, USA releases 4500 kg (4.5 tons) of CO2 [73], compared to 7500 kg (7.5 tons) if the battery is made in Asia. As a guideline, a petrol or diesel car will release ~7 to 10 tons of CO2. Roughly, the same energy must make one EV plus the energy associated with mining and making lithium batteries. In terms of operation, about 2.3 kg of CO2 are released by a petrol car for each liter of gasoline consumption. An electric car with a range of 300 miles between charges must be equipped with a battery of at least 60 kWh. About 150 kg of CO2 is released per 1 kiloWatt hour (kWh) of battery capacity. About 9 tons (9000 kg) of CO2 will be emitted to make the battery; thus, 16–19 tons of CO2 are released for making an EV. Unlike petrol-powered cars, EVs do not release CO2 during running; however, their batteries require recharging and the generation source for electricity plays an important role. EVs are only as green as their power sources, as demonstrated by the life cycle analysis (LCA) for one EV and its petrol equivalent counterpart in British Columbia, Canada [74]. In brief, the deployment of the EV for 150,000 km would reduce the emission of CO2 from ~393 gCO2/km to ~203 gCO2/km. A breakdown of CO2 emissions associated with five major categories is shown in Table 7. About 87% of the electricity in B.C. is produced from hydroelectric sources and 5% from biomass, and 4% from wind energy [75]. A slightly better scenario is observed for operating EVs in Norway, compared to the most efficient petrol cars [76] (Figure 2). Such a result is expected as hydro accounts for ~92 percent of electricity output in the Nordic country and 6.4 percent from wind power [77]. A similar scenario is also observed in France, where nuclear power makes up the largest portion of electricity generation, at ~78% [78]. The reduction of CO2 with EVs in Germany is somewhat disappointing as their deployment offers no reduction of the release of this greenhouse gas. In 2020, Germany generated electricity from wind (27%), nuclear (12%), natural gas (12%), solar (10%), biomass (9.3%), hydroelectricity but 24% from coal [79].

Table 7.
A comparison of CO2 emissions from the manufacturing and operation of an EV versus a petrol car.
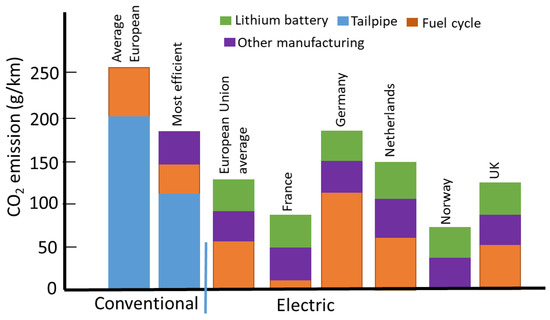
Figure 2.
Life cycle emission of electric and conventional vehicles in Europe (2015) with a mileage of 150,000 km [Redrawn from Ref. [76], open access].
The LCA is also conducted to compare the release of CO2 for ICEV (internal combustion engine vehicles) versus BEV (battery vehicles) in Europe, the USA, China, and India. The reduction of CO2 in Europe is about 3.5-folds, compared with 2.5-fold in the USA. The situation in China is less promising and only a modest result is observed for India (Figure 3). HEVs (hybrid electric vehicles) only reduce life cycle emissions by about 20%, and PHEVs (plug-in hybrid EVs) are a little better in Europe (25–27% lower than gasoline), a little worse in China (6–12% lower than gasoline), and adequate in the US (42–46% lower than gasoline). Therefore, the development focuses on BEV (battery electric vehicle). In terms of CO2 reduction, an EV in Europe is 66–69% lower than an ICEV, compared to 60–68% over its lifetime in the US. In China and India, the magnitude is only 37–45% and 19–34%, respectively [80].
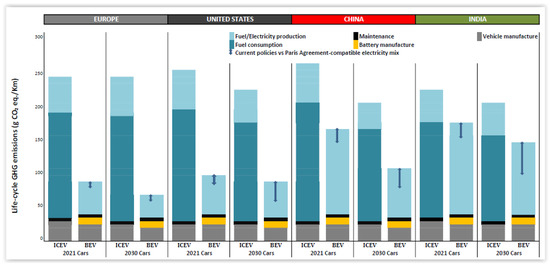
Figure 3.
Life cycle analysis of medium-size gasoline internal combustion engine vehicle (ICEV) versus battery-powered engine vehicle (BEV) [redrawn from Ref. [80], open access].
6. Technical Challenges and Social Aspects
6.1. Recycling of Spent LiBs
A representative car battery in Nissan Leaf with 50 kWh (normal range: 40–60 kWh with 350 V lithium-ion) will lose about 20% of its original capacity after ten years of service [81]. Spent batteries are often reused for stationary energy storage or boats. For recycling, spent batteries are shredded into a powdered mixture, liquefied in a smelter (pyrometallurgy), or subject to acid (hydrometallurgy), followed by precipitation. Considerable efforts must be made for the economical recycling of spent batteries, e.g., the elimination of the shredding stage. Cathode materials can be separated using ultrasound sonication as cavitation at the electrode interface to break the adhesive bond rapidly to delaminate the electrode within seconds [82]. However, the transportation of heavy EV batteries and storage batteries to a recycling center is also costly. In Australia, only 2–3% of spent LiBs were collected and sent offshore for recycling in 2019, compared to <5% in the European Union and the US [83]. Low recycling rates stem from several factors such as technical constraints, logistic issues, economic barriers, and regulatory gaps. Some technical challenges for recycling LiBs are summarized in Table 8.

Table 8.
Technical challenges for recycling lithium-ion batteries.
The explosive sales of EVs can be attributed to their lower cost and extended ranges with tolerable charging times. Most of the governments of developed countries offer a lubricative financial incentive for the purchase of EVs to promote sustainable and environmentally friendly mobility [84] (Table 9).

Table 9.
Countries and financial incentives for the purchase of EVs.
The recycling of spent LiBs will reduce the life cycle costs and recover other expensive metals besides lithium. Spent LiBs should be recycled because lithium and other metals account for over 50% of a battery’s cost and they are not renewable. Cobalt, nickel, and other metals are toxic and contaminate soil and groundwater if they are simply going into landfills. Environmental concern is also extended to lithium fluoride salts (LiPF6), commonly used in a battery’s electrolyte. A typical LiB has the following components [83]: 31% of cathode materials (Li-Co cobalt oxide, Li-Ni, Co-Al oxide, etc.), 22% of anode materials (main graphite), 17% of a copper current collector, 15% of an electrolyte solution, 8% of an aluminum current collector, 5% of carbon black binder, and 3% of a plastic separator. Of important relevance is the estimation of the energy consumed and air emissions generated when recovering LiMn2O4, aluminum, and copper by three different processes: hydrometallurgical, intermediate physical, and direct physical recycling [85]. Energy and greenhouse gas emissions are about 75 MJ/kg battery and 5.1 kg CO2/kg battery, whereas battery assembly is only attributed to 6% of the total required energy.
6.2. Dark Sides of Lithium Mining and Extraction
Except for the mining of lithium in Australia, countries with abundant minerals suffer from political and economic instability. As an example, war-torn Afghanistan has vast natural resources of lithium, cobalt, rare-earth minerals, and lithium in hard rock. Another pitiful case is the mining of cobalt in DR Congo, the poorest and most unstable country. China has lithium, vast rare-earth minerals, and coal, whereas Russia possesses significant nickel, manganese, iron, and other strategic metals and minerals. The United States, Russia, and China might also have 29.79 million tons of recoverable lithium [86]. A recently revised number of total lithium reserves from brine and hard rock is up to 54.1 million tons: hard rock lithium resources (12.8–30.7 million tons) and brine field (21.3–65.3 million tons) [87]. US President Joe Biden invoked the Defense Production Act—a 1950 law that boosts domestic production of the rare metals and materials used in EVs (nytimes.com (accessed on 31 Mrach 2022)). Recycling EV materials will be critical for reducing the demands of lithium and other strategic materials, which are also required for other applications.
Waste generated from mining cobalt, other metals, and rare earth metals can pollute the environment, food, and water besides their adverse effect on crop yields and human health. Community loses communal land, farmland, and homes and local people often cross international borders into Zambia to buy food. A legal case has been launched against Apple, Google, Dell, Microsoft, and Tesla as children were killed or critically injured while mining for cobalt in DR Congo. Like other heavy metals, cobalt is toxic, and it must be handled cautiously [87]. The mining and extraction of cobalt have been linked to power corruption, environmental destruction, and child labor. Working environments are hostile and unsafe [88], resulting in several fatalities and injuries from tunnel collapses. There is also a pending legal suit in Portugal against a subsidiary of London-based mining company Savannah Resources for alleged “improper appropriation” of land. This biggest lithium producer in Europe soon produces higher-grade lithium from reserves of 60,000 tons, sufficient for making 5–7.5 million LiBs for EVs [89].
The mining of lithium in less developed countries also has several environmental impacts. In South America, lithium extraction from salt brines contaminates the local water basins and local people might be exposed to contaminated water. All mining areas must be completely emptied to eliminate obstructions; therefore, trees are cut down and all other life forms are wiped out. The operation also uses heavy machinery and vehicles that consume enormous energy and produce toxic gases, including CO2. The energy required for lithium or extraction is based on CO2-emitting fossil fuels for economic reasons and availability. With hard rock mining, 15 tons of CO2 are emitted per ton of lithium, associated with massive mining wastes. About 500,000 gallons of water are needed to yield one ton of lithium [90]. A severe lack of water might diminish farming activities in lithium mining areas, which are located in dry, hot, and mountainous regions. Such mining and extraction activities are not sustainable, and they are not allowed in countries with high standards of environmental regulation. Thus, advanced, cheap mining methods to extract lithium from seawater should be explored. The current lithium extraction method pumps up brine from the underground pool and allows the sun to evaporate about 95% of the water in the evaporation pool. It is of uttermost importance to reduce water usage together with geothermal energy with zero-emission. Several companies have worked in this direction with numerous patented technologies [91]. The plant in Germany’s Rhine Valley (Vulcan Energy Resources) produces sufficient lithium for 400 million EVs. Nonetheless, the cost of lithium carbonate recovered from salt ponds is 30–50% less than that of hard rock mining [92,93].
Another important source of lithium is geothermal fluids [94,95,96]; however, only 35 samples over 2000 geothermal wells have concentrations greater than 20 ppm (mg/L) [94,95] with an estimated amount of 2 M tons [97]. Among different processes, the most promising approach is the adsorption of lithium using inorganic sorbents, organic resins, solvents, and polymers. Chemical precipitation and membrane-dependent processes are also two viable procedures [63]. In brief, the recovery of lithium from geothermal brines is technically feasible but further developments are still needed to evaluate their cost and environmental sustainability. In addition, significant improvements are needed to improve sorbent stability and suppress interfering species (alkali metals, rare earth metals, metalloids, etc.). Membranes can be designed to retain lithium selectively based on size exclusion, membrane surface charge, or other physicochemical properties [98]. Of interest is the use of nanofiltration for the separation of lithium from Mg2+ and other divalent cations [99,100,101,102,103,104,105]. A commercial membrane (Desal-5 DL 2540C membrane, GE Osmonics) can exclusively retain 61–67% of the Mg2+ in the presence of Li+ [101,102]. The membrane surface charge can be modified to impart separation selectivity [98,100]. Commercial nanofiltration membranes can separate divalent cations from lithium; thus, their potential use to process geothermal brines is highly anticipated.
7. Trends and Future Possibilities
7.1. Advances in Battery Technology
The lifetime of LiBs is always the most critical factor for the performance of EVs. Manufacturing also adds to batteries’ eco-footprint, as the production of LIBs is processed at 800 to 1000 °C, which is only cost-effective by burning fossil fuels or coal. Back in 2019, Tesla announced that the company was ready to install million-mile batteries in its cars. The battery capacity deserves a brief comment here considering the first Audi duo EV introduced in 1983 had a capacity of 8 kW.h. Thus far, several EVs have a capacity of 100 kW.h such as Tesla Model S, Volvo 40 series, Ford Mustang Mach-E (99 kW.h), and even up to 200 kWh for Tesla Roadster [106]. The degradation of EV batteries depends on many factors but generally loses its capacity over time. Fast charging, e.g., with Tesla Superchargers or other 480-volt fast chargers also causes battery degradation due to more heat generation. X-ray scans have been performed to examine lithium-ion prototype pouch battery cells, subjected to 1500 cycles, which is the equivalent of 120,000 miles. The scan revealed cracking and mechanical degradation with excess electrolyte sucking up into the electrode assembly cracks, resulting in capacity loss [107]. Electrolyte decomposition of lithium-ion batteries due to thermal stress is another important factor [108]. Such results are not expected as LiBs can be charged in an environment up to 113° F (45 °C) and discharged in temperatures as high as 140° F or 60 °C [109]. EVs equipped with LiBs can run in cold climates with temperatures down to −20 °C.
As industry and political leaders move toward decarbonization and eventually reverse human-caused global warming, the transport sector is mandated to favor EVs over petrol vehicles. Unfortunately, information related to the implementation of EVs is insufficient, and a subject for further investigation. According to the International Energy Agency (IEA), at least 1 million tons of lithium, equivalent to all lithium reserves in China, are needed to make 125 million EVs by 2030 worldwide [110]. There is an impetus to make a green battery to replace LiB. Salt-based batteries developed by the University of Nottingham are recyclable. The number of charge–discharge cycles is higher using sulfide electrodes without battery degradation. Meanwhile, efforts should focus on LiB with an extended lifetime. As an example, long-lasting batteries are feasible if molybdenum and sulfur are added to make them. Silicon anodes can hold 10 times higher charge, compared to current graphite anodes and the perfecting design of silicon-based anodes is well underway. A solid-state battery with a solid electrolyte should be explored as it is smaller and stores more energy. However, they are expensive and difficult to mass manufacture. Nonetheless, solid electrolytes are inflammable and nontoxic, significantly improving the safety of EVs. NEI offers Lithium Tin Phosphorus Sulfide (Li10SnP2S12)—and oxide, phosphate, and polymer-based electrolyte material compositions [111]. Generally, solid electrolytes are not very chemically stable while in contact with highly reactive lithium metal, resulting in their degradation over time. A solid-state battery is often cracked and short circuit due to the formation of dendrites, which leak from the anode to the electrolyte. Solid-state batteries are still very expensive and suffer from a higher failure rate after repeated charging.
Ceramic-electrolyte cells of Volkswagen and its partner QuantumScape are claimed to be safer, lighter, and charge faster with a respectable driving range of 500 miles [112]. Of importance is the design of an anode made of lithium metal, instead of conventional graphite [113]. The fabrication is based on “mixed ionic-electronic conductors” and “electron and Li-ion insulators”, which are chemically stable in contact with lithium metal. The replacement of lithium-metal anodes with a thin layer of silver-carbon provides a higher capacity with long-lasting features and makes the battery safer. These new solid-state battery packs will last over 500 miles with 1000 recharges, corresponding to a total mileage of at least 500,000 miles. Solid-state battery packs are 50% smaller than LiBs. Solid-state technology is still under investigation and development, but the super-performance of lithium-metal batteries is considered a big reward. During charging, some energy is dissipated as heat or thermal loss because the battery components resist electricity transmission. The internal resistance is more pronounced on high power charges [114]. Thus, batteries must be designed to support quick charging and higher temperatures. Perhaps, the long charging time because of this resistance is one of the most important drawbacks of current EVs.
Although several electrode materials and electrolytes have been attempted, lithium-ion batteries still exhibit the highest energy density of 200–735 (Wh/L) and specific power of 350–3000 (W/kg) with an operating temperature of −20 to 60 °C. The second best in this category is Li-MH (nickel-metal-hydride) of 100–300 (Wh/L) and 250–1000 (W/kg); however, its operating temperature is 0–50 °C. Other types of batteries such as Ni-Cd, Zn-Br2, Na-NiCl, and Na-S, have significantly lower energy densities and specific powers and they cannot function at temperatures below freezing. In this aspect, Pb-PbO2 has an operating temperature of −20 to 45 °C, however, its specific energy storage is ~ 30–40 Whg−1, compared to other batteries as discussed later. Thus, the application of Pb-PbO23 batteries is limited in advanced systems such as EVs [115]. In terms of electrode materials, of notice is the use of graphene, which has been used extensively in electrochemistry [116]. Graphene is barely heated, so it withstands fast charges with insignificant power losses. A graphene battery is claimed to have a driving range of 800 km and 5 min of charging with high-power plugging [117].
Aluminum-air batteries are recyclable with the highest specific energy (1300–8000 W.h/kg) and produce electricity from the oxygen–aluminum reaction (4Al + 3O2 + 6H2O → 4Al(OH)3 +2.71 V). With potassium hydroxide as the electrode, a potential difference of 1.2 V is created by these reactions. The battery offers mileage up to 1600 km [118] and its price is decreasing significantly from the current level of 300 EUR/kWh [119]. Lithium-air requires a constant supply of oxygen, which reacts with lithium, yielding specific energy of 12 kWh/kg [120]. Lithium is oxidized at the anode (Anode: Li Li+ + 1e−), whereas oxygen is reduced at the cathode to induce a current flow (Li+ + 1e− + O2 LiO2). The Li2O2 has ~40.1 MJ/kg = 11.14 kWh/kg of lithium, which is very close to that of gasoline, ~46.8 MJ/kg. Sodium-air (Na2O2) batteries can improve the autonomy of EVs equipped with LiBs at least thirteen times [121]. The use of sodium (ionic charge of 1+ and ionic diameter of 1.98 Å) is appealing because it is the sixth most abundant and inexpensive element. A detailed description of the sodium-air is available elsewhere [122]. Magnesium-ion (Mg-Ion) with 6.2 kWh/L [123] has been investigated by the Advanced Research Projects Agency Energy (ARPAE), Toyota, and NASA (The National Aeronautics and Space Administration, USA) [124,125].
Many EVs are equipped with permanent magnet-based motors, typically made with neodymium and dysprosium. China is the key producer of rare-earth metals worldwide and has absolute control of such materials. As expected, most Chinese vehicle models utilize permanent magnet motors. For the West, there are always problems with such foreign sources and supply chain security. Another environmental concern is the contamination of rare earth metals with radioactive materials, e.g., thorium. Separating the materials requires toxic compounds like sulfate, ammonia, and hydrochloric acid. Processing 1 ton of rare earth metals can produce up to 2000 tons of toxic wastes [126]. Renault’s Zoe replaces magnets with copper windings and Bentley also develops rotors with no magnets or copper. BMW’s new 5th generation drivetrain has no rare earth metals and Audi has also opted for an aluminum rotor induction motor for the e-tron. The fabrication of anodes for LiBs from alloy materials, transition metals, silicon-based compounds, and carbon-based compounds has been addressed by Nzereogu et al. [127]. Similarly, a review of LIB applications in electric vehicles is available elsewhere [128].
The charging modes are excluded in this review but deserve a brief note here as they are different from continent to continent. North America and Japan use the SAE-J1772 standard for electric connectors [129]), whereas Europe and China have the IEC-62196 standard [130] for EV charging. China also promotes the GB/T-20234, 3-2015, which offers both AC and DC charging modes [131]. Tesla’s DC superchargers have a maximum power of 145 kWh but are currently limited to 120 kWh [132], comparable to a Mode 3 fast charging. The emerging topic of wireless charges has been addressed by several authors [133,134,135]. Several authors [136,137] also discuss the use of solar photovoltaic modules for EV charging, including economic and environmental impacts as solar energy can contribute to a significant quantity of energy demand. Table 10 provides a comparison of the performance of different car batteries, which might be useful to potential EV buyers.

Table 10.
Technical data analysis of different batteries for EVs.
Both the lifetime of cycles and specific energy are quoted as the highest values reported. Further information on the technical analysis of different batteries is available elsewhere [142,143]. Of notice is the development of LiFePO4F as a promising material for lithium batteries [144,145].
7.2. Research in Battery Manufacturing Technology
As discussed earlier, active studies on LiBs have focused on electrode materials, robust electrolytes, cobalt-free batteries, or even the replacement of lithium with other materials. There is insignificant progress in the manufacturing technology of LiBs albeit it contributes about 25% of the cost of LiBs [146]. The current manufacturing process has over 12 steps, which are time-consuming and costly [147]. Solvent-free manufacturing has emerged as an effective method to replace conventional solvent coating and drying, two lengthy key steps in electrode fabrication. Research must also focus on the design of thick electrodes, large cells, compact modules, and other manufacturing innovations to build a higher-energy battery system with minimal volume and weight. Other two major concerns are the risks of shorting and fire caused by flammable organic electrolytes inside the LiB pack. With improved manufacturing technology, new battery materials with lower costs are anticipated; however, it is still challenging to fulfill the US Department of Energy’s ultimate target of 80 USD/kWh [148].
7.3. Second-Life Applications of Spent LiBs
A typical EV LiB pack should last about 200,000–250,000 km [149]; however, this mileage might be compromised by increasingly adopted fast charging at >50 kW [150]. When the pack loses 15–20% of its original capacity, the car’s performance is affected in terms of acceleration, recharging, and driving range [151]. From an economic viewpoint, spent batteries can directly be used for stationary applications as lower current density from the battery pack is not a deciding factor. Battery modules with similar capacity and life can be re-assembled for stationary usage [152], such as utility-scale grids, solar energy, building, telecommunication tower storage, area, etc. Of importance are stadium events with high electricity demand, ranging from 200 kW to over 3000 kW [153]. EVs have been promoted as a model of sustainability and CO2 reduction during their operation; however, essential materials and processes used in their manufacturing, electricity for recharge, and subsequent component recycling are attributed to the circles of sustainability. In this context, EVs can be recharged by electricity generated by solar and wind power and stored in spent LiBs. In the long term, financial incentives might be needed to advocate the deployment of second-life EV batteries for public, residential, commercial, and industrial activities and buildings. China ended the purchase of lead-acid batteries in 2018 and has deployed in second-life LiBs for about 2 million telecom tower base stations (54 GWh battery storage demand). About 75% of spent EV batteries should last for several years and they will be sent to recycling to recover all the valued components [154], so there is sufficient time for developing economical technology for battery recycling. The recycling of spent LiBs by different processes with a focus on hydrometallurgy was reviewed by Lv et al. [155], consisting of 100 cited papers. A review of the LCA of electric vehicles is also available from the literature [156] to cover a broad subject range of manufacturing batteries, recycling efficiency, sustainable resource development, environment, and economy.
7.4. Safety Issues and State-of-Charge (SOC)
The design of LiBs should address two important safety points: short circuitry and potential battery explosion. In a battery pack, multiple cells are connected in series to achieve the target voltage level; thus, short-circuiting can be problematic [157]. LiBs have an organic electrolyte, usually consisting of one or a mixture of organic carbonates together and a Li-ion salt, e.g., LiPF6, as discussed earlier. Battery failure can release a combustible mixture to trigger a thermal runaway event [158,159,160]. Battery failure occurs when LiBs are subject to overcharge, external and internal short-circuit [161], mechanical abuse, and heat exposure. The explosion occurs when three carbonates (dimethyl carbonate, ethyl methyl carbonate, and diethyl carbonate) often used in LiBs are subject to 373 K, and 100 kPa absolute pressure [162]. As the flammable electrolyte is a potential hazard [163], the issue can be circumvented by using solid-state electrolytes as mentioned earlier. LG Chem reported the development of fire-retardant plastic for EV batteries very recently [164] (April 2022). This highly heat-resistant plastic can delay flame propagation for about 7 min, which is critical for the safe evacuation of vehicle occupants. In terms of fire delay, this plastic retardant with dimensional stability is 45 times better than currently used plastics. LiBs exhibit high reactivity to water; thus, fires are hard to extinguish with water.
As the safety of the LiB is still a critical issue, LiBs need to be monitored and managed by the Battery Management Systems (BMS) [165], and accurate battery state-of-charge (SOC) estimated by the BMS is necessary for the power application LiB [166]. Three different approaches have been used to obtain SOC: direct measurement (coulomb counting method and open-circuit voltage, OCV); data-driven estimation; and model-based estimation [167,168]. Although this subject is beyond the scope of this paper, a brief discussion is included here to highlight the important aspects of this issue. In brief, the OCV method can estimate the OVC value [169], the battery must be idled for over 1 h before measurement. Therefore, it is not suitable for SOC estimation in operation and the measurement is also susceptible to temperature and reproductive quality [170,171]. The data-driven estimation method is based on the input-output data with a fuzzy controller, the neural network [172,173], and the support vector machine (SVM) [174]. This approach requires extensive training and vigorous computation [175], which are difficult in online adaptation.
Three models can be used for LiBs [157,176,177,178,179], electrochemical models; equivalent circuit models, and electrochemical impedance models, which are expressed as state equations. Several state observers have been applied to the models separately such as extended Kalman filter, unscented Kalman filter, sliding mode observer, particle filter, and H-infinity observer. Some shortcomings, including calculation errors of the Kalman filter (an optimized autoregressive data processing algorithm), are noted [180]. The Kalman algorithm is based only on white noise, not noise characteristics in practical applications, resulting in estimation errors. Of significance is an improved feedforward-long short-term memory (FF-LSTM) modeling method, which can offer an accurate whole-life-cycle SOC prediction as current, voltage, and temperature variations are taken into account [181].
Lastly, fast charging is an essential stakeholder concern for practical applications of EVs; however, fast charging at high charging currents might cause the degradation of LiBs [182]. A recent review by Al-Saadi et al. [183] of several batteries with different chemistries: lithium nickel manganese cobalt (NMC); lithium iron phosphate (LFP); and lithium titanate oxide (LTO). Several studies investigate the effect of fast charging on the degradation of battery cells [15,16,183,184], but most tests are carried out in static conditions. The value of capacity fade after 1% is noticeably affected by battery capacity (kW.h), whereas the charger power (200–600 kW.h) does not affect LTO and LFP. LTO exhibits the lowest degradation under fast-charging profiles, whereas NMC is mostly degraded. Detailed information on such effects is available from Al-Saadi et al. [183].
8. Conclusions
There will be no future for internal combustion engine vehicles (ICEVs) if the world decides to ban petrol-powered vehicles. The West again wants to promote electric cars at the expense of poor people living near lithium and other metal mines. The strategic deployment of increasing EVs requires an enormous amount of lithium, the main component of LiBs. However, Europe and even the USA have relied on imports from Chile and other countries with high lithium reserves. More recycling of LiBs must be implemented to reduce the requirement for virgin lithium. Improved battery technologies are under investigation to fabricate smaller and more powerful LiBs with extended driving ranges and short charging times. These factors, with affordable cost, will ultimately dictate the future of EVs. Regardless of the advanced and clean technology for mining and extraction of metals, people in mining areas will lose their community and a long-established way of life for the comfort of urban people. Therefore, recycling Li-ion batteries could help to circumvent harmful environmental practices. For the extra needed lithium, the manufacturers of EVs must initiate several mining projects across Europe or the USA. Based on current technology, the Li extraction from concentrated brine costs 30% to 50% less than that from mined ores. The oceans might have up to 2.6 × 1011 tons of lithium from seawater and advanced technology will make its recovery economically feasible. Nonetheless, politicians must consider a holistic approach to the transport sector as an outright ban on internal combustion engine cars might not be realistic. Is it a wise idea to rid of oil dependency for a dependency on lithium and other metals? In addition, lithium batteries are also used in grid-scale storage, polymers, ceramics, metallurgy, pharmaceuticals, and aerospace applications. Together with innovations in battery technology and effective recycling, global lithium utilization becomes more effective and reduces the demand for virgin lithium beyond 2050. It is always problematic to depend on a single supply chain from foreign sources. Therefore, advanced technology is still needed to recycle spent batteries and manufacture compact batteries with high capacities. Such optimally designed EVs are capable of mitigating greenhouse gas emissions and free from fossil energy consumption. Battery Management Systems (BMS) will get better with more powerful computers and software to monitor and manage the operation of LiBs.
Author Contributions
J.H.T.L. Conceptualization, writing—original draft preparation, C.T. and D.T.-T. reviewing, J.H.T.L. editing and revising the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lithium Battery Safety (University of Washington). Available online: https://www.ehs.washington.edu/system/files/resources/lithium-battery-safety.pdf (accessed on 25 October 2022).
- Straubel, J.B. Driving Range for the Model S Family. Available online: https://www.tesla.com/en_CA/blog/driving-range-model-s-family (accessed on 18 October 2022).
- Berjoza, D.; Jurgena, I. Effects of change in the weight of electric vehicles on their performance characteristics. Agron. Res. 2017, 15, 952–963. [Google Scholar]
- Carbon Dioxide Emissions from Electricity (World Nuclear Association). Available online: https://www.world-nuclear.org/information-library/energy-and-the-environment/carbon-dioxide-emissions-from-electricity.aspx (accessed on 18 October 2022).
- Yang, S.; Zhang, F.; Ding, H.; He, P.; Zhou, H. Lithium metal extraction from seawater. Joule 2018, 2, 1648–1651. [Google Scholar] [CrossRef] [Green Version]
- Go All out with the All-New 2023 Outlander PHEV. Available online: https://www.mitsubishicars.com/2023-outlander-phev-release-date (accessed on 6 October 2022).
- Plötz, P.; Moll, C.; Bieker, G.; Mock, P.; Li, Y. Real-World Usage of Plug-In Hybrid Electric Vehicles: Fuel Consumption, Electric Driving, and CO2 Emissions; Technical Report; International Council on Clean Transportation Europe (ICCT): Washington, DC, USA, 2020; Available online: https://theicct.org/publication/real-world-usage-of-plug-in-hybrid-electric-vehicles-fuel-consumption-electric-driving-and-co2-emissions/ (accessed on 6 October 2022).
- The Car Guide. 2014 Toyota Prius PHV: To Plug in or Not to Plug in? 2014. Available online: https://www.guideautoweb.com/en/articles/21152/2014-toyota-prius-phv-to-plug-in-or-not-to-plug-in/ (accessed on 6 October 2021).
- Hyundai NEXO Press Kit. Available online: https://www.hyundai.news/eu/models/electrified/nexo/press-kit.html (accessed on 6 October 2022).
- insideEVs. 2019 BMW i3, i3 REx, i3s & i3s REx: Full Specs. 2019. Available online: https://insideevs.com/news/339970/2019-bmw-i3-i3-rex-i3s-amp-i3s-rex-full-specs/ (accessed on 6 October 2022).
- Yong, J.Y.; Ramachandaramurthy, V.K.; Tan, K.M.; Mithulananthan, N. A review on the state-of-the-art technologies of electric vehicle, its impacts and prospects. Renew. Sustain. Energy Rev. 2015, 49, 365–385. [Google Scholar] [CrossRef]
- Richardson, D.B. Electric vehicles and the electric grid: A review of modeling approaches, Impacts, and renewable energy integration. Renew. Sustain. Energy Rev. 2013, 19, 247–254. [Google Scholar] [CrossRef]
- Habib, S.; Kamran, M.; Rashid, U. Impact analysis of vehicle-to-grid technology and charging strategies of electric vehicles on distribution networks—A review. J. Power Sources 2015, 277, 205–214. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F.; Liu, X.; Peng, Y.; Wang, Q. A review on electric vehicles interacting with renewable energy in smart grid. Renew. Sustain. Energy Rev. 2015, 51, 648–661. [Google Scholar] [CrossRef]
- Hawkins, T.R.; Gausen, O.M.; Strømman, A.H. Environmental impacts of hybrid and electric vehicles—A review. Int. J. Life Cycle Assess. 2012, 17, 997–1014. [Google Scholar] [CrossRef]
- Shuai, W.; Maillé, P.; Pelov, A. Charging electric vehicles in the smart city: A survey of economy-driven approaches. IEEE Trans. Intell. Transp. Syst. 2016, 17, 2089–2106. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Morais, H.; Sousa, T.; Lind, M. Electric vehicle fleet management in smart grids: A review of services, optimization and control aspects. Renew. Sustain. Energy Rev. 2016, 56, 1207–1226. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.M.; Ramachandaramurthy, V.K.; Yong, J.Y. Integration of electric vehicles in smart grid: A review on vehicle to grid technologies and optimization techniques. Renew. Sustain. Energy Rev. 2016, 53, 720–732. [Google Scholar] [CrossRef]
- Rahman, I.; Vasant, P.M.; Singh, B.S.M.; Abdullah-Al-Wadud, M.; Adnan, N. Review of recent trends in optimization techniques for plug-in hybrid, and electric vehicle charging infrastructures. Renew. Sustain. Energy Rev. 2016, 58, 1039–1047. [Google Scholar] [CrossRef]
- Mahmud, K.; Town, G.E.; Morsalin, S.; Hossain, M. Integration of electric vehicles and management in the internet of energy. Renew. Sustain. Energy Rev. 2018, 82, 4179–4203. [Google Scholar] [CrossRef]
- Das, H.; Rahman, M.; Li, S.; Tan, C. Electric vehicles standards, charging infrastructure, and impact on grid integration: A technological review. Renew. Sustain. Energy Rev. 2020, 120, 109618. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Foley, A.M.; Zülke, A.; Berecibar, M.; Nanini-Maury, E.; Van Mierlo, J.; Hoster, H.E. Data-driven health estimation and lifetime prediction of lithium-ion batteries: A review. Renew. Sustain. Energy Rev. 2019, 113, 109254. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Hu, X.; Lucu, M.; Widanage, W.D. Gaussian process regression with automatic relevance determination kernel for calendar aging prediction of lithium-ion batteries. IEEE Trans. Ind. Inform. 2020, 16, 3767–3777. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, K.; Liu, K.; Lin, X.; Dey, S.; Onori, S. Advanced fault diagnosis for lithium-ion battery systems: A review of fault mechanisms, fault features, and diagnosis procedures. IEEE Ind. Electron. Mag. 2020, 14, 65–91. [Google Scholar] [CrossRef]
- The Barents Observer. Available online: https://thebarentsobserver.com/en/industry-and-energy/2021/01/longyearbyen-shut-down-coal-power-plant (accessed on 6 October 2022).
- Energy in India Today. Available online: https://www.iea.org/reports/india-energy-outlook-2021/energy-in-india-today (accessed on 6 October 2022).
- China Sector Analysis: Energy. Available online: https://www.globalxetfs.com/china-sector-analysis-energy/#:~:text=Background%20of%20the%20Energy%20Sector%20in%20China&text=This%20distribution%20network%20relies%20on,China’s%20power%20generation%20of%202021 (accessed on 6 October 2022).
- Electricity Production and Distribution. Available online: https://afdc.energy.gov/fuels/electricity_production.html#:~:text=According%20to%20the%20U.S.%20Energy,biomass%2C%20wind%2C%20and%20geothermal (accessed on 6 October 2022).
- Shafique, M.; Azam, A.; Rafiq, M.; Luo, X. Life cycle assessment of electric vehicles and internal combustion engine vehicles: A case study of Hong Kong. Res. Transp. Econ. 2022, 91, 101112. [Google Scholar] [CrossRef]
- Shafique, M.; Luo, X. Environmental life cycle assessment of battery electric vehicles from the current and future energy mix perspective. J. Environ. Manag. 2022, 303, 114050. [Google Scholar] [CrossRef] [PubMed]
- The DRC Mining Industry: Child Labor and Formalization of Small-Scale Mining. Available online: https://www.wilsoncenter.org/blog-post/drc-mining-industry-child-labor-and-formalization-small-scale-mining#:~:text=Cobalt%20is%20an%20essential%20raw,%2C%20environmental%20abuses%2C%20and%20corruption (accessed on 6 October 2022).
- Shafique, M.; Rafiq, M.; Azam, A.; Luo, X. Material flow analysis for end-of-life lithium-ion batteries from battery electric vehicles in the USA and China. Resour. Conser. Recyc. 2022, 178, 106061. [Google Scholar] [CrossRef]
- Nickel–Metal Hydride Battery. Available online: https://en.wikipedia.org/wiki/Nickel%E2%80%93metal_hydride_battery (accessed on 18 October 2022).
- BU-705: How to Recycle Batteries (Battery University). Available online: https://batteryuniversity.com/article/bu-705-how-to-recycle-batteries (accessed on 18 October 2022).
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Meng, F.; McNeice, J.; Zadeh, S.S.; Ghahreman, A. Review of lithium production and recovery from minerals, brines, and lithium-ion batteries. Miner. Process. Extr. Met. Rev. 2019, 42, 123–141. [Google Scholar] [CrossRef]
- US Geological Survey. Mineral Commodities Summary 2021; U.S. Geological Survey: Reston, VA, USA, 2021.
- Castor, S.B.; Henry, C.D. Lithium-rich claystone in the McDermitt Caldera, Nevada, USA: Geologic, mineralogical, and geochemical characteristics and possible origin. Minerals 2020, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- US Geological Survey. Mineral Commodities Summary 2020; U.S. Geological Survey: Reston, VA, USA, 2020; p. 204.
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium resources and production: Critical assessment and global projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Xu, P.; Hong, J.; Qian, X.M.; Xu, Z.W.; Xia, H.; Tao, X.C.; Xu, Z.Z.; Ni, Q.Q. Materials for lithium recovery from salt lake brine. J. Mater. Sci. 2021, 56, 16–63. [Google Scholar] [CrossRef]
- Harvard International Review. Available online: https://hir.harvard.edu/lithium-triangle/ (accessed on 18 October 2022).
- Australian Mineral Facts. Available online: https://www.ga.gov.au/education/classroom-resources/minerals-energy/australian-mineral-facts (accessed on 18 October 2022).
- STT, Storage & Transfer. Available online: https://www.sttsystems.com/solutions/lithium-extraction/ (accessed on 18 October 2022).
- Albemarle Corporation. Lithium Resources. 2020. Available online: https://www.albemarle.com/businesses/lithium/resources--recycling/lithium-resources (accessed on 6 October 2020).
- What Is Lithium Extraction and How Does It Work? Samco: Buffalo, NY, USA, 2018; Available online: https://samcotech.com/what-is-lithium-extraction-and-how-does-it-work/ (accessed on 6 October 2022).
- Boryta, D.A. Removal of boron from lithium chloride brine. U.S. Patent 4,261,960, 14 April 1981. [Google Scholar]
- Brown, P.M.; Jacob, S.R.; Boryta, D.A. Production of Highly Pure Lithium Chloride from Impure Brines. U.S. Patent 4,271,131, 2 June 1981. [Google Scholar]
- Aral, A.; Vecchio-Sadus, A. Lithium: Environmental Pollution and Health Effects; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Dapcevich, M. Does Viral Photo Show a ‘Toxic’ Lithium Extraction Field? 2022. Available online: https://www.snopes.com/fact-check/photo-lithium-extraction-mines/ (accessed on 6 October 2022).
- Els, F. All the Mines Tesla Needs to Build 20 Million Cars a Year. Available online: https://www.mining.com/all-the-mines-tesla-needs-to-build-20-million-cars-a-year/ (accessed on 18 October 2022).
- US Cobalt Spot Price. Available online: https://ycharts.com/indicators/us_cobalt_spot_price (accessed on 18 October 2022).
- The Cost of Cobalt. Available online: https://www.aljazeera.com/program/people-power/2021/4/1/the-cost-of-cobalt (accessed on 18 October 2022).
- Li, W.; Lee, S.; Manthiram, A. High-nickel NMA: A cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries. Adv. Mater. 2020, 32, 200271. [Google Scholar] [CrossRef] [PubMed]
- Electrek. Available online: https://electrek.co/2022/04/22/tesla-using-cobalt-free-lfp-batteries-in-half-new-cars-produced/#:~:text=April%2022,Tesla%20is%20already%20using%20cobalt%2Dfree%20LFP%20batteries%20in,of%20its%20new%20cars%20produced&text=Tesla%20confirmed%20that%20nearly%20half,%2Dphosphate%20(LFP)%20batteries (accessed on 6 October 2022).
- Reserves of Cobalt Worldwide in 2021, by Country (in Metric Tons). Available online: https://www.statista.com/statistics/264930/global-cobalt-reserves/ (accessed on 6 October 2022).
- Volkswagen. Available online: https://www.volkswagenag.com/en/news/stories/2020/03/lithium-mining-what-you-should-know-about-the-contentious-issue.html#:~:text=The%20total%20global%20reserves%20are,the%20production%20volume%20in%202018.&text=Where%20is%20the%20most%20lithium,and%20Argentina%20(6%2C200%20tons) (accessed on 6 October 2022).
- Nickel Reserves: Top 8 Countries (Updated 2022). Available online: https://investingnews.com/daily/resource-investing/base-metals-investing/nickel-investing/nickel-reserves-by-country/ (accessed on 6 October 2022).
- Manganese Reserves by Country. Available online: https://investingnews.com/daily/resource-investing/battery-metals-investing/manganese-investing/manganese-reserves/ (accessed on 6 October 2022).
- Lithium Facts. Available online: https://www.nrcan.gc.ca/our-natural-resources/minerals-mining/minerals-metals-facts/lithium-facts/24009 (accessed on 18 October 2022).
- Lithium Data Sheet—Mineral Commodity Summaries 2020. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-lithium.pdf (accessed on 18 October 2022).
- Stringfellow, W.T.; Dobson, P.F. Technology for the recovery of lithium from geothermal brines. Energies 2021, 14, 6805. [Google Scholar] [CrossRef]
- Lithium Mining in China. Available online: http://www.lithiummine.com/lithium-mining-in-china (accessed on 6 October 2022).
- California’s Lithium Rush for ev Batteries Hinges on Taming Toxic, Volcanic Brine. Available online: https://www.forbes.com/sites/alanohnsman/2022/08/31/californias-lithium-rush-electric-vehicles-salton-sea/?sh=79f919694f63 (accessed on 6 October 2022).
- Lithium Shortages: Threat or Opportunity? Available online: https://www.mining-technology.com/analysis/lithium-price-challenges/#:~:text=Supply%20and%20demand,300%25%20between%202021%20and%202030 (accessed on 6 October 2022).
- A Guide to the 6 Main Types of Lithium Batteries. Available online: https://dragonflyenergy.com/types-of-lithium-batteries-guide/ (accessed on 6 October 2022).
- Tesla Model S. Available online: https://en.wikipedia.org/wiki/Tesla_Model_S (accessed on 6 October 2022).
- Plungis, J. How Long Do Electric Car Batteries Last? 2021. Available online: https://www.carfax.com/blog/how-long-do-electric-car-batteries-last#:~:text=All%20EV%20batteries%20will%20lose,years%20to%20about%20132%20miles (accessed on 6 October 2022).
- Tucker, S. Warranty Coverage for Hybrid and EV Batteries. 2022. Available online: https://www.kbb.com/car-advice/hybrid-ev-battery-warranty/#:~:text=Federal%20law%20requires%20automakers%20to,standard%20in%20all%2050%20states (accessed on 6 October 2022).
- BU-802: What Causes Capacity Loss? Available online: https://batteryuniversity.com/article/bu-802-what-causes-capacity-loss (accessed on 6 October 2022).
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode degradation in lithium-ion batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef] [Green Version]
- Manufacturing the Battery for One Electric Car Produces the Same Amount of Carbon Dioxide as Running a Petrol Car for Eight Years. Available online: https://www.politifact.com/factchecks/2021/may/11/viral-image/producing-electric-cars-battery-does-not-emit-same/ (accessed on 22 October 2022).
- Kukreja, B. Life Cycle Analysis of Electric Vehicles; Quantifying the Impact. 2018. Available online: https://sustain.ubc.ca/sites/default/files/2018-63%20Lifecycle%20Analysis%20of%20Electric%20Vehicles_Kukreja.pdf (accessed on 6 October 2022).
- Canada’s Renewable Power—British Columbia. Available online: https://www.cer-rec.gc.ca/en/data-analysis/energy-commodities/electricity/report/canadas-renewable-power/provinces/renewable-power-canada-british-columbia.html (accessed on 18 October 2022).
- Lutsey, N.P.; Hall, D. Effects of Battery Manufacturing on Electric Vehicle Life-Cycle Greenhouse Gas Emissions. February 2018. Project: Electric Vehicle Life Cycle. International Council on Clean Transportation. Available online: https://www.researchgate.net/publication/323118874_Effects_of_battery_manufacturing_on_electric_vehicle_life-cycle_greenhouse_gas_emissions (accessed on 6 October 2022).
- Bruna Alves, B. Distribution of Electricity Production in Norway 2020, by Source. 2022. Available online: https://www.statista.com/statistics/1025497/distribution-of-electricity-production-in-norway-by-source/#:~:text=In%202020%2C%20hydro%20accounted%20for,142%20terawatt%2Dhours%20in%202020 (accessed on 6 October 2022).
- Nuclear Energy in France. 2007. Available online: https://franceintheus.org/spip.php?article637 (accessed on 6 October 2022).
- Electricity Sector in Germany. Available online: https://en.wikipedia.org/wiki/Electricity_sector_in_Germany#:~:text=The%20top%20producers%20were%20the,%25%20biomass%2C%203.7%25%20hydroelectricity (accessed on 6 October 2022).
- Bieker, G. A global Comparison of the Life-Cycle Greenhouse Gas Emissions of Combustion Engine and Electric Passenger Cars. 2021. Available online: https://theicct.org/publication/a-global-comparison-of-the-life-cycle-greenhouse-gas-emissions-of-combustion-engine-and-electric-passenger-cars/ (accessed on 6 October 2022).
- Castelvecchi, D. Electric cars and batteries: How will the world produce enough? Nature 2021, 596, 336–339. [Google Scholar] [CrossRef]
- Lei, C.; Aldous, I.; Hartley, J.M.; Thompson, D.L.; Scott, S.; Hanson, R.; Anderson, P.A.; Kendrick, E.; Sommerville, R.; Ryder, K.S.; et al. Lithium ion battery recycling using high-intensity ultrasonication. Green Chem. 2021, 23, 4710–4715. [Google Scholar] [CrossRef]
- Jacoby, M. It’s Time to Get Serious about Recycling Lithium-Ion Batteries. C&EN 2019, 97. Available online: https://cen.acs.org/materials/energy-storage/time-serious-recycling-lithium/97/i28 (accessed on 4 October 2022).
- Sanguesa, J.A.; Torres-Sanz, V.; Garrido, P.; Martinez, F.J.; Marquez-Barja, J.M. A review on electric vehicles: Technologies and challenges. Smart Cities 2021, 4, 372–404. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Greenhouse gas emissions of automotive lithium-ion batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Lithium Recovery from Brines. Available online: https://www.lenntech.com/processes/lithium-recovery.htm (accessed on 6 October 2022).
- Understanding Cobalt’s Human Cost. 2021. Available online: https://www.sciencedaily.com/releases/2021/12/211217113232.htm#:~:text=Waste%20generated%20from%20mining%20cobalt,were%20unsafe%2C%20unfair%20and%20stressful (accessed on 6 October 2022).
- Apple and Google Named in US Lawsuit over Congolese Child Cobalt Mining Deaths. Available online: https://www.theguardian.com/global-development/2019/dec/16/apple-and-google-named-in-us-lawsuit-over-congolese-child-cobalt-mining-deaths (accessed on 6 October 2022).
- Portuguese Community Files Legal Action against Lithium Mining Company. Available online: https://www.reuters.com/article/portugal-lithium-idUSL8N2Z33JZ (accessed on 6 October 2022).
- The Spiralling Environmental Cost of Our Lithium Battery Addiction. 2018. Available online: https://www.wired.co.uk/article/lithium-batteries-environment-impact (accessed on 6 October 2022).
- Davis, E.P. EU Faces Green Paradox over Electric Vehicles and Lithium Mining. 2018. Available online: https://dialogochino.net/en/uncategorised/eu-faces-green-paradox-over-electric-vehicles-lithium-mining/ (accessed on 6 October 2022).
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Abe, Y. Rare Metal Series—Current Status of Lithium Resources; JOGMEC Mineral Resources Report; Japan Oil, Gas and Metals National Corporation (JOGMEC): Chiba, Japan, 2010.
- Neupane, G.; Wendt, D.S. Assessment of mineral resources in geothermal brines in the US. In Proceedings of the 42nd Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 13–15 February 2017; Stanford University: Stanford, CA, USA, 2017. [Google Scholar]
- Neupane, G.; Wendt, D.S. Potential economic values of minerals in brines of identified hydrothermal systems in the US. Trans. Geotherm. Resour. Counc. 2017, 41, 1938–1956. [Google Scholar]
- Simmons, S.; Kirby, S.; Verplanck, P.; Kelley, K. Strategic and critical elements in produced geothermal fluids from Nevada and Utah. In Proceedings of the 43rd Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 12–14 February 2018; Stanford University: Stanford, CA, USA, 2018. [Google Scholar]
- McKibben, M.; Elders, W.A.; Raju, A.S.K. Chapter 7—Lithium and other geothermal mineral and energy resources beneath the Salton Sea. In Crisis at the Salton Sea: The Vital Role of Science; University of California Riverside Salton Sea Task Force, Environmental Dynamics and GeoEcology (EDGE) Institute, University of California, Riverside: Riverside, CA, USA, 2021. [Google Scholar]
- Zhang, Y.; Wang, L.; Sun, W.; Hu, Y.H.; Tang, H.H. Membrane technologies for Li+/Mg2+ separation from salt-lake brines and seawater: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 7–23. [Google Scholar] [CrossRef]
- Tian, L.; Ma, W.; Han, M. Adsorption behavior of Li+ onto nano-lithium ion sieve from hybrid magnesium/lithium manganese oxide. Chem. Eng. J. 2010, 156, 134–140. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Zhou, A.; He, X.; Sun, Y.; Zhang, J. A positively charged composite nanofiltration membrane modified by EDTA for LiCl/MgCl2 separation. Sep. Purif. Technol. 2017, 186, 233–242. [Google Scholar]
- Somrani, A.; Hamzaoui, A.; Pontie, M. Study on lithium separation from salt lake brines by nanofiltration (NF) and low pressure reverse osmosis (LPRO). Desalination 2013, 317, 184–192. [Google Scholar] [CrossRef]
- Wen, X.; Ma, P.; Zhu, C.; He, Q.; Deng, X. Preliminary study on recovering lithium chloride from lithium-containing waters by nanofiltration. Sep. Purif. Technol. 2006, 49, 230–236. [Google Scholar] [CrossRef]
- Bi, Q.; Zhang, Z.; Zhao, C.; Tao, Z. Study on the recovery of lithium from high Mg2+/Li+ ratio brine by nanofiltration. Water Sci. Technol. 2014, 70, 1690–1694. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-Y.; Cai, L.-J.; Nie, X.-Y.; Song, X.; Yu, J.-G. Separation of magnesium and lithium from brine using a Desal nanofiltration membrane. J. Water Process Eng. 2015, 7, 210–217. [Google Scholar] [CrossRef]
- Yang, G.; Shi, H.; Liu, W.; Xing, W.; Xu, N. Investigation of Mg2+/Li+ separation by nanofiltration. Chin. J. Chem. Eng. 2011, 19, 586–591. [Google Scholar] [CrossRef]
- Tesla Roadster. Available online: https://ev-database.uk/car/1167/Tesla-Roadster#:~:text=The%20battery%20of%20the%20Tesla,on%20a%20fully%20charged%20battery (accessed on 6 October 2022).
- Thompson, L.M.; Harlow, J.E.; Eldesoky, A.; Bauer, M.K.G.; Cheng, J.H.; Stone, W.S.; Taskovic, T.; McFarlane, C.R.M.; Dahn, J.R. Study of electrolyte and electrode composition changes vs time in aged Li-ion cells. J. Electrochem. Soc. 2021, 168, 020532. [Google Scholar] [CrossRef]
- Henschel, J.; Peschel, C.; Günter, F.; Reinhart, G.; Winter, M.; Nowak, S. Reaction product analysis of the most active “inactive” material in lithium-ion batteries—The electrolyte. ii: Battery operation and additive impact. Chem. Mater. 2019, 31, 9977–9983. [Google Scholar] [CrossRef]
- Geantil, P. The Importance of Industrial Battery Temperature Control. 2021. Available online: https://www.fluxpower.com/blog/the-importance-of-industrial-battery-temperature-control#:~:text=In%20general%2C%20lithium%2Dion%20batteries%20can%20be%20charged%20in%20an,high%20as%20140%C2%B0F (accessed on 6 October 2022).
- Global EV Outlook 2018. Available online: https://www.iea.org/reports/global-ev-outlook-2018 (accessed on 6 October 2022).
- NANOMYTE® Solid Electrolytes. Available online: https://www.neicorporation.com/products/batteries/solid-state-electrolyte/ (accessed on 6 October 2022).
- Nichols, D. Next Gen EV Batteries Will Deliver 500-Mile Range. Available online: https://www.greencars.com/news/next-gen-ev-batteries-will-deliver-500-mile-range (accessed on 6 October 2022).
- Chandler, D. New Electrode Design May Lead to More Powerful Batteries. MIT News. 2020. Available online: https://news.mit.edu/2020/solid-batteries-lithium-metal-electrode-0203 (accessed on 6 October 2022).
- Schweiger, H.G.; Obeidi, O.; Komesker, O.; Raschke, A.; Schiemann, M.; Zehner, C.; Gehnen, M.; Keller, M.; Birke, P. Comparison of several methods for determining the internal resistance of lithium ion cells. Sensors 2010, 10, 5604–5625. [Google Scholar] [CrossRef] [Green Version]
- Oliveri, R.L.; Insinga, M.G.; Pisana, S.; Patella, B.; Aiello, G.; Inguanta, R. High-performance lead-acid batteries enabled by Pb and PbO2: Nanostructured electrodes: Effect of operating temperature. Appl. Sci. 2021, 11, 6357. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luong, J.H.T. Recent advances in electrochemical biosensing schemes using graphene and graphene-based nanocomposites. Carbon 2015, 84, 519–550. [Google Scholar] [CrossRef]
- García, F. La Española Graphenano Presenta una Batería que Dura 800 Kilómetros. 2016. Available online: https://www.elmundo.es/motor/2016/02/11/56bc7d6aca4741e31e8b461f.html (accessed on 6 October 2022).
- Anthony, S.; Extremetech. Aluminium-Air Battery Can Power Electric Vehicles for 1000 Miles will Come to Production Cars in 2017. Available online: https://www.extremetech.com/extreme/151801-aluminium-air-battery-can-power-electric-vehicles-for-1000-miles-will-come-to-production-cars-in-2017 (accessed on 6 October 2022).
- Energy Storage Inter-Platform Group. State of the Art of Energy Storage Regulations and Technology. Available online: http://www.futured.es/wp-content/uploads/2016/06/GIA-Maqueta_eng.pdf (accessed on 6 October 2022).
- Kosivi, J.; Gomez, J.; Nelson, R.; Kalu, E.E.; Weatherspoon, M.H. Non-Paste Based Composite Cathode Electrode for Lithium Air Battery; ECS Meeting Abstracts; The Electrochemical Society: Pennington, NJ, USA, 2014; p. 206. [Google Scholar]
- Brown, N.; Cleantechnica. Sodium-Air Batteries May Best Lithium-Air Batteries. 2013. Available online: https://cleantechnica.com/2013/03/20/sodium-air-batteries-may-best-lithium-air-batteries/ (accessed on 6 October 2022).
- Bi, X.; Wang, R.; Yuan, Y.; Zhang, D.; Zhang, T.; Ma, L.; Wu, T.; Shahbazian-Yassar, R.; Amine, K.; Lu, J. From sodium–oxygen to sodium–air battery: Enabled by sodium peroxide dihydrate. Nano Lett. 2020, 20, 4681–4686. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Fichtner, M. Magnesium–Sulfur Battery: Its Beginning and Recent Progress. MRS Commun. 2017, 7, 770–784. [Google Scholar]
- Seeker. Supercharged! Battery Power for the Future. Available online: https://www.seeker.com/supercharged-battery-power-for-the-future-1766230400.html (accessed on 6 October 2022).
- Andrews, J. A Multiscale Approach to Magnesium Intercalation Batteries: Safer, Lighter, and Longer-Lasting. Available online: https://www.nasa.gov/directorates/spacetech/strg/nstrf_2017/Magnesium_Intercalation_Batteries/ (accessed on 6 October 2022).
- Edmondson, J. Will Rare-Earths be Eliminated in Electric Vehicle Motors? Available online: https://www.idtechex.com/en/research-article/will-rare-earths-be-eliminated-in-electric-vehicle-motors/21972 (accessed on 6 October 2022).
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Rangarajan, S.S.; Sunddararaj, S.P.; Sudhakar, A.V.V.; Shiva, C.K.; Subramaniam, U.; Collins, R.; Senjyu, T. Lithium-ion batteries—The crux of electric vehicles with opportunities and challenges. Clean Technol. 2022, 4, 908–930. [Google Scholar] [CrossRef]
- J1850_201510; Vehicle Architecture for Data Communications Standards—Class B Data Communications Network Interface. SAE International: Warrendale, PA, USA, 2009.
- IEC 62196-1:2014; Plugs, Socket-Outlets, Vehicle Couplers and Vehicle Inlets—Conductive Charging of Electric Vehicles—Part 1: General Requirements. IEC: Geneva, Switzerland, 2014.
- GB/T 20234.3-2015 (GB/T20234.3-2015). Available online: https://www.chinesestandard.net/PDF.aspx/GBT20234.3-2015 (accessed on 6 October 2022).
- Tesla. Tesla Official Website. 2019. Available online: https://www.tesla.com/en_eu/supercharger (accessed on 6 October 2022).
- Manshadi, S.D.; Khodayar, M.E.; Abdelghany, K.; Üster, H. Wireless charging of electric vehicles in electricity and transportation networks. IEEE Trans. Smart Grid 2018, 9, 4503–4512. [Google Scholar] [CrossRef]
- Dai, J.; Ludois, D.C. A Survey of wireless power transfer and a critical comparison of inductive and capacitive coupling for small gap applications. IEEE Trans. Power Electron. 2015, 30, 6017–6029. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Gao, F.; Wang, S.; Deng, J. A family of compensation topologies for capacitive power transfer converters for wireless electric vehicle charger. Appl. Energy 2020, 260, 114156. [Google Scholar] [CrossRef]
- Nohara, J.; Omori, H.; Yamamoto, A.; Kimura, N.; Morizane, T. A miniaturized single-ended wireless ev charger with new high power-factor drive and natural cooling structure. In Proceedings of the 2018 IEEE International Power Electronics and Application Conference and Exposition (PEAC), Shenzhen, China, 4–7 November 2018; pp. 1–6. [Google Scholar]
- Bhatti, A.R.; Salam, Z.; Aziz, M.J.B.A.; Yee, K.P.; Ashique, R.H. Electric vehicles charging using photovoltaic: Status and technological review. Renew. Sustain. Energy Rev. 2016, 54, 34–47. [Google Scholar] [CrossRef]
- Calise, F.; Cappiello, F.L.; Cartenì, A.; d’Accadia, M.D.; Vicidomini, M. A novel paradigm for a sustainable mobility based on electric vehicles, photovoltaic panels and electric energy storage systems: Case studies for Naples and Salerno (Italy). Renew. Sustain. Energy Rev. 2019, 111, 97–114. [Google Scholar] [CrossRef]
- Zakeri, B.; Syri, S. Electrical energy storage systems: A comparative life cycle cost analysis. Renew. Sustain. Energy Rev. 2015, 42, 569–596. [Google Scholar] [CrossRef]
- Liu, J.; Hu, C.; Kimber, A.; Wang, Z. Uses, Cost-benefit analysis, and markets of energy storage systems for electric grid applications. J. Energy Storage 2020, 32, 101731. [Google Scholar] [CrossRef]
- Elio, J.; Phelan, P.; Villalobos, R.; Milcarek, R.J. A review of energy storage technologies for demand-side management in industrial facilities. J. Clean. Prod. 2021, 307, 127322. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, B.; Sun, Q.; Wennersten, R. Application of energy storage in integrated energy systems—A solution to fluctuation and uncertainty of renewable energy. J. Energy Storage 2022, 52, 104812. [Google Scholar] [CrossRef]
- Andujar, J.M.; Segura, F.; Rey, J.; Vivas, F.J. Batteries and hydrogen storage: Technical analysis and commercial revision to select the best option. Energies 2022, 15, 6196. [Google Scholar] [CrossRef]
- Chen, D.; Shao, G.-Q.; Li, B.; Zhao, G.-G.; Li, J.; Liu, J.-H.; Gao, Z.-S.; Zhang, H.-F. Synthesis, crystal structure and electrochemical properties of LiFePO4F cathode material for Li-ion batteries. Electrochim. Acta 2014, 147, 663–668. [Google Scholar] [CrossRef]
- Ramesh, T.N.; Lee, K.T.; Ellis, B.L.; Nazar, L.F. Tavorite lithium iron fluorophosphate cathode materials: Phase transition and electrochemistry of LiFePO4F–Li2FePO4F. Electrochem. Solid-State Lett. 2010, 13, A43–A47. [Google Scholar] [CrossRef] [Green Version]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Energy Storage Grand Challenge Roadmap. 2020. Available online: https://www.energy.gov/energy-storage-grandchallenge/downloads/energy-storage-grandchallenge-roadmap (accessed on 18 October 2022).
- Gao, Y.; Jiang, J.; Zhang, C.; Zhang, W.; Ma, Z.; Jiang, Y. Lithium-ion battery aging mechanisms and life model under different charging stresses. J. Power Source 2017, 356, 103–114. [Google Scholar] [CrossRef]
- Hawkins, T.R.; Singh, B.; Majeau-Bettez, G.; Strømman, A.H. Comparative environmental life cycle assessment of conventional and electric vehicles. J. Ind. Ecol. 2012, 17, 53–64. [Google Scholar] [CrossRef]
- Saxena, S.; Le Floch, C.; MacDonald, J.; Moura, S. Quantifying EV battery end-of-life through analysis of travel needs with vehicle powertrain models. J. Power Source 2015, 282, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Sanghai, B.; Sharma, D.; Baidya, K.; Raja, M. Refurbished and Repower: Second Life of Batteries from Electric Vehicles for Stationary Application; SAE Technical Paper 2019-26-0156; SAE: Warrendale, PA, USA, 2019. [Google Scholar]
- Robb, J. Making Stadiums and Arenas More Resilient and Energy Efficient, Publication No. WP701001EN/CSSC-868, Eaton xStorage Buildings White Paper. March 2018. Available online: https://www.eaton.com/content/dam/eaton/markets/buildings/resiliency/documents/xstorage-peak-shaving-whitepaper.pdf (accessed on 25 October 2022).
- Melin, H.E. Circular Opportunities in the Lithium-Ion Industry. Creation Inn: London, UK, 2017. [Google Scholar]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Xia, X.; Li, P. A review of the life cycle assessment of electric vehicles: Considering the influence of batteries. Sci. Total Environ. 2022, 814, 152870. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, S.; Wu, B.; Fernandez, C.; Xiong, X.; Coffieken, J. A state-of-charge estimation method of the power lithium-ion battery in complex conditions based on adaptive square root extended Kalman filter. Energy 2021, 219, 119603. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H. Experimental analysis of thermal runaway in 18650 cylindrical Li-Ion cells using an accelerating rate calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Liu, K.; Wang, Z.; Tong, X.; Guo, L. Theoretical analysis of lithium-ion battery failure characteristics under different states of charge. Fire Mater. 2018, 42, 680–686. [Google Scholar] [CrossRef]
- Crafts, C.C.; Doughty, D.H.; McBreen, J.; Roth, E.P. Advanced Technology Development Program for Lithium-Ion Batteries: Thermal Abuse Performance of 18650 Li-ion Cells. 2004. Available online: https://doi.org/10.2172/918751 (accessed on 19 October 2022).
- Henriksen, M.; Vaagsaether, K.; Lundberg, J.; Forseth, S.; Bjerketvedt, D. Explosion characteristics for Li-ion battery electrolytes at elevated temperatures. J. Hazard. Mater. 2019, 371, 1–7. [Google Scholar] [CrossRef]
- Hess, S.; Wohlfahrt-Mehrens, M.; Wachtler, M. Flammability of Li-Ion battery electrolytes: Flash point and self-extinguishing time measurements. J. Electrochem. Soc. 2015, 162, A3084–A3097. [Google Scholar] [CrossRef]
- LG Chem Develops Fire Retardant Plastic for EV Batteries. Available online: https://www.just-auto.com/news/lg-chem-develops-fire-retardant-plastic-for-ev-batteries/#:~:text=New%20material%20has%20the%20longest,in%20electric%20vehicles%20(EVs) (accessed on 20 October 2022).
- Zhu, J.; Darma, M.S.D.; Knapp, M.; Sørensen, D.R.; Heere, M.; Fang, Q.; Wang, X.; Dai, H.; Mereacre, L.; Senyshyn, A.; et al. Investigation of lithium-ion battery degradation mechanisms by combining differential voltage analysis and alternating current impedance. J. Power Sources 2020, 448, 227575. [Google Scholar] [CrossRef]
- Zhu, J.; Knapp, M.; Darma, M.S.D.; Fang, Q.; Wang, X.; Dai, H.; Wei, X.; Ehrenberg, H. An improved electro-thermal battery model complemented by current dependent parameters for vehicular low temperature application. Appl. Energy 2019, 248, 149–161. [Google Scholar] [CrossRef]
- Wang, Q.-K.; He, Y.-J.; Shen, J.-N.; Hu, X.-S.; Ma, Z.-F. State of charge-dependent polynomial equivalent circuit modeling for electrochemical impedance spectroscopy of lithium-ion batteries. IEEE Trans. Power Electron. 2018, 33, 8449–8460. [Google Scholar] [CrossRef]
- Han, L.; Jiao, X.H.; Zhang, Z. Recurrent neural network-based adaptive energy management control strategy of plug-in hybrid electric vehicles considering battery aging. Energies 2020, 13, 202. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.L.; Hu, X.; Wei, Z.; Li, Y.; Jiang, Y. Modified Gaussian process regression models for cyclic capacity prediction of lithium-ion batteries. IEEE Trans. Transp. Electrif. 2019, 5, 1225–1236. [Google Scholar] [CrossRef]
- Tagade, P.; Hariharan, K.S.; Ramachandran, S.; Khandelwal, A.; Naha, A.; Kolake, S.M.; Han, S.H. Deep Gaussian process regression for lithium-ion battery health prognosis and degradation mode diagnosis. J. Power Sources 2020, 445, 227281. [Google Scholar] [CrossRef]
- Dong, G.; Yang, F.; Wei, Z.; Wei, J.; Tsui, K.-L. Data-driven battery health prognosis using adaptive Brownian motion model. IEEE Trans. Ind. Inf. 2020, 16, 4736–4746. [Google Scholar] [CrossRef]
- Liu, K.; Ashwin, T.R.; Hu, X.; Lucu, M.; Dhammika, W.W. An evaluation study of different modelling techniques for calendar ageing prediction of lithium-ion batteries. Renew. Sustain. Energy Rev. 2020, 131, 110017. [Google Scholar] [CrossRef]
- Tian, J.P.; Xiong, R.; Yu, Q.Q. Fractional-Order model-based incremental capacity analysis for degradation state recognition of lithium-ion batteries. IEEE Trans. Ind. Electron. 2019, 66, 1576–1584. [Google Scholar] [CrossRef]
- Xiong, R.; Li, L.; Li, Z.; Yu, Q.; Mu, H. An electrochemical model based degradation state identification method of lithium-ion battery for all-climate electric vehicles application. Appl. Energy 2018, 219, 264–275. [Google Scholar] [CrossRef]
- Ouyang, T.; Xu, P.; Chen, J.; Su, Z.; Huang, G.; Chen, N. A novel state of charge estimation method for lithium-ion batteries based on bias compensation. Energy 2021, 226, 120348. [Google Scholar] [CrossRef]
- Ghannoum, A.; Nieva, P. Graphite lithiation and capacity fade monitoring of lithium ion batteries using optical fibers. J. Energy Storage 2020, 28, 101233. [Google Scholar] [CrossRef]
- Park, K.Y.; Park, J.-W.; Seong, W.M.; Yoon, K.; Hwang, T.-H.; Ko, K.-H.; Han, J.H.; Yang, J.; Kang, K. Understanding capacity fading mechanism of thick electrodes for lithium-ion rechargeable batteries. J. Power Sources 2020, 468, 228369. [Google Scholar] [CrossRef]
- Li, X.Y.; Huang, Z.; Tian, J.; Tian, Y. State-of-charge estimation tolerant of battery aging based on a physics-based model and an adaptive cubature Kalman filter. Energy 2021, 220, 119767. [Google Scholar] [CrossRef]
- Guo, Y.J.; Yang, Z.; Liu, K.; Zhang, Y.; Feng, W. A compact and optimized neural network approach for battery state-of-charge estimation of energy storage system. Energy 2021, 219, 119529. [Google Scholar] [CrossRef]
- Kadiyala, P.G.; Bryson, A.E.; Schmidt, S.F. Discrete square root filtering: A survey of current techniques. IEEE Trans. Automat. Control 1971, 16, 727–735. [Google Scholar]
- Wang, S.; Takyi-Aninakwa, P.; Jin, S.; Yu, C.; Fernandez, C.; Stroe, D.-I. An improved feedforward-long short-term memory modeling method for the whole-life-cycle state of charge prediction of lithium-ion batteries considering current-voltage-temperature variation. Energy 2022, 254, 124224. [Google Scholar] [CrossRef]
- Al-Saadi, M.; Olmos, J.; Saez-de-Ibarra, A.; Van Mierlo, J.; Berecibar, M. Fast charging impact on the lithium-ion batteries’ lifetime and cost-effective battery sizing in heavy-duty electric vehicles applications. Energies 2022, 15, 1278. [Google Scholar] [CrossRef]
- Hasan, M.; Avramis, N.; Ranta, M.; Saez-De-Ibarra, A.; El Baghdadi, M.; Hegazy, O. Multi-objective energy management and charging strategy for electric bus fleets in cities using various ECO strategies. Sustainability 2021, 13, 47865. [Google Scholar] [CrossRef]
- Mathieu, R.; Briat, O.; Gyan, P.; Vinassa, J.-M. Comparison of the impact of fast charging on the cycle life of three lithium-ion cells under several parameters of charge protocol and temperatures. Appl. Energy 2020, 283, 116344. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).