Effect of Liquid Hot Water Pretreatment on Hydrolysates Composition and Methane Yield of Rice Processing Residue
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Analytical Methods
2.3. Pretreatment Conditions
2.4. Anaerobic Digestion
2.4.1. Biochemical Methane Potential (BMP) Test
2.4.2. Kinetic Models
3. Results and Discussion
3.1. Substrate Composition
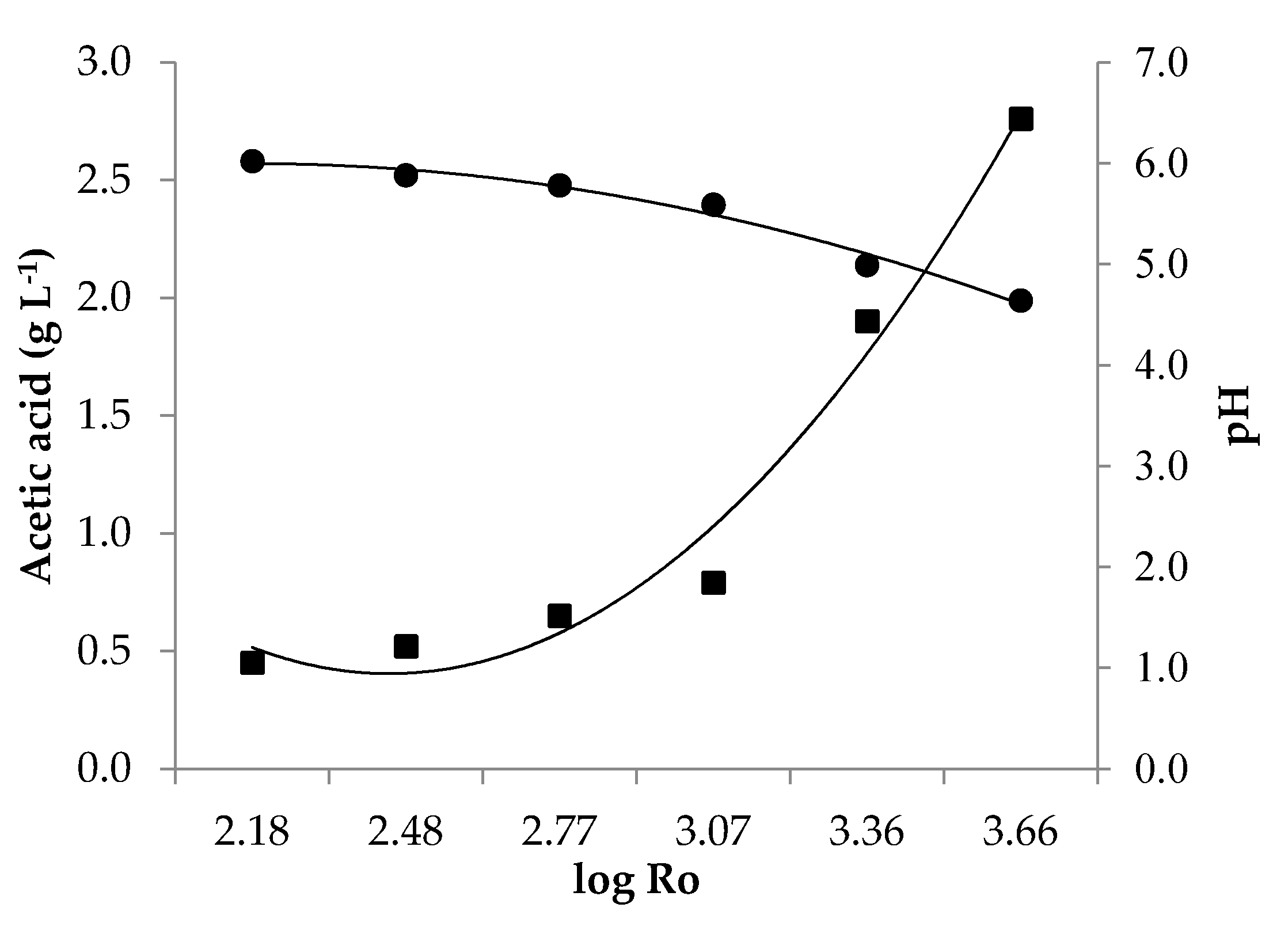
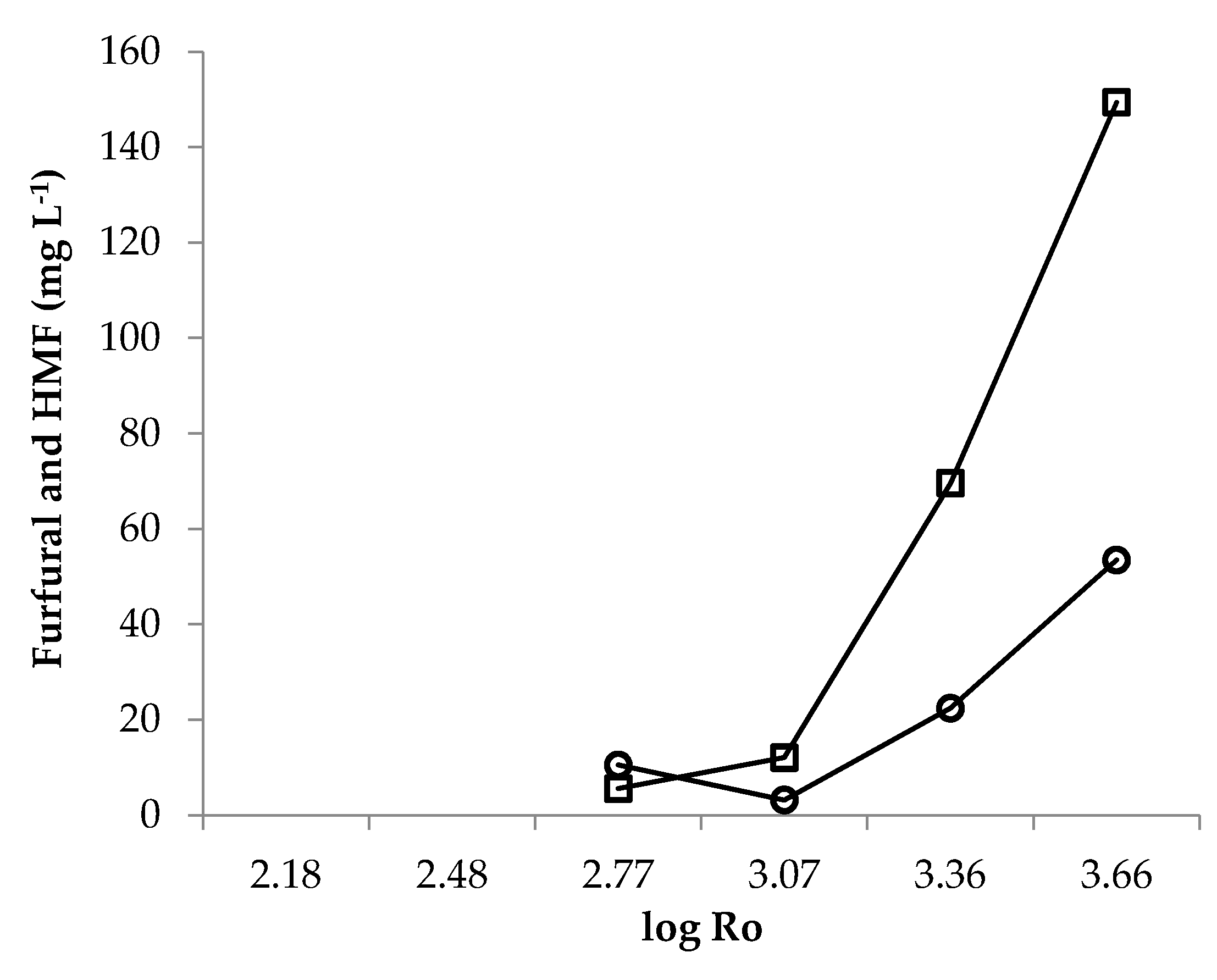
3.2. Effect of LHW Pretreatment on Hydrolysates Composition
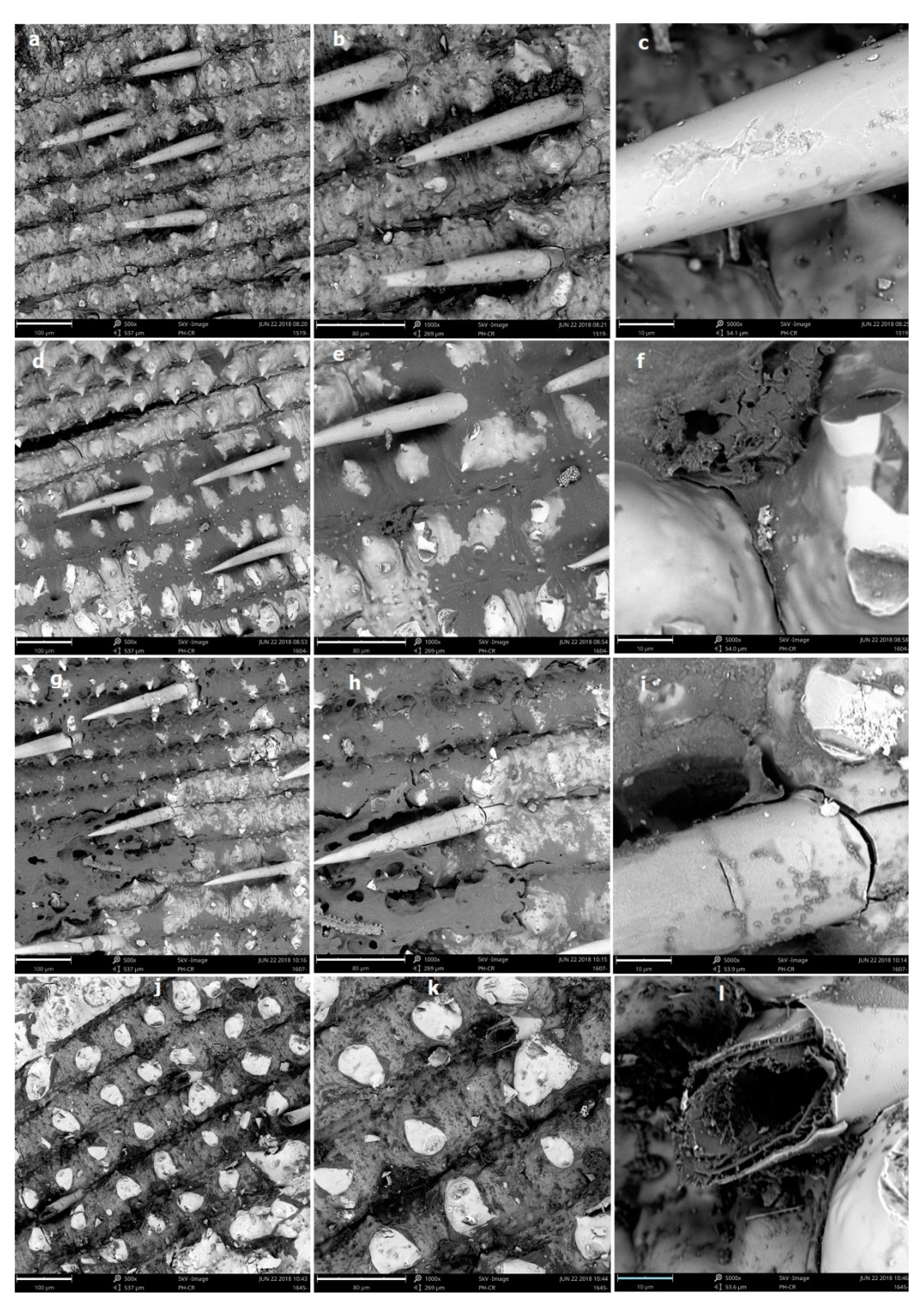
3.3. Effect of LHW Pretreatment on Structural Changes
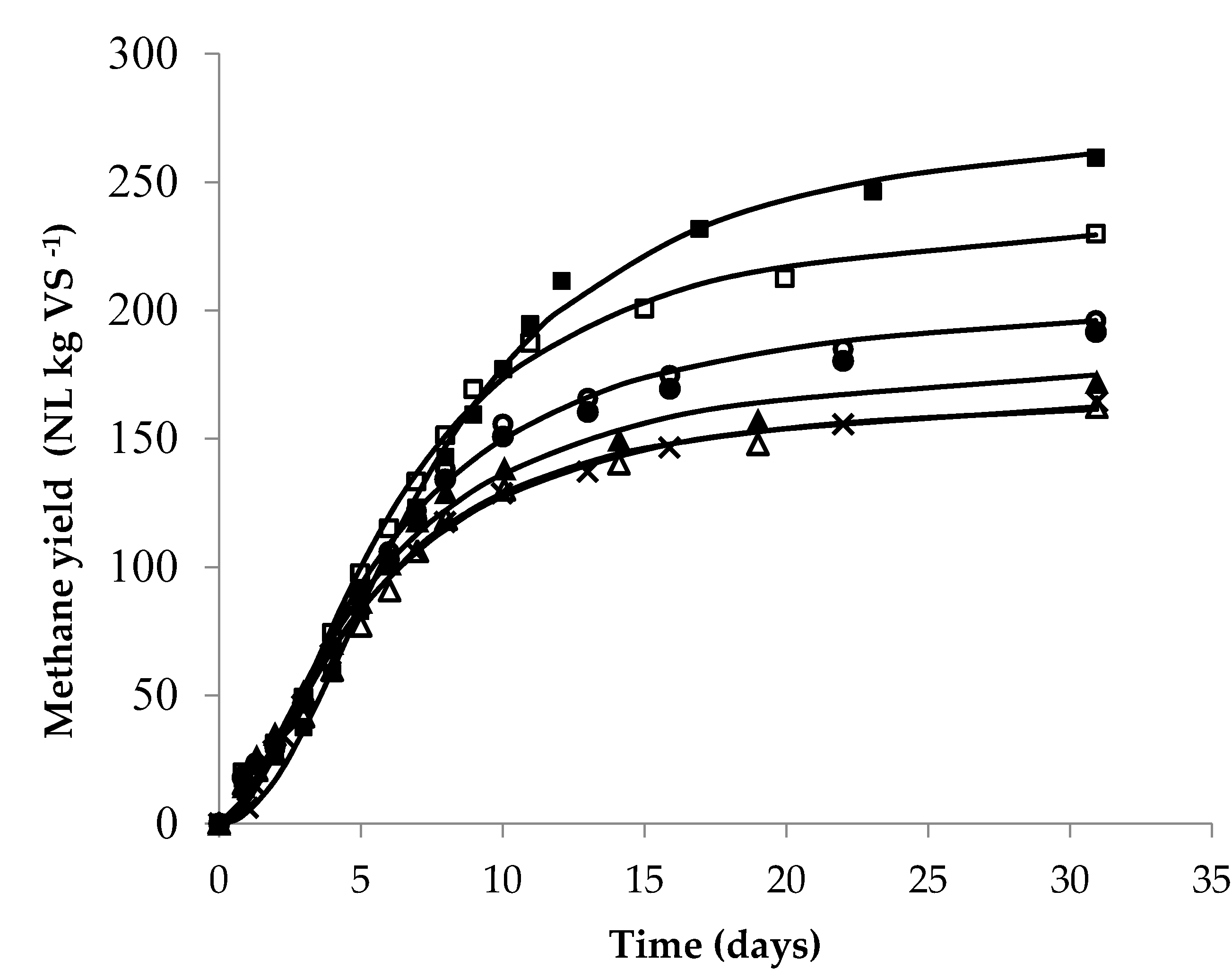
3.4. Effect of LHW Pretreatment on Methane Yield
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Food Outlook—Biannual Report on Global Food Markets. Available online: http://www.fao.org3ca4526enca4526en.pdf (accessed on 18 September 2019).
- IRRI By-products. International Rice Research Institute (IRRI) Rice Knowledge Bank. Available online: http://www.knowledgebank (accessed on 17 January 2020).
- Contreras, L.M. Digestión Anaerobia de Residuos de la Agroindustria Arrocera Cubana Para la Producción de Biogás [Anaerobic Digestion of Residues from Rice Agro-Industry Residues for Biogas Production]. Ph.D.Thesis, Universidad Central “Marta Abreu”, de Las Villas, Santa Clara, Cuba, 2013. [Google Scholar]
- Liu, Q.; Pan, S.; Long, Z.; Li, Z.; Du, L.; Wei, Y. Assessment of Fresh and Dry Rice Straw for Biogas Potential by Anaerobic Digestion. Bioenerg. Res. 2020, 13, 845–852. [Google Scholar] [CrossRef]
- Mofijur, M.; Mahlia, T.M.I.; Logeswaran, J.; Anwar, M.; Silitonga, A.S.; Rahman, S.M.A.; Shamsuddin, A.H. Potential of Rice Industry Biomass as a Renewable Energy Source. Energies 2019, 12, 4116. [Google Scholar] [CrossRef]
- Contreras, L.M.; Schelle, H.; Sebrango, C.R.; Pereda, I. Methane potential and biodegradability of rice straw, rice husk and rice residues from the drying process. Water Sci. Technol. 2012, 65, 1142–1149. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.-I. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Ahmad, F.; Silva, E.L.; Varesche, M.B.A. Hydrothermal processing of biomass for anaerobic digestion—A review. Renew. Sust. Energ. Rev. 2018, 98, 108–124. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Lignocellulosic Biomass in the Bioethanol Production Process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef]
- Fernandez-Cegri, V.; De la Rubia, M.A.; Raposo, F.; Borja, R. Effect of hydrothermal pretreatment of sunflower oil cake on biomethane potential focusing on fibre composition. Bioresour. Technol. 2012, 123, 424–429. [Google Scholar] [CrossRef] [PubMed]
- López González, L.M.; Pereda Reyes, I.; Dewulf, J.; Budde, J.; Heiermann, M.; Vervaeren, H. Effect of liquid hot water pre-treatment on sugarcane press mud methane yield. Bioresour. Technol. 2014, 169, 284–290. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, C. Hydrothermal processing of lignocellulosic materials. Holz. Als. Roh. Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, Y.; Huang, H.; Khunjar, W.; Wang, Z.-W. Recalcitrant dissolved organic nitrogen formation in thermal hydrolysis pretreatment of municipal sludge. Environ. Int. 2020, 138, 105629. [Google Scholar] [CrossRef]
- Antwi, E.; Engler, N.; Nelles, M.; Schüch, A. Anaerobic digestion and the effect of hydrothermal pretreatment on the biogas yield of cocoa pods residues. Waste Manag. 2019, 88, 131–140. [Google Scholar] [CrossRef]
- Ziemiński, K.; Romanowska, I.; Kowalska-Wentel, M.; Cyran, M. Effects of hydrothermal pretreatment of sugar beet pulp for methane production. Bioresour. Technol. 2014, 166, 187–193. [Google Scholar] [CrossRef]
- Wang, D.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Luo, T.; Mei, Z. Can hydrothermal pretreatment improve anaerobic digestion for biogas from lignocellulosic biomass? Bioresour. Technol. 2018, 249, 117–124. [Google Scholar] [CrossRef]
- Budde, J.; Heiermann, M.; Suárez-Quiñones, T.; Plöchl, M. Effects of thermobarical pretreatment of cattle waste as feedstock for anaerobic digestion. Waste Manag. 2014, 34, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoon, Y.-M.; Han, S.K.; Kim, D.; Kim, H. Effect of hydrothermal pre-treatment (HTP) on poultry slaughterhouse waste (PSW) sludge for the enhancement of the solubilization, physical properties, and biogas production through anaerobic digestion. Waste Manag. 2017, 64, 327–332. [Google Scholar] [CrossRef] [PubMed]
- VDLUFA. Bestimmung von flüchtigen stickstoffhaltigen Basen B. Durch Destillation. In Die chemische Untersuchung von Futtermitteln, Methodenbuch Band III [Determination of volatile nitrogenous bases B. By distillation. In The Chemical Analysis of Feed, Method Book, 3rd ed.; VDLUFA: Darmstadt, Germany, 2006. [Google Scholar]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Kaplonek, W.; Nadolny, K. Advanced Desktop SEM Used for Measurement and Analysis of The Abrasive Tool’s Active Surface. Acta Microsc. 2013, 22, 278–288. [Google Scholar]
- Wirth, B.; Krebs, M.; Andert, J. Anaerobic degradation of increased phenol concentrations in batch assays. Environ. Sci. Pollut. Res. 2015, 22, 19048–19059. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. A 1987, 321, 526–533. [Google Scholar]
- VDI. Standard Procedures 4630. Fermentation of Organic Materials. Characterisation of the Substrates, Sampling, Collection of Material Data, Fermentation Tests; Verein Deutscher Ingenieure. Beuth Verlag: Berlin, Germany, 2006. [Google Scholar]
- Buswell, A.M.; Mueller, H.F. Mechanism of Methane Fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- O’Rourke, J.T. Kinetics of Anaerobic Treatment at Reduced Temperatures. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 1968. [Google Scholar]
- Park, B.-D.; Wi, S.G.; Lee, K.H.; Singh, A.P.; Yoon, T.-H.; Kim, Y.S. Characterization of anatomical features and silica distribution in rice husk using microscopic and micro-analytical techniques. Biomass Bioenergy 2003, 25, 319–327. [Google Scholar] [CrossRef]
- Wu, J.; Elliston, A.; Le Gall, G.; Colquhoun, I.J.; Collins, S.R.A.; Wood, I.P.; Dicks, J.; Roberts, I.N.; Waldron, K.W. Optimising conditions for bioethanol production from rice husk and rice straw: Effects of pre-treatment on liquor composition and fermentation inhibitors. Biotechnol. Biofuels 2018, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yano, S.; Inoue, H.; Inoue, S.; Endo, T.; Sawayama, S. Pretreatment of Rice Straw by a Hot-Compressed Water Process for Enzymatic Hydrolysis. Appl. Biochem. Biotech. 2010, 160, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Trably, E.; Barakat, A.; Quemeneur, M.; Steyer, J.P.; Carrère, H. Do by-products of thermochemical treatment of lignocellulosic materials inhibit anaerobic mixed cultures?. Overview of recent findings. In Proceedings of the 13th World Congress on Anaerobic Digestion. Recovering (bio) Resources for the World, Santiago de Compostela, España, 25–28 June 2013. [Google Scholar]
- Fijalkowski, M.; Adach, K.; Petráň, A.; Kroisová, D. Rice Husks—Structure, Composition and Possibility of Use them at Surface Treatment. Mater. Sci. Forum. 2016, 844, 153–156. [Google Scholar] [CrossRef]
- Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Comparative study of various pretreatment reagents on rice husk and structural changes assessment of the optimized pretreated rice husk. Bioresour. Technol. 2013, 135, 116–119. [Google Scholar] [CrossRef]
- Ang, T.N.; Ngoh, G.; Chua, A.; Lee, M. Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses. Biotechnol. Biofuels 2012, 5, 67. [Google Scholar] [CrossRef]
- Ullah, Z.; Man, Z.; Khan, A.S.; Muhammad, N.; Mahmood, H.; Ben Ghanem, O.; Ahmad, P.; Hassan Shah, M.-U.; Mamoon Ur, R.; Raheel, M. Extraction of valuable chemicals from sustainable rice husk waste using ultrasonic assisted ionic liquids technology. J. Clean. Prod. 2019, 220, 620–629. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Rajput, A.A.; Zeshan; Visvanathan, C. Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J. Envrion. Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.; Zhang, C.; Wang, F.; Qiu, L.; Guo, X.; Xu, F. Liquid hot water pretreatment to enhance the anaerobic digestion of wheat straw-effects of temperature and retention time. Env. Sci. Pollut. Res. Int. 2019, 26, 29424–29434. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Huang, H.; Mei, Z.; Shen, F.; Ge, Y.; Hu, G.; Meng, X. Hydrothermal pretreatment of rice straw at relatively lower temperature to improve biogas production via anaerobic digestion. Chin. Chem. Lett. 2019, 30, 1219–1223. [Google Scholar] [CrossRef]
- He, L.; Huang, H.; Zhang, Z.; Lei, Z.; Lin, B.-L. Energy Recovery from Rice Straw through Hydrothermal Pretreatment and Subsequent Biomethane Production. Energ. Fuel 2017, 31, 10850–10857. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, X.; Zhang, Q.; Li, Y. Comparison of liquid hot water and alkaline pretreatments of giant reed for improved enzymatic digestibility and biogas energy production. Bioresour. Technol. 2016, 216, 60–68. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Grigatti, M.; Barbanti, L.; Angelidaki, I. Effects of hydrothermal pre-treatments on Giant reed (Arundo donax) methane yield. Bioresour. Technol. 2013, 147, 152–159. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef]
- Chun, J.; Lee, J.H. Recent Progress on the Development of Engineered Silica Particles Derived from Rice Husk. Sustainability 2020, 12, 10683. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K. Energy Recovery Together with Amorphous Nanosilica Production from Rice Straw via Dry Anarobic Digestion. Bioresources 2018, 13, 1872–1884. [Google Scholar] [CrossRef]

| Parameters | Units | Average Values * |
|---|---|---|
| pH | 6.07 | |
| TS105 | % FM | 93.88 |
| VS | %TS | 61.28 |
| Ash | %TS | 38.72 |
| Crude protein | %TS | 7.85 |
| Hemicellulose | %TS | 18.31 |
| Cellulose | %TS | 19.09 |
| Lignin | %TS | 6.97 |
| C | %TS | 34.32 |
| H | %TS | 3.34 |
| N | %TS | 1.26 |
| S | %TS | 0.10 |
| C/N | 27.33 |
| T (°C) | t (min) | log Ro | Residual RD (%) | pH | Acetic Acid (g L−1) | Propionic Acid (g L−1) | HMF (mg L−1) | Furfural (mg L−1) |
|---|---|---|---|---|---|---|---|---|
| 140 | 10 | 2.18 | 61 | 6.02 | 0.45 | 0.10 | n.d. | n.d. |
| 140 | 20 | 2.48 | 63 | 5.88 | 0.52 | 0.09 | n.d. | n.d. |
| 160 | 10 | 2.77 | 59 | 5.78 | 0.65 | 0.13 | 10.53 | 5.58 |
| 160 | 20 | 3.07 | 55 | 5.59 | 0.79 | 0.12 | 7.91 | 12.08 |
| 180 | 10 | 3.36 | 37 | 4.99 | 1.90 | 0.24 | 22.44 | 69.58 |
| 180 | 20 | 3.66 | 32 | 4.64 | 2.76 | 0.26 | 53.47 | 149.40 |
| log (Ro) | yexp (L CH4 kg−1VS) | % CH4 | η (%) | ymax (L CH4 kg−1VS) | Rm (L CH4 kg−1VS d−1) | λ (Days) |
|---|---|---|---|---|---|---|
| 0.00 | 164 e ± 6.60 | 50.63 | 40 | 169 e ± 6.99 | 18.06 c ± 0.74 | 0.52 bc ± 0.01 |
| 2.18 | 162 e ± 6.06 | 49.62 | 39 | 171 e ± 5.23 | 17.00 d ± 0.25 | 0.47 cd ± 0.05 |
| 2.48 | 167 d ± 6.53 | 50.88 | 40 | 185 d ± 4.49 | 18.51 c ± 0.11 | 0.29 d ± 0.08 |
| 2.77 | 196 c ± 1.61 | 50.27 | 47 | 208 c ± 2.20 | 19.55 bc ± 0.27 | 0.46 cd ± 0.04 |
| 3.07 | 191 c ± 4.59 | 50.21 | 46 | 203 c ± 5.69 | 18.84 b ± 0.05 | 0.41 cd ± 0.05 |
| 3.36 | 230 b ± 5.53 | 49.60 | 55 | 240 b ± 5.74 | 22.27 a ± 0.65 | 0.71 b ± 0.17 |
| 3.66 | 260 a ± 3.79 | 49.12 | 63 | 276 a ± 4.06 | 22.07 a ± 0.74 | 1.32 a ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López González, L.M.; Heiermann, M. Effect of Liquid Hot Water Pretreatment on Hydrolysates Composition and Methane Yield of Rice Processing Residue. Energies 2021, 14, 3254. https://doi.org/10.3390/en14113254
López González LM, Heiermann M. Effect of Liquid Hot Water Pretreatment on Hydrolysates Composition and Methane Yield of Rice Processing Residue. Energies. 2021; 14(11):3254. https://doi.org/10.3390/en14113254
Chicago/Turabian StyleLópez González, Lisbet Mailin, and Monika Heiermann. 2021. "Effect of Liquid Hot Water Pretreatment on Hydrolysates Composition and Methane Yield of Rice Processing Residue" Energies 14, no. 11: 3254. https://doi.org/10.3390/en14113254
APA StyleLópez González, L. M., & Heiermann, M. (2021). Effect of Liquid Hot Water Pretreatment on Hydrolysates Composition and Methane Yield of Rice Processing Residue. Energies, 14(11), 3254. https://doi.org/10.3390/en14113254






