Abstract
Ethanol organosolv fractionation combined with ball milling was conducted on three major agricultural residues: Rice husk (RH), rice straw (RS), and barley straw (BS). The highest lignin extraction yields of RH, RS, and BS were 55.2%, 53.1%, and 59.4% and the purity of lignin recovered was 99.5% for RH and RS, and 96.8% for BS, with similar chemical characteristics, i.e., low molecular weight distributions (1453–1817 g/mol) and poly dispersity index (1.15–1.28). However, considering the simultaneous production of hemicellulose-derived sugars, distinctive fractionation behaviors were shown for the three agricultural residues. The highest hemicellulose-derived sugar yield was 73.8% when RH was fractionated at 170 °C for 30 min. Meanwhile, very low sugar yields of 31.9% and 35.7% were obtained from RS and BS, respectively. The highest glucan-to-glucose conversion yield from enzymatic hydrolysis of fractionated RH reached 85.2%. Meanwhile, the enzymatic digestibility of the fractionated RS and BS was 60.0% and 70.5%, respectively. Consequently, the fractionation efficiency for RH can be improved with fine refinement. For the case of RS, other fractionation process should be applied to achieve effective fractionation performance.
1. Introduction
Due to the enormous challenges posed by increasingly severe climate change, lignocellulosic biomass, a renewable and sustainable resource, has been considered as a promising alternative to the finite oil reserves as it can be used to produce alternative fuels and chemicals [1]. The serious environmental problems associated with the rapidly increasing use of fossil fuels are currently increasing the need for greater use of renewable resources, such as lignocellulosic biomass. Because lignocellulosic biomass is abundant and reproducible, various efforts to use it for the production of industrial bio-based products have attracted great attention over the past decades [2,3].
Recently, numerous efforts have been made to study how to effectively utilize various lignocellulosic biomass, including those obtained from agricultural residues, forest residues, and energy crops [4]. It is assumed that one of the aforementioned various agricultural resources must offer an important renewable source that is a byproduct of crop cultivation and has a structure that is more accessible by enzymes or other chemicals compared to other biomass and is therefore expected to play an important role in future biorefineries. At present, large amounts of agricultural products are cultivated all over the world, and appropriate planning is required to manage the large amounts of agricultural residues produced. Some of the agricultural byproducts produced are used in animal feeding, animal bedding, soil mulching, composting, and household fuels, but most of it is generally incinerated and disposed on-farm, which has a negative impact on human health and the environment [5].
According to a Statista (Hamburg, Germany) report on worldwide 2017/2018 paddy rice production, there are two major regions of South and East Asia. In detail, China and India are two major rice producers in the world. In 2018, China’s rice production was more than 290 million metric tons (MMT), accounting for one-third of the world’s rice production (495.9 MMT worldwide). For reference, Chinese rice production is nearly twice that of India (169.5 MMT) and hundreds of times that of Korea (5.3 MMT) [6]. The global production volume of barley amounted to 142.4 MMT in the 2017/2018 crop year. This statistic shows the European Union (EU), the leading producer, produced over 56 MMT of barley that year [7]. A factsheet from FAO (Food and Agriculture Organization of the United Nations) on rice production reports a “rice straw (lignocellulose)-to-grain (starch)” ratio of 1.1 for most currently planted rice varieties, and rice husk accounts for about 20 wt.% of grain, and is one of the major agro-industrial by-products produced worldwide [2]. Although the straw-to-grain ratio of barley is affected by factors, such as the plant height, spike length, and grain weight, it is generally considered to be approximately 0.75 [8].
The agricultural residues mentioned above, rice straw (RS), rice husk (RH), and barley straw (BS), are fibrous residues generated after the harvest of the principal grains used by mankind, and they are produced at a greater amount than other biomass types. It is a raw material that does not compete with food and is relatively easy to collect and transport using existing infrastructure.
One of the more efficient uses of agricultural by-products (lignocellulosic biomass) into fuels and chemicals is through the biorefining process. Agricultural by-product feedstock is separated into three main constituents (cellulose, hemicellulose, and lignin), each of which is converted into fuel, chemicals, and other materials through a variety of thermochemical and biochemical processes [2]. However, the physicochemical linkage of cellulose–hemicellulose–lignin is a major factor affecting the rate and yield of enzymatic saccharification and makes the biodegradation of lignocellulosic very slow or extremely difficult in nature. It is believed that the extensive and complex interactions that occur in the molecular and macromolecular networks between cellulose, hemicellulose, and lignin in the plant cell wall matrix contribute to the difference in their reactivity during pretreatment. Information on the composition of the plant cell walls of various plant resources is very importance in developing process technologies that make the best use of lignocellulosic biomass and the added value through mechanical, chemical, and biological pretreatment. [1].
Typically, 60% to 75% (based on weight) of lignocellulosic biomass is composed of polysaccharides (cellulose and hemicellulose), which can be hydrolyzed to produce five different monosaccharides, such as glucose, xylose, arabinose, galactose, and mannose, and then further utilized as a substrate in microbial fermentative for the production of value-added products [9]. In the conversion of biomass, hydrolysis of polysaccharides to monosaccharides, in particular cellulose to glucose is a major hurdle to achieve complete utilization of biomass. Following cellulose, hemicellulose is a macromolecule composed mainly of xylose, but other sugars with a lower molecular weight than that of cellulose, such as mannose, galactose, rhamnose, and arabinose, are also contained. These water–carbohydrates can be converted into various industrial chemicals and fuels, such as ethanol, hydrocarbon substitutes, and starting monomers, for the production of biopolymers [10,11]. In addition to cellulose and hemicellulose, lignin is a complex macromolecule that accounts for a significant portion of the plant cell wall, and lignin is the second most abundant biopolymer on earth after cellulose [12]. Basically, lignin monomers are divided into three aromatic alcohols: p-coumaryl, coniferyl, and sinapyl [13]; these three-unit monomers are linked by various types of ethers and carbon–carbon bonds to form large polymers [14]. Despite the many benefits of lignin, such as abundance and high potential, i.e., high carbon content and biodegradability, purification of lignin from lignocellulosic biomass still remains difficult and current process technology development is still unsatisfactory. Thus, the efficient separation and utilization of lignin remains a challenging part of research and investment in bioremediation processes despite its high potential as a carbon source for future biochemical industries [15,16].
Therefore, the development of an effective process that separates the lignin and cellulose fractions from lignocellulosic biomass is necessary for an economically feasible biomass-refining industry. The main requirement for an economically viable process is to generate highly pure lignin, and minimal waste compounds using efficient processing [17]. In general, the fractionation methods for lignocellulosic biomass mainly include physical, chemical, and biological methods and/or their combinations [18]. These varied processes have different impacts on the structure of the lignocellulosic biomass after processing, and result in a significant impact on the downstream biomass conversion process in terms of sugar recovery yields, toxicity of hydrolysates, enzymatic hydrolysis rate and yield, microbial fermentation yield, and waste treatment costs incurred [19]. Among them, thermochemical fractionation results in the production of severe sugar-degraded products and various toxic compounds in the liquid hydrolysate, such as 5-hydroxymethylfurfural (HMF), furfural, formic acid, and acetic acid [20]. Organosolv fractionation has been known to provide a high recovery yield of lignin-derived and hemicellulose-derived sugars; however, fine optimization of the reaction conditions used for the hydrolysis is important to ensure high monomeric sugar recovery and minimal release of potential inhibitors to microbial fermentation. Therefore, an effective lignin separation process needs to be developed to reduce the toxicity of hemicellulosic hydrolysate during fractionation, which makes the high value-added material production from biorefining more economically viable.
In recent years, the conventional ball milling pretreatment method has received a lot of interest as an effective physical pretreatment method, combining a proven chemical pretreatment and a ball milling pretreatment process, and has been proposed to increase the enzymatic hydrolysis of biomass. [18,21,22,23,24]. Previous studies have reported that ball milling pretreatment methods increase the accessible surface area of biomass and effectively reduce the crystallinity and polymerization degree of cellulose [25,26,27]. Among the chemical pretreatment methods, organosolv fractionation using organic solvents is known as an efficient method for effectively depolymerizing lignin from lignocellulosic biomass. In general, the organosolv fractionation method employs organic solvent, mainly ethanol, which can be recovered and reused [28,29]. The advantage of using organosolv fractionation method is that highly pure lignin can be obtained in a liquid form, which can be used for high-value products, such as adhesives, fibers, and biodegradable polymers [30,31]. In our previous work [32], ball milling combined with organosolv fractionation was reported as an effective method in terms of increased glucan retention in solid residues and lignin recovery, which resulted in significantly improved enzyme digestibility on herbaceous biomass. After the organosolv pretreatment, a cellulose-rich solid cake was obtained, whereas most of the lignin and part of the hemicellulose were recovered by dissolution in an organic solvent and recovered. Obviously, lignin removal leads to an increased surface area, making cellulose more accessible to enzymes [33].
In this study, ethanol organosolv fractionation combined with ball milling was conducted on various agricultural residues. Three major agricultural residues, RH, RS, and BS, were chosen to characterize the fractionation behaviors. The effectiveness of the fractionation process was evaluated in terms of the glucan retention, hemicellulose-derived sugar recovery, and lignin recovery yield. The enzymatic digestibility of the residual solids and byproduct formation were also determined.
2. Materials and Methods
2.1. Raw Materials
Three different agricultural residues—RH, RS, and BS—were provided by the National Institute of Crop Science (NICS), Rural Development Administration (RDA, Wanju, Jeolla-bukdo, Korea). The samples were ground by a commercial blender (Blender 7012s, Waring Commercial, CT, USA) and then sieved to a nominal size of 14–45 mesh (from 0.36 to 1.40 mm). The ground biomass was placed in a convection oven at 45 ± 5 °C for 48 h, and then stored in an automatic dehumidification desiccator until use. The average moisture content of the dried residues was 4.0% to 5.5% during the experiments. Ethanol (cat. no. E7023), sulfuric acid (cat. no. 258105), tetrahydrofuran (cat. no. 401757), pyridine (cat. no. 270970), chloroform-d (cat. no. 151858), cyclohexanol (cat. no. 105899), chromium (III) acetylacetonate (cat. no. 574082), and 2-chloro-4,4,5,5-tetramethyl-1,2,3-dioxaphospholane (cat. no. 447536) were purchased from Sigma–Aldrich Korea (Yongin, Gyeongdo, Korea).
2.2. Experimental Setup and Operation
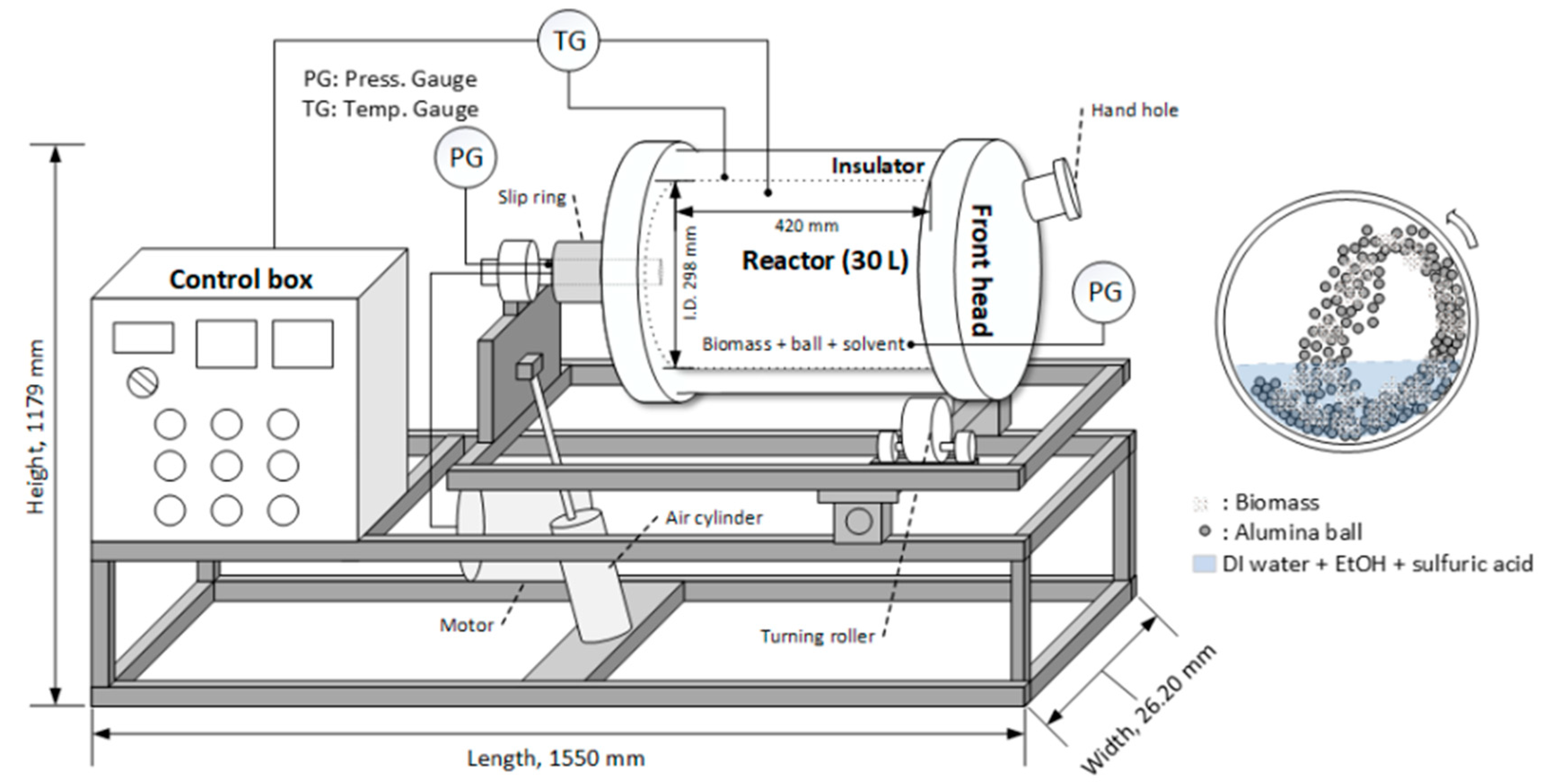
An illustration of a bench-scale ball milling reactor is shown in Figure 1. The ball milling reactor of this system was constructed using SS-316L material with a wall thickness of 0.01 m (inner diameter 0.30 m, depth 0.42 m, and internal volume 30 L).
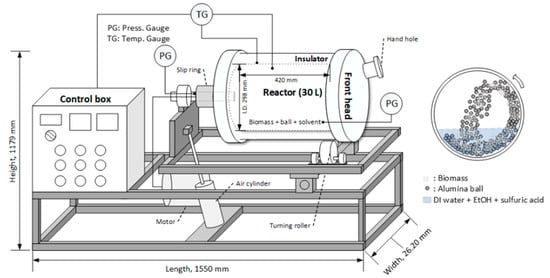
Figure 1.
Diagram of the temperature-controlled and tilted bench-scale (30 L) ball milling reactor system.
The reactor system can be operated at pressures up to 1.96 MPa, 200 °C temperature, and 6.28 rad/s, and metal spiral gaskets were installed at the connection between the reactor and the head at the front and rear to maintain the pressure. The reactor can be rotated using a 3-phase, 380 V, 0.37 kW-riven motor, with a slip ring with a rotating connector that can supply power or signals to the rotating equipment without twisting the power cables. The front head was equipped with a 0.075 m diameter hole for supplying reactants, biomass, balls, and solvents and discharging the product, and the reactor was heated and controlled by a 10 kW mantle-type electric heater. The high-temperature high-pressure ball mill reactor has a tilting system, and because of its heavy weight, the reactor body is operated by placing the reactor on a frame with a rotating roller. The spherical shape alumina ball (HD, 0.01 m diameter, and 3600 kg/m3 density) was purchased from NIKKATO Co. (Osaka, Japan). Before operating the reactor, alumina balls, biomass, and ethanol (60% v/v, 0.25% sulfuric acid w/v) were put together in the reactor, where the ratio of the ball:biomass:solvent was 30:1:10 (w/w/v). The reactor was preheated while rotating for 1 h until the target temperature (170 or 190 °C) was reached. When the target temperature was reached, the reaction was maintained while maintaining the temperature for a reaction time of 30, 60, or 90 min. Upon completion of the reaction, the sugar and lignin in the liquid sample were analyzed for the sugar yield and lignin separation. For the lignin content analysis, the sample was evaporated at 55 °C for 3 h, and then diluted three times with DI (deionized) water to precipitate the lignin. Water was then evaporated at 55 °C for 4 h, and acid hydrolysis using sulfuric acid was performed by adding highly concentrated sulfuric acid (72%), finally making 4.0% sulfuric acid to analyze the sugar and lignin in the sample, which was then subjected to an autoclave for 1 h at 121 °C. The lignin precipitate obtained above was washed with ethanol (60%, v/v) and water, dried at 45 °C in a convection oven, and subjected to compositional analysis. All experiments and analyses were repeated three times.
2.3. Characterization of Organosolv-Separated Lignin
Characterization of organosolv-separated lignin was performed and the average molar mass (Mn), weight average molar mass (Mw), and poly-dispersity (PD) of the organosolv-separated lignin were determined by GPC (gel permeation chromatography, Ultimate 3000 model, Thermo Fisher Scientific Inc., Waltham, USA). For molecular weight measurement, 3.0 mg of the acetylated lignin sample were dissolved in 2.0 mL of tetrahydrofuran (THF) and then filtered using a 0.45-µm PTFE (polytetrafluoroethylene) syringe filter to remove impurities. A Shodex column (KF-806L model) with a Shodex guard column (KF-G model) and refractive index (RI) detector (RefractoMax 520 model) using THF as a mobile phase (1.0 mL/min) with injection volumes of 20 µL were used in the GPC system. For the FT-IR (Fourier transform infrared) spectroscopy analysis using the IRSpirit-L/T model (Shimadzu Inc., Kyoto, Japan), the attenuation total reflectance (ATR) method was used to determine the characteristic absorption peak of the chemical functional groups in organosolv-fractional lignin. Mid-IR spectra were collected by averaging 40 scans from 4000 to 500 cm−1 at a resolution of 1 cm−1. 31P NMR (AVANCE 600, Bruker, Germany) spectra were collected at 242.88 MHz for 256 scans with a 2 s pulse delay. For quantitative 31P NMR analysis, 20 mg of lignin sample were weighed and dissolved into 400 µL of solution A and 150 µL of solution B in a 5-mL vial: Solution A (pyridine:chloroform-d (CDCl3) = 1.6:1 (v/v), solution B (mixture of solution A (25 mL), cyclohexanol (100 mg), and chromium (III) acetylacetonate (90 mg)). The prepared liquid sample was mixed well for 5 min using a vortex mixer, and 70 µL of 2-chloro-4,4,5,5-tetramethyl-1,2,3-dioxaphospholane (TMDP) were then added to the solution and placed in the 31P NMR system.
2.4. Solid and Liquid Composition Analysis of Untreated and Pretreated Samples
The carbohydrates and lignin contents in both solid and liquid samples were determined by following the NREL (National Renewable Energy Laboratory, Golden, CO, USA) LAPs (laboratory analytical procedures) [34,35]. The extractive analysis was firstly carried out by a two-step extraction method using water followed by ethanol. For the composition analysis of extractive-free and fractionated solids, a two-step acid hydrolysis following NREL-LAP was applied to determine the carbohydrate and lignin contents. To quantify the monomeric sugars and organic acid in the samples, an HPLC (high-performance liquid chromatography) system (LC-10A, Shimadzu Inc., Kyoto, Japan) with a refractive index detector (RID-10A, Shimadzu Inc., Kyoto, Japan) was used. HPLC equipped with an Aminex HPX-87P carbohydrate column (Bio-Rad Inc., Hercules, USA) was used for sugar analysis and HPLC-grade highly purified water was used as the mobile phase at a flow rate of 0.6 mL/min. For the Aminex HPX-87P column, the operating temperature of the column was maintained at 85 °C. On the other hand, Aminex HPX-87H (cat. No. 125-0098, Bio-Rad Inc., Hercules, USA) was used for organic acid analysis, where 5 mM sulfuric acid was used as a mobile phase at a volumetric flow rate of 0.6 mL/min. The column temperature was kept at 65 °C for organic acid analysis.
2.5. Enzymatic Digestibility Test
The enzymatic digestibility of untreated and treated solids was measured following NREL-LAP [36]. Enzymatic digestibility tests of samples were carried out using a 250-mL Erlenmeyer flask, which was incubated and shaken at 50 °C and 150 rpm in a shaking incubator (model BF-175SI, BioFree Co., Ltd., Seoul, Korea). Commercial cellulase enzyme (Cellic®CTec2, Novozymes Inc., A/S Bagsvaerd, Denmark) was used at the enzyme loading of 15 FPU/g-glucan. To prepare the testing sample in the 250-mL flask with 100 mL of working volume, the initial glucan loading was 1.0% (w/v) with 50 mM sodium citrate buffer (pH = 4.8). As antibiotics prevent microbial contamination, 1.0 mL of sodium azide (20 mg sodium azide /mL) was added. Samples were taken at appropriate sampling times (6, 12, 24, 36, 48, 60, and 72 h) and then were subjected to HPLC analysis using an HPX-87H column (Bio-Rad Laboratories Inc., Hercules, CA, USA). The total released glucose in the flask at 72 h of hydrolysis was used to calculate the enzymatic digestibility based on the glucan content in the untreated sample.
3. Results and Discussion
3.1. Compositions of Raw Agricultural Residues Used in This Study
Compositional analysis was performed on three lignocellulosic biomasses through five individual replicates. The compositional analysis results of the three untreated biomasses are presented in Table 1. For all the samples, the error values are represented as standard deviations. Among the three samples, BS was analyzed to have the highest carbohydrate content: 41.5% of glucan and 21.4% of hemicellulose-derived sugar polymer, i.e., xylan, arabinan, and galactan. On the other hand, RS and RH contained 32.9% and 35.6% of glucan and 20.6% and 15.3% of hemicellulose-derived sugar, respectively. The carbohydrate contents of the three biomass samples were found to be consistent with previous reports [37,38,39]. Unlike the carbohydrate contents, RH had the highest lignin (acid soluble + acid insoluble) content of 23.1%, compared to the 12.6% and 18.6% observed for RS and BS, respectively. It is also noteworthy that RS and RH showed higher ash contents (14.1% and 15.7%, respectively) than BS (7.4%). This is in line with the current findings on the extraction of minerals (silica) from rice byproducts [40,41,42]. These chemical compositional differences were assumed to be due to the properties of each residue. Therefore, it is important to understand the fractionation reaction behaviors upon the properties of each biomass types.

Table 1.
Chemical compositions of untreated agricultural residues based on an oven-dry biomass.
3.2. Lignin Extraction Yields and Purities for Various Fractionation Conditions
One important component in lignocellulosic biomass is lignin, which is one of the most abundant and renewable aromatic resources in the world [43]. The fractionation reaction conditions in the presence of the appropriate catalyst may affect the lignin’s solubilization and extraction behavior, i.e., this fractionation process should be able to break the intermolecular bonding between lignin and hemicellulose and therefore give relatively pure lignin [44].
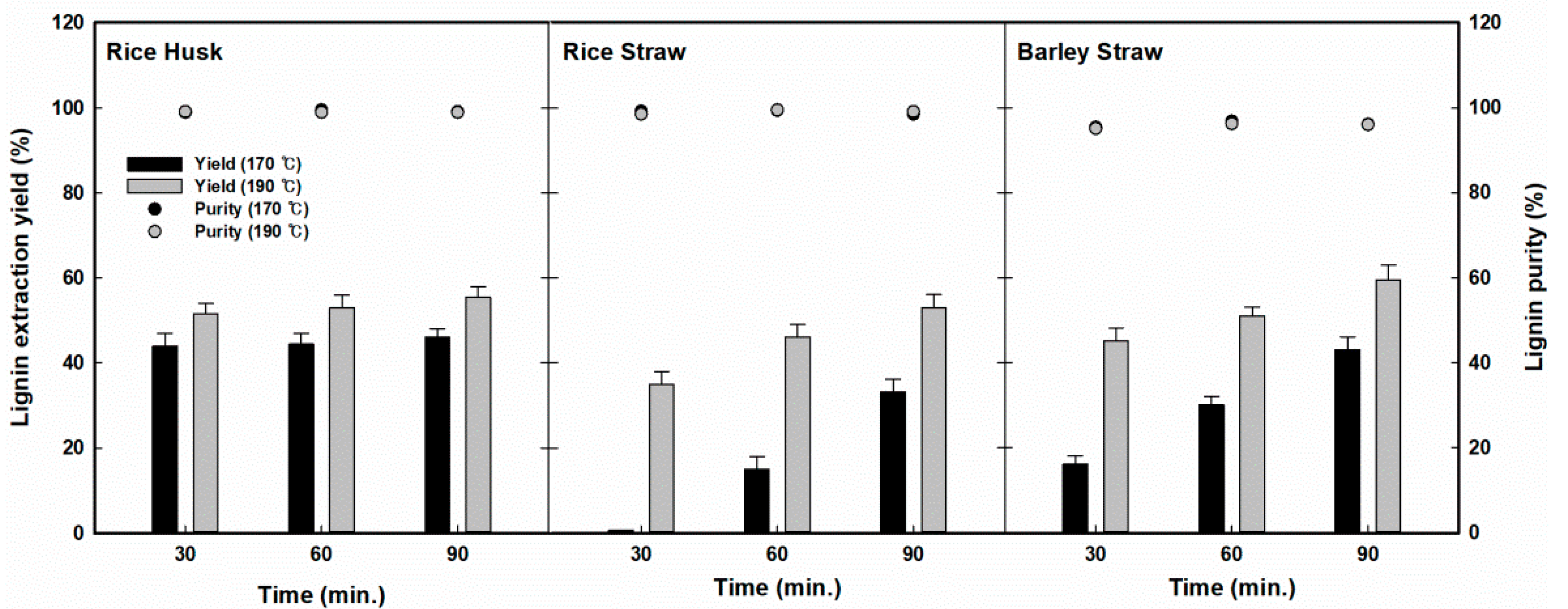
First, chemical fractionation using organosolv was conducted to extract lignin from three agricultural residues (RH, RS, and BS) by applying various temperatures and reaction times. The fractionation reaction was tested at elevated temperatures (170–190 °C) for extended reaction times (30–90 min), and the solubilization of chemical components during the organosolv fractionation reaction was investigated, as shown in Figure 2. For RH, the lignin recovery yields in the liquid hydrolysate ranged from 43.8% (170 °C, 30 min) to 55.2% (190 °C, 90 min). For the tested reaction conditions, lignin recovery showed a significantly broad distribution, in the ranges of 0.6% to 53.1% for RS and 16.3% to 59.4% for BS. In all three residues, the lignin extraction yield tended to increase for increased reaction severity (at higher temperatures and for longer reaction times). It was observed that the highest yields of RH, RS, and BS were 55.2%, 53.1%, and 59.4% at 190 °C for 90 min of reaction, respectively.
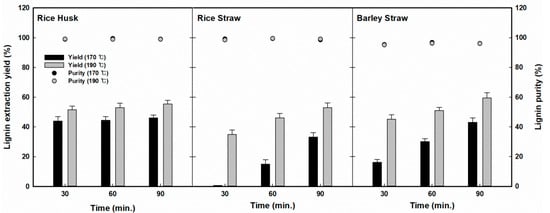
Figure 2.
Changes in lignin extraction yield and purity profiles on three agricultural residues (rice husk, rice straw, and barley straws) with various fractionation conditions.
Different lignin extraction behaviors were observed for various reaction conditions. First, in the case of RH, no significant change was observed for lignin extraction for the various reaction conditions. On the other hand, in the case of RS, significant lignin extraction occurred at 170 °C and, as the temperature was increased to 190 °C, the lignin extraction yield increased further to 53.0%, and the effect of the reaction time was also very significant. In particular, in the fractionation of BS, the yield of lignin extraction increased monotonically depending on the severity of the reaction conditions, which was unlikely in the fractionation of RH or RS. On the other hand, highly pure lignin was recovered from all three residues, with a 95.4% to 96.8% content in BS, slightly lower than that of the other residues (98.9% to 99.5% in RH and 98.5% to 99.5% in RS). It was speculated that the precipitation of lignin by the addition of water to the fractionated liquid resulted in this high purity of the lignin because of its low solubility in water.
3.3. Compositional Changes in Solid Residues after Fractionation
The compositional changes in the treated solid residues observed for various tested reaction conditions are presented in Table 2. Under the tested fractionation conditions, the solid remaining after fractionation (weight (g) of the remaining treated solid residue/weight (g) of untreated solid input) were 60.7% to 58.6% at 170 °C and 55.0% to 48.5% at 190 °C for the raw RH as the reaction time increased from 30 to 90 min. The glucan contents in the treated RH solid increased from 35.6% to 54.0% to 55.4% at 170 °C and increased to 55.6% to 54.1% at 190 °C with an increased reaction time, which corresponded to 32.8% to 32.4% and 30.5% to 26.2% (the values were calculated considering the remaining solid), respectively, based on the untreated RH. In the case of RH, the glucan content decreased slightly as the reaction time increased at the reaction temperature of 190 °C, which may indicate that the decomposition of glucan is somewhat sensitive to the reaction time. Meanwhile, the XMG (Xylan + Mannan + Galactan) contents decreased sharply from 13.6% of untreated RH to 4.6% to 3.3% at 170 °C and 1.9% to 0.7% at 190 °C in treated RH as the reaction time increased, which corresponded to 2.8% to 1.9% and 1.0% to 0.4% of the untreated RH, respectively. In addition, the residual lignin contents decreased from 22.7% of untreated RH to 12.3% to 13.0% at 170 °C and 8.7% to 8.2% at 190 °C in treated RH as the reaction time increased, which corresponded to 7.5% to 7.6% and 4.8% to 4.0% on the basis of the untreated RH. Consequently, approximately 91% and 74% to 86% of the glucan was retained in the treated solids after the organosolv fractionation of RH at 170 and 190 °C, respectively, while about 80% to 86% and 92% to 97% of hemicellulose (XMG) and approximately 67% and 79% to 82% of lignin was dissolved in the fractionation liquid at 170 °C and 190 °C, respectively. The results suggested that more than two-thirds of the major components of RH can be separated in the form of a solid and liquid by applying optimized pretreatment conditions.

Table 2.
Effects of the reaction conditions on the solid remaining and fractionation performances for three agricultural residues.
The glucan contents of fractionated RS increased from 32.9% to 39.2% to 43.1% at 170 °C and increased to 46.7% to 51.8% at 190 °C with an increasing reaction time while the XMG contents in fractionated RS decreased from 16.2% to 18.3% to 14.6% at 170 °C and to 14.7% to 10.3% at 190 °C. On the other hand, the lignin contents increased slightly from 11.7% to 14.3% to 11.2% at 170 °C, and decreased to 11.0% to 9.0% at 190 °C with an increasing reaction time. This change in the content of each component was due to the different hydrolysis reaction rates for each component during the fractionation reaction. Consequently, approximately 88% to 97% and 91% to 96% of the glucan was retained in the fractionated solids through the organosolv fractionation of RS at 170 and 190 °C, respectively, while about 8% to 39% and 39% to 64% of XMG and 0% to 35% and 37% to 56% of lignin was dissolved into the fractionation liquid at 170 °C and 190 °C, respectively. Thus, RS showed higher glucan retention, but the fractionation effect of XMG and lignin was not significant. Therefore, for RS, the organosolv fractionation process may not be an appropriate method for the effective fractionation of XMG and lignin.
In the case of BS, the glucan contents in the fractionated solid increased from 41.5% to 51.9% to 61.2% at 170 °C and increased to 61.3% to 70.2% at 190 °C. The XMG contents in fractionated BS decreased in all samples from 16.2% to 18.3% to 14.6% at 170 °C and 14.7% to 10.3% at 190 °C. However, the lignin contents decreased slightly from 18.1% to 18.4% to 15.1% at 170 °C, and to 14.2% to 9.4% at 190 °C for an increased reaction time. Approximately 89% to 95% and 87% to 92% of the glucan was retained in the fractionated BS solids at 170 °C and 190 °C, respectively, while about 23% to 49% and 51% to 73% of XMG and 18% to 47% and 45% to 64% of lignin were dissolved in the liquid hydrolysate at 170 and 190 °C, respectively. In the case of BS, glucan retention in the fractionated solid was the highest (95%) for all tested samples. In addition, the fractionation of XMG and lignin, even though it was lower than that of RH, was affected significantly by the changing reaction conditions.
3.4. Sugar Recovery and Decomposition Behavior in the Fractionated Hydrolysate
During the course of the fractionation processing, a xylose-rich liquid sample was generated by the hydrolysis of hemicellulose. In addition, usually, more severe reaction conditions make xylose undergo a condensation reaction, resulting in furfural and formic acid, which serve as strong inhibitors in the following conversion steps using enzymes and microbes [45]. To determine the characteristic behavior of sugars and other byproducts during reaction, the yields of hemicellulose-derived sugar (mainly xylose (XMG)) and byproducts, i.e., furfural and formic acid and acetic acid, were analyzed since both furfural and formic acid are the degraded products derived from hemicellulose, and they are well-known inhibitors in the bioconversion step, and acetic acid is usually formed by deacetylation of hemicellulose in the chemical fractionation reaction [45].
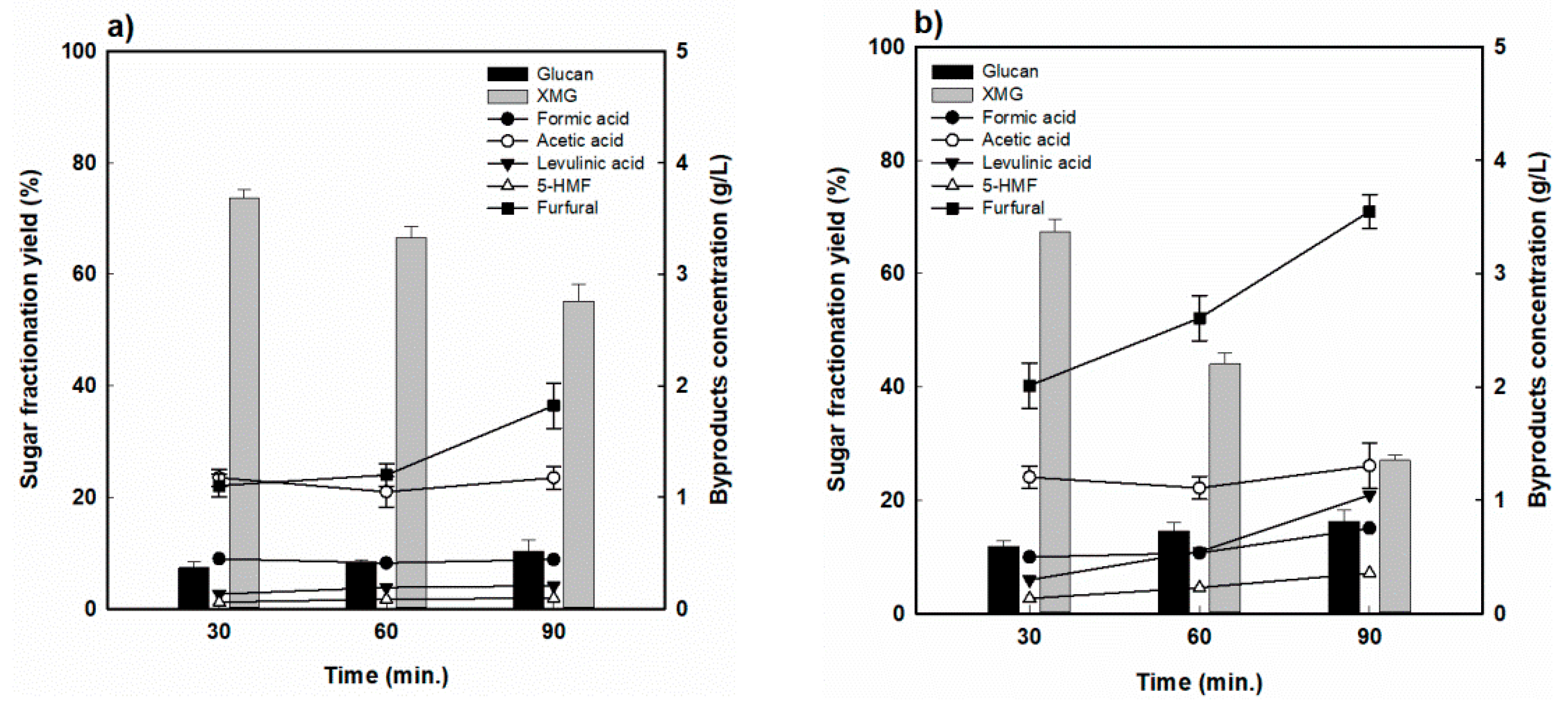
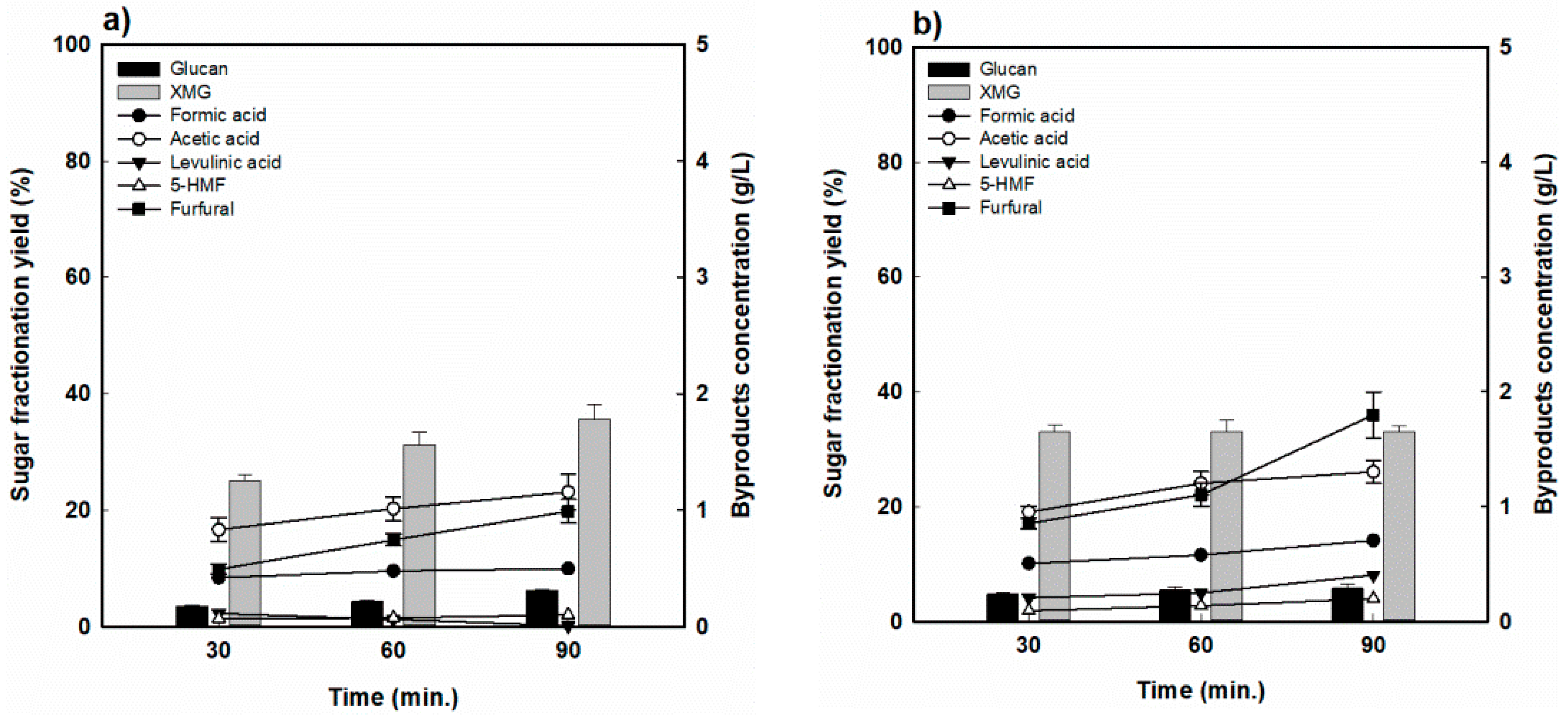
For RH, the hydrolysis yields of hemicellulose for various reaction times at the reaction temperature of 170 °C are shown in Figure 3. The highest hemicellulose-derived sugar (XMG) yield was 73.8% when RH was fractionated at 170 °C for 30 min, and decreased to 55.4% as the reaction time was extended to 90 min (Figure 3a). As shown in Figure 3b, a similar behavior was observed at 190 °C. However, it dramatically dropped from 67.5% at 30 min to 27.0% at 90 min, which was attributed to the decomposition of hemicellulose-derived sugar into furfural and formic acid under the harsher reaction conditions. Indeed, as the reaction time was prolonged, the formation of sugar decomposition products also increased, with furfural concentrations increasing from 1.1 to 1.9 g/L, while the formic acid concentration remained almost constant at 0.4 to 0.45 g/L at 170 °C. As seen in Figure 3b, the concentrations of furfural and formic acid increased from 2.0 to 3.5 g/L and from 0.5% to 0.8%, respectively, at 190 °C. On the other hand, the acetic acid concentration was measured to be approximately 1.2 g/L, and there was no significant change under the various reaction conditions. Levulinic acid and HMF concentrations were at relatively low levels (<0.3%).
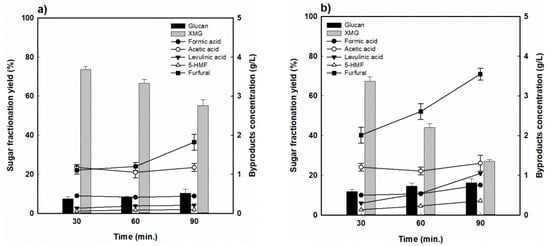
Figure 3.
Hemicellulose-derived sugar yield and concentration of sugar-decomposed products in rice husk-fractionated hydrolysate (a) 170 °C and (b) 190 °C.
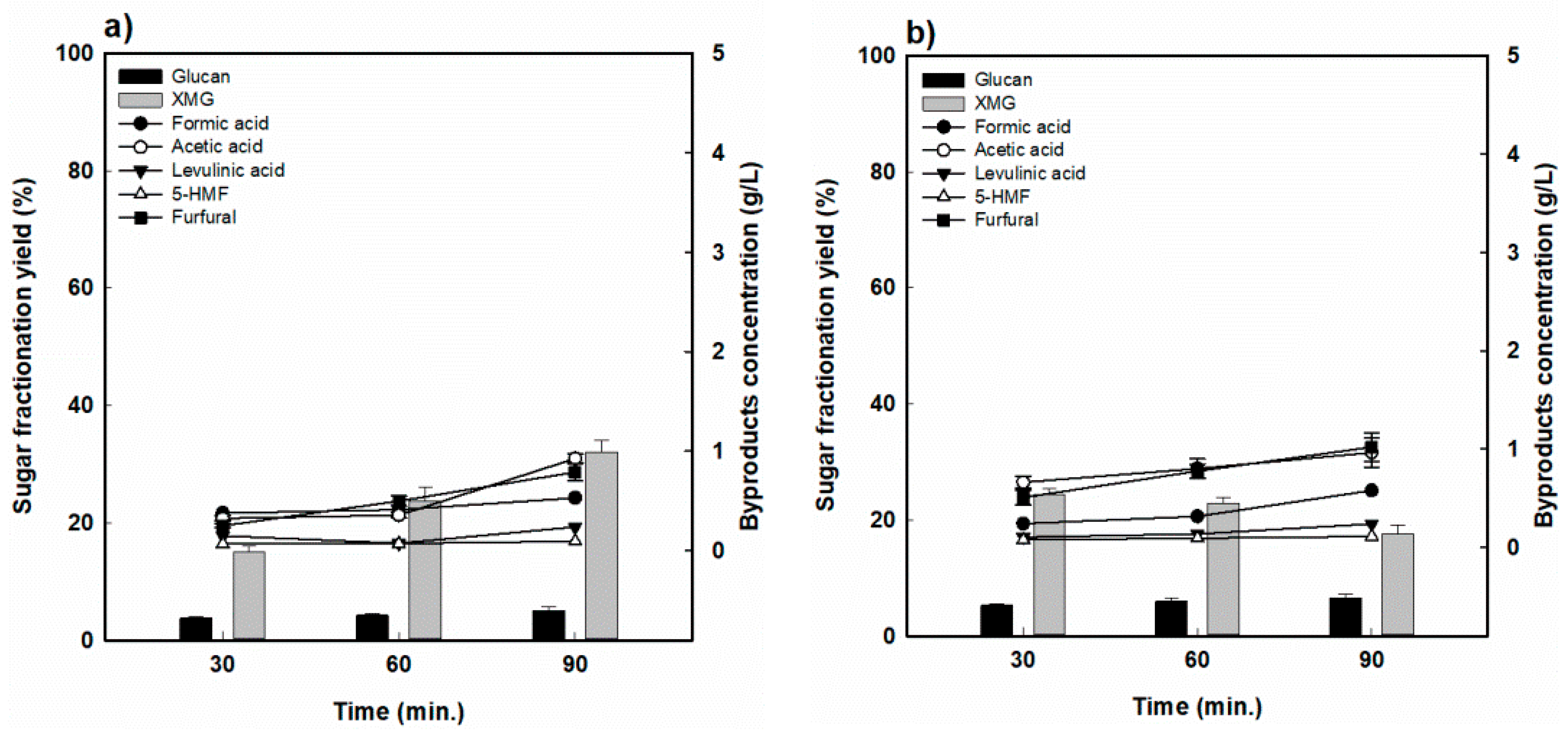
Figure 4 shows the sugar recovery and formation of decomposition products, such as the RS hydrolysate, as a function of the reaction time ((a) 170 °C and (b) 190 °C). The yield of XMG increased slightly over the tested reaction time to 15.2% to 31.9% at 170 °C, but the XMG yield decreased substantially from 24.4% to 17.64% at 190 °C. Therefore, in the case of RS, it was seen that the fractionation process under the tested conditions was not effective for the production of hemicellulose-derived sugar.
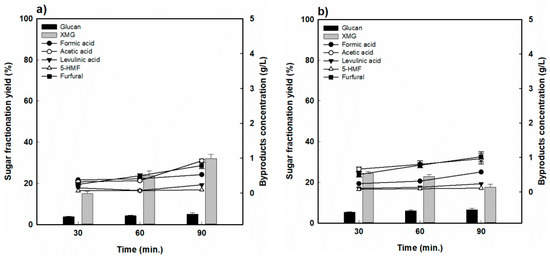
Figure 4.
Hemicellulose-derived sugar yield and concentration of sugar-decomposed products in rice straw-fractionated hydrolysate (a) 170 °C and (b) 190 °C.
In the case of BS (Figure 5), the XMG yield was relatively lower than that of RH for all the reaction conditions, and the values obtained exhibited a similar trend. A higher XMG yield (approximately 35%) was achieved at 170 °C for 90 min of reaction. At the lower temperature (170 °C), the yield increased gradually as the reaction time increased from 25.2% to 35.7%. However, at the higher temperature (190 °C), the XMG yield remained constant (approximately 33%) in spite of the reaction time. The concentration of byproducts showed a distinct trend in which more sugar was decomposed under more severe reaction conditions. At 170 °C, the furfural concentration increased from 0.49 to 0.97 g/L with the increased reaction time, and the formic acid concentration also increased from 0.42 to 0.49 g/L. At higher temperatures, the furfural and formic acid concentrations increased from 0.8 to 1.8 g/L and from 0.5 to 0.70 g/L, respectively. In addition, the acetic acid concentrations showed a monotonic increase from 0.8 to 1.2 g/L at 170 °C and 0.9 to 1.3 g/L at 190 °C with an increase in the reaction time even though the hydrolytic sugar yield was not high. The lowered levels of levulinic acid (0.1–0.4 g/L) and HMF (0.1–0.2 g/L) at each tested temperature were believed to be due to lower glucose production in the fractionated hydrolyzate (Figure 5a,b).
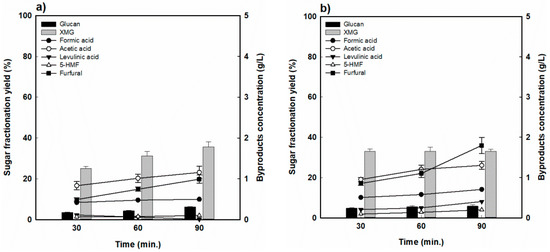
Figure 5.
Hemicellulose-derived sugar yield and concentration of sugar-decomposed products in barley straw-fractionated hydrolysate (a) 170 °C and (b) 190 °C.
3.5. Enzymatic Digestibility of Fractionated Solid Residues
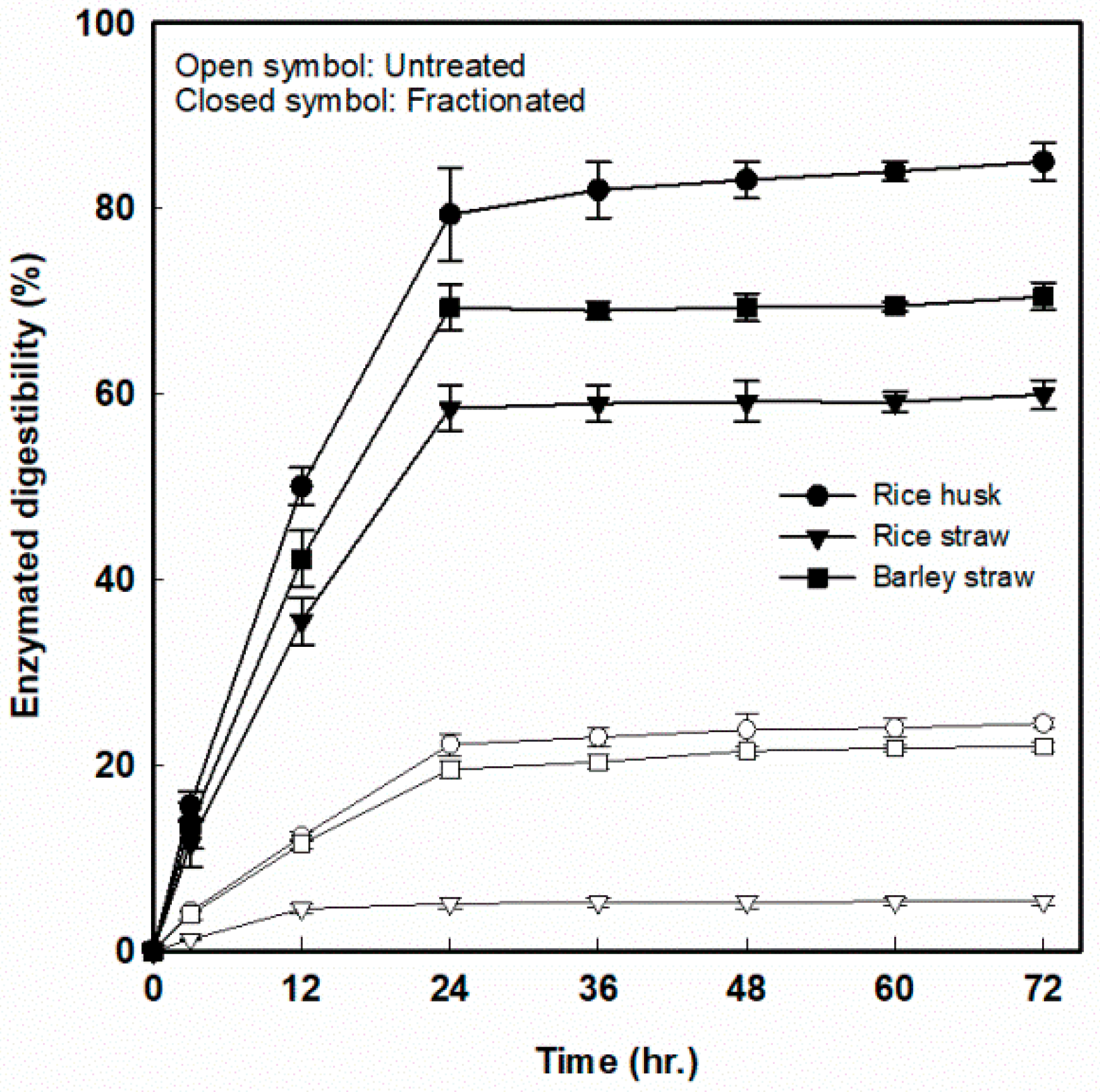
To evaluate the digestibility of fractionated solid for cellulose-derived sugar (glucose) production, an enzymatic hydrolysis was conducted with cellulase loading of 15 FPU/g-glucan. The enzymatic hydrolysis rates of the fractionated solid residues were compared with the reaction rates of untreated RH, RS, and BS. As shown in Figure 6, the highest digestibility (85.2% at 72 h of hydrolysis) was observed from the fractionated RH, and the highest digestibility of RS and BS was 60.0% and 70.5%, respectively, at the specific reaction condition, which resulted in the highest lignin extraction. The increase of the glucose production in enzyme-digested fractionated solid residues was higher than that of untreated RH, RS, and BS by 3.5-, 11.1-, and 3.2-fold, respectively. In the case of fractionated RS, the pretreatment effect seems to be very large despite the lowest degree of enzymatic hydrolysis. This is due to the fundamentally low enzymatic digestibility of 5.4% in untreated rice straw. On the other hand, the enzymatic digestibility of untreated RH and BS was 24.5% and 22.0%, respectively. These results suggest that the factor that greatly affects the enzyme hydrolysis reaction was the removal of components other than cellulose from the lignocellulosic biomass.
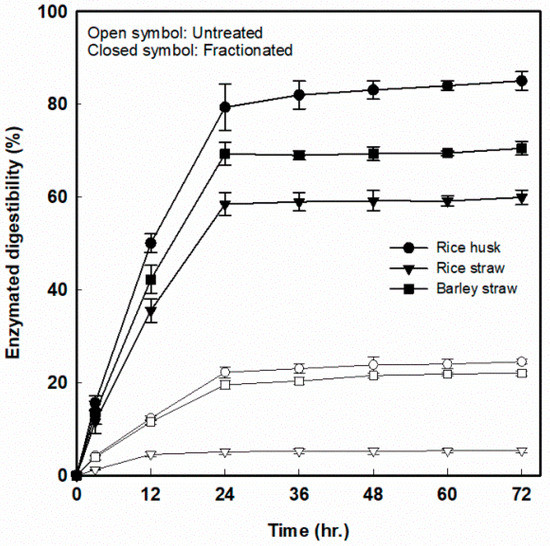
Figure 6.
The 72-h enzymatic digestibility of three fractionated agricultural residues with cellulase loadings of 15 FPU/g-glucan for cellulose-derived sugar production.
3.6. Chemical Characteristics of Extracted Lignin from RS, RH, and BS
The hydroxyl groups of lignin extracted under the fractionation conditions—ethanol concentration of 60%, reaction temperature of 190 °C, and reaction time of 90 min—that showed the highest lignin extraction yields on each agricultural residues were quantified by 31P NMR spectrum and are shown in Table 3, which compares the contents of p-hydroxylphenyl, guaiacyl, and syringyl units (referred to as H, G, and S, respectively) in each residue. In the RH lignin, H, G, and S contents were 0.55, 1.49, and 0.90 mmol/g, respectively, which were found to be higher than those obtained from RS or BS. In the aliphatic hydroxyl content of each extracted lignin, RH lignin showed the lowest content of 0.93 mmol/g, and the RS and BS lignins were present at 1.84 and 1.66 mmol/g, respectively. In contrast, the phenolic hydroxyl content of RH lignin had the highest value of 2.94 mmol/g and RS and BS lignin had corresponding contents of 2.19 and 1.67 mmol/g, respectively. It is generally known that the aliphatic hydroxyl can be reduced by a mechanism involving the loss of γ-methylol groups while removing formaldehyde when the β-aryl ether bonds are cleaved by ethanol [46]. In addition, according to Baucher and Sun’s reports, phenolic hydroxyl units exist in biomass, forming lignin/phenolic–carbohydrate units owing to the combination of ester and ether with hemicellulose [47,48]. For this reason, the lower aliphatic hydroxyl group and higher phenolic hydroxyl group contents in the treated biomass samples may be related to the degree of hemicellulose-derived sugar release from RH.

Table 3.
Structural characteristics of extracted lignin fractions from three agricultural residues.
The Mn, Mw, and polydispersity index (PDI) of extracted lignin from RS, RH, and BS were determined by GPC. In the organosolv process, as the reaction conditions become more severe, smaller lignin fragments were formed, which resulted in the decreased molecular weight of lignin. At the same fractionation conditions, the Mn of RH, RS, and BS were 1497, 1523, and 1703 g/mol, respectively. The PDI represents the molecular weight distribution of lignin, which is considered to be an important factor when lignin forms polymers [49]. As shown in Table 3, the PDI values of organosolv lignin ranged from 1.15 to 1.28, indicating a fairly uniform molecular weight distribution. Therefore, it was confirmed that organosolv-extracted lignin from three agricultural residues had high purity (more than 95%) and a low molecular weight (1453–1817 g/mol) and low PDI (1.15–1.28).
4. Conclusions
To address the importance of lignin valorization in the biorefinery industry, an organosolv fractionation involving ball milling was conducted at a bench scale on three agricultural residues. Lignin extracted from RH, RS, and BS showed relatively high purity (99.5% for RH and RS, and 96.8% for BS) and similar chemical characteristics. However, considering the simultaneous production of hemicellulose-derived sugars, different fractionation behaviors were shown for the three agricultural residues. Among the three tested agricultural residues, it was concluded that more effective results were obtained for RH considering its performance, including the lignin recovery (55.2%), lignin purity (99.5%), hemicellulose-derived sugar yield (73.8%), and glucan-to-glucose yield (85.2%). Fine refinements in this process can improve the fractionation efficiency of RH. Based on the results, it is speculated that the fractionation efficiency for RH can be improved with fine refinements. Furthermore, it is considered that the range of reaction conditions should be shifted for effective BS fractionation. Unfortunately, for the case of RS, other fractionation processes should be applied to achieve effective fractionation performance.
Author Contributions
T.H.K. (Tae Hoon Kim) as the first author conducted all experiments, summarized the data, and drafted the manuscript. H.K. as the co-author conducted experiments and analyzed the data. T.H.K. (Tae Hyun Kim) and K.K.O., as the co-corresponding authors, equally contributed; i.e., designed the reactor system as well as the overall study and experiments, interpreted the results, wrote the manuscript, and finalized the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Technology Development Program to Solve Climate Changes of the National Research Foundation (NRF) funded by the Ministry of Science and ICT (2017M1A2A2087627).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barakat, A.; Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review. Bioresour. Technol. 2013, 134, 362–373. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Matsakas, L.; Gao, Q.; Jansson, S.; Rova, U.; Christakopoulos, P. Green conversion of municipal solid wastes into fuels and chemicals. Electron. J. Biotechnol. 2017, 26, 69–83. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Zacharopoulou, M.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Topakas, E. Sequential high gravity ethanol fermentation and anaerobic digestion of steam explosion and organosolv pretreated corn stover. Bioresour. Technol. 2017, 244, 1129–1136. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Taherzadeh, M.J. Energy recovery from industrial crop wastes by dry anaerobic digestion: A review. Ind. Crops Prod. 2019, 129, 673–687. [Google Scholar] [CrossRef]
- Statista. Available online: http://www.statista.com/statistics/255937/leading-rice-producers-worldwide/ (accessed on 16 October 2019).
- Statista. Available online: http://www.statista.com/statistics/271973/world-barley-production-since-2008/ (accessed on 16 October 2019).
- Kim, S.H.; Gregory, J.M. Straw to Grain Ratio Equation for Combine Simulation. J. Biosyst. Eng. 2015, 40, 314–319. [Google Scholar] [CrossRef]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hägerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Lidén, G.; Zacchi, G. Bio-ethanol–The fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Price, J.T.; Konduri, M.K.; Fatehi, P. Water soluble kraft lignin–acrylic acid copolymer: Synthesis and characterization. Green Chem. 2015, 17, 4355–4366. [Google Scholar] [CrossRef]
- Achinivu, E.C.; Howard, R.M.; Li, G.; Gracz, H.; Henderson, W.A. Lignin extraction from biomass with protic ionic liquids. Green Chem. 2014, 16, 1114–1119. [Google Scholar] [CrossRef]
- Vishtal, A.G.; Kraslawski, A. Challenges in industrial applications of technical lignins. Bio. Res. 2011, 6, 3547–3568. [Google Scholar]
- Hu, J.J.; Zhang, Q.G.; Lee, D.J. Kraft lignin biorefinery: A perspective. Bioresour. Technol. 2018, 247, 1181–1183. [Google Scholar] [CrossRef]
- Løhre, C.; Kleinert, M.; Barth, T. Organosolv extraction of softwood combined with lignin-to-liquid-solvolysis as a semi-continuous percolation reactor. Biomass Bioenergy 2017, 99, 147–155. [Google Scholar] [CrossRef]
- Yuan, Z.; Long, J.; Wang, T.; Shu, R.; Zhang, Q.; Ma, L. Process intensification effect of ball milling on the hydrothermal pretreatment for corn straw enzymolysis. Energy Convers. 2015, 101, 481–488. [Google Scholar] [CrossRef]
- Castro, R.C.A.; Fonseca, B.F.; Santos, H.T.L.; Ferreira, I.S.; Mussatto, S.I.; Roberto, I.C. Alkaline deacetylation as a strategy to improve sugars recovery and ethanol production from rice straw hemicellulose and cellulose. Ind. Crops Prod. 2017, 106, 65–73. [Google Scholar] [CrossRef]
- Mussatto, S.I. (Ed.) Biomass pretreatment with acids. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 169–185. [Google Scholar]
- Barakat, A.; Chuetor, S.; Monlau, F.; Solhy, A.; Rouau, X. Eco-friendly dry chemo-mechanical pretreatments of lignocellulosic biomass: Impact on energy and yield of the enzymatic hydrolysis. Appl. Energy 2014, 113, 97–105. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Hirata, S.; Hassan, M.A. Combined pretreatment using alkaline hydrothermal and ball milling to enhance enzymatic hydrolysis of oil palm mesocarp fiber. Bioresour. Technol. 2014, 169, 236–243. [Google Scholar] [CrossRef]
- Kim, S.M.; Dien, B.S.; Tumbleson, M.E.; Rausch, K.D.; Singh, V. Improvement of sugar yields from corn stover using sequential hot water pretreatment and disk milling. Bioresour. Technol. 2016, 216, 706–713. [Google Scholar] [CrossRef]
- Deng, A.; Ren, J.; Wang, W.; Li, H.; Lin, Q.; Yan, Y.; Sun, R.; Liu, G. Production of xylo-sugars from corncob by oxalic acid-assisted ball milling and microwave-induced hydrothermal treatments. Ind. Crops Prod. 2016, 79, 137–145. [Google Scholar] [CrossRef]
- Inoue, H.; Yano, S.; Endo, T.; Sakaki, T.; Sawayama, S. Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Biotechnol. Biofuels 2008, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Inoue, H.; Endo, T.; Yano, S.; Bon, E.P.S. Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 2010, 101, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Huang, H.; Zhang, H.; Zhang, L.; Yan, L.; Chen, J. Ball milling pretreatment of corn stover for enhancing the efficiency of enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2010, 162, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Arato, C.; Gilkes, N.; Gregg, D.; Mabee, W.; Pye, K.; Xiao, Z.; Zhang, X.; Saddler, J. Biorefining of softwoods using ethanol organosolv pulping: Preliminary evaluation of process streams for manufacture of fuel-grade ethanol and co-products. Biotechnol. Bioeng. 2005, 90, 473–481. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Torre, M.; Moral, A.; Hernández, M.; Cabeza, E.; Tijero, A. Organosolv lignin for biofuel. Ind. Crops Prod. 2013, 45, 58–63. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, B.H.; Im, D.J.; Lee, J.H.; Oh, K.K. Combined Ball Milling and Ethanol Organosolv Pretreatment to Improve the Enzymatic Digestibility of Three Types of Herbaceous Biomass. Energies 2018, 11, 2457. [Google Scholar] [CrossRef]
- Koo, B.W.; Kim, H.Y.; Park, N.; Lee, S.M.; Yeo, H.; Choi, I.G. Organosolv pretreatment of Liriodendron tulipifera and simultaneous saccharification and fermentation for bioethanol production. Biomass Bioenergy 2011, 35, 1833–1840. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; NREL/TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; NREL/TP-510-42629; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Carolina, C.M.; Arturo, J.G.; Mahmoud, E.H. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process Saf. Environ. Prot. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Mor, S.; Manchanda, C.K.; Kansal, S.K.; Ravindra, K. Nanosilica extraction from processed agricultural residue using green technology. J. Clean. Prod. 2017, 143, 1284–1290. [Google Scholar] [CrossRef]
- Shen, Y. Rice husk silica derived nanomaterials for sustainable applications. Renew. Sustain. Energy Rev. 2017, 80, 453–466. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, J.H.; Lee, J.W.; Lee, H.S.; Chang, J.H.; Sang, B.I. Preparation of high purity silica originated from rice husks by chemically removing metallic impurities. J. Ind. Eng. Chem. 2017, 50, 79–85. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Nicolini, A.; Gelosia, M.; Coccia, V.; Petrozzi, A. Lignin as co-product of second generation bioethanol production from ligno-cellulosic biomass. Energy Procedia 2014, 45, 52–60. [Google Scholar] [CrossRef]
- Mesa, L.; Gonzalez, E.; Cara, C.; González, M.; Castro, E.; Mussattoc, S.I. The effect of organosolv pretreatment variables on enzymatic hydrolysis of sugarcane bagasse. Chem. Eng. J. 2011, 168, 1157–1162. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ryu, H.J.; Oh, K.K. Acid-catalyzed hydrothermal severity on the fractionation of agricultural residues for xylose-rich hydrolyzates. Bioresour. Technol. 2013, 132, 84–90. [Google Scholar] [CrossRef]
- Yáñez-S, M.; Matsuhiro, B.; Nuñez, C.; Pan, S.; Hubbell, C.A.; Sannigrahi, P.; Ragauskas, A.J. Physicochemical characterization of ethanol organosolv lignin (EOL) from Eucalyptus globulus: Effect of extraction conditions on the molecular structure. Polym. Degrad. Stab. 2014, 110, 184–194. [Google Scholar] [CrossRef]
- Baucher, M.; Monties, B.; Van Montagu, M.; Boerjan, W. Biosynthesis and genetic engineering of lignin. Crit. Rev. Plant Sci. 1998, 17, 125–197. [Google Scholar] [CrossRef]
- Sun, R.C.; Sun, X.F.; Wang, S.Q.; Zhu, W.; Wang, X.Y. Ester and ether linkages between hydroxycinnamic acids and lignins from wheat, rice, rye, and barley starws, maize stems, and fast-growing poplar wood. Ind. Crops Prod. 2002, 15, 179–188. [Google Scholar] [CrossRef]
- McClelland, D.J.; Motagamwala, A.H.; Li, Y.; Rover, M.R.; Wittrig, A.M.; Wu, C.; Huber, G.W. Functionality and molecular weight distribution of red oak lignin before and after pyrolysis and hydrogenation. Green Chem. 2017, 19, 1378–1389. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).