Use of Co-Solvents in Hydrothermal Liquefaction (HTL) of Microalgae
Abstract
1. Introduction
1.1. HTL of Algal Biomass in Pure Alcohols
1.2. HTL of Algal Biomass with Alcohol Co-Solvents
1.2.1. Ethanol
1.2.2. Isopropyl Alcohol (IPA)
1.3. HTL of Algal Biomass with Glycol Co-Solvents
1.3.1. Ethylene Glycol (EG)
1.3.2. Glycerol
2. Materials and Methods
2.1. Materials
2.2. HTL Process
2.3. Characterization of Products
3. Results
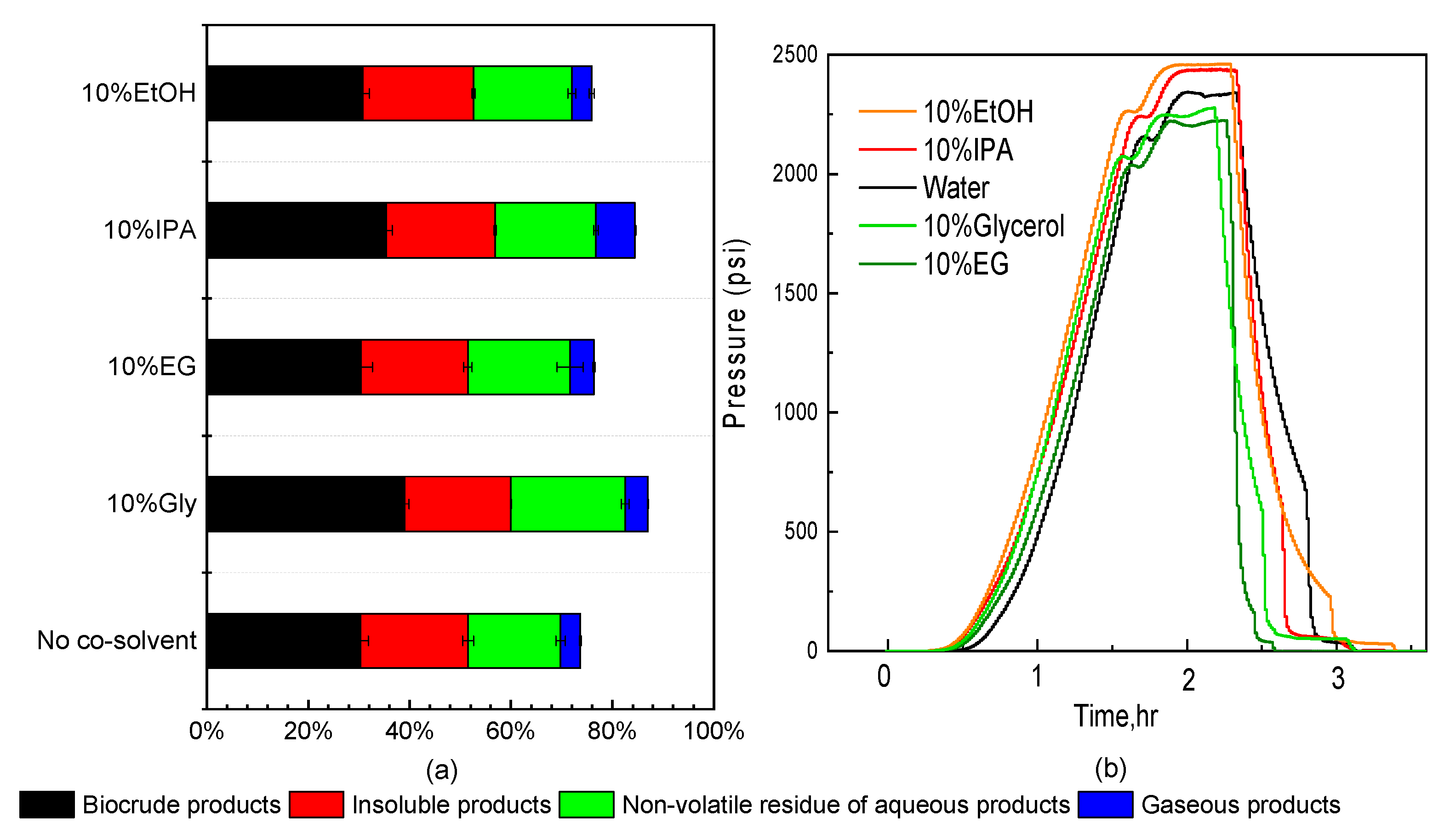
3.1. Effect of Co-Solvent Inclusion on Product Distributions
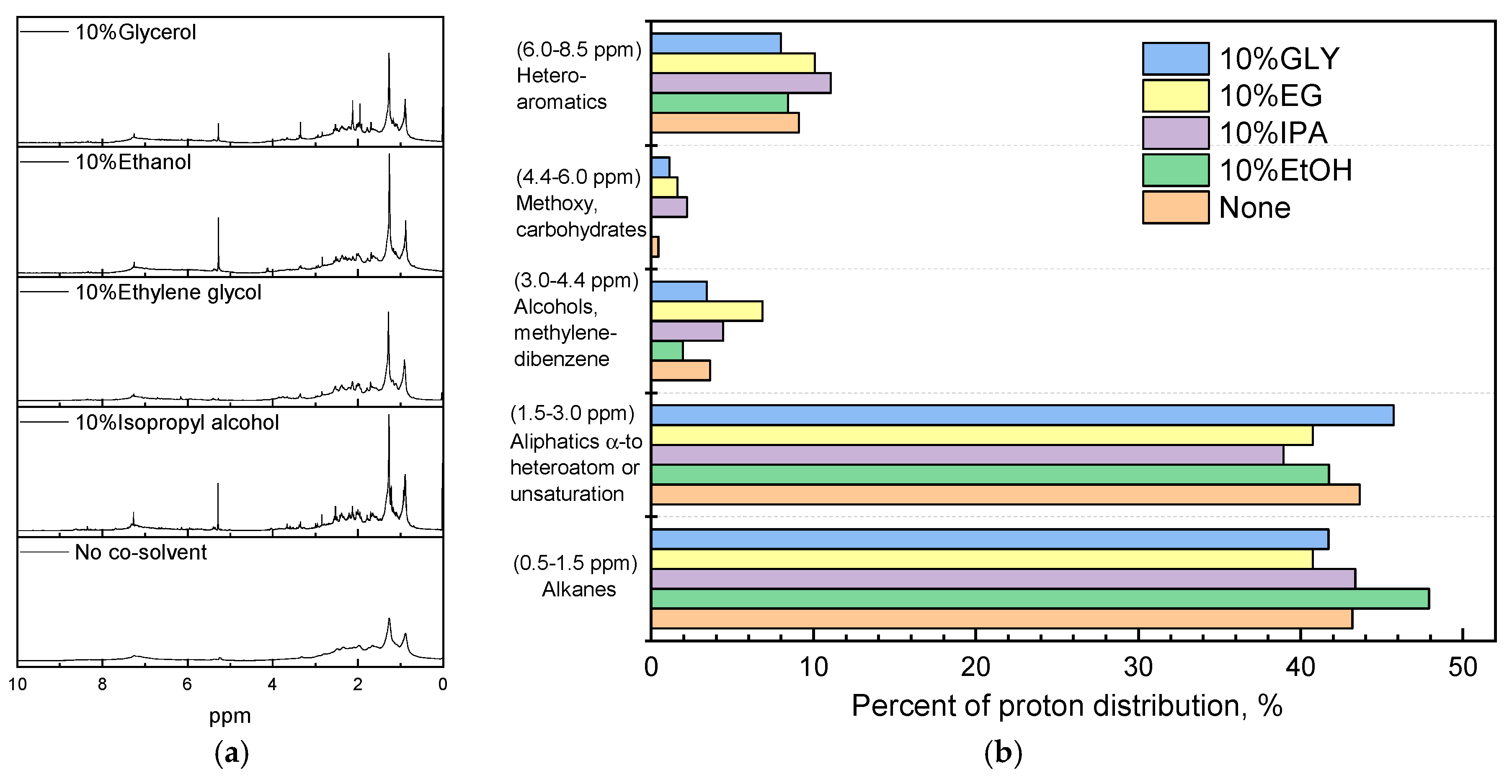
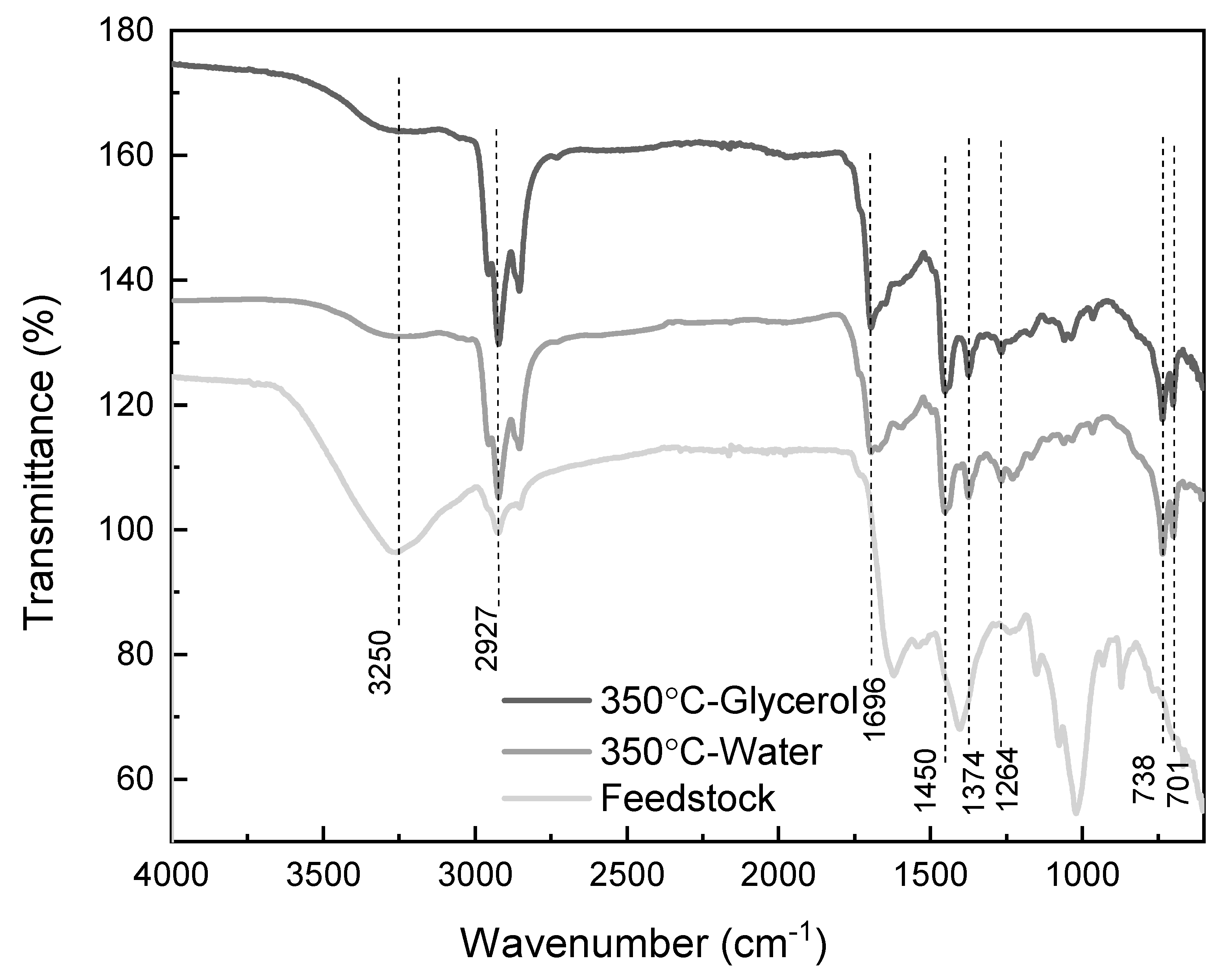
3.2. Effect of Co-Solvent Inclusion on Biocrude Properties
3.3. Effect of Varying Co-Solvent Concentration on Product Yield
3.4. Effect of Varying Co-Solvent Concentration on Biocrude Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jena, U.; Das, K. Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae. Energy Fuels 2011, 25, 5472–5482. [Google Scholar] [CrossRef]
- Jin, B.; Duan, P.; Zhang, C.; Xu, Y.; Zhang, L.; Wang, F. Non-catalytic liquefaction of microalgae in sub-and supercritical acetone. Chem. Eng. J. 2014, 254, 384–392. [Google Scholar] [CrossRef]
- Jin, Y.; Ruan, X.; Cheng, X.; Lü, Q. Liquefaction of lignin by polyethyleneglycol and glycerol. Bioresour. Technol. 2011, 102, 3581–3583. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Jin, B.; Xu, Y.; Yang, Y.; Bai, X.; Wang, F.; Zhang, L.; Miao, J. Thermo-chemical conversion of Chlorella pyrenoidosa to liquid biofuels. Bioresour. Technol. 2013, 133, 197–205. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Jasiūnas, L.; Casamassima, L.; Singh, S.; Jensen, T.; Rosendahl, L.A. Synergetic hydrothermal co-liquefaction of crude glycerol and aspen wood. Energy Convers. Manag. 2015, 106, 886–891. [Google Scholar] [CrossRef]
- Lemoine, F.; Maupin, I.; Lemée, L.; Lavoie, J.M.; Lemberton, J.L.; Pouilloux, Y.; Pinard, L. Alternative fuel production by catalytic hydroliquefaction of solid municipal wastes, primary sludges and microalgae. Bioresour. Technol. 2013, 142, 1–8. [Google Scholar] [CrossRef]
- Aysu, T.; Durak, H. Assessment of avocado seeds (Persea americana) to produce bio-oil through supercritical liquefaction. Biofuels Bioprod. Biorefin. 2015, 9, 231–257. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Mordechaiev, I.; Sliman, T.; Tavor, D. Glycerol−glycerol triacetate mixtures as green reaction mediums. Can. J. Chem. 2014, 92, 240–242. [Google Scholar] [CrossRef]
- Wagner, J.L.; Perin, J.; Coelho, R.S.; Ting, V.P.; Chuck, C.J.; Teixeira Franco, T. Hydrothermal Conversion of Lipid-Extracted Microalgae Hydrolysate in the Presence of Isopropanol and Steel Furnace Residues. Waste Biomass Valoriz. 2018, 9, 1867–1879. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Grigoras, I.F.; Hoffmann, J.; Toor, S.S.; Daraban, I.M.; Jensen, C.U.; Iversen, S.B.; Madsen, R.B.; Glasius, M.; Arturi, K.R. Continuous hydrothermal co-liquefaction of aspen wood and glycerol with water phase recirculation. Appl. Energy 2016, 162, 1034–1041. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Vlachos, D.G. Liquid phase catalytic transfer hydrogenation of furfural over a Ru/C catalyst. Appl. Catal. A Gen. 2014, 480, 17–24. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, Q. Alcell® lignin solubility in ethanol–water mixtures. J. Appl. Polym. Sci. 1995, 57, 1441–1446. [Google Scholar] [CrossRef]
- Feng, S.; Wei, R.; Leitch, M.; Xu, C.C. Comparative study on lignocellulose liquefaction in water, ethanol, and water/ethanol mixture: Roles of ethanol and water. Energy 2018, 155, 234–241. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.-T.; Zhang, P.; Luo, Z.; Zhang, Y. Hydrothermal liquefaction of Chlorella pyrenoidosa in sub-and supercritical ethanol with heterogeneous catalysts. Bioresour. Technol. 2013, 133, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Zeb, H.; Choi, J.; Kim, Y.; Kim, J. A new role of supercritical ethanol in macroalgae liquefaction (Saccharina japonica): Understanding ethanol participation, yield, and energy efficiency. Energy 2017, 118, 116–126. [Google Scholar] [CrossRef]
- Brand, S.; Susanti, R.F.; Kim, S.K.; Lee, H.-S.; Kim, J.; Sang, B.-I. Supercritical ethanol as an enhanced medium for lignocellulosic biomass liquefaction: Influence of physical process parameters. Energy 2013, 59, 173–182. [Google Scholar] [CrossRef]
- Cheng, S.; D’cruz, I.; Wang, M.; Leitch, M.; Xu, C. Highly efficient liquefaction of woody biomass in hot-compressed alcohol−water co-solvents. Energy Fuels 2010, 24, 4659–4667. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Hao, S.; Luo, G.; Zhang, S.; Chen, J. Effect of glycerol as co-solvent on yields of bio-oil from rice straw through hydrothermal liquefaction. Bioresour. Technol. 2016, 220, 471–478. [Google Scholar] [CrossRef]
- Alhassan, Y.; Pali, H.S.; Kumar, N.; Bugaje, I.M. Co-liquefaction of whole Jatropha curcas seed and glycerol using deep eutectic solvents as catalysts. Energy 2017, 138, 48–59. [Google Scholar] [CrossRef]
- Biswas, B.; Arun Kumar, A.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Effects of temperature and solvent on hydrothermal liquefaction of Sargassum tenerrimum algae. Bioresour. Technol. 2017, 242, 344–350. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, X.; Zeng, G.; Wang, J.; Li, H.; Zhou, C.; Pei, X.; You, Q.; Chen, L. Thermochemical liquefaction characteristics of microalgae in sub- and supercritical ethanol. Fuel Process. Technol. 2011, 92, 147–153. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Deng, S.; Cooke, P.; Munson-McGee, S.; Rhodes, I.; Lammers, P.; Nirmalakhandan, N. Optimization of direct conversion of wet algae to biodiesel under supercritical methanol conditions. Bioresour. Technol. 2011, 102, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Kim, B.; Lee, J.W. Concurrent production of biodiesel and chemicals through wet in situ transesterification of microalgae. Bioresour. Technol. 2015, 193, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, B.; Chang, Y.K.; Lee, J.W. Wet in situ transesterification of microalgae using ethyl acetate as a co-solvent and reactant. Bioresour. Technol. 2017, 230, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Reddy, H.; Muppaneni, T.; Schaub, T.; Holguin, F.O.; Cooke, P.; Lammers, P.; Nirmalakhandan, N.; Li, Y.; Lu, X.; et al. In situ ethyl ester production from wet algal biomass under microwave-mediated supercritical ethanol conditions. Bioresour. Technol. 2013, 139, 308–315. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.; Son, J.; Lee, J.W. Catalyst-free production of alkyl esters from microalgae via combined wet in situ transesterification and hydrothermal liquefaction (iTHL). Bioresour. Technol. 2017, 244, 423–432. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.; Rosendahl, L. Continuous hydrothermal liquefaction of biomass: A critical review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Schideman, L.; Funk, T.; Wang, Z. Hydrothermal liquefaction of low lipid content microalgae into bio-crude oil. Trans. ASABE 2011, 54, 239–246. [Google Scholar] [CrossRef]
- Costanzo, W.; Hilten, R.; Jena, U.; Das, K.; Kastner, J.R. Effect of low temperature hydrothermal liquefaction on catalytic hydrodenitrogenation of algae biocrude and model macromolecules. Algal Res. 2016, 13, 53–68. [Google Scholar] [CrossRef]
- Yin, S.; Dolan, R.; Harris, M.; Tan, Z. Subcritical hydrothermal liquefaction of cattle manure to bio-oil: Effects of conversion parameters on bio-oil yield and characterization of bio-oil. Bioresour. Technol. 2010, 101, 3657–3664. [Google Scholar] [CrossRef]
- Harry, I.; Ibrahim, H.; Thring, R.; Idem, R. Catalytic subcritical water liquefaction of flax straw for high yield of furfural. Biomass Bioenergy 2014, 71, 381–393. [Google Scholar] [CrossRef]
- Ocfemia, K.; Zhang, Y.; Funk, T. Hydrothermal processing of swine manure into oil using a continuous reactor system: Development and testing. Trans. ASABE 2006, 49, 533–541. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Pruvost, J.; Legrand, J.; Lepine, O.; Tazerout, M.; Bengoa, C. Hydrothermal liquefaction of Nannochloropsis oceanica in different solvents. Bioresour. Technol. 2016, 214, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Chumpoo, J.; Prasassarakich, P. Bio-Oil from Hydro-Liquefaction of Bagasse in Supercritical Ethanol. Energy Fuels 2010, 24, 2071–2077. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Zhang, P.; Hua, D.; Yang, M.; Li, C.; Chen, Z.; Liu, J. Direct liquefaction of Dunaliella tertiolecta for bio-oil in sub/supercritical ethanol–water. Bioresour. Technol. 2012, 124, 190–198. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, B.; He, Z.; Wang, S.; Salih, O.; Wang, Q. Study on co-liquefaction of Spirulina and Spartina alterniflora in ethanol-water co-solvent for bio-oil. Energy 2018, 155, 1093–1101. [Google Scholar] [CrossRef]
- Ji, C.; He, Z.; Wang, Q.; Xu, G.; Wang, S.; Xu, Z.; Ji, H. Effect of operating conditions on direct liquefaction of low-lipid microalgae in ethanol-water co-solvent for bio-oil production. Energy Convers. Manag. 2017, 141, 155–162. [Google Scholar] [CrossRef]
- Meng, Y.; Bao, G.; Wang, H.; Yang, Z.; Xie, J. Thermochemical liquefaction characteristics of Cyanobacteria in subcritical and supercritical ethanol–water mixture. Int. J. Energy Res. 2017, 41, 1460–1473. [Google Scholar] [CrossRef]
- Peng, X.; Ma, X.; Lin, Y.; Wang, X.; Zhang, X.; Yang, C. Effect of process parameters on solvolysis liquefaction of Chlorella pyrenoidosa in ethanol–water system and energy evaluation. Energy Convers. Manag. 2016, 117, 43–53. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y. Hydrothermal liquefaction of microalgae in an ethanol–water co-solvent to produce biocrude oil. Energy Fuels 2014, 28, 5178–5183. [Google Scholar] [CrossRef]
- Lu, J.; Boughner, E.C.; Liotta, C.L.; Eckert, C.A. Nearcritical and supercritical ethanol as a benign solvent: Polarity and hydrogen-bonding. Fluid Ph. Equilibria 2002, 198, 37–49. [Google Scholar] [CrossRef]
- Jo, H.; Prajitno, H.; Zeb, H.; Kim, J. Upgrading low-boiling-fraction fast pyrolysis bio-oil using supercritical alcohol: Understanding alcohol participation, chemical composition, and energy efficiency. Energy Convers. Manag. 2017, 148, 197–209. [Google Scholar] [CrossRef]
- Bondesgaard, M.; Becker, J.; Xavier, J.; Hellstern, H.; Mamakhel, A.; Iversen, B.B. Guide to by-products formed in organic solvents under solvothermal conditions. J. Supercrit. Fluids 2016, 113, 166–197. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y.; Tavor, D. Glycerol as solvent and hydrogen donor in transfer hydrogenation–dehydrogenation reactions. Tetrahedron Lett. 2009, 50, 5951–5953. [Google Scholar] [CrossRef]
- Jae, J.; Zheng, W.; Lobo, R.F.; Vlachos, D.G. Production of dimethylfuran from hydroxymethylfurfural through catalytic transfer hydrogenation with ruthenium supported on carbon. ChemSusChem 2013, 6, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Heitz, M.; Brown, A.; Chornet, E. Solvent effects on liquefaction: Solubilization profiles of a Canadian prototype wood, Populus deltoides, in the presence of different solvents. Can. J. Chem. Eng. 1994, 72, 1021–1027. [Google Scholar] [CrossRef]
- Rezzoug, S.-A.; Capart, R. Liquefaction of wood in two successive steps: Solvolysis in ethylene-glycol and catalytic hydrotreatment. Appl. Energy 2002, 72, 631–644. [Google Scholar] [CrossRef]
- Zou, S.; Wu, Y.; Yang, M.; Li, C.; Tong, J. Thermochemical catalytic liquefaction of the marine microalgae Dunaliella tertiolecta and characterization of bio-oils. Energy Fuels 2009, 23, 3753–3758. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a green solvent for high product yields and selectivities. Environ. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Wallace, C.W.; Wang, L.; Cheng, D. Enhanced bio-oil production from swine manure co-liquefaction with crude glycerol. Energy Convers. Manag. 2011, 52, 1004–1009. [Google Scholar] [CrossRef]
- Kim, K.H.; Jo, Y.J.; Lee, C.G.; Lee, E. Solvothermal liquefaction of microalgal Tetraselmis sp. biomass to prepare biopolyols by using PEG#400-blended glycerol. Algal Res. 2015, 12, 539–544. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, S.; Yuan, Z.; Xu, C.; Bassi, A. Investigation of aqueous phase recycling for improving bio-crude oil yield in hydrothermal liquefaction of algae. Bioresour. Technol. 2017, 239, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, Z.; Zhang, Y.; Li, B.; Lu, Q.; Ma, Y.; Shen, R.; Zhu, Z. Improved production and quality of biocrude oil from low-lipid high-ash macroalgae Enteromorpha prolifera via addition of crude glycerol. J. Clean. Prod. 2017, 142, 749–757. [Google Scholar] [CrossRef]
- Vasilakos, N.P.; Austgen, D.M. Hydrogen-donor solvents in biomass liquefaction. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 304–311. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Dai, W.; Xu, Y. Liquefaction of sawdust in hot compressed ethanol for the production of bio-oils. Process Saf. Environ. Prot. 2012, 90, 333–338. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.I.; Hakim, M.A.Q.M.A.; Al-Jabri, H.M.S.J.; Alghasal, G.S.H.S. A comparative study of the growth of Tetraselmis sp. in large scale fixed depth and decreasing depth raceway ponds. Bioresour. Technol. 2016, 216, 114–120. [Google Scholar] [CrossRef]
- Han, Y.; Hoekman, S.K.; Cui, Z.; Jena, U.; Das, P. Hydrothermal liquefaction of marine microalgae biomass using co-solvents. Algal Res. 2019, 38, 101421. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- ASTM International Standards. D445: Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity); ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- ASTM International Standards. D446-07. Standard Specifications and Operating Instructions for Glass Capillary Kinematic Viscometers; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Elliott, D.C.; Neuenschwander, G.G.; Hart, T.R.; Rotness, L.J.; Zacher, A.H.; Fjare, K.; Dunn, B.; McDonald, S.; Dassor, G. Hydrothermal Liquefaction of Agricultural and Biorefinery Residues Final Report–CRADA# PNNL/277; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2010. [Google Scholar]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Lievens, C.; Mourant, D.; He, M.; Gunawan, R.; Li, X.; Li, C.-Z. An FT-IR spectroscopic study of carbonyl functionalities in bio-oils. Fuel 2011, 90, 3417–3423. [Google Scholar] [CrossRef]
- Singh, R.; Bhaskar, T.; Balagurumurthy, B. Effect of solvent on the hydrothermal liquefaction of macro algae Ulva fasciata. Process Safety Environ. Prot. 2015, 93, 154–160. [Google Scholar] [CrossRef]
- Kunaver, M.; Jasiukaityte, E.; Čuk, N.; Guthrie, J.T. Liquefaction of wood, synthesis and characterization of liquefied wood polyester derivatives. J. Appl. Polym. Sci. 2010, 115, 1265–1271. [Google Scholar] [CrossRef]
- Beauchet, R.; Pinard, L.; Kpogbemabou, D.; Laduranty, J.; Lemee, L.; Lemberton, J.L.; Bataille, F.; Magnoux, P.; Ambles, A.; Barbier, J. Hydroliquefaction of green wastes to produce fuels. Bioresour. Technol. 2011, 102, 6200–6207. [Google Scholar] [CrossRef] [PubMed]
- Biller, P.; Madsen, R.B.; Klemmer, M.; Becker, J.; Iversen, B.B.; Glasius, M. Effect of hydrothermal liquefaction aqueous phase recycling on bio-crude yields and composition. Bioresour. Technol. 2016, 220, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Minami, E.; Saka, S. Comparison of the decomposition behaviors of hardwood and softwood in supercritical methanol. J. Wood Sci. 2003, 49, 73–78. [Google Scholar] [CrossRef]






| Solvent | Density, g/cm3 | Boiling Point, °C | Critical Temperature, °C | Critical Pressure | Solubility with DCM | Relative Polarity [58] | |
|---|---|---|---|---|---|---|---|
| psi | MPa | ||||||
| Water | 0.998 | 100 | 374 | 3205 | 22 | Immiscible | 1.000 |
| Ethanol | 0.79 | 78.3 | 241 | 913 | 6.3 | Miscible | 0.654 |
| IPA | 0.79 | 82.3 | 235 | 690 | 4.8 | Miscible | 0.546 |
| EG | 1.11 | 197.3 | 447 | 1189 | 8.2 | Immiscible | 0.790 |
| Glycerol | 1.26 | 290.0 | 577 | 1087 | 7.5 | Immiscible | 0.812 |
| Co- Solvent | Biocrude Product Yield, % | Insoluble Product Yield, % | Yield of NVR of Aqueous Product, % | Gaseous Product Yield, % | Total Yield, % | pH of Aqueous Products | HHV of Biocrude, (MJ/kg) | HHV of Insolubles, (MJ/kg) | Viscosity of Biocrude 1, (mm2/s @40 °C) |
|---|---|---|---|---|---|---|---|---|---|
| None | 31.0 ± 2.1 | 21.2 ± 1.1 | 18.3 ± 0.9 | 3.9 ± 0.1 | 74.4 | 8.5 ± 0.1 | 33.3 ± 2.1 | 5.7 ± 0.9 | 14.7 |
| 10% IPA | 35.4 ± 1.1 | 21.4 ± 0.2 | 19.8 ± 0.5 | 7.8 ± 0.1 | 84.5 | 8.5 ± 0.3 | 32.9 ± 2.3 | 4.6 ± 1.1 | 5.3 ± 0.6 |
| 10% EG | 30.4 ± 2.3 | 21.1 ± 0.8 | 20.2 ± 2.6 | 4.7 ± 0.3 | 76.3 | 8.3 ± 0.2 | 34.4 ± 4.1 | 5.6 ± 0.3 | 26.1 |
| 10% EtOH | 30.7 ± 1.4 | 21.9 ± 0.3 | 19.4 ± 0.8 | 4.0 ± 0.5 | 75.9 | 8.5 ± 0.0 | 34.2 ± 0.0 | 5.4 ± 1.2 | 24.5 |
| 10% GLY | 39.0 ± 0.8 | 20.9 ± 0.1 | 22.6 ± 0.8 | 4.5 ± 0.1 | 87.0 | 8.1 ± 0.0 | 35.3 ± 1.8 | 4.8 ± 0.4 | 32.9±13.4 |
| Co- Solvent | Biocrude Product Yield, % | Insoluble Product Yield, % | Yield of NVR of Aqueous Product, % | Gaseous Product Yield, % | Total Yield, % | pH of Aqueous Products | HHV of Biocrude, (MJ/kg) | HHV of Insolubles, (MJ/kg) | Viscosity of Biocrude 1, (mm2/s @40 °C) |
|---|---|---|---|---|---|---|---|---|---|
| None | 31.0 ± 2.1 | 21.2 ± 1.1 | 18.3 ± 0.9 | 3.9 ± 0.1 | 74.4 | 8.5 ± 0.1 | 33.3 ± 2.1 | 5.7 ± 0.9 | 14.7 |
| 10% IPA | 35.4 ± 1.1 | 21.4 ± 0.2 | 19.8 ± 0.5 | 7.8 ± 0.1 | 84.5 | 8.5 ± 0.3 | 32.9 ± 2.3 | 4.6 ± 1.1 | 5.3 ± 0.6 |
| 20% IPA | 28.9 ± 1.3 | 21.4 ± 0.5 | 18.7 ± 0.2 | 9.5 ± 0.3 | 78.5 | 8.6 ± 0.0 | 36.1 ± 0.3 | 2.1 ± 0.0 | 102.4 ± 6.9 |
| 30% IPA | 35.4 ± 2.2 | 21.1 ± 0.0 | 15.8 ± 0.0 | 8.6 ± 0.2 | 80.8 | 8.6 ± 0.0 | 35.4 ± 0.4 | 1.1 ± 0.2 | 40.0 ± 15.8 |
| 10% EG | 30.4 ± 2.3 | 21.1 ± 0.8 | 20.2 ± 2.6 | 4.7 ± 0.3 | 76.3 | 8.3 ± 0.2 | 34.4 ± 4.1 | 5.6 ± 0.3 | 26.1 |
| 20% EG | 38.6 ± 1.6 | 20.8 ± 0.3 | 27.3 ± 3.3 | 5.7 ± 0.1 | 92.4 | 7.9 ± 0.0 | 32.7 ± 0.6 | 5.8 ± 0.2 | 10.6 ± 0.9 |
| 30% EG | 41.1 ± 1.1 | 21.6 ± 0.9 | 30.9 ± 1.3 | 5.1 ± 0.1 | 98.7 | 7.6 ± 0.0 | 32.8 ± 0.2 | 4.8 ± 0.4 | 16.5 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Hoekman, K.; Jena, U.; Das, P. Use of Co-Solvents in Hydrothermal Liquefaction (HTL) of Microalgae. Energies 2020, 13, 124. https://doi.org/10.3390/en13010124
Han Y, Hoekman K, Jena U, Das P. Use of Co-Solvents in Hydrothermal Liquefaction (HTL) of Microalgae. Energies. 2020; 13(1):124. https://doi.org/10.3390/en13010124
Chicago/Turabian StyleHan, Yang, Kent Hoekman, Umakanta Jena, and Probir Das. 2020. "Use of Co-Solvents in Hydrothermal Liquefaction (HTL) of Microalgae" Energies 13, no. 1: 124. https://doi.org/10.3390/en13010124
APA StyleHan, Y., Hoekman, K., Jena, U., & Das, P. (2020). Use of Co-Solvents in Hydrothermal Liquefaction (HTL) of Microalgae. Energies, 13(1), 124. https://doi.org/10.3390/en13010124








