Abstract
The production of biofuels from renewable sources is a major challenge in research. Methanol, ethanol, dimethyl ether (DME), synthetic natural gas (SNG), and hydrogen can be produced from syngas which is the result of the gasification of biomasses. Syngas composition varies according to the gasification technology used (such as fixed bed reactors, fluidized bed reactors, entrained flow reactors), the feedstock characteristics, and the operating parameters. This paper presents a review of the predominant biomass gasification technologies and biofuels obtained from syngas by biomass gasification.
1. Introduction
Currently, there is a growing interest in renewable energy sources because of the cost and the environmental impact of crude oil. The use of renewable sources also is becoming increasingly important because of other environmental concerns such as greenhouse gas emissions (GHG) [1,2,3]. Biomass could be exploited to produce biofuels such as methanol, ethanol, dimethyl ether (DME), synthetic natural gas (SNG), hydrogen, etc. Several governments have launched programs to promote renewable sources, many with a specific focus on biofuels. The European Union has the goal of a 10% share of biofuels in the transport industry by 2020 [4]; however, in the US, biofuels production is expected to reach 36 billion gallons by 2022 [5]. Industrial plants are increasingly focusing their activities on biogas production for power generation or on biomethane upgrading for grid injection. Biogas production is a simple and consolidated technology with a low level of organic transformation into biogas, approximately 5–10 wt. %, dependent on the biomass type as well as on the operative conditions [6,7]. Biodiesel and bioethanol are other biofuels which could be produced with mature technologies, but in both cases, the biomasses used are in competition with the food chain (vegetable oils, cereals, beets, and sugar cane), arising several ethical and social issues [8]. A solution to avoid food/no-food competition is the use of lignocellulosic biomass, which is a residual or derivative from agro-industrial wastes. These second generation biofuels do not compete with food production [9,10,11]. The purpose of this review is to provide a critical overview of biofuels synthesized from syngas by biomass gasification [12]. The production of high value-added biofuels like methanol, bio-hydrogen, ethanol, DME, SNG and biofuels via Fischer-Tropsch (FT) [13,14,15,16] will be addressed in terms of thermodynamics and kinetics. Studies by E.U. International Energy Agency and U.S. Department of Energy show that it is possible to obtain a 50% CO2 reduction by 2050, bringing biofuel use to 26% [17]. Biofuels might represent a viable way for sustainable development and economic growth in the near future. In 2011, approximately 3.4 million workers were already employed in this industry [18,19].
2. Syngas Production via Gasification Technologies
Gasification is a key process for the thermo-chemical conversion of biomass. In the presence of a gasifying agent (GA), biomass is converted to a multifunctional gaseous mixture, usually called syngas or synthesis gas, which can be used for the production of energy (heat and/or electricity generation), chemicals (ammonia), and biofuels. Furthermore, a solid residue after biomass conversion (Char) is generally found [20,21,22,23]. Syngas consists of a mixture of CO, H2, CO2, CH4 (primary components) and H2O, H2S, NH3, tar, and other trace species (secondary components), with a composition dependent on feedstock type and characteristics, operating conditions (i.e., GA, gasifier temperature and pressure, type of bed materials), and gasification technology [24,25,26,27].
According to the International Energy Agency (IEA) Bioenergy Task 33E—Thermal Gasification of Biomass database [28], there are 114 operational biomass gasification plants globally, 14 idle/on hold biomass gasification plants, and 13 under construction/planned biomass gasification plants. This results in a total number of 141 plants, with the following end use of the syngas produced (Figure 1): 106 plants for power production, with global electric power produced from biomass-derived syngas ≅ 356 MW and global thermal power produced from biomass-derived syngas ≅ 185 MW; 24 plants for liquid fuel production (methanol, ethanol, DME, FTS, diesel, gasoline), with global production of liquid fuel from biomass-derived syngas ≅ 750,000 t/year; 8 plants for gaseous fuel production (SNG and H2), with global production of gaseous fuel from biomass-derived syngas ≅ 3.2 × 108 Nm3/year; 7 plants for chemical production (various), with global production of chemical from biomass-derived syngas ≅ 9000 t/year. It is worth highlighting that in four plants, syngas is used for both power production and fuel production.
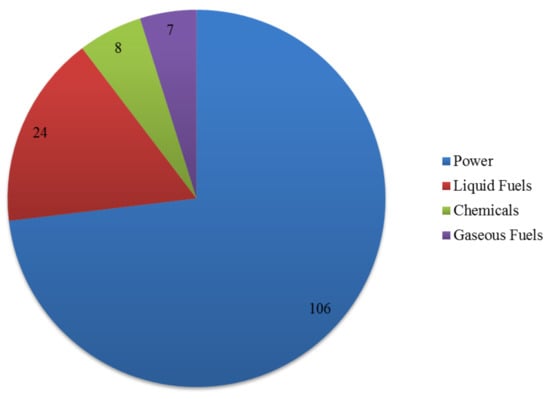
Figure 1.
Number of biomass gasification plants (operational/idle/on hold/under construction/planned) as function of biomass-derived syngas end use (adapted from IEA T33 database [28]).
Through an analysis of the number of biomass gasification plants that are operational/idle/on hold/under construction/planned as a function of start-up year for each end use considered (Figure 2), it is possible to observe that the use of syngas for power production increased in the period 1985–2009, achieving a maximum number of plants (12). After this period, use decreased, with only four plants opened in 2016 and two plants in 2017, with one new plant planned in 2018 and one planned in 2019. This trend may be due to the recent termination of public funds, which were allocated for energy production from renewable sources by national governments. However, an opposite trend can be observed for liquid fuels as an end use of syngas. Since 2007, the number of biomass gasification plants where the syngas produced is used for liquid fuel production has increased. Four new plants are planned as a result of the continuous improvement of the technological maturity of the processes. For both gaseous fuels and chemicals, the trend seems to be almost constant with time. Although no new plants are planned for 2018/2019 for chemical production, one new plant is planned in 2018 and one new plant is planned in 2019 for gaseous fuel production. A list of plants of biofuel production is reported in Section 3.
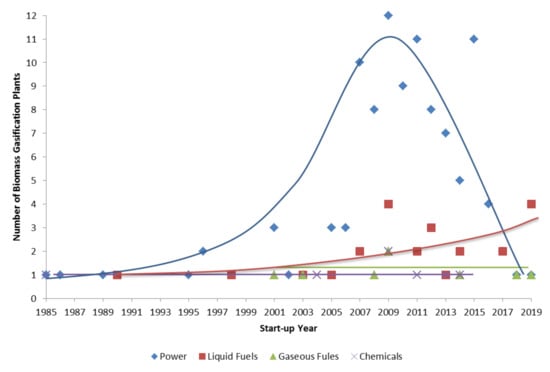
Figure 2.
Trend of number of biomass gasification plants (operational/idle/on hold/under construction/planned) as a function of start-up time—trend lines are qualitative (self-processed data from IEA T33 database [28]).
Usually, gasification is divided into four steps: drying (endothermic step), pyrolysis (endothermic step), oxidation (exothermic stage), and reduction (endothermic stage). Tar-reforming can also be added as a step to produce light hydrocarbons from large tar molecules [20,21,23,29,30]. A simplified gasification reaction is reported below (Equation (1)) [21] and the main reactions are collected in Table 1 [20,21,23,29,30,31].

Table 1.
Main reactions of the gasification process.
The heat required for the gasification process can be auto-thermally provided by exothermic combustion reactions or allo-thermally provided from external sources [32,33].
2.1. Gasification Parameters
Short overviews of the effects of the main types and characteristics of the biomass fed and the main gasification parameters on process performance is summarized in Table 2 and Table 3, respectively [20,21,24,25,32,34,35,36,37].

Table 2.
Effect of feedstock characteristics on gasification process performance.

Table 3.
Effect of operating conditions on the gasification process performance.
Proximate analysis, elemental analysis and higher heating value of various biomass types/typologies are listed in Table 4.

Table 4.
Chemical and physical properties of several biomass types/typologies [76,77,78,79,80,81,82,83,84,85].
Cellulose, hemicellulose and lignin contents of several biomasses are listed in Table 5.

Table 5.
Main components of several biomass types [86,87].
Composition and lower heating value of syngas, produced by several biomasses through different operative conditions (GA, equivalent ratio, steam to biomass ratio (SB), and temperature) and gasifiers (fluidized bed and fixed bed) are reported in Table 6.

Table 6.
Composition of syngas from several biomass types [25,43,44,88,89,90,91].
2.2. Gasification Reactors
Biomass gasification technologies can be classified into three types: fixed bed gasifiers, fluidized bed gasifiers, and entrained flow gasifiers [20].
Fixed bed gasifiers are considered the best choice for small-scale power generation plants of 10 MW [42]. They are classified as updraft and downdraft gasifiers [32]. In the former, biomass is supplied from the top, while the GA t is supplied from the bottom (counter-current). In the latter, the biomass and GA are introduced from the top (co-current) (Figure 3). The operating principle of updraft and downdraft gasifiers is shown in Figure 3 [24]. For updraft reactors, the sequence of the biomass is drying, pyrolysis, and reduction, finally arriving at the combustion zone, with syngas drawn out from the top. For downdraft reactors, both biomass and GA are supplied in the drying zone, going the through pyrolysis, combustion, and reduction, with syngas drawn out from the bottom. It is worth noting that in the downdraft configuration, gaseous products from pyrolysis are sent to the reduction zone, while in the updraft configuration, they are directly found in the syngas.
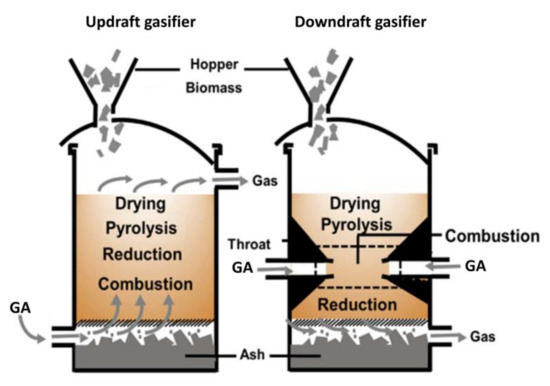
Figure 3.
Fixed bed gasifier schematization (adapted from Sikarwar et al. [32]).
Updraft gasifiers offer high thermal efficiency attributable to range of factors including good contact between the biomass and GA, small pressure drop, slight slag formation, as well as simple and robust design. Their main drawbacks include a high content of tar in syngas as well as limited flexibility in loading and process operation [20,21,41]. Operating temperature varies from a minimum of 650–700 °C to a maximum of 950–1150 °C [24,92,93,94]. Several research groups [24,92,94] have investigated syngas composition from several biomass updraft gasifiers with different gasification conditions, such as biomass type, gasification temperature, GA, and equivalence ratio (ER), highlighting that:
- H2 composition varies from a minimum of 1.6–3% v/v (biomass type = mesquite wood; gasification temperature ≅ 1150 °C; GA = air; ER = 2.7) to a maximum of 30–50% v/v (biomass type = cedar wood; gasification temperature = 650–950 °C; GA = oxygen; ER = 0–0.3);
- CO composition varies from a minimum of 13–21% v/v (biomass type = mesquite wood; gasification temperature ≅ 1150 °C; GA = air; ER = 2.7) to a maximum of 22–25% v/v (biomass type = cedar wood; gasification temperature = 650–950 °C; GA = oxygen; ER = 0–0.3);
- CO2 composition varies from a minimum of 9–12% v/v (biomass type = juniper wood; gasification temperature ≅ 1050 °C; GA = air; ER = 2.7) to a maximum of 25–30% v/v (biomass type = cedar wood; gasification temperature = 650–950 °C; GA = oxygen; ER = 0–0.3);
- CH4 composition varies from a minimum of 1.5–1.8% v/v (biomass type = juniper wood; gasification temperature ≅ 1050 °C; GA = air; ER = 2.7) to a maximum of 8–10% v/v (biomass type = cedar wood; gasification temperature = 650–950 °C; GA = oxygen; ER = 0–0.3);
- Higher Heating Value varies from a minimum of 2.4–3.5 MJ/Nm3 (biomass type = mesquite wood; gasification temperature ≅ 1150 °C; GA = air; ER = 2.7) to a maximum of 6.5–12.1% v/v (biomass type = cedar wood; gasification temperature = 650–950 °C; GA = oxygen; ER = 0–0.3).
Aljbour and Kawamoto investigated the effect of gasification conditions such as residence time, ER, S/C ratio, and gasification temperature on tar concentrations in the syngas, with cedar wood used as biomass feedstock to an updraft gasifier [93]. They found a variation of tar content from ≅30 g/Nm3 to less than 1 g/Nm3, highlighting that higher temperatures, along with sufficient contact time, can contribute to Polycyclic Aromatic Hydrocarbons reduction. Moreover, PAH conversion can be slightly increased by steam, while PAH contents can be greatly reduced by increasing the ERs.
Downdraft gasifiers produce low-tar and low-particulate syngas [95] but their main drawbacks include a difficult control of temperature [24], moreover biomass with low moisture content (<20–25% w/w) [21,96] and with low ash content [97,98] is required, as well as homogeneity of biomass input [20,41]. Operating temperature varies from a minimum of 900 °C to a maximum of 1000–1050 °C [99].
Several research groups [24,99,100,101] investigated syngas composition from several biomass downdraft gasifiers with different gasification conditions, such as biomass type, gasification temperature, GA, and equivalence ratio (ER), highlighting that:
- H2 composition varies from a minimum of 8–12% v/v (biomass type = wood waste; gasification temperature = 900–1050 °C; GA = air; ER = 0.20–0.35) to a maximum of ≅21% v/v (biomass type = eucalyptus wood; gasification temperature = 950 °C; GA = air (two-stage air and premixed air/gas supply); ER = 0.27);
- CO composition varies from a minimum of ≅14% v/v (biomass type = eucalyptus wood; gasification temperature = 950 °C; GA = air; ER = 0.27) to a maximum of ≅23% v/v (biomass type = hazelnut shells; gasification temperature = 1000 °C; GA = air; ER = 0.35);
- CO2 composition varies from a minimum of 5–8% v/v (biomass type = wood waste; gasification temperature = 900–1050 °C; GA = air; ER = 0.20–0.35) to a maximum of ≅11% v/v (biomass type = hazelnut shells; gasification temperature = 1000 °C; GA = air; ER = 0.35);
- CH4 composition varies from a minimum of 1–3% v/v (biomass type = wood waste; gasification temperature = 900–1050 °C; GA = air; ER = 0.20–0.35) to a maximum of ≅4% v/v (biomass type = hazelnut shells; gasification temperature = 1000 °C; GA = air; ER = 0.35);
- Higher Heating Value varies from a minimum of 4.5 MJ/Nm3 (biomass type = wood waste; gasification temperature = 900–1050 °C; GA = air; ER = 0.20–0.35) to a maximum of 6.5% v/v (biomass type = eucalyptus wood; gasification temperature = 950 °C; GA = air (two-stage air and premixed air/gas supply); ER = 0.27).
In terms of downdraft gasifiers, Jordan and Akay [102] and Jaojaruek et al. [101] investigatd syngas tar concentration. This research group observed a variation of tar content in the range 0.376–0.40 g/Nm3 (biomass type = bagasse; gasification temperature = 1040 °C; GA = air; ER = 0.26). The latter research group found a variation of tar content from 0.0432 g/Nm3 (biomass type = eucalyptus wood; gasification temperature = 950 °C; GA = air (two-stage air and premixed air/gas supply); ER = 0.27–1.27 g/Nm3 (biomass type = eucalyptus wood; gasification temperature = 950 °C; GA = air; ER = 0.27).
Fluidized bed gasifiers are a popular choice for large scale power plants because they can be easily scaled up [21]. They are classified as bubbling fluidized bed gasifiers and dual bed gasifiers with separated chambers [21,103]. Both are based on the principle of fluidization of a solid bed. In bubbling fluidized bed gasifiers (fluidization/ GA speed = 2–3 m/s), the GA also acts as a fluidization agent and is supplied from the bottom; accordingly, gasification occurs within the bed (Figure 4). In dual bed gasifiers, gasification occurs in two steps [37,104]. Combustion is first carried out in a combustion chamber, generating the heat required for gasification. Next, pyrolysis and gasification occur in the presence of high speed gas (5–10 m/s), which is carried out in a bubbling fluidized bed gasifier. Separation between syngas and bed material occurs via a cyclone separator at the outlet of the reactor [20,40] (Figure 4).
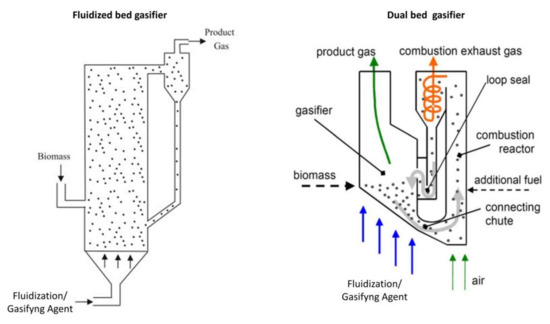
Figure 4.
Fluidized bed gasifier schematization (adapted from Loha et al. [112]; Koppatz et al. [103]).
Fluidized bed gasifiers are characterized by high mass and heat transfer rate, which secures constant temperatures all over the gasifier and high tolerability to diverse biomass feedstock types. Moreover, catalysts can be used as part of the gasifier bed to enhance tar removal [21,105,106,107,108]. Operating temperature varies from a minimum of 700 °C to a maximum of 900 °C with syngas composition of 30–60% v/v H2, 10–25% v/v CO, 15–20% v/v CO2, and 8-12% v/v CH4 for bubbling fluidized bed gasifiers [109,110] and of 22–27% v/v H2, 27–40% v/v CO, 39–42% v/v CO2, and 7–9% v/v CH4 for circulating fluidized bed gasifiers [111].
Entrained flow gasifiers are useful for large scale plants [113]. Thanks to the high operating temperature and the use of oxygen as GA, tar compounds are almost completely converted which is a great advantage for biomass gasification. However, when air is used as a GA, for example, in small-scale units, temperatures decrease which results in tar content growth [114]. As reported by Basu [42], a slurry prepared with mixing biomass and water may be used to facilitate feeding into the reactor.
On the other hand, entrained flow gasifiers require fine powder fuel (0.1–1 mm), despite the high energy cost for biomass size reduction is a great drawback for biomass gasification [20,37]. Therefore, a biomass pre-treatment via torrefaction is usually required for entrained flow gasifiers, allowing the aforementioned drawback to be overcome [115,116,117]. However, as reported by several authors, they are mainly operated as co-gasifiers, suppling both biomass and coal [118,119,120].
Entrained flow gasifiers are classified into two families: top-fed gasifiers and side-fed gasifiers [32]. A top-fed gasifier is a vertical cylinder reactor where fine particles (pulverized) and the GA are co-currently fed from the top in the form of a jet. Thermo-chemical conversion is performed by an inverted burner. Syngas is taken from the side of the lower section while slag is extracted from the bottom of the reactor (Figure 5). In a side-fed gasifier, the pulverized fed and the GA are co-currently fed by nozzles installed in the lower reactor, resulting in an appropriate mixing of biomass and GA. Syngas is extracted from the top and the slag from the bottom of the vessel (Figure 5).
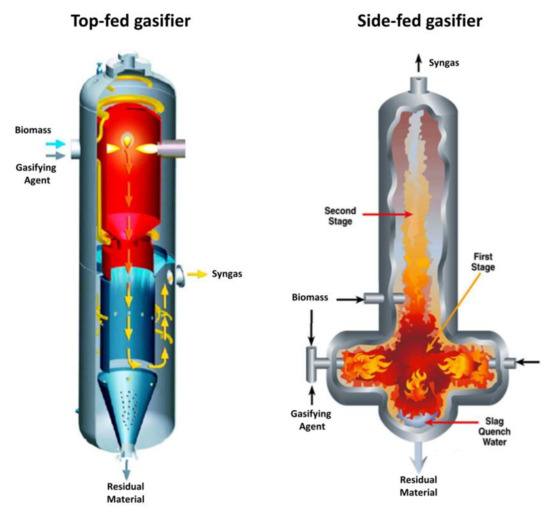
Figure 5.
Entrained flow gasifier schematization (adapted from Basu [42] and NETL [121]).
For both configurations, pressurized fuel into the gasifier is usually provided by a pneumatic feeding system [20,37]. They are highly efficient, with a standard operating temperature and pressure in the range 1300–1500 °C and 20–70 bar, respectively [40].
Hernández and colleagues [45] investigated the effect of particle sizes in the range 0.5–8 mm on syngas composition by feeding a top-fed entrained flow gasifier with dealcoholised marc of grape. Experiments were carried out, using air as GA and at gasification temperature and pressure of 1050 °C and 3 bar, respectively. They observed that the lower the particle sizes, the higher the syngas quality. At a particle size of 0.5 mm, the best composition of syngas (≅9% v/v H2, ≅14% v/v CO, ≅16% v/v CO2, 3% v/v CH4, ≅58% v/v N2) was found.
Briesemeister et al. [114] investigated the effects of operating temperature (900–1300 °C) and equivalence ratio of an air-blown entrained-flow gasifier on tar formation by using air as the GA. They observed tar -oading reduction to less than 0.2 g/Nm3 at 1300 °C.
The main characteristics and performance of gasifiers are summarized below (Table 7).

Table 7.
Characteristics and performance of gasification reactors (adapted from [35,40,45,95]).
3. Biofuels from Syngas
Biomass-derived syngas is used as a raw material in different thermochemical processes for the production of second-generation biofuels [124], both liquid, (such as methanol, ethanol, dimethylether (DME), and Fischer-Tropsch diesel) and gaseous (such as hydrogen and synthetic natural gas (SNG)) [125,126]. In particular, the type of biomass and its production process strongly influences their composition and heating value [127]. The production of liquid biofuel as an energy carrier could be very cost-effective because it would take the same infrastructure, storage system, and transportation used for Liquefied Petroleum Gas [128,129,130].
A list of worldwide second-generation biofuel plants, including Start-up Year, Technology Readiness Level (TRL) and Scale, Fed material, Output stream flow, Technology and Country, elaborated from the International Energy Agency (IEA) Bioenergy Task 33E - Thermal Gasification of Biomass database [28] is reported in Table 8. Notably in terms of TRL-Scale, 18 plants are characterized by a TRL higher than “4-5 Pilot”. Specifically, 14 plants are characterized by TRL as “4-5 Pilot”, 11 plants as “6-7 Demonstration”, five plants as “8 First-of-a-kind commercial demo” and two plants as “9 Commercial”.

Table 8.
Worldwide second-generation biofuel plants (self-processed data from IEA T33 database [28]).
Spath and Dayton [131] carried out a techno-economic screening for the production of fuels and chemicals from biomass-derived syngas, identifying several syngas conversion routes to methanol and its derivatives, such as DEM, ethanol, FT synthesis, hydrogen, and SNG, as described in Figure 6.
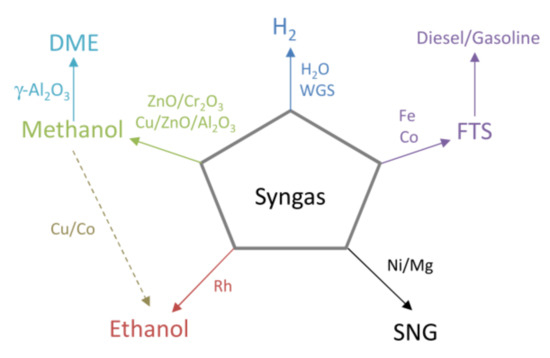
Figure 6.
Syngas conversion routes to second-generation biofuels (adapted from Spath and Dayton [131]).
Syngas conversion condition (in terms of pressure, temperature, and catalyst) as well as its composition (in terms of H2/CO and CO2) in different biofuels are described in Table 9. Notably, in order to enhance the biofuel production process, the production of syngas has to be carried out in operative conditions which fit conditions required for its end use as much as possible.

Table 9.
Syngas conversion condition and syngas composition (H2/CO and CO2) for different biofuels [21,22,36,131,132,133,134,135,136,137,138,139,140,141,142].
When biomass-derived syngas is used for biofuel production, the cleaning of the raw gas is needed strictly in order to remove contaminants and potential catalyst poisons as well as to achieve the qualitative composition required by the biofuel production process [36,143]. Several papers focused on biomass-derived syngas cleaning for end use applications were recently published [143,144,145]. Syngas contaminants are categorized as particulate matter (PM), condensable hydrocarbons (tars), alkaline metals (Na + K), nitrogen (NH3 + HCN), sulphur (H2S + COS + CS2), and halides (HCl + HBr + HF) [143]. Syngas downstream process and cleaning levels required are reported in Table 10.

Table 10.
Syngas purity as a function of the downstream process [36,131,139,143,146,147,148,149,150,151,152].
In addition to syngas cleaning, conditioning operations can be required to adjust syngas composition to meet the specifications of the downstream process in terms of H2/CO ratio, H2/CO2 ratio, and CO2 content, if necessary. In particular, the steam-reforming step and the WGS reaction are used to convert residual tar, light hydrocarbons, and methane to CO and H2 as well as to achieve the targets H2/CO and H2/CO2 required by the fuel production process, respectively. After H2/CO and H2/CO2 adjustments, if necessary, the CO2 removal step is carried out through physical or chemical steps [22,153].
3.1. Methanol
Methanol is an alcohol predominantly used for the production of several chemical compounds such as olefins as well as for fuels such as gasoline over zeolite catalysts [131]. In the chemical industry, it is used for the production of formaldehyde and acetic acid that are intermediate for several products (such as plywood, foams, resins, and plastics). In the fuel sector, methanol is used to produce methyl tert-butyl ether (MTBE) which is used as an anti-knock instead of lead-based substances. Responsible for increasing the octane number of gasoline, MTBE also improves combustion by limiting the emission of harmful unburned products [154,155,156]. Methanol is a flammable substance, highly soluble in water as well as in several organics solvents such as ethers, alcohols, etc. Historically, methanol has been produced via a catalytic process using natural gas and steam as feeding. This is a two-step process; in the first step, methane is reformed by using steam at about 600–650 °C and nickel-based catalysts in order to increase the CO + H2 yield. These catalysts are often doped with potassium [154,157] in order to avoid char formation which could reduce the active metal surface, reducing the catalytic effect on the reaction. The product of steam reforming reaction is syngas, which is composed of a mixture of hydrogen and carbon monoxide with a stoichiometric ratio of 3:1, as reported below:
In the second step, syngas is converted to methanol by using predominantly copper-based on alumina support catalysts [158,159,160] through an exothermic equilibrium limited synthesis process at pressures in the range of 50–100 bar and temperatures in the range 200–300 °C, according to the following reactions [131,161,162,163]:
A ratio (H2 − CO2)/(CO + CO2) slightly above two is usually used in order to favour kinetics and to control by-products [131,164]. The main reaction for methanol production is the reaction based on CO and H2 (Equation 3); however, Wender highlighted the effect of a methanol production promoter by carbon dioxide. Thus, in presence of CO2, the rate of the reaction between CO and H2 increased approximately by a factor of 100.
As a result of the exothermic nature of the reactions, a low temperature helps to increase the conversion. Furthermore, this is a reaction where there is a decreasing amount of mole numbers and by increasing the pressure, the reaction yield also increases. The choice of process temperature close to 250 °C is not attributable to the thermodynamics of reaction (preferred at lower temperature); it is a result of the higher performance of the catalysts in these operating conditions [165].
When syngas is used as feed stream, methanol production starts from the second step.
3.2. Ethanol
Similar to methanol, ethanol is an alcohol that has predominant use as a solvent, a reagent for chemical-pharmaceutical industry, or as a fuel. Nowadays, ethanol (like bioethanol) is often associated to the biofuel context; this a result of its use as a fuel in cars, especially in the US, or its use in place of MTBE or other anti-knock in combination with other fuels [13]. For several years, ethanol was produced through two predominant ways: alcoholic fermentation of sugars contained in the sugar cane or through the use of agricultural crops with high carbohydrate content, such as cereals [165,166]. However, this led to raise the issue regarding the competition between fuel production and human food [167,168]. To avoid this problem, several research centres and corporations have focused on lignocellulosic in bioethanol production for the past decade. This has led to an increase of cost production as a result of the required pre-treatment step before fermentation [169,170].
Another viable alternative for producing ethanol uses syngas derived from biomass gasification [171,172,173]. Ethanol from syngas is directly obtained by employing ad hoc catalysts such as Mo, Rh, K, Cu, Zn, and Fe [174,175,176] and this process is facilitated at pressure in the range of 1–100 bar and temperatures of about 230–300 °C [177]. The predominant reaction for ethanol production from syngas consists in CO hydrogenation (Equation (6)); moreover, ethanol also can be produced by CO2 hydrogenation (Equation (7)), which are both processes exothermic [139,178]:
Beginning with syngas, ethanol production also can be carried out through methanol synthesis followed by methanol homologation, according to the following exothermic reactions catalysed by Cu/Co catalysts [21,139]:
Based on Equations (6), (8) and (9), one observees that for each mole of ethanol, 2 moles of carbon monoxide and 4 moles of hydrogen are required. At the same time, if the syngas also contains carbon dioxide, the stoichiometric ratio between hydrogen and carbon dioxide is three. For MoS2 and Rh catalysts, which are the mainly used on industrial scale, the activity of both catalysts is inhibited by CO2 in the syngas; however, the specific CO2 concentration levels which allow this effect to be avoided are not clear [177]. Philips and colleagues [140] suggested a CO2 concentration of 5% for MoS2 catalyst, while van der Heijden and colleagues suggested <1 and <5 mol % of CO2 for the Rh- and MoS2-catalysts [139].
Clearly, the main issue of ethanol synthesis from syngas is the H2/CO ratio. This ratio in the syngas may be closer to one, resulting from an occurrence of side reactions, such as WGS, which reduce the H2/CO ratio from 2 to ≅1.0 [20,139].
3.3. Dimethylether (DME)
Dimethylether (DME) is an ether used in several applications, e.g., spray propellant, paints, insecticides, glues, and adhesives [163]. Thanks to its chemical-physical properties, it is used as both anti-knock and automotive fuels [132,179,180,181].
DME is produced through a two-stage process: first one is the methanol synthesis followed by the methanol dehydration (Equation (10)). By means of acid catalysts, such as γ-Al2O3 [182,183,184], or the addition of additives such as ferrite or tungsten to Cu/ZnO/Al2O3 catalysts [185,186], the following methanol synthesis reaction occurs [21,187]:
DME production also can be carried out in a single-step synthesis starting from syngas through the use of bifunctional catalysts (CuO–ZnO–MnO and zeolite) operated at 30–70 bar and 200–300°C, according to the following reactions [141,188]:
Ateka and colleagues [141] pointed out that DME yield decreases with the increase of CO2 concentration in the feed; however, for CO2/(CO + CO2) higher than 0.5, an asymptotic trend can be observed.
3.4. Fischer-Tropsch Synthesis (FTS)
In the last decades, Fischer-Tropsch Synthesis has been studied for the valorisation of syngas produced by agro-industrial gasification in order to have a biofuel with near zero carbon emission, thanks to the potential use of biomass as feed [189,190,191]. FTS is used for the production of several biofuels such as gasoline, kerosene, and diesel fuel. Accordingly, it is possible to produce fuels with linear chains and with a high grade of purity [192] and simultaneously without sulphur, nitrogen, or aromatics [193,194]. At present, it is considered to be the most complete technology for transportation biofuel production [21]
FT Synthesis produces several hydrocarbons, paraffin, and olefins such as methane, ethane, ethylene, LPG (C3–C5), fuel (C5–C12), gasoline (C13–C22), and waxes (C23–C33). Their distribution depends on the type of the catalyst used as well as by the process parameters, such as temperature, pressure, feed gas composition, and residence time [195,196,197,198]. The set of reactions is described below [199,200]:
where n is the number of carbon atoms and m is the same for hydrogen atoms contained in the produced hydrocarbon.
Co and Fe catalysts are often used for these reactions in the range of temperatures between 475 K and 625 K at pressure in the range 15–40 bar. In particular, cobalt catalysts improve performance in terms of conversion when compared with iron catalysts; however, iron catalysts guarantee a higher production in terms of olefin and alcohols than Co catalysts which give more paraffinic molecules [201].
C20+ linear HCs, C5+ paraffins and medium weight olefins, which are further processed to generate usable liquid transportation fuels, are the most desired products obtained via FTS [21].
3.5. Hydrogen
At present, hydrogen is predominantly used in chemical and oil industries: ≅61% of H2 produced worldwide is used for ammonia synthesis process, ≅23% for oil refining, and ≅10 for methanol synthesis. Moreover, ≅4% of global H2 produced is used for merchant users and ≅3% for other application [202]. In particular, H2 is considered a valuable and clean alternative to fossil fuel that feeds low temperature fuel cells, such as proton exchange membrane (PEM), and allows electric energy conversion, avoiding pollutant and greenhouse gas emissions [38,203]. Notably, H2 for fuel cells is considered a near-term technology [121]. For example, H2 purity higher than 98–99.9% v/v for application in ammonia synthesis (N2:H2 = 1:3 mol/mol) is required [204]; H2 use in PEM technology requires high purity grade (99.99% v/v—ISO 14687) with gas compositions such as: <0.5–4.5 ppmv CO, <20 ppm CO2; <0.25 ppmv H2S; <1–10 ppmv NH3 [148].
Currently, the predominant feedstock for H2 production consists in steam reforming of hydrocarbons (≅95%) which has the significant drawback of greenhouse gas emissions [38]. In order to make the production of H2 more sustainable, a renewable eco-friendly alternative to fossil fuel is required. A potential hydrogen source of the future is believed to be biomass [203].
As reported in the previous section, hydrogen is a component of syngas, from a minimum of ≅5–10% v/v to a maximum of ≅40–50% v/v, depending on gasifier type, biomass feed, and operating conditions. Biomass gasification using steam as a GA results in syngas with H2 content higher than 40% v/v, reducing tar production [205,206]. In order to increase H2 concentration, syngas is reformed via catalysed reactions such as the steam reforming of methane and higher hydrocarbons as well as the WGS reaction [125,207,208], using several catalysts, such as Ni, Fe, and Mo catalysts at temperature in the range 200–1100 °C and pressure between 1 and 30 bar [207,209,210,211]:
There are several technologies for hydrogen/CO2 separation in the syngas both in bed and out bed, such as polymeric membranes, chemical and physical adsorption of carbon dioxide, temperature swing adsorption (TSA), and pressure swing adsorption (PSA); however, pressure swing adsorption has been considered the most economical technology in several cases [201].
Soukup et al. [212] reported a product gas with a H2 content of 70% v/v using a dual fluidized bed gasification system with CO2 adsorption along with suitable catalysts.
An example of platform of hydrogen from biomass is the project “Hydrogen from biomass for Industry” [208], according to which the production of hydrogen was carried out by several steps, beginning with syngas produced via steam gasification of biomass; this was followed by steam gasification, CO-shift stage, CO2-separation with a pressurized water scrubber, a PSA system, a steam reformer, and advanced gas cleaning components [22] with H2 purity > 98–99% v/v.
Fail and colleagues [148] investigated hydrogen production by using a pilot plant fed with syngas produced by steam gasification of biomass. The pilot plant consisted of several units for syngas conditioning (WGS reactor, wet scrubber operated with rapeseed oil methyl ester, pressure swing adsorption (PSA) for hydrogen purification), resulting in H2 purity > 99.97% v/v.
Gasification via water in supercritical condition (SWC = 22.1 MPa and 374 °C) is a valuable way to process wet biomass, producing hydrogen-rich syngas [213,214]. Demirbas [215] investigated the effect of operating temperatures (650–700 K) on hydrogen production from biomass gasification in supercritical water condition, observing an increase of hydrogen content from 6.6% to 9.4% with the temperature increasing from 650 to 700 K.
3.6. Synthetic Natural Gas (SNG)
SNG production by syngas represents an interesting way for biofuels production; this is a result of the infrastructures, distribution, and sales, which are identical to those used for methane [216,217,218,219]. A review of SNG production was recently carried out by Rönsch et al. [220], in which a comparison among several catalysts was performed, highlighting the main metal for methanation catalysts. Synthesis of methane by syngas could be achieved by using the same catalysts used in the steam reforming reactions, mainly Ni on alumina; however, other catalysts, such as Ru, Co, and Fe, can be used [221]. The reactions involved in the SNG production by syngas are showed below:
Both carbon monoxide hydrogenation and carbon dioxide hydrogenation are exothermic reactions; therefore, continuous cooling of the reactor is necessary to guarantee a temperature of 250–300 °C, i.e., the activation temperature of the catalysts. In order to increase the performance of these reactions, the operative pressure must be in the range between 15 and 25 bars [222]. The Achilles' heel for these reactions is the low hydrogen content in the syngas, which is lower than the stoichiometric value [218]. Moreover, CO2 conversion is inhibited when CO content increases over a certain value. [223]; for example, Weatherbee and Bartholomew reported a strong inhibition of methane production at CO concentration higher than 0.012%, using Ni-based catalysts. [224].
Another issue for SNG production is char formation, in particular because of the low process temperature:
Char formation could cause deactivation of Ni-based catalysts, thus decreasing the performance of methane production [225].
After the dewatering step, the gas produced by SNG process consists of methane and carbon dioxide, usually in equimolar composition. In these operative conditions, thanks to the high pressure of SNG, CO2 separation is considered economically feasible for the production of SNG with a high grade of methane purity [226].
4. Conclusions
In this manuscript, a critical overview is presented of gasification technologies and second-generation biofuels synthesized from syngas by biomass gasification, such as methanol, ethanol, dimethyl ether, Fischer-Tropsch Synthesis, hydrogen, and synthetic natural gas. Synthesis of biofuels from syngas is a feasible and effective way for confronting worldwide energy requirements and GHG emission at the same time.
The main parameters affecting syngas production and composition, such as gasification technologies (fixed bed reactors, fluidized bed reactors, entrained flow reactors), feedstock characteristics (biomass type, moisture content, particle size, ash content), and operating gasification conditions (bed material, temperature, pressure, GA, equivalence ratio, SB) are explored. As shown, syngas composition strictly depends on feedstock, technology, and operating parameters.
Purity of syngas in order to produce second-generation biofuels is highlighted, in terms of particulate matter, condensable hydrocarbons, alkaline metals, nitrogen, sulphur, and halides. Syngas cleaning requirements depend on downstream processes, operating conditions, catalysts, and main reaction mechanisms.
Synthesis of second-generation biofuels from biomass-derived syngas requires the optimization of the gasification process, specifically fed biomass, gasifier type and operating conditions, as well as syngas cleaning and conditioning. In order to define the concept of a whole synthesis chain, gasification process optimization, in terms of proper ratio of syngas components and of contaminant removal, has to be related to the type of the biofuel production process, in terms of catalyst and operating conditions. Notably, parameters has to be identified and defined according to end use, such as operating pressure of gasifiers in order to have syngas at proper downstream pressure, specified composition (such as H2/CO and CO2), and required syngas purity.
Author Contributions
Antonio Molino, Vincenzo Larocca and Simeone Chianese wrote the paper; Dino Musmarra supervised the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Reijnders, L. Conditions for the sustainability of biomass based fuel use. Energy Policy 2006, 34, 863–876. [Google Scholar] [CrossRef]
- Türe, S.; Uzun, D.; Türe, I.E. The potential use of sweet sorghum as a non-polluting source of energy. Energy 1997, 22, 17–19. [Google Scholar] [CrossRef]
- European Union. European Parliament Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009. Off. J. Eur. Union 2009, 140, 16–62. [Google Scholar] [CrossRef]
- USA Energy Independence. Security Act of 2007. Public Law 2007, 2007, 110–140. [Google Scholar]
- Molino, A.; Nanna, F.; Ding, Y.; Bikson, B.; Braccio, G. Biomethane production by anaerobic digestion of organic waste. Fuel 2013, 103, 1003–1009. [Google Scholar] [CrossRef]
- Molino, A.; Migliori, M.; Ding, Y.; Bikson, B.; Giordano, G.; Braccio, G. Biogas upgrading via membrane process: Modelling of pilot plant scale and the end uses for the grid injection. Fuel 2013, 107, 585–592. [Google Scholar] [CrossRef]
- Laursen, W. Students take a green initiative. Chem. Eng. 2005, 774–775, 32–34. [Google Scholar]
- Simpson-Holley, M.; Higson, A.; Evans, G. Bring on the biorefinery. Chem. Eng. 2007, 795, 46–49. [Google Scholar]
- Rajagopalan, S.; Datar, R.; Lewis, R.S. Formation of ethanol from carbon monoxide via a new microbial catalyst. Biomass Bioenergy 2002, 23, 487–493. [Google Scholar] [CrossRef]
- Eisberg, N. Harvesting energy. Chem. Ind. 2006, 17, 24–25. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels securing the planet’s future energy needs. Energy Convers. Manag. 2009, 50, 2239–2249. [Google Scholar] [CrossRef]
- He, J.; Zhang, W. Research on ethanol synthesis from syngas. J. Zhejiang Univ. A 2008, 9, 714–719. [Google Scholar] [CrossRef]
- Van der Drift, A.; Boerrigter, H. Synthesis gas from biomass for fuels and chemicals. In Proceedings of the SYNBIOS Conference Stock, Stockholm, Sweden, 18–20 May 2005; pp. 1–31. [Google Scholar]
- Drzyzga, O.; Revelles, O.; Durante-Rodríguez, G.; Díaz, E.; García, J.L.; Prieto, A. New challenges for syngas fermentation: Towards production of biopolymers. J. Chem. Technol. Biotechnol. 2015, 90, 1735–1751. [Google Scholar] [CrossRef]
- Molino, A.; Migliori, M.; Blasi, A.; Davoli, M.; Marino, T.; Chianese, S.; Catizzone, E.; Giordano, G. Municipal waste leachate conversion via catalytic supercritical water gasification process. Fuel 2017, 206, 155–161. [Google Scholar] [CrossRef]
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Roland Verhé, E. Renewable Bioresources: Scope and Modification for Non-Food Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 19, ISBN 0470021047. [Google Scholar]
- Baer, P.; Brown, M.A.; Kim, G. The job generation impacts of expanding industrial cogeneration. Ecol. Econ. 2015, 110, 141–153. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Asadullah, M. Barriers of commercial power generation using biomass gasification gas: A review. Renew. Sustain. Energy Rev. 2014, 29, 201–215. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H.; Inayat, A.; Khasri, A. Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Molino, A.; Iovane, P.; Donatelli, A.; Braccio, G.; Chianese, S.; Musmarra, D. Steam gasification of refuse-derived fuel in a rotary kiln pilot plant: Experimental tests. Chem. Eng. Trans. 2013, 32, 337–342. [Google Scholar]
- Rodríguez-Olalde, N.E.; Mendoza-Chávez, E.; Castro-Montoya, A.J.; Saucedo-Luna, J.; Maya-Yescas, R.; Rutiaga-Quiñones, J.G.; Ponce Ortega, J.M. Simulation of syngas production from lignin using guaiacol as a model compound. Energies 2015, 8, 6705–6714. [Google Scholar] [CrossRef]
- IEA Task33 International Energy Agency (IEA) Bioenergy Task 33E—Thermal Gasification of Biomass Database. Available online: http://task33.ieabioenergy.com/# (accessed on 20 February 2018).
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- De Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic steam gasification of biomass: Catalysts, thermodynamics and kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef] [PubMed]
- Udomsirichakorn, J.; Salam, P.A. Review of hydrogen-enriched gas production from steam gasification of biomass: The prospect of CaO-based chemical looping gasification. Renew. Sustain. Energy Rev. 2014, 30, 565–579. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Lange, J.P. Lignocellulose conversion: An introduction to chemistry, process and economics. Biofuels Bioprod. Biorefin. 2007, 1, 39–48. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global challenges in the sustainable development of biomass gasification: An overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Silva, V.; Rouboa, A. Co-gasification and recent developments on waste-to-energy conversion: A review. Renew. Sustain. Energy Rev. 2018, 81, 380–398. [Google Scholar] [CrossRef]
- Göransson, K.; Söderlind, U.; He, J.; Zhang, W. Review of syngas production via biomass DFBGs. Renew. Sustain. Energy Rev. 2011, 15, 482–492. [Google Scholar] [CrossRef]
- Siedlecki, M.; de Jong, W.; Verkooijen, A.H.M. Fluidized bed gasification as a mature and reliable technology for the production of bio-syngas and applied in the production of liquid transportation fuels—A review. Energies 2011, 4, 389–434. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. A critical review on biomass gasification, co-gasification, and their environmental assessments. Biofuel Res. J. 2016, 3, 483–495. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2010; ISBN 9780123749888. [Google Scholar]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air-steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xiao, B.; Guo, X.; Hu, Z.; Liu, S.; He, M. Hydrogen-rich gas from catalytic steam gasification of biomass in a fixed bed reactor: Influence of particle size on gasification performance. Int. J. Hydrogen Energy 2009, 34, 1260–1264. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Bula, A. Gasification of biomass wastes in an entrained flow gasifier: Effect of the particle size and the residence time. Fuel Process. Technol. 2010, 91, 681–692. [Google Scholar] [CrossRef]
- Jand, N.; Foscolo, P.U. Decomposition of wood particles in fluidized beds. Ind. Eng. Chem. Res. 2005, 44, 5079–5089. [Google Scholar] [CrossRef]
- Rapagn, S.; Mazziotti di Celso, G. Devolatilization of wood particles in a hot fluidized bed: Product yields and conversion rates. Biomass Bioenergy 2008, 32, 1123–1129. [Google Scholar] [CrossRef]
- Van der Drift, A.; Boerrigter, H.; Coda, B. Entrained Flow Gasification of Biomass—Ash Behaviour, Feeding Issues, and System Analyses; ECN-C-04-039; ECN: Petten, The Netherlands, 2004. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2002, 24, 125–140. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Gil, J.; Aznar, M.P.; Caballero, M.A.; Francés, E.; Corella, J. Biomass Gasification in Fluidized Bed at Pilot Scale with Steam-Oxygen Mixtures. Product Distribution for Very Different Operating Conditions. Energy Fuels 1997, 11, 1109–1118. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G.; Van Paasen, S.V.B.; Bergman, P.C.A.; Kiel, J.H.A. Catalytic decomposition of biomass tars: Use of dolomite and untreated olivine. Renew. Energy 2005, 30, 565–587. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Leckner, B.; Villanueva Perales, A.; Nilsson, S.; Fuentes Cano, D. Improving the performance of fluidized bed biomass/waste gasifiers for distributed electricity: A new three-stage gasification system. Appl. Therm. Eng. 2013, 50, 1453–1462. [Google Scholar] [CrossRef]
- Kirnbauer, F.; Wilk, V.; Hofbauer, H. Performance improvement of dual fluidized bed gasifiers by temperature reduction: The behavior of tar species in the product gas. Fuel 2013, 108, 534–542. [Google Scholar] [CrossRef]
- Abuadala, A.; Dincer, I. Investigation of a multi-generation system using a hybrid steam biomass gasification for hydrogen, power and heat. Int. J. Hydrogen Energy 2010, 35, 13146–13157. [Google Scholar] [CrossRef]
- Demirbas, A. Hydrogen-rich gases from biomass via pyrolysis and air-steam gasification. Energy Sources Part A 2009, 31, 1728–1736. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L.; Jones, D.D.; Hanna, M.A. Contemporary issues in thermal gasification of biomass and its application to electricity and fuel production. Biomass Bioenergy 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Knight, R.A. Experience with raw gas analysis from pressurized gasification of biomass. Biomass Bioenergy 2000, 18, 67–77. [Google Scholar] [CrossRef]
- Mathieu, P.; Dubuisson, R. Performance analysis of a biomass gasifier. Energy Convers. Manag. 2002, 43, 1291–1299. [Google Scholar] [CrossRef]
- Rapagnà, S.; Jand, N.; Kiennemann, A.; Foscolo, P.U. Steam-gasification of biomass in a fluidised-bed of olivine particles. Biomass Bioenergy 2000, 19, 187–197. [Google Scholar] [CrossRef]
- Lv, P.; Yuan, Z.; Ma, L.; Wu, C.; Chen, Y.; Zhu, J. Hydrogen-rich gas production from biomass air and oxygen/steam gasification in a downdraft Gasifier. Renew. Energy 2007, 32, 2173–2185. [Google Scholar] [CrossRef]
- Gil, J.; Corella, J.; Aznar, M.P.; Caballero, M.A. Biomass gasification in atmospheric and bubbling fluidized bed: Effect of the type of gasifying agent on the product distribution. Biomass Bioenergy 1999, 17, 389–403. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Q.; Zhao, H.; Cao, X.; Mei, Q.; Luo, Z.; Cen, K. Biomass-oxygen gasification in a high-temperature entrained-flow gasifier. Biotechnol. Adv. 2009, 27, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Ruoppolo, G.; Miccio, F.; Brachi, P.; Picarelli, A.; Chirone, R. Fluidized bed gasification of biomass and biomass/coal pellets in oxygen and steam atmosphere. Chem. Eng. Trans. 2013, 32, 595–600. [Google Scholar] [CrossRef]
- Narvaez, I.; Orıo, A.; Aznar, M.P.; Corella, J. Biomass Gasification with Air in an Atmospheric Bubbling Fluidized Bed. Effect of Six Operational Variables on the Quality of produced raw gas. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar] [CrossRef]
- Kumar, A.; Eskridge, K.; Jones, D.D.; Hanna, M.A. Steam-air fluidized bed gasification of distillers grains: Effects of steam to biomass ratio, equivalence ratio and gasification temperature. Bioresour. Technol. 2009, 100, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Dincer, I.; Rosen, M.A. Steam and air fed biomass gasification: Comparisons based on energy and exergy. Int. J. Hydrog. Energy 2012, 37, 16446–16452. [Google Scholar] [CrossRef]
- Bhavanam, A.; Sastry, R.C. Biomass Gasification Processes in Downdraft Fixed Bed Reactors: A Review. Int. J. Chem. Eng. Appl. 2011, 2, 425–433. [Google Scholar] [CrossRef]
- Hanping, C.; Bin, L.; Haiping, Y.; Guolai, Y.; Shihong, Z. Experimental investigation of biomass gasification in a fluidized bed reactor. Energy Fuels 2008, 22, 3493–3498. [Google Scholar] [CrossRef]
- Palancar, M.C.; Serrano, M.; Aragón, J.M. Testing the technological feasibility of FLUMOV as gasifier. Powder Technol. 2009, 194, 42–50. [Google Scholar] [CrossRef]
- Wang, Z.; He, T.; Qin, J.; Wu, J.; Li, J.; Zi, Z.; Liu, G.; Wu, J.; Sun, L. Gasification of biomass with oxygen-enriched air in a pilot scale two-stage gasifier. Fuel 2015, 150, 386–393. [Google Scholar] [CrossRef]
- Hamad, M.A.; Radwan, A.M.; Heggo, D.A.; Moustafa, T. Hydrogen rich gas production from catalytic gasification of biomass. Renew. Energy 2016, 85, 1290–1300. [Google Scholar] [CrossRef]
- Sharma, S.; Sheth, P.N. Air-steam biomass gasification: Experiments, modeling and simulation. Energy Convers. Manag. 2016, 110, 307–318. [Google Scholar] [CrossRef]
- Pindoria, R.; Megaritis, A.; Herod, A.; Kandiyoti, R. A two-stage fixed-bed reactor for direct hydrotreatment of volatiles from the hydropyrolysis of biomass: Effect of catalyst temperature, pressure and catalyst ageing time on product characteristics. Fuel 1998, 77, 1715–1726. [Google Scholar] [CrossRef]
- Hein, D.; Karl, J. Conversion of biomass to heat and electricity. In Landolt-Börnstein Numerical Data and Functional Relationships in Science and Technology; Springer: Berlin, Germany, 2006; pp. 374–413. [Google Scholar]
- Franco, C.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. The study of reactions influencing the biomass steam gasification process. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Gañan, J.; Abdulla, A.A.K.; Miranda, A.B.; Turegano, J.; Correia, S.; Cuerda, E.M. Energy production by means of gasification process of residuals sourced in Extremadura (Spain). Renew. Energy 2005, 30, 1759–1769. [Google Scholar] [CrossRef]
- Aqsha, A.; Tijani, M.M.; Moghtaderi, B.; Mahinpey, N. Catalytic pyrolysis of straw biomasses (wheat, flax, oat and barley) and the comparison of their product yields. J. Anal. Appl. Pyrolysis 2017, 125, 201–208. [Google Scholar] [CrossRef]
- Miranda, M.T.; Arranz, J.I.; Román, S.; Rojas, S.; Montero, I.; López, M.; Cruz, J.A. Characterization of grape pomace and pyrenean OAK pellets. Fuel Process. Technol. 2011, 92, 278–283. [Google Scholar] [CrossRef]
- Serrano, C.; Monedero, E.; Lapuerta, M.; Portero, H. Effect of moisture content, particle size and pine addition on quality parameters of barley straw pellets. Fuel Process. Technol. 2011, 92, 699–706. [Google Scholar] [CrossRef]
- Roy, M.M.; Corscadden, K.W. An experimental study of combustion and emissions of biomass briquettes in a domestic wood stove. Appl. Energy 2012, 99, 206–212. [Google Scholar] [CrossRef]
- Sharara, M.A.; Holeman, N.; Sadaka, S.S.; Costello, T.A. Pyrolysis kinetics of algal consortia grown using swine manure wastewater. Bioresour. Technol. 2014, 169, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Strong, P.J.; Xu, S.; Liu, S.; Lu, K.; Sheng, K.; Guo, J.; Che, L.; He, L.; et al. Thermal properties of biochars derived from Waste biomass generated by agricultural and forestry sectors. Energies 2017, 10, 469. [Google Scholar] [CrossRef]
- Gillespie, G.D.; Everard, C.D.; Fagan, C.C.; McDonnell, K.P. Prediction of quality parameters of biomass pellets from proximate and ultimate analysis. Fuel 2013, 111, 771–777. [Google Scholar] [CrossRef]
- Font Palma, C. Modelling of tar formation and evolution for biomass gasification: A review. Appl. Energy 2013, 111, 129–141. [Google Scholar] [CrossRef]
- Bergman, P.C.; Veringa, H.J. Combined Torrefaction and Pelletisation; ECN: Petten, The Netherlands, 2005. [Google Scholar]
- Mohammed, M.A.A.; Salmiaton, A.; Wan Azlina, W.A.K.G.; Mohammad Amran, M.S.; Fakhru’L-Razi, A. Air gasification of empty fruit bunch for hydrogen-rich gas production in a fluidized-bed reactor. Energy Convers. Manag. 2011, 52, 1555–1561. [Google Scholar] [CrossRef]
- Li, J.; Yin, Y.; Zhang, X.; Liu, J.; Yan, R. Hydrogen-rich gas production by steam gasification of palm oil wastes over supported tri-metallic catalyst. Int. J. Hydrogen Energy 2009, 34, 9108–9115. [Google Scholar] [CrossRef]
- Chang, A.C.C.; Chang, H.F.; Lin, F.J.; Lin, K.H.; Chen, C.H. Biomass gasification for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 14252–14260. [Google Scholar] [CrossRef]
- Skoulou, V.; Zabaniotou, A.; Stavropoulos, G.; Sakelaropoulos, G. Syngas production from olive tree cuttings and olive kernels in a downdraft fixed-bed gasifier. Int. J. Hydrogen Energy 2008, 33, 1185–1194. [Google Scholar] [CrossRef]
- Aljbour, S.H.; Kawamoto, K. Bench-scale gasification of cedar wood—Part I: Effect of operational conditions on product gas characteristics. Chemosphere 2013, 90, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Aljbour, S.H.; Kawamoto, K. Bench-scale gasification of cedar wood—Part II: Effect of Operational conditions on contaminant release. Chemosphere 2013, 90, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Annamalai, K.; Ansley, R.J.; Mirik, M. Updraft fixed bed gasification of mesquite and juniper wood samples. Energy 2012, 41, 454–461. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Iribarren, D.; Susmozas, A.; Petrakopoulou, F.; Dufour, J. Environmental and exergetic evaluation of hydrogen production via lignocellulosic biomass gasification. J. Clean. Prod. 2014, 69, 165–175. [Google Scholar] [CrossRef]
- Rajvanshi, A. Biomass Gasification. In Alternative Energy in Agriculture; CRC Press: Boca Raton, FL, USA, 1986; ISBN 0-8493-6348-9. [Google Scholar]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef]
- Sheth, P.N.; Babu, B.V. Experimental studies on producer gas generation from wood waste in a downdraft biomass gasifier. Bioresour. Technol. 2009, 100, 3127–3133. [Google Scholar] [CrossRef] [PubMed]
- Olgun, H.; Ozdogan, S.; Yinesor, G. Results with a bench scale downdraft biomass gasifier for agricultural and forestry residues. Biomass Bioenergy 2011, 35, 572–580. [Google Scholar] [CrossRef]
- Jaojaruek, K.; Jarungthammachote, S.; Gratuito, M.K.B.; Wongsuwan, H.; Homhual, S. Experimental study of wood downdraft gasification for an improved producer gas quality through an innovative two-stage air and premixed air/gas supply approach. Bioresour. Technol. 2011, 102, 4834–4840. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.A.; Akay, G. Occurrence, composition and dew point of tars produced during gasification of fuel cane bagasse in a downdraft gasifier. Biomass Bioenergy 2012, 42, 51–58. [Google Scholar] [CrossRef]
- Koppatz, S.; Pfeifer, C.; Rauch, R.; Hofbauer, H.; Marquard-Moellenstedt, T.; Specht, M. H2 rich product gas by steam gasification of biomass with in situ CO2 absorption in a dual fluidized bed system of 8 MW fuel input. Fuel Process. Technol. 2009, 90, 914–921. [Google Scholar] [CrossRef]
- Miccio, F.; Piriou, B.; Ruoppolo, G.; Chirone, R. Biomass gasification in a catalytic fluidized reactor with beds of different materials. Chem. Eng. J. 2009, 154, 369–374. [Google Scholar] [CrossRef]
- Bartels, M.; Lin, W.; Nijenhuis, J.; Kapteijn, F.; van Ommen, J.R. Agglomeration in fluidized beds at high temperatures: Mechanisms, detection and prevention. Prog. Energy Combust. Sci. 2008, 34, 633–666. [Google Scholar] [CrossRef]
- Ruoppolo, G.; Miccio, F.; Chirone, R. Fluidized bed cogasification of wood and coal adopting primary catalytic method for tar abatement. Energy Fuels 2010, 24, 2034–2041. [Google Scholar] [CrossRef]
- Miccio, F.; Picarelli, A.; Ruoppolo, G. Increasing tar and hydrocarbons conversion by catalysis in bubbling fluidized bed gasifiers. Fuel Process. Technol. 2016, 141, 31–37. [Google Scholar] [CrossRef]
- Vaccaro, S.; Musmarra, D.; Petrecca, M. A technique for measurement of the jet penetration height in fluidized beds by pressure signal analysis. Powder Technol. 1997, 92, 223–231. [Google Scholar] [CrossRef]
- Song, T.; Wu, J.; Shen, L.; Xiao, J. Experimental investigation on hydrogen production from biomass gasification in interconnected fluidized beds. Biomass Bioenergy 2012, 36, 258–267. [Google Scholar] [CrossRef]
- Meng, X.; de Jong, W.; Fu, N.; Verkooijen, A.H.M. Biomass gasification in a 100 kWth steam-oxygen blown circulating fluidized bed gasifier: Effects of operational conditions on product gas distribution and tar formation. Biomass Bioenergy 2011, 35, 2910–2924. [Google Scholar] [CrossRef]
- Ngo, S.I.; Nguyen, T.D.B.; Lim, Y.; Song, B.H.; Lee, U.D.; Choi, Y.T.; Song, J.H. Performance evaluation for dual circulating fluidized-bed steam gasifier of biomass using quasi-equilibrium three-stage gasification model. Appl. Energy 2011, 88, 5208–5220. [Google Scholar] [CrossRef]
- Loha, C.; Gu, S.; De Wilde, J.; Mahanta, P.; Chatterjee, P.K. Advances in mathematical modeling of fluidized bed gasification. Renew. Sustain. Energy Rev. 2014, 40, 688–715. [Google Scholar] [CrossRef]
- Wang, P.; Massoudi, M. Slag behavior in gasifiers. Part I: Influence of coal properties and gasification conditions. Energies 2013, 6, 784–806. [Google Scholar] [CrossRef]
- Briesemeister, L.; Kremling, M.; Fendt, S.; Spliethoff, H. Air-Blown Entrained-Flow Gasification of Biomass: Influence of Operating Conditions on Tar Generation. Energy Fuels 2017, 31, 10924–10932. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Couhert, C.; Salvador, S.; Commandr, J.M. Impact of torrefaction on syngas production from wood. Fuel 2009, 88, 2286–2290. [Google Scholar] [CrossRef]
- Brachi, P.; Chirone, R.; Miccio, F.; Miccio, M.; Ruoppolo, G. Entrained-flow gasification of torrefied tomato peels: Combining torrefaction experiments with chemical equilibrium modeling for gasification. Fuel 2018, 220, 744–753. [Google Scholar] [CrossRef]
- Kajitani, S.; Zhang, Y.; Umemoto, S.; Ashizawa, M.; Hara, S. Co-gasification reactivity of coal and woody biomass in high-temperature gasification. Energy Fuels 2010, 24, 145–151. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Serrano, C. Co-gasification of biomass wastes and coal-coke blends in an entrained flow gasifier: An experimental study. Energy Fuels 2010, 24, 2479–2488. [Google Scholar] [CrossRef]
- Valero, A.; Usón, S. Oxy-co-gasification of coal and biomass in an integrated gasification combined cycle (IGCC) power plant. Energy 2006, 31, 1643–1655. [Google Scholar] [CrossRef]
- National Energy Technology Laboratory Commercial Gasifiers. Available online: https://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/fmb (accessed on 29 January 2018).
- González-Vázquez, M.P.; García, R.; Pevida, C.; Rubiera, F. Optimization of a bubbling fluidized bed plant for low-temperature gasification of biomass. Energies 2017, 10, 306. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Leckner, B. Estimation of gas composition and char conversion in a fluidized bed biomass gasifier. Fuel 2013, 107, 419–431. [Google Scholar] [CrossRef]
- Demirbas, A. Competitive liquid biofuels from biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biofuels. Prog. Energy Combust. Sci. 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Matas Güell, B.; Sandquist, J.; Sørum, L. Gasification of Biomass to Second Generation Biofuels: A Review. J. Energy Resour. Technol. 2012, 135, 14001. [Google Scholar] [CrossRef]
- Lens, P.; Westermann, P.; Haberbauer, M.; Moreno, A. Biofuels for Fuel Cells: Renewable Energy from Biomass Fermentation; IWA Publishing: London, UK, 2005. [Google Scholar]
- Adachi, Y.; Komoto, M.; Watanabe, I.; Ohno, Y.; Fujimoto, K. Effective utilization of remote coal through dimethyl ether synthesis. Fuel 2000, 79, 229–234. [Google Scholar] [CrossRef]
- Sardesai, A.; Tartamella, T.; Lee, S. CO2/dimethyl ether (DME) feed mixtures in the DME-to-hydrocarbons (DTH) process. In Proceedings of the 12th Annual International Pittsburgh Coal Conference, Pittsburgh, PA, USA, 11–15 September 1995. [Google Scholar]
- Caldeira-Pires, A.; da Luz, S.M.; Palma-Rojas, S.; Rodrigues, T.O.; Silverio, V.C.; Vilela, F.; Barbosa, P.C.; Alves, A.M. Sustainability of the biorefinery industry for fuel production. Energies 2013, 6, 329–350. [Google Scholar] [CrossRef]
- Spath, P.L.; Dayton, D.C. Preliminary Screening—Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass-Derived Syngas; National Renewable Energy Laboratory: Golden, CO, USA, 2003.
- Zhang, W. Automotive fuels from biomass via gasification. Fuel Process. Technol. 2010, 91, 866–876. [Google Scholar] [CrossRef]
- Sauciuc, A.; Abosteif, Z.; Weber, G.; Potetz, A.; Rauch, R.; Hofbauer, H.; Schaub, G.; Dumitrescu, L. Influence of operating conditions on the performance of biomass-based Fischer-Tropsch synthesis. Biomass Convers. Biorefin. 2012, 2, 253–263. [Google Scholar] [CrossRef]
- Ciferno, J.P.; Marano, J.J. Benchmarking Biomass Gasification Technologies for Fuels, Chemicals and Hydrogen Production; US Department of Energy, National Energy Technology Laboratory: Albany, OR, USA, 2002.
- Dowaki, K.; Genchi, Y. Life cycle inventory analysis on Bio-DME and/or Bio-MeOH products through BLUE tower process. Int. J. Life Cycle Assess. 2009, 14, 611–620. [Google Scholar] [CrossRef]
- Srinivas, S.; Malik, R.K.; Mahajani, S.M. Fischer-Tropsch synthesis using bio-syngas and CO2. Energy Sustain. Dev. 2007, 11, 66–71. [Google Scholar] [CrossRef]
- Riedel, T.; Schulz, H.; Schaub, G.; Jun, K.W.; Hwang, J.S.; Lee, K.W. Fischer-Tropsch on iron with H2/CO and H2/CO2 as synthesis gases: The episodes of formation of the Fischer-Tropsch regime and construction of the catalyst. Top. Catal. 2003, 26, 41–54. [Google Scholar] [CrossRef]
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Fratalocchi, L.; Lietti, L. CO2 hydrogenation to hydrocarbons over Co and Fe-based Fischer-Tropsch catalysts. Catal. Today 2016, 277, 161–170. [Google Scholar] [CrossRef]
- Van der Heijden, H.; Ptasinski, K.J. Exergy analysis of thermochemical ethanol production via biomass gasification and catalytic synthesis. Energy 2012, 46, 200–210. [Google Scholar] [CrossRef]
- Phillips, S.; Jechura, J.; Dayton, D.; Eggeman, T. Thermochemical Ethanol via Indirect Gasification and Mixed Alcohol Synthesis of Lignocellulosic Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2007.
- Ateka, A.; Sánchez-Contador, M.; Ereña, J.; Aguayo, A.T.; Bilbao, J. Catalyst configuration for the direct synthesis of dimethyl ether from CO and CO2 hydrogenation on CuO-ZnO-MnO/SAPO-18 catalysts. In Reaction Kinetics, Mechanisms and Catalysis; Springer: Berlin, Germany, 2018. [Google Scholar]
- Saravanan, K.; Ham, H.; Tsubaki, N.; Bae, J.W. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts. Appl. Catal. B Environ. 2017, 217, 494–522. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Nexant, I.; San Francisco, C.; Aden, N.T.M.A. Survey and Down-Selection of Acid Gas Removal Systems for the Thermochemical Conversion of Biomass to Ethanol with a Detailed Analysis of an MDEA System; National Renewable Energy Laboratory: Golden, CO, USA, 2009.
- Reyes Valle, C.; Villanueva Perales, A.L.; Vidal-Barrero, F.; Gómez-Barea, A. Techno-economic assessment of biomass-to-ethanol by indirect fluidized bed gasification: Impact of reforming technologies and comparison with entrained flow gasification. Appl. Energy 2013, 109, 254–266. [Google Scholar] [CrossRef]
- Fail, S.; Diaz, N.; Benedikt, F.; Kraussler, M.; Hinteregger, J.; Bosch, K.; Hackel, M.; Rauch, R.; Hofbauer, H. Wood gas processing to generate pure hydrogen suitable for PEM fuel cells. ACS Sustain. Chem. Eng. 2014, 2, 2690–2698. [Google Scholar] [CrossRef]
- Loipersböck, J.; Lenzi, M.; Rauch, R.; Hofbauer, H. Hydrogen production from biomass: The behavior of impurities over a CO shift unit and a biodiesel scrubber used as a gas treatment stage. Korean J. Chem. Eng. 2017, 34, 2198–2203. [Google Scholar] [CrossRef]
- Aldana, P.A.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Wang, G.; Xu, S.; Wang, C.; Zhang, J.; Fang, Z. Desulfurization and tar reforming of biogenous syngas over Ni/olivine in a decoupled dual loop gasifier. Int. J. Hydrog. Energy 2017, 42, 15471–15478. [Google Scholar] [CrossRef]
- Haro, P.; Johnsson, F.; Thunman, H. Improved syngas processing for enhanced Bio-SNG production: A techno-economic assessment. Energy 2016, 101, 380–389. [Google Scholar] [CrossRef]
- Atsonios, K.; Kougioumtzis, M.A.; Panopoulos, K.D.; Kakaras, E. Alternative thermochemical routes for aviation biofuels via alcohols synthesis: Process modeling, techno-economic assessment and comparison. Appl. Energy 2015, 138, 346–366. [Google Scholar] [CrossRef]
- Tsubaki, N.; Ito, M.; Fujimoto, K. A New Method of Low-Temperature Methanol Synthesis. J. Catal. 2001, 197, 224–227. [Google Scholar] [CrossRef]
- Santangelo, D.L.O.; Ahón, V.R.R.; Costa, A.L.H. Optimization of methanol synthesis loops with quench reactors. Chem. Eng. Technol. 2008, 31, 1767–1774. [Google Scholar] [CrossRef]
- Kralj, A.K.; Glavič, P. Multi-criteria optimization in a methanol process. Appl. Therm. Eng. 2009, 29, 1043–1049. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Bulushev, D.A.; Beloshapkin, S.; Ross, J.R.H. The effect of potassium on the activity and stability of Ni-MgO-ZrO2 catalysts for the dry reforming of methane to give synthesis gas. Catal. Today 2011, 178, 132–136. [Google Scholar] [CrossRef]
- Supp, E. How to Produce Methanol from Coal; Springer: Berlin, Germany, 2013. [Google Scholar]
- Cybulski, A. Liquid-phase methanol synthesis: Catalysts, mechanism, kinetics, chemical equilibria, vapor-liquid equilibria, and modeling—A review. Catal. Rev. 1994, 36, 557–615. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Zhang, Y.; Ding, W.; Chen, S.; Fang, W.; Yang, Y. Promoting effect of an aluminum emulsion on catalytic performance of Cu-based catalysts for methanol synthesis from syngas. Fuel Process. Technol. 2010, 91, 723–728. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, L.; Guo, Q.; Fan, H.; Zheng, H.; Xie, K. Influence of the calcination on the activity and stability of the Cu/ZnO/Al2O3 catalyst in liquid phase methanol synthesis. Fuel 2010, 89, 1348–1352. [Google Scholar] [CrossRef]
- Guo, X.; Li, L.; Liu, S.; Bao, G.; Hou, W. Preparation of CuO/ZnO/Al2O3 catalysts for methanol synthesis using parallel-slurry-mixing method. J. Fuel Chem. Technol. 2007, 35, 329–333. [Google Scholar] [CrossRef]
- Meshkini, F.; Taghizadeh, M.; Bahmani, M. Investigating the effect of metal oxide additives on the properties of Cu/ZnO/Al2O3 catalysts in methanol synthesis from syngas using factorial experimental design. Fuel 2010, 89, 170–175. [Google Scholar] [CrossRef]
- Dybkjaer, I.; Christensen, T.S. Syngas for Large Scale Conversion of Natural Gas to Liquid Fuels. Stud. Surf. Sci. Catal. 2001, 136, 435–440. [Google Scholar]
- Larocca, V.; Molino, A.; Petrone, M.T.; Barisano, D.; Giordano, G.; Braccio, G. Synthesis of Methanol from Biomass-derived syngas: Modelling and sizing of a bench-scale reactor. Int. J. Energy Technol. 2010, 2, 1–7. [Google Scholar]
- Taherzadeh, M. Ethanol from Lignocelluloce: Physiological Effects of Inhibitors and Fermentation Strategies; Chalmers University of Technology: Gothenburg, Sweden, 1999. [Google Scholar]
- Ajanovic, A. Biofuels versus food production: Does biofuels production increase food prices? Energy 2011, 36, 2070–2076. [Google Scholar] [CrossRef]
- Zhang, Z.; Lohr, L.; Escalante, C.; Wetzstein, M. Food versus fuel: What do prices tell us? Energy Policy 2010, 38, 445–451. [Google Scholar] [CrossRef]
- Goldemberg, J. Ethanol for a Sustainable Energy Future. Science 2007, 315, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.J.; Hall, C.A.S.; Herendeen, R.A.; Hagens, N.; Costanza, R.; Mulder, K.; Lynd, L.; Greene, N.; Dale, B.; Laser, M.; et al. Energy Returns on Ethanol Production. Science 2006, 312, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Liu, P. Mechanism of ethanol synthesis from syngas on Rh(111). J. Am. Chem. Soc. 2009, 131, 13054–13061. [Google Scholar] [CrossRef] [PubMed]
- Spivey, J.J.; Egbebi, A. Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas. Chem. Soc. Rev. 2007, 36, 1514. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.A.; Gogate, M.R.; Davis, R.J. Fe-promotion of supported Rh catalysts for direct conversion of syngas to ethanol. J. Catal. 2009, 261, 9–16. [Google Scholar] [CrossRef]
- Pan, X.; Fan, Z.; Chen, W.; Ding, Y.; Luo, H.; Bao, X. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mater. 2007, 6, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.W.; Márquez, M.A.; McCutchen, M.S. Alcohol synthesis in a high-temperature slurry reactor. Catal. Today 1997, 36, 255–263. [Google Scholar] [CrossRef]
- Tien-Thao, N.; Zahedi-Niaki, M.H.; Alamdari, H.; Kaliaguine, S. Conversion of syngas to higher alcohols over nanosized LaCo0.7Cu0.3O3 perovskite precursors. Appl. Catal. A 2007, 326, 152–163. [Google Scholar] [CrossRef]
- Subramani, V.; Gangwal, S.K. A review of recent literature to search for an efficient catalytic process for the conversion of syngas to ethanol. Energy Fuels 2008, 22, 814–839. [Google Scholar] [CrossRef]
- Benito, M.; Sanz, J.L.; Isabel, R.; Padilla, R.; Arjona, R.; Daza, L. Bio-ethanol steam reforming: Insights on the mechanism for hydrogen production. J. Power Sources 2005, 151, 11–17. [Google Scholar] [CrossRef]
- Kulawska, M.; Skrzypek, J. Kinetics of the synthesis of higher aliphatic alcohols from syngas over a modified methanol synthesis catalyst. Chem. Eng. Process. 2001, 40, 33–40. [Google Scholar] [CrossRef]
- Goldemberg, J.; Johansson, T.B.; Reddy, A.K.N.; Williams, R.H. A global clean cooking fuel initiative. Energy Sustain. Dev. 2004, 8, 5–12. [Google Scholar] [CrossRef]
- Larson, E.D.; Yang, H. Dimethyl ether (DME) from coal as a household cooking fuel in China. Energy Sustain. Dev. 2004, 8, 115–126. [Google Scholar] [CrossRef]
- Gunda, A.; Tartamella, T.; Gogate, M.; Lee, S. Dimethyl ether synthesis from CO2-rich syngas in the LPDME process. Fuel Energy Abstr. 1997, 38, 75. [Google Scholar]
- Ramos, F.S.; Farias, A.M.D.; Borges, L.E.P.; Monteiro, J.L.; Fraga, M.A.; Sousa-Aguiar, E.F.; Appel, L.G. Role of dehydration catalyst acid properties on one-step DME synthesis over physical mixtures. Catal. Today 2005, 101, 39–44. [Google Scholar] [CrossRef]
- Sai Prasad, P.S.; Bae, J.W.; Kang, S.H.; Lee, Y.J.; Jun, K.W. Single-step synthesis of DME from syngas on Cu-ZnO-Al2O3/zeolite bifunctional catalysts: The superiority of ferrierite over the other zeolites. Fuel Process. Technol. 2008, 89, 1281–1286. [Google Scholar] [CrossRef]
- Bae, J.W.; Kang, S.H.; Lee, Y.J.; Jun, K.W. Synthesis of DME from syngas on the bifunctional Cu-ZnO-Al2O3/Zr-modified ferrierite: Effect of Zr content. Appl. Catal. B Environ. 2009, 90, 426–435. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, W.; Yin, L.; Xie, K. Liquid-phase preparation of catalysts used in slurry reactors to synthesize dimethyl ether from syngas: Effect of heat-treatment atmosphere. Fuel Process. Technol. 2009, 90, 1442–1446. [Google Scholar] [CrossRef]
- Galvita, V.V.; Semin, G.L.; Belyaev, V.D.; Yurieva, T.M.; Sobyanin, V.A. Production of hydrogen from dimethyl ether. Appl. Catal. A 2001, 216, 85–90. [Google Scholar] [CrossRef]
- Ogawa, T.; Inoue, N.; Shikada, T.; Ohno, Y. Direct Dimethyl Ether Synthesis. J. Nat. Gas Chem. 2003, 12, 219–227. [Google Scholar]
- Sunde, K.; Brekke, A.; Solberg, B. Environmental impacts and costs of hydrotreated vegetable oils, transesterified lipids andwoody BTL-A review. Energies 2011, 4, 845–877. [Google Scholar] [CrossRef]
- Ohno, Y. Recent Situation and Future Development of DME Direct Synthesis Technology; Japan DME Forum: Tokyo, Japan, 2002. [Google Scholar]
- Demirbas, A. Global biodiesel strategies. Energy Educ. Sci. Technol. 2006, 17, 27–63. [Google Scholar]
- Tijmensen, M.J.A.; Faaij, A.P.C.; Hamelinck, C.N.; Van Hardeveld, M.R.M. Exploration of the possibilities for production of Fischer Tropsch liquids and power via biomass gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Exergetic optimisation of a production process of Fischer-Tropsch fuels from biomass. Fuel Process. Technol. 2005, 86, 375–389. [Google Scholar] [CrossRef]
- Dry, M.E. Fischer-Tropsch reactions and the environment. Appl. Catal. A 1999, 189, 185–190. [Google Scholar] [CrossRef]
- Dry, M.E. High quality diesel via the Fischer-Tropsch process—A review. J. Chem. Technol. Biotechnol. 2002, 77, 43–50. [Google Scholar] [CrossRef]
- Demirbas, A. Recent advances in biomass conversion technologies. Energy Educ. Sci. Technol. 2000, 6, 77–83. [Google Scholar]
- Sie, S.T.; Krishna, R. Fundamentals and selection of advanced Fischer-Tropsch reactors. Appl. Catal. A 1999, 186, 55–70. [Google Scholar] [CrossRef]
- Ahn, V.R.; Costa, E.F.; Monteagudo, J.E.P.; Fontes, C.E.; Biscaia, E.C.; Lage, P.L.C. A comprehensive mathematical model for the Fischer-Tropsch synthesis in well-mixed slurry reactors. Chem. Eng. Sci. 2005, 60, 677–694. [Google Scholar] [CrossRef]
- Rapagna, S.; Jand, N.; Foscolo, P.U. Catalytic Gasification of Biomass Rich Gas to Produce. Int. J. Hydrogen Energy 1998, 23, 551–557. [Google Scholar] [CrossRef]
- Li, S.; Krishnamoorthy, S.; Li, A.; Meitzner, G.D.; Iglesia, E. Promoted Iron-Based Catalysts for the Fischer-Tropsch Synthesis: Design, Synthesis, Site Densities, and Catalytic Properties. J. Catal. 2002, 206, 202–217. [Google Scholar] [CrossRef]
- Schulz, H. Short history and present trends of Fischer-Tropsch synthesis. Appl. Catal. A 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. “Renewable” hydrogen: Prospects and challenges. Renew. Sustain. Energy Rev. 2011, 15, 3034–3040. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C. Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of thermochemical conversion processes of biomass to hydrogen-rich gas mixtures. Energy Sources Part A 2016, 38, 2971–2976. [Google Scholar] [CrossRef]
- Saxena, R.C.; Seal, D.; Kumar, S.; Goyal, H.B. Thermo-chemical routes for hydrogen rich gas from biomass: A review. Renew. Sustain. Energy Rev. 2008, 12, 1909–1927. [Google Scholar] [CrossRef]
- Chianese, S.; Loipersböck, J.; Malits, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Musmarra, D. Hydrogen from the high temperature water gas shift reaction with an industrial Fe/Cr catalyst using biomass gasification tar rich synthesis gas. Fuel Process. Technol. 2015, 132, 39–48. [Google Scholar] [CrossRef]
- Muller, S.; Kotik, J.; Proll, T.; Rauch, R.; Hofbauer, H. Hydrogen from biomass for industry—Biomass gasification for integration in refineries. In Proceedings of the International Conference Polygeneration Strategies, Wien, Austria, 30 August–1 September 2011. [Google Scholar]
- Stelmachowski, M.; Nowicki, L. Fuel from the synthesis gas-the role of process engineering. Appl. Energy 2003, 74, 85–93. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Goodman, D.W. CO-free production of hydrogen via stepwise steam reforming of methane. J. Catal. 2000, 192, 316–321. [Google Scholar] [CrossRef]
- Chianese, S.; Fail, S.; Binder, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Blasi, A.; Musmarra, D. Experimental investigations of hydrogen production from CO catalytic conversion of tar rich syngas by biomass gasification. Catal. Today 2016, 277, 182–191. [Google Scholar] [CrossRef]
- Soukup, G.; Pfeifer, C.; Kreuzeder, A.; Hofbauer, H. In situ CO2 capture in a dual fluidized bed biomass steam gasifier—Bed material and fuel variation. Chem. Eng. Technol. 2009, 32, 348–354. [Google Scholar] [CrossRef]
- Lu, Y.J.; Jin, H.; Guo, L.J.; Zhang, X.M.; Cao, C.Q.; Guo, X. Hydrogen production by biomass gasification in supercritical water with a fluidized bed reactor. Int. J. Hydrogen Energy 2008, 33, 6066–6075. [Google Scholar] [CrossRef]
- Molino, A.; Migliori, M.; Macrì, D.; Valerio, V.; Villone, A.; Nanna, F.; Iovane, P.; Marino, T. Glucose gasification in super-critical water conditions for both syngas production and green chemicals with a continuous process. Renew. Energy 2016, 91, 451–455. [Google Scholar] [CrossRef]
- Demirbaş, A. Hydrogen production from biomass via supercritical water extraction. Energy Sources 2005, 27, 1409–1417. [Google Scholar] [CrossRef]
- Gassner, M.; Maréchal, F. Thermo-economic optimisation of the polygeneration of synthetic natural gas (SNG), power and heat from lignocellulosic biomass by gasification and methanation. Energy Environ. Sci. 2012, 5, 5768. [Google Scholar] [CrossRef]
- Duret, A.; Friedli, C.; Maréchal, F. Process design of Synthetic Natural Gas (SNG) production using wood gasification. J. Clean. Prod. 2005, 13, 1434–1446. [Google Scholar] [CrossRef]
- Gassner, M.; Maréchal, F. Thermo-economic process model for thermochemical production of Synthetic Natural Gas (SNG) from lignocellulosic biomass. Biomass Bioenergy 2009, 33, 1587–1604. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Kuznecova, I.; Gusca, J. Property based ranking of CO and CO2 methanation catalysts. Energy Procedia 2017, 128, 255–260. [Google Scholar] [CrossRef]
- Molino, A.; Braccio, G. Synthetic natural gas SNG production from biomass gasification—Thermodynamics and processing aspects. Fuel 2015, 139, 425–429. [Google Scholar] [CrossRef]
- Mills, G.A.; Steffgen, F.W. Catalytic methanation. Catal. Rev. 1974, 8, 159–210. [Google Scholar] [CrossRef]
- Weatherbee, G.D.; Bartholomew, C.H. Hydrogenation of CO2 on group VIII metals. II. Kinetics and mechanism of CO2 hydrogenation on nickel. J. Catal. 1982, 77, 460–472. [Google Scholar] [CrossRef]
- Dayton, D. Review of the Literature on Catalytic Biomass Tar Destruction: Milestone Completion Report; National Renewable Energy Laboratory: Golden, CO, USA, 2002.
- Vitasari, C.R.; Jurascik, M.; Ptasinski, K.J. Exergy analysis of biomass-to-synthetic natural gas (SNG) process via indirect gasification of various biomass feedstock. Energy 2011, 36, 3825–3837. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).