Abstract
The growing demand for energy is particularly important to engineers with respect to how the energy produced by heat and power plants can be used efficiently. Formerly, performance evaluation of thermal power plants was done through energy analysis. However, the energy method does not account for irreversibilities within the system. An effective method to measure and improve efficiency of thermal power plant is exergy analysis. Exergy analysis is used to evaluate the performance of a system and its main advantage is enhancement of the energy conversion process. It helps identify the main points of exergy destruction, the quantity and causes of this destruction, as well as show which areas in the system and components have potential for improvements. The current study is a comprehensive review of exergy analyses applied in the solid fuels heat and power sector, which includes coal, biomass and a combination of these feedstocks as fuels. The methods for the evaluation of the exergy efficiency and the exergy destruction are surveyed in each part of the plant. The current review is expected to advance understanding of exergy analysis and its usefulness in the energy and power sectors: it will assist in the performance assessment, analysis, optimization and cost effectiveness of the design of heat and power plant systems in these sectors.
1. Introduction
The worldwide demand for, and consumption of energy and power are expected to increase in future years due to the expansion of urbanization, the rapid rate of industrialization and the continuous improvements being made to the standard of living [1,2]. Humankind currently uses 410 × 1018 joules per annum, which is equal to the energy content of over 90,000 billion litres of oil, i.e., commercially-traded energy [3]. Nevertheless, the rate of consumption is constrained by the available resources [4,5] and a consequence of energy sources being limited is that their efficient use requires thermal processes to be optimized, with special emphasis being placed on the energy associated with exhaust gases and other forms of waste heat [6]. The energy sector needs not only to be effective in order to meet the increasing demands for heat and electricity from society, but also to utilize the resources that are available for their production by improving the efficiency of plants.
Normally, performance assessment of a system is carried out using the concept of the first law of thermodynamics, which is based on the conservation of energy [1,7]. However, using energy alone in the efficiency analysis of processes is bound to lead to misconceptions, misevaluations and poor decisions [8]: it only embraces information of the inputs and outputs of the energy in the process and excludes its quality [9]. The use of second law analysis allows the quality of the energy to be determined, and the irreversibilities quantified, as a result of the entropy that is generated and which causes inefficiency in the process [1]. Second law analysis is often based on the concept of “exergy”, also known as “available energy”, “availability” or “useful energy” [8]. This enables the main sources of loss to be identified and provides directions for improving performance within the system [7].
Exergy is the maximum amount of work that can be obtained from a stream of matter, heat or work as it is brought into equilibrium with the environment [10]. The reference environment of temperature, pressure and mixture of substances found in abundance in nature must be defined: it is given a zero exergy (i.e., “dead state”) [11]. Exergy analysis has been widely used in the evaluation, simulation and design of thermo-chemical and thermo-mechanical systems [12]. Its application reaches beyond technical analysis, as it is also used in thermo-economic, environmental and sustainability analyses of industrial systems [13]: exergy analysis allows for the thermodynamic assessment of energy conservation because it provides the tool for making a clear distinction between the energy lost to the environment and internal irreversibilities in the process [6]. It also represents quantitatively the useful energy, i.e., the work content of the great variety of streams (comprising mass, heat, work), that enters into the system and accounts for the exergy destroyed during a process, which is proportional to the entropy generated [14]. This destruction of exergy, or irreversibility, is a yardstick by which losses in the plant are determined and compared [7].
The energy in solid fuels can be converted into useful products through biological/biochemical and thermochemical processes [15,16]. In terms of faster reaction rate and reduction in larger amount and volume of solid materials, the thermochemical processes are more efficient than the biological methods [17]. The three main thermochemical conversion methods are combustion, pyrolysis and gasification [18]. The combustion process is a commercially viable option [19] and the most widely used method for solid fuels conversion into heat and power [15,20].
In the literature, only a few papers have taken a comprehensive view of exergy analysis on the biomass-based fuels and coal-fired heat and power plant. Saidur et al. [21] reviewed the application of exergy analysis to different biomass fuels. Their investigations were based on biomass gasification rather than conversion through combustion processes. Kaushik et al. [18] examined energy and exergy analysis of coal-fired thermal power plants. Though their studies were based on thermochemical conversion of the fuel using a combustion method, they only examined the design conditions of existing plants. To the best knowledge of the authors, there is no review of exergy analysis of biomass and coal co-fired heat and power plants. Therefore, the aim of this study is to review exergy analysis in the heat and power sector, with respect to comparing performance, making assessments of, and suggesting improvements for, coal-fired, biomass-fired and co-fired coal and biomass heat and power plants.
2. Exergy Analysis
The aim of exergy analysis is to detect and evaluate the thermodynamic imperfections of a process quantitatively and indicate possible ways of improving it [22]. Thermodynamic imperfection, or irreversibility, is a function of the generation of entropy. Exergy analysis enables system designers and engineers to identify the parts with the highest entropy generation, providing them with key points on which to focus: they can then increase the efficiency of the system and, simultaneously, lower the negative impact exerted on the environment [23]. Achieving these two objectives involves making evaluations of, and optimizing, each component in the entire system using the mass, energy, entropy and exergy flow. For a steady-state process, these balances are expressed below [23,24].
The mass balance equation can be written in the rate form of Equation (1):
where and are the mass flow rates of the fluid entering and exiting the system, respectively.
The energy rate balance for a steady-state system is expressed as Equation (2):
where the energy rate entering and exiting the system is and , respectively, is the heat rate into the system and is the work transfer rate performed by the system.
The entropy rate balance equation is given by Equation (3):
where is the entropy rate of a flow and is the entropy generation rate.
The exergy rate balance for a system is calculated using Equation (4):
where is the heat transfer rate at temperature through the boundary at position j, is the exergy destruction rate and is the temperature of the reference environment.
Rearranging the exergy balance in Equation (4), the irreversible (or exergy destruction) rate can be expressed by Equation (5) thus:
Assuming there are no heat losses since the insulation of each component in the system is good, the exergy associated with the heat transfer rate in the components is zero [25], and Equation (5) becomes:
The exergy destruction rate due to irreversibility in a system can also be given as Equation (7):
The exergy rate associated with a flowing stream of matter contains physical, chemical, kinetic and potential exergy [26] according to Equation (8):
where and are exergy due to kinetic and potential energy, respectively, is the specific physical exergy and is the specific chemical exergy. Assuming that the changes in velocity and elevation of the flowing stream are negligible, then and can be discarded in the calculation of changes in exergy. The exergy flow rate of a stream is shown in Equation (9):
The specific physical exergy, is exergy due to the differences in the temperature and pressure of a system with respect to the reference environment [27]: it can be expressed by Equation (10) thus:
where and are the specific enthalpy and the entropy respectively at the temperature of the reference environment.
The specific chemical exergy, depends on the chemical composition of a substance in its particular state, and if it is in equilibrium with the reference environment [28]. For solid fuels, the specific chemical exergy can be estimated based on the elemental compositions of the fuel [29,30,31,32,33,34,35,36,37].
The second law of efficiency or exergy efficiency, , of any system can be defined as the ratio of the exergy transfer rate associated with the output to the exergy transfer rate associated with the input of the system [3]. It is the best variable for evaluating the performance of a thermal system and its components [38], and is expressed here in Equation (11):
where represents the fuel exergy rate, while and represent the exergy rate of the product and the rate of exergy loss from the system. If the heat losses from the components are neglected, then the exergy loss is zero [38] and Equation (11) can be rewritten as Equation (12):
The first law efficiency or energy efficiency of a system is defined as the ratio of energy output rate to the energy input rate to system and is calculated using Equation (13) [18].
where and are energy output and energy input rate respectively. is the rate of energy loss.
3. Cycle Analysis of a Solid Fuel Fired Power Plant Using the Exergy Method
The performance evaluation of the whole plant is done based on the components of the system. A detailed overview of the methods used for exergy analysis of each component of the solid fuel plant is given here using a modified Rankine cycle incorporated with feedwater heaters.
Generally, solid based fuels operate under thermodynamic cycles by using a working fluid in vapour form for the generation of power known as the “vapour power cycle” or the Rankine cycle. This cycle consists of four processes: reversible adiabatic pumping, constant-pressure heat transfer in the boiler, reversible adiabatic expansion in the turbine and constant-pressure heat transfer in the condenser [39]. Modifications of the Rankine cycle for optimal performance lead to the formation of the reheat, superheat and regenerative cycles, with the addition of feed water heaters and de-aerators. A process flow diagram of an advanced, modified Rankine cycle based on a solid-fuel combined heat and power plant is shown in Figure 1 [40], and includes both the heating of feedwater and reheating of steam.
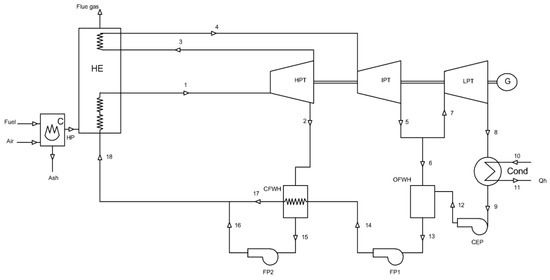
Figure 1.
Flow diagram of solid fuel-fired heat and power plant model (modified from [40]).
Exergy analysis is applied to each component of the plant in order to evaluate the system’s performance at steady state. The parts of the plant in question are: the combustion part of the boiler, heat exchangers in the boiler (HE), high pressure turbine (HPT), intermediate pressure turbine (IPT), low pressure turbine (LPT), condenser (Cond), condensate extraction pump (CEP), open feed water heater (OFWH), feed water pump 1 (FP1), closed feed water heater (CFWH) and feed water pump 2 (FP2). A detailed analysis is obtained by considering the mass, energy, entropy and exergy flow rate in the control volume of each individual system as well as the overall plant.
3.1. Boiler
The boiler is divided into two parts: the combustor and the heat exchanger [12], as presented in Figure 1. If these are both assumed to be adiabatic, operating at steady state with negligible changes in their kinetic and potential energy, then each analysis in the boiler can be made by considering the mass, energy, entropy and exergy balance using the input and output conditions of the flows.
3.1.1. Boiler Combustor (C)
The exergy balance rate in the boiler combustor is given in Equation (14):
The exergy destruction rate for the boiler combustor is then calculated by Equation (15) thus:
The exergy efficiency is expressed as Equation (16):
3.1.2. Boiler Heat Exchanger (HE)
The exergy balance rate for the boiler heat exchanger can be written as Equation (17):
The exergy destruction rate is:
The exergy efficiency is given as:
The overall boiler exergy efficiency is:
3.2. High Pressure Turbine (HPT)
The exergy balance rate for the high pressure turbine is:
The exergy destruction rate in the system is expressed as:
The exergy efficiency is written as follows:
3.3. Intermediate Pressure Turbine (IPT)
The exergy balance rate is:
The exergy destruction rate is:
The exergy efficiency is:
3.4. Low Pressure Turbine (LPT)
The exergy balance rate is:
The exergy destruction rate is:
The exergy efficiency is:
3.5. Condenser
The exergy balance rate is:
The exergy destruction rate is:
The exergy efficiency is:
3.6. Condensate Extraction Pump (CEP)
The exergy balance rate is:
The exergy destruction rate is:
The exergy efficiency is:
3.7. Open Feed Water Heater (OFWH)
The exergy balance rate is:
The exergy destruction rate is:
The exergy efficiency is:
3.8. Feed Pump (FP1)
The exergy balance rate is:
The exergy destruction rate is:
The exergy efficiency is:
3.9. Closed Feed Water Heater (CFWH)
The exergy balance rate is:
The exergy destruction rate is:
The exergy efficiency is:
3.10. Feed Pump (FP2)
The exergy balance rate is:
The exergy destruction rate is:
The exergy efficiency is:
For a combined heat and power plant, the overall exergy efficiency can be written as [41,42,43]:
where is the exergy flow rate associated to the heat produced, . Here, only the useful products have been included in comparison to the exergy input. The exergy of ash and flue gas are discarded as they do not represent a product flow.
4. Application of Exergy Analysis in Solid Fuel-Fired Heat and Power Plants
The use of exergy analysis on energy conversion processes has increased in the past years and has incorporated studies of different types of heat and power plant systems for improving the efficiency of existing power plants together with developing systems and systems under design for maximizing utilization of the energy produced. In this study, we have reviewed the application of exergy analysis as an evaluator of performance in coal-fired, biomass-fired and coal-biomass co-combustion-fired power plants.
4.1. Coal-Fired Heat and Power Plants
Coal supplies about 45% of the global electricity demand [44]. It is likely to continue as a key component of the fuel mix in the generation of power even though these plants account for over 28% of the total global emissions of carbon dioxide [45]. In order to maximize the utility of coal used in the production of energy, considerable efforts need to be made to enhance the capacity and efficiency of plants whilst simultaneously reducing their environmental impact and costs of power generation [46]. Improving both the efficiency and cost effectiveness of power plants can be achieved by reducing the thermodynamic inefficiencies associated with the system that result in a reduction of the CO2 emission per MW of electricity generated [47].
Exergy analysis has been proven to be a better way of measuring the efficiency of coal and reducing its environmental footprint by considering the effect of irreversibility in the process. A number of studies have been reported on the performance assessment and efficiency improvement of coal-based power plants using exergy analysis. Table 1 shows a summary of recent studies along with the most important conclusions that can be drawn from the application of exergy analysis when evaluating coal-fired heat and power plants.

Table 1.
Previous studies of exergy analysis applied to coal-fired heat and power plants.
These results identify the boiler as being the component of the plant with the highest exergy destruction as a result of the entropy generated due to irreversible combustion reactions. Exergy destruction in the combustor section of the boiler has been attributed to the chemical reaction between the fuel and air, while the large temperature difference between the combustion gases and the feedwater causes exergy destruction in the heat transfer section. The loss in the boiler in this particular case is over 50% of the total exergy destruction. As a result of the irreversibilities identified using exergy analysis, the exergetic efficiency is lower than the energetic efficiency, as can be seen in Table 1. The exergy efficiency of the power plants studied ranges from 17% to 38%. The results of the exergy destruction, heat loss and entropy generation in each component of a coal thermal power plant are summarized in Table 2 [23]. The energy analysis shows that highest energy loss occurs in the condenser, whereas the actual exergy destruction is in the boiler according to exergy analysis. The results also show that exergy destruction increases with increase in enropy generation. Hence, exergy analysis acounts for the entropy generated within the system, therefore, total exergy destruction is more than the heat loss.

Table 2.
Results from the analysis of a coal-fired thermal plant [23].
Whilst the reference temperature does not have a noticeable effect on the energy efficiency [23], it does affect the exergy efficiency slightly, as shown in Table 3. This indicates that the surroundings of the system affect its performance when exergy analysis is used. Even though variations in the reference temperature, do not affect the overall exergy results significantly, it is important in determining the optimal operation condition in a given plant design [26]. An increase in the ambient temperature has a greater effect on the condenser compared to other components, as indicated in Table 4 [49].

Table 3.
Variation in energy and exergy efficiencies at different reference temperatures [23].

Table 4.
The exergy rate of fuel and irreversibility rates of a power plant, kW, at different reference temperatures [49].
Taniguchi et al. [63] conducted exergy analyses of coal combustion processes with air temperatures entering the combustion chamber higher than the ambient temperature. They found that an increase in the temperature of the combustion air increases exergy efficiency. The decrease in the amount of excess air reduces flue gas losses and improves the combustion temperature [48]. Feedwater heaters can be installed to decrease the temperature difference between the flue gases and the working fluid [51]; both of these measures decrease the irreversibilities in the boiler. Operation of a power plant at full load has been shown to increase the combustion efficiency of the system and the exergy efficiency of the plant [52,53], indicating that power plants operating at their rated capacity are more economical than when operating at part loads [23]. The performance of the boiler system and the exergy efficiency increase with an increase in the steam pressure and temperature and number of feed water heaters, but a decrease in pressure in the condenser and reheater [23,51,52], while utilization of the rejected heat from the condensers as employed in the cogeneration systems improves the overall efficiency of the system [54].
The adoption of fluidized bed combustion firing technologies has been suggested as a means of improving the performance of energy conversion systems since (i) their heating surfaces located in the combustion chamber have high heat transfer rates and (ii) their combustion efficiency is superior to conventional firing systems [55]. Moreover, a fluidized bed boiler has the capacity of burning fuel mixtures with widely differing characteristics. Its low combustion temperature minimizes NOx, and the usage of adsorbent in the bed permits the capture of sulphur [64].
4.2. Heat and Power Plants Fired by Biomass-Based Fuels
Biomass energy is derived from plant and animal material, such as wood and wood waste, agricultural crops and their waste by-products, solid municipal refuse, animal offal, waste from food processing units, aquatic plants and algae [65]. The resource known as biomass can be considered as being renewable material in which the energy of sunlight is stored in the form of chemical bonds; when the bonds between adjacent carbon, hydrogen and oxygen molecules are broken by digestion, combustion or decomposition, these substances can release their stored chemical energy [66].
The reduction in the use of coal fuels, and the need to find alternatives to fossil fuels in order to decrease CO2 emissions, have attracted more interest in using biomass fuels as the energy carrier since biomass is perceived as being a carbon-neutral source [67,68]: biomass is thus regarded a suitable source of energy [69]. However, the overall efficiencies of biomass-fired power plants are relatively low [19,70]. Only a few papers in the literature have discussed exergy analysis applied to biomass-based heat and power plants—these are summarized in Table 5.

Table 5.
Previous studies of exergy analysis applied to biomass-fired heat and power plants.
Li et al. [67] used conventional exergy analysis to find the sources of irreversibilities and to identify exergy destruction in the various different components of the biomass boiler. They also used advanced exergy analysis to provide comprehensive information about the avoidable exergy destruction for each component, as well as for the whole system. Their results showed that a combustion chamber with a higher degree of heat absorption has a higher exergy in the specified boiler components and that, in a biomass boiler system, the combustion process is where most of the exergy destruction that is avoidable can be found.
Kamate and Gangavati [71] applied exergy analysis to two types of steam turbines to examine the effective utilization of cogeneration power plants in the sugar industry. They found that the efficiency of the plant using a non-condensing steam turbine (back pressure steam turbine) with energy and exergy efficiencies of 0.863 and 0.307 respectively, was higher than that of a plant using an extraction (condensing) steam turbine with energy and exergy efficiencies of 0.682 and 0.260 respectively, because the former does not reject heat in the condensation process. However, when a greater amount of electricity is needed, the latter is preferred.
The generation of entropy occurs mainly in the combustion process, which prompted Baloyi et al. [72] to examine the change in its rate as a function of the air to fuel (AF) ratio in an adiabatic combustor, using wood as the source of fuel. They showed that the entropy generation rate reaches a minimum at an AF of 4.9 and equivalence ratio of 1.64.
The performances of biomass multi-generation and cogeneration power plants have also been evaluated. Soltani et al. [28] investigated a biomass multi-generation energy system that produces electricity, steam, hot water, district heating and timber heating: significant increases in both the energy and exergy efficiencies were observed in the biomass multi-generation systems compared to conventional systems. A fuel energy savings ratio of 8.2% was reported by Kamate and Gangavati [43] for a biomass cogeneration plant over the generation of heat and power in two separate plants.
Different methods have been applied to calculate the efficiency of the incineration of municipal solid waste. Solheimslid et al. [73] used the chemical exergy of solid biomass by employing correlations, the chemical exergy obtained from the combustion equation and the absolute entropy to determine the exergy efficiency of municipal waste in a combined heat and power plant, and found both results to be in good agreement. Grosso et al. [74] examined the energy recovery efficiency, reported in the Waste Frame Directive (Directive 2008/98/EC), which accounts for the production of both power and heat. According to the directive, the energy recovery efficiency must be equal to, or exceed, 0.60 for waste incineration plants to be classified as energy recovery, rather than waste disposal, units. They analysed and compared the energy recovery efficiency to the exergy efficiency in the form of energy recovery criteria for different types of waste incineration plants in Europe, and found out that only the exergy efficiency can be considered a reliable measure.
4.3. Biomass and Coal Co-Fired Heat and Power Plant
Co-firing biomass in coal-fired boilers is regarded as being the most cost-effective approach for utilising biomass to generate power [75] because it requires little initial investment: the combustion technologies used in biomass co-firing plants are similar to those used in existing coal-fired plants [76]. Three different methods are used in biomass co-firing technology: direct, indirect and parallel co-firing. In the first method, biomass is fed directly into a boiler furnace with coal whilst the second entails a combination of gasification and combustion: the biomass is gasified and the product gas is fed into a boiler furnace containing the coal. The third method involves the biomass being burnt in a separate boiler to generate steam, which is then used in a power plant together with coal [77]. Selecting the appropriate co-firing option depends on the type of biomass available and site-specific factors, such as the types of coal handling equipment used and the arrangement of the coal firing systems installed [78,79].
Biomass co-fired with coal in traditional coal-fired boilers presents one combination of utilising fossil and renewable energy that derives the greatest benefit from both types of fuel; it leads to an effective reduction in CO2 and SOx emissions, and often NOx emissions too. It represents an attractive alternative for reducing emissions of greenhouse gas from coal-fired boilers [80]. Coal-biomass co-firing prevents the concentration of chlorine, which can otherwise result in the formation of harmful alkaline and chlorine compounds on the heat transfer surfaces in boilers [81]. Progress has been made over the past years in developing the co-ultilization of biomass fuels in coal-fired boiler plants [82]. Exergy analysis can nevertheless be used to evaluate performance in order to identify both the magnitude and the locations of imperfections in the process, with the aim of improving the efficiency of the plant. Reports pertaining to exergy analyses of biomass co-combustion processes are very few and far between: Table 6 shows a summary of previous work performed in this field.

Table 6.
Previous studies of exergy analysis applied to coal-biomass co-combustion heat and power plants
Biomass co-combustion is considered as a measure for reducing CO2 emissions. However, the exergy losses due to irreversibility from biomass co-firing are larger than for coal-based power plants. This irrervisibility has led to decreases in the exergy efficiencies of both the boiler and the overall co-combustion plant [83]: the gas exiting the furnace has a lower temperature due to a reduction in the exergy input to the plant. Applying biomass co-firing to a fluidized bed shows that the velocity of the fluidized bed does not influence the exergy efficiency [84].
The Soma coal thermal power plant in Turkey was modified to operate as both a direct and parallel co-firing biomass plant; performance evaluation shows that biomass parallel combustion performs better, from both technical and environmental aspects, than direct co-firing which suffers from problems of corrosion and fouling in the boiler [25]. As a result of the direct contact that occurs between biomass and coal in the direct co-firing method, the alkali metals and chlorine from the biomass reduced the melting temperature of the ash: the result was slagging at the furnace walls of the boiler and a possible decrease in the efficiency of the plant [85].
5. Discussion
The performance assessment of energy from solid fuels used for generation of heat and power has been reviewed. An effective utilization of this energy in the heat and power plant is needed: as the fuel conversion efficiencies investigated are low. The use of energy efficiency to evaluate the performance of the system is not adequate as the energy method does not identify degradation of the energy quality during the energy conversion processes. As a result of this inaccuracy, the energy efficiencies are higher than the exergy efficiencies.
The difference between the energy and exergy efficiencies is observed in the heat and power plant, Figure 2, while little variation is seen in the power plant, Figure 3, where the data used is collected from Table 1, Table 5 and Table 6. The produced heat, often distributed as water around 100 °C has a low energy quality (low exergy) but represents rather high energy content.
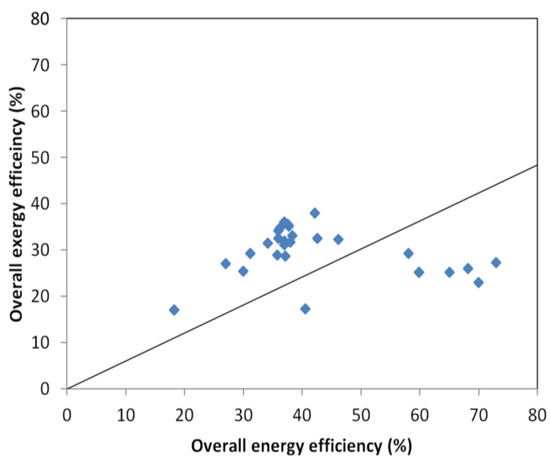
Figure 2.
Variation of exergy and energy efficiency in different combined heat and power plants.
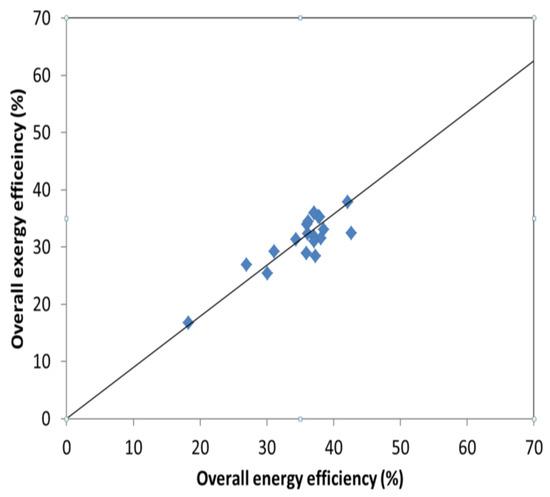
Figure 3.
Variation of exergy and energy efficiency in different power plants.
The performance of the whole plant is based on the individual components of the system. Therefore, identification of the component with highest inefficiencies is the first step for performance improvement of the overall plant. According to the energy analysis, the major energy losses in a power plant are due to the heat rejection in the condenser as a result of the large enthalpy difference between the turbine and the condenser: here, second law analysis shows that less than 6% of the total exergy loss stems from the condenser while as much as 69% of the total energy loss is found in this component [62]. From the exergy analysis, the highest degree of exergy destruction occurs in the boiler (combustion and heat transfer) with over 50% of the total irreversibility in the plant. Figure 4 shows the effect of boiler efficiency on the performance of the overall plant, where the plant data is taken from Table 1, Table 5 and Table 6. The result indicates that an increase in the boiler efficiency will increase the overall exergy efficiency of the plant.
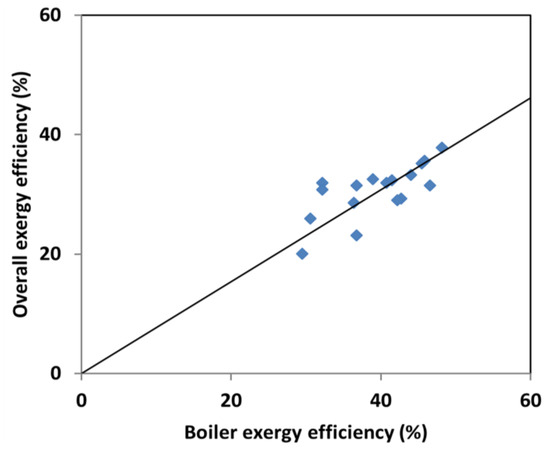
Figure 4.
Effect of the boiler efficiency on the overall plant exergy efficiency.
The performance evaluations of the solid fuel-fired heat and power plants reviewed, shows in general, that the coal-fired plant has highest exergy efficiency compared with the other solid fuels. This is as a result of higher operating temperature and pressure. However, the CO2 emissions associated with the system have an impact on the environment as a greenhouse gas. The emissions can be reduced by integrating the plant with carbon capture and storage [86]. But adopting this technology means extra cost and more energy is consumed during the process, which leads to reduction in the efficiency of the plant [47].
The biomass-based fuels on the other hand account for about 14% of the energy utilize in the world [87]. It remains the main source of energy for more than half of the world’s population [88]. Although, biomass has a lower efficiency than coal; it is a suitable and renewable energy option that provides clean gas fuels presently and in the future [89,90].
Co-combustion of biomass and coal could decrease the consumption rate of coal as well as reduce the environmental impact from coal-fired plant. Biomass contains only a small amount of nitrogen and sulphur, which will reduce NO2 and SO2 emissions associated with coal [91]. Co-firing also gives higher exergy efficiency than the biomass-based plant. However, co-firing of biomass in the existing coal-fired plant decreases the boiler and overall exergy efficiency due to increased moisture content in the biomass, which reduces the furnace exit gas temperature [83]. Moreover, it increases corrosion and ash deposition in the system, and if co-utilization of biomass fuel in coal-fired plant is not carefully designed, it will involve risk of power outages [80].
Different improvement measures have been suggested by the past studies in order to reduce exergy destruction. Though excess air is needed for complete combustion, the amount should be minimized because an increase in excess air will reduce the adiabatic flame temperature and decrease the exergy efficiency of the boiler [67] as well as the overall exergy efficiency of the plant [53]. Installation of feedwater heaters will decrease the temperature difference of the flue gas and feedwater, and will reduce irreversibilities encountered in the boiler heat exchanger. The decrease in pressure of the condenser as well as increase in steam pressure and temperature will reduce exergy destruction and increase the overall system performance. However, increase in the temperature is limited by the boiler tube’s oxidation temperature and allowable stress [67]. Moreover, the benefit of higher revenue as a result of increase in performance of the plant due to the increase in temperature and installation of feedwater heaters should be balanced against the increase in the capital cost to ensure that the pay-back period on the investment is favourable [23]. A plant operating at its full capacity is shown to be more economical and with higher exergy efficiency than those operating at part loads [52]. Because at full capacity, the heat absorbed in the combustion chamber will increase together with the efficiency of the boiler. However, this may not always be true for the combined heat and power plant: here, extraction ratio had a significant influence on the performance of the plant. As the plant with the smallest extraction ratio will have the highest exergy efficiency and lowest energy efficiency [42].
The use of advanced exergy-based method for evaluation of inefficiencies in the thermal conversion systems should be recommended. This method accounts for the avoidable and unavoidable exergy destruction associated with the plant and interaction between the components. The unavoidable part of exergy destruction cannot be improved, even using the best possible solution with available technology [44], as a result of limitation in the design specifications of the plant. The efforts to improve the plant should then be concentrated on the avoidable part so that the real thermodynamic inefficiencies and their causes can be identified [92].
6. Conclusions
Exergy analysis is a reliable method that can be used for the design, optimization, performance evaluation and calculation of efficiency of a solid fuel-fired heat and power plant. The exergetic method enables the main sources of loss to be identified, quantifies the irreversibilities that result from the entropy generated and provides direction for improving performance in the system. The application of exergy analysis should be extended to biomass-based and biomass co-combustion fired power plants so that important improvements can be made, because limited research work has been carried out in these sectors. Turkey and India are the two major countries where exergy analysis has been applied to solid fuel power plants, the majority of which are coal-fired. The results of the present review indicate that extensive research should focus on the combustion and heat transfer processes in boilers in order to optimise the performance of solid fuel-fired heat and power plants.
Acknowledgments
Financial support from University of Borås, Sweden and TETFund Nigeria through Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria is greatly appreciated.
Author Contributions
Francis Chinweuba Eboh is responsible for the literature survey and writing of the manuscript. Peter Ahlström and Tobias Richards supervised the research work.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| energy rate (kW) | |
| exergy rate (kW) | |
| ex | specific exergy (kJ/kg) |
| h | specific enthalpy (kJ/kg) |
| irreversibility rate or exergy destruction rate (kW) | |
| mass flow rate (kg/s) | |
| heat transfer rate (kW) | |
| entropy rate (kW/K) | |
| s | specific entropy (kJ/kgK) |
| T | temperature (K) |
| work transfer rate (kW) | |
| Subscripts | |
| a | air |
| f | fuel |
| fg | flue gas |
| hp | hot product |
| L | lost |
| p | product |
| pl | plant |
| e | exit |
| o | out |
| gen | generation |
| i | input |
| 0 | reference environment or dead state |
| Superscripts | |
| Ch | chemical |
| Ke | kinetic energy |
| Pe | potential energy |
| Ph | physical |
| Abbreviations | |
| B | boiler |
| C | combustor |
| CEP | condensate extraction pump |
| CFWH | closed feed water heater |
| CHP | combined heat and power |
| Cond | condenser |
| FP | feed water pump |
| HE | heat exchanger |
| HPT | high pressure turbine |
| IPT | intermediate pressure turbine |
| LPT | low pressure turbine |
| OFWH | open feed water heater |
| HP | hot product |
| WTP | waste-to-energy |
| Greek letter | |
| efficiency |
References
- Gupta, M.K.; Kaushik, S.C.; Ranjan, K.R.; Panwar, N.L.; Reddy, V.S.; Tyagi, S.K. Thermodynamic performance evaluation of solar and other thermal power generation systems: A review. Renew. Sustain. Energy Rev. 2015, 50, 567–582. [Google Scholar] [CrossRef]
- Pazheri, F.R.; Othman, M.F.; Malik, N.H. A review on global renewable electricity scenario. Renew. Sustain. Energy Rev. 2014, 31, 835–845. [Google Scholar] [CrossRef]
- Ranjan, K.R.; Kaushik, S.C. Energy, exergy and thermo-economic analysis of solar distillation systems: A review. Renew. Sustain. Energy Rev. 2013, 27, 709–723. [Google Scholar] [CrossRef]
- Luis, P.; Van der Bruggen, B. Exergy analysis of energy-intensive production processes: Advancing towards a sustainable chemical industry. J. Chem. Technol. Biotechnol. 2014, 89, 1288–1303. [Google Scholar] [CrossRef]
- Hinderink, A.P.; Arons, J.D.; Van der Kooi, H. On the efficiency and sustainability of the process industry. Green Chem. 1999, 1, G176–G180. [Google Scholar] [CrossRef]
- Gallo, W.L.R.; Milanez, L.F. Choice of a reference state for exergetic analysis. Energy 1990, 15, 113–121. [Google Scholar] [CrossRef]
- Ray, T.K.; Datta, A.; Gupta, A.; Ganguly, R. Exergy-based performance analysis for proper O&M decisions in a steam power plant. Energy Convers. Manag. 2010, 51, 1333–1344. [Google Scholar]
- Gaggioli, R.A.; Wepfer, W.J. Exergy economics. Energy 1980, 5, 823–837. [Google Scholar] [CrossRef]
- Luis, P. Exergy as a tool for measuring process intensification in chemical engineering. J. Chem. Technol. Biotechnol. 2013, 88, 1951–1958. [Google Scholar] [CrossRef]
- Rosen, M.A.; Dincer, I. On exergy and environmental impact. Int. J. Energy Res. 1997, 21, 643–654. [Google Scholar] [CrossRef]
- Richards, T.; Pavletic, C.; Pettersson, J. Efficiencies of NaOH production methods in a Kraft pulp mill. Int. J. Energy Res. 2009, 33, 1341–1351. [Google Scholar] [CrossRef]
- Saidur, R.; Ahamed, J.U.; Masjuki, H.H. Energy, exergy and economic analysis of industrial boilers. Energy Policy 2010, 38, 2188–2197. [Google Scholar] [CrossRef]
- Dewulf, J.; Van Langenhove, H.; Muys, B.; Bruers, S.; Bakshi, B.R.; Grubb, G.F.; Paulus, D.M.; Sciubba, E. Exergy: Its potential and limitations in environmental science and technology. Environ. Sci. Technol. 2008, 42, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Bejan, A. Fundamentals of exergy analysis, entropy generation minimization, and the generation of flow architecture. Int. J. Energy Res. 2002, 26, 545–565. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Sudheer, P.D.V.N.; David, Y.; Chae, C.G.; Kim, Y.J.; Baylon, M.G.; Baritugo, K.-A.; Kim, T.W.; Kim, M.-S.; Na, J.G.; PARK, S.J. Advances in the biological treatment of coal for synthetic natural gas and chemicals. Korean J. Chem. Eng. 2016, 33, 2788–2801. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Kaushik, S.C.; Reddy, V.S.; Tyagi, S.K. Energy and exergy analyses of thermal power plants: A review. Renew. Sustain. Energy Rev. 2011, 15, 1857–1872. [Google Scholar] [CrossRef]
- Naik, R.J.; Gupta, B.; Sharma, G.S. Exergy analysis of 4.5 MW biomass-based steam power plant. J. Human Soc. Sci. 2012, 1, 1–4. [Google Scholar]
- Demirbas, A. Combustion systems for biomass fuel. Energy Sources A Recovery Util. Environ. Eff. 2007, 29, 303–312. [Google Scholar] [CrossRef]
- Saidur, R.; Boroumandjazi, G.; Mekhilef, S.; Mohammed, H.A. A review on exergy analysis of biomass based fuels. Renew. Sustain. Energy Rev. 2012, 16, 1217–1222. [Google Scholar] [CrossRef]
- Szargut, J.; Morris, D.R.; Steward, F.R. Exergy Analysis of Thermal, Chemical, and Metallurgical Processes; Hemisphere: New York, NY, USA, 1988. [Google Scholar]
- Regulagadda, P.; Dincer, I.; Naterer, G.F. Exergy analysis of a thermal power plant with measured boiler and turbine losses. Appl. Therm. Eng. 2010, 30, 970–976. [Google Scholar] [CrossRef]
- Dincer, I.; Rosena, M.A. Exergy, Environment and Sustainable Development; Elsevier: Oxford, UK, 2007. [Google Scholar]
- Amirabedin, E.; McIlveen-Wright, D. A feasibility study of co-firing biomass in the thermal power plant at Soma in order to reduce emissions: An exergy approach. Int. J. Environ. Res. 2013, 7, 139–154. [Google Scholar]
- Rosen, M.A.; Dincer, I. Effect of varying dead-state properties on energy and exergy analyses of thermal systems. Int. J. Therm. Sci. 2004, 43, 121–133. [Google Scholar] [CrossRef]
- Ganapathy, T.; Alagumurthi, N.; Gakkhar, R.P.; Murugesan, K. Exergy analysis of operating lignite-fired thermal power plant. J. Eng. Sci. Technol. Rev. 2009, 2, 123–130. [Google Scholar]
- Soltani, R.; Dincer, I.; Rosen, M.A. Thermodynamic analysis of a novel multigeneration energy system based on heat recovery from a biomass CHP cycle. Appl. Therm. Eng. 2015, 89, 90–100. [Google Scholar] [CrossRef]
- Rant, Z. Towards the estimation of specific exergy of fuels. Allg. Wärmetech. 1961, 10, 172–176. (In German) [Google Scholar]
- Szargut, J.; Styrylska, T. Approximate evaluation of the exergy of fuels (in German). Brennst. Warme Kraft 1964, 16, 589–596. [Google Scholar]
- Eisermann, W.; Johnson, P.; Conger, W.L. Estimating thermodynamic properties of coal, char, tar and ash. Fuel Process. Technol. 1980, 3, 39–53. [Google Scholar] [CrossRef]
- Shieh, J.H.; Fan, L.T. Estimation of energy (enthalpy) and exergy (availability) contents in structurally complicated materials. Energy Sources 1982, 6, 1–46. [Google Scholar] [CrossRef]
- Ikumi, S.; Luo, C.D.; Wen, C.Y. Method of estimating entropies of coals and coal liquids. Can. J. Chem. Eng. 1982, 60, 551–555. [Google Scholar] [CrossRef]
- Bilgen, S.; Kaygusuz, K. The calculation of the chemical exergies of coal-based fuels by using the higher heating values. Appl. Energy 2008, 85, 776–785. [Google Scholar] [CrossRef]
- Song, G.; Shen, L.; Xiao, J. Estimating specific chemical exergy of biomass from basic analysis data. Ind. Eng. Chem. Res. 2011, 50, 9758–9766. [Google Scholar] [CrossRef]
- Song, G.; Xiao, J.; Zhao, H.; Shen, L. A unified correlation for estimating specific chemical exergy of solid and liquid fuels. Energy 2012, 40, 164–173. [Google Scholar] [CrossRef]
- Eboh, F.C.; Ahlström, P.; Richards, T. Estimating the specific chemical exergy of municipal solid waste. Energy Sci. Eng. 2016, 4, 217–231. [Google Scholar] [CrossRef]
- Tsatsaronis, G.; Park, M.-H. On avoidable and unavoidable exergy destructions and investment costs in thermal systems. Energy Convers. Manag. 2002, 43, 1259–1270. [Google Scholar] [CrossRef]
- Borgnakke, C.; Sonntag, R. Fundamentals of Thermodynamics; John Wiley & Sons, Inc.: Danvers, MA, USA; Chichester, UK, 2008. [Google Scholar]
- Singh, O. Applied Thermodynamics; New Age International (P) Ltd.: New Delhi, India, 2003. [Google Scholar]
- Erdem, H.H.; Dagdas, A.; Sevilgen, S.H.; Cetin, B.; Akkaya, A.V.; Sahin, B.; Teke, I.; Gungor, C.; Atas, S. Thermodynamic analysis of an existing coal-fired power plant for district heating/cooling application. Appl. Therm. Eng. 2010, 30, 181–187. [Google Scholar] [CrossRef]
- Liao, C.; Ertesvåg, I.S.; Zhao, J. Energetic and exergetic efficiencies of coal-fired CHP (combined heat and power) plants used in district heating systems of China. Energy 2013, 57, 671–681. [Google Scholar] [CrossRef]
- Kamate, S.; Gangavati, P. Energy and exergy analysis of a 44-MW bagasse-based cogeneration plant in India. Cogener. Distrib. Gener. J. 2010, 25, 35–51. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Morosuk, T.; Tsatsaronis, G. Advanced thermodynamic analysis and evaluation of a supercritical power plant. Energies 2012, 5, 1850–1863. [Google Scholar] [CrossRef]
- Coal Industry Advisory Board: IEA Programme of Power Generation from Coal. Measuring and Reporting Efficiency Performance and CO2 Emissions, OECD/IEA. 2010. Available online: https://www.iea.org/ciab/papers/power_generation_from_coal.pdf (accessed on 5 July 2016).
- Fu, C.; Anantharaman, R.; Jordal, K.; Gundersen, T. Thermal efficiency of coal-fired power plants: From theoretical to practical assessments. Energy Convers. Manag. 2015, 105, 530–544. [Google Scholar] [CrossRef]
- Olaleye, A.K.; Wang, M.; Kelsall, G. Steady state simulation and exergy analysis of supercritical coal-fired power plant with CO2 capture. Fuel 2015, 151, 57–72. [Google Scholar] [CrossRef]
- Gürtürk, M.; Oztop, H.F. Exergy analysis of a circulating fluidized bed boiler cogeneration power plant. Energy Convers. Manag. 2016, 120, 346–357. [Google Scholar] [CrossRef]
- Kopac, M.; Hilalci, A. Effect of ambient temperature on the efficiency of the regenerative and Reheat Çatalağzı power plant in Turkey. Appl. Therm. Eng. 2007, 27, 1377–1385. [Google Scholar] [CrossRef]
- Eskin, N.; Gungor, A.; Özdemir, K. Effects of operational parameters on the thermodynamic performance of FBCC steam power plant. Fuel 2009, 88, 54–66. [Google Scholar] [CrossRef]
- Srinivas, T.; Gupta, A.V.S.S.K.S.; Reddy, B.V. Generalized thermodynamic analysis of steam power cycles with ‘n’ number of feedwater heaters. Int. J. Thermodyn. 2007, 10, 177–185. [Google Scholar]
- Sengupta, S.; Datta, A.; Duttagupta, S. Exergy analysis of a coal-based 210 MW thermal power plant. Int. J. Energy Res. 2007, 31, 14–28. [Google Scholar] [CrossRef]
- Pattanayak, L.; Sahu, J.N. Steady state modeling on energy and exergy analysis of a pulverized coal fired thermal power plant. Asia-Pac. J. Chem. Eng. 2015, 10, 876–884. [Google Scholar] [CrossRef]
- Rosen, M.A. Energy and exergy-based comparison of coal-fired and nuclear steam power plants. Int. J. Exergy 2001, 3, 180–192. [Google Scholar] [CrossRef]
- Erdem, H.H.; Akkaya, A.V.; Cetin, B.; Dagdas, A.; Sevilgen, S.H.; Sahin, B.; Teke, I.; Gungor, C.; Atas, S. Comparative energetic and exergetic performance analyses for coal-fired thermal power plants in Turkey. Int. J. Therm. Sci. 2009, 48, 2179–2186. [Google Scholar] [CrossRef]
- Rosen, M.A.; Tang, R. Assessing and improving the efficiencies of a steam power plant using exergy analysis. Part 2: Improvements from modifying reheat pressure. Int. J. Exergy 2006, 4, 377–390. [Google Scholar] [CrossRef]
- Khoodaruth, A.; Aljundi, I.H. Performance analysis of a grate stroker coal-fired power plant based on the second law of thermodynamics. Int. J. Exergy 2015, 1, 84–103. [Google Scholar] [CrossRef]
- Nazrul Islam, A.K.M.; Alam, F.; Ashraful Islam, M. Energy and exergy analysis of a coal-based thermal power plant. In Proceedings of the 6th Bangladesh Society of Mechanical Engineers International Conference on Thermal Engineering, Dhaka, Bangladesh, 19–21 December 2014.
- Ashok, K.T.; Chandramouli, R.; Jothikumar, K. Exergy analysis of a coal based 63 MW circulating fluidized bed boiler—A case study. J. Appl. Sci. 2014, 14, 1515–1521. [Google Scholar]
- Habib, M.A.; Said, S.A.M.; Al-Bagawi, J.J. Thermodynamic performance analysis of the Ghazlan power plant. Energy 1995, 20, 1121–1130. [Google Scholar] [CrossRef]
- Callak, M.; Balkan, F.; Hepbasli, A. Avoidable and unavoidable exergy destructions of a fluidized bed coal combustor and a heat recovery steam generator. Energy Convers. Manag. 2015, 98, 54–58. [Google Scholar] [CrossRef]
- Mahamud, R.; Khan, M.M.K.; Rasul, M.G.; Leinster, M.G. Exergy analysis and efficiency improvement of a coal-fired thermal power in Queensland. In Thermal Power Plants: Advanced Applications; Rasul, M.G., Ed.; lnTech: Rijeka, Croatia, 2013; Chapter 1. [Google Scholar]
- Taniguchi, H.; Mouri, K.; Nakahara, T.; Arai, N. Exergy analysis on combustion and energy conversion processes. Energy 2005, 30, 111–117. [Google Scholar] [CrossRef]
- Oktay, Z. Investigation of coal-fired power plants in Turkey and a case study: Can plant. Appl. Therm. Eng. 2009, 29, 550–557. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (Part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Li, C.; Gillum, C.; Toupin, K.; Donaldson, B. Biomass boiler energy conversion system analysis with the aid of exergy-based methods. Energy Convers. Manag. 2015, 103, 665–673. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Biomass energy and the environmental impacts associated with its production and utilization. Renew. Sustain. Energy Rev. 2010, 14, 919–937. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (Part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Athari, H.; Soltani, S.; Seyed Mahmoudi, S.M.; Rosen, M.A.; Morosuk, T. Exergoeconomic analysis of a biomass post-firing combined-cycle power plant. Energy 2014, 77, 553–561. [Google Scholar] [CrossRef]
- Kamate, S.C.; Gangavati, P.B. Exergy analysis of cogeneration power plants in sugar industries. Appl. Therm. Eng. 2009, 29, 1187–1194. [Google Scholar] [CrossRef]
- Baloyi, J.; Bello-Ochende, T.; Meyer, J.P. Thermodynamic optimisation and computational analysis of irreversibilities in a small-scale wood-fired circulating fluidised bed adiabatic combustor. Energy 2014, 70, 653–663. [Google Scholar] [CrossRef]
- Solheimslid, T.; Harneshaug, H.K.; Lümmen, N. Calculation of first-law and second-law-efficiency of a Norwegian combined heat and power facility driven by municipal waste incineration—A case study. Energy Convers. Manag. 2015, 95, 149–159. [Google Scholar] [CrossRef]
- Grosso, M.; Motta, A.; Rigamonti, L. Efficiency of energy recovery from waste incineration, in the light of the new waste framework directive. Waste Manag. 2010, 30, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Tillman, D.A. Biomass co-firing: The technology, the experience, the combustion consequences. Biomass Bioenergy 2000, 19, 365–384. [Google Scholar] [CrossRef]
- Khorshidi, Z.; Ho, M.T.; Wiley, D.E. Techno-economic study of biomass co-firing with and without CO2 capture in an Australian black coal-fired power plant. Energy Procedia 2013, 37, 6035–6042. [Google Scholar] [CrossRef]
- Nussbaumer, T. Combustion and co-combustion of biomass: Fundamentals, technologies, and primary measures for emission reduction. Energy Fuels 2003, 17, 1510–1521. [Google Scholar] [CrossRef]
- Basu, P.; Butler, J.; Leon, M.A. Biomass co-firing options on the emission reduction and electricity generation costs in coal-fired power plants. Renew. Energy 2011, 36, 282–288. [Google Scholar] [CrossRef]
- Obernberger, I.; Thek, I. The Pellet Handbook; Earthscan: London, UK, 2010. [Google Scholar]
- Baxter, L.; Koppejan, J. Biomass-Coal Co-Combustion: Opportunity for Affordable Renewable Energy. 2004. Available online: http://www.ieabcc.nl/publications/paper_cofiring.pdf (accessed on 1 July 2016).
- The European Bioenergy Networks (EUBIONET): Biomass Co-Firing- an Efficient Way to Reduce Greenhouse Gas Emissions. 2003. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/2003_cofiring_eu_bionet.pdf (accessed on 8 July 2003).
- Al-Mansour, F.; Zuwala, J. An evaluation of biomass co-firing in Europe. Biomass Bioenergy 2010, 34, 620–629. [Google Scholar] [CrossRef]
- Mehmood, S.; Reddy, B.V.; Rosen, M.A. Exergy analysis of a biomass co-firing based pulverized coal power generation system. Int. J. Green Energy 2015, 12, 461–478. [Google Scholar] [CrossRef]
- Martín, C.; Villamañán, M.A.; Chamorro, C.R.; Otero, J.; Cabanillas, A.; Segovia, J.J. Low-grade coal and biomass co-combustion on fluidized bed: Exergy analysis. Energy 2006, 31, 330–344. [Google Scholar] [CrossRef]
- De, S.; Assadi, M. Impact of cofiring biomass with coal in power plants—A techno-economic assessment. Biomass Bioenergy 2009, 33, 283–293. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Kaygusuz, K.; Türker, M.F. Biomass energy potential in Turkey. Renew. Energy 2002, 26, 661–678. [Google Scholar] [CrossRef]
- Zeng, X.; Ma, Y.; Ma, L. Utilization of straw in biomass energy in China. Renew. Sustain. Energy Rev. 2007, 11, 976–987. [Google Scholar] [CrossRef]
- Li, C.; Liu, D.; Ramaswamy, S.; Yan, J. Biomass energy and products: Advanced technologies and applications. Appl. Energy 2015, 157, 489–490. [Google Scholar] [CrossRef]
- Wang, J.-J.; Yang, K.; Xu, Z.-L.; Fu, C. Energy and exergy analyses of an integrated CCHP system with biomass air gasification. Appl. Energy 2015, 142, 317–327. [Google Scholar] [CrossRef]
- Gungor, A. Simulation of co-firing coal and biomass in circulating fluidized beds. Energy Convers. Manag. 2013, 65, 574–579. [Google Scholar] [CrossRef]
- Tsatsaronis, G.; Morosuk, T.; Koch, D.; Sorgenfrei, M. Understanding the thermodynamic inefficiencies in combustion processes. Energy 2013, 62, 3–11. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).