Abstract
Infertility is a worldwide health issue defined by the World Health Organization (WHO) as the inability to establish a pregnancy after 12 months or more of regular and unprotected sexual intercourse. Male infertility etiology can be related to either congenital or acquired factors. The therapeutical approach to male infertility depends on the underlying causes and includes medical and surgical treatments. In recent studies, the potential role of nerve growth factor (NGF) in male reproductive physiology has been proposed. It has been hypothesized that neurotrophins might be involved in testis morphogenesis and regulation of several aspects of spermatogenesis. Moreover, it has been shown that NGF exerts its role on gonadotropin-releasing hormone (GnRH) neurons through the activation of the PKC/p–ERK1/2/p–CREB cascade, which leads to the activation of hypothalamic cells and the consequent activation of hypothalamus–pituitary–gonadal axis (HPG) with the secretion of GnRH. Lastly, it has been shown that the physiology of mature sperm is affected by both exogenous and endogenous NGF. The NGF impact on the HPG axis and its effect on GnRH neurons might be exploited in the therapy of male hypogonadism or used as a protective strategy against gonadal dysfunction related to chemotherapeutic agents. Moreover, the improving effect of NGF on sperm motility and vitality could be useful to enhance assisted reproduction outcomes. NGF could be supplemented to cryopreserved sperm samples to counteract the oxidative stress induced by the frozen and thawing processes. Indeed, the potential clinical applications of NGF in male infertility treatment have been discussed.
1. Introduction
During the past years, there has been growing interest in the understanding of male infertility causes and treatments. Infertility has an important social impact because it can affect mental health, being related to depression, anxiety disorders, and other psychological diseases. According to the WHO, the quality of life and psychological health depend directly on sexual health. Many factors can be related to male infertility, such as hormonal disease, obesity, diabetes, ejaculatory disorders, urogenital infections, testicular trauma, chemo/radiotherapy, or surgical treatments.
Recent findings reported the implication of nerve growth factor (NGF) in male reproductive pathophysiology. A wide number of animal and human studies demonstrated the involvement of NGF in spermatogenesis, testis morphogenesis, and the hypothalamus–pituitary–gonadal (HPG) axis and its improving effect on sperm traits. Therefore, NGF might be exploited in male infertility treatment, suggesting potential new strategies for male infertility therapy.
1.1. Male Infertility
1.1.1. Epidemiology
Infertility is a worldwide health issue defined by the World Health Organization (WHO) as the inability to establish a pregnancy after 12 months or more of regular and unprotected sexual intercourse [1,2]. Infertility affects approximately 15% of reproductive-aged couples and 186 million individuals globally [3]. Data suggest that in about 50% of infertility cases, “male factor” infertility has an important role and is solely responsible in 20–30% of cases [4]. Infertility is related to psychological distress, leading to depression and anxiety disorders, and has an important social impact [5,6].
1.1.2. Etiology
The etiology of male infertility can be related to a variety of factors, which can be distinguished into congenital or acquired [2]. Both congenital and acquired causes of male infertility can be classified into three categories: pretesticular, post-testicular, and testicular causes [7]. Among the pretesticular causes of male infertility, hypogonadotropic hypogonadism (HH) is one of the main causes [8]. Patients affected by HH have a deficit of LH and FSH secretion, which can be due either to a pituitary or hypothalamic dysfunction [9,10].
The hyposecretion of LH and FSH compromises normal spermatogenesis and production of testosterone, being responsible for infertility [11,12]. Other pretesticular causes of male infertility are coital disorders, such as ejaculatory disorders (e.g., anejaculation, retrograde ejaculation) [13,14] and erectile dysfunction [15]. It should be noted that some authors included coital disorders among the post-testicular causes of male infertility [16]. Post-testicular causes of male infertility also include primarily all the obstructions of the seminal tract [17,18]. Infections of the urogenital tract are also among the post-testicular causes of male infertility; in fact, microorganisms and leucocytes in the semen might damage sperm motility [19,20]. Moreover, activated leucocytes might produce reactive oxygen radicals (ROS), inducing sperm cell dysfunction [21].
Furthermore, inflammatory diseases of the accessory glands (e.g., prostatitis) and autoimmune reaction against the spermatozoa are included in the post-testicular causes of male infertility [22]. Post-testicular diseases might also be congenital, as in the case of CAVD (congenital absence of the vas deferens) [23]. Lastly, male infertility can be due to primitive testicular dysfunction, which causes impaired sperm production [24].
Testicular causes of male infertility include orchitis, testicular trauma, torsion, cryptorchidism (congenital or acquired) [25,26,27], systemic diseases, iatrogenic forms, and genetic abnormalities. Varicocele can be considered a cofactor of defective sperm production because it is associated with testicular atrophy and Leydig cell dysfunction [28]. In about 50% of cases, the etiology of male infertility still remains unknown (idiopathic infertility) [29]. Idiopathic infertility is probably, in most cases, related to genetic causes, considering that more than one thousand genes are involved in spermatogenesis [30].
1.1.3. Treatments
The therapeutical approach to male infertility depends on the underlying causes and includes medical and surgical treatments [31]. Medical treatment of male infertility mainly involves hormonal treatment [32], which is based on the use of gonadotropin-releasing hormone (GnRH), gonadotropins, testosterone, dopamine agonists, aromatase inhibitors (AI), and selective estrogen receptor modulators (SERMs) [33].
Men affected by hypogonadotropic hypogonadism related to a reduced secretion of GnRH from the hypothalamus can be treated with GnRH therapy [34]. The pulsatile administration of GnRH stimulates the anterior pituitary to release gonadotropins [35], with the re-establishment of the hypothalamus–pituitary–gonadal (HPG) axis leading to high levels of testosterone and stimulation of Sertoli cells by the FSH [36]. In about 85% of patients treated with pulsatile GnRH, spermatogenesis is induced [37,38,39].
The administration of gonadotropins can be useful in men with hypogonadotropic hypogonadism related to pituitary dysfunction. It has been shown that gonadotropin therapy induces spermatogenesis in about 80% of patients [40,41]. Dopamine agonists are used for the treatment of male infertility associated with prolactin-secreting pituitary adenoma [42]. SERMs are indicated for the treatment of idiopathic infertility [43]. Their action is based on the inhibition of central estrogen feedback upregulating the production of pituitary gonadotropins [44]. Surgical therapy for male infertility is indicated mainly in men with obstructive azoospermia (OA) [45,46].
When treatment has failed or no specific treatment is available for the condition underlying male infertility, assisted reproductive technologies (ARTs) are indicated [47]. Among them, the most used and successful are intrauterine insemination (IUI), in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI) [48,49].
IUI is a technique that involves the introduction of spermatozoa through the cervix using a catheter [50]. This technique is indicated in case of mild male infertility or unexplained infertility [51]. However, IUI needs a good semen quality and thus it is not fully indicated for “idiopathic oligozoospermia and asthenozoospermia or in men affected by retrograde ejaculation and anejaculation [51,52].
IVF facilitates fertilization by bringing the spermatozoa close to the oocyte [52] occurring outside the female body. IVF techniques usually include (i) a transvaginal ovum retrieval whereby a small needle is inserted through the back of the vagina and guided via ultrasound into the ovarian follicles to collect the fluid that contains the eggs and (ii) an embryo transfer whereby one or several embryos are placed into the uterus of the female with the intent to establish a pregnancy.
ICSI is the treatment of choice in case of IVF failure [53] and it consists of the injection of a single spermatozoon directly into the oocyte’s cytoplasm [54]. It can be used with ejaculated sperm, epididymal sperm, or testicular sperm. Retrieved epididymal or testicular spermatozoa are often cryopreserved to avoid repeated aspirations or biopsy in case of ART failure [55].
2. NGF
2.1. Neurotrophins
Neurotrophins are a family of growth factors mainly involved in the regulation of neuronal survival, function, and plasticity within the central and peripheral nervous systems. Neurotrophins include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4) [56]. These factors exert their effects by binding two major receptor types: the p75 neurotrophin receptor (p75NTR) and the tropomyosin-related tyrosine kinase (Trk) receptors. P75NTR is a low-affinity receptor to which all neurotrophins can bind; neurotrophins, in the absence of Trk receptors, can bind to p75NTR, which acts as a death receptor.
Trk receptors are a family of three receptors including TrkA, which functions mainly as a receptor for NGF, TrkB (as a receptor for BDNF and NT-4), and TrkC (as a receptor for NT-3) [57]. Neurotrophins binding to Trk receptors activate different intracellular signaling cascades, including Ras/mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase/protein kinase B-mammalian target of rapamycin (PI3K/Akt-mTOR), and phosphoinositide-specific phospholipase C-γ (PLC-γ) pathways [58,59,60]. The activation of the Ras/MAPK and PI3K/Akt pathways is involved in promoting neuronal differentiation and survival [61,62]. PLC-γ pathways trigger the intracellular release of calcium ions from the endoplasmic reticulum, leading to the activation of calcium-dependent proteins and the expression of transcription factors and ion channels [63,64,65].
Among the neurotrophin family, nerve growth factor (NGF) was the first to be discovered. It was identified in the early 1950s by Rita Levi-Montalcini in mouse sarcoma cultures [66]. NGF shows neuroprotective and neurotrophic effects in the central nervous system and regulates the survival and maturation of developing neurons within the peripheral nervous system [67,68]. Moreover, NGF is involved in the neural response to damage in nociceptive sensory neurons, Schwann cells, and α motor neurons [69,70,71,72]. Neurotrophins are mainly known for their neurotrophic role, but they also exert a variety of effects outside the nervous system [73,74]. Changes in NGF in the serum and plasma were shown during the beginning and progression of many pathologies, including neurological, psychiatric and immune diseases [75,76], and physiopathological conditions, such as cardiometabolic disruptions [77,78], oxidant circumstances [79,80], stressful events [81,82], alcohol addiction [83,84,85], and aging [86,87,88].
Indeed, the expression of neurotrophin receptors in several non-neuronal tissues and the involvement of neurotrophins in essential non-neuronal functions, such as the maintenance of immune cells, has been demonstrated. In particular, NGF induces the differentiation of B lymphocytes [89] and is involved in the maintenance of neutrophils, peritoneal mast cells, and B lymphocytes [90,91,92].
2.2. NGF Expression in the Reproductive System
It is known that NGF plays a pivotal role in regulating neuronal cell growth and survival; increasing evidence shows that NGF also exerts a variety of effects on non-neuronal cells. In recent studies, the expression of NGF and its receptors (TrkA and p75NTR) outside the nervous system has been demonstrated, in particular in the male reproductive system [93,94,95,96], leading to the hypothesis that NGF could have a role in the reproductive system [97,98]. Moreover, pieces of evidence show that the testis and brain share a common embryonic origin [99,100], explaining the expression of neural receptors in sperm cells [101].
Ayer-LeLievre et al., in 1988, demonstrated that in testis and epididymis of rats and mice, NGF and TrkA were expressed [102]. Successively, a large body of studies confirmed these results. The presence of NGF was first detected in the prostate of guinea pigs [103], rabbits, and bulls [104]. In a study conducted on golden hamsters, it was found that NGF expression is greater in the caudal portion of the epididymis than in the other regions [105]. Many studies conducted on a variety of species, such as camelids, llamas, rabbits, and alpacas, demonstrated the presence of NGF and its receptors in seminal plasma [106,107,108,109,110]. In 2010, Li et al. reported the presence of NGF, TrkA, and p75NTR in the spermatozoa’s tail and head. Moreover, they showed that oligo/asthenozoospermic men had lower levels of NGF in their semen compared to fertile men [111]. All these findings suggest the potential role of NGF in male reproductive physiology [112,113].
3. NGF Specific Functions in the Male Reproductive System
3.1. NGF’s Role in Testis Morphogenesis
It has been demonstrated that neurotrophins are involved in the morphogenetic process regulating local cell–cell interactions in many tissues, such as kidney, tooth, dermatome, and ovary [114,115,116,117]. Moreover, neurotrophins play a role in germ cell survival and differentiation [118]. Therefore, it has been hypothesized that neurotrophins might be implicated in testis morphogenesis. The expression of neurotrophins in postnatal testis has been shown [102,119,120].
The expression of p75NTR in the mesenchymal tissue that surrounds the testis cord has been observed [121,122]. Levine et al. studied the effects of neurotrophins on seminiferous cord formation using the Trk-specific inhibitor K252a and the inhibitory TrkC-IgG antagonist [123]. They demonstrated that the inhibition of the neurotrophin pathway resulted in an inhibition of testis cord formation, suggesting that neurotrophins play a crucial role in testis morphogenesis [124].
Cupp et al. studied the role of TrkA and TrkC in the process of testis development, finding that TrkA is involved in the early stages of testis morphogenesis, whereas TrkC is involved in the later stages, both being implicated in the regulation of the germ cells number [125].
3.2. NGF Role in Spermatogenesis
Spermatogenesis involves a complex system of processes that lead to the production of mature spermatozoa in the seminiferous tubules (ST) [126,127]. The spermatogenic process requires three crucial steps: the mitotic division of spermatogonia [128]; meiosis I, with the generation of spermatocytes, and meiosis II, with the generation of spermatids [129]; and spermiogenesis, culminating in the production of mature spermatozoa [130]. Spermatogenesis is regulated by both endocrine and paracrine mechanisms [131,132].
The hormonal control is mediated by follicle-stimulating hormone (FSH) and testosterone [133]. The paracrine mechanism of regulation of spermatogenesis is mediated by Sertoli cells and germ cells [134], which are involved in the production of a variety of regulatory factors [135,136]. The proteins secreted by germ cells exert their effect on Sertoli cells, stimulating them to produce many molecules involved in the process of spermatogenesis, such as ABP [137], transferrin [138,139], IL-1a [140], SGP-2/1 [141], inhibin [142], and ceruloplasmin [143]. Moreover, germ cells inhibit the production of 17b-estradiol [144].
The process of spermatogenesis requires a synergic and/or redundant action of regulatory molecules in order to occur correctly [145,146]. Among these molecules involved in spermatogenesis regulation, there is growing attention on neurotrophins. The presence of different neurotrophins in mammalian testis has been demonstrated [147], but among them, the NGF is the only one that showed a potential role in spermatogenesis.
Many studies indicated the impact of NGF on several aspects of spermatogenesis. The presence of NGF protein [148] and mRNA [102] in germ cells has been shown, and its mitogen activity has been demonstrated [118]. NGF shows different effects, such as guaranteeing the physiological integrity of seminiferous epithelial cells [149], stimulating DNA synthesis within the seminiferous tubules [150], and inducing the secretion of ABP from Sertoli cells [120]. These effects are exerted through the binding between NGF and its receptors, p75NT and Trk [119,151], which are expressed on Sertoli cells.
The presence of NGF receptors on Sertoli cells in animals’ testis suggested the role of NGF in spermatogenesis regulation. Subsequently, the presence of NGF mRNA and p75NTR mRNA and protein in human testis was demonstrated [152,153].
3.3. The Impact of NGF on the Hypothalamus-Pituitary-Gonadal (HPG) Axis
Spermatogenesis is regulated by the hypothalamus–pituitary–gonadal (HPG) axis [154]. The hypothalamus produces gonadotropin-releasing hormone (GnRH) and releases it in a pulsatile manner [155]. The pulsatile secretion of neuropeptide is essential for stimulating the gonadotropic cells of the anterior pituitary to synthesize and secrete LH and FSH [156].
The stimulus of LH and FSH on testis cells activates two crucial endocrine signals: LH stimulates the production of testosterone from Leydig cells [157], and FSH induces the production of ABP (androgen-binding protein) and inhibin from Sertoli cells [158]. FSH and LH have a pivotal role in regulating spermatogenesis, mainly because they mediate the production of Sertoli factors, respectively, in a direct or indirect (through a testosterone–androgen receptor) way [159]. The HPG axis is finely regulated by a negative feedback mechanism [160].
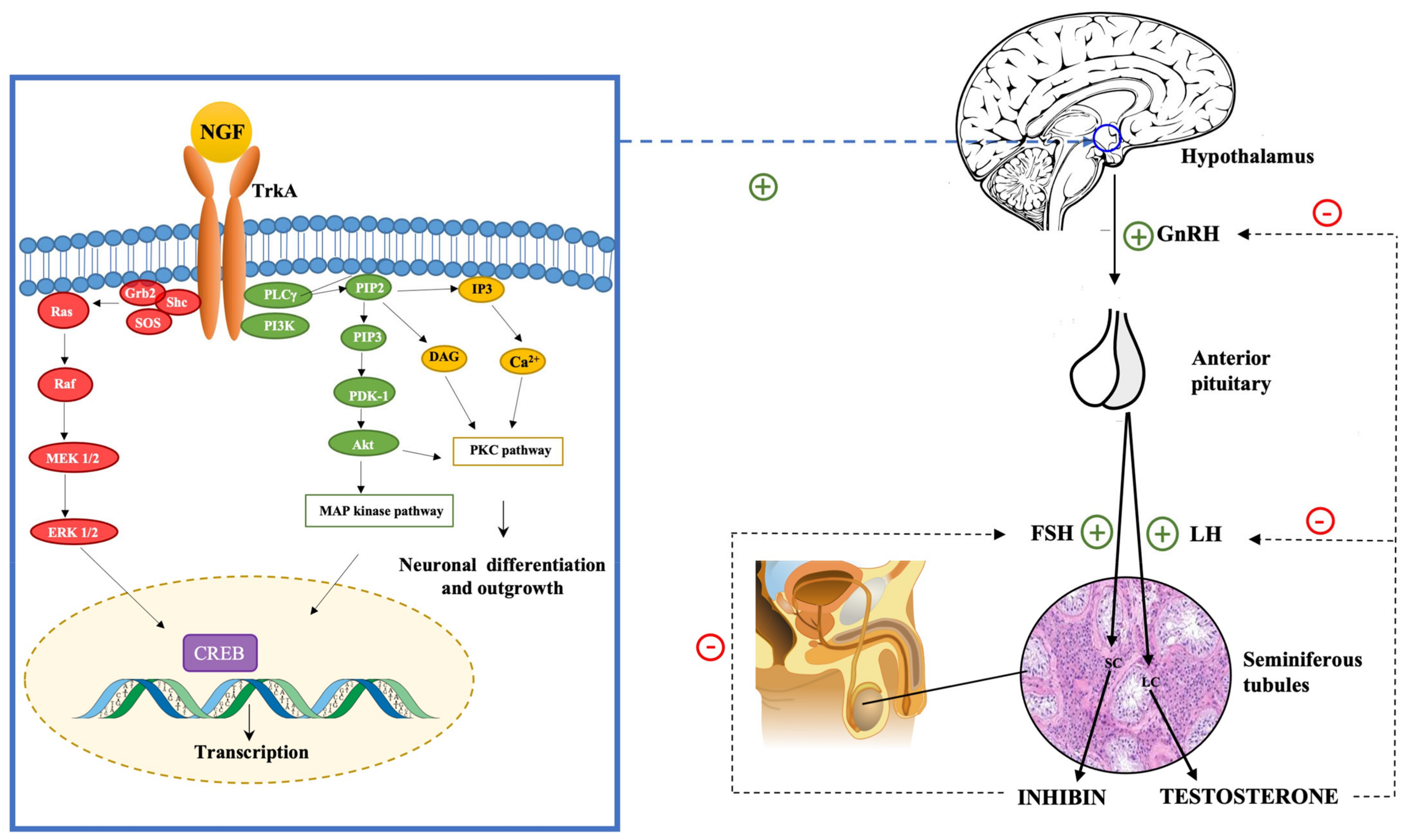
Testosterone, in high levels, inhibits the hypothalamic secretion of GnRH and the pituitary secretion of LH [161]. Inhibin acts on the anterior pituitary, inhibiting the production of FSH [162]. The NGF is involved in the regulation of the hypothalamus–pituitary axis [163,164]. Luo et al. in 2018 demonstrated that NGF can activate GnRH neurons in the hypothalamus and, thus, regulate the hypothalamic secretion of GnRH [165]. Moreover, they found that by using TrkA inhibitors, the effects of NGF on the HPG axis were extinguished. This finding led to the hypothesis that the impact of NGF on the hypothalamic secretion of GnRH was mediated by the TrkA receptor (see Figure 1).
Figure 1.
NGF regulates the hypothalamus–pituitary–gonadal (HPG) axis stimulating the hypothalamic production of GnRH. The binding of the NGF to the TrkA receptor activates many intracellular pathways, including the PI3Kinase pathway, which leads to the activation of Akt kinase, the Ras pathway, which leads to MAP kinase activation, and the PLC pathway, which leads to the activation of PKC. These intracellular pathways are involved in regulating the expression of neuronal survival and differentiation genes and the transcription of GnRH gene and neuropeptide production.
The binding of NGF to TrkA activates many intracellular signaling cascades, such as MAPK, PI3K, and PLC-γ-PKC pathways [166]. GnRH transcription and neuropeptide production in GT1-7 cells are regulated by PKC, PKA, and MAPK pathways [167,168]. TrkA activation leads to the activation of ERK1/2 and ERK5. These kinases phosphorylate and activate CREB, Elk-1, and MEF2, which are transcription factors involved in regulating the expression of neuronal survival and differentiation genes [61,169]. The NGF exerts its role on GnRH neurons through the activation of the PKC/p-ERK1/2/p-CREB cascade, which leads to the activation of hypothalamic cells and the consequent activation of the HPG axis with the secretion of GnRH [170,171].
3.4. NGF Effects on Sperm Traits
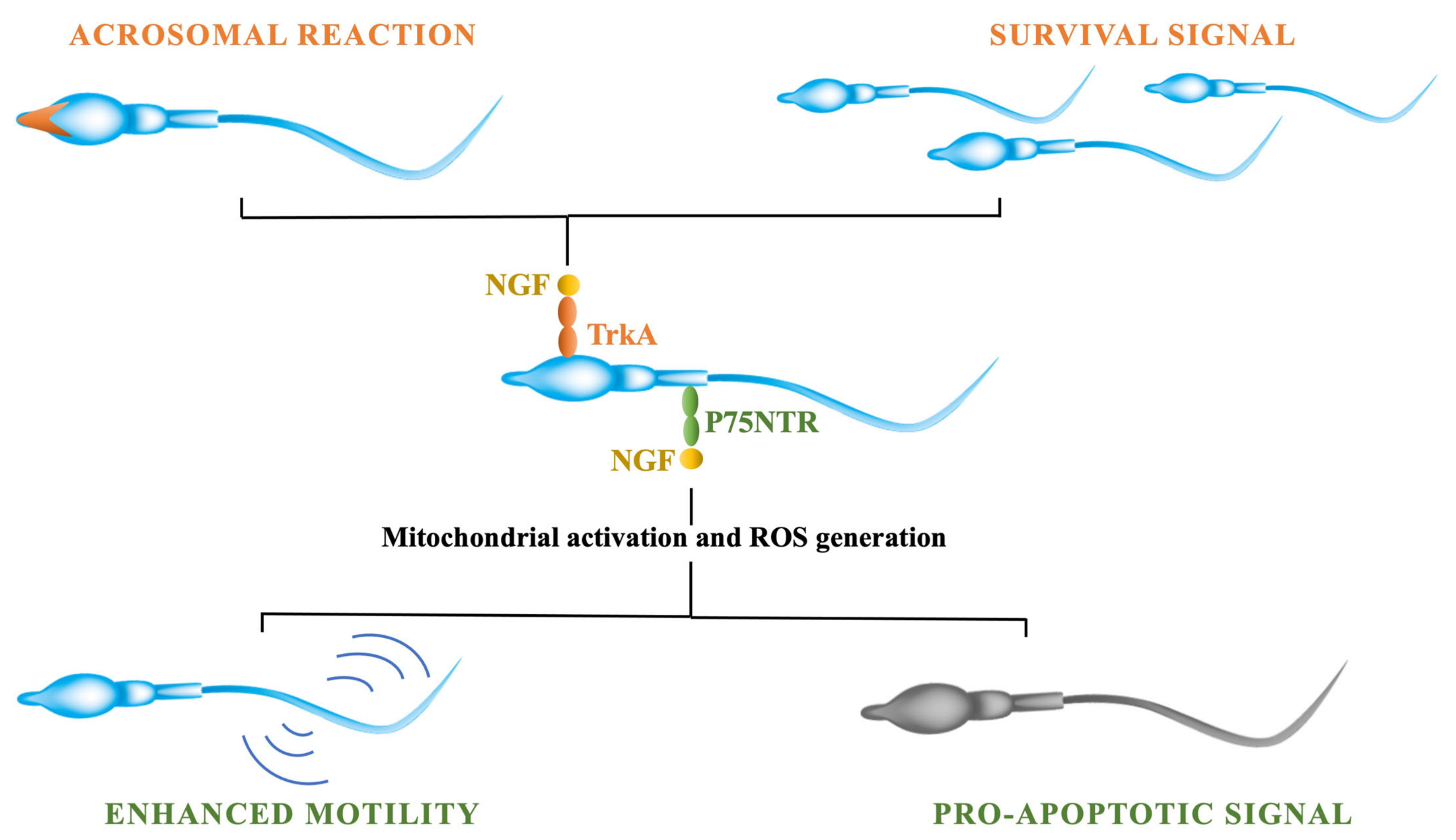
Various studies demonstrated that nerve growth factor (NGF) exerts a variety of effects on mature sperm traits [172]. the expression of NGF receptors in sperm cells has been demonstrated: p75NTR is mainly in the midpiece and tail, whereas TrKA is expressed in the head and acrosome [173]. In 2010, two different studies reported the presence of NGF and the expression of TrkA receptor in animal sperm. One group studied hamster sperm, demonstrating that the NGF stimulates acrosome reaction and increases sperm motility (see Figure 2).
Figure 2.
NGF exerts its effects on sperm cells through its binding to its two receptors: TrkA and p75NTR. The activation of the TrkA receptor is involved in stimulating cells’ survival and modulating the acrosomal reaction through the activation of the kinases’ pathway. The binding of the NGF to p75NTR receptor, instead, is involved in modulating sperm cells’ apoptosis by triggering the mitochondrial activity and the production of ROS. Moreover, the activation of p75NTR is involved in modulating sperm motility through the activation of the sperm’s respiratory chain.
This stimulating effect of NGF on sperm motility was found to be time- and dose-dependent. The other group studied bovine sperm, demonstrating the presence of NGF and TrkA receptors in ejaculated sperm. They showed that exogenous NGF had positive effects on sperm viability and apoptosis, although the NGF did not affect acrosome reaction [174]. Two years later, a study on human sperm was published; it reported that NGF has an improving effect on human sperm motility traits, such as straight-line velocity, curvilinear velocity, average path velocity, linearity, and beat-cross frequency [175]. After incubating the sperm in vitro with NGF, the sperm motility increased in a dose-dependent manner [176]. Bezerra et al. studied the proteome of seminal plasma in rabbits and suggested that NGF expression might increase sperm motility [177].
According to the literature, NGF improves sperm motility and vigor [175,178]. Other studies showed that NGF also has an improving effect on the motility of cryopreserved sperm. It has been demonstrated that adding NGF in cryopreserved samples improves human sperm motility and viability, decreases apoptosis, and increases nitric oxide (NO) concentration [178]. Parthipan et al. compared the NGF levels in bovine sperm of “good” and “poor” donors and observed that NGF levels were higher in “good” sperm donors. Furthermore, they demonstrated that NGF exerts positive effects on sperm kinetic traits after thawing (e.g., sperm speed, linearity, straightness, and mitochondrial membrane potential) [179].
A recent study carried out on llamas suggests that the NGF contained in sperm has a seminal plasma origin; in fact, the NGF has been detected in ejaculated sperm, whereas it is not present in epididymal germ cells [180]. The NGF exerts its main effects on sperm traits through binding to its receptors, TrkA and p75NTR [181]. The activation of these receptors might also be involved in spermatogenesis and testicular development; in fact, the expression of NGF receptors in Leydig and Sertoli cells has been demonstrated [182].
The activation of the TrkA receptor leads to the activation of kinases (e.g., MAPK) that are involved in modulating the acrosomal reaction. Following the binding of the NGF to the TrkA, PI3kinase activation occurs, leading to an increase in cell survival [174]. The activation of p75NTR, instead, is important mainly in modulating capacitation, sperm motility, and apoptosis. It has been shown that by blocking p75NTR, the reduction of sperm track speed occurred. That was consistent with the high number of receptors expressed in the midpiece, which is the site of energy production by mitochondria. NGF at a 100 ng/mL dose significantly improved motility rate in comparison to lower doses; higher doses (125 and 150 ng/mL) did not further increased sperm motility [183]. The binding of the NGF to p75NTR activates the sperm’s respiratory chain, which is responsible for the sperm’s propulsive force [184]. As a consequence of this mitochondrial activation, the generation of reactive oxygen species (ROS) occurs [185,186]. Therefore, the activation of sperm motility and capacitation might be considered a preapoptotic status [187] associated with ROS generation [188]. The NGF is involved in modulating sperm apoptosis and capacitation by triggering mitochondrial activity and the production of ROS [189].
The NGF might have a pivotal role in modulating the survival and senescence of spermatozoa depending on which receptor pathway is activated: p75NTR proapoptotic or TrkA prosurvival. An in vitro study on rabbit sperm reported that the blocking of the TrKA resulted in a reduced number of motile cells, due to the positive impact of NGF–TrKA on the survival rate of sperm [189]. Two different P75NTR signaling pathways have been found: one including TrkA activation and the other focused only on P75NTR activity via NFkB activation [183]. Indeed, in the absence of TrkA, P75NTR can induce apoptosis [190]. These findings suggest that the role of NGF depends on the balance between its two receptors. The different “death–survival”pathways activated by NGF receptors’ interactions were determined by the concentration (ratio) of P75NTR to TrkA [191].
4. New Therapeutical Opportunities
4.1. The Potential Role of NGF in Male Infertility Treatment
Accumulating evidence has shown that NGF plays a role in the reproductive system [192,193,194,195,196]. Studies on Leydig cells’ development showed that NGF promotes the proliferation of stem Leydig cells (SLCs), progenitor Leydig cells (PLCs), and immature Leydig cells (ILCs) and induces PLCs to differentiate. These results suggested that the NGF pathway might be a potential target for the development of new therapies for diseases related to the dysfunction of Leydig cells, such as partial androgen deficiency of the aging male (PADAM) [197]. The standard treatment for testosterone deficiency syndrome, also known as hypogonadism, is testosterone supplementation therapy (TST) [198].
Exogenous testosterone can be administrated through several routes of delivery (e.g., nasal, buccal, subdermal, intradermal, and intramuscular), with the aim of increasing serum levels of testosterone and, thus, reducing signs and symptoms of hypogonadism [199]. However, testosterone therapy is related to several potential side effects. In fact, TST is not indicated in patients with a high risk of prostate cancer or important cardiovascular diseases [200]. Moreover, exogenous testosterone exerts inhibitory feedback on the HPG axis, resulting in the inhibition of intratesticular production of testosterone [201].
Alternative therapies are pulsatile GnRH administration and gonadotropin therapy, which are safer and more effective [202]. A further alternative therapeutic option for hypogonadism treatment might be represented by NGF administration. Luo et al. in 2018 studied the effects of NGF on hypogonadism. They demonstrated that NGF administration significantly increased serum levels of testosterone and gonadotropins, improved sperm quality, and restored fertility in aging mice [165]. NGF is able to exert these effects through the activation of GnRH neurons. Moreover, they suggested that the more convenient way to deliver NGF to the hypothalamus might be intranasal administration, associated with the liposome encapsulation of the protein [203,204,205]. In a successive study, published in 2021, Luo et al. showed that NGF administration could rescue spermatogenesis in azoospermic mice [206].
They induced azoospermia in mice by treating them with busulfan injections. Busulfan is a chemotherapy drug used to manage the allogeneic transplantation of hematopoietic progenitor cells in patients affected by chronic myeloid leukemia (CML) [207]. Busulfan administration is associated with gonadal dysfunction [208]. It has been shown that a single dose of busulfan injected in mice induced the alteration of testosterone and LH levels and destroyed differentiated spermatogonia, resulting in azoospermia [209,210]. In their study, Luo et al. showed that NGF nasal administration increased sexual hormone levels, enhanced sperm quality, and induced spermatogonia differentiation. NGF therapy resulted to restore spermatogenesis in azoospermic mice. These results suggest that NGF therapy might be used as a protective strategy against gonadal dysfunction related to busulfan treatment or other anticancer agents (see Table 1).

Table 1.
Potential NGF therapeutical applications in male infertility.
4.2. The Effect of NGF Addition to Cryopreserved Sperm
Cryopreservation of human semen is a procedure that can be useful in a variety of conditions [211]. Indeed, it may be useful before surgical treatment for infertility or before cytotoxic treatment for malignant diseases, such as radiotherapy or chemotherapy. Indeed, such therapies may lead to ejaculatory dysfunctions or testicular failure [212,213]; thus, sperm cryopreservation is an effective route to preserve male fertility [214]. Cryopreservation is used also for the storage of donors’ semen until the screening for infectious diseases confirms the negativity [215] (see Table 2).

Table 2.
Potential NGF application in semen preservation.
Moreover, cryopreservation plays an important role in the management of male infertility [217]. It is used for the storage of sperm obtained from azoospermic men after testicular sperm extraction in order to avoid to repeat biopsies or aspirations [218]. The frozen semen can be used in assisted reproduction treatments, such as intrauterine insemination, in vitro fertilization, and ICSI [219,220,221]. However, cryopreservation can cause sperm cell damage. Both the processes of freezing and thawing expose spermatozoa to acute stresses [222] (see Table 2). A large number of studies were conducted to examine cryodamage in sperm cells [223,224,225]. It has been observed that spermatozoa after thawing showed a decreased motility and viability in comparison to the prefrozen state [226]. A correlation between the decrease of spermatozoa motility and mitochondrial damage after thawing has been demonstrated [227].
Cryopreservation can cause lipid peroxidation in the sperm membrane and induce an enhancement in reactive oxygen species concentration [228]. Elevated levels of reactive oxygen species (ROS) compromise sperm motility [229,230,231] and viability [232], DNA integrity [233], and acrosomal structure [234,235]. Cryopreservation leads to sperm apoptosis, affecting mitochondrial membrane properties and enhancing ROS generation [236] (see Table 2). It has been suggested that oxidative stress and apoptosis-inducing factors are responsible for DNA alteration in spermatozoa during the process of cryopreservation [237,238]. In order to protect sperm cells against cryodamage, different additives are used. The supplements used are mainly antioxidants, antifreeze proteins, and cryoprotectants [239,240].
Many studies have demonstrated that adding antioxidants to freezing samples might be useful to counteract ROS action and improve sperm functions [241]. It has been demonstrated that brain-derived neurotrophic factor (BDNF) supplementation to frozen-thawed human spermatozoa improves sperm parameters, showing a protective effect against oxidative stress and apoptosis [242]. It has been shown that NGF exerts an improving effect on cells’ viability [243]. Furthermore, it has been proposed that the addition of exogenous NGF to an incubation medium might improve sperm viability [174].
Among the sperm traits, one of the most important for fertilization is sperm motility. The oxidative stress induced by the cryopreservation process can lead to chromosomal and DNA damage, resulting in a decrease in sperm motility [244,245]. Supplementing frozen sperm with antioxidants, such as vitamin E and NGF, resulted in a significant enhancement of post-thaw motility [175,246]. Saeednia et al. studied the effects of exogenous NGF addition (0.5, 1, and 5 ng/mL) to cryopreserved semen samples. They showed that exogenous NGF significantly improved spermatozoa viability and motility, demonstrating the cryoprotective effect of NGF [178].
4.3. NGF Supplementation in Assisted Reproduction
The in vitro fertilizing capacity of spermatozoa is mainly correlated to two sperm parameters: vitality and progressive motility (PM) [247]. Both sperm traits are associated with an improved outcome of assisted reproductive technology, especially IVF and IUI, which require effective sperm motility [248]. Therefore, in order to increase the ART success rate, it might be useful to enhance sperm vitality and progressive motility [249,250,251]. A variety of studies were conducted to investigate the improving effect of different substances on sperm motility and vitality and thus their potential role as supplements to sperm processing media. It has been shown that caffeine, pentoxifylline, and 2-deoxyadenosine improve sperm motility [252], but they exert noxious effects on embryonic development [253].
Many authors suggested that sperm motility and vitality are positively affected by NGF, based on the detection of NGF and its receptors in spermatozoa and testis [254]. In a recent study, it has been demonstrated that supplementing IVF medium with NGF could enhance embryonic cleavage rates and blastocyst hatching rates in bovines, leading to an improvement in IVF outcomes. Nevertheless, in this study both the spermatozoa and the oocytes were exposed to NGF, and the authors suggested that NGF can act on the oocyte in a direct way [255]. Subsequently, in 2021, the improving effects of IGF-I and NGF on human spermatozoa’s motility and vitality were studied. In this study, the authors showed that incubating spermatozoa in a medium supplemented with IGF-I or NGF can enhance in vitro vitality and progressive motility of spermatozoa, suggesting their potential role in improving assisted reproduction outcomes [216].
5. Conclusions
In conclusion, the latest studies have shown that neurotrophins, especially NGF, are implicated in the male reproductive system. Some studies were conducted to investigate the possible role of NGF in the management of male infertility. Indeed, it has been demonstrated that NGF has an improving effect on male infertility in aging mice and is able to restore fertility in male mice with busulfan-induced infertility. Moreover, some studies showed the potential application of NGF in ARTs. In fact, NGF might be supplemented to IVF medium in order to improve IVF outcomes. In addition, it could be used as an antioxidant supplement for cryopreserved semen samples in order to reduce sperm cells’ cryodamage. Further studies are required to reach a better understanding of the involvement of NGF in male reproductive system pathophysiology and, hence, its potential role in the management of male infertility.
Author Contributions
Conceptualization, G.F. and M.F.; investigation, F.F., G.F. and M.F.; writing—original draft preparation, C.P., F.F., G.F. and M.F.; writing—review and editing, C.P., F.F., G.F. and M.F.; visualization, L.T., G.B., F.T., C.B., A.M., M.R., S.F., A.G., C.P. and M.F.; supervision, C.P., F.F., G.F. and M.F.; project administration, L.T., G.B. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank IBBC-CNR, and Sapienza University of Rome, Rome, Italy.
Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise.
Abbreviations
| ABP | androgen-binding protein |
| AKT | protein kinase B |
| ARTs | assisted reproductive technologies |
| BDNF | brain-derived neurotrophic factor |
| CAVD | congenital absence of the vas deferens |
| CML | chronic myeloid leukemia |
| CREB | c-AMP-response element binding protein |
| DAG | diacylglycerol |
| EdU | 5-ethynyl-2′-deoxyuridine |
| ERK | extracellular signal-regulated kinase |
| FSH | follicle-stimulating hormone |
| GnRH | gonadotropin-releasing hormone |
| GRB2 | growth factor receptor-bound protein 2 |
| HH | hypogonadotropic hypogonadism |
| HPG | hypothalamus–pituitary–gonadal |
| ICSI | intracytoplasmic sperm injection |
| ILCs | immature Leydig cells |
| IP3 | inositol triphosphate |
| IUI | intrauterine insemination |
| IVF | in vitro fertilization |
| LC | Leydig cell |
| LH | luteinizing hormone |
| MAPK | mitogen-activated protein kinase |
| MEK | mitogen-activated protein kinase |
| NGF | nerve growth factor |
| NO | nitric oxide |
| NT-3 | neurotrophin-3 |
| NT-4 | neurotrophin-4 |
| OA | obstructive azoospermia |
| PADAM | partial androgen deficiency of the aging male |
| PDK | 3-phosphoinositide-dependent kinase 1 |
| PI3K | phosphoinositide 3-kinase |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PIP3 | phosphatidylinositol 3,4,5-trisphosphate |
| PKC | protein kinase C |
| PLC-γ | phosphoinositide-specific phospholipase C-γ |
| PLCs | progenitor Leydig cells |
| PM | progressive motility |
| RAF | rapidly accelerated fibrosarcoma |
| ROS | reactive oxygen species |
| SC | Sertoli cell |
| SERMs | selective estrogen receptor modulators |
| SHC | src honology and collagen |
| SLCs | stem Leydig cells |
| TST | testosterone supplementation therapy |
| WHO | World Health Organization |
References
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Fainberg, J.; Kashanian, J.A. Recent advances in understanding and managing male infertility. F1000Research 2019, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.E.; McLain, A.; Louis, J.F.; King, R.B.; Trumble, A.C.; Sundaram, R.; Louis, G.B. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil. Steril. 2013, 99, 1324–1331.e1. [Google Scholar] [CrossRef]
- Bak, C.W.; Seok, H.H.; Song, S.H.; Kim, E.S.; Her, Y.S.; Yoon, T.K. Hormonal imbalances and psychological scars left behind in infertile men. J. Androl. 2012, 33, 181–189. [Google Scholar] [CrossRef]
- Wu, A.K.; Elliott, P.; Katz, P.P.; Smith, J.F. Time costs of fertility care: The hidden hardship of building a family. Fertil. Steril. 2013, 99, 2025–2030. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Adonakis, G.; Kaponis, A.; Mamoulakis, C.; Takenaka, A.; Sofikitis, N. Pre-Testicular, Testicular, and Post-Testicular Causes of Male Infertility. In Endocrinology of the Testis and Male Reproduction; Simoni, M., Huhtaniemi, I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–47. [Google Scholar] [CrossRef]
- Lenzi, A.; Balercia, G.; Bellastella, A.; Colao, A.; Fabbri, A.; Foresta, C.; Galdiero, M.; Gandini, L.; Krausz, C.; Lombardi, G.; et al. Epidemiology; diagnosis, and treatment of male hypogonadotropic hypogonadism. J. Endocrinol. Investig. 2009, 32, 934–938. [Google Scholar] [CrossRef]
- Huhtaniemi, I.; Alevizaki, M. Mutations along the hypothalamic-pituitary-gonadal axis affecting male reproduction. Reprod. Biomed. Online 2007, 15, 622–632. [Google Scholar] [CrossRef]
- Bianco, S.D.C.; Kaiser, U.B. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat. Rev. Endocrinol. 2009, 5, 569–576. [Google Scholar] [CrossRef]
- Whitcomb, R.W.; Crowley, W.F. Male hypogonadotropic hypogonadism. Endocrinol. Metab. Clin. N. Am. 1993, 22, 125–143. [Google Scholar] [CrossRef]
- Fraietta, R.; Zylberstejn, D.S.; Esteves, S.C. Hypogonadotropic hypogonadism revisited. Clinics 2013, 68 (Suppl. S1), 81–88. [Google Scholar] [CrossRef]
- Aust, T.R.; Lewis-Jones, D.I. Retrograde ejaculation and male infertility. Hosp. Med. 2004, 65, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, N. Ejaculatory dysfunction as a cause of infertility. Reprod. Med. Biol. 2012, 11, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Maggi, M. Sexual dysfunction and male infertility. Nat. Rev. Urol. 2018, 15, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Wiser, H.J.; Sandlow, J.; Köhler, T.S. Causes of male infertility. In Male Infertility; Parekattil, S., Agarwal, A., Eds.; Springer: New York, NY, USA, 2012; pp. 3–14. [Google Scholar] [CrossRef]
- Wong, T.W.; Straus, F.H.; Jones, T.M.; Warner, N.E. Pathological aspects of the infertile testis. Urol. Clin. N. Am. 1978, 5, 503–530. [Google Scholar] [CrossRef]
- Cocuzza, M.; Alvarenga, C.; Pagani, R. The epidemiology and etiology of azoospermia. Clinics 2013, 68, 15–26. [Google Scholar] [CrossRef]
- Merino, G.; Carranza-Lira, S.; Murrieta, S.; Rodriguez, L.; Cuevas, E.; Moran, C. Bacterial infection and semen characteristics in infertile men. Syst. Biol. Reprod. Med. 1995, 35, 43–47. [Google Scholar] [CrossRef]
- Diemer, T.; Huwe, P.; Ludwig, M.; Hauck, E.W.; Weidner, W. Urogenital infection and sperm motility. Andrologia 2003, 35, 283–287. [Google Scholar] [CrossRef]
- Krausz, C.; Mills, C.; Rogers, S.; Tan, S.L.; Aitken, R.J. Stimulation of oxidant generation by human sperm suspensions using phorbol esters and formyl peptides: Relationships with motility and fertilization in vitro. Fertil. Steril. 1994, 62, 599–605. [Google Scholar] [CrossRef]
- Restrepo, B.; Cardona Maya, W. Antisperm antibodies and fertility asociation. Actas Urol. Esp. 2013, 37, 571–578. [Google Scholar] [CrossRef]
- Bieth, E.; Hamdi, S.M.; Mieusset, R. Genetics of the congenital absence of the vas deferens. Hum. Genet. 2021, 140, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Minhas, S.; Dhillo, W.S.; Jayasena, C.N. Male infertility due to testicular disorders. J. Clin. Endocrinol. Metab. 2021, 106, E442–E459. [Google Scholar] [CrossRef] [PubMed]
- Villumsen, A.L.; Zachau-Christiansen, B. Spontaneous alterations in position of the testes. Arch. Dis. Child. 1966, 41, 198–200. [Google Scholar] [CrossRef]
- Wohlfahrt-Veje, C.; Boisen, K.A.; Boas, M.; Damgaard, I.N.; Kai, C.M.; Schmidt, I.M.; Chellakooty, M.; Suomi, A.-M.; Toppari, J.; Skakkebaek, N.E.; et al. Acquired cryptorchidism is frequent in infancy and childhood. Int. J. Androl. 2009, 32, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Main, K.M.; Skakkebæk, N.E.; Virtanen, H.E.; Toppari, J. Genital anomalies in boys and the environment. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Kantartzi, P.D.; Goulis, C.D.; Goulis, G.D.; Papadimas, I. Male infertility and varicocele: Myths and reality. Hippokratia 2007, 11, 99–104. [Google Scholar]
- Ferlin, A.; Arredi, B.; Foresta, C. Genetic causes of male infertility. Reprod. Toxicol. 2006, 22, 133–141. [Google Scholar] [CrossRef]
- Linn, E.; Ghanem, L.; Bhakta, H.; Greer, C.; Avella, M. Genes Regulating Spermatogenesis and Sperm Function Associated With Rare Disorders. Front. Cell Dev. Biol. 2021, 9, 634536. [Google Scholar] [CrossRef]
- Leaver, R.B. Male infertility: An overview of causes and treatment options. Br. J. Nurs. 2016, 25, S35–S40. [Google Scholar] [CrossRef]
- Kamischke, A.; Nieschlag, E. Analysis of medical treatment of male infertility. Hum. Reprod. 1999, 14, 1–23. [Google Scholar] [CrossRef]
- Dabaja, A.A.; Schlegel, P.N. Medical treatment of male infertility. Transl. Androl. Urol. 2014, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, L.B.; Boepple, P.A.; Pralong, F.P.; Crowley, W.F. Adult-Onset Idiopathic Hypogonadotropic Hypogonadism—A Treatable Form of Male Infertility. N. Engl. J. Med. 1997, 336, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.M.; Hansen, J.R. Gonadotropin-releasing hormone and its analogs. Iowa Med. 1986, 76, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, M.; Nieschlag, E. Hormone substitution in male hypogonadism. Mol. Cell. Endocrinol. 2000, 161, 73–88. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Frisch, L.; Conn, P.M. Gonadotropin-releasing hormone (GnRH) antibodies formation in hypogonadotropic azoospermic men treated with pulsatile GnRH-Diagnosis and possible alternative treatment. Fertil. Steril. 1988, 50, 622–629. [Google Scholar] [CrossRef]
- Wei, C.; Long, G.; Zhang, Y.; Wang, T.; Wang, S.; Liu, J.; Ma, D.; Liu, X. Spermatogenesis of male patients with congenital hypogonadotropic hypogonadism receiving pulsatile gonadotropin-releasing hormone therapy versus gonadotropin therapy: A systematic review and meta-analysis. World J. Mens Health 2020, 38, 654–665. [Google Scholar] [CrossRef]
- Liu, L.; Banks, S.M.; Barnes, K.M.; Sherins, R.J. Two-year comparison of testicular responses to pulsatile gonadotropin-releasing hormone and exogenous gonadotropins from the inception of therapy in men with isolated hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 1988, 67, 1140–1145. [Google Scholar] [CrossRef]
- Madhukar, D.; Rajender, S. Hormonal treatment of male infertility: Promise and pitfalls. J. Androl. 2009, 30, 95–112. [Google Scholar] [CrossRef]
- Burgués, S.; Calderón, M.D. Subcutaneous self-administration of highly purified follicle stimulating hormone and human chorionic gonadotrophin for the treatment of male hypogonadotrophic hypogonadism. Hum. Reprod. 1997, 12, 980–986. [Google Scholar] [CrossRef]
- Dabbous, Z.; Atkin, S.L. Hyperprolactinaemia in male infertility: Clinical case scenarios. Arab J. Urol. 2018, 16, 44–52. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; Barbagallo, F.; Calogero, A.E.; La Vignera, S. Effects of the selective estrogen receptor modulators for the treatment of male infertility: A systematic review and meta-analysis. Expert Opin. Pharmacother. 2019, 20, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.R.; Siddhanti, S.; Ciaccia, A.V.; Plouffe, L. A pharmacological review of selective oestrogen receptor modulators. Hum. Reprod. Update 2000, 6, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.; Tanrikut, C. Surgical management of male infertility: An update. Transl. Androl. Urol. 2014, 3, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Schiff, J.D.; Ramírez, M.L.; Bar-Chama, N. Medical and Surgical Management Male Infertility. Endocrinol. Metab. Clin. N. Am. 2007, 36, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Tournaye, H. Male factor infertility and ART. Asian J. Androl. 2012, 14, 103–108. [Google Scholar] [CrossRef]
- Duran, H.E.; Morshedi, M.; Kruger, T.; Oehninger, S. Intrauterine insemination: A systematic review on determinants of success. Hum. Reprod. Update 2002, 8, 373–384. [Google Scholar] [CrossRef]
- Huang, J.Y.J.; Rosenwaks, Z. Assisted reproductive techniques. Methods Mol. Biol. 2014, 1154, 171–231. [Google Scholar] [CrossRef]
- Aboulghar, M.; Baird, D.T.; Collins, J.; Evers, J.L.H.; Fauser, B.C.J.M.; Lambalk, C.B.; Somigliana, E.; Sunde, A.; Tarlatzis, B.; Crosignani, P.G.; et al. Intrauterine insemination. Hum. Reprod. Update 2009, 15, 265–277. [Google Scholar] [CrossRef]
- Keck, C.; Gerber-Schafer, C.; Wilhelm, C.; Vogelgesang, D.; Breckwoldt, M. Intrauterine insemination for treatment of male infertility. Int. J. Androl. Suppl. 1997, 20, 55–64. [Google Scholar]
- Hasler, J.F.; Barfield, J.P. Vitro Fertilization. In Bovine Reproduction; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021; pp. 1124–1141. [Google Scholar] [CrossRef]
- Palermo, G.D.; Neri, Q.V.; Hariprashad, J.J.; Davis, O.K.; Veeck, L.L.; Rosenwaks, Z. ICSI and its outcome. Semin. Reprod. Med. 2000, 18, 161–169. [Google Scholar] [CrossRef]
- O’Neill, C.L.; Chow, S.; Rosenwaks, Z.; Palermo, G.D. Development of ICSI. Reproduction 2018, 156, F51–F58. [Google Scholar] [CrossRef] [PubMed]
- Justice, T.; Christensen, G. Sperm Cryopreservation Methods. Methods Mol. Biol. 2013, 927, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. Neurotrophic Factors: An Overview. Methods Mol. Biol. 2018, 1727, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. Keeping track of neurotrophin receptors. Cell 1991, 65, 915–918. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Casaccia-Bonnefil, P.; Carter, B.D.; Dobrowsky, R.T.; Chao, M.V. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature 1996, 383, 716–719. [Google Scholar] [CrossRef]
- Friedman, W.J.; Greene, L.A. Neurotrophin signaling via Trks and p75. Exp. Cell Res. 1999, 253, 131–142. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Yuan, X.-B.; Jin, M.; Xu, X.; Song, Y.-Q.; Wu, C.-P.; Poo, M.-M.; Duan, S. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat. Cell Biol. 2003, 5, 38–45. [Google Scholar] [CrossRef]
- Rose, C.R.; Blum, R.; Pichler, B.; Lepier, A.; Kafitz, K.W.; Konnerth, A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature 2003, 426, 74–78. [Google Scholar] [CrossRef]
- Toledo-Aral, J.J.; Brehm, P.; Halegoua, S.; Mandel, G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron 1995, 14, 607–611. [Google Scholar] [CrossRef]
- Minichiello, L.; Calella, A.M.; Medina, D.L.; Bonhoeffer, T.; Klein, R.; Korte, M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 2002, 36, 121–137. [Google Scholar] [CrossRef]
- Nahum, L.H. the Nerve Growth Factor (Ngf). Conn. Med. 1964, 28, 508–512. [Google Scholar] [PubMed]
- Fiore, M.; Amendola, T.; Triaca, V.; Tirassa, P.; Alleva, E.; Aloe, L. Agonistic encounters in aged male mouse potentiate the expression of endogenous brain NGF and BDNF: Possible implication for brain progenitor cells’ activation. Eur. J. Neurosci. 2003, 17, 1455–1464. [Google Scholar] [CrossRef][Green Version]
- Ciafrè, S.; Ferraguti, G.; Tirassa, P.; Iannitelli, A.; Ralli, M.; Greco, A.; Chaldakov, G.N.; Rosso, P.; Fico, E.; Messina, M.P.; et al. Nerve growth factor in the psychiatric brain. Riv. Psichiatr. 2020, 55, 4–15. [Google Scholar] [CrossRef]
- Wood, S.J.; Pritchard, J.; Sofroniew, M.V. Re-expression of Nerve Growth Factor Receptor after Axonal Injury Recapitulates a Developmental Event in Motor Neurons: Differential Regulation when Regeneration is Allowed or Prevented. Eur. J. Neurosci. 1990, 2, 650–657. [Google Scholar] [CrossRef]
- Verge, V.M.K.; Richardson, P.M.; Benoit, R.; Riopelle, R.J. Histochemical characterization of sensory neurons with high-affinity receptors for nerve growth factor. J. Neurocytol. 1989, 18, 583–591. [Google Scholar] [CrossRef]
- Ruit, K.G.; Osborne, P.A.; Schmidt, R.E.; Johnson, E.M.; Snider, W.D. Nerve growth factor regulates sympathetic ganglion cell morphology and survival in the adult mouse. J. Neurosci. 1990, 10, 2412–2419. [Google Scholar] [CrossRef]
- Heumann, R.; Lindholm, D.; Bandtlow, C.; Meyer, M.; Radeke, M.J.; Misko, T.P.; Shooter, E.; Thoenen, H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: Role of macrophages. Proc. Natl. Acad. Sci. USA 1987, 84, 8735–8739. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Stankulov, I.S.; Fiore, M.; Ghenev, P.I.; Aloe, L. Nerve growth factor levels and mast cell distribution in human coronary atherosclerosis. Atherosclerosis 2001, 159, 57–66. [Google Scholar] [CrossRef]
- Aloe, L.; Alleva, E.; Fiore, M. Stress and nerve growth factor: Findings in animal models and humans. Pharmacol. Biochem. Behav. 2002, 73, 159–166. [Google Scholar] [CrossRef]
- Schulte-Herbruggen, O.; Braun, A.; Rochlitzer, S.; Jockers-Scherubl, M.C.; Hellweg, R. Neurotrophic factors—A tool for therapeutic strategies in neurological, neuropsychiatric and neuroimmunological diseases? Curr. Med. Chem. 2007, 14, 2318–2329. [Google Scholar] [CrossRef]
- Bruscolini, A.; Sacchetti, M.; La Cava, M.; Nebbioso, M.; Iannitelli, A.; Quartini, A.; Lambiase, A.; Ralli, M.; de Virgilio, A.; Greco, A. Quality of life and neuropsychiatric disorders in patients with Graves’ Orbitopathy: Current concepts. Autoimmun. Rev. 2018, 17, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Chaldakov, G.N.; Fiore, M.; Tonchev, A.B.; Aloe, L. Neuroadipology: A novel component of neuroendocrinology. Cell Biol. Int. 2010, 34, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Tore, F.; Tonchev, A.; Fiore, M.; Tuncel, N.; Atanassova, P.; Aloe, L.; Chaldakov, G. From Adipose Tissue Protein Secretion to Adipopharmacology of Disease. Immunol. Endocr. Metab. Agents Med. Chem. 2007, 7, 149–155. [Google Scholar] [CrossRef]
- Carito, V.; Ceccanti, M.; Tarani, L.; Ferraguti, G.; Chaldakov, G.N.; Fiore, M. Neurotrophins’ Modulation by Olive Polyphenols. Curr. Med. Chem. 2016, 23, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Petrella, C.; Di Certo, M.G.; Gabanella, F.; Barbato, C.; Ceci, F.M.; Greco, A.; Ralli, M.; Polimeni, A.; Angeloni, A.; Severini, C.; et al. Mediterranean Diet, Brain and Muscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021, 28, 7595–7613. [Google Scholar] [CrossRef]
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Tirassa, P.; Iannitelli, A.; Ralli, M.; Vitali, M.; Ceccanti, M.; Chaldakov, G.N.; et al. Nerve Growth Factor, Stress and Diseases. Curr. Med. Chem. 2020, 28, 2943–2959. [Google Scholar] [CrossRef]
- Rosso, P.; Iannitelli, A.; Pacitti, F.; Quartini, A.; Fico, E.; Fiore, M.; Greco, A.; Ralli, M.; Tirassa, P. Vagus nerve stimulation and Neurotrophins: A biological psychiatric perspective. Neurosci. Biobehav. Rev. 2020, 113, 338–353. [Google Scholar] [CrossRef]
- Carito, V.; Ceccanti, M.; Ferraguti, G.; Coccurello, R.; Ciafrè, S.; Tirassa, P.; Fiore, M. NGF and BDNF Alterations by Prenatal Alcohol Exposure. Curr. Neuropharmacol. 2019, 17, 308–317. [Google Scholar] [CrossRef]
- Ciafrè, S.; Ferraguti, G.; Greco, A.; Polimeni, A.; Ralli, M.; Ceci, F.M.; Ceccanti, M.; Fiore, M. Alcohol as an early life stressor: Epigenetics, metabolic, neuroendocrine and neurobehavioral implications. Neurosci. Biobehav. Rev. 2020, 118, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Iannitelli, A.; Carito, V.; Tirassa, P.; Chaldakov, G.N.; Messina, M.P.; et al. Nerve Growth Factor in Alcohol Use Disorders. Curr. Neuropharmacol. 2020, 19, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Triaca, V.; Amendola, T.; Tirassa, P.; Aloe, L. Brain NGF and EGF administration improves passive avoidance response and stimulates brain precursor cells in aged male mice. Physiol. Behav. 2002, 77, 437–443. [Google Scholar] [CrossRef]
- Chaldakov, G.; Fiore, M.; Tonchev, A.; Dimitrov, D.; Pancheva, R.; Rancic, G.; Aloe, L. Homo obesus: A Metabotrophin-Deficient Species. Pharmacol. Nutr. Insight Curr. Pharm. Des. 2007, 13, 2176–2179. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Otten, U.; Ehrhard, P.; Peck, R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc. Natl. Acad. Sci. USA 1989, 86, 10059–10063. [Google Scholar] [CrossRef] [PubMed]
- Torcia, M.; Bracci-Laudiero, L.; Lucibello, M.; Nencioni, L.; Labardi, D.; Rubartelli, A.; Cozzolino, F.; Aloe, L.; Garaci, E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell 1996, 85, 345–356. [Google Scholar] [CrossRef]
- Kannan, Y.; Usami, K.; Okada, M.; Shimizu, S.; Matsuda, H. Nerve growth factor suppresses apoptosis of murine neutrophils. Biochem. Biophys. Res. Commun. 1992, 186, 1050–1056. [Google Scholar] [CrossRef]
- Horigome, K.; Bullock, E.D.; Johnson, E.M. Effects of nerve growth factor on rat peritoneal mast cells. Survival promotion and immediate-early gene induction. J. Biol. Chem. 1994, 269, 2695–2702. [Google Scholar] [CrossRef]
- Shaoxia, P.U.; Changwei, Q.U.; Zhi, L.I.; Yansen, L.I.; Chunmei, L.I. Expression of nerve growth factor (NGF) and its receptors TrkA and p75 in the reproductive organs of laying hens. Rev. Bras. Cienc. Avic. 2016, 18, 187–192. [Google Scholar] [CrossRef]
- Perrard, M.-H.; Vigier, M.; Damestoy, A.; Chapat, C.; Silandre, D.; Rudkin, B.B.; Durand, P. Β-Nerve Growth Factor Participates in an Auto/Paracrine Pathway of Regulation of the Meiotic Differentiation of Rat Spermatocytes. J. Cell. Physiol. 2007, 210, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Artico, M.; Bronzetti, E.; Saso, L.; Felici, L.M.; D’Ambrosio, A.; Forte, F.; Grande, C.; Ortolani, F. Immunohistochemical profile of some neurotransmitters and neurotrophins in the seminiferous tubules of rats treated by lonidamine. Eur. J. Histochem. 2007, 51, 19–24. [Google Scholar] [PubMed]
- Adams, G.P.; Ratto, M.H. Ovulation-inducing factor in seminal plasma: A review. Anim. Reprod. Sci. 2013, 136, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Jia, L.; Zhang, Y.; Ji, W.; Li, H. Activation of the NGF/TrkA signaling pathway attenuates diabetic erectile dysfunction. Oncotarget 2017, 8, 105692–105702. [Google Scholar] [CrossRef] [PubMed]
- Spinnler, K.; Fröhlich, T.; Arnold, G.J.; Kunz, L.; Mayerhofer, A. Human tryptase cleaves pro-nerve growth factor (Pro-NGF): Hints of local, mast cell-dependent regulation of NGF/PRO-NGF action. J. Biol. Chem. 2011, 286, 31707–31713. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, P.; Wu, C.; Yu, L.; Zhao, S.; Gu, X. In silico analysis indicates a similar gene expression pattern between human brain and testis. Cytogenet. Genome Res. 2003, 103, 58–62. [Google Scholar] [CrossRef]
- Graves, J.A.M. Review: Sex Chromosome Evolution and the Expression of Sex-Specific Genes in the Placenta. Placenta 2010, 31, S27–S32. [Google Scholar] [CrossRef]
- Ramírez-Reveco, A.; Villarroel-Espíndola, F.; Rodríguez-Gil, J.E.; Concha, I.I. Neuronal signaling repertoire in the mammalian sperm functionality. Biol. Reprod. 2017, 96, 505–524. [Google Scholar] [CrossRef]
- Ayer-LeLievre, C.; Olson, L.; Ebendal, T.; Hallbook, F.; Persson, H. Nerve growth factor mRNA and protein in the testis and epididymis of mouse and rat. Proc. Natl. Acad. Sci. USA 1988, 85, 2628–2632. [Google Scholar] [CrossRef]
- Harper, G.P.; Barde, Y.A.; Burnstock, G.; Carstairs, J.R.; Dennison, M.E.; Suda, K.; Vernon, C.A. Guinea pig prostate is a rich source of nerve growth factor. Nature 1979, 279, 160–162. [Google Scholar] [CrossRef]
- Harper, G.P.; Thoenen, H. The Distribution of Nerve Growth Factor in the Male Sex Organs of Mammals. J. Neurochem. 1980, 34, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.Z.; Tanaka, A.; Watanabe, G.; Matsuda, H.; Taya, K. Effect of NGF on the motility and acrosome reaction of golden hamster spermatozoa in vitro. J. Reprod. Dev. 2010, 56, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Arai, K.Y.; Shimizu, K.; Kojima, C.; Itoh, M.; Watanabe, G.; Taya, K. Cellular localization of NGF and its receptors trkA and p75LNGFR in male reproductive organs of the Japanese monkey, Macaca fuscata fuscata. Endocrine 2006, 29, 155–160. [Google Scholar] [CrossRef]
- Levanti, M.B.; Germanà, A.; de Carlos, F.; Ciriaco, E.; Vega, J.A.; Germanà, G. Effects of increased nerve growth factor plasma levels on the expression of TrkA and p75NTR in rat testicles. J. Anat. 2006, 208, 373–379. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, V.K.; Singh, S.; Hariprasad, G.R.; Mal, G.; Srinivasan, A.; Yadav, S. Proteomic identification of camel seminal plasma: Purification of β-nerve growth factor. Anim. Reprod. Sci. 2013, 136, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Druart, X.; Rickard, J.; Mactier, S.; Kohnke, P.; Kershaw-Young, C.; Bathgate, R.; Gibb, Z.; Crossett, B.; Tsikis, G.; Labas, V.; et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteomics 2013, 91, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.P.; Glanville, R.W.; Thoenen, H. The purification of nerve growth factor from bovine seminal plasma. Biochemical characterization and partial amino acid sequence. J. Biol. Chem. 1982, 257, 8541–8548. [Google Scholar] [CrossRef]
- Li, C.; Zheng, L.; Wang, C.; Zhou, X. Absence of nerve growth factor and comparison of tyrosine kinase receptor A levels in mature spermatozoa from oligoasthenozoospermic, asthenozoospermic and fertile men. Clin. Chim. Acta 2010, 411, 1482–1486. [Google Scholar] [CrossRef]
- Adams, G.P.; Ratto, M.H.; Silva, M.E.; Carrasco, R.A. Ovulation-inducing factor (OIF/NGF) in seminal plasma: A review and update. Reprod. Domest. Anim. 2016, 51, 4–17. [Google Scholar] [CrossRef]
- Seidl, K.; Buchberger, A.; Erck, C. Expression of nerve growth factor and neurotrophin receptors in testicular cells suggest novel roles for neurotrophins outside the nervous system. Reprod. Fertil. Dev. 1996, 8, 1075–1087. [Google Scholar] [CrossRef]
- Brill, G.; Kahane, N.; Carmeli, C.; Von Schack, D.; Barde, Y.A.; Kalcheim, C. Epithelial-mesenchymal conversion of dermatome progenitors requires neural tube-derived signals: Characterization of the role of Neurotrophin-3. Development 1995, 121, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Newman Hirshfield, A.; Malamed, S.; Ojeda, S.R. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: Changes at the time of folliculogenesis. Endocrinology 1995, 136, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Luukko, K. Neurotrophins in odontogenesis. Int. J. Dev. Biol. 1995, 39, 195–202. [Google Scholar] [PubMed]
- Ojeda, S.R.; Dissen, G.A.; Junier, M.P. Neurotrophic factors and female sexual development. Front. Neuroendocrinol. 1992, 13, 120–162. [Google Scholar]
- Onoda, M.; Pflug, B.; Djakiew, D. Germ cell mitogenic activity is associated with nerve growth factor-like protein(s). J. Cell. Physiol. 1991, 149, 536–543. [Google Scholar] [CrossRef]
- Persson, H.; Lievre, C.A.-L.; Söder, O.; Villar, M.J.; Metsis, M.; Olson, L.; Ritzen, M.; Hökfelt, T. Expression of β-nerve growth factor receptor mRNA in Sertoli cells downregulated by testosterone. Science 1990, 247, 704–707. [Google Scholar] [CrossRef]
- Lonnerberg, P.; Soder, O.; Parvinen, M.; Ritzen, E.M.; Persson, H. β-Nerve growth factor influences the expression of androgen-binding protein messenger ribonucleic acid in the rat testis. Biol. Reprod. 1992, 47, 381–388. [Google Scholar] [CrossRef]
- Wheeler, E.F.; Bothwell, M. Spatiotemporal patterns of expression of NGF and the low-affinity NGF receptor in rat embryos suggest functional roles in tissue morphogenesis and myogenesis. J. Neurosci. 1992, 12, 930–945. [Google Scholar] [CrossRef]
- Russo, M.A.; Odorisio, T.; Fradeani, A.; Rienzi, L.; De Felici, M.; Cattaneo, A.; Siracusa, G. Low-affinity nerve growth factor receptor is expressed during testicular morphogenesis and in germ cells at specific stages of spermatogenesis. Mol. Reprod. Dev. 1994, 37, 157–166. [Google Scholar] [CrossRef]
- Levine, E.; Cupp, A.S.; Skinner, M.K. Role of neurotropins in rat embryonic testis morphogenesis (Cord formation). Biol. Reprod. 2000, 62, 132–142. [Google Scholar] [CrossRef][Green Version]
- Cupp, A.S.; Kim, G.H.; Skinner, M.K. Expression and action of neurotropin-3 and nerve growth factor in embryonic and early postnatal rat testis development. Biol. Reprod. 2000, 63, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Cupp, A.S.; Tessarollo, L.; Skinner, M.K. Testis developmental phenotypes in neurotropin receptor trkA and trkC null mutations: Role in formation of seminiferous cords and germ cell survival. Biol. Reprod. 2002, 66, 1838–1845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neto, F.T.L.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef]
- De Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13, 1–8. [Google Scholar] [CrossRef]
- Larose, H.; Kent, T.; Ma, Q.; Shami, A.N.; Harerimana, N.; Li, J.Z.; Hammoud, S.S.; Handel, M.A. Regulation of meiotic progression by Sertoli-cell androgen signaling. Mol. Biol. Cell 2020, 31, 2841–2862. [Google Scholar] [CrossRef]
- Hess, R.A.; De Franca, L.R. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [CrossRef]
- Holdcraft, R.W.; Braun, R.E. Hormonal regulation of spermatogenesis. Int. J. Androl. 2004, 27, 335–342. [Google Scholar] [CrossRef]
- Rossi, P.; Dolci, S. Paracrine mechanisms involved in the control of early stages of mammalian spermatogenesis. Front. Endocrinol. 2013, 4, 181. [Google Scholar] [CrossRef]
- Sofikitis, N.; Giotitsas, N.; Tsounapi, P.; Baltogiannis, D.; Giannakis, D.; Pardalidis, N. Hormonal regulation of spermatogenesis and spermiogenesis. J. Steroid Biochem. Mol. Biol. 2008, 109, 323–330. [Google Scholar] [CrossRef]
- Griswold, M.D. 50 years of spermatogenesis: Sertoli cells and their interactions with germ cells. Biol. Reprod. 2018, 99, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Meinhardt, A.; Nieschlag, E. Paracrine regulation of cellular interactions in the testis: Factors in search of a function. Eur. J. Endocrinol. 1997, 137, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Iliadou, P.K.; Tsametis, C.; Kaprara, A.; Papadimas, I.; Goulis, D.G. The sertoli cell: Novel clinical potentiality. Hormones 2015, 14, 504–514. [Google Scholar] [CrossRef]
- Galdieri, M.; Monaco, L.; Stefanini, M. Secretion of Androgen Binding Protein by Sertoli Cells Is Influenced by Contact with Germ Cells. J. Androl. 1984, 5, 409–415. [Google Scholar] [CrossRef]
- Le Magueresse, B.; Jégou, B. In vitro effects of germ cells on the secretory activity of sertoli cells recovered from rats of different ages. Endocrinology 1988, 122, 1672–1680. [Google Scholar] [CrossRef]
- Onoda, M.; Djakiew, D. A 29,000 Mr protein derived from round spermatids regulates Sertoli cell secretion. Mol. Cell. Endocrinol. 1993, 93, 53–61. [Google Scholar] [CrossRef]
- Haugen, T.B.; Landmark, B.F.; Josefsen, G.M.; Hansson, V.; Högset, A. The mature form of interleukin-1α is constitutively expressed in immature male germ cells from rat. Mol. Cell. Endocrinol. 1994, 105, R19–R23. [Google Scholar] [CrossRef]
- Onoda, M.; Djakiew, D. Pachytene spermatocyte protein(s) stimulate sertoli cells grown in bicameral chambers: Dose-dependent secretion of ceruloplasmin, sulfated glycoprotein-1, sulfated glycoprotein-2, and transferrin. Vitr. Cell. Dev. Biol.-Anim. 1991, 27, 215–222. [Google Scholar] [CrossRef]
- Pineau, C.; Sharpe, R.M.; Saunders, P.T.K.; Gérard, N.; Jégou, B. Regulation of Sertoli cell inhibin production and of inhibin α-subunit mRNA levels by specific germ cell types. Mol. Cell. Endocrinol. 1990, 72, 13–22. [Google Scholar] [CrossRef]
- Onoda, M.; Djakiew, D. Modulation of Sertoli cell secretory function by rat round spermatid protein(s). Mol. Cell. Endocrinol. 1990, 73, 35–44. [Google Scholar] [CrossRef]
- Le Magueresse, B.; Jegou, B. Possible involvement of germ cells in the regulation of oestradiol-17,β and ABP secretion by immature rat sertoli cells (in vitro studies). Biochem. Biophys. Res. Commun. 1986, 141, 861–869. [Google Scholar] [CrossRef]
- Vigier, M.; Weiss, M.; Perrard, M.H.; Godet, M.; Durand, P. The effects of FSH and of testosterone on the completion of meiosis and the very early steps of spermiogenesis of the rat: An in vitro study. J. Mol. Endocrinol. 2004, 33, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Hakovirta, H.; Kaipia, A.; Söder, O.; Parvinen, M. Effects of activin-A, inhibin-A, and transforming growth factor-β1 on stage-specific deoxyribonucleic acid synthesis during rat seminiferous epithelial cycle. Endocrinology 1993, 133, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Olson, L.; Ayer-LeLievre, C.; Ebendal, T.; Seiger, Å. Nerve growth factor-like immunoreactivities in rodent salivary glands and testis. Cell Tissue Res. 1987, 248, 275–286. [Google Scholar] [CrossRef]
- MacGrogan, D.; Desprès, G.; Romand, R.; Dicou, E. Expression of the β-nerve growth factor gene in male sex organs of the mouse, rat, and guinea pig. J. Neurosci. Res. 1991, 28, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Seidl, K.; Holstein, A.F. Organ culture of human seminiferous tubules: A useful tool to study the role of nerve growth factor in the testis. Cell Tissue Res. 1990, 261, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Parvinen, M.; Pelto-Huikko, M.; Soder, O.; Schultz, R.; Kaipia, A.; Mali, P.; Toppari, J.; Hakovirta, H.; Lönnerberg, P.; Ritzén, E.M. Expression of β-nerve growth factor and its receptor in rat seminiferous epithelium: Specific function at the onset of meiosis. J. Cell Biol. 1992, 117, 629–641. [Google Scholar] [CrossRef]
- Djakiew, D.; Pflug, B.; Dionne, C.; Onoda, M. Postnatal expression of nerve growth factor receptors in the rat testis. Biol. Reprod. 1994, 51, 214–221. [Google Scholar] [CrossRef]
- MacGrogan, D.; Saint-André, J.-P.; Dicou, E. Expression of Nerve Growth Factor and Nerve Growth Factor Receptor Genes in Human Tissues and in Prostatic Adenocarcinoma Cell Lines. J. Neurochem. 1992, 59, 1381–1391. [Google Scholar] [CrossRef]
- Robinson, L.L.L.; Townsend, J.; Anderson, R.A. The human fetal testis is a site of expression of neurotrophins and their receptors: Regulation of the germ cell and peritubular cell population. J. Clin. Endocrinol. Metab. 2003, 88, 3943–3951. [Google Scholar] [CrossRef]
- Plant, T.M. The hypothalamo-pituitary-gonadal axis. J. Endocrinol. 2015, 226, T41–T54. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.M.; Yang, W.X. Molecular regulation of hypothalamus-pituitary-gonads axis in males. Gene 2014, 551, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Stamatiades, G.A.; Kaiser, U.B. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol. Cell. Endocrinol. 2018, 463, 131–141. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of follicle-stimulating hormone in spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef] [PubMed]
- Tilbrook, A.J.; Clarke, I.J. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol. Reprod. 2001, 64, 735–742. [Google Scholar] [CrossRef]
- Corradi, P.F.; Corradi, R.B.; Greene, L.W. Physiology of the Hypothalamic Pituitary Gonadal Axis in the Male. Urol. Clin. N. Am. 2016, 43, 151–162. [Google Scholar] [CrossRef]
- Luisi, S.; Florio, P.; Reis, F.M.; Petraglia, F. Inhibins in female and male reproductive physiology: Role in gametogenesis, conception, implantation and early pregnancy. Hum. Reprod. Update 2005, 11, 123–135. [Google Scholar] [CrossRef]
- Scaccianoce, S.; Cigliana, G.; Nicolai, R.; Muscolo, L.A.; Porcu, A.; Navarra, D.; Perez-Polo, R.; Angelucci, L. Hypothalamic involvement in the activation of the pituitary-adrenocortical axis by nerve growth factor. Neuroendocrinology 1993, 58, 202–209. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Pareek, V.; Faiq, M.A.; Narayan, R.K.; Raza, K.; Prasoon, P.; Sharma, V.K. Neurotrophin mediated HPA axis dysregulation in stress induced genesis of psychiatric disorders: Orchestration by epigenetic modifications. J. Chem. Neuroanat. 2019, 102, 101688. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Y.; Zhang, T.; Su, Z.; Yu, D.; Lin, Q.; Chen, H.; Zhang, Q.; Xiang, Q.; Xue, W.; et al. Nasal delivery of nerve growth factor rescue hypogonadism by up-regulating GnRH and testosterone in aging male mice. EBioMedicine 2018, 35, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Marlin, M.C.; Li, G. Biogenesis and Function of the NGF/TrkA Signaling Endosome. Int. Rev. Cell Mol. Biol. 2015, 314, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Higa-Nakamine, S.; Maeda, N.; Toku, S.; Yamamoto, H. Involvement of protein kinase D1 in signal transduction from the protein kinase C pathway to the tyrosine kinase pathway in response to gonadotropin-releasing hormone. J. Biol. Chem. 2015, 290, 25974–25985. [Google Scholar] [CrossRef]
- Sasson, R.; Dearth, R.K.; White, R.S.; Chappell, P.E.; Mellon, P.L. Orexin A induces GnRH gene expression and secretion from GT1-7 hypothalamic GnRH neurons. Neuroendocrinology 2007, 84, 353–363. [Google Scholar] [CrossRef]
- Riccio, A.; Ahn, S.; Davenport, C.M.; Blendy, J.A.; Ginty, D.D. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 1999, 286, 2358–2361. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Chrivia, J.C.; Latchman, D.S. Nerve growth factor up-regulates the transcriptional activity of CBP through activation of the p42/p44(MAPK) cascade. J. Biol. Chem. 1998, 273, 32400–32407. [Google Scholar] [CrossRef][Green Version]
- Wortzel, I.; Seger, R. The ERK cascade: Distinct functions within various subcellular organelles. Genes Cancer 2011, 2, 195–209. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, A.; Abad, P.; Arias-Alvarez, M.; Rebollar, P.G.; Bautista, J.M.; Lorenzo, P.L.; García-García, R.M. Recombinant rabbit beta nerve growth factor production and its biological effects on sperm and ovulation in rabbits. PLoS ONE 2019, 14, e0219780. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X. The potential roles of neurotrophins in male reproduction. Reproduction 2013, 145, R89–R95. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Yi, K.; Ma, Y.; Zhang, W.; Zhou, X. Detection of nerve growth factor (NGF) and its specific receptor (TrkA) in ejaculated bovine sperm, and the effects of NGF on sperm function. Theriogenology 2010, 74, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-G.; Lin, K.; Xu, X.-B.; Zhang, S.-C.; Wang, N.; Fan, M. Evidence for the involvement of NGF in human sperm motility. J. Biomed. Sci. Eng. 2012, 5, 534–541. [Google Scholar] [CrossRef][Green Version]
- Lin, K.; Ding, X.-F.; Shi, C.-G.; Zeng, D.; QuZong, S.; Liu, S.-H.; Wu, Y.; LuoBu, G.; Fan, M.; Zhao, Y.-Q. Nerve growth factor promotes human sperm motility in vitro by increasing the movement distance and the number of A grade spermatozoa. Andrologia 2015, 47, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.; Arruda-Alencar, J.; Martins, J.; Viana, A.; Neto, A.V.; Rêgo, J.; de Oliveira, R.V.; Lobo, M.; Moreira, A.; Moreira, R.; et al. Major seminal plasma proteome of rabbits and associations with sperm quality. Theriogenology 2019, 128, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Saeednia, S.; Bahadoran, H.; Amidi, F.; Asadi, M.H.; Naji, M.; Fallahi, P.; Nejad, N.A. Nerve growth factor in human semen: Effect of nerve growth factor on the normozoospermic men during cryopreservation process. Iran. J. Basic Med. Sci. 2015, 18, 292–299. [Google Scholar] [PubMed]
- Parthipan, S.; Selvaraju, S.; Somashekar, L.; Arangasamy, A.; Sivaram, M.; Ravindra, J.P. Spermatozoal transcripts expression levels are predictive of semen quality and conception rate in bulls (Bos taurus). Theriogenology 2017, 98, 41–49. [Google Scholar] [CrossRef]
- Sari, L.M.; Zampini, R.; Argañaraz, M.E.; Carretero, M.I.; Fumuso, F.G.; Barraza, D.E.; Ratto, M.; Apichela, S.A. Expression of β-NGF and high-affinity NGF receptor (TrKA) in llama (Lama glama) male reproductive tract and spermatozoa. Mol. Reprod. Dev. 2018, 85, 934–944. [Google Scholar] [CrossRef]
- Cacialli, P. Expression of Nerve Growth Factor and Its Receptor TrkA in the Reproductive System of Adult Zebrafish. Vet. Sci. 2022, 9, 225. [Google Scholar] [CrossRef]
- Hong, W.F.; Su, Z.; Xiao, X. The Roles of Nerve Growth Factor and Its Receptors in Leydig Cells Development and Function. Int. J. Sci. 2018, 5, 176–183. [Google Scholar]
- Castellini, C.; Mattioli, S.; Bosco, A.D.; Cotozzolo, E.; Mancinelli, A.C.; Rende, M.; Stabile, A.M.; Pistilli, A. Nerve growth factor receptor role on rabbit sperm storage. Theriogenology 2020, 153, 54–61. [Google Scholar] [CrossRef]
- Suarez, S.S. Control of hyperactivation in sperm. Hum. Reprod. Update 2008, 14, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Curry, B.J. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxidants Redox. Signal. 2011, 14, 367–381. [Google Scholar] [CrossRef]
- Ecroyd, H.W.; Jones, R.C.; Aitken, R.J. Endogenous redox activity in mouse spermatozoa and its role in regulating the tyrosine phosphorylation events associated with sperm capacitation. Biol. Reprod. 2003, 69, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Weese, D.L.; Peaster, M.L.; Himsl, K.K.; Leach, G.E.; Lad, P.M.; Zimmern, P.E. Stimulated reactive oxygen species generation in the spermatozoa of infertile men. J. Urol. 1993, 149, 64–67. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Bosco, A.D.; Collodel, G.; Pistilli, A.; Stabile, A.M.; Macchioni, L.; Mancuso, F.; Luca, G.; Rende, M. In vitro effect of nerve growth factor on the main traits of rabbit sperm. Reprod. Biol. Endocrinol. 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Willnow, T.E.; Petersen, C.M. p75NTR--live or let die. Curr. Opin. Neurobiol. 2005, 15, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Mattioli, S.; Cotozzolo, E.; Pistilli, A.; Rende, M.; Bartolini, D.; Di Sante, G.; Menchetti, L.; Bosco, A.D.; Stabile, A.M. The Effect of Interaction NGF/p75(NTR) in Sperm Cells: A Rabbit Model. Cells 2022, 11, 1035. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, A.; Arias-Alvarez, M.; Timón, P.; Bautista, J.M.; Rebollar, P.G.; Lorenzo, P.L.; Garcia-Garcia, R.M. Characterization of β-Nerve Growth Factor-TrkA system in male reproductive tract of rabbit and the relationship between β-NGF and testosterone levels with seminal quality during sexual maturation. Theriogenology 2019, 126, 206–213. [Google Scholar] [CrossRef]
- Maranesi, M.; Zerani, M.; Leonardi, L.; Pistilli, A.; Arruda-Alencar, J.; Stabile, A.; Rende, M.; Castellini, C.; Petrucci, L.; Parillo, F.; et al. Gene Expression and Localization of NGF and Its Cognate Receptors NTRK1 and NGFR in the Sex Organs of Male Rabbits. Reprod. Domest. Anim. 2015, 50, 918–925. [Google Scholar] [CrossRef]
- Chaves, R.N.; Alves, A.M.C.V.; Lima, L.F.; Matos, H.M.T.; Rodrigues, A.P.R.; Figueiredo, J.R. Role of nerve growth factor (NGF) and its receptors in folliculogenesis. Zygote 2013, 21, 187–197. [Google Scholar] [CrossRef]
- D’Angelo, A.; Ceccanti, M.; Petrella, C.; Greco, A.; Tirassa, P.; Rosso, P.; Ralli, M.; Ferraguti, G.; Fiore, M.; Messina, M.P. Role of neurotrophins in pregnancy, delivery and postpartum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Streiter, S.; Fisch, B.; Sabbah, B.; Ao, A.; Abir, R. The importance of neuronal growth factors in the ovary. Mol. Hum. Reprod. 2015, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Yang, Y.; Liu, H.; Zhang, Q.; Xiang, Q.; Ge, R.; Su, Z.; Huang, Y. NGF induces adult stem Leydig cells to proliferate and differentiate during Leydig cell regeneration. Biochem. Biophys. Res. Commun. 2013, 436, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.A.; Brannigan, R.E. Testosterone deficiency. JAMA-J. Am. Med. Assoc. 2019, 322, 1116. [Google Scholar] [CrossRef]
- Barbonetti, A.; D’Andrea, S.; Francavilla, S. Testosterone replacement therapy. Andrology 2020, 8, 1551–1566. [Google Scholar] [CrossRef]
- Grech, A.; Breck, J.; Heidelbaugh, J. Adverse effects of testosterone replacement therapy: An update on the evidence and controversy. Ther. Adv. Drug Saf. 2014, 5, 190–200. [Google Scholar] [CrossRef]
- Majzoub, A.; Sabanegh, E. Testosterone replacement in the infertile man. Transl. Androl. Urol. 2016, 5, 859–865. [Google Scholar] [CrossRef]
- Kim, E.D.; Crosnoe, L.; Bar-Chama, N.; Khera, M.; Lipshultz, L.I. The treatment of hypogonadism in men of reproductive age. Fertil. Steril. 2013, 99, 718–724. [Google Scholar] [CrossRef]
- Meng, T.; Cao, Q.; Lei, P.; Bush, A.I.; Xiang, Q.; Su, Z.; He, X.; Rogers, J.T.; Chiu, I.-M.; Zhang, Q.; et al. Tat-haFGF14–154 Upregulates ADAM10 to Attenuate the Alzheimer Phenotype of APP/PS1 Mice through the PI3K-CREB-IRE1α/XBP1 Pathway. Mol. Ther.-Nucleic Acids 2017, 7, 439–452. [Google Scholar] [CrossRef]
- Lou, G.; Zhang, Q.; Xiao, F.; Xiang, Q.; Su, Z.; Zhang, L.; Yang, P.; Yang, Y.; Zheng, Q.; Huang, Y. Intranasal administration of TAT-haFGF14-154 attenuates disease progression in a mouse model of Alzheimer’s disease. Neuroscience 2012, 223, 225–237. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, R.; Garcia, A.A.; Braschi, C.; Capsoni, S.; Maffei, L.; Berardi, N.; Cattaneo, A. Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc. Natl. Acad. Sci. USA 2005, 102, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Y.; Ji, X.; He, W.; Fan, J.; Huang, Y.; Wang, Y. NGF Rescues Spermatogenesis in Azoospermic Mice. Reprod. Sci. 2021, 28, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Begna, K.; Abdelatif, A.; Schwager, S.; Hanson, C.; Pardanani, A.; Tefferi, A. Busulfan for the treatment of myeloproliferative neoplasms: The Mayo Clinic experience. Blood Cancer J. 2016, 6, e427. [Google Scholar] [CrossRef]
- Qu, N.; Itoh, M.; Sakabe, K. Effects of chemotherapy and radiotherapy on spermatogenesis: The role of testicular immunology. Int. J. Mol. Sci. 2019, 20, 957. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, X.; Fan, C.; Li, Y.; Pu, Y.; Cao, H.; Zhang, X. Establishment of recipient model for spermatogonial stem cells transplantation in Kunming mice. Tissue Cell 2014, 46, 249–254. [Google Scholar] [CrossRef]
- Skurikhin, E.G.; Pakhomova, A.V.; Pershina, O.V.; Ermolaeva, L.A.; Ermakova, N.N.; Krupin, V.A.; Pan, E.S.; Kudryashova, A.I.; Rybalkina, O.Y.; Zhdanov, V.V.; et al. Regenerative Potential of Stem and Progenitor Cells from Ischemic Testes of C57Bl/6 Mice in Culture and in the Model of Spermatogenesis Suppression Caused by Busulfan. Bull. Exp. Biol. Med. 2017, 162, 400–405. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Jang, T.H. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Agarwal, A.; Cho, C.L.; Esteves, S.C.; Majzoub, A. Development of treatment strategies in men with vulnerable sperm. Transl. Androl. Urol. 2017, 6, S476–S478. [Google Scholar] [CrossRef]
- Dohle, G.R. Male infertility in cancer patients: Review of the literature. Int. J. Urol. 2010, 17, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Anger, J.T.; Gilbert, B.R.; Goldstein, M. Cryopreservation of sperm: Indications, methods and results. J. Urol. 2003, 170, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, B.; Tiptiri-Kourpeti, A.; Metallinou, C. IGF-I and NGFβ enhance in vitro progressive motility and vitality of human spermatozoa. Reprod. Med. Biol. 2021, 20, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Holoch, P.; Wald, M. Current options for preservation of fertility in the male. Fertil. Steril. 2011, 96, 286–290. [Google Scholar] [CrossRef]
- Di Santo, M.; Tarozzi, N.; Nadalini, M.; Borini, A. Human sperm cryopreservation: Update on techniques, effect on DNA integrity, and implications for ART. Adv. Urol. 2012, 2012, 854837. [Google Scholar] [CrossRef]
- Agca, Y.; Critser, J.K. Cryopreservation of spermatozoa in assisted reproduction. Semin. Reprod. Med. 2002, 20, 15–23. [Google Scholar] [CrossRef]
- Muller, I.; Oude Ophuis, R.J.A.; Broekmans, F.J.M.; Lock, T.M.T.W. Semen cryopreservation and usage rate for assisted reproductive technology in 898 men with cancer. Reprod. Biomed. Online 2016, 32, 147–153. [Google Scholar] [CrossRef]
- Hourvitz, A.; Goldschlag, D.E.; Davis, O.K.; Gosden, L.V.; Palermo, G.D.; Rosenwaks, Z. Intracytoplasmic sperm injection (ICSI) using cryopreserved sperm from men with malignant neoplasm yields high pregnancy rates. Fertil. Steril. 2008, 90, 557–563. [Google Scholar] [CrossRef]
- Talaei, T.; Esmaeelpour, T.; Aekiyash, F.; Bahmanpour, S. Effects of cryopreservation on plasma membrane glycoconjugates of human spermatozoa. Iran. J. Reprod. Med. 2010, 8, 119–124. [Google Scholar]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Oehninger, S.; Duru, N.K.; Srisombut, C.; Morshedi, M. Assessment of sperm cryodamage and strategies to improve outcome. Mol. Cell Endocrinol. 2000, 169, 3–10. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm cryodamage in ruminants: Understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef] [PubMed]
- Nijs, M.; Creemers, E.; Cox, A.; Janssen, M.; Vanheusden, E.; Castro-Sanchez, Y.; Thijs, H.; Ombelet, W. Influence of freeze-thawing on hyaluronic acid binding of human spermatozoa. Reprod. Biomed. Online 2009, 19, 202–206. [Google Scholar] [CrossRef]
- Ozkavukcu, S.; Erdemli, E.; Isik, A.; Oztuna, D.; Karahuseyinoglu, S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J. Assist. Reprod. Genet. 2008, 25, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Mazzilli, F.; Rossi, T.; Sabatini, L.; Pulcinelli, F.M.; Rapone, S.; Dondero, F.; Gazzaniga, P.P. Human sperm cryopreservation and reactive oxygen species (ROS) production. Acta Eur. Fertil. 1995, 26, 145–148. [Google Scholar] [PubMed]
- Taylor, K.; Roberts, P.; Sanders, K.; Burton, P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod. Biomed. Online 2009, 18, 184–189. [Google Scholar] [CrossRef]
- McLaughlin, E.A.; Ford, W.C.L.; Hull, M.G.R. Motility characteristics and membrane integrity of cryopreserved human spermatozoa. J. Reprod. Fertil. 1992, 95, 527–534. [Google Scholar] [CrossRef]
- Critser, J.K.; Arneson, B.W.; Aaker, D.V.; Huse-Benda, A.R.; Ball, G.D. Cryopreservation of human spermatozoa. II. Postthaw chronology of motility and of zona-free hamster ova penetration. Fertil. Steril. 1987, 47, 980–984. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2011, 2011, 686137. [Google Scholar] [CrossRef]
- Thomson, L.K.; Fleming, S.D.; Aitken, R.J.; De Iuliis, G.N.; Zieschang, J.A.; Clark, A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef]
- Cross, N.L.; Hanks, S.E. Effects of cryopreservation on human sperm acrosomes. Hum. Reprod. 1991, 6, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jiang, S.; Su, J.; Zhang, J.; Bao, X.; Ding, R.; Shi, P.; Li, S.; Wu, C.; Zhao, G.; et al. The effects of cryopreservation on the acrosome structure, enzyme activity, motility, and fertility of bovine, ovine, and goat sperm. Anim. Reprod. 2020, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial dysfunction and oxidative stress caused by cryopreservation in reproductive cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Said, T.M.; Gaglani, A.; Agarwal, A. Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 2010, 21, 456–462. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A.; Linfor, J.J.; Meyers, S.A. Reactive Oxygen Species and Cryopreservation Promote DNA Fragmentation in Equine Spermatozoa. J. Androl. 2003, 24, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank 2016, 17, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Mode of action of cryoprotectants for sperm preservation. Anim. Reprod. Sci. 2016, 169, 2–5. [Google Scholar] [CrossRef]
- Bahmyari, R.; Zare, M.; Sharma, R.; Agarwal, A.; Halvaei, I. The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: A systematic review and meta-analysis. Andrologia 2020, 52, e13514. [Google Scholar] [CrossRef]
- Najafi, A.; Asadi, E.; Moawad, A.R.; Mikaeili, S.; Amidi, F.; Adutwum, E.; Safa, M.; Sobhani, A.G. Supplementation of freezing and thawing media with brain-derived neurotrophic factor protects human sperm from freeze-thaw-induced damage. Fertil. Steril. 2016, 106, 1658–1665.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dicou, E.; Djakiew, D. Characterization of nerve growth factor precursor protein expression in rat round spermatids and the trophic effects of nerve growth factor in the maintenance of Sertoli cell viability. Mol. Cell. Endocrinol. 1997, 127, 129–136. [Google Scholar] [CrossRef]
- Oberoi, B.; Kumar, S.; Talwar, P. Study of human sperm motility post cryopreservation. Med. J. Armed. Forces India 2014, 70, 349–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Connell, M.; McClure, N.; Lewis, S.E.M. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.; Wang, R.U.N.; Hellstrom, W.J.; Sikka, S.C. Effect of Cryoprotective Additives and Cryopreservation Protocol on Sperm Membrane Lipid Peroxidation and Recovery of Motile Human Sperm. J. Androl. 1993, 14, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Fisch, B.; Kraicer, R.K.; Amit, S.; Zukerman, Z.; Ovadia, J.; Tadir, Y. The relationship between sperm parameters and fertilizing capacity in vitro: A predictive role for swim-up migration. J. Vitr. Fertil. Embryo Transf. 1990, 7, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Dcunha, R.; Hussein, R.S.; Ananda, H.; Kumari, S.; Adiga, S.K.; Kannan, N.; Zhao, Y.; Kalthur, G. Current Insights and Latest Updates in Sperm Motility and Associated Applications in Assisted Reproduction. Reprod. Sci. 2022, 29, 7–25. [Google Scholar] [CrossRef]
- Bongso, A.; Ng, S.-C.; Mok, H.; Lim, M.N.; Teo, H.L.; Wong, P.; Ratnam, S. Improved sperm concentration, motility, and fertilization rates following Ficoll treatment of sperm in a human in vitro fertilization program. Fertil. Steril. 1989, 51, 850–854. [Google Scholar] [CrossRef]
- Hosseini, A.; Khalili, M.A. Improvement of motility after culture of testicular spermatozoa: The effects of incubation timing and temperature. Transl. Androl. Urol. 2017, 6, 271–276. [Google Scholar] [CrossRef]
- Rajfer, J. Enhancement of sperm motility in assisted reproduction. Rev. Urol. 2006, 8, 88. [Google Scholar]
- Mbizvo, M.T.; Johnston, R.C.; Baker, G.H.W. The effect of the motility stimulants, caffeine, pentoxifylline, and 2- deoxyadenosine on hyperactivation of cryopreserved human sperm. Fertil. Steril. 1993, 59, 1112–1117. [Google Scholar] [CrossRef]
- Scott, L.; Smith, S. Human sperm motility-enhancing agents have detrimental effects on mouse oocytes and embryos. Fertil. Steril. 1995, 63, 166–175. [Google Scholar] [CrossRef]
- Weng, Q.; Shi, Z.Q.; Tukada, J.; Watanabe, G.; Taya, K. Immunodetection of NGF, trkA, p75 and inhibin α-Subunit in interstitial cells of golden hamsters treated with hCG. J. Reprod. Dev. 2009, 55, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Amiss, E.; Stewart, J.W.; Negrón-Pérez, V.M.; Jones, K.; Haines, H.; Rhoads, M.L.; Lima, F.S. 117 Supplementation of IVF medium with nerve growth factor improved bovine embryonic cleavage rates during summer months. Reprod. Fertil Dev. 2020, 32, 185. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).