Abstract
The article systematizes information about the sources of β-glucan, its technological functions and practical aspects of its use in dairy and milk-based products. According to the analysis of scientific information, the main characteristics of β-glucan classifications were considered: the source of origin, chemical structure, and methods of obtention. It has been established that the most popular in the food technology of dairy products are β-glucans from oat and barley cereal, which exhibit pronounced technological functions in the composition of dairy products (gel formation, high moisture-binding capacity, increased yield of finished products, formation of texture, and original sensory indicators). The expediency of using β-glucan from yeast and mushrooms as a source of biologically active substances that ensure the functional orientation of the finished product has been revealed. For the first time, information on the use of β-glucan of various origins in the most common groups of dairy and milk-based products has been systematized. The analytical review has scientific and practical significance for scientists and specialists in the field of food production, in particular dairy products of increased nutritional value.
1. Introduction
β-glucan is a polysaccharide found naturally in the cell walls of cereals, yeasts, seaweeds, bacteria, and fungi. The physicochemical, functional, and technological properties of β-glucan are extremely different, depending on the source of origin []. This polysaccharide is used in the therapeutic, cosmetic, fitness, and professional sports fields [,]. Interest in β-glucan has arisen because it is a powerful immunostimulant, prebiotic, and dietary fiber [,]. Interest in the use of β-glucan in the food industry is associated not only with its positive impact on the health of consumers but also with its functional and technological properties, which significantly improve the consumer characteristics of food products.
The use of oat β-glucan in the food industry became possible when the EFSA confirmed in 2010 that the daily consumption of oat β-glucan in the amount of 3 g can reduce the risk of coronary disease and have a positive effect on the cardiovascular system, provided that a diet with low saturated fat content is followed []. β-glucan made from yeast has been recognized as a novel ingredient and authorized for release since 2011 [].
β-glucan, as polysaccharide, built only from glucose monomers is versatile and multifunctional, but its characteristics, strongly dependent on botanical origin, is still unsufficiently investigated, especially considering the mutual interactions of different recipe components. β-glucan is a very promising techno-functional ingredient for food industry, but the data characterizing its properties are too dispersed in the scientific literature and need to be organized to be useful for food professionals. Special attention is paid to research on the rheological properties of β-glucan because of its ability to form gels and increase the viscosity of products during technological processing due to its high structuring ability, which can be useful when researching low-fat food systems []. The regularities of the effect of molecular weight, pH, temperature, shear rate, and the duration of the breaking spatial bonds process on the structuring ability and thixotropy of food systems with β-glucan remain debatable issues and require further study [].
Triton Market Research concluded that the β-glucan market was assessed to be worth 340.63 million USD in 2018 and is predicted to generate a revenue of approximately 656.65 million USD by 2027, which includes the dairy industry as one of the branches that needs β-glucan as a technological ingredient.
In market conditions with a growing demand for healthy food and low-calorie products (most often due to a decrease in the mass fraction of fat), the use of ingredients capable of imitating the organoleptic properties of high-fat analogs, as well as improving the physicochemical properties of dairy and milk-based products, is relevant []. Fat significantly affects the quality indicators of food products, so reducing its content or excluding it from the composition of food products inevitably leads to a deterioration in their quality [].
Scientists have repeatedly proven that polysaccharides, in particular β-glucan, are technologically effective ingredients in dairy and milk-based products, which allows them to be used both individually and in combination with proteins of various origins, pectins, and other substances [,]. Different methods of processing β-glucan, intermediate raw materials with it, or products in the recipe in which it is contained can give it new functional and technological properties. However, the number of studies devoted to the understanding of the β-glucan specifics used in dairy product technologies is quite small, which confirms the need for further development in this scientific direction [].
Because of this, the organization of scientific knowledge about the chemical structure and properties of β-glucan from different sources in dairy and milk-based products will help advance these technologies in line with current food trends.
Considering the fact that the use of β-glucan in the food industry, and in particular the dairy, is a promising direction, and the existing information in this area is not systematized, the analysis of all existing recommendations, methods, and advantages of using this polysaccharide of various origins in the composition of certain types of dairy products is relevant. The results of this analytical review can have scientific and practical significance in the field of food science and the industrial production of dairy products.
2. The Characteristics of β-Glucan of Various Origins
2.1. Sources of β-Glucan
The amount of β-glucan that can be obtained from natural sources depends on the method and degree of purification, technology, and growing conditions (for grain crops), as well as on the source of β-glucan itself, which determines its chemical structure and functional and technological properties. The information on the content of β-glucan in the main raw materials, systematized by the authors based on an analysis of research works, is given in Table 1.

Table 1.
β-glucan content with different sources of origin.
One of the most famous sources of β-glucan is cereal crops: oats, barley, rye, wheat, etc., which explains the largest number of research papers with this raw material []. However, this information is not enough to answer all of the questions that scientists and food technologists have about how β-glucan can be used in different food systems. The possibility of using other types of β-glucan in the technology of dairy products, in particular their impact on organoleptic indicators, as well as bioavailability and the impact on the human body, are still an important question.
Without special processing methods, up to 6% of β-glucan can be obtained from grain crops, depending on the type of plant (Table 1). Increasing the yield of β-glucan is possible with the use of genotyping technology, which has found application for such grain crops as oats, barley, and wheat and provides raw materials with the average content of this polysaccharide at a level of 6–9% for oats and barley and 1–1.5% for wheat [,,]. Separate patented methods of genotyping can allow increasing the content of β-glucan even more. Thus, Stephen Alan Jobling and his colleagues invented a method of obtaining wheat grain with a β-glucan content of more than 3% [], which is based on recombinant DNA technology.
2.2. Chemical Structure
The chemical structure of β-glucan in cereals is an unbranched polysaccharide formed from glycopyranose residues connected by β-(1→4) bonds and isolated β-(1→3) bonds []. Isolated β-(1→4) bonds are not found in the structure of grain β-glucans. Most β-(1→4) linkages are arranged in groups of two or three []. The main structural fragment is cepicellotriose and cellotetraose residues connected by single β-(1→3) bonds []. The main chain of β-glucan thus resembles the structure of cellulose but has a bend in the position of the β-(1→3) linkage, as a result of which the strong hydrogen bonds that are present in cellulose are destroyed []. This explains the solubility of β-glucan from cereals in water.
The extraction of β-glucan from bacterial cell walls is one technological innovation that lacks sufficient scientific data []. Thus, Gemilang Lara Utama et al. [] state that the β-(1→3)-glucan component in the cell walls of yeasts and bacteria such as Xanthomonas campertris and Bacillus sp. is much smaller than that of the fungus, as a result of which the yield of β-glucan from such a source is lower. However, such representatives of bacteria are usually able to produce structuring homo- or heteropolysaccharides capable of forming gels in food systems [].
Bacterial β-glucan have a straight and unbranched β-(1→3)-D-glucan structure, while β-glucan from seaweed can contain a straight chain of β-(1→3) residues or a straight chain of β-(1→6)-linked glucosyl side branches [,].
It should be noted that the content of β-glucan in some species of microalgae, such as Euglena, can reach 90%, which usually depends on the quality of the raw material and the method of extraction []. Using excess irradiation of Euglena microalgae [] or culturing cells in a certain growth medium and special conditions [], it is possible to obtain the maximum yield of β-glucan at a level of 90%, while extraction by traditional methods provides a yield of 20–70%, depending on which morphological part of the microalgae was used.
The content of β-glucan in yeast, such as Saccharomyces cerevisiae, is higher than in cereal crops and is 55–65% [,]. Their chemical structure is represented by a complex of linear β-(1→3) chains with residual straight chains connected to them by long branches connected through β-(1→6) bonds [].
Edible mushrooms can also be a source of β-glucan. However, most mushrooms do not have a high content of this polysaccharide (up to 1%); although some of them, such as Gyrophora esculenta, contain more than 40% β-glucan.
The rest of the fungi vary in β-glucan content, from those containing it at the level of wheat to those containing up to 20% or more. They are mostly looked at as a source of β-glucan in the biomedical and pharmaceutical fields. Krzysztof Sobieralski et al. [] proved that mushrooms are extremely promising for obtaining a biologically active form of β-glucan, which can be a component in the formulation of functional food products, in particular dairy products []. It can be explained by the fact that these compounds have another structure, water solubility, and molecular mass, which determine their medicinal properties. β-glucans made from mushroms show a very wide range of health-supporting activity.
β-glucan of fungal origin contain β-(1→6)-linked chains extending from a β-(1→3) backbone. It should be noted that the basic structure of β-glucans also depends on the fungal source: fungal β-glucans have short β-(1→6)-linked chains, while yeast has β-(1→6) side chains with additional β-(1→3) chains [,]. These differences affect the properties of β-glucan as an immunoprotector and also further determine its ability to suppress the development of pathogenic microflora []. However, β-glucans made from mushrooms are not registered for medical use, so studies that will confirm the possibility of their further use are ongoing.
The chemical structure of β-glucan of various types is illustrated in Figure 1.
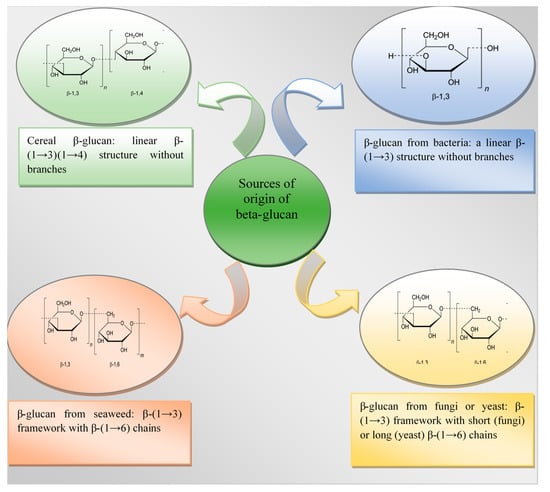
Figure 1.
Chemical structure of β-glucan depending on the source of origin [,].
2.3. Techno-Functional Properties
According to the scientific database PubMed, the number of scientific works on the search term “β-glucan” is 18,039 articles (from 1953 to 2021, inclusive), of which the results of 4283 works were published in the last 5 years, which indicates that it is generally understudied, because interest in β-glucan increased only during the last 2–3 years.
β-glucan is currently not widely used in the food industry [], which is due to the insufficient awareness of manufacturers regarding its functional and technological properties. Most experts associate β-glucan with a biologically active additive that is used for therapeutic purposes or as part of various diets for people with diabetes, obesity, and cardiovascular diseases. This makes sense because there have been a lot more clinical studies on the chemical makeup of β-glucan and how it affects the way the human body works (Figure 2) than on how it is used in food technology [].
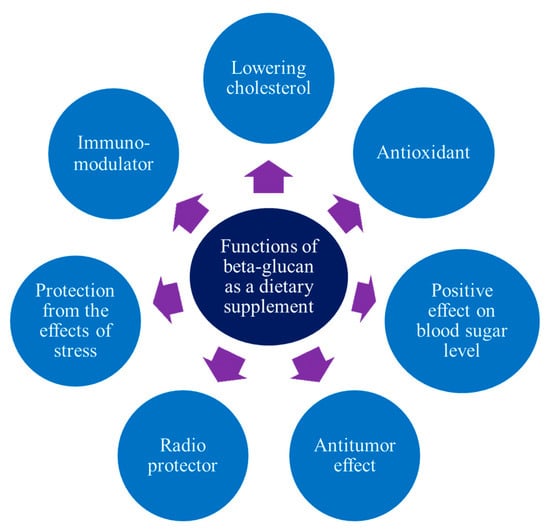
Figure 2.
Functional properties of the β-glucan of various types.
The composition of foods such as yeast, mushrooms, seaweed, and cereals (oat, barley, rye) includes complex carbohydrates, polysaccharides called β-glucans []. They were discovered and studied in the middle of the 20th century almost simultaneously in the USA and Japan. The use of medicinal mushrooms in Asian medicine has a long tradition. In a detailed study of the biological effects of these fungi, it was found that the presence of β-glucans in them is an important factor in nonspecific immunomodulation [,].
Most research on the use of β-glucan, as a technological ingredient, has been conducted in the field of bread and bakery products []. As for dairy products, the most common application for their use are in yogurts and yogurt drinks, fermented and non-fermented sports drinks, white-brined cheeses, and ice cream.
This mostly concerns the use of oat and barley β-glucan [], while β-glucan of yeast, bacteria, and fungi is little-researched, for example, in ice cream technology. At the same time, this ingredient opens a new field for scientific developments, considering its potential technological properties in the composition of many dairy and milk-based products, such as formation of a texture, original sensory properties, and others (Figure 3).
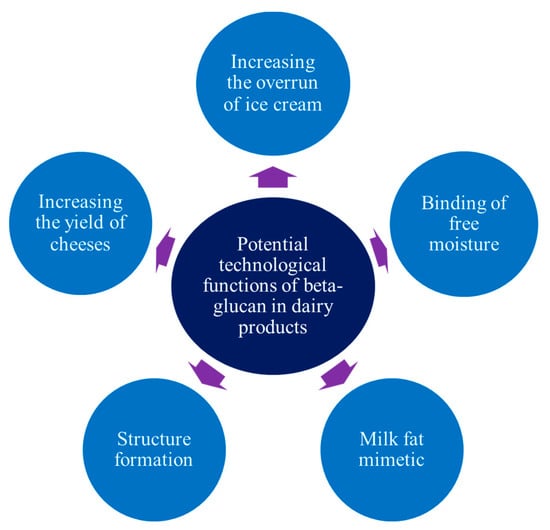
Figure 3.
Technological properties of the β-glucan of different types.
2.4 Recommended Dosage of β-glucan for Dairy Products
The authors systematized the recommended dosage ranges of β-glucan of various origins for dairy and milk-based products based on the analysis of scientific developments using this additive (Table 2, Table 3 and Table 4). In the next parts of this article, we will talk in more depth about the techno-functional properties of β-glucan in dairy products at the following doses.

Table 2.
Recommended doses of β-glucan of various origins in fermented and non-fermented drinks and fermented milk products.

Table 3.
Recommended dosages of β-glucan of different origins in cheeses and cheese-like products.

Table 4.
Recommended doses of β-glucan of various origins in ice cream and frozen desserts.
It should be noted that the given data (Table 2, Table 3 and Table 4) are generalized ranges of β-glucan content in dairy products. When planning an experiment using this additive, it is necessary to take into account not only its origin and chemical structure but also the recipe composition of the product and the compatibility of other components with β-glucan [], as well as the purpose of its use—to achieve a certain technological effect and/or provide the product with a functional orientation.
3. The Use of β-Glucan in the Technology of Dairy Drinks and Fermented Milk Products
3.1. Non-Fermented Milk Drinks
The production of chocolate milk is usually associated with the use of carrageenan as a stabilizer capable of reducing the gravitational settling of cocoa powder particles. Bandana Chatterjee and Tinkal Patel [] proved that the use of oat β-glucan in the amount of 3% in combination with carrageenan increases the viscosity, has a favorable effect on the sensory indicators of chocolate milk, and also enriches the product with dietary fiber, which allows positioning such a drink as functional.
In the technology of milk with fillers—banana, chocolate, vanilla, strawberry—it is possible to use barley β-glucan, since it has a chemical structure similar to oat β-glucan. In addition, it is possible to completely replace carrageenan with β-glucan as it is an effective structure-former, which has been proven in experimental work with meat emulsions [].
Oat β-glucan can act as a substitute for guar gum. Eva Vasquez-Orejarena et al. [] developed a composition for a high-protein milk drink in which oat flour was used as a stabilizer and a source of β-glucan. It was established that the combination of milk protein isolate in the amount of 2.5% and 1.9% of oat flour (0.75 g oat β-glucan per 1 serving) provided high-suspension stability for the drink (> 80%) and viscosity inherent in liquid drinks (< 50 mPa·s) with stabilizers []. An increase in the amount of oat flour led to a significant increase in the viscosity of protein drinks (from 51 to 100 mPa·s), which negatively affects their taste perception. Researchers have repeatedly drawn the attention of manufacturers to the fact that the design of recipes for new drinks, in particular milk drinks, with dietary fiber, such as β-glucan, requires a systematic approach to determining the rational dose of the additive []. The functional properties of dietary fiber and marketing promotion make it a popular product, which immediately affects the desire of manufacturers to include it in the composition of products and position them as healthy or enriched []. However, exceeding the rational dose of β-glucan in cereals leads to the formation of a sandy consistency of the product, an aftertaste of oatmeal, or dryness in the mouth [].
The combination of a protein ingredient with cereal β-glucan has been studied in the composition of a milk-based functional drink []. Orange-juice-based drink samples contained 0.5% barley β-glucan and from 0 to 1.5% whey protein isolate. Due to the protein and β-glucan content, the drink becomes more structured, less acidic, and somewhat loses its orange taste. This is partly explained by the ability of β-glucan to neutralize the sour taste, which is confirmed by the results of the sensory evaluation of pasta with β-glucan [], but the mechanism of this action requires additional research. β-glucan prevents the delamination of food systems due to its structuring ability, which is confirmed by the stability of the physicochemical parameters during storage. Similar data are given by Marika Lyly et al. [], based on the results of the study of the β-glucan effects with different degrees of purification on the viscosity of food systems classified as beverages. Thus, preparations with a cereal β-glucan content in the range of 13.4–13.7% for an excess of 0.5% lead to the excessive viscosity of food systems, which makes them unsuitable for positioning as liquid drinks, while preparations with a higher degree of purification (21.9–34.2%) allow their use in amounts up to 2%, provided that traditional structure-formers, such as starch, carrageenan, and guar gum are excluded.
3.2. Fermented Dairy Products
The use of oat β-glucan in fermented milk drinks (kefir, yogurts, rhazhenka, acidophilic milk, etc.) is promising due to the fact that it allows the improvement of the physicochemical parameters of the product, in particular, to increase viscosity, reduce acidity, prevent consistency defects (the separation of free water and the delamination of the product) [], as well as provide original taste properties. Thus, it was indicated that 0.6% of oat β-glucan in the composition of kefir, yogurt, and fermented milk drinks based on buttermilk and skimmed milk significantly increases the viscosity, especially in yogurt []. Furthermore, it was found that kefir drink and fermented milk had the best taste properties, while yogurt acquired a pronounced extraneous aftertaste of rice porridge. A dose of 0.6% oat β-glucan excessively increases the viscosity of the drink, which makes it impossible for the fermentation process to take place effectively. Xiaoqing Qu et al. [] claim that a dose of oat β-glucan at the level of 0.3% somewhat changes the chemical structure of three-dimensional mesh structure of yogurt due to the fact that oat β-glucan slows down the interaction with casein, which shortens the fermentation process by 16 min and increases the taste properties of the product. At the same time, a study by other scientists indicates that oat β-glucan in the amount of 1.4% does not provide proper structuring of yogurt and leads to a liquid consistency of the product [], which can be explained by a specific combination of lactic acid cultures [], a reduced fermentation temperature (36 °C), and an increased dose of polysaccharide, which was due to the desire to position the product as functional in terms of its dietary fiber content, but without a scientific explanation of the product formulation, it is impossible. Thus, the addition of oat β-glucan to milk before fermentation slows protein aggregation due to phase separation between milk proteins and β-glucan, which leads to a decrease in gelation []. Therefore, when using cereal β-glucan with relatively high content, it is advisable to additionally use probiotic strains of microorganisms that will ensure proper gel formation. Furthermore, the increased amount of oat β-glucan has a positive effect on the growth and development of microorganisms such as L. Paracasei [].
The influence of cereal β-glucan on the development and vital activity of probiotic organisms in the composition of fermented milk and milk-based drinks was also noted by other scientists. María Isabel Chávez de la Vega et al. [] reported that oat β-glucan affects the proteolytic activity of Lb. Rhamnosus GG during milk fermentation. In order to achieve the maximum impact on the development of Lb. Rhamnosus GG, β-glucan content should be 22.46 g per 1 liter of milk []. Poorva Sharma et al. [] established that the number of bacterial cells of Lactobacillus acidophilus and Lactobacillus bulgaricus during fermentation of mixtures with whey protein concentrate (70%) and oat β-glucan significantly increases during the first 10 hours of the process. Similar are the conclusions regarding the significant influence of oat β-glucan on the vital activity of Lactobacillus plantarum B28 in the composition of a probiotic drink made from oats [].
Most scientists confirm that one of the main advantages of using cereal β-glucans as part of fermented milk products is their effect on syneresis [], which is especially relevant in the production of low-fat or skimmed fermented milk products. The difference in the degree of influence of oat or barley β-glucan on the viscosity of yogurts [] can be explained by the composition of their formulations. It was established that, in the presence of starch, the hydrophobicity of the hydrogen bonds of amylose and β-glucan occurs, which leads to the destabilization of the spatial network and, as a result, the liquid consistency of the drink []. This shows once again that the ingredients in products with β-glucan need to be backed up by science.
The use of β-glucan from baker’s yeast in the technology of milk drinks allows obtaining healthy products. Eunice Mah et al. [] developed a milk drink with 0.1% β-glucan from dispersed yeast, which was included in the diet of marathon runners. It has been established that the consumption of such a drink during the 91st day reduces the symptoms of a cold a few days after intense exertion, which allows reducing training gaps after a marathon and recovering strength sooner. Eunice Mah et al. [] investigated soluble and insoluble β-glucan from Wellmune® brand yeast at 0.1% in a milk drink that improved symptoms in marathon runners. The results of the conducted research confirm that not all β-glucan is able to show immunomodulating properties. In particular, this is more characteristic of β-glucans from fungi and yeast []. The yeast preparation, Wellmune®, is widely used in various fields of the food industry and pharmaceuticals [,], but it is understudied in the technology of milk drinks, in particular for therapeutic and medicinal purposes.
The use of brewer’s yeast as a source of β-glucan is rational not only from the point of view of improving the physicochemical parameters of the product but also from the point of view of implementing the principles of sustainable development of brewing enterprises []. Brewer’s yeast is a by-product of beer production, the volume of which is extremely large, which can have a negative impact on the environment [,].
β-glucan from brewer’s yeast was studied in the range of 0 to 2% in the composition of skimmed milk yogurt []. It was established that its dose at a level of 1.5% improves the rheological properties, in particular, it allows to obtain the viscosity value and consistency characteristic of yogurt with high-fat content. The possibility of using β-glucan from brewer’s yeast as a milk fat replacer was investigated by Anna Piotrowska et al. [] in the formulation of yogurt with 3% fat. Among the range of β-glucan (0.15–0.9%) chosen for the study, the best dose was 0.3%, which provided a rich milky taste, viscous consistency, and milky smell. Such a result of the sensory evaluation was possible only because the yogurt was not low-fat.
In another study, it was found that yeast β-glucan in low-fat yogurt is an effective thickener and reduces fermentation time by 25%, which can be explained by the property of β-glucan from brewer’s yeast to form small-sized clusters in the yogurt matrix []. Increasing its dose to 0.8% helps to improve the sensory properties of the product but does not significantly affect the physicochemical properties, such as syneresis, titrated acidity, and viscosity.
The source of β-glucan can be yeast sediment obtained during the production of wines [], for example, Viorica wine (Moldova), the amount of which was determined by the laboratory method and was (28.17 ± 0.32)% []. Its addition to low-fat yogurt in the amount of 0.2–0.5% provides a reduction in the fermentation process by 1 hour (total time: 5 hours), which is due to the gel-forming ability of β-glucan []. However, this amount does not significantly affect the rheological parameters and is not able to act as a substitute for milk fat, which was also reported in other experiments [].
The extraction of β-glucan from edible mushrooms and its use in the food industry is a promising direction in the development of technologies for healthy food products containing biologically active substances, vitamins, and mineral complexes in their chemical composition [].
Bernadetta Hozová et al. [] investigated the quality of fruit yogurts with β-glucan hydrogels from Pleurotus ostreatus pleura and Lentinus edodes lentinan. The experiment demonstrated the ability of these β-glucan hydrogels in the amount of 0.5 mL per 150 mL of yogurt to suppress the development of coliform bacteria, yeast, and mold, but no positive effect on the vital activity of sourdough cultures was noted. Such data correlates with the scientific statements of other scientists regarding the ability of β-glucans from edible mushrooms to inhibit the growth of pathogenic microorganisms and exert an anti-allergic, antioxidant effect [].
β-glucan extraction from Pleurotus citrinopileatus mushrooms was investigated in low-fat yogurt technology []. Scientists have developed pasty β-glucan and used it in yogurts in the amount of 0.3–0.5%. According to the results of the experiment, a rational dose of β-glucan of 0.3% was established, the excess of which reduced the viscosity and sensory properties.
The use of β-glucan from Ganoderma lucidum mushrooms as part of therapeutic yogurt allows for protecting the body of children aged 3–5 years from infectious diseases [], and its consumption in the amount of 1% with Plukenetia volubilis seeds in yogurt based on skimmed milk powder significantly improves organoleptic properties, even though there was some reduction in rheological characteristics []. This dose allows to simulate the presence of fat in yogurt and brings it closer to the control sample in terms of taste perception.
In general, the use of β-glucan from edible mushrooms in dairy beverage technologies is quite limited due to the lack of information on its properties in food systems based on dairy raw materials, as well as the complex production technology (paste- or gel-like form).
The use of β-glucan from microalgae in food technology is also poorly researched. One such representative is Euglena gracilis, which contains a significant amount of β-glucan (> 50%) []. The use of this microalgae became possible when the NDA in 2020 recognized the safety of dried whole cells of Euglena gracilis as an innovative ingredient, which confirmed the regulation of the European Commission []. The permissible levels of use of Euglena gracilis in fermented milk drinks are as follows: yogurt–no more than 150 mg/100 g, yogurt drinks–no more than 93.75 mg/100 g [].
The effect of biologically active substances of Euglena gracilis on the vital activity of lactobacilli is known, but scientists have not yet determined what role β-glucan plays in this process. Junjie Dai et al. [] believe that β-glucan support for the growth of bacteria such as Lactobacillus acidophilus is mediated because it is not a major probiotic molecule in Euglena []. This data may be important in the development of fermented milk drink formulations with β-glucan from microalgae Euglena gracilis.
The effectiveness of bacterial β-glucan was evaluated in yogurt by Niamh Kearney et al. [], who used the strain Lactobacillus paracasei NFBC 338 containing the Pediococcus parvulus glycosyltransferase gene responsible for β-glucan production. This technology makes it possible to reduce the syneresis of the fermented clot due to the high moisture-binding capacity of β-glucan and to improve the texture of yogurt due to the increase in viscosity. However, the influence of β-glucan on the vital activity of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus was not detected, although the recombinant probiotic culture maintained high viability (> 108 CFU mL−1) during 28 days of yogurt storage. The improvement in the structure of yogurts when using different lactobacteria as a source of β-glucan can be explained by their production of exopolysaccharides, in particular β-glucan capable of inhibiting casein aggregation, which increases its stability and affects the viscosity of the final product [].
4. The Use of β-Glucan in Cheese and Cheese-like Products Technology
4.1. Oat and Barley β-Glucan
β-glucan use of various origins in cheese technology is not as popular as in dairy beverages, which is due to the specific properties of β-glucan in these food systems. Scientists are becoming more interested in it, though, because it can structure mixtures during the fermentation of milk curd and actively bind whey, which leads to a higher yield of the finished product [,].
The most studied in the technology of cheese and cheese-like product manufacturing is cereal β-glucan, which, despite its ability to imitate the taste of milk fat, has certain limitations due to the deterioration of sensory indicators of protein-containing products []. Thus, Pantelis Volikakis et al. [] investigated the effect of oat β-glucan in amounts of 0.7 and 1.4% in the technology of white-brined cheese with a reduced fat content (70% lower than in the full-fat analog) on quality indicators. The ability of β-glucan to reduce active acidity and increase the yield of the finished product by preventing whey separation during 90 days of storage was noted, which undoubtedly affects the texture of the product []. However, its use leads to a significant deterioration in taste and appearance. Other studies have also reported the possibility of an aftertaste of oat flour and a gray color, which significantly impairs the consumer properties of the product. Thus, the use of cereal β-glucan in cheese technology should be limited and should be combined with natural dyes and/or food flavor fillers to make a product with an interesting and unique taste and smell []. The fat replacer "Nutrim" based on oat β-glucan in the form of a hydrocolloid suspension was studied in samples of low-fat cheddar cheese (mass fraction of fat: 3.47 and 6.84%) []. Although the control sample had a higher yield of finished product compared to the experimental samples, an improvement in cheese texture was noted. Using scanning microscopy, it has been proven that a β-glucan-based milk fat replacer contributes to the formation of a uniform texture with small moisture droplets, which is associated with the high fat- and water-holding capacity of the polysaccharide presented in the form of a suspension [,].
The combination of β-glucan and phytosterols makes it possible to obtain an effective replacer for milk fat in low-fat cream cheese technology []. β-glucan provides a significant increase in viscosity, while phytosterols reduce the coefficient of friction, which contributes to the easy and plastic spreading of the cheese. This combination makes it possible to effectively use the advantages of both components and obtain a low-fat product with sensory properties similar to a high-fat counterpart. The combination of phytosterols, β-glucan, and the probiotic culture of L. Rhamnosus allowed for an increase in the content of diacetyl compounds, which contribute to the formation of the buttery taste of the low-fat cream swirl []. In addition, the combination of β-glucan and phytosterols provides an open product structure with evenly distributed cell walls of L. Rhamnosus in a casein matrix [].
That is why the search for options for combining polysaccharides with other technological ingredients has extraordinary practical value, especially in the technology of low-fat products [,].
Barley β-glucan has been investigated in the composition of functional low-fat Dahi cheese based on buffalo milk []. The mass fraction of the additive at a level of 0.5% ensures the most harmonious combination of individual organoleptic indicators. Adding more barley β-glucan makes the product less similar to traditional cheese because it makes the structure too hard. This happens when β-glucan binds too much whey [], and it also changes the color of the cheese, which has been seen in other studies [,]. R. Elsanhoty et al. [] note the possibility of using up to 5% of barley β-glucan in low-fat labneh cheese technology, which effectively masks fat reduction (up to 50%) and significantly improves the viability of probiotic cultures Lactobacillus acidophilus LA-5 and Bifidobacteria lactis Bb12 included in the composition of the starter preparation. An interesting detail of the obtained data is the excellent microbiological indicators, despite the increased yield of the finished product due to the retention of whey []. Other scientists have reported on the ability of barley β-glucan to influence the fermentation time of milk cheese mixtures because, at a high degree of gelation between the polysaccharide and the casein micelles of milk, it is significantly reduced [], which, presumably, can be one of the aspects of enzyme preparation economy and requires additional experimental studies.
The high gelling ability of barley β-glucan has also been investigated in the technology of curd, in particular, due to the urgency of finding natural functional and technological ingredients capable of reducing the volume of raw material losses during production. Carmen M. Tudorica et al. [] established that barley β-glucan not only reduces the loss of raw materials due to effective water retention [] but also increases the viscous and elastic characteristics of curd, increases the yield of the finished product, and significantly reduces the duration of the technological process due to the high water-binding capacity and the structuring ability of β-glucan. Furthermore, unlike oat β-glucan, it acts like milk fat, which gives the product a better taste and makes it possible to use more of it [].
Another method of producing cottage cheese with cereal β-glucan is also known []. It was established that, with a dosage of β-glucan in the amount of 0.5%, the product acquires excellent physicochemical indicators, and its production becomes more economically profitable than the analog without β-glucan. This is due to the increase in the nutritional value of cottage cheese and the presence of dietary fiber in it, which makes it a functional product attractive to the consumer. The addition of β-glucan also increases the yield of the product and the content of calcium, phosphorus, and vitamins B2 and C in it by preventing the separation of whey, which is also a source of biologically active substances []. Another important effect of β-glucan is a concern for the environment, which helps to reduce the number of secondary dairy resources [,].
The use of barley β-glucan in the technology of pasta filata cheeses requires small doses, which is associated with a negative effect on the elasticity of the cheese mass in the process of kneading and forming products, as well as melting during the use of the finished product []. A rational dose of barley β-glucan (0.2%) was established in the technology of low-fat mozzarella, which ensures the high elasticity of the cheese mass due to an increase in the mass fraction of moisture in the product and masks the lack of fat [].
4.2. β-Glucan from Yeast
Although yeast β-glucan has a strong immunostimulant effect and helps protect the body from infectious and viral diseases, its use in cheese technology is problematic because yeast is an undesirable component of the microflora in such products and can cause a variety of texture defects, such as swelling and the appearance of cracks [,]. However, β-glucans are more likely to be used in a line of alternative cheeses and products that taste like cheese that are meant to help vegans, vegetarians, people with high cholesterol, and people who like to try new things in their diets.
Kerry Group P. L. C. (Ireland) offers for use high-quality β-glucan from baker’s yeast, which can be used in the production of cheeses, fermented milk products, and sports drinks []. The advantage of this β-glucan is its high content of vitamin B12, which is especially important for vegans and vegetarians because it is the most deficient in people who do not consume food of animal origin []. "Hyeast Biotech" (China) offers a highly purified preparation of yeast β-glucan, which has a pronounced immunoprotective effect []. Such an additive is widely used in vegan pasty cheeses or other cheese substitutes that have a texture and taste similar to their dairy counterparts without the specific odor of yeast due to the high degree of purity []. Saccharomyces cerevisiae has a significant potential to produce β-glucan. However, its microstructural mesh can be much smaller than certain bacterial species, which should be considered when choosing a food system []. Considering that Saccharomyces cerevisiae is popular for use in the production of pizza, cheese casseroles, and pasta due to their cheesy taste, β-glucan isolated from them can have a positive effect on the taste properties of cheeses. The preservation capacity of Saccharomyces cerevisiae biomass was also reported, which may be related to the content of protein peptides [,]. Such a property can be interesting, for example, in the production of cheese with mold.
4.3. β-Glucan from Microorganisms
The isolation of β-glucan is also possible from such microorganisms as Pediococcus parvulus 2.6, Aspergillus spp., Oenococcus oeni IOEB0205, Xanthomonas campestris, Lactobacillus diolivorans G77, Lasiodiplodia theobromae, Botryosphaeria rhodina, and Bacillus natto [,].
β-glucan produced from X. campestris has the smallest dimensions of the microstructural network, which affects the presence of biologically active substances. It is somewhat higher in β-glucans from S. cerevisiae and B. natto, and the lowest biological activity in β-glucan is from A. oryzae.
Bacillus subtilis natto is used in Asian countries in the technology of fermented products, in particular cheese-like products based on legumes []. Cyclic β-glucans from Agrobacterium, Bradyrhizobium, and Rhizobium spp. are considered more soluble and bioavailable, but they have not been studied in cheese technology, which outlines the scope of scientific interest in them.
4.4. β-Glucan from Edible Mushrooms
The use of β-glucan from edible mushrooms is limited due to insufficient awareness of their immunomodulating and preventive properties and a lack of scientific data on their use in cheese technology []. However, considering the fact that cereal β-glucan is not widely used in the composition of these products, edible mushrooms can be a promising source of β-glucans [].
In addition, β-glucan extracts from the edible mushroom Pleurotus ostreatus in the amount of 0.4% were used in the production of non-fat cheese based on sheep’s milk []. In general, the texture of the cheese was improved by the β-glucan use of Pleurotus ostreatus, but for this, the duration of ripening must be at least 180 days. The most important advantage of its use is the possibility of reducing the mass fraction of fat by up to 50% in cheese, which does not affect the change in organoleptic indicators. This is partly due to the ability of β-glucan to mimic the taste of milk fat [] and, on the other hand, to the pasty form of β-glucan obtained from Pleurotus ostreatus []. β-glucan in a form other than powder may pose some problems, but this technology has a place and needs to be improved because of the demand for high-quality, low-fat functional products that taste like their high-fat counterparts [].
Kondyli et al. [] continued a series of experiments with β-glucan from Pleurotus ostreatus in the technology of a functional pasty cheese-like product based on sheep’s milk. At a mass fraction of 0.4%, β-glucan did not significantly affect the biological value of the finished product, but allowed the improvement of its structure by increasing viscosity []. However, due to the high moisture-binding capacity of β-glucan, the pasty cheese-like product contains more moisture than its counterparts, which affects the water activity in this food system and, accordingly, the duration and storage conditions of the finished product. In addition, the product was distinguished by a bright and attractive color. Khorshidian et al. [] recommended not exceeding the dose of β-glucan in dairy products by more than 1%. However, other scientists proved that the permissible dose of β-glucan in yogurt can be 1.5% [], which outlines the contradiction between the existing scientific data and requires clarification for each product technology individually [].
Expanding the possibilities of using β-glucan from edible mushrooms is also promising from the point of view of cost. Schizophylum commune Fr and Auricularia auricula Judae are the mushrooms with the highest β-glucan content and, at the same time, the cheapest species among them []. Furthermore, in terms of how they work, some types of fungi are like baker’s yeast (Saccharomyces cerevisiae) [,]. Therefore, the use of β-glucan of various origins in the composition of cheeses and cheese-like products should be rational and take into account their advantages in these food systems. First of all, we are talking about improving rheological characteristics, which is especially relevant in low-fat or fat-free products [], as well as enriching the chemical composition with biologically active substances, giving the product a functional focus [,,]. On the other hand, the use of β-glucan as a milk fat mimetic is somewhat limited, as it impairs the taste and color of some types of cheese, which requires its combination with fat replacers, for example, based on protein ingredients (Simplesse D-100, etc.) []. It is also important that some types of β-glucan can be useful in the production of alternative milk cheeses for certain groups of the population, which requires additional experimental studies.
5. The Use of β-Glucan in the Technology of Ice Cream and Frozen Desserts
5.1. Oat and Barley β-glucan
Traditional ice cream is a high-calorie product with a fairly high content of sugars (up to 15–16% sucrose and 4.2–5.5% lactose) and fat (up to 16%), which limits its use for people who are overweight, lactose-intolerant, diabetic or who follow low-fat diets [,]. Considering the fact that this dessert is common in most countries of the world, scientists are developing new types of ice cream with reduced fat, milk sugar, probiotics, protein, and sour milk content []. A sharp drop in taste quality in low-fat or non-fat frozen desserts is a big problem that needs a complex solution [,]
Scientists have said many times that polysaccharides can act like they do not have any fat, and when they are combined with protein ingredients and treated specially, they become good replacements for milk fat [].
Oat β-glucan is similar to guar gum in its technological and functional properties [], which allows it to be used in ice cream recipes not only as a milk fat mimetic but also to partially or completely replace the stabilizer.
Marek Aljewicz et al. [] investigated the possibility of reducing the mass fraction of fat in classic ice cream from 10 to 2.5% using highly purified oat β-glucan. A dose of β-glucan at the level of 1% provides a product that is maximally close to the control sample with high-fat content in terms of sensory indicators []. Oat β-glucan increases the overrun and viscosity of ice cream mixes. However, in the case of excessive structuring, the aeration of mixtures with air during freezing may deteriorate, which will reduce overrun and increase the hardness of ice cream. Due to its high moisture-binding and water- and fat-holding capacity, β-glucan in excess effectively structures mixtures, which impairs the uniform distribution of the air phase in the thickness of the product [,]. In order to increase the aeration of mixtures during freezing, it is advisable to use inulin, which can reduce the hardness of ice cream, which is one of the recommendations for the use of β-glucan in frozen desserts [,].
Lazaridou et al. [] also dealt with the issue of reducing the effect of β-glucan hardness in food systems. It was established that the addition of polyols to barley β-glucan solutions slows cryostructuring and leads to the formation of weaker and less thermostable cryogels, compared to control systems without polyols. On the other hand, mechanical deformation tests revealed an increase in the hardness and strength of β-glucan cryogels with the inclusion of polyols in the following order: sucrose, fructose < glucose, xylose < sorbitol, which requires further scientific research in the technology of frozen desserts.
The use of cereal β-glucan in the amount of less than 0.5% in ice cream technology may not be justified in general because such a dose will not make it possible to achieve a technological effect. It is well known that using 0.4% barley β-glucan in the production of ice cream based on buffalo milk with 4.17% fat not only produces the desired result but also reduces overall quality, particularly due to the unsatisfactory texture of the product [,]. A similar conclusion was reached by another group of scientists, who determined the dose of oat β-glucan at the level of 0.6% to be the most acceptable among the range of 0.1–0.6% for use in low-fat ice cream, which provides ice cream with a rich milky taste due to increased viscosity, which prevents the defect of a watery and empty taste [].
Rahil Rezaei et al. [] reported that oat β-glucan was able to regulate the textural properties of frozen soy yogurt by increasing viscosity. In addition, an overall increase in quality was observed when the fermentation process of the mixture was prolonged since the presence of soybeans is an inhibitory factor for the duration of fermentation of such mixtures []. With higher concentrations of oat β-glucan in the technology of frozen soy yogurt, the duration and temperature of ice cream mixture ripening are subject to the refinement of technological modes, which will significantly affect the quality of the product. The introduction of β-glucan in the amount of up to 1–2% allows reducing the duration of ripening from 24 to 13 h (at a temperature of 2 °C), which ensures the high viscosity and moderate hardness of the product after freezing []. An increase in temperature up to 6 °C does not make it possible to reach the optimal viscosity value within 24 hours and negatively affects the quality of the frozen dessert.
Ice cream is a complex dispersed system in which the air phase is distributed within fairly stable air bubbles in a partially frozen dispersion medium, which influences the formation of ice cream quality characteristics such as overrun, resistance to melting, and texture []. Because of this, the authors think it’s important to study in detail the microstructure of ice cream made with oat β-glucan (Figure 4). This is a scientific first and can help experts decide where to go next with research on different kinds of ice cream made with β-glucan. Scientists found that a dose of β-glucan at the level of 0.75–1% contributes to the structuring of low-fat milk–vegetable ice cream mixtures, which is explained by an increase in low-energy bonds between functional groups of polysaccharide macromolecules []. The greater number of areas of consecutive cellotriose units that "cross-link" β-glucan macromolecules into the gel matrix [] increases the time of ultimate failure and, as a result, causes the formation of a plastic ice cream texture. The microstructure of milk–vegetable ice cream is characterized by the presence of an additional framework of microbubbles, which wraps larger air inclusions and stabilizes them (Figure 4). Other scientists [] also looked at β-glucan’s ability to make foam, but this work [] is the first to describe how this polysaccharide can make foam with a complex structure.
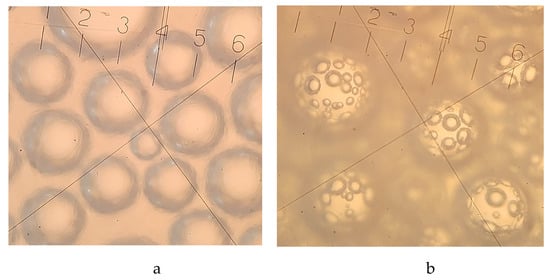
Figure 4.
Microstructure of milk–vegetable ice cream: a-without β-glucan, b-with 1% oat β-glucan [].
In another work, it was indicated that, with the help of microscopy, it is possible to determine the limited dose of oat β-glucan in the recipe composition of ice cream []. Thus, in the composition of low-fat sour milk ice cream, the optimal amount of oat β-glucan is 0.75%, which ensures an even distribution of the air phase inside the ice cream, a creamy consistency, and increased overrun. However, increasing the dosage of β-glucan to 1% leads to an excessive increase in the viscosity of the ice cream mixture and the corresponding complication of its air saturation during freezing, which is confirmed by the results of microscopy, which indicates the rapid destruction of the junctions of air bubbles.
5.2. β-Glucan of Bacterial Origin
As in the case of cereal β-glucan use, β-glucan of bacterial origin also leads to an increase in the resistance of ice cream to melting, which is probably related to the formation of a stable polysaccharide matrix, inside which molecules retain free moisture. However, Marek Aljewicz et al. [] reported that a dose of β-glucan isolated from Agrobacterium sp. at a level of 1% provides the same resistance to melting value as 0.5% highly purified oat β-glucan, which suggests a less pronounced ability of bacterial β-glucan to retain free moisture. This can be a technological advantage of bacterial β-glucan because the resulting ice cream will be less hard than when using cereal β-glucan. For a significant decrease in the mass fraction of fat in ice cream, β-glucan from Agrobacterium sp. cannot completely mask its absence, which may somewhat limit its use.
The source of β-glucan can be bacteria such as Alcaligenes spp., Agrobacterium spp., Paenibacillus spp., Rhizobium spp., Saccharomyces cerevisiae, Candida spp., fungi such as Aureobasidium pullulan, and Poria cocos. However, β-glucan from bacteria and fungi has not yet been explored in ice cream production. Scientists should look into whether or not they could be used to make frozen desserts because their chemical makeup includes biologically active substances and complexes that can reveal protective functions in the human body. Triveni P. Shukla and Gregory J. Halpern [] proposed a way to reduce the mass fraction of fat in ice cream by replacing it with an emulsified liquid shortening composition containing a gel of dietary fiber, water, and lipid, as well as additional bioactive components, including yeast β-glucan []. Using yeast β-glucan makes it possible to make low-calorie ice cream that also has health benefits []. This type of ice cream will be in high demand among modern consumers.
Microalgae Nannochloropsis oculata, Diacronema vlkianum, and Porphyridium cruentum are usually spray-dried or obtained from biomass, and they are used in food technology as stabilizers, hydrocolloids, and dyes. It is known that they contain proteins and carbohydrates, of which from 14–21 to 40% are β-glucans [,], which explains the high technological efficiency of powdered supplements in the production of ice cream. Turkish scientists used the powder of microalgae Nannochloropsis oculata, Porphyridium cruentum, and Diacronema vlkianum in ice cream formulation in an amount from 0.1 to 0.3% [], which ensured the production of ice cream with an attractive color and an increased content of biologically active substances, in particular, phenolic compounds. The best-tasting ice cream was made with Porphyridium cruentum microalgae.
Therefore, in the process of developing recipes for ice cream with β-glucan, it should be taken into account that such additives are aimed at producing a product with original color and taste and giving it a functional status due to enrichment with bioactive compounds [,]. To achieve a technological effect, for example, improving rheological characteristics, it is advisable to combine them with other polysaccharides or additives [].
6. Conclusions
1. A review of the scientific literature on the use of polysaccharides, in particular β-glucans of various types, in the dairy industry indicates their prospects and a limited number of scientific studies in this direction, which outlines the scope of scientific interests.
2. The most studied types of dairy and milk-based products, wherein β-glucan is used, are fermented and non-fermented drinks, especially yogurts, as well as low-fat cheeses. Meanwhile, the technology of ice cream, frozen desserts, and cheese products is the least-researched, especially for β-glucans produced from yeast, fungi, and bacteria.
3. The main functions of oat and barley β-glucans are technological (formation of texture, imitation of the taste of milk fat, increase in viscosity of drinks, improvement of rheological characteristics) and biological (reduction of cholesterol level, positive effect on the intestinal tract, etc.), and β-glucans from yeast and edible mushrooms have pronounced biological functions (positive effect on the immune system, etc.). The properties and functions of β-glucan from bacteria are the least-studied.
4. Based on a review of the scientific literature, the chemical structure of β-glucan of various origins is illustrated, and the recommended doses of β-glucan in dairy and milk-based products are systematized. So, for milk drinks, the total dose of β-glucan is in the range of 1.9–3.0%; for fermented milk products 0.1–0.5%; cheeses and cheese-like products: 0.2–1.4%; ice cream and frozen desserts: 0.5–2.0%.
5. The advantages of oat β-glucan are the ability to form a secondary foam structure in ice cream, to prevent the separation of free moisture during the production of fermented milk products and cheeses, and to act as a milk fat mimetic in low-fat products. Exceeding the recommended content ranges, β-glucan can destroy the structure of the product, give it an undesirable aftertaste or uncharacteristic consistency, etc., which indicates the need to comply with the existing recommendations for the use of β-glucan in the composition of certain types of dairy products.
6. The information systematized in the article has scientific and practical significance for scientists and specialists in the field of food technologies for the further innovative development of dairy technologies and dairy products of increased nutritional value.
Author Contributions
Conceptualization, A.M. and M.B.-O.; writing—original draft preparation, A.M.; writing—review and editing, T.O.; K.N. and M.B.-O.; visualization, A.M.; supervision, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
The project is financed by the program of the Minister of Education and Science named the “Regional Initiative of Excellence” in the years 2019–2023, project number 026/RID/2018/19, the amount of financing PLN 9,542,500.00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nishantha, M.D.; Diddugoda, J.; Nie, X.; Weining, S. Beta-Glucan: An Overview of its Properties, Health Benefits, Genetic Background and Practical Applications. Sch. J. Agric. Vet. Sci. 2018, 5, 130–140. [Google Scholar] [CrossRef]
- Pillai, R.; Redmond, M.; Röding, J. Anti-Wrinkle Therapy: Significant New Findings in the Non-Invasive Cosmetic Treatment of Skin Wrinkles with Beta-Glucan. Int. J. Cosmet. Sci. 2005, 27, 292. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Choi, J.-S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012, 851362. [Google Scholar] [CrossRef] [PubMed]
- Ciecierska, A.; Drywień, M.E.; Hamulka, J.; Sadkowski, T. Nutraceutical functions of beta-glucans in human nutrition. Rocz. Panstw. Zakl. Hig. 2019, 70, 315–324. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to oat beta glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1885. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the safety of ‘yeast beta-glucans’ as a Novel Food ingredient. EFSA J. 2011, 9, 2137. [Google Scholar] [CrossRef]
- Wang, L.; Ye, F.; Li, S.; Wei, F.; Chen, J.; Zhao, G. Wheat flour enriched with oat β-glucan: A study of hydration, rheological and fermentation properties of dough. J. Cereal Sci. 2017, 75, 143–150. [Google Scholar] [CrossRef]
- Nishantha, M.D.L.C.; Zhao, X.; Jeewani, D.C.; Bian, J.; Nie, X.; Weining, S. Direct comparison of β-glucan content in wild and cultivated barley. Int. J. Food Prop. 2018, 21, 2218–2228. [Google Scholar] [CrossRef]
- Lim, J.; Inglett, G.E.; Lee, S. Response to Consumer Demand for Reduced-Fat Foods; Multi-Functional Fat Replacers. Jpn. J. Food Eng. 2010, 11, 147–152. [Google Scholar] [CrossRef]
- Mamat, H.; Hill, S.E. Effect of fat types on the structural and textural properties of dough and semi-sweet biscuit. J. Food Sci. Technol. 2014, 51, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Sapiga, V.; Polischuk, G.; Breus, N.; Osmak, T. Polyfunctional Properties of Oat β-Glucan in the Composition of Milk-Vegetable Ice Cream. Ukr. Food J. 2021, 10, 691–702. [Google Scholar] [CrossRef]
- Kamath, R.; Basak, S.; Gokhale, J. Recent trends in the development of healthy and functional cheese analogues-a review. LWT 2022, 155, 112991. [Google Scholar] [CrossRef]
- Amer, E.M.; Saber, S.; Markeb, A.A.; Elkhawaga, A.; Mekhemer, I.; Zohri, A.-N.; Abujamel, T.; Harakeh, S.; Abd-Allah, E. Enhancement of β-Glucan Biological Activity Using a Modified Acid-Base Extraction Method from Saccharomyces cerevisiae. Molecules 2021, 26, 2113. [Google Scholar] [CrossRef] [PubMed]
- Ragaee, S.M.; Campbell, G.L.; Scoles, G.J.; McLeod, J.G.; Tyler, R.T. Studies on Rye (Secale cereale L.) Lines Exhibiting a Range of Extract Viscosities. 1. Composition, Molecular Weight Distribution of Water Extracts, and Biochemical Characteristics of Purified Water-Extractable Arabinoxylan. J. Agric. Food Chem. 2001, 49, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Anttila, H.; Sontag-Strohm, T.; Salovaara, H. Viscosity of beta-glucan in oat products. Agric. Food Sci. 2004, 13, 80–87. [Google Scholar] [CrossRef]
- Henry, R. Genetic and environmental variation in the pentosan and β-glucan contents of barley, and their relation to malting quality. J. Cereal Sci. 1986, 4, 269–277. [Google Scholar] [CrossRef]
- Henry, R.J.; Brown, A.H.D. Variation in the Carbohydrate Composition of Wild Barley (Hordeum spontaneum) Grain. Plant Breed. 1987, 98, 97–103. [Google Scholar] [CrossRef]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef]
- Rakha, A.; Åman, P.; Andersson, R. How Does the Preparation of Rye Porridge Affect Molecular Weight Distribution of Extractable Dietary Fibers? Int. J. Mol. Sci. 2011, 12, 3381–3393. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2019, 8, 1. [Google Scholar] [CrossRef]
- Colasuonno, P.; Marcotuli, I.; Cutillo, S.; Simeone, R.; Blanco, A.; Gadaleta, A. Effect of barley chromosomes on the β-glucan content of wheat. Genet. Resour. Crop Evol. 2020, 67, 561–567. [Google Scholar] [CrossRef]
- Phuwadolpaisarn, P. Comparison of β-Glucan Content in Milled Rice, Rice Husk and Rice Bran from Rice Cultivars Grown in Different Locations of Thailand and the Relationship between β-Glucan and Amylose Contents. Molecules 2021, 26, 6368. [Google Scholar] [CrossRef]
- Demirbas, A. β-Glucan and mineral nutrient contents of cereals grown in Turkey. Food Chem. 2005, 90, 773–777. [Google Scholar] [CrossRef]
- Jung, T.-D.; Shin, G.-H.; Kim, J.-M.; Choi, S.-I.; Lee, J.-H.; Lee, S.J.; Park, S.J.; Woo, K.S.; Oh, S.K.; Lee, O.-H. Comparative Analysis of γ-Oryzanol, β-Glucan, Total Phenolic Content and Antioxidant Activity in Fermented Rice Bran of Different Varieties. Nutrients 2017, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Niba, L.L.; Hoffman, J. Resistant starch and β-glucan levels in grain sorghum (Sorghum bicolor M.) are influenced by soaking and autoclaving. Food Chem. 2003, 81, 113–118. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, L.; Liu, M.; Guo, G.; Wu, B. Analysis of β-d-glucan biosynthetic genes in oat reveals glucan synthesis regulation by light. Ann. Bot. 2021, 127, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.A.; Fincher, G.B. Current challenges in cell wall biology in the cereals and grasses. Front. Plant Sci. 2012, 3, 130. [Google Scholar] [CrossRef] [PubMed]
- Marcotuli, I.; Houston, K.; Schwerdt, J.G.; Waugh, R.; Fincher, G.B.; Burton, R.A.; Blanco, A.; Gadaleta, A. Genetic Diversity and Genome Wide Association Study of β-Glucan Content in Tetraploid Wheat Grains. PLoS ONE 2016, 11, e0152590. [Google Scholar] [CrossRef]
- Luo, J.; Chen, D.; Mao, X.; He, J.; Yu, B.; Cheng, L.; Zeng, D. Purified β-glucans of Different Molecular Weights Enhance Growth Performance of LPS-challenged Piglets via Improved Gut Barrier Function and Microbiota. Animals 2019, 9, 602. [Google Scholar] [CrossRef]
- Aboushanab, S.A.S.; Vyrova, D.V.; Selezneva, I.S.; Ibrahim, M.N.G. The potential use of β-Glucan in the industry, medicine and cosmetics. AIP Conf. Proc. 2019, 2174, 020198. [Google Scholar] [CrossRef]
- Sekar, A.; Kim, M.; Jeong, H.C.; Kim, K. Strain Selection and Optimization of Mixed Culture Conditions for Lactobacillus pentosus K1-23 with Antibacterial Activity and Aureobasidium pullulans NRRL 58012 Producing Immune-Enhancing β-Glucan. J. Microbiol. Biotechnol. 2018, 28, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure inSaccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-G.; Shim, Y.Y.; Choi, S.-O.; Park, W.-M. New Method Development for Nanoparticle Extraction of Water-Soluble β-(1→3)-d-Glucan from Edible Mushrooms, Sparassis crispa and Phellinus linteus. J. Agric. Food Chem. 2009, 57, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Ubiparip, Z.; De Doncker, M.; Beerens, K.; Franceus, J.; Desmet, T. β-Glucan phosphorylases in carbohydrate synthesis. Appl. Microbiol. Biotechnol. 2021, 105, 4073–4087. [Google Scholar] [CrossRef] [PubMed]
- Manzi, P.; Pizzoferrato, L. Beta-glucans in edible mushrooms. Food Chem. 2000, 68, 315–318. [Google Scholar] [CrossRef]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Kim, Y.-S. Water-solubility of β-Glucans in Various Edible Mushrooms—Research Note. Prev. Nutr. Food Sci. 2005, 10, 294–297. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef]
- Bobadilla, F.; Rodriguez-Tirado, C.; Imarai, M.; Galotto, M.J.; Andersson, R. Soluble β-1,3/1,6-glucan in seaweed from the southern hemisphere and its immunomodulatory effect. Carbohydr. Polym. 2013, 92, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Desamero, M.J.; Yasuda, K.; Nakashima, A.; Suzuki, K.; Chambers, J.K.; Uchida, K.; Ogawa, R.; Hachimura, S.; Nakayama, J.; et al. Effects of orally administered Euglena gracilis and its reserve polysaccharide, paramylon, on gastric dysplasia in A4gnt knockout mice. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J. Appl. Phycol. 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Jobling, S.A.; Belobrajdic, D.P.; Bird, A.R. Wheat having high levels of beta-glucan. WIPO Patent WO 2015017901 A1, 12 February 2015. [Google Scholar]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [CrossRef]
- Cho, K.C.; White, P.J. Enzymatic analysis of β-glucan content in different oat genotypes. Cereal Chem. 1993, 70, 539–542. [Google Scholar]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef]
- Okobira, T.; Miyoshi, K.; Uezu, K.; Sakurai, K.; Shinkai, S. Molecular Dynamics Studies of Side Chain Effect on the β-1,3-d-Glucan Triple Helix in Aqueous Solution. Biomacromolecules 2008, 9, 783–788. [Google Scholar] [CrossRef]
- Tupe, S.G.; Deshmukh, S.K.; Zambare, R.B.; Tripathi, A.A.; Deshpande, M.V. Biopolymers from Fungi and Their Applications. In Fungal Biopolymers and Biocomposites; Springer: Singapore, 2022; pp. 3–14. [Google Scholar] [CrossRef]
- Utama, G.L.; Dio, C.; Lembong, E.; Cahyana, Y.; Balia, R.L. Microorganism-based β-glucan production and their potential as antioxidant. Sys. Rev. Pharm. 2020, 11, 868–873. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Suzuki, T.; Kusano, K.; Kondo, N.; Nishikawa, K.; Kuge, T.; Ohno, N. Biological Activity of High-Purity β-1,3-1,6-Glucan Derived from the Black Yeast Aureobasidium pullulans: A Literature Review. Nutrients 2021, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Watanabe, K.; Taira, S.; Kasubuchi, M.; Li, X.; Irie, J.; Itoh, H.; Kimura, I. Barley β-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS ONE 2018, 13, e0196579. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Vismara, R.; Passarelli, V.; Gualtieri, P. Paramylon (β-1,3-glucan) content in wild type and WZSL mutant of Euglena gracilis. Effects of growth conditions. J. Appl. Phycol. 2001, 13, 59–65. [Google Scholar] [CrossRef]
- Tuse, D.; Marquez, L.; Hokama, L.A. Production of beta-1, 3-glucan in Euglena. U.S. Patent 5,084,386 A, 28 January 1993. [Google Scholar]
- Sobieralski, K.; Siwulski, M.; Lisiecka, J.; Jedryczka, M.; Sas-Golak, I.; Fruzynska-Jozwiak, D. Fungi-derived β-glucans as a component of functional food. Acta Sci. Pol. Hortorum Cultus. 2012, 11, 111–128. [Google Scholar]
- Camilli, G.; Tabouret, G.; Quintin, J. The Complexity of Fungal β-Glucan in Health and Disease: Effects on the Mononuclear Phagocyte System. Front. Immunol. 2018, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Keen, C.L.; Gershwin, M.E. Mushrooms, Tumors, and Immunity: An Update. Exp. Biol. Med. 2004, 229, 393–406. [Google Scholar] [CrossRef]
- De Graaff, P.; Govers, C.; Wichers, H.; Debets, R. Consumption of β-glucans to spice up T cell treatment of tumors: A review. Expert Opin. Biol. Ther. 2018, 18, 1023–1040. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Sharifinia, M.; Ghaedi, G. β-glucan as a promising food additive and immunostimulant in aquaculture industry. Ann. Anim. Sci. 2022, 22, 817–827. [Google Scholar] [CrossRef]
- Zou, Y.; Liao, D.; Huang, H.; Li, T.; Chi, H. A systematic review and meta-analysis of beta-glucan consumption on glycemic control in hypercholesterolemic individuals. Int. J. Food Sci. Nutr. 2015, 66, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Andrzej, K.M.; Małgorzata, M.; Sabina, K.; Horbańczuk, O.K.; Rodak, E. Application of rich in β-glucan flours and preparations in bread baked from frozen dough. Food Sci. Technol. Int. 2020, 26, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Patel, T. Increased Sensory Quality and Consumer Acceptability by Fortification of Chocolate Flavored Milk with Oat Beta Glucan. Int. J. Clin. Biomed. Res. 2016, 2, 25–28. [Google Scholar]
- Vasquez-Orejarena, E.; Simons, C.T.; Litchfield, J.H.; Alvarez, V.B. Functional Properties of a High Protein Beverage Stabilized with Oat-β-Glucan. J. Food Sci. 2018, 83, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Temelli, F.; Bansema, C.; Stobbe, K. Development of an Orange-flavored Barley β-Glucan Beverage with Added Whey Protein Isolate. J. Food Sci. 2004, 69, 237–242. [Google Scholar] [CrossRef]
- Mah, E.; Kaden, V.N.; Kelley, K.M.; Liska, D.J. Beverage Containing Dispersible Yeast β-Glucan Decreases Cold/Flu Symptomatic Days After Intense Exercise: A Randomized Controlled Trial. J. Diet. Suppl. 2020, 17, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Liutkevičius, A.; Speičienė, V.; Alenčikienė, G.; Mieželienė, A.; Kaminskas, A.; Abaravičius, J.A.; Vitkus, D.; Jab, V. Oat β-glucan in milk products: Impact on human health. Agric. Food 2015, 3, 74–81. [Google Scholar]
- Qu, X.; Nazarenko, Y.; Yang, W.; Nie, Y.; Zhang, Y.; Li, B. Effect of Oat β-Glucan on the Rheological Characteristics and Microstructure of Set-Type Yogurt. Molecules 2021, 26, 4752. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Waszkiewicz-Robak, B.; Swiderski, F. Possibility of beta-glucan from spent brewer’s yeast addition to yoghurts. Pol. J. Food Nutr. Sci. 2009, 59, 299–302. [Google Scholar]
- Hozová, B.; Kuniak, Ľ.; Kelemenová, B. Application of β-d-glucans isolated from mushrooms Pleurotus ostreatus (pleuran) and Lentinus edodes (lentinan) for increasing the bioactivity of yoghurts. Czech J. Food Sci. 2004, 22, 204–214. [Google Scholar] [CrossRef]
- Henao, S.L.D.; Urrego, S.A.; Cano, A.M.; Higuita, E.A. Randomized Clinical Trial for the Evaluation of Immune Modulation by Yogurt Enriched with β-Glucans from Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), in Children from Medellin, Colombia. Int. J. Med. Mushrooms 2018, 20, 705–716. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of dried whole cell Euglena gracilis as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, e06100. [Google Scholar] [CrossRef]
- Mejri, W.; Bornaz, S.; Sahli, A. Formulation of non-fat yoghurt with β-glucanfrom spent brewer’s yeast. J. Hyg. Eng. Des. 2014, 8, 163–173. [Google Scholar]
- Chirsanova, A.I.; Boistean, A.V.; Chiseliță, N.; Siminiuc, R. Impact of yeast sediment beta-glucans on the quality indices of yoghurt. Food Syst. 2021, 4, 12–18. [Google Scholar] [CrossRef]
- Pappa, E.C.; Kondyli, E.; MacNaughtan, W.; Kakouri, A.; Nesseris, K.; Israilides, C. Quality and Sensory Properties of Reduced Fat Yoghurt Made with Addition of β-Glucans. Food Nutr. Sci. 2018, 9, 390–402. [Google Scholar] [CrossRef]
- Volikakis, P.; Biliaderis, C.G.; Vamvakas, C.; Zerfiridis, G.K. Effects of a commercial oat-β-glucan concentrate on the chemical, physico-chemical and sensory attributes of a low-fat white-brined cheese product. Food Res. Int. 2004, 37, 83–94. [Google Scholar] [CrossRef]
- Kondyli, E.; Pappa, E.C.; Kremmyda, A.; Arapoglou, D.; Metafa, M.; Eliopoulos, C.; Israilides, C. Manufacture of Reduced Fat White-Brined Cheese with the Addition of β-Glucans Biobased Polysaccharides as Textural Properties Improvements. Polymers 2020, 12, 2647. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, D.; Khatkar, S.K.; Chawla, R.; Panwar, H.; Kapoor, S. Effect of β-glucan fortification on physico-chemical, rheological, textural, colour and organoleptic characteristics of low fat dahi. J. Food Sci. Technol. 2017, 54, 2684–2693. [Google Scholar] [CrossRef] [PubMed]
- Elsanhoty, R.; Zaghlol, A.; Hassanein, A. The Manufacture of Low Fat Labneh Containing Barley β-Glucan 1-Chemical Composition, Microbiological Evaluation and Sensory Properties. Curr. Res. Dairy Sci. 2009, 1, 1–12. [Google Scholar] [CrossRef]
- Melnikova, E.I.; Bogdanova, E.V.; Bolgova, M.S.; Samojlenko, A.V. Method for producing cottage cheese enriched with beta-glucan. Russia Patent RU 2645253 C2, 19 February 2018. [Google Scholar]
- Vithanage, C.R.; Mishra, V.K.; Vasiljevic, T.; Shah, N.P. Use of β-glucan in development of low-fat Mozzarella cheese. Milchwiss. -Milk Sci. Int. 2008, 130, 48–51. [Google Scholar]
- Kondyli, E.; Pappa, E.C.; Arapoglou, D.; Metafa, M.; Eliopoulos, C.; Israilides, C. Effect of Fortification with Mushroom Polysaccharide β-Glucan on the Quality of Ovine Soft Spreadable Cheese. Foods 2022, 11, 417. [Google Scholar] [CrossRef]
- Aljewicz, M.; Majcher, M.; Nalepa, B. A Comprehensive Study of the Impacts of Oat β-Glucan and Bacterial Curdlan on the Activity of Commercial Starter Culture in Yogurt. Molecules 2020, 25, 5411. [Google Scholar] [CrossRef]
- Shibani, F.; Asadollahi, S.; Eshaghi, M. The effect of beta-glucan as a fat substitute on the sensory and physico-chemical properties of low-fat ice cream. J. Food Saf. Processing 2021, 1, 71–84. [Google Scholar]
- Mykhalevych, A.; Sapiga, V.; Polischuk, G.; Osmak, T. Functional and technological properties of oat beta-glucan in acidophilic-whey ice cream. Food Environ. Saf. 2022, 21, 116–128. [Google Scholar] [CrossRef]
- Rezaei, R.; Khomeiri, M.; Kashaninejad, M.; Mazaheri-Tehrani, M.; Aalami, M. Potential of β-d-glucan to enhance physicochemical quality of frozen soy yogurt at different aging conditions. Iran. Food Sci. Technol. Res. J. 2019, 15, 1–12. [Google Scholar]
- Durmaz, Y.; Kilicli, M.; Toker, O.S.; Konar, N.; Palabiyik, I.; Tamtürk, F. Using spray-dried microalgae in ice cream formulation as a natural colorant: Effect on physicochemical and functional properties. Algal Res. 2020, 47, 101811. [Google Scholar] [CrossRef]
- Kivelä, R.; Pitkänen, L.; Laine, P.; Aseyev, V.; Sontag-Strohm, T. Influence of homogenisation on the solution properties of oat β-glucan. Food Hydrocoll. 2010, 24, 611–618. [Google Scholar] [CrossRef]
- Vasquez Mejia, S.M.; de Francisco, A.; Manique Barreto, P.L.; Damian, C.; Zibetti, A.W.; Mahecha, H.S.; Bohrer, B.M. Incorporation of β-glucans in meat emulsions through an optimal mixture modeling systems. Meat Sci. 2018, 143, 210–218. [Google Scholar] [CrossRef]
- Lumaga, R.B.; Azzali, D.; Fogliano, V.; Scalfi, L.; Vitaglione, P. Sugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a β-glucan-enriched beverage. Food Funct. 2012, 3, 67–75. [Google Scholar] [CrossRef]
- Chakraborty, P.; Witt, T.; Harris, D.; Ashton, J.; Stokes, J.R.; Smyth, H.E. Texture and mouthfeel perceptions of a model beverage system containing soluble and insoluble oat bran fibres. Food Res. Int. 2019, 120, 62–72. [Google Scholar] [CrossRef]
- Lyly, M.; Ohls, N.; Lähteenmäki, L.; Salmenkallio-Marttila, M.; Liukkonen, K.-H.; Karhunen, L.; Poutanen, K. The effect of fibre amount, energy level and viscosity of beverages containing oat fibre supplement on perceived satiety. Food Nutr. Res. 2010, 54, 2149. [Google Scholar] [CrossRef]
- Jaworska, D.; Królak, M.; Przybylski, W.; Jezewska-Zychowicz, M. Acceptance of Fresh Pasta with β-Glucan Addition: Expected Versus Perceived Liking. Foods 2020, 9, 869. [Google Scholar] [CrossRef]
- Lazaridou, A.; Serafeimidou, A.; Biliaderis, C.G.; Moschakis, T.; Tzanetakis, N. Structure development and acidification kinetics in fermented milk containing oat β-glucan, a yogurt culture and a probiotic strain. Food Hydrocoll. 2014, 39, 204–214. [Google Scholar] [CrossRef]
- Lyly, M.; Salmenkallio-Marttila, M.; Suortti, T.; Autio, K.; Poutanen, K.; Lähteenmäki, L. Influence of Oat β-Glucan Preparations on the Perception of Mouthfeel and on Rheological Properties in Beverage Prototypes. Cereal Chem. 2003, 80, 536–541. [Google Scholar] [CrossRef]
- Kontogiorgos, V.; Tosh, S.; Wood, P. Phase behaviour of high molecular weight oat β-glucan/whey protein isolate binary mixtures. Food Hydrocoll. 2009, 23, 949–956. [Google Scholar] [CrossRef]
- de la Vega, M.I.C.; Alatorre-Santamaría, S.; Gómez-Ruiz, L.; García-Garibay, M.; Guzmán-Rodríguez, F.; González-Olivares, L.G.; Cruz-Guerrero, A.E.; Rodríguez-Serrano, G.M. Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lactobacillus rhamnosus GG in Milk Fermentation: Optimization by Response Surface. Fermentation 2021, 7, 210. [Google Scholar] [CrossRef]
- Sharma, P.; Trivedi, N.; Gat, Y. Development of functional fermented whey–oat-based product using probiotic bacteria. 3 Biotech 2017, 7, 272. [Google Scholar] [CrossRef]
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a new oat-based probiotic drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef]
- Kaur, R.; Riar, C.S. Sensory, rheological and chemical characteristics during storage of set type full fat yoghurt fortified with barley β-glucan. J. Food Sci. Technol. 2020, 57, 41–51. [Google Scholar] [CrossRef]
- Jirdehi, S.; Qajarbeygi, Z.; Khaksar, P. Effect of prebiotic β-glucan composite on physical, chemical, rheological and sensory properties of set-type low-fat Iranian yoghurt. Egypt J. Basic Appl. Sci. 2013, 3, 205–210. [Google Scholar]
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.A.; Singh, G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94–103. [Google Scholar] [CrossRef]
- Talbott, S.; Talbott, J. Effect of BETA 1, 3/1, 6 GLUCAN on Upper Respiratory Tract Infection Symptoms and Mood State in Marathon Athletes. J. Sports Sci. Med. 2009, 8, 509–515. [Google Scholar]
- McFarlin, B.K.; Carpenter, K.C.; Davidson, T.; McFarlin, M.A. Baker’s Yeast Beta Glucan Supplementation Increases Salivary IgA and Decreases Cold/Flu Symptomatic Days After Intense Exercise. J. Diet. Suppl. 2013, 10, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation. 2020, 6, 123. [Google Scholar] [CrossRef]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-Products in the Malting and Brewing Industries—Re-Usage Possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef]
- Raikos, V.; Grant, S.B.; Hayes, H.; Ranawana, V. Use of β-glucan from spent brewer’s yeast as a thickener in skimmed yogurt: Physicochemical, textural, and structural properties related to sensory perception. J. Dairy Sci. 2018, 101, 5821–5831. [Google Scholar] [CrossRef]
- Ministerul Agriculturii și Industriei Alimentare. Raport «Privind utilizarea mijloacelor financiare ale Fondul viei și vinului». Available online: https://madrm.gov.md/sites/default/files/Documente%20atasate%20Advance%20Pagines/Raport%20FVV-2017-2019-ONVV-MADRM.pdf. (accessed on 7 July 2022).
- Dönmez, Ö.; Mogol, B.A.; Gökmen, V. Syneresis and rheological behaviors of set yogurt containing green tea and green coffee powders. J. Dairy Sci. 2017, 100, 901–907. [Google Scholar] [CrossRef]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef]
- Kubala, L.; Ruzickova, J.; Nickova, K.; Sandula, J.; Ciz, M.; Lojek, A. The effect of (1→3)-β-d-glucans, carboxymethylglucan and schizophyllan on human leukocytes in vitro. Carbohydr. Res. 2003, 338, 2835–2840. [Google Scholar] [CrossRef]
- Vanegas-Azuero, A.-M.; Gutiérrez, L.-F. Physicochemical and sensory properties of yogurts containing sacha inchi (Plukenetia volubilis L.) seeds and β-glucans from Ganoderma lucidum. J. Dairy Sci. 2018, 101, 1020–1033. [Google Scholar] [CrossRef]
- Danilov, R.A.; Ekelund, N.G.A. Effects of pH on the growth rate, motility and photosynthesis inEuglena gracilis. Folia Microbiol. 2001, 46, 549–554. [Google Scholar] [CrossRef]
- The European Parliament; The Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001; OJ L 327, 11.12.2015; The European Parliament and The Council of the European Union: Brussel, Belgium, 2015; pp. 1–22. [Google Scholar]
- Dai, J.; He, J.; Chen, Z.; Qin, H.; Du, M.; Lei, A.; Zhao, L.; Wang, J. Euglena gracilis Promotes Lactobacillus Growth and Antioxidants Accumulation as a Potential Next-Generation Prebiotic. Front. Nutr. 2022, 9, 864565. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Sasaki, K.; Sasaki, D.; Yasuda, K.; Suzuki, K.; Kondo, A. The alga Euglena gracilis stimulates Faecalibacterium in the gut and contributes to increased defecation. Sci. Rep. 2021, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- Kearney, N.; Stack, H.M.; Tobin, J.T.; Chaurin, V.; Fenelon, M.A.; Fitzgerald, G.F.; Ross, R.; Stanton, C. Lactobacillus paracasei NFBC 338 producing recombinant beta-glucan positively influences the functional properties of yoghurt. Int. Dairy J. 2011, 21, 561–567. [Google Scholar] [CrossRef]
- Li, X.-W.; Lv, S.; Shi, T.-T.; Liu, K.; Li, Q.-M.; Pan, L.-H.; Zha, X.-Q.; Luo, J.-P. Exopolysaccharides from yoghurt fermented by Lactobacillus paracasei: Production, purification and its binding to sodium caseinate. Food Hydrocoll. 2020, 102, 105635. [Google Scholar] [CrossRef]
- Singh, M.; Kim, S.; Liu, S.X. Effect of Purified Oat β-Glucan on Fermentation of Set-Style Yogurt Mix. J. Food Sci. 2012, 77, E195–E201. [Google Scholar] [CrossRef]
- Sahan, N.; Yasar, K.; Hayaloglu, A.A.; Karaca, O.B.; Kaya, A. Influence of fat replacers on chemical composition, proteolysis, texture profiles, meltability and sensory properties of low-fat Kashar cheese. J. Dairy Res. 2008, 75, 1–7. [Google Scholar] [CrossRef]
- Santipanichwong, R.; Suphantharika, M. Carotenoids as colorants in reduced-fat mayonnaise containing spent brewer’s yeast β-glucan as a fat replacer. Food Hydrocoll. 2007, 21, 565–574. [Google Scholar] [CrossRef]
- Konuklar, G.; Inglett, G.E.; Carriere, C.J.; Felker, F.C. Use of a beta-glucan hydrocolloidal suspension in the manufacture of low-fat Cheddar cheese: Manufacture, composition, yield and microstructure. Int. J. Food Sci. Technol. 2004, 39, 109–119. [Google Scholar] [CrossRef]
- Mishra, N. Cereal β Glucan as a Functional Ingredient. In Innovations in Food Technology; Mishra, P., Mishra, R.R., Adetunji, C.O., Eds.; Springer: Singapore, 2020; pp. 109–122. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, Y.; Luo, J.; Wang, P.; Liu, B.; Du, Y.; Jiao, Y.; Hu, Y.; Chen, C.; Ren, F.; et al. Effect of anthocyanin-absorbed whey protein microgels on physicochemical and textural properties of reduced-fat Cheddar cheese. J. Dairy Sci. 2021, 104, 228–242. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. Texture and lubrication properties of functional cream cheese: Effect of β-glucan and phytosterol. J. Texture Stud. 2018, 49, 11–22. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. The viability of probiotic Lactobacillus rhamnosus (non-encapsulated and encapsulated) in functional reduced-fat cream cheese and its textural properties during storage. Food Control 2019, 100, 8–16. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. Flavour profiles of functional reduced-fat cream cheese: Effects of β-glucan, phytosterols, and probiotic L. rhamnosus. LWT 2019, 105, 16–22. [Google Scholar] [CrossRef]
- Giha, V.; Ordoñez, M.J.; Villamil, R.A. How does milk fat replacement influence cheese analogue microstructure, rheology, and texture profile? J. Food Sci. 2021, 86, 2802–2815. [Google Scholar] [CrossRef] [PubMed]
- Karp, S.; Wyrwisz, J.; Kurek, M.A. The impact of different levels of oat β-glucan and water on gluten-free cake rheology and physicochemical characterisation. J. Food Sci. Technol. 2020, 57, 3628–3638. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.; Tudorica, C.M. The Role of Complex Carbohydrates and Non-Starch Polysaccharides in the Regulation of Postprandial Glucose and Insulin Responses in Cereal Foods. J. Nutraceuticals Funct. Med. Foods. 2003, 4, 49–55. [Google Scholar] [CrossRef]
- Tudoricặ, C.M.; Kuri, V.; Brennan, C.S. Nutritional and Physicochemical Characteristics of Dietary Fiber Enriched Pasta. J. Agric. Food Chem. 2002, 50, 347–356. [Google Scholar] [CrossRef]
- Tudorica, C.M.; Jones, T.E.R.; Kuri, V.; Brennan, C.S. The effects of refined barleyβ-glucan on the physico-structural properties of low-fat dairy products: Curd yield, microstructure, texture and rheology. J. Sci. Food Agric. 2004, 84, 1159–1169. [Google Scholar] [CrossRef]
- Caseiro, C.; Dias, J.N.R.; de Andrade Fontes, C.M.G.; Bule, P. From Cancer Therapy to Winemaking: The Molecular Structure and Applications of β-Glucans and β-1, 3-Glucanases. Int. J. Mol. Sci. 2022, 23, 3156. [Google Scholar] [CrossRef] [PubMed]
- Polischuk, G.; Kochubey-Lytvynenko, O.; Osmak, T.; Kuzmik, U.; Bass, O.; Mykhalevych, A.; Sapiga, V. Scientific explanation of composition of acidophilic-whey ice cream, enriched with protein. Food Environ. Saf. J. 2021, 20, 13–20. [Google Scholar] [CrossRef]
- Neelima; Rao, P.S.; Sharma, R.; Rajput, Y.S. Direct estimation of sialic acid in milk and milk products by fluorimetry and its application in detection of sweet whey adulteration in milk. J. Dairy Res. 2012, 79, 495–501. [Google Scholar] [CrossRef]
- Osmak, T.; Mleko, S.; Bass, O.; Mykhalevych, A.; Kuzmyk, U. Enzymatic hydrolysis of lactose in concentrates of reconstituted demineralized whey, intended for ice cream production. Ukr. Food J. 2021, 10, 277–288. [Google Scholar] [CrossRef]
- Samuelsen, A.B.C.; Schrezenmeir, J.; Knutsen, S.H. Effects of orally administered yeast-derived beta-glucans: A review. Mol. Nutr. Food Res. 2014, 58, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.F.M.; McSweeney, P.L.H.; Sheehan, J.J. Split defect and secondary fermentation in Swiss-type cheeses—A review. Dairy Sci. Technol. 2010, 90, 3–26. [Google Scholar] [CrossRef]
- Kerry Health and Nutrition Institute. Available online: https://khni.kerry.com/ (accessed on 12 July 2022).
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.C.; La Ferrera, G.M.G.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef] [PubMed]
- Hyeast Biotech. Available online: https://www.hiyeast.com/ (accessed on 13 July 2022).
- Kholts-Shitinger, C.; Klapkholts, S.; Varadan, R.; Kazino, M.; Braun, P.; Ajzen, M.; Kon, E.; Privot, D. Non-dairy cheese replica comprising a coacervate. Russia Patent RU 2672489 C2, 15 November 2018. [Google Scholar]
- Utama, G.L.; Dio, C.; Sulistiyo, J.; Chye, F.Y.; Lembong, E.; Cahyana, Y.; Verma, D.K.; Thakur, M.; Patel, A.R.; Singh, S. Evaluating comparative β-glucan production aptitude of Saccharomyces cerevisiae, Aspergillus oryzae, Xanthomonas campestris, and Bacillus natto. Saudi J. Biol. Sci. 2021, 28, 6765–6773. [Google Scholar] [CrossRef]
- Pereira, P.R.; Freitas, C.S.; Paschoalin, V.M.F. Saccharomyces cerevisiae biomass as a source of next-generation food preservatives: Evaluating potential proteins as a source of antimicrobial peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4450–4479. [Google Scholar] [CrossRef] [PubMed]
- Abbas, C.A. Production of Antioxidants, Aromas, Colours, Flavours, and Vitamins by Yeasts. In Yeasts in Food and Beverages; Querol, A., Fleet, G., Eds.; Springer: Berlin, Heidelberg, 2006; Volume 10, pp. 285–334. [Google Scholar] [CrossRef]
- El Ghany, K.A.; Hamouda, R.A.; Mahrous, H.; Elhafe, E.A.; Ahmed, F.A.H.; Hamza, H.A. Description of Isolated LAB Producing β-glucan from Egyptian Sources and Evaluation of its Therapeutic Effect. Int. J. Pharmacol. 2016, 12, 801–811. [Google Scholar] [CrossRef]
- Pérez-Ramos, A.; Mohedano, M.L.; Pardo, M.; López, P. β-Glucan-Producing Pediococcus parvulus 2.6: Test of Probiotic and Immunomodulatory Properties in Zebrafish Models. Front. Microbiol. 2018, 9, 1684. [Google Scholar] [CrossRef] [PubMed]
- Hitosugi, M.; Hamada, K.; Misaka, K. Effects of Bacillus subtilis var. natto products on symptoms caused by blood flow disturbance in female patients with lifestyle diseases. Int. J. Gen. Med. 2015, 8, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.; Naraian, R.; Singh V., K. Medicinal properties of Pleurotus species (Oyster mushrooms): A review. World J. Fungal Plant Biol. 2012, 3, 1–12. [Google Scholar]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.F.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. β-Glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Yousefi, M.; Shadnoush, M.; Mortazavian, A.M. An Overview of β-Glucan Functionality in Dairy Products. Curr. Nutr. Food Sci. 2018, 14, 280–292. [Google Scholar] [CrossRef]
- Chaikliang, C.; Wichienchot, S.; Youravoug, W.; Graidist, P. Evaluation on prebiotic properties of β-glucan and oligo-β-glucan from mushrooms by human fecal microbiota in fecal batch culture. Funct. Foods Health Dis. 2015, 5, 395–405. [Google Scholar] [CrossRef]
- Lam, K.-L.; Cheung, P.C.-K. Non-digestible long chain beta-glucans as novel prebiotics. Bioact. Carbohydrates Diet. Fibre 2013, 2, 45–64. [Google Scholar] [CrossRef]
- Brennan, C.S.; Tudorica, C.M. Carbohydrate-based fat replacers in the modification of the rheological, textural and sensory quality of yoghurt: Comparative study of the utilisation of barley beta-glucan, guar gum and inulin. Int. J. Food Sci. Technol. 2008, 43, 824–833. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Oat Beta-Glucan: Its Role in Health Promotion and Prevention of Diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Belemets, T.; Radzievskaya, I.; Tochkova, O.; Yushchenko, N.; Kuzmyk, U.; Mykhalevych, A. Evaluation of oxidity resistance of milk-containing products based on blending of vegetable oils. Technol. Audit Prod. Reserv. 2021, 1, 26–33. [Google Scholar] [CrossRef]
- Polishchuk, G.; Kuzmyk, U.; Osmak, T.; Kurmach, M.; Bass, O. Analysis of the nature of the composition substances of sour-milk dessert with plant-based fillers. East. -Eur. J. Enterp. Technol. 2021, 6, 68–73. [Google Scholar] [CrossRef]
- Belemets, T.; Kuzmyk, U.; Gryshchenko, R.; Osmak, T. Determination of optimal technological parameters of obtaining stevia extract in technology of sour dairy desserts. East. -Eur. J. Enterp. Technol. 2022, 4, 60–67. [Google Scholar] [CrossRef]
- Bandini, L.G.; Vu, D.; Must, A.; Cyr, H.; Goldberg, A.; Dietz, W.H. Comparison of High-Calorie, Low-Nutrient-Dense Food Consumption among Obese and Non-Obese Adolescents. Obes. Res. 1999, 7, 438–443. [Google Scholar] [CrossRef]
- Sapiga, V.; Polischuk, G.; Osmak, T.; Mykhalevych, A.; Maslikov, M. Scientific explanation of the composition and technological modes of manufacture of dairy ice cream with vegetable puree. Ukr. J. Food Sci. 2019, 7, 83–91. [Google Scholar] [CrossRef]
- Akbari, M.; Eskandari, M.H.; Davoudi, Z. Application and functions of fat replacers in low-fat ice cream: A review. Trends Food Sci. Technol. 2019, 86, 34–40. [Google Scholar] [CrossRef]
- Venables, A.; Frangella, J.; Poulterer, B.; Ruszkay, T. Frozen desserts and methods for manufacture thereof. U.S. Patent 20,070,098,868 A1, 3 May 2007. [Google Scholar]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; Salas-de la Cruz, D.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Aljewicz, M.; Florczuk, A.; Dąbrowska, A. Influence of β-Glucan Structures and Contents on the Functional Properties of Low-Fat Ice Cream During Storage. Pol. J. Food Nutr. Sci. 2020, 70, 233–240. [Google Scholar] [CrossRef]
- Fan, R.; Zhou, D.; Cao, X. Evaluation of oat β-glucan-marine collagen peptide mixed gel and its application as the fat replacer in the sausage products. PLoS ONE 2020, 15, e0233447. [Google Scholar] [CrossRef]
- BahramParvar, M.; Tehrani, M.M. Application and Functions of Stabilizers in Ice Cream. Food Rev. Int. 2011, 27, 389–407. [Google Scholar] [CrossRef]
- Kurek, M.A.; Wyrwisz, J.; Wierzbicka, A. Optimization of beta-glucan and water content in fortified wheat bread using Response Surface Methodology according to staling kinetics. LWT 2017, 75, 352–357. [Google Scholar] [CrossRef]
- Lazaridou, A.; Vaikousi, H.; Biliaderis, C. Effects of polyols on cryostructurization of barley β-glucans. Food Hydrocoll. 2008, 22, 263–277. [Google Scholar] [CrossRef]
- Abdel-Haleem, A.M.H.; Awad, R.A. Some quality attributes of low fat ice cream substituted with hulless barley flour and barley ß-glucan. J. Food Sci. Technol. 2015, 52, 6425–6434. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Rehman, S.; Zubair, H.; Saeed, H.M.; Kousar, S.; Shahid, M. Effect of Skim Milk in Soymilk Blend on the Quality of Ice Cream. Pak. J. Nutr. 2003, 2, 305–311. [Google Scholar] [CrossRef]
- Su, L.-W.; Cheng, Y.-H.; Hsiao, F.S.-H.; Han, J.-C.; Yu, Y.-H. Optimization of Mixed Solid-state Fermentation of Soybean Meal by Lactobacillus Species and Clostridium butyricum. Pol. J. Microbiol. 2018, 67, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.; Kamińska-Dwórznicka, A.; Galus, S.; Jakubczyk, E. Effects of Different Ingredients and Stabilisers on Properties of Mixes Based on Almond Drink for Vegan Ice Cream Production. Sustainability 2021, 13, 12113. [Google Scholar] [CrossRef]
- Burkus, Z.; Temelli, F. Stabilization of emulsions and foams using barley β-glucan. Food Res. Int. 2000, 33, 27–33. [Google Scholar] [CrossRef]
- Shukla, T.; Halpem, G. Ice creams comprising emulsified liquid shortening compositions comprising dietary fiber gel, water and lipid. WIPO Patent WO 2005046357 A1, 29 June 2005. [Google Scholar]
- Casas-Arrojo, V.; Decara, J.; de Los Ángeles Arrojo-Agudo, M.; Pérez-Manríquez, C.; Abdala-Díaz, R.T. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (S.F.Gray) Nägeli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Cebreros, A.H.; Ibarra-Castro, L.; Martínez-Brown, J.M.; Velasco-Blanco, G.; Martínez-Téllez M., A.; Medina-Jasso M., A.; Nieves-Soto, M.; Quintana-Zavala, D. Potential of Nannochloropsis in beta glucan production. In Nannochloropsis: Biology, Biotechnological Potential and Challenges; Nova Science Publishers: New York, NY, USA, 2017; pp. 181–225. [Google Scholar]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef]
- Wong, J.F.; Hong, H.J.; Foo, S.C.; Yap, M.K.K.; Tan, J.W. A review on current and future advancements for commercialized microalgae species. Food Sci. Hum. Wellness 2022, 11, 1156–1170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).