Abstract
We assessed the efficacy of the lung immune prognostic index (LIPI) in predicting the progression of pathological T3 renal cell carcinoma (RCC). The LIPI scores of patients with pathological T3 RCC were calculated in the pre- and post-operative phases. Patients were divided into zero-point, one-point, and two-point groups according to their LIPI score and into the upstage and non-upstage groups according to the pre- and post-operative increase in LIPI score. Overall survival (OS) was evaluated using Kaplan–Meier curves stratified by group. Univariate and multivariate analyses of OS were performed via Cox proportional hazard regression analysis. LIPI scores were calculated in 80 patients wherein blood sampling data were available. The upstage and non-upstage groups comprised eight and seventy-two patients, respectively. Kaplan–Meier curves showed a significant difference in the pre- to post-operative LIPI score upstage group. LIPI score change was a poor prognostic factor using univariate analysis (OS: hazard ratio (HR) = 4.10, 95% confidence interval (CI) = 1.07–15.61, p = 0.038) and multivariate analysis (OS: HR = 4.38, 95% CI = 1.13–16.89, p = 0.031). An increase in the LIPI score in the pre-operative phase was a poor prognostic factor for pathological T3 RCC.
1. Introduction
The advent of immune checkpoint inhibitors (ICIs) for cancer treatment represents a major shift in the chemotherapy era. They have resulted in significant changes in treatment regimens since the early days of chemotherapy. ICIs have been used for treating a wide range of cancers; numerous studies have been conducted to identify the factors that predict their efficacy in different cancer types. The lung immune prognostic index (LIPI), which was a score calculated by combining two blood-based parameters—derived neutrophil-to-lymphocyte ratio (dNLR) and lactate dehydrogenase (LDH)—has been studied as a predictor of patient responses to ICIs in non-small cell lung cancer cases [1,2,3,4,5]. Studies on LIPI have also been conducted for other cancer types, including renal cell carcinoma (RCC), to assess the predictive value of ICI in terms of response, overall survival (OS), and recurrence-free survival (RFS) [6,7,8,9].
Adjuvant treatment is now available for managing patients with RCC who are considered to be at a high risk of recurrence, as indicated by the KEYNOTE 564 trial [10,11,12,13,14,15]. Patients diagnosed with pathological T3 (pT3) have worse prognoses than those with T1 and T2, thus requiring more careful monitoring [16]. However, there are currently no tumor markers that reflect the disease status of RCC. The use of ICIs also exposes the patient to the risk of developing side effects. If serious side effects occur, there is a risk that the choice of treatment options at the time of relapse will be limited. We have therefore focused on LIPI score as a means of selecting patients for whom adjuvant treatment is more likely to be recommended and investigated whether changes in LIPI scores could be used to predict the progression of pT3 RCC.
In this study, we evaluated the pre- and post-operative LIPI scores in patients with pT3 RCC as well as changes in LIPI scores between the two periods. We also assessed whether LIPI scores and their degree of change could be associated with disease progression.
2. Methods
2.1. Patients
From January 2014 to July 2024, 545 patients with suspected renal cell carcinoma underwent surgical treatment at Jichi Medical University Hospital. In terms of surgical technique, partial or total resection, laparoscopic surgery, open surgery, and robotic surgery were selected according to tumor size and patient background. Of these, 85 patients diagnosed with pT3 were listed and 80 patients with appropriate blood samples were included (thirty-nine, thirty, six, and five patients who underwent open total nephrectomy, laparoscopic total nephrectomy, robot-assisted partial nephrectomy, and robot-assisted total nephrectomy, respectively). This study was a single-center, retrospective study.
2.2. Ethical Considerations
This study was approved by the Institutional Review Board of Jichi Medical University Hospital (approval no. A22-023). Written informed consent was obtained from all participants prior to their inclusion in the study.
2.3. Endpoints
The primary outcome was OS divided by the pre- and post-operative LIPI score and changes in LIPI scores between the two periods. OS was defined as the time from surgery until death.
2.4. Calculation of LIPI Score
LIPI score was calculated based on the patients’ blood test results. LIPI score was assessed by two parameters: dNLR and LDH. It was assigned on the basis of a dNLR (neutrophils/(leukocytes–neutrophils)) of >3 according to the cutoff used in the largest study published to date on ICI use in patients with cancer. The upper limit of LDH was 222 IU/L [1,17]. This LDH cutoff value was adopted by our institution, similar to what has been used in previous studies [6,18]. The LIPI score was assigned as follows: 2 points, dNLR > 3 and LDH > 222 U/L; 1 point, dNLR > 3 or LDH > 222 U/L; and 0 points, neither criterion met. Based on the LIPI score, the patients were stratified into three groups: the zero-point, one-point, and two-point groups.
2.5. Data Collection
The LIPI score calculated from each patient’s pre-operative blood sample (taken within one month pre-operatively) was defined as the pre-operative score, whereas the score calculated using the patient’s post-operative blood sample (taken within three months post-operatively) was defined as the post-operative score (Figure 1). The group was divided into two groups by LIPI score, one with zero points and the other with one or two points. Changes in LIPI scores between the pre- to post-operative scores were calculated and classified into two groups: upstage (0–1, 0–2, and 1–2) and non-upstage (other patterns).
Figure 1.
Timing of blood sampling used to calculate the patients’ LIPI scores. LIPI: lung immune prognostic index.
2.6. Statistical Analysis
The OS between pre- and post-operative LIPI scores and between the upstage and non-upstage groups were assessed using the Kaplan–Meier method and compared via the log-rank test. The associations of OS with clinical parameters, including pathology results and LIPI score changes, were assessed using Cox’s proportional hazard regression analysis. The parameters included pre- and post-operative LIPI scores, as well as the changes thereof. The Fuhrman and WHO/International Society of Urological Pathology (ISUP) classifications were divided into grades 3/4 and others. Pre- and post-operative levels of serum C-reactive protein (CRP), which is considered a prognostic predictor of RCC, were also included with a cutoff of 0.5 [19,20,21]. Regarding univariate comparisons, categorical data were compared using the Chi-squared test; continuous data were compared using the Mann–Whitney U test. All statistical analyses were performed using JMP Pro, version 17 (SAS Institute, Cary, NC, USA). Statistical significance was set at p < 0.05.
3. Results
The patients’ background characteristics are presented in Table 1 (n = 80). None were treated with pre-operative chemotherapy; nine cases were treated with pembrolizumab as a post-operative adjuvant therapy.

Table 1.
Characteristics of the patient population (n = 80).
The LIPI scores and cases at the pre- and post-operative phases are shown in Figure 2. Pre- and post-operative dNLR, LDH, and LIPI scores for all cases are shown in Table S1. The pre-operative LIPI scores were 0 points in sixty cases, 1 point in sixteen cases, and 2 points in four cases. The post-operative LIPI scores were 0 points in sixty-seven cases, 1 point in eleven cases, and 2 points in two cases. The change in LIPI score from the pre- to post-operative periods corresponded to eight cases in the upstage group and 72 in the non-upstage group; the distribution of pre- and post-operative LIPI scores in each group is presented in Table S2. The distribution of post-operative LIPI scores showed statistically significant differences, with a trend towards higher LIPI scores in the upstage group. In contrast, the non-upstage group had a higher proportion of cases of pre-operative LIPI scores of 2 points.
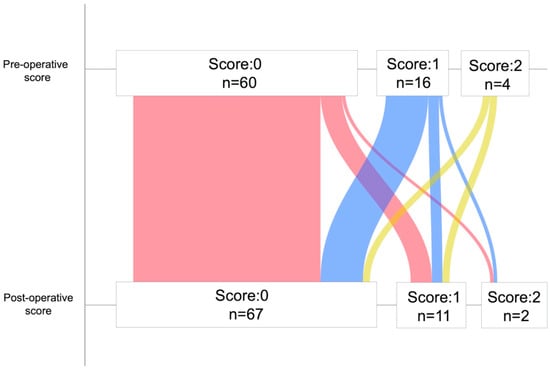
Figure 2.
Number of scores and cases at the pre-operative phase and post-operative phase.
The OS was assessed using the Kaplan–Meier method; the pre- and post-operative LIPI scores as well as the change in LIPI score pre- to post-operative in two groups were compared using the log-rank test. The median OS for the pre-operative LIPI score was not reached in the 0-point group (95% confidence interval (CI) = 104—not reached (NR)) and the 1 point and 2-point groups (95% CI = 47—NR) (Figure 3A). The median OS was also NR in the 0 point group (95% CI = 104—NR) and the 1-point and 2-point groups (95% CI = 12—NR) in terms of post-operative LIPI score (Figure 3B). Considering the pre- to post-operative period, there were eight cases in the upstage group and seventy-two in the non-upstage group in terms of LIPI score change. The median OS was NR in the upstage group (95% CI = 104—NR) and non-upstage group (95% CI = 12—NR) (Figure 3C).
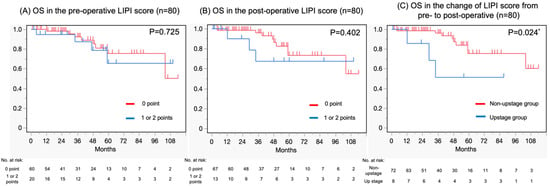
Figure 3.
Kaplan–Meier curves depicting (A) OS in the pre-operative LIPI score, (B) OS in the post-operative LIPI score, and (C) OS in the change of LIPI score pre- to post-operative. OS, overall survival; LIPI, lung immune prognostic index. * Statistical significance.
The log-rank test was used for evaluating the difference in OS between the two groups; no significant difference was found in pre-operative LIPI (zero points vs. one and two points) (p = 0.725), nor was a significant difference found in post-operative LIPI (zero points vs. one and two points) (p = 0.402). On the other hand, there was a statistically significant difference in the change in LIPI score from pre- to post-surgery (upstage group vs. non-upstage group) (p = 0.024).
Univariate and multivariate analyses of OS were performed using Cox proportional hazard regression (Table 2). Regarding OS, our univariate analysis identified sarcomatoid change and LIPI score change (pre- to post-operative) as poor prognostic factors. Our multivariate analysis of these factors identified both LIPI score change (pre- to post-operative) (hazard ratio (HR) = 4.38, 95% CI = 1.13–16.89, p = 0.031) and sarcomatoid change (HR = 8.71, 95% CI = 1.64–46.19, p = 0.010) as independent factors for poor prognosis.

Table 2.
Univariate and multivariate Cox proportional hazard regression analyses of OS in the study population (n = 80).
4. Discussion
In this study, we investigated whether pre- and post-operative LIPI scores and the changes in these scores correlated with OS in patients with pT3 RCC. The change in LIPI score pre- to post-operative was found to highly correlate with OS. The results suggested that the change in LIPI scores could be used to predict the course of the disease, as well as a pathological diagnosis.
LIPI was first reported to represent a predictor of patient responses to ICIs, mainly in patients with non-small-cell lung cancer [1]. Its prognostic value in terms of ICIs has since been demonstrated in other cancers as well [6], including RCC [22,23] (Table S3). Several studies have reported an association between LIPI score and both ICI and tyrosine kinase inhibitors in metastatic RCC cases [24,25,26]. Although the literature on LIPI is scattered and suggests an association with renal cancer progression and response to drug treatment, the number of reports examining changes associated with surgical treatment is limited. Therefore, this study examined how the LIPI score changes as a result of surgery.
The dNLR of LIPI has recently been studied in various ways. Although the neutrophil-to-leukocyte ratio (NLR) appears to represent a prognostic factor in patients with non-small cell lung carcinoma, it may be even more relevant than NLR because it includes monocytes and other granulocyte subpopulations [27,28]. High dNLR is associated with shorter survival times in patients with several tumor types—including melanoma, as well as pancreatic, bladder, and kidney cancer [17,29,30,31].
A number of studies have examined LIPI scores in blood test results at single points in time, such as before surgery or after ICI treatment. However, this study focused on how LIPI scores changed over the course of the RCC disease. This is because we hypothesized that the blood test results do not necessarily reflect the tumor environment only at the particular time point of sampling. Infections, inflammation, hematologic disorders, and oral steroid use, for instance, can also cause elevated white blood cell counts. We therefore suggest that performing multiple tests to make more accurate diagnoses is important. In this study, we evaluated the association with OS using only pre- and post-operative LIPI scores; however, no significant results were found. This study was also validated with respect to progression-free survival (PFS), without showing statistical significance in pre- and post-operative LIPI scores or in pre- and post-operative LIPI score changes. This difference in OS and PFS outcomes could be due to differences in treatment intervention and efficacy or owing to the site of recurrence.
This study only investigated LIPI scores in pT3 RCC. However, we recognize that other reports have evaluated this parameter in RCCs of the ≥pT3, N1-2, and M0 classifications as well—all of which are considered high risk [32]. In the KEYNOTE 564 trial, pT3 RCC was considered appropriate for treatment via adjuvant therapy, owing to its high risk of recurrence [10,11,12]. Several reports have shown that RCC with a pathological diagnosis of T3 has a worse prognosis compared with the T1 and T2 types [30,31,32]. Considering that T4 RCC cases are exceedingly rare, this study only included cases of RCC with pathological diagnoses of T3 following surgical treatment. In real clinical situations, how treatment is managed for patients with T3 renal cancer is very important. As not all patients would opt for adjuvant treatment, this study focuses on changes in the LIPI score to improve patient selection and treatment outcomes.
The lack of suitable tumor markers makes accurately monitoring RCC progression difficult. Several studies have reported that serum CRP level is associated with the progression and recurrence of RCC [33,34,35]. This present study also evaluated serum CRP levels in terms of OS using univariate analyses. Using univariate analyses, pre- and post-operative CRP was not an independent predictor of poor prognosis. In clinical practice, CRP level can be influenced by a number of factors; consequently, it is not considered to accurately reflect early-stage tumor effects. Therefore, monitoring disease progression using several prognostic markers over multiple time points is important.
This study has a few limitations that are worth noting. Selection bias might have occurred because the data were retrospectively obtained from a single center. In this study, the pathology of RCC was classified as clear cell type or other, and abnormal growth patterns such as rhabdoid changes and necrosis, which can impact prognosis, were not considered [36]. Both the Fuhrman and ISUP grading systems were used for nuclear grading. Although one of these systems is standardized, the system used at the time of diagnosis was also considered so as to reduce the burden on the pathologists [37].
5. Conclusions
In this study, patients with pT3 RCC wherein the LIPI scores were elevated from the pre-operative phase were found to generally have poor disease prognoses. There were no specific tumor markers for RCC, making accurately monitoring the microenvironment of this tumor type difficult. Monitoring changes in LIPI scores might thereby help manage the treatment course in patients with this deadly malignancy and might also be useful for selecting patients to whom more adjuvant therapy should be given.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol32060335/s1, Table S1: The results of dNLR, LDH and LIPI (pre- and post-operatively) in 80 patients and the change in LIPI scores; Table S2: Pre- and post-operative LIPI scores divided into upstage and non-upstage; Table S3: Some of the previous studies on LIPI and their results.
Author Contributions
H.H. and T.S. contributed to the study conception and design, data analysis and interpretation, and drafting of the first version of the manuscript. M.K., E.-i.T., and S.A. contributed to the data acquisition and helped draft the manuscript. H.K. and T.F. supervised the study, helped draft the manuscript, and critically revised it for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the institutional review board of Jichi Medical University Hospital (approval no. A22-023, 20230123) and conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Informed Consent Statement
Written informed consent was obtained from all of the patients prior to their participation in the study.
Data Availability Statement
The dataset analyzed in this study is available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ICIs | Immune checkpoint inhibitors |
| LIPI | Lung Immune Prognostic Index |
| RCC | Renal cell carcinoma |
| OS | Overall survival |
| pT3 | Pathological T3 |
| dNLR | Derived neutrophil-to-leukocyte ratio |
| NLR | Neutrophil-to-leukocyte ratio |
| LDH | Lactate dehydrogenase |
| ISUP | International Society of Urological Pathology |
| CRP | C-reactive protein |
| CI | Confidence interval |
| NR | Not reached |
| HR | Hazard ratio |
References
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, A. Correlation of Lung Immune Prognostic Index with Efficacy of PD-1/PD-L1 Inhibitor Combined with Chemotherapy and Prognosis in Patients with Advanced Non-Small Cell Lung Cancer. Am. J. Clin. Oncol. 2023, 46, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Auclin, E.; Roulleaux Dugage, M.; Gorria, T.; Vauchier, C.; Laguna, J.; Lupinacci, L.; Crous, C.; Naigeon, M.; Oudard, S.; Besse, B.; et al. Validation of the Lung Immune Prognostic Index in patients with untreated advanced non-small cell lung cancer: Post hoc analysis of the IMpower 130, 131 and 150 trials. Lung Cancer 2025, 199, 108039. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Sun, S.; Lu, H.; Wu, J.; Wu, J.; Wu, Z.; Xi, J.; Liao, W.; Wang, Y. Neutrophil-to-lymphocyte ratio-based prognostic score can predict outcomes in patients with advanced non-small cell lung cancer treated with immunotherapy plus chemotherapy. BMC Cancer 2025, 25, 697. [Google Scholar] [CrossRef]
- Herranz-Bayo, E.; Chara-Velarde, L.; Cassinello-Espinosa, J.; Gimeno-Ballester, V.; Artal-Cortés, A.; Moratiel-Pellitero, A.; Alcácera-López, A.; Navarro-Expósito, F.; Riesco-Montes, B.; Clemente-Andujar, M. Lung immune prognostic index (LIPI) as a prognostic factor in patients with extensive-stage small cell lung cancer treated with first-line chemoimmunotherapy. Clin. Transl. Oncol. 2025, 27, 1484–1492. [Google Scholar] [CrossRef]
- Meyers, D.E.; Stukalin, I.; Vallerand, I.A.; Lewinson, R.T.; Suo, A.; Dean, M.; North, S.; Pabani, A.; Cheng, T.; Heng, D.Y.C.; et al. The Lung Immune Prognostic Index Discriminates Survival Outcomes in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors. Cancers 2019, 11, 1713. [Google Scholar] [CrossRef]
- Pierro, M.; Baldini, C.; Auclin, E.; Vincent, H.; Varga, A.; Martin Romano, P.; Vuagnat, P.; Besse, B.; Planchard, D.; Hollebecque, A.; et al. Predicting Immunotherapy Outcomes in Older Patients with Solid Tumors Using the LIPI Score. Cancers 2022, 14, 5078. [Google Scholar] [CrossRef]
- Sun, T.; Guo, Y.; Sun, B.; Chen, L.; Ren, Y.; Zhu, L.; Zhang, L.; Liu, Y.; Zheng, C. Association of the pretreatment lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced hepatocellular carcinoma. Eur. J. Med. Res. 2023, 28, 225. [Google Scholar] [CrossRef]
- Carril, L.; Colomba, E.; Romero-Ferreiro, C.; Cerbone, L.; Ratta, R.; Barthélémy, P.; Vindry, C.; Cherifi, F.; Boughalem, E.; Linassier, C.; et al. Efficacy of first-line combination therapy in metastatic renal cell carcinoma (mRCC) patients (pts) with poor performance status (PS). J. Clin. Oncol. 2022, 40 (Suppl. 6), 320. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sarwar, N.; et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Chang, Y.H.; Lee, J.L.; Sarwar, N.; et al. Overall survival with adjuvant pembrolizumab in renal-cell carcinoma. N. Engl. J. Med. 2024, 390, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Gurney, H.; Chang, Y.H.; Lee, J.L.; et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Chen, Y.; Wang, L.; Panian, J.; Dhanji, S.; Derweesh, I.; Rose, B.; Bagrodia, A.; McKay, R. Treatment Landscape of Renal Cell Carcinoma. Curr. Treat. Options Oncol. 2023, 24, 1889–1916. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sarwar, N.; et al. Pembrolizumab as post nephrectomy adjuvant therapy for patients with renal cell carcinoma: Results from 30-month follow-up of KEYNOTE-564. J. Clin. Oncol. 2022, 40 (Suppl. 6), 290. [Google Scholar] [CrossRef]
- Ding, K.; Yang, Z.; Zhang, D.; Sun, L. Efficacy Assessment of Post-nephrectomy Adjuvant Therapies in Patients with Renal Cell Carcinoma. Ann. Surg. Oncol. 2024, 31, 3894–3905. [Google Scholar] [CrossRef]
- Patel, S.H.; Uzzo, R.; Larcher, A.; Peyronnet, B.; Lane, B.; Pruthi, D.; Reddy, M.; Capitanio, U.; Joshi, S.; Noyes, S.; et al. Oncologic and Functional Outcomes of Radical and Partial Nephrectomy in pT3a Pathologically Upstaged Renal Cell Carcinoma: A Multi-institutional Analysis. Clin. Genitourin. Cancer 2020, 18, e723–e729. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Ascierto, P.A.; Pigozzo, J.; Del Vecchio, M.; Maio, M.; Antonini Cappellini, G.C.; Guidoboni, M.; Queirolo, P.; Savoia, P.; Mandalà, M.; et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann. Oncol. 2016, 27, 732–738. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Y.; Ye, Q.; Wang, Y.; Min, L.; Luo, Y.; Zhou, Y.; Tu, C. Lung immune prognostic index could predict metastasis in patients with osteosarcoma. Front. Surg. 2022, 9, 923427. [Google Scholar] [CrossRef]
- Carril-Ajuria, L.; Lavaud, P.; Dalban, C.; Negrier, S.; Gravis, G.; Motzer, R.J.; Chevreau, C.; Tannir, N.M.; Oudard, S.; McDermott, D.F.; et al. Validation of the Lung Immune Prognostic Index (LIPI) as a prognostic biomarker in metastatic renal cell carcinoma. Eur. J. Cancer 2024, 204, 114048. [Google Scholar] [CrossRef]
- Obayashi, K.; Miki, J.; Fukuokaya, W.; Yanagisawa, T.; Kimura, S.; Tsuzuki, S.; Kimura, T.; Egawa, S. The prognostic value of the preoperative lung immune prognostic index in patients with urothelial bladder cancer undergoing radical cystectomy. Int. J. Clin. Oncol. 2022, 27, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef]
- Carril-Ajuria, L.; Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Hammers, H.J.; Plimack, E.R.; Donskov, F.; Rini, B.I.; Jiang, R.; Lee, C.W.; et al. Prognostic value of the lung immune prognostic index in patients with untreated advanced renal cell carcinoma (aRCC) receiving nivolumab plus ipilimumab (N+I) or sunitinib (SUN) in the CheckMate 214 trial. J. Clin. Oncol. 2022, 40, 4538. [Google Scholar] [CrossRef]
- Silva, C.A.C.; Zoppi, S.; Reni, A.; Piccinno, G.; Lahmar, I.; Cerbone, L.; Carril-Ajuria, L.; Colomba, E.; Flippot, R.; Sow, C.; et al. The Lung Immune Prognostic Index (LIPI) stratifies prognostic groups and correlates with gut microbiota (GM) in patients with advanced renal cell carcinoma (RCC). J. Clin. Oncol. 2023, 41, 719. [Google Scholar] [CrossRef]
- Paramanathan, A.; Saxena, A.; Morris, D.L. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg. Oncol. 2014, 23, 31–39. [Google Scholar] [CrossRef]
- Gu, X.B.; Tian, T.; Tian, X.J.; Zhang, X.J. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: A meta-analysis. Sci. Rep. 2015, 5, 12493. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Takagi, T.; Hikichi, T.; Konno, N.; Sugimoto, M.; Watanabe, K.O.; Nakamura, J.; Waragai, Y.; Kikuchi, H.; Takasumi, M.; et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol. Lett. 2016, 11, 3441–3445. [Google Scholar] [CrossRef]
- Amato, R.J.; Flaherty, A.; Zhang, Y.; Ouyang, F.; Mohlere, V. Clinical prognostic factors associated with outcome in patients with renal cell cancer with prior tyrosine kinase inhibitors or immunotherapy treated with everolimus. Urol. Oncol. 2014, 32, 345–354. [Google Scholar] [CrossRef]
- van Kessel, K.E.M.; de Haan, L.M.; Fransen van de Putte, E.E.; van Rhijn, B.W.; de Wit, R.; van der Heijden, M.S.; Zwarthoff, E.C.; Boormans, J.L. Elevated derived neutrophil-to-lymphocyte ratio corresponds with poor outcome in patients undergoing pre-operative chemotherapy in muscle-invasive bladder cancer. Bladder Cancer 2016, 2, 351–360. [Google Scholar] [CrossRef]
- Ishiyama, Y.; Kondo, T.; Yoshida, K.; Iizuka, J.; Takagi, T. Prognostic value of the lung immune prognostic index on recurrence after radical surgery for high-risk renal cell carcinoma. Cancers 2024, 16, 776. [Google Scholar] [CrossRef]
- Veccia, A.; Falagario, U.; Martini, A.; Marchioni, M.; Antonelli, A.; Simeone, C.; Cormio, L.; Capitanio, U.; Mir, M.C.; Derweesh, I.; et al. Upstaging to pT3a in patients undergoing partial or radical nephrectomy for cT1 renal tumors: A systematic review and meta-analysis of outcomes and predictive factors. Eur. Urol. Focus. 2021, 7, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, Z.A.; Capitanio, U.; Pruthi, D.; Ghali, F.; Larcher, A.; Patel, D.N.; Eldefrawy, A.; Patel, S.; Cotta, B.H.; Bradshaw, A.W.; et al. Risk factors for upstaging, recurrence, and mortality in clinical T1-2 renal cell carcinoma patients upstage to pT3a disease: An international analysis utilizing the 8th edition of the tumor-node-metastasis staging criteria. Urology 2020, 138, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.M.; Lebastchi, A.H.; Chipollini, J.; Niemann, A.; Mehra, R.; Morgan, T.M.; Miller, D.C.; Palapattu, G.S.; Hafez, K.S.; Sexton, W.; et al. Multi-institutional survival analysis of incidental pathologic T3a upstaging in clinical T1 renal cell carcinoma following partial nephrectomy. Urology 2018, 117, 95–100. [Google Scholar] [CrossRef]
- Komai, Y.; Saito, K.; Sakai, K.; Morimoto, S. Increased preoperative serum C-reactive protein level predicts a poor prognosis in patients with localized renal cell carcinoma. BJU Int. 2007, 99, 77–80. [Google Scholar] [CrossRef]
- De Martino, M.; Klatte, T.; Seemann, C.; Waldert, M.; Haitel, A.; Schatzl, G.; Remzi, M.; Weibl, P. Validation of serum C-reactive protein (CRP) as an independent prognostic factor for disease-free survival in patients with localised renal cell carcinoma (RCC). BJU Int. 2013, 111, E348–E353. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, Y.; Kondo, T.; Ishihara, H.; Yoshida, K.; Iizuka, J.; Tanabe, K.; Takagi, T. C-reactive protein kinetics to predict recurrence of high-risk renal cell carcinoma after radical surgery. Int. J. Clin. Oncol. 2022, 27, 969–976. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Cheville, J.C.; Thompson, R.H.; Lohse, C.M.; Boorjian, S.A.; Leibovich, B.C.; Costello, B.A. Impact of rhabdoid differentiation on prognosis for patients with Grade 4 renal cell carcinoma. Eur. Urol. 2015, 68, 5–7. [Google Scholar] [CrossRef]
- Kim, H.; Inomoto, C.; Uchida, T.; Furuya, H.; Komiyama, T.; Kajiwara, H.; Kobayashi, H.; Nakamura, N.; Miyajima, A. Verification of the International Society of Urological Pathology recommendations in Japanese patients with clear cell renal cell carcinoma. Int. J. Oncol. 2018, 52, 1139–1148. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).