Evaluating the Cost-Effectiveness of Cervical Cancer Screening and Treatment in Western Romania
Abstract
1. Introduction
2. Materials and Methods
- Squamous carcinoma (which accounts for about 85% of all cases).
- Adenocarcinoma (which accounts for about 10–15% of all cases).
The Artificial Intelligence Algorithms
3. Results
3.1. Data Analysis and Statistics
3.2. Artificial Intelligence
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asandului, L.; Caunic, R.-E.; Coţofrei, P. Assessment of the Efficiency of Public Hospitals in Romania. Sci. Ann. Econom. Bus. 2023, 7, 567–583. [Google Scholar] [CrossRef]
- Petre, I.; Barna, F.; Gurgus, D.; Tomescu, L.C.; Apostol, A.; Petre, I.; Furau, C.; Năchescu, M.L.; Bordianu, A. Analysis of the Healthcare System in Romania: A Brief Review. Healthcare 2023, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Mosadeghrad, A.M. Factors Influencing Healthcare Service Quality. Int. J. Health Policy Manag. 2014, 3, 77–89. [Google Scholar] [CrossRef]
- Cohn, D.E. Surgery or Chemoradiation for Stage IB Cervical Cancer? How Cost Effectiveness Impacts a Complex Decision. J. Gynecol. Oncol. 2014, 25, 79. [Google Scholar] [CrossRef][Green Version]
- Shiroiwa, T.; Shimozuma, K.; Fukuda, T. Treatment Costs for Breast Cancer in Japan: Large Claim Database Analysis. Value Health 2014, 17, A735. [Google Scholar] [CrossRef]
- Zhou, Y.; Rassy, E.; Coutte, A.; Achkar, S.; Espenel, S.; Genestie, C.; Pautier, P.; Morice, P.; Gouy, S.; Chargari, C. Current Standards in the Management of Early and Locally Advanced Cervical Cancer: Update on the Benefit of Neoadjuvant/Adjuvant Strategies. Cancers 2022, 14, 2449. [Google Scholar] [CrossRef]
- Boncz, I.; Endrei, D.; Ágoston, I.; Kovács, G.; Vajda, R.; Csákvári, T.; Sebestyén, A. Annual Health Insurance Cost of Breast Cancer Treatment in Hungary. Value Health 2014, 17, A735. [Google Scholar] [CrossRef][Green Version]
- Radu, C.-P.; Pana, B.C.; Pele, D.T.; Costea, R.V. Evolution of Public Health Expenditure Financed by the Romanian Social Health Insurance Scheme From 1999 to 2019. Front. Public Health 2021, 9, 795869. [Google Scholar] [CrossRef]
- Todor, R.D.; Bratucu, G.; Moga, M.A.; Candrea, A.N.; Marceanu, L.G.; Anastasiu, C.V. Challenges in the Prevention of Cervical Cancer in Romania. Int. J. Environ. Res. Public Health 2021, 18, 1721. [Google Scholar] [CrossRef]
- Arbyn, M.; Antoine, J.; Mägi, M.; Smailyte, G.; Stengrevics, A.; Suteu, O.; Valerianova, Z.; Bray, F.; Weiderpass, E. Trends in Cervical Cancer Incidence and Mortality in the Baltic Countries, Bulgaria and Romania. Int. J. Cancer 2011, 128, 1899–1907. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global Cancer Statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Chen, L.; Melamed, A.; Tergas, A.I.; Khoury-Collado, F.; Hou, J.Y.; Clair, C.M.S.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; et al. Cost of Care for the Initial Management of Cervical Cancer in Women with Commercial Insurance. Am. J. Obstet. Gynecol. 2021, 224, e1–e286. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.-L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses 2023, 15, 1440. [Google Scholar] [CrossRef]
- Liang, L.A.; Tanaka, L.F.; Radde, K.; Bussas, U.; Ikenberg, H.; Heideman, D.A.M.; Meijer, C.J.L.M.; Blettner, M.; Klug, S.J. Population-Based Age- and Type-Specific Prevalence of Human Papillomavirus among Non-Vaccinated Women Aged 30 Years and above in Germany. BMC Infect. Dis. 2024, 24, 1008. [Google Scholar] [CrossRef] [PubMed]
- Kedzia, W.; Józefiak, A.; Pruski, D.; Rokita, W.; Marek, S. Human papilloma virus genotyping in women with CIN 1. Ginekol. Pol. 2010, 81, 664–667. [Google Scholar]
- Ring, L.L.; Munk, C.; Galanakis, M.; Tota, J.E.; Thomsen, L.T.; Kjaer, S.K. Incidence of Cervical Precancerous Lesions and Cervical Cancer in Denmark from 2000 to 2019: Population Impact of Multi-cohort Vaccination against Human Papillomavirus Infection. Int. J. Cancer 2023, 152, 1320–1327. [Google Scholar] [CrossRef]
- Berkhof, J.; Bogaards, J.A.; Demirel, E.; Diaz, M.; Sharma, M.; Kim, J.J. Cost-Effectiveness of Cervical Cancer Prevention in Central and Eastern Europe and Central Asia. Vaccine 2013, 31, H71–H79. [Google Scholar] [CrossRef]
- Jewell, E.L.; Smrtka, M.; Broadwater, G.; Valea, F.; Davis, D.M.; Nolte, K.C.; Valea, R.; Myers, E.R.; Samsa, G.; Havrilesky, L.J. Utility Scores and Treatment Preferences for Clinical Early-Stage Cervical Cancer. Value Health 2011, 14, 582–586. [Google Scholar] [CrossRef]
- Kim, K.; Kang, S.B.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S. Comparison of Chemoradiation with Radiation as Postoperative Adjuvant Therapy in Cervical Cancer Patients with Intermediate-Risk Factors. Eur. J. Surg. Oncol. (EJSO) 2009, 35, 192–196. [Google Scholar] [CrossRef]
- Yen, C.-H.; Yang, C.-C.; Ho, S.-Y.; Lee, S.-W.; Chen, C.-C.; Shieh, L.-T. Impact of Adjuvant Hysterectomy for Bulky Cervical Cancer after Definitive Chemoradiotherapy with Insufficient Brachytherapy Dose: A Retrospective Analysis. Ther. Radiol. Oncol. 2023, 7, 3. [Google Scholar] [CrossRef]
- Li, M.; Hu, M.; Wang, Y.; Yang, X. Adjuvant Chemoradiotherapy versus Radiotherapy in Cervical Cancer Patients with Intermediate-Risk Factors: A Systematic Review and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 238, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, I.M.; Legianawati, D.; Sinuraya, R.K.; Suwantika, A.A. Cost-Effectiveness Analysis of Chemoradiation and Radiotherapy Treatment for Stage IIB and IIIB Cervical Cancer Patients. Int. J. Women’s Health 2021, 13, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, G.M.; Lauer, J.A.; Zelle, S.; Baeten, S.; Baltussen, R. Cost Effectiveness of Strategies to Combat Breast, Cervical, and Colorectal Cancer in Sub-Saharan Africa and South East Asia: Mathematical Modelling Study. BMJ 2012, 344, e614. [Google Scholar] [CrossRef]
- Chai, Y.L.; Shi, F.; Wang, J.; Su, J.; Yang, Y.; Ma, H.; Liu, Z.; Wang, T.; Wang, J.; Zhou, X.; et al. Cost-Effectiveness of Radical Hysterectomy with Adjuvant Radiotherapy versus Radical Radiotherapy for FIGO Stage IIB Cervical Cancer. OncoTargets Ther. 2016, 9, 349–354. [Google Scholar] [CrossRef]
- Hillner, B.E.; Smith, T.J. Efficacy and Cost Effectiveness of Adjuvant Chemotherapy in Women with Node-Negative Breast Cancer: A Decision-Analysis Model. N. Engl. J. Med. 1991, 324, 160–168. [Google Scholar] [CrossRef]
- Aswathy, S.; Quereshi, M.A.; Kurian, B.; Leelamoni, K. Cervical Cancer Screening: Current Knowledge & Practice among Women in a Rural Population of Kerala, India. Indian J. Med. Res. 2012, 136, 205–210. [Google Scholar]
- Bruni, L.; Serrano, B.; Roura, E.; Alemany, L.; Cowan, M.; Herrero, R.; Poljak, M.; Murillo, R.; Broutet, N.; Riley, L.M.; et al. Cervical Cancer Screening Programmes and Age-Specific Coverage Estimates for 202 Countries and Territories Worldwide: A Review and Synthetic Analysis. Lancet Glob. Health 2022, 10, e1115–e1127. [Google Scholar] [CrossRef]
- Screening for Cervical Cancer. CA Cancer J. Clin. 2020, 70, 347–348. [CrossRef]
- Wu, Z.; Li, T.; Han, Y.; Jiang, M.; Yu, Y.; Xu, H.; Yu, L.; Cui, J.; Liu, B.; Chen, F.; et al. Development of Models for Cervical Cancer Screening: Construction in a Cross-Sectional Population and Validation in Two Screening Cohorts in China. BMC Med. 2021, 19, 197. [Google Scholar] [CrossRef]
- Kojalo, U.; Tisler, A.; Parna, K.; Kivite-Urtane, A.; Zodzika, J.; Stankunas, M.; Baltzer, N.; Nygard, M.; Uuskula, A. An Overview of Cervical Cancer Epidemiology and Prevention in the Baltic States. BMC Public Health 2023, 23, 660. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Mittal, S.; Mandal, R.; Basu, P. Screening Technologies for Cervical Cancer: Overview. Cytojournal 2022, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Mitteldorf, C.A.T.S. Cervical Cancer Screening: From Pap Smear to Future Strategies. J. Bras. Patol. Med. Lab. 2016, 52, 238–245. [Google Scholar] [CrossRef]
- Rodriguez, J.; Viveros-Carreño, D.; Pareja, R. Adjuvant Treatment after Radical Surgery for Cervical Cancer with Intermediate Risk Factors: Is It Time for an Update? Int. J. Gynecol. Cancer 2022, 32, 1219–1226. [Google Scholar] [CrossRef]
- Agosti, J.M.; Goldie, S.J. Introducing HPV Vaccine in Developing Countries—Key Challenges and Issues. N. Engl. J. Med. 2007, 356, 1908–1910. [Google Scholar] [CrossRef]
- Boutayeb, A.; Boutayeb, S. The Burden of Non Communicable Diseases in Developing Countries. Int. J. Equity Health 2005, 4, 2. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. Human Papillomavirus and Related Diseases in the World. Summary Report; ICO: Barcelona, Spain; IARC: Lyon, France, 2023. [Google Scholar]
- Cancer Today. World Health Organization 2023. Available online: https://gco.iarc.fr/today/data-sources-methods (accessed on 11 April 2023).
- Hou, X.; Shen, G.; Zhou, L.; Li, Y.; Wang, T.; Ma, X. Artificial Intelligence in Cervical Cancer Screening and Diagnosis. Front. Oncol. 2022, 12, 851367. [Google Scholar] [CrossRef]
- Mercioni, M.A.; Holban, Ş.; Dumache, R. Novel Activation Function Design for Multiple Sclerosis Detection in Medical Imaging. In Proceedings of the 2024 E-Health and Bioengineering Conference (EHB), IASI, Romania, 14–15 November 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Mercioni, M.A.; Căleanu, C.D. Computer Aided Diagnosis for Contrast-Enhanced Ultrasound Using a Small Hybrid Transformer Neural Network. In Proceedings of the 2024 International Symposium on Electronics and Telecommunications (ISETC), Timisoara, Romania, 7–8 November 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Mercioni, M.A.; Holban, S. Uterine Corpus Endometrial Carcinoma Prediction from Genomic Analysis with Machine Learning. In Proceedings of the 2024 International Conference on Development and Application Systems (DAS), Suceava, Romania, 23–25 May 2024; pp. 121–126. [Google Scholar] [CrossRef]
- Mercioni, M.A.; Căleanu, C.D.; Sîrbu, C.L. Computer Aided Diagnosis for Contrast-Enhanced Ultrasound Using Transformer Neural Network. In Proceedings of the 2023 25th International Symposium on Symbolic and Numeric Algorithms for Scientific Computing (SYNASC), Nancy, France, 11–14 September 2023; pp. 256–259. [Google Scholar] [CrossRef]
- Kenner, B.; Chari, S.T.; Kelsen, D.; Klimstra, D.S.; Pandol, S.J.; Rosenthal, M.; Rustgi, A.K.; Taylor, J.A.; Yala, A.; Abul-Husn, N.; et al. Artificial Intelligence and Early Detection of Pancreatic Cancer: 2020 Summative Review. Pancreas 2021, 50, 251–279. [Google Scholar] [CrossRef]
- Bitkina, O.V.; Park, J.; Kim, H.K. Application of Artificial Intelligence in Medical Technologies: A Systematic Review of Main Trends. Digit. Health 2023, 9, 20552076231189331. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial Intelligence with Multi-Functional Machine Learning Platform Development for Better Healthcare and Precision Medicine. Database 2020, 2020, baaa010. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J.; Liu, Y.; Zhang, Y.; Liu, S.; Chaikovska, T.; Liu, C. Artificial Intelligence in Cervical Cancer Research and Applications. Acadlore Trans. AI Mach. Learn. 2023, 2, 99–115. [Google Scholar] [CrossRef]
- Viñals, R.; Jonnalagedda, M.; Petignat, P.; Thiran, J.-P.; Vassilakos, P. Artificial Intelligence-Based Cervical Cancer Screening on Images Taken during Visual Inspection with Acetic Acid: A Systematic Review. Diagnostics 2023, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Meza Ramirez, C.A.; Greenop, M.; Almoshawah, Y.A.; Martin Hirsch, P.L.; Rehman, I.U. Advancing Cervical Cancer Diagnosis and Screening with Spectroscopy and Machine Learning. Expert Rev. Mol. Diagn. 2023, 23, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ye, Q.; Yang, X.; Chen, J.; Ma, H.; Xia, J.; Del Ser, J.; Yang, G. AI-Based Medical e-Diagnosis for Fast and Automatic Ventricular Volume Measurement in Patients with Normal Pressure Hydrocephalus. Neural Comput. Applic 2023, 35, 16011–16020. [Google Scholar] [CrossRef]
- Songsaeng, D.; Nava-apisak, P.; Wongsripuemtet, J.; Kingchan, S.; Angkoondittaphong, P.; Phawaphutanon, P.; Supratak, A. The Diagnostic Accuracy of Artificial Intelligence in Radiological Markers of Normal-Pressure Hydrocephalus (NPH) on Non-Contrast CT Scans of the Brain. Diagnostics 2023, 13, 2840. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Lee, S.; Song, J.-Y.; Lee, J.-K.; Lee, N.-W. Role of Artificial Intelligence Interpretation of Colposcopic Images in Cervical Cancer Screening. Healthcare 2022, 10, 468. [Google Scholar] [CrossRef]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial Intelligence in Cancer Imaging: Clinical Challenges and Applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunović, H. Artificial Intelligence in Retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar] [CrossRef]
- Nakisige, C.; De Fouw, M.; Kabukye, J.; Sultanov, M.; Nazrui, N.; Rahman, A.; De Zeeuw, J.; Koot, J.; Rao, A.P.; Prasad, K.; et al. Artificial Intelligence and Visual Inspection in Cervical Cancer Screening. Int. J. Gynecol. Cancer 2023, 33, 1515–1521. [Google Scholar] [CrossRef]
- Lilhore, U.K.; Poongodi, M.; Kaur, A.; Simaiya, S.; Algarni, A.D.; Elmannai, H.; Vijayakumar, V.; Tunze, G.B.; Hamdi, M. Hybrid Model for Detection of Cervical Cancer Using Causal Analysis and Machine Learning Techniques. Comput. Math. Methods Med. 2022, 2022, 1–17. [Google Scholar] [CrossRef]
- Rayner, M.; Welp, A.; Stoler, M.H.; Cantrell, L.A. Cervical Cancer Screening Recommendations: Now and for the Future. Healthcare 2023, 11, 2273. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Arbyn, M.; Bergeron, C.; Bosch, F.X.; Dillner, J.; Jit, M.; Kim, J.; Poljak, M.; Nieminen, P.; Sasieni, P.; et al. Cervical Screening: ESGO-EFC Position Paper of the European Society of Gynaecologic Oncology (ESGO) and the European Federation of Colposcopy (EFC). Br. J. Cancer 2020, 123, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.L.M.; Muñoz, N. Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Khanna, N.N.; Maindarkar, M.A.; Viswanathan, V.; Fernandes, J.F.E.; Paul, S.; Bhagawati, M.; Ahluwalia, P.; Ruzsa, Z.; Sharma, A.; Kolluri, R.; et al. Economics of Artificial Intelligence in Healthcare: Diagnosis vs. Treatment. Healthcare 2022, 10, 2493. [Google Scholar] [CrossRef]
- Hendrix, N.; Veenstra, D.L.; Cheng, M.; Anderson, N.C.; Verguet, S. Assessing the Economic Value of Clinical Artificial Intelligence: Challenges and Opportunities. Value Health 2022, 25, 331–339. [Google Scholar] [CrossRef]
- Rizkya, I.; Syahputri, K.; Sari, R.M.; Siregar, I.; Utaminingrum, J. Autoregressive Integrated Moving Average (ARIMA) Model of Forecast Demand in Distribution Centre. IOP Conf. Ser. Mater. Sci. Eng. 2019, 598, 012071. [Google Scholar] [CrossRef]
- Taylor, S.J.; Letham, B. Forecasting at Scale. Am. Stat. 2018, 72, 37–45. [Google Scholar] [CrossRef]
- Linear Regression. In Springer Texts in Statistics; Springer International Publishing: Cham, Switzerland, 2022; pp. 37–79. ISBN 978-3-030-73791-7.
- Chai, T.; Draxler, R.R. Root Mean Square Error (RMSE) or Mean Absolute Error (MAE)? Geosci. Model Dev. 2014, 7, 1247–1250. [Google Scholar] [CrossRef]
- Poldrack, R.A.; Huckins, G.; Varoquaux, G. Establishment of Best Practices for Evidence for Prediction: A Review. JAMA Psychiatry 2020, 77, 534. [Google Scholar] [CrossRef]
- Hirth, J. Disparities in HPV Vaccination Rates and HPV Prevalence in the United States: A Review of the Literature. Hum. Vaccines Immunother. 2019, 15, 146–155. [Google Scholar] [CrossRef]
- Sakamoto, T.; Furukawa, T.; Lami, K.; Pham, H.H.N.; Uegami, W.; Kuroda, K.; Kawai, M.; Sakanashi, H.; Cooper, L.A.D.; Bychkov, A.; et al. A Narrative Review of Digital Pathology and Artificial Intelligence: Focusing on Lung Cancer. Transl. Lung Cancer Res. 2020, 9, 2255–2276. [Google Scholar] [CrossRef] [PubMed]
- Disease Diagnosis with Medical Imaging Using Deep Learning. In Lecture Notes in Networks and Systems; Springer International Publishing: Cham, Switzerland, 2022; pp. 198–208. ISBN 978-3-030-98014-6.
- Chiorean, D.M.; Mitranovici, M.-I.; Mureșan, M.C.; Buicu, C.-F.; Moraru, R.; Moraru, L.; Cotoi, T.C.; Cotoi, O.S.; Apostol, A.; Turdean, S.G.; et al. The Approach of Artificial Intelligence in Neuroendocrine Carcinomas of the Breast: A Next Step towards Precision Pathology?—A Case Report and Review of the Literature. Medicina 2023, 59, 672. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Cancer Detection with Popular AI Algorithms: A Brief Review. In IFMBE Proceedings; Springer Nature: Cham, Switzerland, 2024; pp. 467–475. ISBN 978-3-031-62501-5.
- Lara, H.; Li, Z.; Abels, E.; Aeffner, F.; Bui, M.M.; ElGabry, E.A.; Kozlowski, C.; Montalto, M.C.; Parwani, A.V.; Zarella, M.D.; et al. Quantitative Image Analysis for Tissue Biomarker Use: A White Paper From the Digital Pathology Association. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.; Higgins, D.; Mazo Vargas, C.; Watson, W.; Mooney, C.; Rahman, A.; Aspell, N.; Connolly, A.; Aura Gonzalez, C.; Gallagher, W. Future of Biomarker Evaluation in the Realm of Artificial Intelligence Algorithms: Application in Improved Therapeutic Stratification of Patients with Breast and Prostate Cancer. J. Clin. Pathol. 2021, 74, 429–434. [Google Scholar] [CrossRef]
- Arndt, T.; Taube, E.T.; Deubzer, H.E.; Rothe, K.; Calaminus, G.; Sehouli, J.; Pietzner, K. Management of Malignant Dysgerminoma of the Ovary. EJGO 2022, 43, 353. [Google Scholar] [CrossRef]
- Keskin, M.; Savaş-Erdeve, Ş.; Kurnaz, E.; Çetinkaya, S.; Karaman, A.; Apaydın, S.; Aycan, Z. Gonadoblastoma in a Patient with 46, XY Complete Gonadal Dysgenesis. Turk. J. Pediatr. 2016, 58, 538–540. [Google Scholar] [CrossRef]
- Petre, I.; Mercioni, M.A.; Petre, I. Cervical Cancer or Cervical Endometriosis—Case Report. 2nd Int. Congr. Appl. Sci. 2023, 325. [Google Scholar]
- Talukdar, S.; Kumar, S.; Bhatla, N.; Mathur, S.; Thulkar, S.; Kumar, L. Neo-Adjuvant Chemotherapy in the Treatment of Advanced Malignant Germ Cell Tumors of Ovary. Gynecol. Oncol. 2014, 132, 28–32. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Tang, S.; Feng, F.; Liu, C.; Wang, L.; Zhao, W.; Zhang, T.; Yao, Y.; Wang, X.; et al. Impact of endometriosis on risk of ovarian, endometrial and cervical cancers: A meta-analysis. Arch. Gynecol. Obstet. 2019, 299, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Z.; Wei, R.; Li, L. An Analysis of Prognostic Factors in Patients with Ovarian Malignant Germ Cell Tumors Who Are Treated with Fertility-Preserving Surgery. Gynecol. Obstet. Investig. 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Popescu, A.V.; Craina, M.; Pantea, S.; Popescu, M.M.; Popa, Z.; Stelea, L.; Chiriac, V.D.; Petre, I. Cervical Cancer—The Wertheim-Meigs Surgery: Intraoperative Complications. In Proceedings of the 4th Congress of the Romanian Society for Minimal Invasive Surgery in Ginecology/Annual Days of the National Institute for Mother and Child Health Alessandrescu-Rusescu, Bucharest, Romania, 1–3 November 2019; pp. 491–494. [Google Scholar]
- Petre, I.; Toader, D.O.; Petrita, R.; Pinta, A.-R.; Alexa, A.A.; Mercioni, M.A.; Sandulescu, R.-M. Clinical Performance and Safety of Cerviron Vaginal Ovules in the Management of Cervical Lesions Postoperative Care: A National, Multicentric Study. medRxiv 2023. [Google Scholar] [CrossRef]
- Mitranovici, M.-I.; Chiorean, D.M.; Mureșan, M.C.; Buicu, C.-F.; Moraru, R.; Moraru, L.; Cotoi, T.C.; Cotoi, O.S.; Toru, H.S.; Apostol, A.; et al. Diagnosis and Management of Dysgerminomas with a Brief Summary of Primitive Germ Cell Tumors. Diagnostics 2022, 12, 3105. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global Patterns of Cancer Incidence and Mortality Rates and Trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Wszołek, K.; Pruski, D.; Tomczyk, K.; Kampioni, M.; Chmaj-Wierzchowska, K.; Przybylski, M.; Wilczak, M. Women’s Healthcare Services since the COVID-19 Pandemic Outbreak in Poland. Int. J. Environ. Res. Public Health 2021, 19, 180. [Google Scholar] [CrossRef]
- Calendarul Național de Vaccinare. 2022. Available online: https://insp.gov.ro/wp-content/uploads/2022/08/Calendar-de-vaccinare-2022.pdf (accessed on 29 May 2025).
- Bosco, L.; Serra, N.; Fasciana, T.; Pistoia, D.; Vella, M.; Di Gregorio, L.; Schillaci, R.; Perino, A.; Calagna, G.; Firenze, A.; et al. Potential Impact of a Nonavalent Anti HPV Vaccine in Italian Men with and without Clinical Manifestations. Sci. Rep. 2021, 11, 4096. [Google Scholar] [CrossRef]
- Simion, L.; Rotaru, V.; Cirimbei, C.; Gales, L.; Stefan, D.-C.; Ionescu, S.-O.; Luca, D.; Doran, H.; Chitoran, E. Inequities in Screening and HPV Vaccination Programs and Their Impact on Cervical Cancer Statistics in Romania. Diagnostics 2023, 13, 2776. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Prinja, S.; Srinivasan, R.; Rai, B.; Malliga, J.; Jyani, G.; Gupta, N.; Ghoshal, S. Cost Effectiveness of Strategies for Cervical Cancer Prevention in India. PLoS ONE 2020, 15, e0238291. [Google Scholar] [CrossRef]
- Goldie, S.J.; Gaffikin, L.; Goldhaber-Fiebert, J.D.; Gordillo-Tobar, A.; Levin, C.; Mahé, C.; Wright, T.C. Cost-Effectiveness of Cervical-Cancer Screening in Five Developing Countries. N. Engl. J. Med. 2005, 353, 2158–2168. [Google Scholar] [CrossRef]
- Petre, I.; Vernic, C.; Petre, I.; Vlad, C.S.; Sipos, S.I.; Bordianu, A.; Luciana, M.; Dragomir, R.D.; Fizedean, C.M.; Daliborca, C.V. Systematic Review on the Effectiveness and Outcomes of Nivolumab Treatment Schemes in Advanced and Metastatic Cervical Cancer. Diseases 2024, 12, 77. [Google Scholar] [CrossRef]
- Moraru, L.; Mitranovici, M.-I.; Chiorean, D.M.; Moraru, R.; Caravia, L.; Tiron, A.T.; Cotoi, O.S. Adenomyosis and Its Possible Malignancy: A Review of the Literature. Diagnostics 2023, 13, 1883. [Google Scholar] [CrossRef]
- Beal, C.M.; Salmerón, J.; Flores, Y.N.; Torres, L.; Granados-García, V.; Dugan, E.; Lazcano-Ponce, E. Cost Analysis of Different Cervical Cancer Screening Strategies in Mexico. Salud Publica Mex. 2014, 56, 429–501. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.G.; Lince-Deroche, N.; Chibwesha, C.J.; Firnhaber, C.; Smith, J.S.; Michelow, P.; Meyer-Rath, G.; Jamieson, L.; Jordaan, S.; Sharma, M.; et al. Cost-Effectiveness of Cervical Cancer Screening in Women Living with HIV in South Africa: A Mathematical Modeling Study. J. Acquir. Immune Defic. Syndr. 2018, 79, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Hafidz, F.; Icanervilia, A.; Rizal, M.; Listiani, P.; Setyaningsih, H.; Sasanti, M.; Ekawati, F.; Atthobari, J.; Utami, T.; Trirahmanto, A.; et al. Economic Evaluation of Cervical Cancer Screening by HPV DNA, VIA, and Pap Smear Methods in Indonesia. Asian Pac. J. Cancer Prev. 2024, 25, 3015–3022. [Google Scholar] [CrossRef]
- Luo, W. Predicting Cervical Cancer Outcomes: Statistics, Images, and Machine Learning. Front. Artif. Intell. 2021, 4, 627369. [Google Scholar] [CrossRef]
- Devi, S.; Gaikwad, S.; R, H. Prediction and Detection of Cervical Malignancy Using Machine Learning Models. Asian Pac. J. Cancer Prev. 2023, 24, 1419–1433. [Google Scholar] [CrossRef]
- Van Den Akker-van Marle, M.E. Cost-Effectiveness of Cervical Cancer Screening: Comparison of Screening Policies. J. Natl. Cancer Inst. 2002, 94, 193–204. [Google Scholar] [CrossRef]
- Chiorean, D.; Mitranovici, M.-I.; Toru, H.; Cotoi, T.; Tomuț, A.; Turdean, S.; Cotoi, O. New Insights into Genetics of Endometriosis—A Comprehensive Literature Review. Diagnostics 2023, 13, 2265. [Google Scholar] [CrossRef]
- Zimmermann, M.R.; Vodicka, E.; Babigumira, J.B.; Okech, T.; Mugo, N.; Sakr, S.; Garrison, L.P.; Chung, M.H. Cost-Effectiveness of Cervical Cancer Screening and Preventative Cryotherapy at an HIV Treatment Clinic in Kenya. Cost Eff. Resour. Alloc. 2017, 15, 13. [Google Scholar] [CrossRef]
- Burger, E.A.; Ortendahl, J.D.; Sy, S.; Kristiansen, I.S.; Kim, J.J. Cost-Effectiveness of Cervical Cancer Screening with Primary Human Papillomavirus Testing in Norway. Br. J. Cancer 2012, 106, 1571–1578. [Google Scholar] [CrossRef]



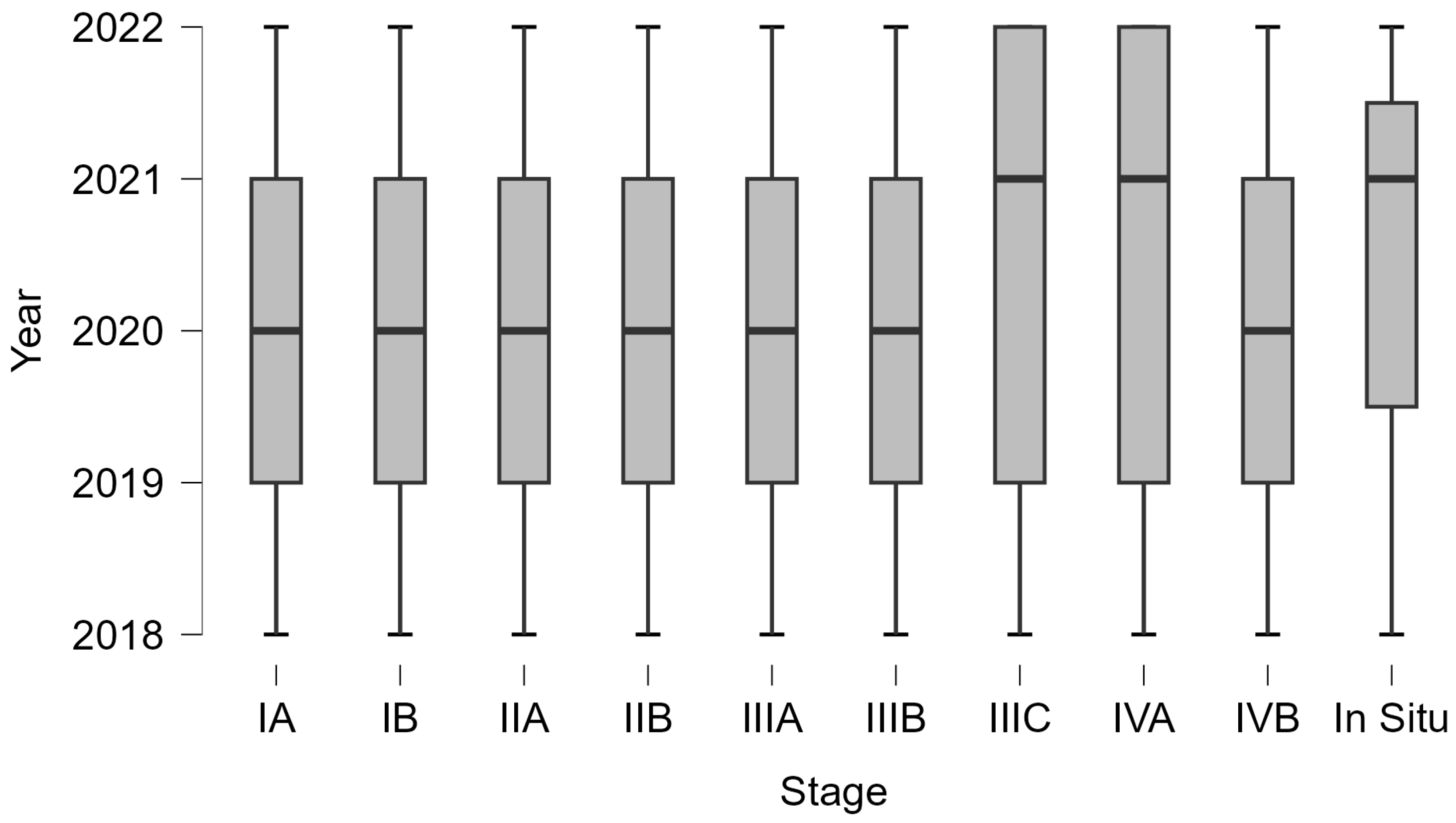


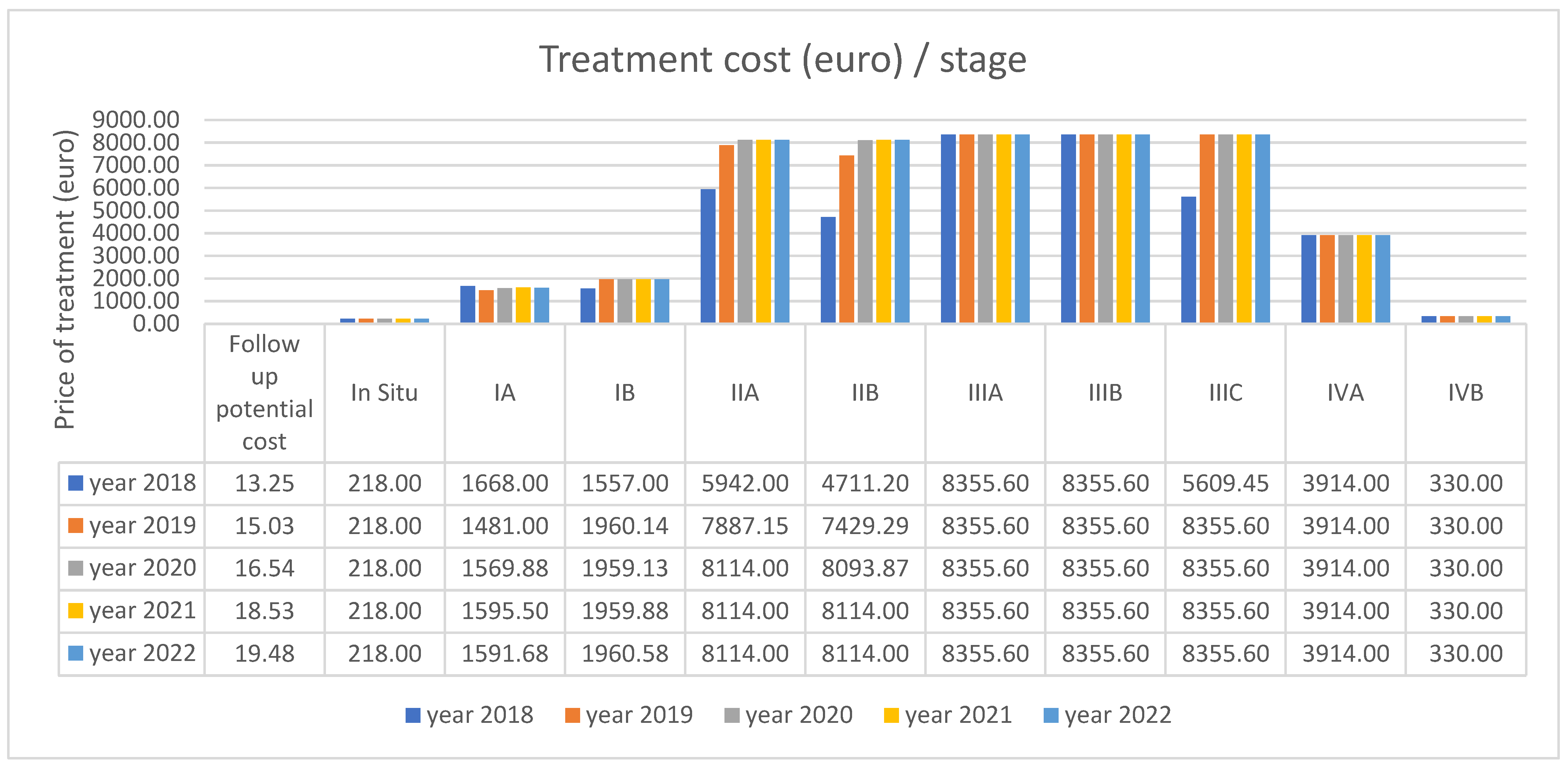

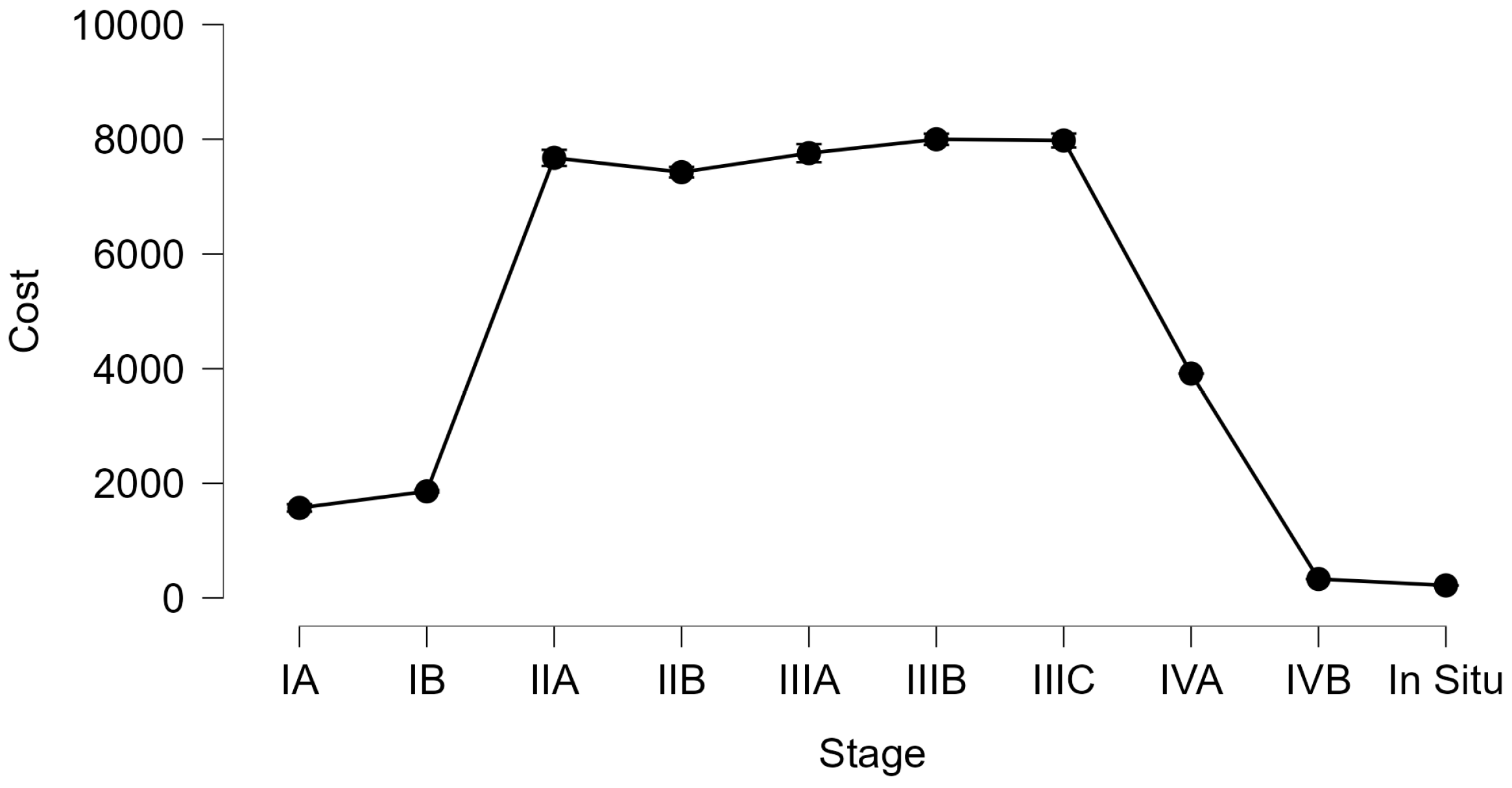











| Cost (€) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| In Situ | IA | IB | IIA | IIB | IIIA | IIIB | IIIC | IVA | IVB | |
| No. | 11 | 97 | 345 | 256 | 865 | 337 | 554 | 378 | 351 | 538 |
| Mean | 218.00 | 1570.78 | 1859.52 | 7675.36 | 7425.57 | 7757.38 | 7999.28 | 7977.82 | 3914.00 | 330.00 |
| Std. Deviation | 0.00 | 323.89 | 203.21 | 1142.49 | 1367.77 | 1470.06 | 1171.35 | 1203 | 0.00 | 0.00 |
| Minimum | 218.00 | 218.00 | 1450.00 | 4711.20 | 4711.20 | 4155.6 | 4155.6 | 4155.6 | 3914.00 | 330.00 |
| Maximum | 218.00 | 1668.00 | 1961.20 | 8114.00 | 8114.00 | 8355.6 | 8355.6 | 8355.6 | 3914.00 | 330.00 |
| Stage and Procedure | Number of Patients | Cost of Treatment (Euro)/Patient |
|---|---|---|
| In Situ—Conization | 11 | 218 |
| Stage IA—under or equal to 45 years—Conization | 5 | 218 |
| Stage IA—under or equal to 45 years—Surgery | 10 | 1450 |
| Stage IA—over 45 years—Conization + HT | 82 | 1668 |
| Stage IB—Surgery | 68 | 1450 |
| Stage IB—Surgery, chemotherapy, brachytherapy | 277 | 1961.2 |
| Stage IIA—Surgery, chemotherapy, brachytherapy | 33 | 4711.2 |
| Stage IIA—Surgery, chemotherapy, radiotherapy | 223 | 8114 |
| Stage IIB—Surgery, chemotherapy, brachytherapy | 175 | 4711.2 |
| Stage IIB—Surgery, chemotherapy, radiotherapy | 690 | 8114 |
| Stage IIIA—Surgery, chemotherapy, radiotherapy, brachytherapy | 289 | 8355.6 |
| Stage IIIA—Chemotherapy, radiotherapy, brachytherapy | 48 | 4155.6 |
| Stage IIIB—Surgery, chemotherapy, radiotherapy, brachytherapy | 507 | 8355.6 |
| Stage IIIB—Chemotherapy, radiotherapy, brachytherapy | 47 | 4155.6 |
| Stage IIIC—Surgery, chemotherapy, radiotherapy, brachytherapy | 344 | 8355.6 |
| Stage IIIC—Chemotherapy, radiotherapy, brachytherapy | 34 | 4155.6 |
| Stage IVA—Chemotherapy, radiotherapy | 351 | 3914 |
| Stage IVB—Chemotherapy | 538 | 330 |
| Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | IA | IB | IIA | IIB | IIIA | IIIB | IIIC | IVA | IVB | |
| No. | 11 | 97 | 345 | 256 | 865 | 337 | 554 | 378 | 351 | 538 |
| Mean | 55.82 | 55.63 | 57.04 | 59.32 | 57.38 | 62.77 | 58.37 | 56.27 | 61.82 | 58.82 |
| Std. Deviation | 5.09 | 9.48 | 10.30 | 11.79 | 11.35 | 8.86 | 10.09 | 10.56 | 8.39 | 12.08 |
| Minimum | 49.00 | 30.00 | 26.00 | 33.00 | 29.00 | 33.00 | 32.00 | 34.00 | 33.00 | 32.00 |
| Maximum | 67.00 | 71.00 | 84.00 | 88.00 | 88.00 | 89.00 | 87.00 | 86.00 | 84.00 | 84.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petre, I.; Negru, Ș.M.; Buleu, F.; Moleriu, R.D.; Mercioni, M.A.; Petre, I.; Bordianu, A.; Turi, V.; Marc, L.; Popa, D.I.; et al. Evaluating the Cost-Effectiveness of Cervical Cancer Screening and Treatment in Western Romania. Curr. Oncol. 2025, 32, 336. https://doi.org/10.3390/curroncol32060336
Petre I, Negru ȘM, Buleu F, Moleriu RD, Mercioni MA, Petre I, Bordianu A, Turi V, Marc L, Popa DI, et al. Evaluating the Cost-Effectiveness of Cervical Cancer Screening and Treatment in Western Romania. Current Oncology. 2025; 32(6):336. https://doi.org/10.3390/curroncol32060336
Chicago/Turabian StylePetre, Ion, Șerban Mircea Negru, Florina Buleu, Radu Dumitru Moleriu, Marina Adriana Mercioni, Izabella Petre, Anca Bordianu, Vladiana Turi, Luciana Marc, Daian Ionel Popa, and et al. 2025. "Evaluating the Cost-Effectiveness of Cervical Cancer Screening and Treatment in Western Romania" Current Oncology 32, no. 6: 336. https://doi.org/10.3390/curroncol32060336
APA StylePetre, I., Negru, Ș. M., Buleu, F., Moleriu, R. D., Mercioni, M. A., Petre, I., Bordianu, A., Turi, V., Marc, L., Popa, D. I., & Vlad, D. C. (2025). Evaluating the Cost-Effectiveness of Cervical Cancer Screening and Treatment in Western Romania. Current Oncology, 32(6), 336. https://doi.org/10.3390/curroncol32060336






