Abstract
Biliary tract cancer (BTC) is a group of rare and aggressive malignancies with a dismal prognosis. There is currently a significant lack in effective treatment options for BTC, with gemcitabine-cisplatin remaining the first-line standard of care treatment for over a decade. A wave of investigational therapies, including new chemotherapy combinations, immunotherapy, and biomarker-driven targeted therapy have demonstrated promising results in BTC, and there is hope for many of these therapies to be incorporated into the Canadian treatment landscape in the near future. This review discusses the emerging therapies under investigation for BTC and provides a perspective on how they may fit into Canadian practice, with a focus on the barriers to treatment access.
1. Background
Biliary tract cancer (BTC) is a group of aggressive malignancies arising from the gallbladder (gallbladder cancer, GBC), intrahepatic or extrahepatic bile ducts (cholangiocarcinoma, iCCA or eCCA), or ampulla of Vater (ampullary cancer) [1]. There is a paucity of literature describing the epidemiology of BTC, with the existing data suggesting that the incidence fluctuates over time and varies by region, sex, ethnicity, and subtype [2,3]. Globally, BTC has been reported to occur at rate between 1 and 4 cases per 100,000 people per year in most regions, with some regions exceeding an age-standardized annual incidence of 15 cases per 100,000 [2]. This qualifies BTC as a rare disease [4]. In Canada, one retrospective study of population-based cancer registries found an age standardized annual incidence rate of 2.1 cases per 100,000 people for gall bladder and extrahepatic cholangiocarcinoma (eCCA) [5]. A higher rate of gallbladder cancer has consistently been reported in women and a higher rate of eCCA has been reported in men [2,5]. As BTC is typically an aggressive disease, with few symptoms in the early stages, the majority are diagnosed in an advanced or metastatic stage where potentially curative surgery is not possible. The 5-year relative survival rate for patients with any-stage BTC is 9–10%, and is only 2% for patients with metastatic disease [6].
Currently, there is a lack of effective therapeutic options for patients with advanced BTC. For patients with resectable tumours, curative intent therapy with surgery followed by adjuvant chemotherapy is the standard of care, resulting in a median overall survival (OS) of over 4 years [7]. Radiotherapy may also be used as neoadjuvant or adjuvant therapy in conjunction with surgical resection, although the optimal methods for delivery of radiotherapy are unclear [8]. Nevertheless, only 20% of BTCs may be eligible for potentially curative resection [9]. Neoadjuvant chemoradiation followed by liver transplantation is an option for unresectable CCA without metastases, with a single-center study from the Mayo Clinic demonstrating a 4-year survival rate of 51% with this method [10]. However, the eligibility criteria to receive this therapy are strict. A similar chemoradiotherapy protocol used at Toronto General Hospital and Princess Margaret Cancer Centre showed a high rate of dropout and disease progression [11]. The current standard of care first-line treatment for patients with unresectable BTC is gemcitabine-cisplatin. This is based on the phase III ABC-02 trial which demonstrated significantly improved OS for gemcitabine-cisplatin compared to gemcitabine alone (up to 8 cycles; median OS 11.7 vs. 8.1 months; hazard ratio [HR] 0.64; 95% confidence interval [CI] 0.52–0.80) [12]. This standard of care has remained unchanged for over a decade as there has been no successful phase III trials to show superior survival benefit.
Following progression on gemcitabine-cisplatin, many patients are not well enough to receive second-line therapy and there is a lack of effective treatment options for those fit to receive subsequent therapy [13]. While there are some promising targeted therapies for BTC, these are not funded in Canada and they could only benefit a small percentage of patients. In addition, patients who are elderly or have poor performance status are unable to tolerate chemotherapy, with their treatment limited to supportive care including decompression of the biliary tree through biliary stenting and ablation techniques [14]. This highlights the significant unmet need for more effective and tolerable treatment options in BTC, particularly in the first-line setting, and given the extremely poor prognosis for patients, it emphasizes the importance of patient-centered outcomes, such as quality of life and progression-free survival (PFS), in therapy selection.
Therapeutic development in BTC has flourished in the last few years, and after many unsuccessful clinical trials, there are finally novel treatments that can provide hope for patients with advanced, unresectable BTC. Several new regimens are expected to enter the treatment landscape in Canada within the next 5–10 years; however, significant barriers to accessing these therapies exist. Most therapies under investigation fit under the categories of new chemotherapy combinations, immunotherapy, and biomarker-driven targeted therapy (Figure 1). This review highlights promising therapies emerging for the treatment of advanced, unresectable BTC and provides a perspective on the barriers to accessing these treatments in Canada.
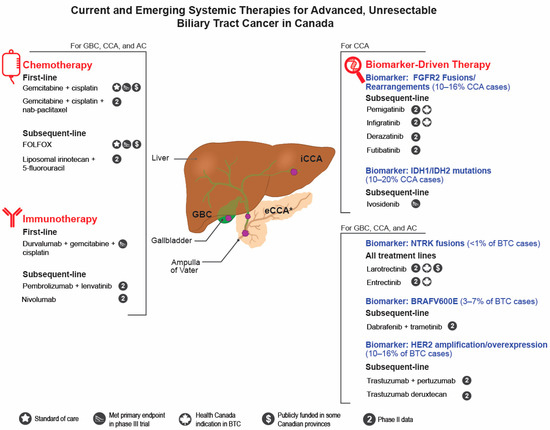
Figure 1.
Current and emerging systemic therapies for advanced, unresectable biliary tract cancer in Canada. AC, Ampullary cancer; BTC, biliary tract cancer; CCA, cholangiocarcinoma; eCCA, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; iCCA, intrahepatic cholangiocarcinoma. * eCCA can be further subdivded into perihilar CCA which is proximal to the origin of the cystic duct and distal CCA which occurs between the cyctic duct and Ampulla of Vater.
2. Emerging Therapies in BTC
2.1. Chemotherapy
A number of chemotherapy combinations have been evaluated in clinical trials for the first-line treatment of advanced or metastatic BTC. None of these trials demonstrated survival outcomes beyond those achieved with gemcitabine-cisplatin in the ABC-02 trial; however, a recent phase II study evaluating albumin-bound paclitaxel added to gemcitabine-cisplatin in advanced BTC reported a median OS of 19.2 months, which compares favorably to the OS reported in the ABC-02 trial [15]. In addition, 20% of patients were down-staged to resectable disease. The phase III SWOG S1815 study evaluating gemcitabine-cisplatin in combination with albumin-bound paclitaxel or placebo is ongoing and will help clarify whether the chemotherapy triplet can achieve meaningful improvements in clinical outcomes over the chemotherapy doublet, without compromising quality of life or substantially increasing toxicity.
Other triplet chemotherapy regimens have demonstrated less encouraging results when compared to gemcitabine-cisplatin. The PRODIGE38 study did not show an improved 6-month PFS rate for modified FOLFIRINOX compared to gemcitabine-cisplatin, which was the primary endpoint of the phase II portion of the study (44.6% vs. 47.3%) [16]. The median PFS and OS were also shorter in the modified FOLFIRINOX arm (median PFS: 6.2 vs. 7.4 months; median OS: 11.7 vs. 13.8 months).
Few randomized controlled trials have demonstrated efficacy of chemotherapy combinations in the second-line setting. The phase III ABC-06 study evaluating modified FOLFOX in patients with advanced BTC following first-line gemcitabine-cisplatin demonstrated a 15% improvement in 6- and 12-month survival rates compared to active symptom control alone [17]. The median OS was 6.2 months for modified FOLFOX versus 5.3 months for the control arm (HR 0.69; 95% CI 0.50–0.97; p = 0.031). This regimen has since become the most common second-line treatment option for patients with advanced BTC in Canada. Most recently, the phase II NIFTY trial, enrolling advanced BTC patients who progressed on first-line gemcitabine-cisplatin, reported a significantly longer median OS with liposomal irinotecan and 5-FU versus 5-FU alone (8.6 months vs. 5.5 months; HR 0.68; 95% CI 0.48–0.98; p = 0.035) [18].
2.2. Immunotherapy
Therapies targeting immune checkpoint pathways, including the programmed death-1 (PD-1)/programmed death-ligand 1(PD-L1) axis, have demonstrated activity in other cancers and have shown success in prolonging PFS and OS in both biomarker-selected and unselected populations [19]. The rationale for investigation of PD-1/PD-L1 targeted immunotherapy in BTC is based on the observation of PD-L1-expressing tumor cells and the presence of tumor-infiltrating CD8 T cells in the tumor microenvironment of BTC, both of which have been recognized as biomarkers of efficacy for PD-1/PD-L1 immunotherapy [20,21].
Single-agent immunotherapy with the anti-PD-1 agents pembrolizumab and nivolumab or the anti-PD-L1 agent durvalumab have demonstrated modest activity in phase II clinical trials of chemorefractory BTC, with overall response rates (ORRs) between 5 to 22% in unselected patients and 41% in patients with microsatellite instability/mismatch repair deficiency [22,23,24,25]. (Table 1) Dual checkpoint inhibition, targeting both the PD-1 and CTLA4 axis have also been explored, with similar outcomes to those achieved in single-agent trials [22,26] (Table 1).

Table 1.
Results reported from phase II/III clinical trials investigating immune checkpoint inhibitors in advanced BTC
There is evidence to suggest that chemotherapy may act synergistically with immunotherapy through several mechanisms of immunomodulation, providing a rationale to explore combination therapies in BTC [32]. Both nivolumab and durvalumab have been evaluated in phase II clinical trials in combination with gemcitabine-cisplatin for patients with unresectable or metastatic BTC. These trials reported median OS results of 10.6 months and 18.1 months, respectively [27,28].
The TOPAZ-1 study was subsequently conducted, which was a phase III, double-blind placebo-controlled trial randomizing patients with unresectable or metastatic BTC to receive gemcitabine-cisplatin (for up to eight cycles) plus durvalumab or gemcitabine-cisplatin (for up to eight cycles) plus placebo as first-line treatment [30]. The study met its primary endpoint showing a statistically significant improvement in OS for durvalumab plus gemcitabine-cisplatin versus placebo plus gemcitabine-cisplatin (median OS 12.9 vs. 11.3 months; HR, 0.76; 95% CI 0.64–0.91; p = 0.021) [31]. Addition of durvalumab to gemcitabine-cisplatin also prolonged PFS (median 7.2 vs. 5.7; HR 0.75; 95% CI 0.63–0.89; p = 0.001) and increased ORR (27% vs. 19%) [30]. Rates of grade 3/4 adverse events were not increased with the addition of durvalumab and 13% of patients experienced any grade immune related events (vs. 5% in the control arm). The triplet combination was well tolerated and quality of life was also maintained [33]. Another triplet chemo-immunotherapy regimen, pembrolizumab, gemcitabine, and cisplatin, is also being evaluated for patients with advanced BTC in the ongoing phase III KEYNOTE-966 trial [34].
Immune checkpoint inhibitors in combination with other novel agents and locoregional therapy are also being studied in BTC. Thus far, results have been reported for a phase II study evaluating the anti-VEGF agent lenvatinib in combination with pembrolizumab in advanced previously treated BTC. In this trial, pembrolizumab-lenvatinib achieved an ORR of 25%, median PFS of 4.9 months, and median OS of 11.0 months [29].
2.3. Biomarker-Driven Targeted Therapies
Several targeted agents have shown encouraging efficacy in patients with advanced BTC who harbor specific genomic alterations, although together, this represents a small fraction of patients with BTC and many of these patients may not be identified due to the limited access to biomarker testing for BTC in Canada. Recently, two agents targeting the Fibroblast Growth Factor Receptor (FGFR) family genes—pemigatinib and infigratinib—have been approved by Health Canada for previously treated, unresectable, or metastatic CCA with FGFR2 gene rearrangements. FGFR2 gene fusions occur in 10−16% of CCA cases, and almost exclusively occur in the intrahepatic subtype. The approval of pemigatinib and infigratinib were based on phase II studies where a median PFS of approximately 7 months was reached in patients with chemo-refractory cholangiocarcinoma with FGFR2 gene fusions [35,36]. (Table 2) A similar median PFS was achieved with erdafitinib in the LUC2001 trial (5.6 months), derazatinib in the FIDES-01 trial (8.0 months), and futibatinib in the FOENIX-CCA2 trial (8.9 months); all phase II studies of patients with advanced iCCA with FGFR2 fusions [37,38,39]. Notably, futibatinib has demonstrated some activity in patients previously treated with anti-FGFR agents, and both derazatinib and futibatinib have demonstrated similar activity in CCA with FGFR2 mutations as those with FGFR2 fusions/rearrangements [40,41]. Several ongoing phase III trials are evaluating first-line FGFR inhibitor therapy in patients with unresectable or metastatic CCA compared with gemcitabine-cisplatin. These include the PROOF (infigratinib, NCT03773302), FIGHT-302 (pemigatinib, NCT03656536), and FOENIX-CCA trials (futibatinib, NCT04093362).

Table 2.
Results reported from phase II/III clinical trials investigating biomarker-driven targeted therapy in >20 patients with advanced BTC.
Although rarely occurring in CCA (<1% of cases), tumors with gene fusions involving the neurotrophic tyrosine receptor kinase (NTRK) family proteins have been reported to respond to TRK inhibitors in a small number of patients evaluated in basket studies (2 of 3 patients with a partial response) [47,48]. Entrectinib and larotrectinib are currently approved by Health Canada for patients with unresectable locally advanced or metastatic solid tumors with NTRK gene fusions and no other satisfactory treatment options.
Activating mutations in isocitrate dehydrogenase 1/2 (IDH1/2) occur in 10–20% of patients with CCA. The phase III ClarIDHy study randomized patients with previously treated advanced CCA harboring IDH1/2 mutations to treatment with the IDH1 inhibitor ivosidinib or placebo [42,49]. Ivosidinib led to a statistically significant improvement in PFS compared with placebo (2.7 vs. 1.4 months; HR, 0.37; 95% CI 0.25–0.54) (Table 2). No significant difference in OS was observed; however, this was likely caused by crossover of patients into the experimental arm. Several other agents targeting IDH1 and IDH2 are under investigation in BTC, including next-generation inhibitors aimed at escaping resistance mechanisms acquired after ivosidenib treatment [50].
Mutations in the BRAF gene have been reported in 3–7% of BTC cases, and are also enriched in those with iCCA. In a basket study exploring the activity of the BRAF and MEK inhibitors dabrafenib and trametinib in rare tumours harboring the BRAFV600E mutation, the cohort of 43 patients with BTC achieved an ORR of 51% and median OS of 14 months [43]. Encouraging activity for this combination was also reported in the NCI-MATCH trial subprotocol H, with 3 of 4 patients with BRAFV600E-mutated BTC achieving a partial response [51].
HER2 amplification or overexpression occurs in 10–16% of gallbladder carcinomas and 5–11% of eCCA [52]. Given the success of anti-HER2 therapies in other solid tumors expressing HER2, several phase I/II studies have evaluated these therapies in BTC [50]. Clinical trials of pertuzumab-trastuzumab and trastuzumab deruxtecan in patients with previously treated metastatic BTC have reported ORRs of 22% and 36%, respectively, and median OS results of 10.9 months and 7.1 months [44,45] (Table 2). Trastuzumab is now being tested in combination with gemcitabine-cisplatin in the phase II BILHER study (NCT03613168). In a study of patients with metastatic treatment-refractory BTC and HER2 mutations, the irreversible HER2 kinase neratinib achieved an ORR of 16% and median OS of 5.4 months [46].
Increasingly sophisticated genomic analyses in BTC are expected to reveal insights on additional biomarkers of treatment response and new therapeutic targets. For example, a multi-omics analysis in iCCA tumor samples identified an IDH mutant-enriched subtype that correlated with hypermethylation and decreased expression of ARID1A, suggesting that inhibition of chromatin modifiers such as EZH2 may be effective in this subtype [53]. Other studies have reported a high frequency of other actionable biomarkers in BTC including PTEN, CDKN2A, and KRAS which warrant further investigation in clinical trials [54,55]. Outside of microsatellite instability, biomarkers that can predict response to immune checkpoint inhibitors have been unsuccessful thus far, likely due to the heterogeneity of immune cells in the tumor microenvironment of BTC. Single-cell RNA sequencing techniques can characterize complex immune cell populations in the tumor microenvironment and have shown promise in identifying biomarkers for immunotherapy response [56]. Together, this emphasizes the continued role precision medicine will play in improving treatment response in BTC.
3. Canadian Perspective on Access to Therapies in BTC
With several recent BTC trials reporting encouraging results, the question on many Canadian oncologists’ minds is which therapies will be made available to our patients and funded by the Canadian provinces. Following Health Canada approval, the decision to provide Canadians with access to drugs through public reimbursement programs is made at the provincial level. Funding decisions are largely aided by recommendations from Health Technology Assessment (HTA) bodies, including the Canadian Agency for Drugs and Technologies in Health (CADTH) and Quebec’s Institut National d’Excellence en Santé et en Services Sociaux (INESSS). These HTA bodies consider cost-effectiveness, patient-based values, and adoption feasibility in addition to clinical benefit, when appraising drugs for reimbursement recommendations [57].
As BTC is a rare, aggressive disease with extremely poor prognosis and limited treatment advances, access to new therapies can be hindered if HTA assessments (specifically clinical value and cost-effectiveness) are measured against the same standards as all oncology drugs. For example, although phase III randomized controlled trials are the gold standard for determining clinical benefit, this trial design may be difficult to achieve in BTC, particularly in the second-line setting, as trial enrollment is challenged by a small overall population of eligible patients. Evaluation of targeted agents that require the selection of even smaller genetically defined subpopulations adds to enrollment difficulty. This challenge is illustrated in the negative recommendation issued by CADTH for the reimbursement of pemigatinib in patients with previously treated CCA with FGFR2 fusions [58]. This recommendation was based on the uncertainty that pemigatinib filled the patient-identified needs of improved tumor response, delayed disease progression, and improved quality of life, given the single-arm, open-label design of the phase II FIGHT-202 trial; despite the acknowledgment that a phase III randomized controlled trial would be unfeasible in this setting.
In contrast to the negative recommendation for pemigatinib, the TRK inhibitor larotrectinib has been issued a conditional positive recommendation for funding by CADTH, for any patient with an advanced solid tumor harboring NTRK fusions who have no other effective treatment options [59]. This followed an initial negative recommendation based on uncertainty in the clinical benefit of larotrectinib given the heterogeneity of patients enrolled in the three single-arm phase I/II trials, among other reasons. The current positive recommendation was based on updated pooled analyses of the aforementioned trials, as well as supportive real-world data. This analysis demonstrated ORRs of 73% in the overall population and median PFS of approximately 33 months. Although, with only two patients with CCA enrolling, it is unclear whether the efficacy results for larotrectinib can be generalizable to patients with BTC. Furthermore, patients with NTRK fusions represent <1% of the population across many cancers, including BTC, and variable access to testing across provinces may impede access to this therapy [60,61].
Another challenge with assessing new therapies in BTC is deciding what constitutes a clinically meaningful benefit. Both the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) have published consensus documents proposing how a clinically meaningful benefit may be measured across different tumor types and scenarios. In ASCO’s publication, working groups for colon, pancreatic, lung, and breast cancer all selected median OS as the primary end point of interest. In general, they deemed an HR between 0.6 to 0.8 and a median OS improvement between 2.5 to 6 months over standard therapy as a clinically meaningful outcome [62]. However, the group acknowledged that the definition of a clinically meaningful benefit is nuanced and may be influenced by other factors including clinical context, effectiveness, toxicity, and patient goals and preferences. Thus, consensus values should not be used to set standards for regulatory approval or funding. It is unclear whether these benchmarks are applicable to BTC, where the inability to design trials with a large sample size, as is common in lung, colon, and breast trials, may prevent such hazard ratios from being achieved.
Response rate, duration of response, and PFS, although not validated as surrogate markers for OS, are also clinically meaningful in BTC. A large, durable response can downstage patients, as was reported in 20% of patients in the phase II trial of albumin-bound paclitaxel plus gemcitabine-cisplatin [15]. This may allow them to receive potentially curative surgery. Retrospective studies have observed similar survival outcomes after surgery in patients with initially unresectable localized iCCA that were down-staged following chemotherapy compared with initially resectable patients [63,64]. In addition, given the small size of the biliary tract, even minimal tumor growth can lead to disease symptoms causing significant deterioration of quality of life and necessitate stent placements or changes. These are associated with complications requiring hospitalization, including bleeding, perforation, cholangitis, and infection [65]. Consideration of these outcomes in future value assessments may better reflect the unique circumstances and needs of patients with BTC.
Quality of life is also of great importance to patients with BTC and must be considered in the value assessment of a therapy [66]. The Magnitude of Clinical Benefit Scale created by an ESMO working group (ESMO-MCBS) incorporates quality of life measures by increasing the clinical benefit score of a drug if quality of life and/or major toxicity is improved, such that drugs demonstrating a smaller magnitude of benefit for efficacy may still be categorized as substantially beneficial if these criteria are met [67].
The prospect of patients with BTC gaining access to new treatments is exciting. However, the only regimen currently being reviewed for Health Canada approval is durvalumab in combination with gemcitabine-cisplatin for first-line treatment of BTC. This is based on results from the phase III TOPAZ-1 trial, which is the first phase III randomized, double-blind, placebo-controlled trial in over a decade to demonstrate a statistically significant improvement in OS, without increasing toxicity or reducing quality of life, compared to gemcitabine-cisplatin alone [30,33]. However, the TOPAZ-1 trial has potential limitations that may impact HTA assessment and use in Canada, particularly if it is assessed against the same standards traditionally used in other cancers. Although an improvement in median OS of 1.6 months and a HR of 0.76 may be a meaningful benefit to some patients and caregivers, it is uncertain whether this outcome alone will meet the value thresholds set by CADTH who have historically issued few positive recommendations for reimbursement where the improvement of median OS was below 2 months [57].
As demonstrated in other clinical trials, median OS alone does not appropriately capture the extended right-sided tail commonly observed in the survival curves for chemoimmunotherapy regimens, which represents a portion of patients with long-term survival [68,69,70]. In such cases, analysis of survival rate at a set milestone is suggested to better capture the incremental effect of the experimental treatment [71]. At the current follow-up of 23 months, the survival curves from TOPAZ-1 show a potential right-tail plateau forming, corresponding to an improved 24-month OS rate for gemcitabine-cisplatin plus durvalumab over gemcitabine-cisplatin plus placebo (23.6% vs. 11.5%) [31]. With an improvement in 2-year OS rate exceeding 10%, durvalumab in combination with gemcitabine-cisplatin has earned a score of 4 points on the ESMO-MCBS, representing a substantial clinical benefit [72,73].
Another potential limitation of the TOPAZ-1 trial comes from the dosing schedule of gemcitabine-cisplatin. In TOPAZ-1, gemcitabine-cisplatin was stopped after eight cycles of therapy in both arms, similar to the dosing regimen used in the ABC-02 trial. However, anecdotally, many Canadian oncologists will give gemcitabine-cisplatin until progression or dose-limiting toxicity (such as neuropathy related to cisplatin), or treatment is continued with gemcitabine monotherapy after cycle 8. This may complicate the implementation of durvalumab for the first-line treatment of BTC specifically in Canada. The practice of giving gemcitabine-cisplatin until progression or toxicity is largely based on studies from other tumor types such as breast cancer, which demonstrate improved survival with continued palliative chemotherapy [74]. One retrospective observational study from Canada suggests that some patients may benefit from continued chemotherapy [75], while other studies have not observed a clear benefit [76]. The ongoing KEYNOTE-966 trial allows gemcitabine to be given with pembrolizumab or placebo beyond eight cycles (until progressive disease or unacceptable toxicity), which may better reflect the practice of some Canadian oncologists [34].
4. Conclusions
The current landscape of systemic treatments for advanced, unresectable BTC is extremely limited in Canada, consisting mainly of chemotherapy options including standard of care first-line treatment with gemcitabine-cisplatin. Although biomarker-driven agents targeted against NTRK (larotrectinib, entrectinib) and FGFR (pemigatinib, and infigratinib) are approved by Health Canada for second-line therapy, there are several barriers to accessing these agents for patients with advanced BTC. These include lack of provincial funding and access to timely biomarker testing. In addition, the pool of patients who harbor FGFR2 or NTRK fusions and who are fit to receive second-line therapy and beyond represent less than 5% of the BTC population. Therefore, there is still a high unmet need for more effective therapies for all patients with BTC, particularly in the first-line setting.
The wave of chemo-immunotherapy and biomarker-driven treatments showing activity in clinical trials for advanced BTC is reminiscent of the therapeutic evolution in non-small cell lung cancer that began 10 years ago, which provided much needed new treatment options for patients with a very poor prognosis. However, in contrast to the rarity of BTC, the high frequency of lung cancer in Canada and throughout the world allowed for large, randomized phase III trials to be done which demonstrated a clear clinical benefit. Given these large trials are challenging to conduct in BTC, different considerations for value assessment beyond median OS are needed. Progression-free survival, response rate, duration of response, and quality of life are particularly important outcomes in BTC. Landmark OS analyses that can capture whether a portion of patients can achieve long-term survival from an experimental treatment are also valuable. To better understand how new therapies might provide value to patients, clinical trials and real-world studies should aim to capture outcomes such as biliary stenting, hospitalizations, and down-staging, and explore whether these correlate with patient quality of life. Together, this may help to shape the definition of a clinically meaningful benefit for patients with BTC and improve access to therapies that meet the needs of patients.
Author Contributions
Conceptualization, V.C.T. and H.J.L.; writing—original draft preparation, S.D.; writing—review and editing, V.C.T., R.R., R.B., E.M.Y., S.D., and H.J.L.; project management, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by AstraZeneca Canada to support medical writing assistance and administrative coordination of this manuscript. The funders did not contribute to the content or writing of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge medical writing support, provided by Sarah Doucette of IMPACT Medicom, which was funded by AstraZeneca Canada.
Conflicts of Interest
V.C.T. has received honoraria and has served on advisory boards for AstraZeneca, Eisai, Incyte, Ipsen, and Roche and has received institutional research and clinical trial funding form AstraZeneca, Basilea, Eisai, Exelixis, Ipsen, Merck, and Roche. R.R. has served as a consultant or on an advisory board for Eisai and Ipsen, has received honoraria from Eisai, BMS, Ipsen and Bayer, and has received research funding from Eisai. R.B. has no relevant conflicts of interest to disclose. E.M.Y. has participated as an investigator on clinical trials supported by Gilead Sciences, Abbvie, Merck, Intercept, Genfit, Pfizer, Novodisc, Allergan, and Madrigal; has received unrestricted research grants from Paladin Labs; and has received honoraria for continuing medical education lectures from Intercept, Gilead Canada, Abbvie Canada, and Merck Canada. S.D. has received funding from AstraZeneca Canada for medical writing services. H.J.L. has received honorarium from Eisai, Taiho, Merck, Lilly, Bristol Meyers Squibb, and Amgen for consultant work and has participated as an investigator on clinical trials supported by Bayer, Bristol Meyers Squibb, Lilly, Roche, AstraZeneca, and Amgen.
References
- Beaulieu, C.; Lui, A.; Yusuf, D.; Abdelaziz, Z.; Randolph, B.; Batuyong, E.; Ghosh, S.; Bathe, O.F.; Tam, V.; Spratlin, J.L. A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada. Curr. Oncol. 2021, 28, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Piñeros, M.; Ferreccio, C.; Adsay, V.; Soerjomataram, I.; Bray, F.; Koshiol, J. Gallbladder and extrahepatic bile duct cancers in the Americas: Incidence and mortality patterns and trends. Int. J. Cancer 2020, 147, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, A.L.; Shiels, M.S.; Jones, G.S.; Pfeiffer, R.M.; Petrick, J.L.; Beebe-Dimmer, J.L.; Koshiol, J. Biliary tract cancer incidence and trends in the United States by demographic group, 1999–2013. Cancer 2019, 125, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; Nestler-Parr, S.; Babela, R.; Khan, Z.M.; Tesoro, T.; Molsen, E.; Hughes, D.A. Rare Disease Terminology and Definitions—A Systematic Global Review: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2015, 18, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Cattelan, L.; Lagacé, F.; Ghazawi, F.M.; Alakel, A.; Grose, E.; Le, M.; Nechaev, V.; Sasseville, D.; Waschke, K.; et al. Epidemiologic trends and geographic distribution of patients with gallbladder and extrahepatic biliary tract cancers in Canada. HPB 2021, 23, 1541–1549. [Google Scholar] [CrossRef]
- American Cancer Society. Survival Rates for Bile Duct Cancer. Available online: https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html (accessed on 9 June 2022).
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Wang, N.; Huang, A.; Kuang, B.; Xiao, Y.; Xiao, Y.; Ma, H. Progress in Radiotherapy for Cholangiocarcinoma. Front. Oncol. 2022, 12, 868034. [Google Scholar] [CrossRef]
- Yoo, C.; Shin, S.H.; Park, J.-O.; Kim, K.-P.; Jeong, J.H.; Ryoo, B.-Y.; Lee, W.; Song, K.-B.; Hwang, D.-W.; Park, J.-H.; et al. Current Status and Future Perspectives of Perioperative Therapy for Resectable Biliary Tract Cancer: A Multidisciplinary Review. Cancers 2021, 13, 1647. [Google Scholar] [CrossRef]
- Duignan, S.; Maguire, D.; Ravichand, C.S.; Geoghegan, J.; Hoti, E.; Fennelly, D.; Armstrong, J.; Rock, K.; Mohan, H.; Traynor, O. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: A single-centre national experience. HPB 2014, 16, 91–98. [Google Scholar] [CrossRef]
- Loveday, B.P.T.; Knox, J.J.; Dawson, L.A.; Metser, U.; Brade, A.; Horgan, A.M.; Gallinger, S.; Greig, P.D.; Moulton, C.-A. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J. Surg. Oncol. 2018, 117, 213–219. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Lamarca, A.; Hubner, R.A.; David Ryder, W.; Valle, J.W. Second-line chemotherapy in advanced biliary cancer: A systematic review. Ann. Oncol. 2014, 25, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, T.; Kahaleh, M. Comparing palliative treatment options for cholangiocarcinoma: Photodynamic therapy vs. radiofrequency ablation. Clin. Endosc. 2022, 55, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef]
- Phelip, J.M.; Desrame, J.; Edeline, J.; Barbier, E.; Terrebonne, E.; Michel, P.; Perrier, H.; Dahan, L.; Bourgeois, V.; Akouz, F.K.; et al. Modified FOLFIRINOX versus CISGEM chemotherapy for Patients with advanced biliary tract cancer (PRODIGE 38 AMEBICA): A randomized phase II study. J. Clin. Oncol. 2022, 40, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Kim, R.; Coppola, D.; Wang, E.; Chang, Y.D.; Kim, Y.; Anaya, D.; Kim, D.W. Prognostic value of CD8CD45RO tumor infiltrating lymphocytes in patients with extrahepatic cholangiocarcinoma. Oncotarget 2018, 9, 23366–23372. [Google Scholar] [CrossRef]
- Spencer, K.R.; Wang, J.; Silk, A.W.; Ganesan, S.; Kaufman, H.L.; Mehnert, J.M. Biomarkers for Immunotherapy: Current Developments and Challenges. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e493–e503. [Google Scholar] [CrossRef]
- Ioka, T.; Ueno, M.; Oh, D.-Y.; Fujiwara, Y.; Chen, J.-S.; Doki, Y.; Mizuno, N.; Park, K.; Asagi, A.; Hayama, M.; et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC). J. Clin. Oncol. 2019, 37, 387. [Google Scholar] [CrossRef]
- Ueno, M.; Chung, H.C.; Nagrial, A.; Marabelle, A.; Kelley, R.K.; Xu, L.; Mahoney, J.; Pruitt, S.K.; Oh, D.Y. Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase II KEYNOTE-158 study. Ann. Oncol. 2018, 29, viii210. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Jackett, L.; Lum, C.; Behren, A.; Palmer, J.; et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients With Advanced Biliary Tract Cancers. JAMA Oncol. 2020, 6, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Sahai, V.; Griffith, K.A.; Beg, M.S.; Shaib, W.L.; Mahalingam, D.; Zhen, D.B.; Deming, D.A.; Dey, S.; Mendiratta-Lala, M.; Zalupski, M. A multicenter randomized phase II study of nivolumab in combination with gemcitabine/cisplatin or ipilimumab as first-line therapy for patients with advanced unresectable biliary tract cancer (BilT-01). J. Clin. Oncol. 2020, 38, 4582. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Kim, T.Y.; Bang, J.-H.; Nam, A.-R.; Lee, Y.; Zhang, Q.; Rebelatto, M.; Li, W.; et al. Phase II study assessing tolerability, efficacy, and biomarkers for durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin (GemCis) in chemo-naïve advanced biliary tract cancer (aBTC). J. Clin. Oncol. 2020, 38, 4520. [Google Scholar] [CrossRef]
- Lin, J.; Yang, X.; Long, J.; Zhao, S.; Mao, J.; Wang, D.; Bai, Y.; Bian, J.; Zhang, L.; Yang, X.; et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg. Nutr. 2020, 9, 414–424. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Oh, D.; He, A.R.; Qin, S.; Chen, L.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. 56P—Updated overall survival (OS) from the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+ GC) in patients (pts) with advanced biliary tract cancer (BTC). Ann. Oncol. 2022, 33 (Suppl. S7), S19–S26. [Google Scholar] [CrossRef]
- Emens, L.A.; Middleton, G. The Interplay of Immunotherapy and Chemotherapy: Harnessing Potential Synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A., III; Okusaka, T.; Vogel, A.; Lee, M.A.; Takahashi, H.; Breder, V.V.; Blanc, J.-F.; Li, J.; Watras, M.; Xiong, J.; et al. Patient-reported outcomes for the phase 3 TOPAZ-1 study of durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. J. Clin. Oncol. 2022, 40, 4070. [Google Scholar] [CrossRef]
- Finn, R.S.; Kelley, R.K.; Furuse, J.; Edeline, J.; Ren, Z.; Su, S.-C.; Malhotra, U.; Siegel, A.B.; Valle, J.W. Abstract CT283: KEYNOTE-966: A randomized, double-blind, placebo-controlled, phase 3 study of pembrolizumab in combination with gemcitabine and cisplatin for the treatment of advanced biliary tract carcinoma. Cancer Res. 2020, 80, CT283. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Javle, M.; Kelley, R.K.; Roychowdhury, S.; Weiss, K.H.; Abou-Alfa, G.K.; Macarulla, T.; Sadeghi, S.; Waldschmidt, D.; Zhu, A.X.; Goyal, L.; et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann. Oncol. 2018, 29, viii720. [Google Scholar] [CrossRef]
- Busset, M.D.; Shaib, W.L.; Mody, K.; Personeni, N.; Damjanov, N.; Harris, W.P.; Bergamo, F.; Brandi, G.; Masi, G.; Halfdanarson, T.R.; et al. Derazantinib for patients with intrahepatic cholangiocarcinoma harboring FGFR2 fusions/rearrangements: Primary results from the phase II study FIDES-01. Ann. Oncol. 2021, 32, S376. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Morizane, C.; Valle, J.W.; Karasic, T.B.; Abrams, T.A.; Kelley, R.K.; Cassier, P.A.; Furuse, J.; et al. Updated results of the FOENIX-CCA2 trial: Efficacy and safety of futibatinib in intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 fusions/rearrangements. J. Clin. Oncol. 2022, 40, 4009. [Google Scholar] [CrossRef]
- Park, J.O.; Feng, Y.-H.; Chen, Y.-Y.; Su, W.-C.; Oh, D.-Y.; Shen, L.; Kim, K.-P.; Liu, X.; Bai, Y.; Liao, H.; et al. Updated results of a phase IIa study to evaluate the clinical efficacy and safety of erdafitinib in Asian advanced cholangiocarcinoma (CCA) patients with FGFR alterations. J. Clin. Oncol. 2019, 37, 4117. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Bahleda, R.; Hierro, C.; Sanson, M.; Bridgewater, J.; Arkenau, H.-T.; Tran, B.; Kelley, R.K.; Park, J.O.; Javle, M.; et al. Futibatinib, an Irreversible FGFR1–4 Inhibitor, in Patients with Advanced Solid Tumors Harboring FGF/FGFR Aberrations: A Phase I Dose-Expansion Study. Cancer Discov. 2022, 12, 402–415. [Google Scholar] [CrossRef]
- Javle, M.M.; Abou-Alfa, G.K.; Macarulla, T.; Personeni, N.; Adeva, J.; Bergamo, F.; Malka, D.; Vogel, A.; Knox, J.J.; Evans, T.R.J.; et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma patients with FGFR2 mutations or amplifications: Interim results from the phase 2 study FIDES-01. J. Clin. Oncol. 2022, 40, 427. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Kawamoto, Y.; Komatsu, Y.; Ueno, M.; Kobayashi, S.; Ikeda, M.; Sasaki, M.; Furuse, J.; Okano, N.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). J. Clin. Oncol. 2022, 40, 4006. [Google Scholar] [CrossRef]
- Harding, J.J.; Piha-Paul, S.A.; Shah, R.H.; Cleary, J.M.; Quinn, D.I.; Brana, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; et al. Targeting HER2 mutation–positive advanced biliary tract cancers with neratinib: Final results from the phase 2 SUMMIT basket trial. J. Clin. Oncol. 2022, 40, 4079. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; Dubois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib inTRKFusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Cowzer, D.; Harding, J.J. Advanced Bile Duct Cancers: A Focused Review on Current and Emerging Systemic Treatments. Cancers 2022, 14, 1800. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.-I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; McShane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and Trametinib in Patients With Tumors With BRAFV600E Mutations: Results of the NCI-MATCH Trial Subprotocol H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef]
- Nam, A.-R.; Kim, J.-W.; Cha, Y.; Ha, H.; Park, J.E.; Bang, J.-H.; Jin, M.H.; Lee, K.-H.; Kim, T.-Y.; Han, S.-W.; et al. Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget 2016, 7, 58007–58021. [Google Scholar] [CrossRef] [PubMed]
- Farshidfar, F.; Zheng, S.; Gingras, M.C.; Newton, Y.; Shih, J.; Robertson, A.G.; Hinoue, T.; Hoadley, K.A.; Gibb, E.A.; Roszik, J.; et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Rep. 2017, 18, 2780–2794. [Google Scholar] [CrossRef]
- Okawa, Y.; Ebata, N.; Kim, N.K.D.; Fujita, M.; Maejima, K.; Sasagawa, S.; Nakamura, T.; Park, W.-Y.; Hirano, S.; Nakagawa, H. Actionability evaluation of biliary tract cancer by genome transcriptome analysis and Asian cancer knowledgebase. Oncotarget 2021, 12, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Fassan, M.; Ruzzenente, A.; Mafficini, A.; Wood, L.D.; Corbo, V.; Melisi, D.; Malleo, G.; Vicentini, C.; Malpeli, G.; et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 2014, 5, 2839–2852. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, Z.; Yao, R.; Cheng, Q.; Li, W.; Wu, R.; Xie, Z.; Zhu, Y.; Qiu, X.; Yang, S.; et al. Single-cell atlas of diverse immune populations in the advanced biliary tract cancer microenvironment. NPJ Precis. Oncol. 2022, 6, 58–62. [Google Scholar] [CrossRef]
- Meyers, D.E.; Jenei, K.; Chisamore, T.M.; Gyawali, B. Evaluation of the Clinical Benefit of Cancer Drugs Submitted for Reimbursement Recommendation Decisions in Canada. JAMA Intern. Med. 2021, 181, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technology in Health. CADTH Reimbursement Recommendation Pemigatinib (Pemazyre). Available online: https://www.cadth.ca/sites/default/files/DRR/2022/PC0252%20Pemazyre%20-%20CADTH%20Final%20Rec.pdf (accessed on 9 June 2022).
- Canadian Agency for Drugs and Technology in Health. CADTH Reimbursement Recommendation Larotrectinib (Vitrakvi). Available online: https://www.cadth.ca/sites/default/files/DRR/2021/PC0221%20Vitrakvi%20-%20CADTH%20Final%20Rec%20KG_NA_Corrected-meta.pdf (accessed on 9 June 2022).
- Demols, A.; Rocq, L.; Charry, M.; De Nève, N.; Verrellen, A.; Ramadhan, A.; Van Campenhout, C.; De Clercq, S.; Salmon, I.; D’Haene, N. NTRK gene fusions in biliary tract cancers. J. Clin. Oncol. 2020, 38, 574. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Detecting and targeting NTRK gene fusions in cholangiocarcinoma: News and perspectives. Expert Rev. Precis. Med. Drug Dev. 2021, 6, 225–227. [Google Scholar] [CrossRef]
- Ellis, L.M.; Bernstein, D.S.; Voest, E.E.; Berlin, J.D.; Sargent, D.; Cortazar, P.; Garrett-Mayer, E.; Herbst, R.S.; Lilenbaum, R.C.; Sima, C.; et al. American Society of Clinical Oncology Perspective: Raising the Bar for Clinical Trials by Defining Clinically Meaningful Outcomes. J. Clin. Oncol. 2014, 32, 1277–1280. [Google Scholar] [CrossRef]
- Riby, D.; Mazzotta, A.D.; Bergeat, D.; Verdure, L.; Sulpice, L.; Bourien, H.; Lièvre, A.; Rolland, Y.; Garin, E.; Boudjema, K.; et al. Downstaging with Radioembolization or Chemotherapy for Initially Unresectable Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 3729–3737. [Google Scholar] [CrossRef]
- Le Roy, B.; Gelli, M.; Pittau, G.; Allard, M.A.; Pereira, B.; Serji, B.; Vibert, E.; Castaing, D.; Adam, R.; Cherqui, D.; et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br. J. Surg. 2018, 105, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Bagul, A.; Pollard, C.; Dennison, A.R. A review of problems following insertion of biliary stents illustrated by an unusual complication. Ann. R. Coll. Surg. Engl. 2010, 92, e27–e31. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Lie, X.; Gwaltney, C.; Rokutanda, N.; Barzi, A.; Melisi, D.; Macarulla, T.; Ueno, M.; Kim, S.T.; Meyers, O.; et al. Understanding Patient Experience in Biliary Tract Cancer: A Qualitative Patient Interview Study. Oncol. Ther. 2021, 9, 557–573. [Google Scholar] [CrossRef] [PubMed]
- European Society for Medical Oncology. ESMO-MCBS Evaluation Forms. Available online: https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms (accessed on 9 June 2022).
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Damuzzo, V.; Agnoletto, L.; Leonardi, L.; Chiumente, M.; Mengato, D.; Messori, A. Analysis of Survival Curves: Statistical Methods Accounting for the Presence of Long-Term Survivors. Front. Oncol. 2019, 9, 453–458. [Google Scholar] [CrossRef]
- European Society for Medical Oncology. ESMO-Magnitude of Clinical Benefit Scale V1.1 Evaluation form 2A for Therapies That Are Not Likely to Be Curative with Primary Endpoint of OS. Available online: https://www.esmo.org/content/download/117387/2059146/1/ESMO-MCBS-Version-1-1-Evaluation-Form-2a-OS-12-Months.pdf (accessed on 9 June 2022).
- European Society for Medical Oncology. ESMO-MCBS Scorecards: Durvalumab. Available online: https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-scorecards/scorecard-350-1 (accessed on 25 September 2022).
- Gennari, A.; Stockler, M.; Puntoni, M.; Sormani, M.; Nanni, O.; Amadori, D.; Wilcken, N.; D’Amico, M.; Decensi, A.; Bruzzi, P. Duration of Chemotherapy for Metastatic Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Oncol. 2011, 29, 2144–2149. [Google Scholar] [CrossRef]
- Doherty, M.K.; McNamara, M.G.; Aneja, P.; McInerney, E.; Moignard, S.; Horgan, A.M.; Jiang, H.; Panzarella, T.; Jang, R.; Dhani, N.; et al. Long term responders to palliative chemotherapy for advanced biliary tract cancer. J. Gastrointest. Oncol. 2017, 8, 352–360. [Google Scholar] [CrossRef]
- Hyung, J.; Kim, B.; Yoo, C.; Kim, K.-P.; Jeong, J.H.; Chang, H.-M.; Ryoo, B.-Y. Clinical Benefit of Maintenance Therapy for Advanced Biliary Tract Cancer Patients Showing No Progression after First-Line Gemcitabine Plus Cisplatin. Cancer Res. Treat. 2019, 51, 901–909. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).