Treatment Algorithm in Cancer-Associated Thrombosis: Updated Canadian Expert Consensus
Abstract
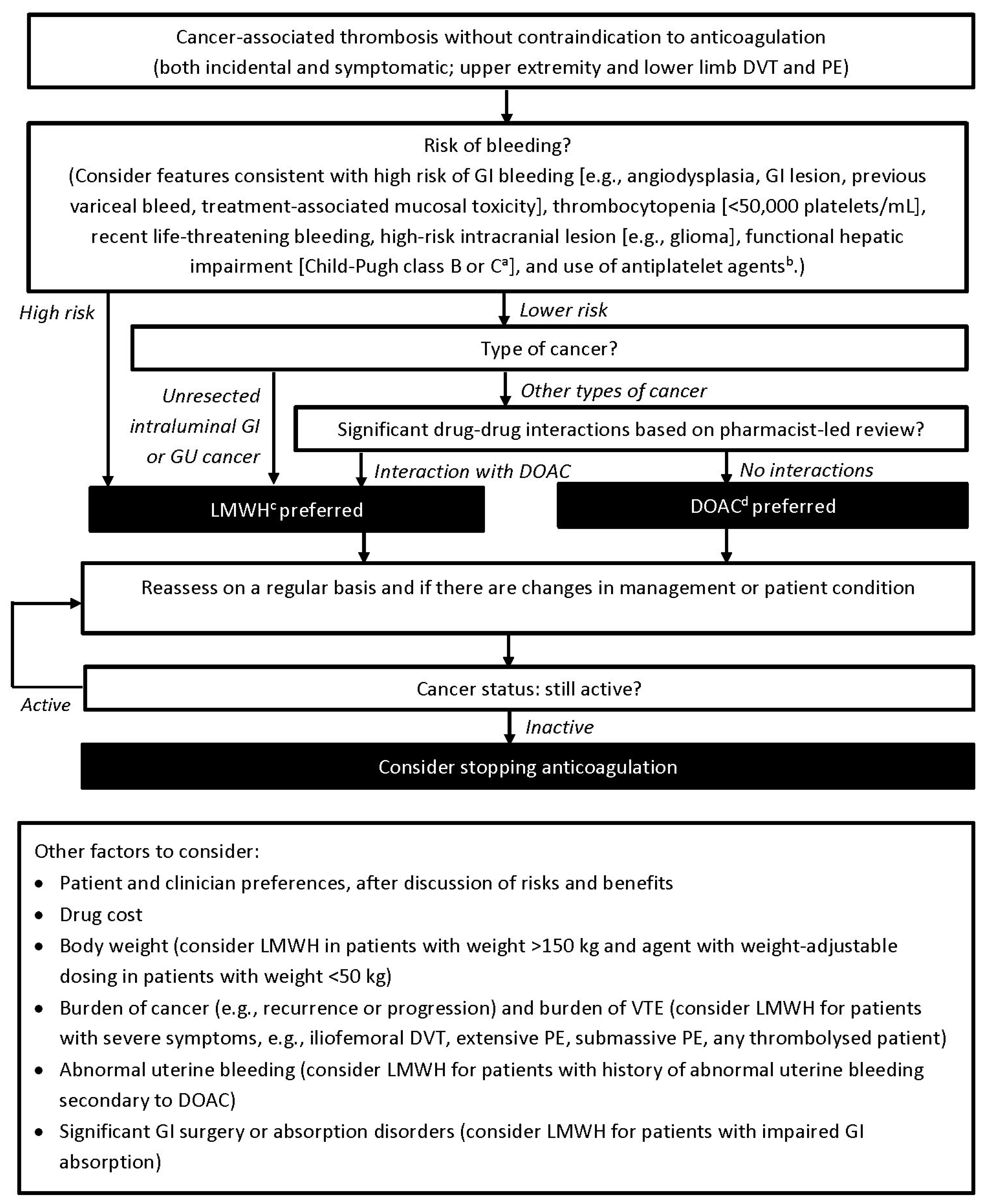
:1. Introduction
2. Methods
2.1. Literature Review
2.2. Revision of Treatment Algorithm
2.3. Role of the Funding Sources
3. Results
4. Discussion
4.1. Efficacy and Safety of Anticoagulants
4.2. Incidental VTE
4.3. Upper Extremity and Catheter-Related VTE
4.4. Risk of Bleeding
4.4.1. Features Consistent with a High Risk of GI Bleeding
4.4.2. Thrombocytopenia
4.4.3. Intracranial Lesions
4.4.4. Hepatic and Renal Impairment
4.4.5. Use of Antiplatelet Agents
4.5. Drug–Drug Interactions
4.6. Other Factors to Consider
4.6.1. Patient and Clinician Preference and Drug Cost
4.6.2. Body Weight
4.6.3. Burden of Cancer and Burden of VTE
4.6.4. Abnormal Uterine Bleeding
4.6.5. Significant GI Surgery or Absorption Disorders
4.7. Reassessing Treatment for Secondary Prophylaxis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Mulder, F.I.; Horváth-Puhó, E.; van Es, N.; van Laarhoven, H.W.M.; Pedersen, L.; Moik, F.; Ay, C.; Büller, H.R.; Sørensen, H.T. Venous thromboembolism in cancer patients: A population-based cohort study. Blood 2021, 137, 1959–1969. [Google Scholar] [CrossRef]
- Horsted, F.; West, J.; Grainge, M.J. Risk of venous thromboembolism in patients with cancer: A systematic review and meta-analysis. PLoS Med. 2012, 9, e1001275. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007, 111, 2339–2346. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, H.T.; Mellemkjaer, L.; Olsen, J.H.; Baron, J.A. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000, 343, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Bohlke, K.; Khorana, A.A.; Kuderer, N.M.; Lee, A.Y.; Arcelus, J.I.; Balaban, E.P.; Clarke, J.M.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J. Clin. Oncol. 2015, 33, 654–656. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar]
- Lee, A.Y.Y.; Kamphuisen, P.W.; Meyer, G.; Bauersachs, R.; Janas, M.S.; Jarner, M.F.; Khorana, A.A.; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: A randomized clinical trial. JAMA 2015, 314, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Mismetti, P.; Schellong, S.; Eriksson, H.; Baanstera, D.; Schnee, J.; Goldhaber, S.Z.; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N. Engl. J. Med. 2009, 361, 2342–2352. [Google Scholar]
- Einstein, Investigators; Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Büller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar] [PubMed] [Green Version]
- Einstein-PE Investigators; Büller, H.R.; Prins, M.H.; Lensing, A.W.A.; Decousus, H.; Jacobson, B.F.; Minar, E.; Chlumsky, J.; Verhamme, P.; Wells, P.; et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 2012, 366, 1287–1297. [Google Scholar]
- Agnelli, G.; Büller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Masiukiewicz, U.; Pak, R.; Thompson, J.; Raskob, G.E.; et al. Oral apixaban for the treatment of acute venous thromboembolism. N. Engl. J. Med. 2013, 369, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Hokusai-VTE Investigators; Büller, H.R.; Decousus, H.; Grosso, M.A.; Mercuri, M.; Middeldorp, S.; Prins, M.H.; Raskob, G.E.; Schellong, S.M.; Schwocho, L.; et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N. Engl. J. Med. 2013, 369, 1406–1415. [Google Scholar] [PubMed] [Green Version]
- Schulman, S.; Kakkar, A.K.; Goldhaber, S.Z.; Schellong, S.; Eriksson, H.; Mismetti, P.; Christiansen, A.V.; Friedman, J.; Le Maulf, F.; Peter, N.; et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014, 129, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Carrier, M.; Blais, N.; Crowther, M.; Kavan, P.; Le Gal, G.; Moodley, O.; Shivakumar, S.; Tagalakis, V.; Wu, C.; Lee, A.Y.Y. Treatment algorithm in cancer-associated thrombosis: Canadian expert consensus. Curr. Oncol. 2018, 25, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef] [Green Version]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Bockenstedt, P.L.; Chesney, C.; Fanikos, J.; Fenninger, R.B.; Fogerty, A.E.; Gao, S.; et al. NCCN guidelines insights: Cancer-associated venous thromboembolic disease, version 2.2018. J. Natl. Compr. Canc. Netw. 2018, 16, 1289–1303. [Google Scholar] [CrossRef] [Green Version]
- Meyer, G.; Marjanovic, Z.; Valcke, J.; Lorcerie, B.; Gruel, Y.; Solal-Celigny, P.; Le Maignan, C.; Extra, J.M.; Cottu, P.; Fargel, D. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch. Intern. Med. 2002, 162, 1729–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deitcher, S.R.; Kessler, C.M.; Merli, G.; Rigas, J.R.; Lyons, R.M.; Fareed, J.; ONCENOX Investigators. Secondary prevention of venous thromboembolic events in patients with active cancer: Enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin. Appl. Thromb. Hemost. 2006, 12, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.D.; Pineo, G.F.; Brant, R.F.; Mah, A.F.; Burke, N.; Dear, R.; Wong, T.; Cook, R.; Solymoss, S.; Poon, M.C.; et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am. J. Med. 2006, 119, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- McBane, R.D., II; Wysokinski, W.E.; Le-Rademacher, J.G.; Zemla, T.; Ashrani, A.; Tafur, A.; Perepu, U.; Anderson, D.; Gundabolu, K.; Kuzma, C.; et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J. Thromb. Haemost. 2020, 18, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Planquette, B.; Bertoletti, L.; Charles-Nelson, A.; Laporte, S.; Grange, C.; Mahé, I.; Pernod, G.; Elias, A.; Couturaud, F.; Falvo, N.; et al. Rivaroxaban vs dalteparin in cancer-associated thromboembolism: A randomized trial. Chest 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Cameron, C.; Delluc, A.; Castellucci, L.; Khorana, A.A.; Lee, A.Y. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer-associated thrombosis: A systematic review and meta-analysis. J. Thromb. Res. 2014, 134, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Davis, W.M.; Ratnaweera, N.; Szefer, E.; Ballantyne Scott, B.; Lee, A.Y.Y. A meta-analysis of case fatality rates of recurrent venous thromboembolism and major bleeding in patients with cancer. J. Thromb. Haemost. 2020, 120, 702–713. [Google Scholar] [CrossRef]
- Caiano, L.; Carrier, M.; Marshall, A.; Young, A.M.; Ageno, W.; Delluc, A.; Wang, T.-F. Outcomes among patients with cancer and incidental or symptomatic venous thromboembolism: A systematic review and meta-analysis. J. Thromb. Haemost. 2021, 19, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.I.; Di Nisio, M.; Ay, C.; Carrier, M.; Bosch, F.T.M.; Segers, A.; Kraaijpoel, N.; Grosso, M.A.; Zhang, G.; Verhamme, P.; et al. Clinical implications of incidental venous thromboembolism in cancer patients. Eur. Respir. J. 2020, 55, 1901697. [Google Scholar] [CrossRef] [PubMed]
- Spirk, D.; Sebastian, T.; Barco, S.; Banyai, M.; Beer, J.H.; Mazzolai, L.; Baldi, T.; Aujesky, D.; Hayoz, D.; Engelberger, R.P.; et al. Clinical Outcomes of Incidental Venous thromboembolism in cancer and noncancer patients: The SWIss Venous ThromboEmbolism Registry (SWIVTER). J. Thromb. Haemost. 2021, 121, 641–649. [Google Scholar] [CrossRef]
- Kovacs, M.J.; Kahn, S.R.; Rodger, M.; Anderson, D.R.; Andreou, R.; Mangel, J.E.; Morrow, B.; Clement, A.M.; Wells, P.S. A pilot study of central venous catheter survival in cancer patients using low-molecular-weight heparin (dalteparin) and warfarin without catheter removal for the treatment of upper extremity deep vein thrombosis (The Catheter Study). J. Thromb. Haemost. 2007, 5, 1650–1653. [Google Scholar] [CrossRef]
- Delluc, A.; Le Gal, G.; Scarvelis, D.; Carrier, M. Outcome of central venous catheter associated upper extremity deep vein thrombosis in cancer patients. Thromb. Res. 2015, 135, 298–302. [Google Scholar] [CrossRef]
- Davies, G.A.; Lazo-Langner, A.; Gandara, E.; Rodger, M.; Tagalakis, V.; Louzada, M.; Corpuz, R.; Kovacs, M.J. A prospective study of rivaroxaban for central venous catheter associated upper extremity deep vein thrombosis in cancer patients (Catheter 2). Thromb Res. 2018, 162, 88–92. [Google Scholar] [CrossRef]
- Vedovati, M.C.; Tratar, G.; Mavri, A.; Mazzetti, M.; Rosa, V.S.; Pierpaoli, L.; Cotugno, M.; Agnelli, G.; Becattini, C. Upper extremities deep vein thrombosis treated with oral direct anticoagulants: A prospective cohort study. Int. J. Cardiol. 2021, 339, 158–163. [Google Scholar] [CrossRef]
- Mulder, F.I.; Bosch, F.T.M.; Young, A.M.; Marshall, A.; McBane, R.D.; Zemla, T.J.; Carrier, M.; Kamphuisen, P.W.; Bossuyt, P.M.M.; Büller, H.R.; et al. Direct oral anticoagulants for cancer-associated venous thromboembolism: A systematic review and meta-analysis. Blood 2020, 136, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Kraaijpoel, N.; Di Nisio, M.; Mulder, F.I.; van Es, N.; Beyer-Westendorf, J.; Carrier, M.; Garcia, D.; Grosso, M.; Kakkar, A.K.; Mercuri, M.F.; et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE Cancer study. Thromb. Haemost. 2018, 118, 1439–1449. [Google Scholar] [CrossRef]
- Ageno, W.; Vedovati, M.C.; Cohen, A.; Huisman, M.; Bauersachs, R.; Gussoni, G.; Becattini, C.; Agnelli, G. Bleeding with apixaban and dalteparin in patients with cancer-associated venous thromboembolism: Results from the CARAVAGGIO study. Thromb. Haemost. 2021, 121, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.E.; Vlazny, D.T.; Casanegra, A.I.; Brunton, N.; Froehling, D.A.; Meverden, R.A.; Hodge, D.O.; Peterson, L.G.; McBane, R.D.; Wysokinski, W.E. Bleeding in patients with gastrointestinal cancer compared to non-gastrointestinal cancer treated with apixaban, rivaroxaban, or enoxaparin for acute venous thromboembolism. Mayo Clin. Proc. 2021, 96, 2793–2805. [Google Scholar] [CrossRef]
- Kamphuisen, P.W.; Lee, A.Y.Y.; Meyer, G.; Bauersachs, R.; Janas, M.S.; Jarner, M.F.; Khorana, A.A.; CATCH Investigators. Clinically relevant bleeding in cancer patients treated for venous thromboembolism from the CATCH study. J. Thromb. Haemost. 2018, 16, 1069–1077. [Google Scholar]
- Samuelson Bannow, B.R.; Lee, A.Y.Y.; Khorana, A.A.; Zwicker, J.I.; Noble, S.; Ay, C.; Carrier, M. Management of anticoagulation for cancer-associated thrombosis in patients with thrombocytopenia: A systematic review. Res. Pr. Thromb. Haemost. 2018, 2, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Samuelson Bannow, B.T.; Lee, A.; Khorana, A.A.; Zwicker, J.I.; Noble, S.; Ay, C.; Carrier, M. Management of cancer-associated thrombosis in patients with thrombocytopenia: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1246–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carney, B.J.; Uhlmann, E.J.; Puligandla, M.; Mantia, C.; Weber, G.M.; Neuberg, D.S.; Zwicker, J.I. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J. Thromb. Haemost. 2019, 17, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leader, A.; Hamulyák, E.N.; Carney, B.J.; Avrahami, M.; Knip, J.J.; Rozenblatt, S.; Beenen, L.F.M.; Yust-Katz, S.; Icht, O.; Coppens, M.; et al. Intracranial hemorrhage with direct oral anticoagulants in patients with brain metastases. Blood Adv. 2020, 4, 6291–6297. [Google Scholar] [CrossRef]
- Swartz., A.W.; Drappatz, J. Safety of direct oral anticoagulants in central nervous system malignancies. Oncologist 2021, 26, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Bayer Inc. XARELTO (Rivaroxaban Tablets) Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00059600.PDF (accessed on 19 May 2021).
- Pfizer Canada ULC. ELIQUIS (Apixaban Tablets) Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00053440.PDF (accessed on 19 May 2021).
- Servier Canada Inc. LIXIANA (Edoxaban Tablets [as Edoxaban Tosylate Monohydrate]) Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00055048.PDF (accessed on 19 May 2021).
- Pfizer Canada ULC. FRAGMIN (Dalteparin Sodium Injection) Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00057038.PDF (accessed on 19 May 2021).
- Sanofi-Aventis Canada Inc. LOVENOX (Enoxaparin Sodium for Injection, Manufacturer’s Standard) Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00054401.PDF (accessed on 19 May 2021).
- LEO Pharma Inc. INNOHEP (Tinzaparin Sodium) Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00040736.PDF (accessed on 19 May 2021).
- Lanas, A.; Wu, P.; Medin, J.; Mills, E.J. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clin. Gastroenterol. Hepatol. 2011, 9, 762–768.e6. [Google Scholar] [CrossRef]
- García Rodríguez, L.A.; Lin, L.J.; Hernández-Díaz, S.; Johansson, S. Risk of upper gastrointestinal bleeding with low-dose acetylsalicylic acid alone and in combination with clopidogrel and other medications. Circulation 2011, 123, 1108–1115. [Google Scholar] [CrossRef] [Green Version]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [Green Version]
- Peixoto de Miranda, E.J.F.; Takahashi, T.; Iwamoto, F.; Yamashiro, S.; Samano, E.; Scarlatelli Macedo, A.V.; Ramacciotti, E. Drug–Drug interactions of 257 antineoplastic and supportive care agents with 7 anticoagulants: A comprehensive review of interactions and mechanisms. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620936325. [Google Scholar] [CrossRef]
- Li, A.; Li, M.K.; Crowther, M.; Vazquez, S.R. Drug-drug interactions with direct oral anticoagulants associated with adverse events in the real world: A systematic review. Thromb. Res. 2020, 194, 240–245. [Google Scholar] [CrossRef]
- Verso, M.; Munoz, A.; Bauersachs, R.; Huisman, M.V.; Mandalà, M.; Vescovo, G.; Becattini, C.; Agnelli, G. Effects of concomitant administration of anticancer agents and apixaban or dalteparin on recurrence and bleeding in patients with cancer-associated venous thromboembolism. Eur. J. Cancer 2021, 148, 371–381. [Google Scholar] [CrossRef]
- Wang, T.F.; Baumann, K.; Leader, A.; Spectre, G.; Lim, M.Y.; Gahagan, A.; Gangaraju, R.; Sanfilippo, K.M.; Mallick, M.; Zwicker, J.I.; et al. Characteristics and outcomes of patients on concurrent direct oral anticoagulants and targeted anticancer therapies—TacDOAC registry: Communication from the ISTH SSC Subcommittee on Hemostasis and Malignancy. J. Thromb. Haemost. 2021, 19, 2068–2081. [Google Scholar] [CrossRef]
- Patel, S.H.; George, T.L.; Wang, T.-F.; Vogt, S.M.; Folefac, E.; Xu, M.; Yang, Y.; Parikh, A.B.; Verschraegen, C.F.; Clinton, S.K.; et al. Increased bleeding risk associated with concurrent vascular endothelial growth factor receptor tyrosine kinase inhibitors and low-molecular-weight heparin. Cancer 2021, 127, 938–945. [Google Scholar] [CrossRef]
- Marcath, L.A.; Xi, J.; Hoylman, E.K.; Kidwell, K.M.; Kraft, S.L.; Hertz, D.L. Comparison of nine tools for screening drug-drug interactions of oral oncolytics. J. Oncol. Pr. 2018, 14, e368–e374. [Google Scholar] [CrossRef] [PubMed]
- Bossaer, J.B.; Thomas, C.M. Drug interaction database sensitivity with oral antineoplastics: An exploratory analysis. J. Oncol. Pr. 2017, 13, e217–e222. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C.; Spencer, F.A.; Gibson, M.; Rush, J.E.; Sanderink, G.; Murphy, S.A.; Ball, S.P.; Antman, E.M.; TIMI 11A Investigators. Influence of patient characteristics and renal function on factor Xa inhibition pharmacokinetics and pharmacodynamics after enoxaparin administration in non-ST-segment elevation acute coronary syndromes. Am. Heart J. 2002, 143, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Wilbur, K.; Burton, E.; Anderson, D.R. Effect of patient weight on the anticoagulant response to adjusted therapeutic dosage of low-molecular-weight heparin for the treatment of venous thromboembolism. Haemostasis 2001, 31, 42–48. [Google Scholar] [CrossRef]
- Hainer, J.W.; Barrett, J.S.; Assaid, C.A.; Fossler, M.J.; Cox, D.S.; Leathers, T.; Leese, P.T. Dosing in heavyweight/obese patients with the LMWH, tinzaparin: A pharmacodynamic study. J. Thromb. Haemost. 2002, 87, 817–823. [Google Scholar]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012, 141, e24S–e43S, Erratum in Chest 2012, 141, 1369 and Chest 2013, 144, 721. [Google Scholar] [CrossRef] [Green Version]
- Easaw, J.C.; Shea-Budgell, M.A.; Wu, C.M.J.; Czaykowski, P.M.; Kassis, J.; Kuehl, B.; Lim, H.J.; MacNeil, M.; Martinusen, D.; McFarlane, P.A.; et al. Canadian consensus recommendations on the management of venous thromboembolism in patients with cancer. Part 2: Treatment. Curr. Oncol. 2015, 22, 144–155. [Google Scholar] [CrossRef]
- Martin, K.A.; Beyer-Westendorf, J.; Davidson, B.L.; Huisman, M.V.; Sandset, P.M.; Moll, S. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: Updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J. Thromb. Haemost. 2021, 19, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Samuelson Bannow, B. Management of heavy menstrual bleeding on anticoagulation. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 533–537. [Google Scholar] [CrossRef]
- Godin, R.; Marcoux, V.; Tagalakis, V. Abnormal uterine bleeding in women receiving direct oral anticoagulants for the treatment of venous thromboembolism. Vasc. Pharm. 2017, 93-95, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Leven, C.; Hoffmann, C.; Roche, C.; Couturaud, F.; Thereaux, J.; Lacut, K. Impact of bariatric surgery on oral anticoagulants pharmacology, and consequences for clinical practice: A narrative review. Fundam. Clin. Pharm. 2021, 35, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.W.; Kessler, C.M.; Goldhaber, S.Z.; Kovacs, M.J.; Monreal, M.; Huisman, M.V.; Bergqvist, D.; Turpie, A.G.; Ortel, T.L.; Spyropoulos, A.C.; et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: The DALTECAN study. J. Thromb. Haemost. 2015, 13, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palomares, L.; Solier-Lopez, A.; Elias-Hernandez, T.; Asensio-Cruz, M.; Blasco-Esquivias, I.; Marin-Barrera, L.; Rodriguez de la Borbolla-Artacho, M.; Praena-Fernandez, J.M.; Montero-Romero, E.; Navarro-Herrero, S.; et al. Tinzaparin in cancer associated thrombosis beyond 6 months: TICAT study. Thromb. Res. 2017, 157, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Marshall, A.; Levine, M.; Hill, C.; Hale, D.; Thirlwall, J.; Wilkie, V.; French, K.; Kakkar, A.; Lokare, A.; Maraveyas, A.; et al. Treatment of cancer-associated venous thromboembolism: 12-month outcomes of the placebo versus rivaroxaban randomization of the SELECT-D trial (SELECT-D: 12m). J. Thromb. Haemost. 2020, 18, 905–915. [Google Scholar] [CrossRef]
- Chee, C.E.; Ashrani, A.A.; Marks, R.S.; Petterson, T.M.; Bailey, K.R.; Melton, L.J.; Heit, J.A. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: A population-based cohort study. Blood 2014, 123, 3972–3978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, R.A.; Al Zaki, A.; Desilet, N.; Szefer, E.; Ratnaweera, N.; Peterson, E.; Lee, A.Y.Y. Patient characteristics and long-term outcomes beyond the first 6 months after a diagnosis of cancer-associated venous thromboembolism. Thomb. Res. 2020, 188, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Büller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Porcari, A.; Raskob, G.E.; Weitz, J.I.; AMPLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N. Engl. J. Med. 2013, 368, 699–708. [Google Scholar] [PubMed]
- Weitz, J.I.; Lensing, A.W.A.; Prins, M.H.; Bauersachs, R.; Beyer-Westerdorf, J.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N. Engl. J. Med. 2017, 376, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health—US National Library of Medicine. API-CAT STUDY for APIxaban Cancer Associated Thrombosis (API-CAT). Available online: https://clinicaltrials.gov/ct2/show/NCT03692065 (accessed on 23 August 2021).
| Reference (Study Name) | Patients (n) | Intervention | Duration (Months) | Major Bleeding (%) b | Recurrent VTE (%) b | Death (%) b |
|---|---|---|---|---|---|---|
| LMWH compared with VKA | ||||||
| Meyer et al. 2002 (CANTHANOX) [24] | 67 | Enoxaparin 1.5 mg/kg daily | 3 | 7 | 3 | 22.7 |
| 71 | VKA | 16 | 4.2 | 11.3 | ||
| Lee et al., 2003 (CLOT) [8] | 336 | Dalteparin 200 IU/kg daily for 1 month, and then 150 IU/kg | 6 | 4 | 9 | 39 |
| 336 | VKA | 6 | 17 | 41 | ||
| Deitcher et al. 2006 (ONCENOX) a [25] | 29 | Enoxaparin 1 mg/kg daily | 3 | 6.5 | 6.9 | 6.5 |
| 32 | Enoxaparin 1.5 mg/kg daily | 11.1 | 6.3 | 19.4 | ||
| 30 | VKA | 2.9 | 10 | 8.8 | ||
| Hull et al. 2006 (LITE) [26] | 100 | Tinzaparin 175 IU/kg daily | 3 | 7 | 6 | 19 |
| 100 | VKA | 7 | 10 | 20 | ||
| Lee et al. 2015 (CATCH) [9] | 449 | Tinzaparin 175 IU/kg daily | 6 | 2.7 | 7.2 | 33 |
| 451 | VKA | 2.4 | 10.5 | 31 | ||
| DOAC compared with LMWH | ||||||
| Raskob et al. 2018 (Hokusai-VTE Cancer) [16] | 522 | LMWH for ≥5 days, and then edoxaban 60 mg daily | 12 | 6.9 | 7.9 | 39.5 |
| 524 | Dalteparin 200 IU/kg daily for 1 month, and then 150 IU/kg | 4.0 | 11.3 | 36.6 | ||
| Young et al. 2018 (SELECT-D) [17] | 203 | Rivaroxaban 15 mg twice daily for 3 weeks, and then 20 mg daily | 6 | 6 | 4 | 25 |
| 203 | Dalteparin 200 IU/kg daily for 1 month, and then 150 IU/kg | 4 | 11 | 30 | ||
| McBane et al. 2020 (ADAM-VTE) [27] | 145 | Apixaban 10 mg twice daily for 7 days, and then 5 mg twice daily | 6 | 0 | 0.7 | 16 |
| 142 | Dalteparin 200 IU/kg daily for 1 month, and then 150 IU/kg | 1.4 | 6.3 | 11 | ||
| Agnelli et al. 2020 (CARAVAGGIO) [18] | 576 | Apixaban 10 mg twice daily for 7 days, and then 5 mg twice daily | 6 | 3.8 | 5.6 | 23.4 |
| 579 | Dalteparin 200 IU/kg daily for 1 month, and then 150 IU/kg | 4.0 | 7.9 | 26.4 | ||
| Planquette et al. 2021 (CASTA-DIVA) [28] | 74 | Rivaroxaban 15 mg twice daily for 3 weeks, and then 20 mg daily | 3 | 1.4 | 6.0 | 25.7 |
| 84 | Dalteparin 200 IU/kg daily for 1 month, and then 150 IU/kg | 3.7 | 9.5 | 23.8 | ||
| Anticoagulant | Creatinine Clearance (mL/min) | |||
|---|---|---|---|---|
| <15 or Dialysis | 15–29 | 30–50 | >50 | |
| LMWH | ||||
| Dalteparin [51] | Dose reduction should be considered a | Dose reduction should be considered a | 200 IU/kg once daily for 1 month, and then 150 IU/kg | 200 IU/kg once daily for 1 month, and then 150 IU/kg |
| Enoxaparin [52] | 100 IU/kg once daily | 100 IU/kg once daily | 100 IU/kg twice daily | 100 IU/kg twice daily |
| Tinzaparin [53] | 175 IU/kg once daily a | 175 IU/kg once daily a | 175 IU/kg once daily | 175 IU/kg once daily |
| DOAC | ||||
| Apixaban [49] | Not recommended | 10 mg twice daily for 7 days, and then 5 mg twice daily b | 10 mg twice daily for 7 days, and then 5 mg twice daily b | 10 mg twice daily for 7 days, and then 5 mg twice daily b |
| Edoxaban [50] | Not recommended | Not recommended | 30 mg once daily (following initial 5–10 days of LMWH) | 60 mg once daily (following initial 5–10 days of LMWH) |
| Rivaroxaban [48] | Not recommended | 15 mg twice daily for 3 weeks, and then 20 mg once daily b | 15 mg twice daily for 3 weeks, and then 20 mg once daily b | 15 mg twice daily for 3 weeks, and then 20 mg once daily b |
| Interacting Drug | Outcome | Proposed Mechanism of Interaction |
|---|---|---|
| Acalabrutinib | ↑ bleeding risk | Weak CYP3A4 inhibitor/antiplatelet effect |
| Amiodarone | ↑ bleeding risk | Weak CYP3A4/P-gp inhibitor |
| Carbamazepine | ↓ antithrombotic efficacy | Strong CYP3A4/P-gp inducer |
| Clarithromycin | ↑ bleeding risk | Strong CYP3A4/P-gp inhibitor |
| Cyclosporine | ↑ bleeding risk | Weak CYP3A4/P-gp inhibitor |
| Diltiazem | ↑ bleeding risk | Moderate CYP3A4/P-gp inhibitor |
| Efavirenz | ↓ antithrombotic efficacy | Moderate CYP3A4 inducer |
| Fluconazole | ↑ bleeding risk | Moderate CYP3A4 inhibitor |
| Ibrutinib | ↑ bleeding risk | Weak CYP3A4/P-gp inhibitor/antiplatelet effect |
| Loperamide | ↑ bleeding risk | Mechanism unclear |
| Miconazole (topical) | ↑ bleeding risk | Mechanism unclear |
| Nevirapine | ↓ antithrombotic efficacy | Weak CYP3A4 inducer |
| Oxcarbazepine | ↓ antithrombotic efficacy | Weak CYP3A4 inducer |
| Phenobarbital | ↓ antithrombotic efficacy | Strong CYP3A4 inducer |
| Phenytoin | ↓ antithrombotic efficacy | Strong CYP3A4/P-gp inducer |
| Quinidine | ↑ bleeding risk | Moderate P-gp inhibitor |
| Rifampicin | ↓ antithrombotic efficacy | Strong CYP3A4/P-gp inducer |
| Ritonavir | ↑ bleeding risk | Strong CYP3A4/P-gp inhibitor |
| Tocilizumab | ↓ antithrombotic efficacy | Indirect P-gp inducer |
| Verapamil | ↑ bleeding risk | Moderate CYP3A4/P-gp inhibitor |
| Reference (Study Name) | Anticoagulant | Age (Years) | Metastatic Cancer (%) | Cancer Therapy (%) | ECOG PS 2 (%) | Top 3 Cancer Types |
|---|---|---|---|---|---|---|
| Lee et al., 2003 (CLOT) [8] | Dalteparin | 62 | 66 | 79 | 35 | Breast Colorectal Lung |
| VKA | 63 | 69 | 77 | 36 | ||
| Lee et al., 2015 (CATCH) [9] | Tinzaparin | 60 | 66 | 51 | 24 | Gynecologic Lung Upper GI |
| VKA | 59 | 63 | 55 | 23 | ||
| Raskob et al., 2018 (Hokusai-VTE Cancer) [16] | Edoxaban | 64 | 52 | 72 | 24 | Colorectal Lung Genitourinary |
| Dalteparin | 64 | 53 | 63 | 24 | ||
| Young et al., 2018 (SELECT-D) [17] | Rivaroxaban | 67 | 58 | 69 | 26 | Colorectal Lung Breast |
| Dalteparin | 67 | 58 | 70 | 21 | ||
| McBane et al., 2020 (ADAM-VTE) [27] | Apixaban | 64 | 65 | 73 | 13 | Colorectal Lung Pancreatic |
| Dalteparin | 64 | 66 | 74 | 8 | ||
| Agnelli et al., 2020 (CARAVAGGIO) [18] | Apixaban | 67 | 68 a | 61 | 19 | Colorectal Lung Breast |
| Dalteparin | 67 | 68 a | 63 | 23 | ||
| Planquette et al., 2021 (CASTA-DIVA) [28] | Rivaroxaban | 69 | 77 | 70 | NR | Colorectal Lung Breast |
| Dalteparin | 71 | 76 | 74 | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrier, M.; Blais, N.; Crowther, M.; Kavan, P.; Le Gal, G.; Moodley, O.; Shivakumar, S.; Suryanarayan, D.; Tagalakis, V.; Wu, C.; et al. Treatment Algorithm in Cancer-Associated Thrombosis: Updated Canadian Expert Consensus. Curr. Oncol. 2021, 28, 5434-5451. https://doi.org/10.3390/curroncol28060453
Carrier M, Blais N, Crowther M, Kavan P, Le Gal G, Moodley O, Shivakumar S, Suryanarayan D, Tagalakis V, Wu C, et al. Treatment Algorithm in Cancer-Associated Thrombosis: Updated Canadian Expert Consensus. Current Oncology. 2021; 28(6):5434-5451. https://doi.org/10.3390/curroncol28060453
Chicago/Turabian StyleCarrier, Marc, Normand Blais, Mark Crowther, Petr Kavan, Grégoire Le Gal, Otto Moodley, Sudeep Shivakumar, Deepa Suryanarayan, Vicky Tagalakis, Cynthia Wu, and et al. 2021. "Treatment Algorithm in Cancer-Associated Thrombosis: Updated Canadian Expert Consensus" Current Oncology 28, no. 6: 5434-5451. https://doi.org/10.3390/curroncol28060453
APA StyleCarrier, M., Blais, N., Crowther, M., Kavan, P., Le Gal, G., Moodley, O., Shivakumar, S., Suryanarayan, D., Tagalakis, V., Wu, C., & Lee, A. Y. Y. (2021). Treatment Algorithm in Cancer-Associated Thrombosis: Updated Canadian Expert Consensus. Current Oncology, 28(6), 5434-5451. https://doi.org/10.3390/curroncol28060453





