COVID-19 Pneumonia on Post-Operative Day 2 after Esophagectomy: Performing Esophago-Gastric Junction Cancer Surgery during the SARS-Cov-2 Second Wave
Abstract
1. Introduction
2. Materials and Methods
3. Case Reports
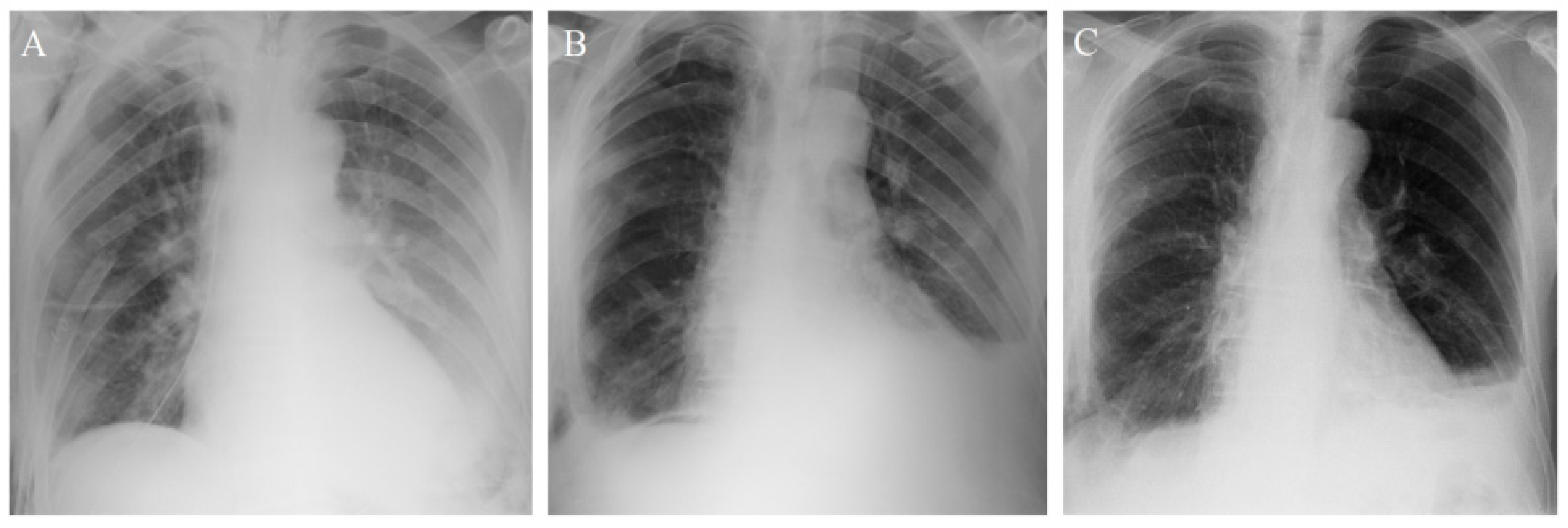
3.1. Patient 1
3.2. Patient 2
3.3. Patient 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- We have therefore made the assessment that #COVID19 can be characterized as a pandemic-@DrTedros #coronavirus pic.twitter.com/JqdsM2051A—World Health Organization (WHO) (@WHO). Available online: https://twitter.com/WHO/status/1237777021742338049?s=20 (accessed on 11 March 2020).
- Bin Traiki, T.A.; AlShammari, S.A.; AlAli, M.N.; Aljomah, N.A.; Alhassan, N.S.; Alkhayal, K.A.; Al-Obeed, O.A.; Zubaidi, A.M. Impact of COVID-19 pandemic on patient satisfaction and surgical outcomes: A retrospective and cross-sectional study. Ann. Med. Surg. 2020, 58, 14–19. [Google Scholar] [CrossRef] [PubMed]
- COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: An international cohort study. Lancet 2020, 396, 27–38. [Google Scholar] [CrossRef]
- Aminian, A.; Safari, S.; Razeghian-Jahromi, A.; Ghorbani, M.; Delaney, C.P. COVID-19 Outbreak and Surgical Practice: Unexpected Fatality in Perioperative Period. Ann. Surg. 2020, 272, e27–e29. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Chen, D.; Paone, D.; Geraci, T.C.; Scheinerman, J.; Bizekis, C.; Zervos, M.; Cerfolio, R.J. Thoracic surgery outcomes for patients with Coronavirus Disease 2019. J. Thorac. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Law, S.; Wong, K.H.; Kwok, K.F.; Chu, K.M.; Wong, J. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann. Surg. 2004, 240, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Di Corpo, M.; Schlottmann, F.; Strassle, P.D.; Nurczyk, K.; Patti, M.G. Treatment Modalities for Esophageal Adenocarcinoma in the United States: Trends and Survival Outcomes. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Reichert, M.; Schistek, M.; Uhle, F.; Koch, C.; Bodner, J.; Hecker, M.; Hörbelt, R.; Grau, V.; Padberg, W.; Weigand, M.A.; et al. Ivor Lewis esophagectomy patients are particularly vulnerable to respiratory impairment-a comparison to major lung resection. Sci. Rep. 2019, 9, 11856. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Management of complications of radical esophagectomy. Indian J. Surg. Oncol. 2013, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Atkins, B.Z.; Shah, A.S.; Hutcheson, K.A.; Mangum, J.H.; Pappas, T.N.; Harpole, D.H., Jr.; D’Amico, T.A. Reducing hospital morbidity and mortality following esophagectomy. Ann. Thorac. Surg. 2004, 78, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Takeda, F.R.; Obregon, C.A.; Navarro, Y.P.; Santo Filho, M.A.; Ribeiro Junior, U.; Sallum, R.A.A.; Cecconello, I. Management of Respiratory Failure Caused by COVID-19 after Thoracoscopic Esophagectomy. Clinics (Sao Paulo) 2021, 76, e2483. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Kelsen, D.P.; Blackstone, E.H. Esophagus and esophagogastric junction. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S.B., Greene, F.L., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer: New York, NY, USA, 2017; pp. 185–202. [Google Scholar]
- Besnier, E.; Tuech, J.J.; Schwarz, L. We Asked the Experts: Covid-19 Outbreak: Is There Still a Place for Scheduled Surgery? “Reflection from Pathophysiological Data”. World J. Surg. 2020, 44, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, T.; Olson, M.T.; Theodorou, D.; Zografos, G.; Singhal, S. Esophageal cancer: Challenges, concerns, and recommendations for management amidst the COVID-19 pandemic. Ann. Gastroenterol. 2020, 33, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e181. [Google Scholar] [CrossRef]
- Ueda, M.; Martins, R.; Hendrie, P.C.; McDonnell, T.; Crews, J.R.; Wong, T.L.; McCreery, B.; Jagels, B.; Crane, A.; Byrd, D.R.; et al. Managing cancer care during the COVID-19 pandemic: Agility and collaboration toward a common goal. J. Natl. Compr. Canc. Netw. 2020, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. for the CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Sjoquist, K.M.; Burmeister, B.H.; Smithers, B.M.; Zalcberg, J.R.; Simes, R.J.; Barbour, A.; Gebski, V.; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011, 12, 681–692. [Google Scholar] [CrossRef]
- Mariette, C.; Dahan, L.; Mornex, F.; Maillard, E.; Thomas, P.A.; Meunier, B.; Boige, V.; Pezet, D.; Robb, W.B.; Brun-Ly, V.L.; et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J. Clin. Oncol. 2014, 32, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Wahed, S.; Chmelo, J.; Navidi, M.; Hayes, N.; Phillips, A.W.; Immanuel, A. Delivering esophago-gastric cancer care during the COVID-19 pandemic in the United Kingdom: A surgical perspective. Dis. Esophagus 2020, 33, doaa091. [Google Scholar] [CrossRef] [PubMed]
| Post-Operative Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SARS-Cov-2 antigen swab test | (+) | (−) | |||||||||||||||||
| RT-PCR of SARS-Cov-2 swab test | (+) | (+) | (+) | ||||||||||||||||
| WBC (K/uL) | 5.11 | 6.08 | 5.64 | 5.26 | 4.20 | 3.31 | 2.34 | 2.49 | 2.87 | ||||||||||
| CRP (mg/L) | 167.2 | 196.8 | 126.3 | 86.0 | 42.7 | 17.4 | 16.5 | 36.5 | 36.7 | ||||||||||
| PCT (ng/mL) | 0.42 | 2.95 | 1.37 | 0.57 | 0.24 | ||||||||||||||
| Chest tube collection (mL/24 h) | 1100 | 850 | 700 | 900 | 450 | 200 | 50 | ||||||||||||
| Hypertension (systolic BP > 140 mmHg) | |||||||||||||||||||
| Atrial fibrillation | |||||||||||||||||||
| Dyspnea | |||||||||||||||||||
| Antimicrobial tx: Meropenem | 1 g i.v./12 h | ||||||||||||||||||
| Jejunostomy enteral feeding (mL/h) | 15 | 30 | 45 | 60 | 60 | 60 | 60 | 60 | 60 | ||||||||||
| Oral intake | |||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurczyk, K.; Chan, C.-E.; Nowak, N.; Skoczylas, T. COVID-19 Pneumonia on Post-Operative Day 2 after Esophagectomy: Performing Esophago-Gastric Junction Cancer Surgery during the SARS-Cov-2 Second Wave. Curr. Oncol. 2021, 28, 1348-1353. https://doi.org/10.3390/curroncol28020128
Nurczyk K, Chan C-E, Nowak N, Skoczylas T. COVID-19 Pneumonia on Post-Operative Day 2 after Esophagectomy: Performing Esophago-Gastric Junction Cancer Surgery during the SARS-Cov-2 Second Wave. Current Oncology. 2021; 28(2):1348-1353. https://doi.org/10.3390/curroncol28020128
Chicago/Turabian StyleNurczyk, Kamil, Chia-En Chan, Norbert Nowak, and Tomasz Skoczylas. 2021. "COVID-19 Pneumonia on Post-Operative Day 2 after Esophagectomy: Performing Esophago-Gastric Junction Cancer Surgery during the SARS-Cov-2 Second Wave" Current Oncology 28, no. 2: 1348-1353. https://doi.org/10.3390/curroncol28020128
APA StyleNurczyk, K., Chan, C.-E., Nowak, N., & Skoczylas, T. (2021). COVID-19 Pneumonia on Post-Operative Day 2 after Esophagectomy: Performing Esophago-Gastric Junction Cancer Surgery during the SARS-Cov-2 Second Wave. Current Oncology, 28(2), 1348-1353. https://doi.org/10.3390/curroncol28020128






