Neoadjuvant Chemotherapy in Breast Cancer: Review of the Evidence and Conditions That Facilitated Its Use during the Global Pandemic
Abstract
1. Introduction
2. Condition #1: An Opportunity and Motivation for Change Was Identified
3. Condition #2: An Engaged Coalition of Influential Stakeholder Representatives Was Formed
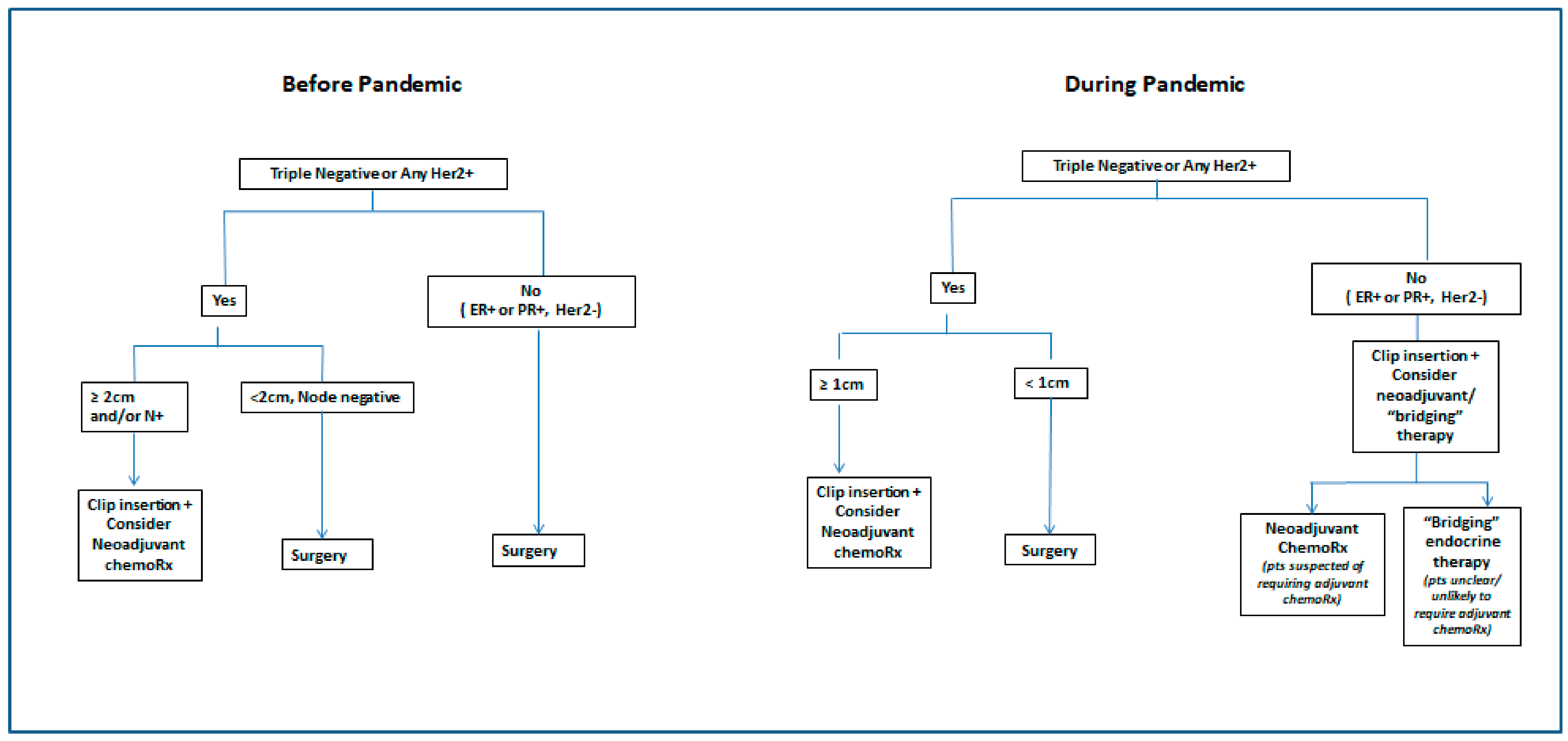
4. Condition #3. Processes for People to Follow Were Clearly Documented and Communicated
5. Condition #4: Obstacles to Action Were Removed and Supportive Background Processes Were Instituted
6. Condition #5: Experiences Were Analysed and Gains Were Consolidated
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Harbeck, N.; Gnant, M. Breast Cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.; Dakhil, S.R.; et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008, 26, 778–785. [Google Scholar] [CrossRef] [PubMed]
- King, T.A.; Morrow, M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 2015, 12, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Zardavis, D.; Piccart, M. Neoadjuvant therapy for breast cancer. Annu. Rev. Med. 2015, 66, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P. Impact of neoadjuvant chemotherapy on locoregional surgical treatment of breast cancer. Ann. Surg. Oncol. 2015, 22, 1425–1433. [Google Scholar] [CrossRef]
- Boughey, J.C.; Ballman, K.V.; Le-Petross, H.T.; McCall, L.M.; Mittendorf, E.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Feliberti, E.; Hunt, K. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: Results from ACOSOG Z1071 (Alliance). Ann. Surg. 2016, 263, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. NEJM N. Eng. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. NEJM N. Eng. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Natori, A.; Ethier, J.L.; Amir, E.; Cescon, D.W. Capecitabine in early breast cancer: A meta-analysis of randomised controlled trials. Eur. J. Cancer 2017, 77, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Lüftner, D.; Bauerfeind, I.; Braun, M.; Brucker, S.Y.; Fasching, P.A.; Felberbaum, R.; Hagemann, F.; Haidinger, R.; Harbeck, N.; Hönig, A.; et al. Treatment of Early Breast Cancer Patients: Evidence, Controversies, Consensus: Focusing on Systemic Therapy—German Experts’ Opinions for the 16th International St. Gallen Consensus Conference (Vienna 2019). Breast Care 2019, 14, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Puig, C.A.; Hoskin, T.L.; Day, C.N.; Habermann, E.; Boughey, J. National Trends in the Use of Neoadjuvant Chemotherapy for Hormone Receptor-Negative Breast Cancer: A National Cancer Data Base Study. Ann. Surg. Oncol. 2017, 24, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Koulis, T.A.; Beecham, K.; Speers, C.; Tyldesley, S.; Voduc, D.; Simmons, C.; Olson, R. Neoadjuvant systemic therapy in breast cancer: Use and trends in radiotherapy practice. Curr. Oncol. 2017, 24, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Waddimba, A.C.; Ogola, G.O.; Fleshman, J.W.; Preskitt, J.T. A systematic review and meta-analysis of surgery delays and survival in breast, lung and colon cancers: Implication for surgical triage during the COVID-19 pandemic. Am. J. Surg. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Berger-Richardson, D.; Ko, G.; Look Hong, N.J. Preparing for the Renaissance: Treating Breast Cancer during the COVID-19 Pandemic and Planning for a Safe Re-Emergence to Routine Surgical Care within a Universal Health Care System. Curr. Oncol. 2020, 27, 163–168. [Google Scholar] [CrossRef]
- Finley, C.; Prashad, A.; Camuso, N.; Daly, C.; Aprikian, A.; Ball, C.; Bentley, J.; Charest, D.; Fata, P.; Helyer, L.; et al. Guidance for management of cancer surgery during the COVID-19 pandemic. Can. J. Surg. 2020, 63, S2–S4. [Google Scholar] [CrossRef]
- Canadian Cancer Society. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/breast/prognosis-and-survival/survival-statistics/?region=on (accessed on 14 July 2020).
- Ontario Health (Cancer Care Ontario). Pandemic Planning Clinical Guideline for Patients with Cancer; Ontario Health (CCO): Toronto, ON, Canada, 2020; Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/64736 (accessed on 14 July 2020).
- American College of Surgeons. ACS Guidelines for Triage and Management of Elective Cancer Surgery Cases during the Acute and Recovery Phases of Coronavirus Disease 2019 (COVID-19) Pandemic. Available online: https://www.facs.org/-/media/files/covid19/acs_triage_and_management_elective_cancer_surgery_during_acute_and_recovery_phases.ashx (accessed on 11 February 2021).
- Society of Surgical Oncology (SSO). Resource for Management Options of Breast Cancer during COVID-19. 2020. Available online: https://www.surgonc.org/wp-content/uploads/2020/03/Breast-Resource-during-COVID-19-3.23.20.pdf (accessed on 11 February 2021).
- Curigliano, G.; Banerjee, S.; Cervantes, A.; Garassino, M.C.; Garrido, P.; Girard, N.; Haanen, J.; Jordan, K.; Lordick, F.; Machiels, J.P.; et al. Managing cancer patients during the COVID-19 pandemic: An ESMO multidisciplinary expert consensus. Ann. Oncol. 2020, 31, 1320–1335. [Google Scholar] [CrossRef]
- American Society of Breast Surgeons (ASBrS). Recommendations for Prioritization, Treatment and Triage of Breast Cancer Patients during the COVID-19 Pandemic: Executive Summary; Ver.1.0 2020; American Society of Breast Surgeons (ASBrS): Columbia, MD, USA, 2020. [Google Scholar]
- Katz, S.; Hawley, S.T.; Hamilton, A.S.; Ward, K.C.; Morrow, M.; Jagsi, R.; Hofer, T.P. Surgeon Influence on Variation in Receipt of Contralateral Prophylactic Mastectomy for Women with Breast Cancer. JAMA Surg. 2018, 153, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, A.; Lee, J.; Gelmon, K.; Poirier, B.; Lu, F.I.; Akra, M.; Boileau, J.F.; Tonkin, K.; Li, H.; Illman, C.; et al. Neoadjuvant therapy for breast cancer: Updates and proceedings from the Seventh Annual Meeting of the Canadian Consortium for Locally Advanced Breast Cancer. Curr. Oncol. 2018, 25, 490–498. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Pease, A.M.; Riba, L.A.; Gruner, R.A.; Tung, N.M.; James, T.A. Oncotype DX® Recurrence Score as a Predictor of Response to Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2019, 26, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Park, K.U.; Chen, Y.; Chitale, D.; Choi, S.; Ali, H.; Nathanson, S.D.; Bensenhaver, J.; Procter, E.; Petersen, L.; Loutfi, R.; et al. Utilization of the 21-Gene Recurrence Score in a Diverse Breast Cancer Patient Population: Development of a Clinicopathologic Model to Predict High-Risk Scores and Response to Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2018, 25, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J.R.; Moran, M.S.; Isakoff, S.J.; Kutzman, S.H.; Willey, S.; Burstein, H.; Bleicher, R.J.; Lyons, J.A.; Sarantou, T.; Baron, P.L.; et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res. Treat. 2020, 181, 487–497. [Google Scholar] [CrossRef]
- Brenes Sánchez, J.M.; Picado, A.L.; Olivares Crespo, M.E.; García Sáenz, J.Á.; De La Plata Merlo, R.M.; De La Muela, M.H. Breast Cancer Management During COVID-19 Pandemic in Madrid: Surgical Strategy. Clin. Breast Cancer 2021, 21, e128–e135. [Google Scholar] [CrossRef]
- Massachusetts General Hospital Clinician Resource: Clinical Re-Entry Tool. Available online: https://www.massgeneral.org/surgical-oncology/about/news-and-events/re-entry-tool-for-breast-surgeons/ (accessed on 11 February 2021).
- Wang, F.; Reid, S.; Zheng, W.; Pal, T.; Meszoely, I.; Mayer, I.A.; Bailey, C.E.; Park, B.H.; Shu, X.O. Sex Disparity Observed for Oncotype DX Breast Recurrence Score in Predicting Mortality Among Patients with Early Stage ER-Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Prachand, V.N.; Milner, R.; Angelos, P.; Posner, M.C.; Fung, J.J.; Agrawal, N.; Jeevanandam, V.; Mathews, J.B. Medically Necessary, Time-Sensitive Procedures: Scoring System to Ethically and Efficiently Manage Resource Scarcity and Provider Risk During the COVID-19 Pandemic. J. Am. Coll. Surg. 2020, 231, 281–288. [Google Scholar] [CrossRef]
- Hussain, T.; Kneeshaw, P.J. Stopping tamoxifen peri-operatively for vte risk reduction: A proposed management algorithm. Int. J. Surg. 2012, 10, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.; Blanchette, P.; Shah, P.S.; Ye, X.Y.; Boldt, R.G.; Fernandes, R.; Vandenberg, T.; Raphael, J. Do all patients with HER2 positive breast cancer require one year of adjuvant trastuzumab? A systematic review and meta-analysis. Breast 2020, 54, 203–210. [Google Scholar] [CrossRef]
- Yu, K.D.; Wang, X.; Chen, W.K.; Fan, L.; Mo, M.; Chen, H. Tailored duration of adjuvant trastuzumab for human epidermal growth factor receptor 2-positive breast cancer. NPJ Precis. Oncol. 2020, 4, 23. [Google Scholar] [CrossRef]
- Manguso, N.; Gangi, A.; Giuliano, A.E. Neoadjuvant Chemotherapy and Surgical Management of the Axilla in Breast Cancer: A Review of Current Data. Oncology 2015, 29, 733–738. [Google Scholar]
- Pilewski, M.; Morrow, M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol. 2017, 3, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, V.; Provenzano, E.; Symmans, W.F.; Boughey, J.C.; Coles, C.; Curigliano, G.; Dixon, J.M.; Esserman, L.J.; Fastner, G.; Kuehn, T.; et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann. Oncol. 2015, 26, 1280–1291. [Google Scholar] [CrossRef]
- Provenzano, E.; Bossuyt, V.; Viale, G.; Cameron, D.; Badve, S.; Denkert, C.; MacGrogan, G.; Penault-Llorca, F.; Boughey, J.; Curigliano, G.; et al. Residual Disease Characterization Working Group of the Breast International Group-North American Breast Cancer Group Collaboration. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: Recommendations from an international working group. Mod. Pathol. 2015, 28, 1185–1201. [Google Scholar] [PubMed]
- Bossuyt, V.; Symmans, W.F. Standardizing of Pathology in Patients Receiving Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2016, 23, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; van der Noordaa, M.; Wei, J.; Osdoit, M.; Reyal, F.; Hamy, A.-S.; Lae, M.; Martin, M.; del Monte, M.; Boughey, J.C.; et al. Residual Cancer Burden after Neoadjuvant Therapy and Long-Term Survival Outcomes in Breast Cancer: A Multi-Center Pooled Analysis. Abstract GS5-01. In Proceedings of the 2019 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 10–14 December 2019. [Google Scholar]
- Yeung, C.; Hilton, J.; Clemons, M.; Mazzarello, S.; Hutton, B.; Haggar, F.; Addison, C.L.; Kuchuck, I.; Zhu, X.; Gelmon, K.; et al. Estrogen, progesterone, and HER2/neu receptor discordance between primary and metastatic breast tumours-a review. Cancer Metastasis Rev. 2016, 35, 427–437. [Google Scholar] [CrossRef]
- Fung-Kee-Fung, M.; Boushey, R.; Morash, R. Exploring a “community of practice” methodology as a regional platform for large-scale collaboration in cancer surgery-the Ottawa approach. Curr. Oncol. 2014, 21, 13–18. [Google Scholar] [CrossRef]
- Dharmarajan, H.; Anderson, J.L.; Kim, S.; Sridharan, S.; Umamaheswar, D.; Ferris, R.L.; Solari, M.G.; Clump, D.A.; Skinner, H.D.; Ohr, J.P.; et al. Transition to a virtual multidisciplinary tumor board during the COVID-19 pandemic: University of Pittsburgh experience. Head Neck 2020, 42, 1310–1316. [Google Scholar] [CrossRef]
- Nicolò Gennaro, N.; Marrari, A.; Bertuzzi, A.F.; Balzarini, L.; Santoro, A.; Politi, L.S. The radiologist empowerment through virtual multidisciplinary tumor boards: The commitment of oncologic care during COVID-19 pandemic. Clin. Imaging 2020, 70, 49–50. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse, T.; Sehdev, S.; Seely, J.; Gravel, D.H.; Clemons, M.; Cordeiro, E.; Arnaout, A. Neoadjuvant Chemotherapy in Breast Cancer: Review of the Evidence and Conditions That Facilitated Its Use during the Global Pandemic. Curr. Oncol. 2021, 28, 1338-1347. https://doi.org/10.3390/curroncol28020127
Tse T, Sehdev S, Seely J, Gravel DH, Clemons M, Cordeiro E, Arnaout A. Neoadjuvant Chemotherapy in Breast Cancer: Review of the Evidence and Conditions That Facilitated Its Use during the Global Pandemic. Current Oncology. 2021; 28(2):1338-1347. https://doi.org/10.3390/curroncol28020127
Chicago/Turabian StyleTse, Tabitha, Sandeep Sehdev, Jean Seely, Denis H. Gravel, Mark Clemons, Erin Cordeiro, and Angel Arnaout. 2021. "Neoadjuvant Chemotherapy in Breast Cancer: Review of the Evidence and Conditions That Facilitated Its Use during the Global Pandemic" Current Oncology 28, no. 2: 1338-1347. https://doi.org/10.3390/curroncol28020127
APA StyleTse, T., Sehdev, S., Seely, J., Gravel, D. H., Clemons, M., Cordeiro, E., & Arnaout, A. (2021). Neoadjuvant Chemotherapy in Breast Cancer: Review of the Evidence and Conditions That Facilitated Its Use during the Global Pandemic. Current Oncology, 28(2), 1338-1347. https://doi.org/10.3390/curroncol28020127






