Abstract
Primary signet ring cell/histiocytoid carcinoma of the eyelid is a rare ocular malignancy and its diagnosis is often delayed. This neoplasm presents as an insidious, diffusely infiltrative mass in the periocular area that later infiltrates the orbit. An exenteration is usually indicated; however, nearly one-third of patients develop local recurrence or metastasis. Morphologically, it resembles signet ring cell carcinoma of the stomach and breast, raising the possibility of mutations in CDH1, the gene encoding E-cadherin. To determine whether primary signet ring cell/histiocytoid carcinoma harbors the CDH1 mutation or other actionable mutations, we analyzed the tumor tissue via next-generation sequencing. We identified only one case of primary signet ring cell carcinoma of the eyelid with adequate DNA quality for sequencing from the pathological archive during the period 2000 to 2020. A comprehensive evaluation including histopathology, immunohistochemistry, and next-generation sequencing assay was performed on tumor tissue. Immunohistochemically, the tumor exhibited E-cadherin membranous staining with the aberrant cytoplasmic staining of β-catenin. Using next-generation sequencing, we demonstrated the mutation in the CDH1 gene. In addition, other clinically actionable mutations including ERBB2 and PIK3CA were also detected. The alterations in other actionable genes indicate a need for larger studies to evaluate the pathogenesis and potential therapies for primary signet ring cell/histiocytoid carcinoma of the eyelid.
1. Introduction
Primary signet ring cell/histiocytoid carcinoma is a rare skin adnexal neoplasm with only fewer than 50 cases reported in the literature [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. While most tumors involve the eyelids, identical neoplasms have rarely been reported in the axilla [27,28,29]. The morphology of the tumor cells resembles carcinoma with a signet ring cell appearance in other solid organs. Previous studies have demonstrated the possible origin of apocrine/eccrine glands with a variable expression of estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR), and Her2-Neu immunohistochemically [2,3,4,24]. However, the genetic alterations of the tumor had not been determined until a recent publication by Raghavan et al., indicated a mutation in the CDH1 gene [30] that has been demonstrated in signet ring cell carcinoma of the stomach, breast lobular carcinoma, and plasmacytoid urothelial carcinoma. In this study, we performed a comprehensive mutational investigation using next-generation sequencing to elucidate the mutational profile of the tumor and expand our current understanding of this rare neoplasm.
2. Materials and Methods
This study was based on the clinical, histologic, immunohistochemical findings and genetic analysis of a case of primary signet-ring cell carcinoma of the eyelid, which was identified in the archival, institutional files of Taipei Veterans General Hospital. Only two cases were identified during the period 2000 to 2020. The case diagnosed in 2003 was excluded because of the poor quality of DNA for molecular analysis. The study patient was diagnosed in 2019. Clinical information was collected from medical records and ophthalmologists. The patient signed an informed consent for the study which was approved by the institutional review board of Taipei Veterans General Hospital. The resected tumor tissue was fixed in 10% formalin and embedded in paraffin. A panel of immunohistochemical stains including CK7, gross cystic disease fluid protein-15 (GCDFP-15), E-cadherin, Her2-Neu, GATA binding protein 3 (GATA3), AR, ER, and PR were performed for differential diagnosis. The antibodies used, their sources, and their dilutions are given in Table 1. For next-generation sequencing analysis, genomic DNA (gDNA) was extracted from formalin-fixed, paraffin-embedded (FFPE) tissue using the RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE (Invitrogen™, Carlsbad, CA. USA) according to the manufacturer’s instructions. The extracted gDNA was quantified with a Qubit® fluorometer (Life Technologies, Carlsbad, CA. USA) using the Qubit® dsDNA HS Assay Kit; 30 ng of gDNA were subjected to library preparation using the Oncomine™ Tumor Mutation Load Assay (Thermo Fisher Scientific, Waltham, MA. USA), which could simultaneously assess the tumor mutation load and mutation signatures across approximately 1.7 Mb and 409 cancer-related genes. The prepared library was subsequently analyzed using the Ion Library TaqMan® Quantitation Kit (Thermo Fisher Scientific) and the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA. USA) to assess the quantity and quality of the DNA library. A total of 50 pM of a DNA library were subjected to automatic template preparation and chip loading on the Ion Chef System (Thermo Fisher Scientific). DNA sequencing was then performed on the Ion GeneStudio S5 system (Thermo Fisher Scientific). Data were analyzed using the Ion ReporterTM Oncomine Tumor Mutation Load workflow (Thermo Fisher Scientific), and the variants were called when a minimum mean coverage of 500 reads was achieved and at least 5% of the variant reads were identified. The called variants were further filtered using the Taiwan Biobank database to exclude likely benign variants (>1% population allele frequency in the Taiwanese population).

Table 1.
Antibodies used in the immunohistochemical study and their results.
3. Results
3.1. Clinical Features
A 60-year-old man, who had been diagnosed as having poorly differentiated adenocarcinoma of the right eyelid at another hospital for three years, presented with progressive proptosis and limitation of extraocular muscle movement. At examination, the right lower eyelid was diffusely thickened with a nodular consistency. An orbit-computed tomographic (CT) scan showed diffuse enhancing soft tissue infiltrating at the right orbit, involving the right lacrimal gland, the retrobulbar and extraconal space, the eyeball, the extraocular muscle, and the orbital apex, with suspicious extension to the optic canal (Figure 1A,B). A magnetic resonance imaging (MRI) scan of the orbit showed tumor growth in the whole right orbital cavity, causing marked proptosis, encasing the right optic nerve and extraocular muscles, and extending into nasolacrimal duct and outside the orbit to the right cheek. A biopsy of the indurated area of the right lower eyelid revealed infiltration by signet-ring cell carcinoma in the subepithelial stroma. Whole-body PET was performed for a systemic workup and showed tumor growth in the right orbit without other fluorodeoxyglucose (FDG)-avid lesions noted elsewhere in the whole body. Orbital exenteration and craniofacial resection were performed, and a histopathologic study showed extensive orbital extension from the eyelid carcinoma.
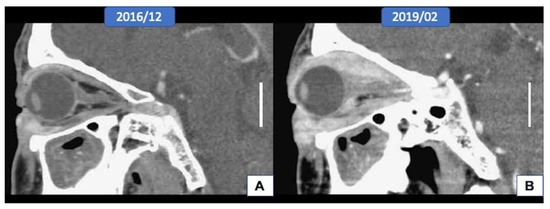
Figure 1.
Radiological features of the tumor (scale bar, 2 cm). (A,B) The orbital computed tomographic scan showed the extension of the tumor from the eyelid to the whole orbital socket after three years.
3.2. Histopathologic Findings
The epidermis of the eyelid was spared, but the full thickness of the dermis was diffusely infiltrated by carcinoma cells. In addition, the tumor extended to the extraocular skeletal muscle, lacrimal gland, sclera, conjunctiva, and part of the cornea. The optic nerve and optic nerve sheath were not involved by the tumor; however, the resection margins in the subcutaneous soft tissue at the cheek area, right orbital apex, and right temporal bone showed tumor cells infiltration (Figure 2A). The neoplastic cells manifested a destructive growth pattern (Figure 2B). Signet ring cell morphology, characterized by abundant eosinophilic cytoplasm and eccentric nuclei, was noted in places (Figure 2C). Some of the neoplastic cells were larger with enlarged nuclei, suggesting a histiocytoid appearance (Figure 2D). Morphologically, this is consistent with the diagnosis of primary signet ring cell/histiocytoid carcinoma of the eyelid. There was no mitotic figures or necrosis identified, which is correlated with its clinically slow-growing behavior. Immunohistochemically, the tumor cells showed a strong-diffuse positivity for CK(AE1/AE3) and CK7 (data not shown). There was moderate-focal reactivity for AR, GATA3, and GCDFP15, whereas no staining was observed with ER and PR (Figure 3A–E). The Her2-Neu stain exhibited moderate complete staining in 40% of the tumor cells on the representative sectioned slide (Figure 3F). The tumor cells showed a preserved of E-cadherin expression. There was aberrant staining for β-catenin being localized in the cytoplasm (Figure 3G,H).
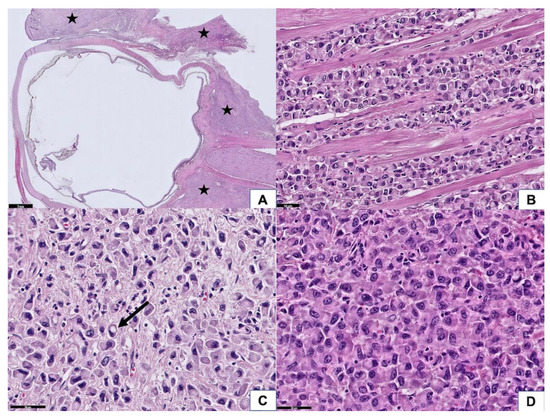
Figure 2.
Histopathological features of the tumor. (A) At scanning power, the tumor was diffusely infiltrated in the eyelid, extraocular muscle, and retrobulbar soft tissue (asterisk). The optic nerve was spared (50× magnification). (B) Tumor cells diffusely infiltrated in the extraocular skeletal muscle, manifesting a destructive growth pattern and causing a limitation of eye movement (200× magnification). (C) A few tumor cells reveal a signet ring cell feature (arrow) (400× magnification). (D) Some of the tumor cells have enlarged nuclei, exhibiting a histiocytoid appearance (400× magnification).
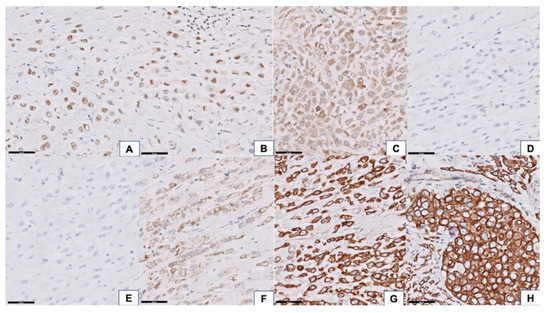
Figure 3.
Immunohistochemical features of the tumor. The tumor cells showed focal moderate positivity for androgen receptor (AR) (A) 400× magnification, GATA binding protein 3 (GATA3), (B) 400× magnification, and gross cystic disease fluid protein-15 (GCDFP15, (C) 400× magnification) and negativity for estrogen receptor (ER), (D) 400× magnification, and progesterone receptor (PR), (E) 400× magnification. Her2-Neu stain exhibited moderate complete staining in 40% of the tumor cells (F) 400× magnification. Preserved of E-cadherin membranous staining, (G) 400× magnification, with aberrant cytoplasmic expression of beta-catenin, (H) 400× magnification, was identified in tumor tissue.
3.3. Next-Generation Sequencing
A total of 29 nonsynonymous (missense, splicing site, insertion, deletion) mutations were detected in the tumor tissue of primary signet ring cell/histiocytoid carcinoma of the eyelid, with an allele frequency ranging from 4.41% to 89.43%. In addition to the mutation in CDH1, the tumor harbored actionable genetic alterations, including ERBB2 and PIK3CA. The molecular characteristics of the detected mutations are given in Table 2. The nucleotide changes of the CDH1, ERBB2, and PIK3CA genes are shown in Figure S1A–C.

Table 2.
Mutational signatures of the patient with primary signet ring cell/histiocytoid carcinoma of the eyelid.
4. Discussion
In contrast to the germline mutations in CDH1 that typify diffuse hereditary gastric cancers [31], our case showed CDH1 mutation with a variant allele frequency (VAF) of 27.08%, suggesting a somatic mutation in CDH1. The splice site mutation of CDH1 detected in our case has been reported in 27% and 10% of hereditary diffuse gastric cancers and lobular breast cancers, respectively [31], and it constitutes one type of truncating mutations of CDH1, which is the most commonly identified mutations in CDH1. Compared with the recent publication by Raghavan et al., they also discovered a spice site mutation of CDH1 (c.531+1 G>T, VAF 23.3%) [30]. Our case represents the second case of CDH1 mutation reported in the literature.
Although CDH1 mutations or promotor hypermethylation result in a loss of E-cadherin protein expression in many cancer types, our case revealed the preservation of membranous expression of E-cadherin. This phenomenon has been reported in a proportion of lobular breast carcinoma [32,33,34]. The protein expressed in these cases appears dysfunctional and is not normally associated with the catenin complex. We also demonstrated this by showing an aberrant cytoplasmic expression pattern for β-catenin, suggesting a failed cadherin–catenin complex formation that is required for the maintenance of cell–cell adhesion.
Previous studies based on the genetic analysis of lobular carcinoma of the breast have proposed an evolutionary pathway of neoplastic cells from the E-cadherin-positive to E-cadherin-negative tumor cells. The former harbored less genetic instability and less proliferative index [32]. Another putative pathway involving E-cadherin downregulation is the activation of the TGF-β pathway. Our case exhibited mutations in CDH1, CDH2 and CDKN2B, all of which are genes downstream of the TGF-β pathway, indicating the potential role of the TGF-β superfamily in the tumorigenesis [35]. In summary, CDH1 alterations and the expression of its encoding protein, E-cadherin, may be modulated by other factors yet to be identified.
Another significant finding is that our case was found to have mutations in targetable kinases, ERBB2 and PIK3CA, similar to the results reported in plasmacytoid urothelial carcinoma [36]. Raghavan et al. also described the presence of targetable mutations in NTRK3, CDKN1B, and PIK3CA in their case of primary signet ring cell carcinoma of the eyelid [30]. Alterations in ERBB2(HER2) have been reported in diverse cancers, including breast and gastric cancer. HER2-targeted agents, such as trastuzumab, have been FDA-approved and led to dramatic improvements in outcomes across different malignancies [37]. Our patient carried the somatic HER2 mutation (p.V777L), which has been reported in breast cancer. This mutation results in activating mutation that is sensitive to the irreversible kinase inhibitor, neratinib [38], which has been approved by the FDA for patients with early-stage HER2-positive breast cancer [39]. The PIK3CA gene encodes the phophatidylinosital-4,5-bisphosphate 3-kinase catalytic subunit alpha [40], a catalytic unit of the PI3-kinase (PI3K) pathway. Recurrent somatic mutations in PIK3CA are frequent in cancer and result in the activation of the PI3K/AKT/MTOR pathway. Mutations in PIK3CA are common in many cancer types and are observed in 20–30% of breast, cervical, and uterine cancers and 10–20% of bladder, gastric, head and neck, and colorectal cancers [41,42]. The FDA-approved PI3K inhibitor, alpelisib, has been used for the treatment of patients with breast cancer under certain conditions. A histopathological resemblance between cutaneous apocrine carcinoma and breast carcinoma is well known. Given the presumed apocrine gland origin of primary signet ring cell/histiocytoid carcinoma and the lack of standard guidelines in adjuvant therapy, one might consider treating it based on the general guidelines for the treatment of breast cancer [29]. Previously, anti-estrogen therapy has been used as an adjuvant therapy, with some success in patients of primary signet ring cell/histiocytoid carcinoma with ER expression [2]. Recently, one patient received anti-androgen therapy because of AR expression and the co-existence of prostate cancer. The anti-androgen treatment slowed the effect of primary signet ring cell/histiocytoid carcinoma and reached a “stable disease” for two years [24]. Whether these mutations or hormonal status have true therapeutic relevance in primary signet ring cell/histiocytoid carcinoma requires further investigation.
Our study has only examined one case, however, one must bear in mind that these neoplasms are very rare and are infrequently encountered as histological specimens. Although mutational analysis via next-generation sequencing has been reported in one other case, our case doubles the knowledge of the tumor genetics and supports CDH1 in the pathogenesis. With more cases reported, a later meta-analysis can be performed to evaluate the impact of these genetic alterations and look for generalizable determinants.
5. Conclusions
Primary signet ring cell/histiocytoid carcinoma of the eyelid is a rare entity that is unfamiliar to general pathologists and is often diagnosed as “poorly-differentiated adenocarcinoma, suggesting metastatic adenocarcinoma”. A thorough systemic workup fails to show metastatic disease, indicating a primary malignancy in the orbital area. The morphology of the signet ring cell is reminiscent of signet ring cell carcinoma of the stomach, signet ring cell variant breast lobular carcinoma, and plasmacytoid urothelial carcinoma, suggesting a potential common genetic alteration in the CDH1 gene. Furthermore, the presence of other clinically actionable alterations in genes such as ERBB2 and PIK3CA and unresectable, local aggressive disease imply that future research should investigate the early use of targeted therapies as a potential treatment for patients with primary signet ring cell/histiocytoid carcinoma of the eyelid.
Supplementary Materials
The following are available online at https://www.mdpi.com/1718-7729/28/1/90/s1, Figure S1: Images of CDH1, ERBB2, and PIK3CA gene at specific loci were taken using Integrative genomics viewer (IGV) software.
Author Contributions
Conceptualization, L.-C.W., C.-C.T., and T.-C.L.; methodology, L.-C.W. and H.-L.H.; software, Y.-C.Y.; validation, Y.-C.Y.; formal analysis, L.-C.W. and Y.-C.Y.; investigation, L.-C.W. and C.-C.T.; resources, C.-C.T., H.-L.H., and T.-Y.C.; data curation, L.-C.W. and Y.-C.Y. writing—original draft preparation, L.-C.W.; writing—review and editing, all authors; visualization, L.-C.W.; supervision, C.-C.T. and T.-Y.C.; project administration, H.-L.H. and T.-Y.C.; funding acquisition, T.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taipei Veterans General Hospital, grant number V108E-008-3(109).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kramer, T.R.; E Grossniklaus, H.; McLean, I.W.; Orcutt, J.; Green, W.R.; Iliff, N.T.; Tressera, F. Histiocytoid variant of eccrine sweat gland carcinoma of the eyelid and orbit: Report of five cases. Ophthalmology 2002, 109, 553–559. [Google Scholar] [CrossRef]
- Requena, L.; Prieto, V.G.; Requena, C.; Requena, C.; Sarasa, J.L.; Manzano, R.; Seco, M.; Rütten, A.; Kazakov, D.V.; Cerroni, L.; et al. Primary signet-ring cell/histiocytoid carcinoma of the eyelid: A clinicopathologic study of 5 cases and review of the literature. Am. J. Surg. Pathol. 2011, 35, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, M.; Uehara, T.; Yoshizawa, A.; Kobayashi, Y.; Momose, M.; Honda, T.; Ota, H. A case of primary signet-ring cell/histiocytoid carcinoma of the eyelid: Immunohistochemical comparison with the normal sweat gland and review of the literature. Am. J. Dermatopathol. 2012, 34, e139–e145. [Google Scholar] [CrossRef]
- Bernárdez, C.; Macías del Toro, E.; Ramírez Bellver, J.L.; Martinez Menchón, T.; Martinez Barba, E.; Molina-Ruiz, A.M.; Requena, L. Primary Signet-Ring Cell/Histiocytoid Carcinoma of the Eyelid: A “Binocle” Presentation of the “Monocle Tumor”. Am. J. Dermatopathol. 2016, 38, 623–627. [Google Scholar] [CrossRef]
- Auw-Haedrich, C.; Boehm, N.; Weissenberger, C. Signet ring carcinoma of the eccrine sweat gland in the eyelid, treated by radiotherapy alone. Br. J. Ophthalmol. 2001, 85, 112–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellman, B.; Gregory, N.A.; Silvers, D.; Fountain, K.S. Sweat gland carcinoma with metastases to the skin: Response to 5-fluorouracil chemotherapy. Cutis 1995, 55, 221–224. [Google Scholar] [PubMed]
- Fb, F.B.; Hofman, V.; Lagier, J.; Gastaud, P.; Santini, J.; Hofman, P. Primary signet ring cell carcinoma of the eccrine sweat gland in the eyelid. Immunohistochemical and ultrastructural study of a case. J. Fr. Ophtalmol. 2002, 25, 547–551. [Google Scholar]
- Bhargava, R.; Beriwal, S.; Dabbs, D.J. Mammaglobin vs GCDFP-15: An immunohistologic validation survey for sensitivity and specificity. Am. J. Clin. Pathol. 2007, 127, 103–113. [Google Scholar] [CrossRef]
- González-Lois, C.; Rodríguez-Peralto, J.L.; Serrano-Pardo, R.; Martínez-González, M.A.; López-Ríos, F. Cutaneous signet ring cell carcinoma: A report of a case and review of the literature. Am. J. Dermatopathol. 2001, 23, 325–328. [Google Scholar] [CrossRef]
- Bouza Gonzalo, E.; Luezas Morcuende, J.; Martínez Montero, J.C.; Cuesta Gil, M.; Fernández De Castro Pombo, J. Eccrine gland adenocarcinoma of the eyelid. Arch. Soc. Esp. Oftalmol. 2001, 76, 739–742. [Google Scholar]
- Grizzard, W.S.; Torezynski, E.; Edwards, W.C. Adenocarcinoma of eccrine sweat glands. Arch Ophthalmol. 1976, 94, 2119–2123. [Google Scholar] [CrossRef]
- Jakobiec, F.A.; Austin, P.; Iwamoto, T.; Trokel, S.L.; Marquardt, M.D.; Harrison, W. Primary infiltrating signet ring carcinoma of the eyelids. Ophthalmology 1983, 90, 291–299. [Google Scholar] [CrossRef]
- Kiyohara, T.; Kumakiri, M.; Kouraba, S.; Tokuriki, A.; Ansai, S. Primary cutaneous signet ring cell carcinoma expressing cytokeratin 20 immunoreactivity. J. Am. Acad. Dermatol. 2006, 54, 532–536. [Google Scholar] [CrossRef][Green Version]
- Langel, D.J.; Yeatts, R.P.; White, W.L. Primary signet ring cell carcinoma of the eyelid: Report of a case demonstrating further analogy to lobular carcinoma of the breast with a literature review. Am. J. Dermatopathol. 2001, 23, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.L.; Heegaard, S.; Clemmensen, O.; Prause, J.U. Signet ring cell carcinoma of the eyelid—The monocle tumour. Apmis 2008, 116, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Rosen, Y.; Kim, B.; Yermakov, V.A. Eccrine sweat gland tumor of clear cell origin involving the eyelids. Cancer 1975, 36, 1034–1041. [Google Scholar] [CrossRef]
- Swinson, B.; Ryan, F.; Barrett, A.W.; Jerjes, W.; Landon, G.; Calonje, E.; Kalavrezos, N. Histiocytoid eccrine sweat gland carcinoma of the eyelid: Report of a case. Clin. Exp. Dermatol. 2006, 31, 786–789. [Google Scholar] [CrossRef]
- Thomas, J.W.; Fu, Y.S.; Levine, M.R. Primary mucinous sweat gland carcinoma of the eyelid simulating metastatic carcinoma. Am. J. Ophthalmol. 1979, 87, 29–33. [Google Scholar] [CrossRef]
- Wollensak, G.; Witschel, H.; Böhm, N. Signet ring cell carcinoma of the eccrine sweat glands in the eyelid. Ophthalmology 1996, 103, 1788–1793. [Google Scholar] [CrossRef]
- Palakkamanil, M.M.; Mahmood, M.N.; Chan, A. Diagnostic and treatment challenges of a case of primary cutaneous signet-ring cell/histiocytoid carcinoma of the eyelid. BMC Ophthalmol. 2020, 20, 410. [Google Scholar] [CrossRef] [PubMed]
- Tanboon, J.; Uiprasertkul, M.; Luemsamran, P. Signet-ring cell/histiocytoid carcinoma of the eyelid: A case report and review of the literature. Am. J. Dermatopathol. 2013, 35, e1–e5. [Google Scholar] [CrossRef]
- Stewart, S.; Houghton, J.; Kamalarajah, S.; Curragh, D. Primary Adnexal Signet-Ring Cell/Histiocytoid Carcinoma. Ophthalmic. Plast Reconstr. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sayan, A.; Mitchell, O.; Taibjee, S.; Ilankovan, V. Unusual case of primary cutaneous signet-ring cell (histocytoid) carcinoma. Br. J. Oral. Maxillofac. Surg. 2020, 58, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Ito, T.; Tanioka, F.; Fukamizu, H.; Tokura, Y. Primary signet-ring cell/histiocytoid carcinoma of the eyelid expressing androgen receptors and treated with bicalutamide. J. Dermatol. 2017, 44, e230–e231. [Google Scholar] [CrossRef] [PubMed]
- Droubi, D.; Zeitouni, N.C.; Skitzki, J.; Bogner, P.N. Primary signet-ring cell carcinoma of the axilla. J. Cutan. Pathol. 2013, 40, 269–273. [Google Scholar] [CrossRef]
- Tan, J.S.; McKelvie, P.A.; Hardy, T.G. Primary signet ring cell carcinoma of the eyelid. Orbit 2013, 32, 399–401. [Google Scholar] [CrossRef]
- Cameselle-Teijeiro, J.; Alfonsín-Barreiro, N.; Allegue, F.; Caeiro, M. Apocrine carcinoma with signet ring cells and histiocytoid features. A potentially confusing axillary tumor. Pathol. Res. Pract. 1997, 193, 713–720; discussion 721–722. [Google Scholar] [CrossRef]
- Kuno, Y.; Numata, T.; Kanzaki, T. Adenocarcinoma with signet ring cells of the axilla showing apocrine features: A case report. Am. J. Dermatopathol. 1999, 21, 37–41. [Google Scholar] [CrossRef]
- Misago, N.; Shinoda, Y.; Okawa, T.; Aoki, S.; Toda, S.; Koike, K.; Narisawa, Y. Histiocytoid and signet-ring cell carcinoma of the axilla: A type of cutaneous apocrine carcinoma equivalent to histiocytoid lobular carcinoma of the breast? Clin. Exp. Dermatol. 2011, 36, 874–877. [Google Scholar] [CrossRef]
- Raghavan, S.S.; Clark, M.; Louie, C.Y.; Jensen, K.C.; Dietrich, B.; Beadle, B.M.; El-Sawy, T.; Baik, F.; Kunder, C.A.; Brown, R.A. Molecular profiling of a primary cutaneous signet-ring cell/histiocytoid carcinoma of the eyelid. J. Cutan. Pathol. 2020, 47, 860–864. [Google Scholar] [CrossRef]
- Oliveira, C.; Pinheiro, H.; Figueiredo, J.; Seruca, R.; Carneiro, F. E-cadherin alterations in hereditary disorders with emphasis on hereditary diffuse gastric cancer. Prog. Mol. Biol. Transl. Sci. 2013, 116, 337–359. [Google Scholar]
- Da Silva, L.; Parry, S.; Reid, L.; Keith, P.; Waddell, N.; Kossai, M.; Clarke, C.; Lakhani, S.R.; Simpson, P.T. Aberrant expression of E-cadherin in lobular carcinomas of the breast. Am. J. Surg. Pathol. 2008, 32, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Berx, G.; Cleton-Jansen, A.M.; Strumane, K.; Keith, P.; Waddell, N.; Kossai, M.; Catherine, C.; Sunil, R.L.; Peter, T.S.; Simpson, P.T.; et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 1996, 13, 1919–1925. [Google Scholar]
- Harigopal, M.; Shin, S.J.; Murray, M.P.; Tickoo, S.K.; Brogi, E.; Rosen, P.P. Aberrant E-cadherin staining patterns in invasive mammary carcinoma. World J. Surg. Oncol. 2005, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, K.; Zaidi, S.; Korkut, A.; Chen, J.; Rao, S.; Gu, S.; Jogunoori, W.; Mishra, B.; Akbani, R.; Mishra, L. A Pan-Cancer Analysis Reveals High-Frequency Genetic Alterations in Mediators of Signaling by the TGF-β Superfamily. Cell Syst. 2018, 7, 422–437.e427. [Google Scholar]
- Al-Ahmadie, H.A.; Iyer, G.; Lee, B.H.; Scott, S.N.; Mehra, R.; Bagrodia, A.; Jordan, E.J.; Gao, S.P.; Ramirez, R.; Cha, E.K.; et al. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nat. Genet. 2016, 48, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Kavuri, S.M.; Searleman, A.C.; Shen, W.; Shen, D.; Koboldt, D.C.; Monsey, J.; Goel, N.; Aronson, A.B.; Li, S.; et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013, 3, 224–237. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208051s004lbl.pdf (accessed on 15 February 2021).
- Volinia, S.; Hiles, I.; Ormondroyd, E.; Nizetic, D.; Antonacci, R.; Rocchi, M.; Waterfield, M.O. Molecular cloning, cDNA sequence, and chromosomal localization of the human phosphatidylinositol 3-kinase p110 alpha (PIK3CA) gene. Genomics 1994, 24, 472–477. [Google Scholar] [CrossRef]
- Weinstein, J.N.; The Cancer Genome Atlas Research Network; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).