Abstract
Background: Diagnosis of ventricular dyssynchrony may play a role in patient selection for cardiac resynchronisation therapy. Various imaging techniques are available for diagnosis of dyssynchrony, including echocardiography and radionuclide imaging. Whether these techniques yield concordant results is not known. Methods: We studied 31 patients with left ventricular systolic dysfunction. Dyssynchrony was evaluated by echocardiography using interventricular mechanical delay (IVMD) and pulsed-wave tissue Doppler imaging (TDI). Dyssynchrony was also measured in the same patients using radionuclide angiography with nuclear phase analysis (NPA). Control subjects with normal systolic function were studied to determine cutoff values for each technique. Results: NPA was more likely to diagnose interventricular dyssynchrony than IVMD (68% vs 26% of patients, p = 0.001). Intraventricular dyssynchrony was more often diagnosed by NPA than TDI (84% vs 42%, p = 0.002). Agreement between echocardiography and NPA was poor in diagnosing inter- and intraventricular dyssynchrony (k < 0.22 for all comparisons). Conclusions: Poor agreement between echocardiography and NPA in diagnosing dyssynchrony may be explained by the inherent differences between these techniques, which are therefore non-interchangeable. Recent data suggest that echocardiography has limited value in predicting response to CRT. As NPA evaluates dyssynchrony differently, it should be evaluated for this application.
Introduction
Detection of mechanical dyssynchrony has been proposed in order to improve patient selection before cardiac resynchronisation therapy (CRT) []. Several imaging techniques exist for the diagnosis of dyssynchrony. Echocardiography is the most frequently used, with M-mode, pulsed-wave Doppler and tissue Doppler imaging (TDI). Magnetic resonance imaging may also quantify ventricular dyssynchrony []. However, the technique is relatively expensive and time-consuming, and cannot be used to study the patient once a device is implanted. Another technique that measures ventricular function and dyssynchrony is radionuclide angiography with nuclear phase analysis (NPA) [], which has been in use for >25 years to assess ventricular contraction. This technique uses 99mTc-labelled erythrocytes to evaluate temporal changes in regional ventricular blood pool activity, thereby allowing evaluation of inter- and intra-ventricular dyssynchrony (Figure 1). Presence of left ventricular dyssynchrony assessed by NPA was predictive of unfavourable outcome in patients with idiopathic dilated cardiomyopathy []. This technique has the advantage over echocardiography of evaluating global ventricular dyssynchrony rather than regional wall motion as with TDI, and unlike MRI may be performed in patients already implanted with CRT [,].
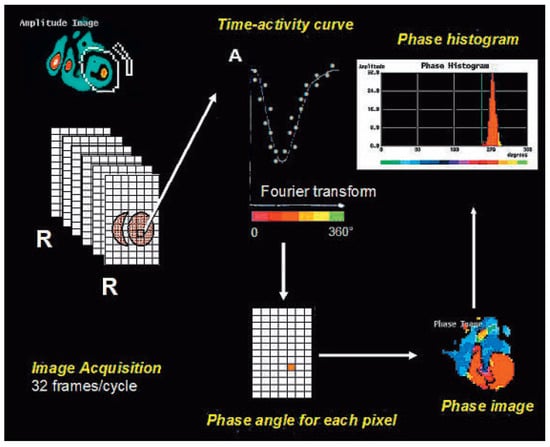
Figure 1.
Principles of nuclear phase analysis (NPA) of radionuclide ventriculography. 32 images are acquired per cardiac cycle (by ECG gating) with planar scintigraphy in the “best septal” LAO projection (for optimal separation of the ventricles). After defining the endsystolic and enddiastolic frames, a region of interest is manually drawn (in this case around the left ventricle). For each pixel, a time-activity curve is plotted using a first harmonic Fourier transform. The maximum change in activity defines the phase angle of the pixel. Colour-coding of each pixel according its phase angle then allows construction of a phase map, the data of which can also be displayed as a phase histogram. This is an example of a left ventricular phase histogram in a normal subject.
There is currently no gold standard for diagnosis of dyssynchrony, and a direct head-to-head comparison of different techniques is lacking. Our aim was therefore to investigate whether echocardiography and NPA provide concordant results for evaluation of inter- and intraventricular dyssynchrony.
Methods
Study Population
The study included 31 patients (66 ± 14 years, 21 males) with a left ventricular ejection fraction (LVEF) of ≤0.35 (measured by radionuclide angiography) and in sinus rhythm. QRS width was not a criterion, since it has previously been shown that mechanical dyssynchrony may be present in patients with a narrow QRS []. Six patients were implanted with a CRT device which was inactivated during the recordings. A control group of 40 subjects (49 ± 13 years, 19 males) with normal left ventricular systolic function (LVEF ≥0.6), sinus rhythm, a narrow QRS (<120 ms), and without structural heart disease was also studied to obtain cutoff values of the echocardiographic parameters, as previously reported []. Another group of 33 subjects (50 ± 14 years, 15 males) without a cardiovascular history, normal left ventricular systolic function, sinus rhythm, and a narrow QRS (<120 ms) was also studied to obtain normal cutoff values for NPA. Patients with bundle branch block were excluded from the control group, as mechanical dyssynchrony may result from the intraventricular conduction delay despite normal global systolic function. The protocol was approved by the institutional ethics committee, and all patients gave informed consent to participation in the study.
Echocardiography
All echocardiographic data were acquired by a single experienced observer (HM) to reduce variability of the recordings, using a Philips Sonos 7500 echocardiograph (Andover, MA) with an S3 probe. Digital echocardiograms were recorded according to the American Society of Echocardiography guidelines [].
Standard echocardiography: The same technique as described in the CARE-HF study [] was used to measure interventricular mechanical delay (IVMD). Pulsed-wave Doppler samples were placed at the leaflet tips of the aortic and pulmonary valves in the apical five-chamber view and the parasternal short-axis view respectively. The aortic and pulmonary pre-ejection intervals were defined as the delays between the onset of the QRS complex and onset of blood flow. The difference between these intervals yielded the IVMD.
TDI: Pulsed-wave TDI samples (with 250 Hz pulsed-rate frequency) were placed on the basal segment of the free wall of the right ventricle and a total of four basal segments of the left ventricle in the apical 2- and 4-chamber views. At each point the delay between QRS onset and onset of the Sm (Systolic motion) wave was measured. Interventricular delay was defined as the maximal absolute delay between measurements of the right and left ventricle []. Intraventricular delay was defined as the maximal difference between any 2 of the 4 measurements of the left ventricle [,,,,].
All recordings were performed at end-expiration during normal breathing. Gain and filter settings were adjusted to optimise the images, and the sweep speed was set to 100 mm/s. A single observer (HM) measured the data using digital callipers with dedicated software (EnconCert, Philips, Andover, MA). Each parameter was averaged over three consecutive beats, and the data were rounded off to the nearest 5 ms for easier interpretation. The same observer repeated the recordings and measurements in 16 patients to assess intra-observer reproducibility.
Radionuclide Angiography and NPA
Standard blood labelling was performed after a blood sample was drawn and labelled with 1 GBq of technetium-99m and then reinjected to the patient intravenously. The ECG was monitored continuously during image acquisition for R-wave gating, with elimination of extrasystolic and post-extrasystolic cycles. Multigated equilibrium blood pool planar scintigrams at 32 frames/cycle (200–250 Kcounts/frame in a 64 × 64 matrix) were acquired using an ADAC-Phillips gamma camera until the number of counts was at least 6 × 106 in the “best-septal” left anterior oblique projection providing optimal right and left ventricular discrimination. This usually required approximately 10 min of acquisition. The right and left ventricular regions of interest in telesystole and telediastole (corresponding to the minimum and maximum blood pool activities respectively) were manually drawn by a single investigator (I.F.) Ventricular dyssynchrony was assessed by NPA as previously described [,,]. The computer assigns a phase angle (between 0 and 360°) to each pixel of the image (Figure 1). A phase histogram is then generated, representing the distribution of the different phases measured in the regions of interest selected. This histogram is colour-coded, allowing identification of the different peaks corresponding to different regions of the ventricles. Each ventricle may be analysed separately, with calculation of the corresponding mean and standard deviation (SD) of the phase histogram which represent the mean time and the spread of regional motion. Interventricular dyssynchrony was measured as the absolute difference between the arithmetic mean phase of each ventricle. Left intraventricular dyssynchrony was calculated as the SD of the left ventricular phase histogram [,].
Phase data were processed using locally-developed customised software (Hermes Medical Solutions, Stockholm, Sweden). Phase angles were converted from 360° angles to milliseconds for easier comparison with echocardiography by the following formula: (phase angle/360)xRR(ms). Analysis was restricted to the ventricular histograms by a cropping tool of the customised software. To avoid discontinuities of the phase histogram, the entire scale was shifted by −100 ms when required, thus placing mean ventricular ejection time in the centre of the phase histogram scale, as previously described [,]. The same investigator blindly performed the measurements twice in 18 patients at different times to assess intra-observer reproducibility.
Statistical Analysis
The Shapiro-Wilk test indicated that the echocardiographic and nuclear data did not have Gaussian distributions. Due to positive skewness of the data, we used the 90th percentile (instead of mean + 2 SD) to obtain cutoff values of the parameters from the control populations. The Mann-Whitney test was used to compare continuous variables between the control and patient groups. We used the McNemar test to compare diagnosis of dyssynchrony obtained by the different techniques. Linear regression was used to correlate values of dyssynchrony between echocardiography and NPA. Agreement was evaluated using the Kappa statistic (with k ≤ 0.2 indicating poor agreement and k = 0.21–0.4 fair agreement). Reproducibility was assessed using the Bland-Altman method. Data are expressed as mean ± SD. A two-sided p value of <0.05 was considered statistically significant.
Results
The patient demographics are shown in Table 1.

Table 1.
Patient population demographics.
Reproducibility of the measurements: 95% limits of agreement were −27 ms to 26 ms for IVMD, −20 ms to 19 ms for TDI interventricular dyssynchrony and −19 ms to 17 ms for TDI intraventricular dyssynchrony. Reproducibility of NPA was similar to that of TDI concerning interventricular dyssynchrony: −18 ms to 22 ms, and slightly less good for intraventricular dyssynchrony: −25 ms to 27 ms. Variations in measurement of NPA parameters were chiefly due to slight differences in regions of interest and in selection of the limits of the phase histograms by the cropping tool.
Normal limits for diagnosis of dyssynchrony: Cutoff echocardiographic values obtained from the control group (rounded off to the nearest 5 ms) were as follows: >30 ms for IVMD, >25 ms for interventricular dyssynchrony by TDI, and >20 ms for left intraventricular dyssynchrony by TDI. For NPA, cutoff values (rounded to the nearest 5 ms) were >20 ms for interventricular dyssynchrony, >30 ms for left intraventricular dyssynchrony (LV phase SD). Results of all parameters of dyssynchrony differed significantly between control and patient groups (Table 2). We also analysed our data by applying previously-reported limits of IVMD >40 ms [], TDI interventricular delay >38 ms [], TDI left intraventricular delay >40 ms [], NPA interventricular delay >30 ms [], and NPA LV phase SD >45 ms [] and >50 ms [].

Table 2.
Results of the different parameters of dyssynchrony in the control and patient groups.
Interventricular dyssynchrony: Diagnosis of interventricular dyssynchrony depended on the imaging technique used and the cutoff applied (Table 3). Agreement between the measurement techniques was poor regardless of whether the cutoffs were derived from the study control population or from previous published reports (k < 0.22 for all comparisons). Correlation between echocardiography and NPA was moderate for IVMD and poor for TDI (Figure 2).

Table 3.
Comparison and agreement between echocardiography and NPA for diagnoses of dyssynchrony in the patient population.
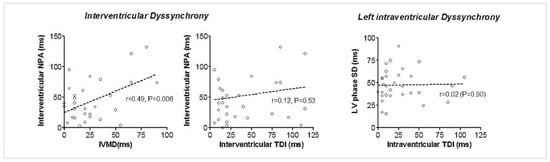
Figure 2.
Correlation between echocardiography and NPA for parameters of interventricular and left intraventricular dyssynchrony.
Intraventricular dyssynchrony: Diagnosis of left intraventricular dyssynchrony was more probable by NPA than by echocardiography, and was highly dependent on the cutoff used (Table 3). Analysing the left ventricular phase maps, 8/31 (26%) patients had evidence of apical dyskinesia (Figure 3). Significant scatter of the measurements obtained from the two techniques resulted in poor correlation for diagnosis of intraventricular dyssynchrony (k < 0.1 for all comparisons). Thus there was no statistically detectable correlation between NPA and TDI for intraventricular dyssynchrony (Figure 2).
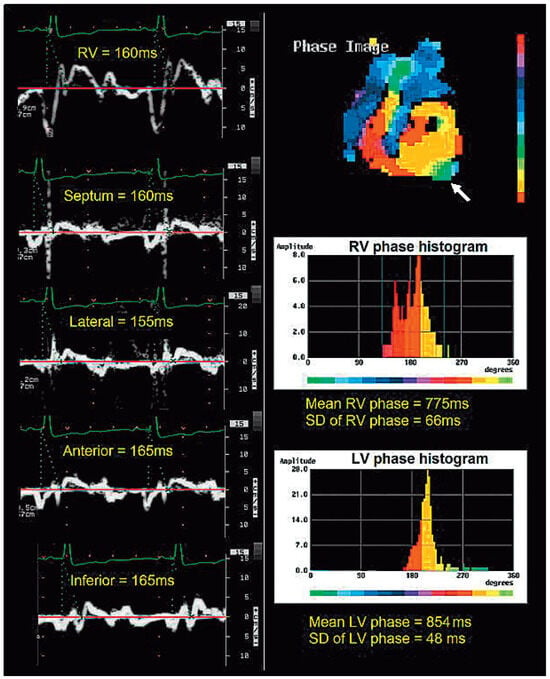
Figure 3.
Discordance between TDI (on the left of the panel) showing absence of inter- or intraventricular dyssynchrony (delays of 5 and 10 ms respectively) and NPA (on the right of the panel) showing both inter- and intraventricular dyssynchrony in the same patient (interventricular delay of 854 − 775 = 79 ms, with a left ventricular phase SD of 48 ms (N < 30 ms). The left ventricular apex is dyskinetic (arrow) shown in green with late phase on the histogram. Note that this patient has a narrow QRS.
An example of discordant diagnosis of inter- and intraventricular dyssynchrony between TDI and NPA in a single patient is shown in Figure 3.
Evaluation of dyssynchrony according to QRS duration: The patient population was divided into two groups according to QRS duration <120 ms (n = 15) or ≥120 ms (n = 16). There was significantly greater interventricular dyssynchrony with echocardiographic parameters in patients with a wide QRS (IVMD 39 ± 27 ms vs 12 ± 9 ms, p = 0.003, and interventricular TDI 59 ± 40 ms vs 27 ± 88 ms, p = 0.034), and a trend towards greater left intraventricular dyssynchrony by TDI (32 ± 30 ms vs 20 ± 23 ms, p = 0.14). There were no differences in dyssynchrony between wide and narrow QRS groups as concerns NPA parameters (interventricular delay 48 ± 40 ms vs 39 ± 27 ms, p = 0.71, and intraventricular LV phase SD 49 ± 20 ms vs 46 ± 14 ms, p = 0.71). Agreement for diagnosis of dyssynchrony between echocardiography and NPA was equally poor in patients with a wide or normal QRS (Table 4).

Table 4.
Agreement between echocardiography and NPA for diagnosis of dyssynchrony in patients with a normal and a wide QRS complex.
Discussion
Our study shows that dyssynchrony in patients with severe systolic dysfunction is more frequently diagnosed with radionuclide angiography than with echocardiography. The former technique showed evidence of interventricular dyssynchrony in 68% of patients and of left intraventricular dyssynchrony in 84% of patients, compared to less than half of patients studied by echocardiography. The prevalence of mechanical dyssynchrony shown by NPA in our patients was higher than previously reported by Fauchier et al. [] where only 37% of patients had interventricular dyssynchrony and 54% left intraventricular dyssynchrony. These differences are due to lower cutoff values for dyssynchrony derived from our larger control population of 33 patients compared to 20 patients in that study [] (higher cutoffs are automatically generated due to larger SD values, despite similar mean values). When we used the same 45 ms cutoff as Fauchier et al. [] for left ventricular phase SD, prevalence of intraventricular dyssynchrony fell to 55% (almost identical to their study). Importantly, agreement between techniques for diagnosing dyssynchrony was no better when we applied cutoffs derived from previously published reports.
The lack of agreement in diagnosis of dyssynchrony between NPA and echocardiography is due to inherent differences in the techniques. First, the physical parameters being measured are different. Pulsed-wave Doppler measures blood flow and TDI measures regional wall motion, whereas NPA analyses regional changes in blood volume. Even though these parameters are interrelated, their relationship is complex. For example, regional changes in blood volume within the left ventricle may not coincide with ventricular outflow, due to redistribution in dyskinetic segments. As another example, TDI may indicate early onset of motion in a segment that is initially stretched while ejection of blood from that region may be very much delayed. Second, pulsed-wave TDI only measures longitudinal wall motion, and ignores radial and circumferential components that also play a part in ejection. Third, echocardiography analyses data from a limited number of points, whereas NPA evaluates dyssynchrony of the entire right and left ventricles. Apical dyskinesia will therefore be missed by TDI samples placed on the basal segments, whereas NPA will indicate dyssynchrony.
Henneman et al. [] reported good correlation between TDI and phase analysis of myocardial perfusion single-photon emission computed tomography (SPECT) for assessment of dyssynchrony. Their results do not contradict our data since SPECT measures myocardial thickening, contrary to radionuclide angiography which assesses changes in blood volume. In another report by the same authors [], phase LV SD measured by SPECT predicted clinical response to CRT with a sensitivity and specificity of 74%, which was less than the 80% sensitivity and specificity previously reported by the same group using TDI []. Recently the PROSPECT trial [] has shown that current echocardiographic techniques have limited value for prediction of response to CRT in patients with a wide QRS, and similarly disappointing results were found for patients with a narrow QRS in the RethinQ trial []. It is therefore unlikely that a technique that is in agreement with these echocardiographic parameters will be able to predict response to CRT. Furthermore, compared to radionuclide angiography, myocardial SPECT has the disadvantages of increased imaging time, inability to evaluate interventricular dyssynchrony and potentially limited feasibility in patients with low myocardial perfusion.
Toussaint et al. [] have shown that baseline interventricular (and not left intraventricular) dyssynchrony assessed by radionuclide angiography and NPA is predictive of a favourable response to CRT. However, left intraventricular dyssynchrony in that study was measured between the apex and the base, and not as the SD of the ventricular phase histogram as in our study and other publications [,]. The main mechanism whereby CRT brings improvement is believed to be correction of left intraventricular dyssynchrony (which increases contractility and reduces mitral regurgitation), rather than of interventricular dyssynchrony (which may nevertheless affect ventricular interdependence) []. Hence prediction of response to CRT by NPA evaluation of left intraventricular dyssynchrony should be reevaluated using the technique described in our report.
Study Limitations
We analysed only pulsed-wave TDI and not colourDoppler TDI, for which many parameters of dyssynchrony have been described. However, as the latter technique is not available in all echocardiographs, pulsed-wave TDI is often used in clinical practice. Pulsed-wave TDI also has better temporal resolution, with a pulsed-rate frequency of 250 Hz compared to approx. 100 Hz with colour-Doppler TDI (depending on the imaging sector used). The results might have been different had we analysed delays to peak Sm by TDI (instead of only to Sm onset). However, we and others have previously shown that this measurement has limited reproducibility [,]. We limited TDI acquisition to four sample volumes of the left ventricle, and had more segments been used agreement with NPA might have been better. The control groups were different for echocardiography and for NPA, and were not matched for age to the patient group, which may therefore have yielded inappropriate cutoff values of dyssynchrony. However, it has previously been shown that dyssynchrony is not related to age in control subjects []. We also used other cutoff values obtained from previous studies in our analysis, which did not affect the results. The population size was relatively limited due to the number of parameters studied in each patient. However, the results very clearly indicated lack of agreement between the different techniques for measuring dyssynchrony, and it is unlikely that the findings would have differed with a larger population. Finally, presence of class III or IV heart failure was not mandatory in our patient population, since our aim was to compare techniques for evaluation of dyssynchrony in patients with systolic dysfunction, and not to evaluate prevalence of dyssynchrony in candidates for CRT.
Conclusions
Whether diagnosis of mechanical dyssynchrony can improve patient selection for CRT remains to be proven by multicentre randomised trials [,,]. The main focus of interest has been echocardiography, but the disappointing results of the PROSPECT [] and RethinQ [] trials have dampened enthusiasm for current echocardiographic parameters. Our study shows that radionuclide angiography yields different results from echocardiography in the diagnosis of dyssynchrony, due to the inherent differences between techniques, and therefore merits further investigation for prediction of response to CRT.
Financial Support
Carine Stettler was supported by a research grant by Medtronic. Haran Burri, MD, was supported by a research grant from the Fondation de Recherche de l’Hôpital de la Tour.
Acknowledgments
Carine Stettler was supported by a research grant by Medtronic. Haran Burri, MD, was supported by a research grant from the Fondation de Recherche de l’Hôpital de la Tour. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology.
Conflicts of Interest
None of the authors has any potential conflict of interest.
References
- Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S; et al. Cardiac resynchronization therapy: Part 1—Issues before device implantation. J Am Coll Cardiol. 2005, 46, 2153–2167.
- Nelson GS, Curry CW, Wyman BT, Kramer A, Declerck J, Talbot M; et al. Predictors of Systolic Augmentation From Left Ventricular Preexcitation in Patients with Dilated Cardiomyopathy and Intraventricular Conduction Delay. Circulation 2000, 101, 2703–2709. [CrossRef]
- Somsen GA, Verberne HJ, Burri H, Ratib O, Righetti A. Ventricular mechanical dyssynchrony and resynchronization therapy in heart failure: A new indication for Fourier analysis of gated blood-pool radionuclide ventriculography. Nucl Med Commun. 2006, 27, 105–112. [CrossRef] [PubMed]
- Fauchier L, Marie O, Casset-Senon D, Babuty D, Cosnay P, Fauchier JP. Interventricular and intraventricular dyssynchrony in idiopathic dilated cardiomyopathy: A prognostic study with fourier phase analysis of radionuclide angioscintigraphy. J Am Coll Cardiol. 2002, 40, 2022–2030.
- Kerwin WF, Botvinick EH, O’Connell JW, Merrick SH, DeMarco T, Chatterjee K; et al. Ventricular contraction abnormalities in dilated cardiomyopathy: Effect of biventricular pacing to correct interventricular dyssynchrony. J Am Coll Cardiol. 2000, 35, 1221–1227. [CrossRef]
- Burri H, Sunthorn H, Somsen A, Zaza S, Fleury E, Shah D; et al. Optimizing sequential biventricular pacing using radionuclide ventriculography. Heart Rhythm. 2005, 2, 960–965. [CrossRef] [PubMed]
- Yu C-M, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart 2003, 54–60.
- Burri H, Muller H, Vieira I, Lerch R. Poor agreement of echographic measures of ventricular dyssynchrony. Eur J Echocardiogr. 2008, 9, 235–240.
- Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ; et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004, 1086–1119.
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L; et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005, 352, 1539–1549. [CrossRef]
- Penicka M, Bartunek J, De Bruyne B, Vanderheyden M, Goethals M, De Zutter M; et al. Improvement of Left Ventricular Function After Cardiac Resynchronization Therapy Is Predicted by Tissue Doppler Imaging Echocardiography. Circulation 2004, 978–983.
- Bader H, Garrigue S, Lafitte S, Reuter S, Jais P, Haissaguerre M; et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol. 2004, 248–256.
- Yu C-M, Lin H, Ho P-C, Yang H. Assessment of Left and Right Ventricular Systolic and Diastolic Synchronicity in Normal Subjects by Tissue Doppler Echocardiography and the Effects of Age and Heart Rate. Echocardiography. 2003, 19–27.
- Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P; et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004, 1834–1840.
- Bordachar P, Lafitte S, Reuter S, Sanders P, Jaïs P, Haïssaguerre M; et al. Echocardiographic parameters of ventricular dyssynchrony validation in patients with heart failure using sequential biventricular pacing. J Am Coll Cardiol. 2004, 2157–2165.
- Fauchier L, Marie O, Casset-Senon D, Babuty D, Cosnay P, Fauchier JP. Reliability of QRS duration and morphology on surface electrocardiogram to identify ventricular dyssynchrony in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2003, 92, 341–344. [CrossRef]
- Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E; et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007, 49, 1708–1714. [CrossRef]
- Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C; et al. Can LV Dyssynchrony as Assessed with Phase Analysis on Gated Myocardial Perfusion SPECT Predict Response to CRT? J Nucl Med. 2007, 48, 1104–1111. [CrossRef]
- Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P; et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004, 44, 1834–1840. [CrossRef]
- Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J; et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008, 117, 2608–2616. [CrossRef] [PubMed]
- Beshai JF, Grimm RA, Nagueh SF, Baker JH, 2nd, Beau SL, Greenberg SM; et al. Cardiac-Resynchronization Therapy in Heart Failure with Narrow QRS Complexes. N Engl J Med 2007.
- Toussaint JF, Lavergne T, Kerrou K, Froissart M, Ollitrault J, Darondel JM; et al. Basal asynchrony and resynchronization with biventricular pacing predict long-term improvement of LV function in heart failure patients. Pacing Clin Electrophysiol. 2003, 26, 1815–1823. [CrossRef] [PubMed]
- Yu C-M, Chau E, Sanderson JE, Fan K, Tang M-O, Fung W-H; et al. Tissue Doppler Echocardiographic Evidence of Reverse Remodeling and Improved Synchronicity by Simultaneously Delaying Regional Contraction After Biventricular Pacing Therapy in Heart Failure. Circulation 2002, 105, 438–445. [CrossRef]
- Jansen AHM, Bracke F, van Dantzig JM, Meijer A, Korsten EHM, Peels KH; et al. Optimization of Pulsed Wave Tissue Doppler to Predict Left Ventricular Reverse Remodeling After Cardiac Resynchronization Therapy. J Am Soc Echocardiogr. 2006, 19, 185–191. [CrossRef]
- Hawkins NM, Petrie MC, MacDonald MR, Hogg KJ, McMurray JJV. Selecting patients for cardiac resynchronization therapy: Electrical or mechanical dyssynchrony? Eur Heart J. 2006, 27, 1270–1281. [CrossRef]
- Burri H, Lerch R. Echocardiography and patient selection for cardiac resynchronization therapy: A critical appraisal. Heart Rhythm. 2006, 3, 474–479. [CrossRef] [PubMed]
- Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J; et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008, 117, 2608–2616. [CrossRef]
© 2010 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.