Abstract
Recurrent surgery in device patients carries a certain risk of infection and should therefore be kept to a minimum. We present the case of a patient in whom a new pacemaker had to be implanted from the left side and the question was, what should be done with the redundant pacemaker on the right side: to explant or not to explant? The answer depends upon the behaviour of the pacemaker at the time of elective replacement indication (ERI) and of end of life (EOL), and differs between the five manufacturers operating in Switzerland. This behaviour is explained in detail and thus can guide cardiologists towards individual decision making.
Case Presentation
In 1991, a 65-year-old male patient was implanted with a dual chamber pacemaker for symptomatic persistent third degree atrioventricular block. Both leads were inserted via the right cephalic vein. Elective device replacement was first necessary in 2000. In 2007, the battery was depleted again. As an insulation defect of the atrial lead was present, lead replacement was planned at the same time. During surgery, the subclavian vein was found to be occluded, resulting in the implantation of a new atrial lead via the right internal jugular vein. In March 2015, the pacemaker battery was once more depleted. As the right ventricular lead now showed a slow, but continuous decrease in impedance (from 510 ohms in 2007 to 330 ohms in 2015), the patient was offered implantation of a new dual chamber pacemaker from the leh side. Extraction of the leads was not considered for various reasons (age, comorbidities and access to one lead via the jugular vein). The patient did not express discomfort from the right-sided old device (Figure 1).
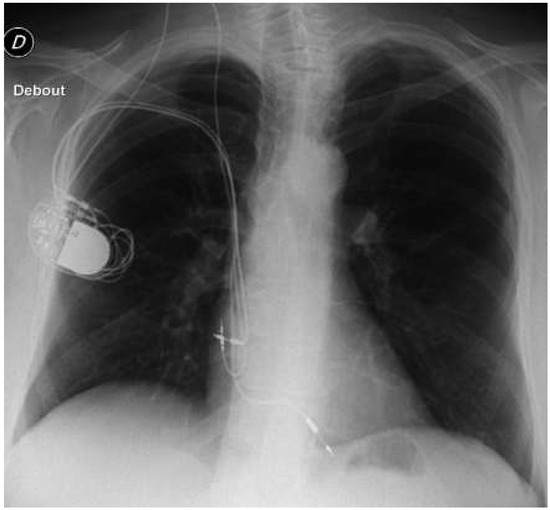
Figure 1.
Chest X ray at presentation.
Pacemaker Problem
The pertinent question was whether the right-sided pacemaker should be leh in place and programmed to an ODO or OOO mode, or if it should be explanted. Arguments against explanting the pacemaker were that there was no obvious clinical need and that there is a statistical 1.4% chance of infection [1], double that associated with a de-novo implant [2]. This had to be balanced against possible interferences between the new pacemaker implanted from the leh side and the old device falling below the “elective replacement indicator” (ERI) and then later going into its “end of life” (EOL) mode.
If the pacing mode remains in ODO or OOO mode, no problems will occur. However, if the mode switched to VOO or DOO, induction of ventricular fibrillation via an “R on T phenomenon” is possible. Albeit rare [3], this is an avoidable life-threatening complication. If the pacemaker switches to a VVI or DDD mode with a fixed rate, this fear is unfounded, but the fixed rate might limit programming of the new device. A final option would be to program the output to its lowest and pulse width to its shortest value.
Depending on the manufacturer, different behaviours at EOL are present. They are presented in alphabetical order in Table 1.

Table 1.
Necessity of pacemaker explantation for different manufacturers.
Biotronik pacemakers
The pacemaker can be programmed to an “off” mode. However, a password given by the manufacturer’s repre- sentative is needed to program this mode. At ERI, this “off” mode is not changed. Programming is only reverted if a back-up mode event occurs, e.g., due to heavy electromagnetic interference, or at “end of life”. However, at EOL pacing output is already so low that no effective pacing occurs. This behaviour applies to pacemakers of all generations as well as to cardiac resynchronisation therapy (CRT) devices.
The pacemaker thus does not need to be explanted.
Boston Scientific pacemakers
Pacing can be programmed “off”. This mode is not affected by EOL. During further depletion, the pace- maker will switch to a storage mode and then cannot pace any longer. This behaviour applies to pacemakers of all generations as well as to CRT devices.
The pacemaker thus does not need to be explanted.
Medtronic pacemakers
At ERI, programming will switch to VVI 65/min with an output of 5.0 V / 1.0 ms. At EOL, the device typically continues to work in VVI 65/min. Sometimes beyond EOL (and there is no way to tell when) there is a moment when the device can reset (“power on reset”) owing to low battery voltage, and resume pacing at random settings and device behaviour. This behaviour applies to pacemakers of all generations as well as to CRT devices.
The pacemaker thus needs to be explanted.
Sorin (LivaNova) pacemakers
If the battery impedance falls below the ERI value, the device switches to VVI 70/min with unipolar pacing of 5.0 V / 0.5 ms, whatever the previous programmed mode was. Sensing is set to 2.2 mV in a unipolar mode. Even if a OOO mode were reprogrammed, the next day the device will again perform an impedance measure- ment and switch back to VVI 70/min. This interferes with standard settings, especially in patients with DDD pacemakers. This behaviour applies to pacemakers of all generations as well as to CRT devices.
The pacemaker thus usually needs to be explanted.
St Jude Medical pacemakers
The programmed pacemaker mode is not affected and remains in the ODO/OVO setting. However, if the pace- maker falls into “back-up” mode, it switches into a VVI mode. Such a switch occurs when the pacemaker notices inconsistencies in its sohware status. In spite of this being a rare phenomenon, St Jude Medical recommends explanting the pacemaker.
The pacemaker thus needs to be explanted.
Conclusion
In our patient, the Medtronic pacemaker was ex- planted. Over a follow-up of 9 months, the course of the patient was uneventful.
Generally, it depends on the specific behaviour of the pacemakers from each manufacturer whether a pacemaker must be explanted or not in such a situation.
Disclosure Statement
Simon von Gunten, Jean-Luc Crevoisier and Tobias Reichlin: none declared. Michael Kühne has served on the speakers’ bureau for Boston Scientific, St Jude Medical and Biotronik. Stefan Osswald has served on the speakers’ bureau for Medtronic, Boston Scientific, Biotronik, St Jude Medical and has received unrestricted grants from Medtronic, Boston Scientific, Biotronik, and St Jude Medical. Christian Sticherling has served on the speakers’ bureau for Medtronic, Biotronik and Sorin and had scientific support from Medtronic, Biotronik, Boston Scientific, St Jude Medical and Sorin. Beat Schaer has served on the speakers’ bureau for Medtronic and Boston Scientific.
References
- Poole, J.E.; Gleva, M.J.; Mela, T.; Chung, M.K.; Uslan, D.Z.; Borge, R.; et al. Complication Rates Associated With Pacemaker or Implantable Cardioverter-Defibrillator Generator Replacements and Upgrade Procedures Results From the REPLACE Registry. Circulation 2010, 122, 1553–U43. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, K.A.; Konstantelias, A.A.; Falagas, M.E. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015, 17, 767–777. [Google Scholar] [PubMed]
- Oupadia, P.; Ramaswamy, K. Images in clinical medicine. “R-on-T” phenomenon. N. Engl. J. Med. 1998, 338, 1812. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.