Summary
Background: The optimal timing of the initial echocardiographic assessment and the influence of microorganisms on echocardiographic findings in patients with infective endocarditis (IE) are not well studied. Methods: In 274 patients with IE, we studied the impact of antibiotic treatment duration of ≤2 days (early, 119 patients) or >2 days (late, 144 patients) prior to diagnostic echocardiography on IE specific findings and on clinical outcomes. Results were stratified for patients with Staphylococcus aureus (SA patients, n = 84) and those with other causative organisms (non-SA patients, n = 190). Results: There were no differences on specific echocardiographic findings between patients with early versus late echocardiography: Presence of vegetations: 91% vs 86%, p = 0.25; size of vegetations: 1.5 ± 0.7 cm vs 1.5 ± 0.8 cm, p = 0.83; paravalvular abscess: 24% vs 24%, p = 0.88, or valve destruction: 44% vs 35%, p = 0.17. There were also no differences in terms of clinical outcomes between the two groups: Heart surgery for IE in 61% vs 53%, p = 0.21, and in-hospital death in 8% vs 11%, p = 0.46. The presence of SA was not associated with specific findings on echocardiography or worse clinical outcomes compared to non-SA patients. Conclusions: In patients with infective endocarditis, the findings of early vs late initial echocardiographic assessment did not differ, and echocardiographic findings did not allow inference on the causing organism. Neither the timing of the initial echocardiographic study nor any organism involved was associated with clinical outcome.
Introduction
Infective endocarditis (IE) is a life threatening condition with in-hospital mortality rates of 15%–20% [1,2,3,4]. Staphylococcus aureus (SA) has been identified as the most common causing microorganism in patients with IE (up to 31% of all patients with IE) and has been associated with worse outcomes compared to other microorganisms. In some series, patients with SA endocarditis had mortality rates of 22%–40% for left sided native valve IE and up to 45% in prosthetic valve IE [5,6,7]. Early diagnosis and prompt initiation of adequate treatment is crucial for favourable outcomes [8].
While the initial management of IE is mainly guided by clinical findings and positive blood cultures, echocardiography is the cornerstone for confirmation of IE. In all patients with SA bacteraemia, transoesophageal echocardiography (TEE) is now indicated, due to its high accuracy in detecting IE [9,10]. Although there is no data on optimal timing of echocardiographic assessment in the case of suspected endocarditis, American and European Guidelines on management of IE recommend echocardiography at the earliest possible opportunity [5,9]. However, increasing overuse of echocardiography in the setting of suspected IE with low pre-test probability has been noted [11,12].
The aims of this study were (1.) to study the influence of IE causing organisms, in particular that of SA, on echocardiographic findings, (2.) to analyse the impact of timing of the initial echocardiographic assessment on IE specific findings and (3.) to study the impact of microorganisms as well as of timing of the initial echocardiography on outcome.
Methods
Patient Population
All patients referred to our echocardiography laboratory for assessment of infective endocarditis between 1995 and 2011 were identified from our prospective echocardiography database. Only patients with echocardiographic findings confirming the diagnosis of IE comprised the study group.
Clinical and Laboratory Data
Baseline clinical and demographic variables as well as information on outcomes were derived from chart review. For all patients the onset of typical symptoms and signs of IE (fever >38 °C, typical vascular and immunological findings) as well as the time of initiation of antibiotic treatment prior to the first echocardiographic examination were carefully assessed. Complete blood count, C-reactive protein levels and serum creatinine levels at hospital admission were recorded. We also reviewed computed tomography exams that were performed within 72 hours of hospital admission for evidence of systemic or (in the case of right-sided IE) infective pulmonary embolisms. The risk factors for IE included pre-existing valvular heart disease, intravenous drug abuse, immunosuppressive drug therapy, diabetes mellitus, human immunodeficiency virus (HIV) infection and the presence of central venous catheters [9]. Based on clinical, laboratory, microbiology and echocardiographic findings the modified Duke criteria were applied to all study subjects [13,14].
Microbiology Data
Data of isolated microorganisms were derived from blood cultures, broad-range polymerase chain reaction [15,16] performed on postoperative valve material as well as from serological testing. If more than one type of microorganism had been isolated, the most likely IE causing organism was determined by an infectious disease specialist. Special attention was paid to the incubation time of blood cultures and the time that had elapsed between positive blood cultures and the echocardiographic study. For the purpose of this study patients were grouped in those with Staphylococcus aureus (SA patients) and those with other microorganisms (non-SA patients) causing IE.
Echocardiography
Transthoracic (TTE) and/or transoesophageal echocardiography (TEE) were used according to clinical setting and image quality. When typical findings of IE were identified on TTE in clinically stable patients TEE was not necessarily performed [9]. Findings consistent with IE were vegetations (defined as oscillating intracardiac masses on a valvular structure, in the path of regurgitant jets or on implanted prosthetic material), valvular and paravalvular abscesses, novel partial dehiscence of a prosthetic valve and/or newly detected valvular regurgitation of ≥ moderate severity [17,18,19]. In all patients in whom vegetations were detected, their maximal length was measured. To assess the impact of echocardiographic timing on findings consistent with IE, patients were further divided into those with early (≤2 days of antibiotic treatment prior to the initial echocardiographic study) and late assessment (>2 days of antibiotic treatment). In the absence of relevant literature, those time frames were chosen arbitrarily. They do however represent typical time frames often discussed in clinical practice.
Clinical Outcomes
We analysed the impact of timing of the initial echocardiographic assessment as well as of microorganisms causing IE on heart surgery for IE and on in-hospital death. Heart surgery for IE was further divided into early (within 6 weeks of the diagnosis) and late surgery.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation or median and range, as appropriate. Categorical variables are expressed as number and percentage. For comparison between groups we used the independent sample T-test, the Mann-Whitney test or chi-square tests, as appropriate. For comparison of 3 or more groups we performed one-way analysis of variance (ANOVA) with Duncan’s posthoc analysis or chi-square test. A two-sided p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS software (release 19, SPSS Inc., Chicago, Illinois).
Results
Patient Population
Of a total of 1,995 patients with suspected infective endocarditis referred for echocardiographic assessment, 327 (16.3%) had findings confirming IE (flow chart Figure 1). 53 of these patients had to be excluded from analysis due to incomplete clinical data. The remaining 274 patients with clinical signs and echocardiographic findings confirming the diagnosis of IE and who were treated accordingly represent the study subjects.
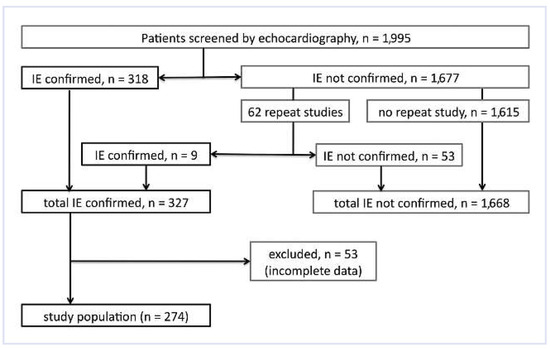
Figure 1.
Flow chart of patient population.
Microbiological Spectrum
The spectrum of organisms involved is given in Figure 2. Of the 236 organisms, 224 (82%) were identified by blood cultures, while 12 (18%) were only detected by PCR or serological analyses.
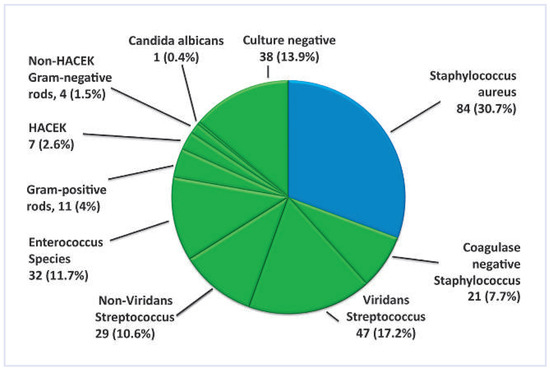
Figure 2.
Overview of IE causing organisms (n = 236). Blue colour = Staphylococcus aures patients; green colour = non-Staphylococcus aureus patients ; HACEK = Haemophilus, Actinobacillus, Cardiobacterium hominis, Eikenella, Kingella.
Clinical Findings and Duke Classification
The baseline characteristics and specific findings for the Duke classification of the 274 patients in the study group are shown in Table 1. Patients in the SA-group had higher CRP and creatinine levels (both p <0.001) as well as more vascular (embolic) complications (p = 0.001) at initial presentation and were more likely to have a history of fever (p = 0.02). When the study population was divided into those with early vs late echocardiographic assessment, the baseline characteristics as in Table 1 did not significantly differ between the two groups, with the only exception of the leucocyte count (early: 12,800 ± 7,100/μl vs late: 10,900 ± 5,700/μl, p = 0.02).

Table 1.
Baseline characteristics of the study population (n = 274).
Since the presence of echocardiographic findings consistent with IE was an inclusion criterion for this study, all patients had at least one, and 66% had two major Duke criteria. Based on the modified Duke classification, 214 (78%) patients would have been classified as definite IE. The remaining 60 (22%) patients qualified as “possible IE”, including the 38 (14%) patients with unidentified microorganism. However, all patients who were analysed in this study were clinically considered to have IE and received a full course of antibiotics.
Time Course of IE
Compared to the non-SA group, SA patients had a shorter time interval from first clinical signs to diagnosis (9 [1–30] days vs 14 [1–180] days, p <0.001) and shorter blood culture incubation times (1 [1–3] days vs 2 [0–34] days, p <0.001). However, once the diagnosis was confirmed by echocardiography, the time to the clinical endpoints (in-hospital death and heart surgery for IE) did not differ significantly between the two groups.
Influence of Microorganisms on Echocardiographic Findings (Table 2)

Table 2.
Sites of infectious endocarditis and specific findings on echocardiography.
More SA infections were found in native tricuspid valves (p = 0.01), whereas overall prosthetic valve IE was more often caused by non-SA organisms (p = 0.006). Paravalvular abscess formation in patients with prosthetic valves was the only IE specific finding more common in the non-SA group compared to the SA group (p = 0.04, most often caused by Enterococcus species). All other findings were not significantly different between the SA and the non-SA groups. Figure 3 illustrates the heterogeneous appearances of IE specific findings caused by the same microorganism (panel 1A/B, 2A/B and 3A/B), and the homogenous appearances of findings caused by different microorganisms (panel 4A/B).
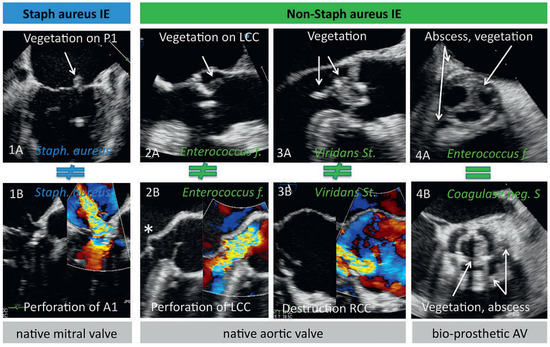
Figure 3.
Spectrum of echocardiographic findings in infective endocarditis. Illustration of the heterogeneous appearances of infective endocarditis specific echocardiographic findings caused by the same microorganism (panel 1A/B, 2A/B and 3A/B), and the homogenous appearances of findings caused by different microorganisms (panel 4A/B). * = septic aneurysm of the anterior mitral leaflet; Staph = Staphylococcus; P1 = segment P1 of the posterior mitral leaflet; A1 = segment A1 of the anterior mitral leaflet; Enterococcus f = Enterococcus faecalis; St = Streptococcus; Coagulase neg S = Coagulase negative staphylococcus; LCC and RCC = left and right coronary cusp, respectively; AV = aortic valve.
Early vs Late Initial Echocardiographic Assessment
The duration of antibiotic therapy before the initial echocardiographic assessment had no significant impact on IE specific findings on echocardiography (Table 3). Sub-analyses for the 57 patients who had no antibiotic treatment at all before their initial echocardiographic study did not reveal significant differences in echocardiographic findings compared to those who had prior antibiotic treatment.

Table 3.
Analysis of early versus late initial echocardiographic assessment.
Influences of Microorganisms and Timing of Echocardiography on Outcome
26 patients (10%) died during the index hospital stay, and heart valve surgery was required in 151 (55%) of patients (110 or 40% were performed early). The outcome of SA patients in terms of the need for heart surgery (51% vs 57%, p = 0.32), the need for early heart surgery for IE (35% vs. 43%, p = .21) or the rate of in-hospital deaths (13% vs 8%, p = 0.18) was not significantly different from patients with non-SA IE. Even when analysing all identified microorganisms separately, no single organism was associated with better or worse clinical outcome. Furthermore, the duration of antibiotic treatment (0–2 days or >2 days) before the initial echocardiographic assessment was not associated with different clinical outcome (Table 3).
Patients with an Initially False Negative Study
In the nine patients in whom typical findings of IE were detected only on follow-up echocardiography, vegetations were smaller compared to those with vegetations on their initial echocardiographic study (0.7 cm vs 1.6 cm, p = 0.04), and fewer had new significant valvular regurgitation (44% vs 79%, p = 0.01). All other echocardiographic findings, organisms involved as well as clinical outcomes were not different compared to those with typical findings on their initial echocardiography.
Discussion
In 274 patients with acute infective endocarditis (IE) the initial echocardiographic findings were not specific for Staphylocouccus aureus (SA) or any other organisms, and the outcome did not differ between SA and non-SA patients. Early vs late initial echocardiographic assessment with regards to the duration of prior antibiotic treatment was not associated with specific echocardiographic findings or clinical outcomes.
Timing of the Initial Echocardiographic Assessment
Little is known about the course of IE specific changes over time, and hence about the influence of timing of the initial echocardiographic assessment on findings or diagnosis of IE. American and European IE guidelines suggest “rapid” echocardiographic assessment in all patients with suspected IE but do not further specify the timeline [5,9]. Indeed there is no evidence on the best timing of echocardiography in literature. It is however known that presence and size of vegetations are relatively stable over time; 70% of vegetations were still present at the end of the antibiotic treatment period, and of these 59% did not show any difference in size [20]. Furthermore, the choice of antibiotic treatment appears to influence the size of vegetations on serial echocardiography; vancomycin-associated treatment was related to a 45% reduction of vegetation size, while penicillin reduced vegetation size only by 5% [21]. In our study, IE specific findings were not significantly different between patients with early (median of 1 day of antibiotic treatment) or late echo assessment (median of 9 days), and late echocardiographic assessment was not associated with a worse outcome. To the best of our knowledge, no similar data concerning timing of the initial echocardiographic study has been published before.
Patients with Initially Negative Echocardiographic Studies
Repeat echocardiography after 7–10 days is indicated in cases with initially negative findings and persistent clinical suspicion for IE [5]. However, there is only limited data on rates of and findings in repeat studies. In the study of Sochowsky et al., repeat studies were positive in 3 out of 7 patients (43%) [22]. Features associated with initially negative studies included challenging echocardiographic conditions such as mitral valve prolapse, ruptured chords, presence of prosthetic valves, small vegetations mistaken for strands and SA endocarditis [10,22,23]. In our series, repeat studies confirmed IE in 9 out of 62 patients (15%), and vegetations of these patients were smaller compared to all other patients. This suggests that they grew later or might have been missed in the initial study due to their small size, despite the use of TEE in the initial assessment of 7 out of 9 patients with repeat studies.
Association Between IE Causing Organisms and Specific Findings on Echocardiography
The ability to infer on a specific organism based on findings on echocardiography is not well studied. While numerous studies on IE have identified the spectrum of involved organisms, most of them did not precisely analyse the association between echocardiographic findings and identified organisms [3,24,25,26]. A recent international prospective study demonstrated that the sites infected with SA and the echocardiographic findings in SA IE are not specific, and a considerable overlap exists for example with coagulase negative staphylococcus [2]. In our study however, SA caused more tricuspid valve IE compared non-SA patients, and significantly more non-SA patients had prosthetic valve infection and prosthetic paravalvular abscess formation compared to SA patients. While relevant differences in echocardiographic findings can be found between SA and non-SA patients, none of the findings was specific for one of the groups, and therefore inference to the IE causing organism based on echocardiographic findings is impossible.
Clinical Implications
Rapid performance of echocardiography in patients with IE is important to confirm the suspected diagnosis, to assess parameters that predict complications and guide management decisions in order to optimise outcome [3,25,26,27,28,29]. But how rapid is rapid? While our study was not designed to give a definite answer to that question, our results indicate that short-term antibiotic treatment does not significantly alter the IE specific findings and hence does not influence the confirmation of IE. In addition, the time period of antibiotic treatment before confirmation of IE by echocardiography did not influence outcome. Therefore, deferring the initial echocardiographic assessment for a few days appears to be safe. Most importantly, this holds true only as long as patients are clinically stable and adequately treated for potential IE. Deferring the confirmation of IE by echocardiography needs to be weighed against the eventually unnecessary exposure to potentially harmful antibiotic treatment. The optimal timing for echocardiographic assessment therefore remains a decision based on individual clinical factors.
The goal of early diagnosis of IE is improving patient outcome. Assuming optimal timing of the initial echocardiographic assessment, it is important to remember which questions can be answered by echocardiography. Echocardiography is key for confirmation of IE and detecting complications at the valvular level that may influence management decisions such as timing of surgery or length of antibiotic treatment [3,8]. However, as emphasised by our study, echocardiography does not allow inference on the IE causing microorganisms. This limitation of echocardiography is important to realise, since long-time survival of patients depends on early and targeted antibiotic treatment [4]. On the other hand, our study demonstrates that many of the clinical findings differed significantly between SA and non-SA patients. Therefore, a precise patient history, including assessment of risk factors for IE, and a meticulous physical examination to detect vascular and immunologic complications may give early clues on the microorganism involved and are a valuable guide for the choice of empiric antibiotic treatment.
Limitations
This analysis has the limitations associated with retrospective studies. In order to best assess the optimal timing for the echocardiographic detection of IE specific alterations, a prospective study with multiple repeat echocardiographic assessments before and during AB treatment would be necessary. However, since TEE is associated with (an albeit low) morbidity and mortality risk [30], multiple repetitive studies would be difficult to justify in view of the current literature.
When dividing our population in SA and non-SA patients, we included culture negative patients in the group of non-SA patients. We may have unintentionally mixed unidentified SA patients with the non-SA patients. If so, this did not have a relevant impact on results. When we analysed the culture negative group separately, it did not differ significantly from the non-SA group.
In this study we did not make a distinction between the echocardiographic modalities used. TEE, for example, is known to have higher sensitivity for detection of paravalvular abscess formation and prosthetic valve endocarditis [31,32]. The modality used in a given patient represented the optimal choice for decision making in the specific situation. However, we cannot exclude that using TEE in each patient might have slightly altered our results.
Our outcome data are limited despite the long period of time covered by this study. Over the 16 years of the study duration, diagnostic accuracy as well as therapeutic strategies have changed. Furthermore, there have been changes in clinical consultants and cardiac surgeons during the study period, potentially influencing management strategies and outcome. The (relatively few) changes in echocardiographers involved may have influenced the accuracy of the operator dependent TTE and TEE. Finally, the influence of new surgical strategies, new antibiotics or new resistance patterns to antibiotics has not been studied.
Conclusions
In patients with infective endocarditis, the findings of early vs late initial echocardiographic assessment did not differ. Echocardiographic findings of infective endocarditis did not allow inference on the causing organism. Neither the timing of the initial echocardiographic study nor any organism involved was associated with clinical outcome.
Funding/Potential Competing Interests
No financial support and no other potential conflict of interest relevant to this article were reported.
References
- Bashore, T.M.; Cabell, C.; Fowler, V., Jr. Update on infective endocarditis. Curr Probl Cardiol. 2006, 31, 274–352. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G.; Jr Bayer, A.S.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Thuny, F.; Di Salvo, G.; Belliard, O.; Avierinos, J.F.; Pergola, V.; Rosenberg, V.; et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005, 112, 69–75. [Google Scholar] [CrossRef]
- Thuny, F.; Grisoli, D.; Collart, F.; Habib, G.; Raoult, D. Management of infective endocarditis: challenges and perspectives. Lancet 2012, 379, 965–975. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Jr. , Bolger, A.F.; Levison, M.E.; et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005, 111, e394–434. [Google Scholar]
- Fowler, V.G., Jr. , Miro, J.M.; Hoen, B.; Cabell, C.H.; Abrutyn, E.; Rubinstein, E.; et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005, 293, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Chirouze, C.; Cabell, C.H.; Fowler, V.G.; Khayat, N.; Olaison, L.; Miro, J.M.; Habib, G.; Abrutyn, E.; Eykyn, S.; Corey, G.R.; et al. Prognostic Factors in 61 Cases of Staphylococcus aureus Prosthetic Valve Infective Endocarditis from the International Collaboration on Endocarditis Merged Database. Clin. Infect. Dis. 2004, 38, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Botelho-Nevers, E.; Thuny, F.; Casalta, J.P.; Richet, H.; Gouriet, F.; Collart, F.; Riberi, A.; Habib, G.; Raoult, D. Dramatic Reduction in Infective Endocarditis–Related Mortality With a Management-Based Approach. Arch. Intern. Med. 2009, 169, 1290–1298. [Google Scholar] [CrossRef]
- Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and by the International Society of Chemotherapy (ISC) for Infection and Cancer; Habib, G. ; Hoen, B.; Tornos, P.; Thuny, F.; Prendergast, B.; Vilacosta, I.; Moreillon, P.; Antunes, M.d.J.; Thilen, U.; et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur. Hear. J. 2009, 30, 2369–2413. [Google Scholar] [CrossRef]
- Fowler, V.G.; Li, J.; Corey, G.; Boley, J.; A Marr, K.; Gopal, A.K.; Kong, L.K.; Gottlieb, G.; Donovan, C.L.; Sexton, D.J.; et al. Role of Echocardiography in Evaluation of Patients With Staphylococcus aureusBacteremia: Experience in 103 Patients. JACC 1997, 30, 1072–1078. [Google Scholar] [CrossRef]
- Greaves, K.; Mou, D.; Patel, A.; Celermajer, D.S. Clinical criteria and the appropriate use of transthoracic echocardiography for the exclusion of infective endocarditis. Heart 2003, 89, 273–275. [Google Scholar] [CrossRef]
- Kuruppu, J.C.; Corretti, M.; Mackowiak, P.; Roghmann, M.-C. Overuse of Transthoracic Echocardiography in the Diagnosis of Native Valve Endocarditis. Arch. Intern. Med. 2002, 162, 1715–1720. [Google Scholar] [CrossRef][Green Version]
- Durack, D.T.; Lukes, A.S.; Bright, D.K.; Service, D.E. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Bosshard, P.P.; Kronenberg, A.; Zbinden, R.; Ruef, C.; Böttger, E.C.; Altwegg, M. Etiologic Diagnosis of Infective Endocarditis by Broad-Range Polymerase Chain Reaction: A 3-Year Experience. Clin. Infect. Dis. 2003, 37, 167–172. [Google Scholar] [CrossRef]
- Rampini, S.K.; Bloemberg, G.V.; Keller, P.M.; Buchler, A.C.; Dollenmaier, G.; Speck, R.F.; et al. Broad-range 16S rRNA gene polymerase chain reaction for diagnosis of culture-negative bacterial infections. Clin Infect Dis. 2011, 53, 1245–1251. [Google Scholar] [CrossRef]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Moura, L.; Popescu, B.A.; Agricola, E.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr. 2010, 11, 223–244. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009, 22, 975–1014. [Google Scholar] [PubMed]
- Vuille, C.; Nidorf, M.; Weyman, A.E.; Picard, M.H. Natural history of vegetations during successful medical treatment of endocarditis. Am. Heart J. 1994, 128, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Rohmann, S.; Erbel, R.; Darius, H.; Makowski, T.; Meyer, J. Effect of antibiotic treatment on vegetation size and complication rate in infective endocarditis. Clin. Cardiol. 1997, 20, 132–140. [Google Scholar] [CrossRef] [PubMed]
- A Sochowski, R.; Chan, K.-L. Implication of negative results on a monoplane transesophageal echocardiographic study in patients with suspected infective endocarditis. JACC 1993, 21, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Shively, B.K.; Gurule, F.T.; Roldan, C.A.; Leggett, J.H.; Schiller, N.B. Diagnostic value of transesophageal compared with transthoracic echocardiography in infective endocarditis. JACC 1991, 18, 391–397. [Google Scholar] [CrossRef]
- Netzer, R.O.-M.; Zollinger, E.; Seiler, C.; Cerny, A. Infective endocarditis: clinical spectrum, presentation and outcome. An analysis of 212 cases 1980-1995. Heart 2000, 84, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Rohmann, S.; Erbel, R.; Darius, H.; Görge, G.; Makowski, T.; Zotz, R.; Mohr-Kahaly, S.; Nixdorff, U.; Drexler, M.; Meyer, J. Prediction of Rapid versus Prolonged Healing of Infective Endocarditis by Monitoring Vegetation Size. J. Am. Soc. Echocardiogr. 1991, 4, 465–474. [Google Scholar] [CrossRef]
- Steckelberg, J.M.; Murphy, J.G.; Ballard, D.; Bailey, K.; Tajik, A.J.; Taliercio, C.P.; Giuliani, E.R.; Wilson, W.R. Emboli in Infective Endocarditis: The Prognostic Value of Echocardiography. Ann. Intern. Med. 1991, 114, 635–640. [Google Scholar] [CrossRef]
- Evangelista, A.; Gonzalez-Alujas, M.T. Echocardiography in infective endocarditis. Heart 2004, 90, 614–617. [Google Scholar] [CrossRef]
- Sanfilippo, A.J.; Picard, M.H.; Newell, J.B.; Rosas, E.; Davidoff, R.; Thomas, J.D.; Weyman, A.E. Echocardiographic assessment of patients with infectious endocarditis: Prediction of risk for complications. JACC 1991, 18, 1191–1199. [Google Scholar] [CrossRef]
- Thuny, F.; Beurtheret, S.; Mancini, J.; Gariboldi, V.; Casalta, J.-P.; Riberi, A.; Giorgi, R.; Gouriet, F.; Tafanelli, L.; Avierinos, J.-F.; et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur. Hear. J. 2009, 32, 2027–2033. [Google Scholar] [CrossRef]
- Daniel, W.G.; Erbel, R.; Kasper, W.; A Visser, C.; Engberding, R.; Sutherland, G.R.; Grube, E.; Hanrath, P.; Maisch, B.; Dennig, K. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991, 83, 817–821. [Google Scholar] [CrossRef]
- Karalis, D.G.; Bansal, R.C.; Hauck, A.J.; Ross, J.J.J.; Applegate, P.M.; Jutzy, K.R.; Mintz, G.S.; Chandrasekaran, K. Transesophageal echocardiographic recognition of subaortic complications in aortic valve endocarditis. Clinical and surgical implications. Circulation 1992, 86, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.G.; Mügge, A.; Martin, R.P.; Lindert, O.; Hausmann, D.; Nonnast-Daniel, B.; Laas, J.; Lichtlen, P.R. Improvement in the Diagnosis of Abscesses Associated with Endocarditis by Transesophageal Echocardiography. New Engl. J. Med. 1991, 324, 795–800. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the author. Attribution-Non-Commercial-NoDerivatives 4.0.