Abstract
Percutaneous mitral valve repair (MVR) using the MitraClip system is a novel and promising technique for the treatment of mitral regurgitation (MR) which involves mechanical edge-to-edge coaptation of the mitral leaflets and has been employed in over 5,000 patients worldwide. Its feasibility and high procedural success rates have been established in several small single- or multi-centric registries and one randomised controlled trial, and cardiovascular outcomes in high- risk surgical patients appear to be superior to historical controls. Overall, the procedure has proven to be safe with exceedingly low rates of fatal or life-threatening complications. Additionally, significant improvements in functional capacity and quality of life have been reported following MitraClip implantation. However, apart from these encouraging results, open questions remain to be addressed, particularly about long- term durability and clinical efficacy, and the selection of the most appropriate candidates for MitraClip implantation. As the experience with this procedure continues to expand, larger studies are expected that will help to further define the role of the MitraClip procedure among established therapies.
Background
Mitral valve disease, particularly mitral regurgitation (MR) is the leading cardiac valve pathology in western societies [1]. The prevalence of moderate to severe MR in a general population aged 75 years and older is in the range of 10% and a further increase may be expected in the future due to the aging of the population. Although in many instances MR may remain silent for a long period of time, its presence generally contributes to an impaired prognosis of the patient, and therefore represents an important target for treatment [2,3,4,5,6,7].
The anatomic aetiologies of MR are multiple and include degenerative pathologies in which the leaflets or chordae are structurally altered (fibroelastic deficiency, leaflet prolapse and flail, or Barlow’s disease). It is generally agreed upon that surgical mitral valve repair (MVR) represents the therapy of choice for the treatment of degenerative MR due to its superior outcomes compared to mitral valve replacement or medical therapy [8,9,10]. Indeed, excellent long-term results have been reported for MVR particularly in degenerative MR with 10-year clinical recurrence rates in the range of 25% and 10-year survival rates of 55–80% [10,11,12]. Functional MR, on the other hand, is a direct consequence of underlying myocardial disease affecting the valvular apparatus by a variety of mechanisms (anular dilation, papillary muscle dysfunction, chordal tethering), and is observed in patients with ischaemic or dilated cardiomyopathy. The role of mitral valve surgery is less well established in this subgroup of MR patients due to the lack of convincing data showing superiority of mitral valve surgery over conservative treatment [13,14,15,16]. Additionally, recurrence rates after surgical MVR of functional MR are high [17].
Nonetheless, many patients that may potentially benefit from MVR, are currently denied surgery because of a high surgical risk, advanced age or comorbidities [18]. Thus, a variety of transcatheter techniques for the treatment of MR have been proposed to avoid the risks of surgery, and some of them are currently undergoing preclinical or clinical evaluation [19,20,21]. Amongst these techniques, percutaneous edge-to-edge MVR using the MitraClip (Abbott Vascular, Abbott Park, Illinois, USA; formerly manufactured by Evalve Inc, Menlo Park, California, USA) is one of the most promising with encouraging results obtained from several single-centre and multi-centric registries and a first randomised trial. However, its role in the management of patients with severe MR and integration with existing therapies for MR is yet to be defined. The present review article summarises the current published literature on percutaneous edge-to-edge MVR emphasising issues of patient selection, indications, procedural success and outcomes.
History and development of the MitraClip
The development of the MitraClip dates back to 1998, when interventional cardiologist Frederick St. Goar started experimenting with percutaneous techniques for MVR [20]. He learned of the Alfieri surgical edge- to-edge mitral repair technique which involves the use of a suture to approximate the edges of the regurgitant mitral valve leaflets thereby restoring leaflet coaptation and creating a double-orifice mitral valve [22]. Given the reported early success of the Alfieri technique, it seemed logical to try to replicate this surgical paradigm by a percutaneous technique [23]. After a series of animal tests using a nitinol wire-based loop to produce leaflet coaptation, the wire-loop was replaced by a rigid, fully invertible, polyester-covered 2-armed clip with tissue stabilising grippers deployed from the atrial side, all very similar to the present-day Mitra- Clip. The other key to the success of the device was a steerable delivery catheter system introduced via the femoral vein that allowed for optimal steering capabilities.
The feasibility of percutaneous MVR with the MitraClip device was first demonstrated in a porcine model. In 14 anesthetised pigs, the clip was advanced via transseptal route into the mitral valve plane, and appropriate grasping of both leaflets creating a double- orifice mitral valve could be documented by echocardiography and postmortem analysis in all animals [24]. A follow-up study in a different group of pigs revealed rapid coverage of the clip with a neointimal layer at 4 weeks after the procedure, further tissue incapsulation at 12 weeks and solid tissue bridging between the arms of the clip at 24 weeks [25] (Figure 1). The first human implant of a MitraClip was performed in June 2003 by Dr. Jose Condado in Caracas Venezuela in a 48-year-old woman with severe MR due to a bileaflet flail. The procedure was performed without complications and after successful clip deployment her MR decreased to <2+ [26]. Most importantly, her symptoms resolved after the procedure and MR has remained mild on echocardiographic follow-up over a period of seven years. The device has received CE mark approval in 2008 and has since been implanted in over 5,000 patients worldwide (Figure 2).
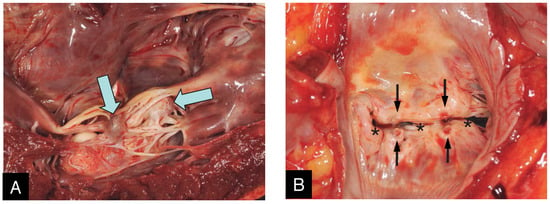
Figure 1.
Macroscopic images in an explanted heart of a 64 year-old gentleman undergoing heart transplant 11 months and 21 days after his MitraClip procedure. From the ventricular side (A) the two clips (arrows) can be seen buried in the subvalvular chordal structures and encapsulated by a thin semitransparent tissue layer. From the atrial side (B), only the indentations from the opposing arms of the MitraClip on the mitral leaflet (black arrows) can be seen dividing the mitral orifice into one central and two commissural openings (asterisks).
The MitraClip procedure
The MitraClip device consists of a percutaneously delivered MRI-compatible cobalt-chromium implant with two arms and two grippers which are used to grasp the opposing edges of the mitral leaflets (Figure 3). The procedure is generally performed under general anaesthesia with fluoroscopic and transoesophageal echocardiographic guidance and haemodynamic monitoring with a Swan-Ganz catheter in the pulmonary artery. The use of X-plane and 3D echocardiography is particularly helpful to visualise mitral valve anatomy and allow orientation of the device in 3D [27]. The device is delivered via transfemoral venous route. After transseptal puncture, the transseptal sheath is exchanged by a steerable 24-F guide catheter (which tapers to 22-F at the interatrial septum) through which the clip delivery system is advanced into the left atrium. Thereafter, the MitraClip device is manoeuvered under echocardiographic guidance, aligned with the origin of the regurgitant jet, and pushed below the level of the mitral leaflets into the left ventricle (LV). Careful consideration must be given to a perpendicular orientation of the clip arms and the leaflet edges before closing the clip (3D echocardiography). After opening the two arms of the clip, the device is retracted with extended arms (Figure 4A) and both leaflets are grasped by closing the grippers. Once adequate leaflet insertion is ascertained with echocardiography, the arms of the Mitra- Clip can be closed to approximate both scallops and restore coaptation. MR is immediately assessed by transoesophageal echocardiography and haemodynamic measurements, and if necessary, the device can be repositioned by reopening the arms and releasing the leaflets. If the desired result is obtained, the MitraClip is deployed and released from the delivery catheter (Figure 4B). Repeat clip insertion can be performed if MR reduction is suboptimal and the result is expected to improve with more than one clip. The only limitation to the number of clips implanted is the development of significant mitral stenosis.
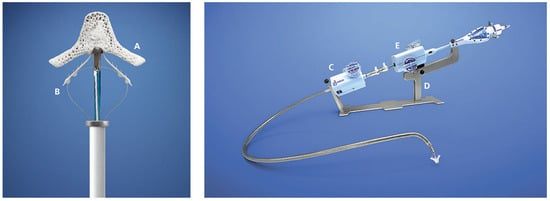
Figure 3.
Close-Up if the MitraClip device (left) and entire MitraClip delivery system (right): The MitraClip device is an MRI-compatible cobalt-chromium implant covered in a polyester fabric to promote tissue growth. Each arm (A) of the device is 4 mm wide and 8 mm long and are shown in the extended state. The grippers (B) are used to grasp the opposing free edges of the mitral leaflets against the arms and improve leaflet coaptation. The 24F steerable transvenous sheath (C) tapers down to 22F at the site of the transseptal puncture. The stabiliser (D) is used to hold the clip delivery system (E) through which the clip is steered and deployed. (Images courtesy of Abbott Vascular. © 2012 Abbott Laboratories. All rights reserved.).
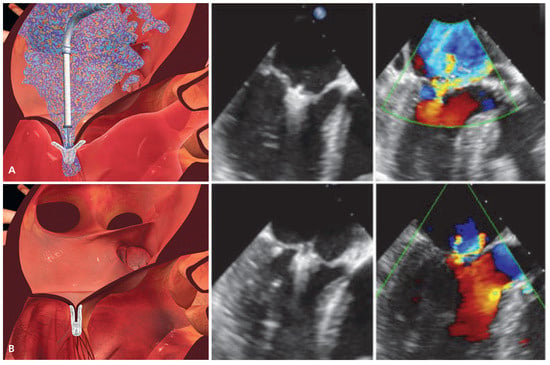
Figure 4.
Schematic and echocardiographic images during MitraClip implantation. (A) After transseptal puncture, the MitraClip device is advanced over a steerable transseptal sheath, aligned with the origin of the MR jet and manoeuvered into the left ventricle. Thereafter, the clip is retracted with extended arms in order to grasp both mitral leaflets. The echocardiographic images shows adequate leaflet insertion, the MR jet is still considerable. (B) After closing both arms of the MitraClip device, the edges of the mitral leaflets are approximated thereby restoring coaptation and creating the typical double orifice mitral valve. After confirming adequate MR reduction on echocardiography, the clip is deployed and released from the delivery system. (Images [A, B] courtesy of Abbott Vascular. © 2012 Abbott Laboratories. All rights reserved.).
Indications and patient selection
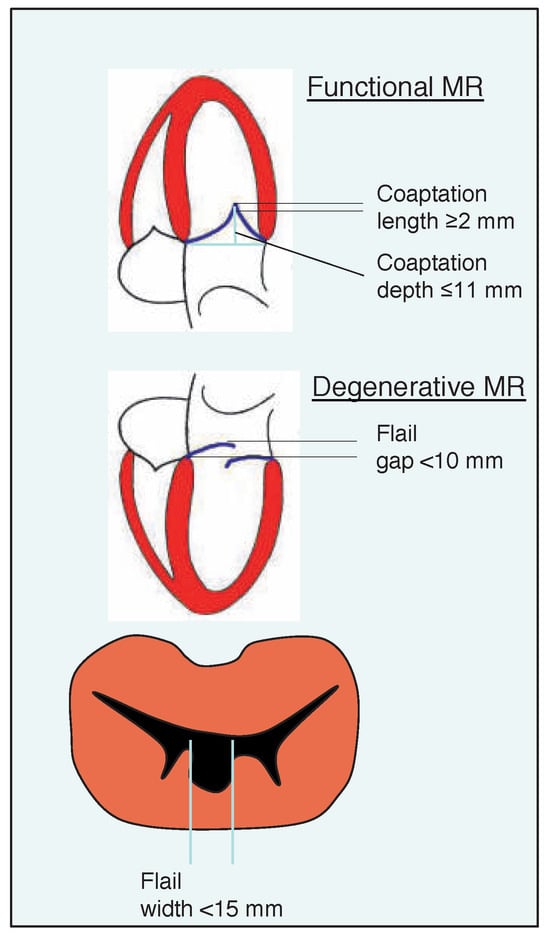
The MitraClip device has been applied to a wide spectrum of degenerative and functional mitral regurgitant pathologies. However, considerable uncertainty still remains about indications and ideally suitable patient populations for percutaneous MVR. Current patient selection criteria are based on the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) programme and include clinical patient characteristics and anatomical features of the mitral valve (Table 1 and Figure 5). EVEREST patient selection criteria appear very stringent which may be related to the fact that per protocol no more than two MitraClips could be implanted per patient in these trials. European registries have reported high procedural success rates in patients with less stringent selection criteria. In the Hamburg registry, patients with mitral valve orifice areas >2 cm2 and more extensively prolapsed or flail leaflets (prolapse width ≤25 mm, flail gap ≤20 mm) were included with excellent procedural success rates [28]. Furthermore, there was no exclusion for very low ejection fraction or an upper limit of LV size. Thus, the main anatomical selection criterion appears to be the ability to properly grasp both leaflets. Treating MR at the medial or lateral commissures is feasible, but requires exceptional care as the ability to manoeuvre the device is limited and the risk of entanglement in the commissural chordae high. Apart from more or less stringent criteria, the role of accurate anatomical characterisation of mitral valve morphology by echocardiography cannot be stressed enough. In the majority of cases, transoesophageal echocardiography (TEE) will be necessary to resolve relevant morphological features and to identify prohibitive factors such as a small mitral valve orifice or extensive immobilisation, thickening or calcification of the leaflets.
Table 1.
Patient selection criteria for the MitraClip procedure (based on inclusion criteria in the EVEREST trial programme [34,35,50]).
Figure 5.
Morphological patient selection criteria for suitability of MitraClip intervention in patients with functional or degenerative mitral regurgitation. (Adapted from [35]: Feldman T, Kar S, Rinaldi M, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST [Endovascular Valve Edge- to-Edge REpair Study] cohort. J Am Coll Cardiol. 2009;54:686–94. © 2009, Elsevier. Reprinted with permission).
Another area of uncertainty relates to the safety of percutaneous MVR in patients with severely reduced LV function and dilated ventricles. Current guidelines discourage the use of mitral valve surgery in patients with functional MR, ejection fraction <30% and no option for revascularisation [9]. In the EVEREST trials, an ejection fraction <25% was an exclusion criterion. The underlying reason for this is the concern that removing the low impedance pop-off valve mechanism may impose a high afterload on a compromised ventricle and result in an acute low-output state [29,30]. However, several reports have documented high procedural success rates and good short-term outcomes of percutaneous MVR in populations with severely reduced LV function rendering it an attractive treatment modality for symptomatic high-risk patients with poor LV’s [31,32,33]. Most importantly, none of the patients treated so far went into an acute low output state after successful MitraClip implantation.
Moreover, MitraClip treatment does not preclude the later use of other device-based therapies that are often applied in patients with heart failure. Access to the left atrium is not restricted for atrial radiofrequency ablation procedures in atrial fibrillation or for device-closure of the left atrial appendage. Additionally, access to the coronary sinus is granted to preserve the possibility of biventricular pacing lead insertion or placement of mitral anuloplasty devices in the future. In fact cardiac resynchronisation therapy (CRT) is an established treatment with proven benefit in patients with congestive heart failure. The MitraClip procedure was shown to be feasible in CRT non-responders and to improve functional class and LV ejection fraction and reduce LV dimensions in about 70% of these patients [33].
Procedural efficacy
The efficacy and short-term outcomes of percutaneous MVR with the MitraClip have been investigated in several single- or multi-centric cohort studies which are summarised in Table 2 [28,34,35,36,37,38,39,40]. These reports are heterogenous with regard to the clinical patient characteristics. As mentioned above, the EVEREST trial programme had stringent morphological inclusion criteria and particularly enrolled patients with degenerative MR, preserved ejection fraction and low surgical risk. On the other hand, in Europe after obtaining CE mark approval in 2008, the MitraClip device has been available for clinical use outside of specified study protocols. As a result, European centres have predominantly enrolled patients with functional MR, reduced LV function and high surgical risk, i.e., those patients in whom surgeons are reluctant to perform mitral valve surgery (Table 2).
Table 2.
Comparative summary of published series of patients treated with MitraClip percutaneous MVR*.
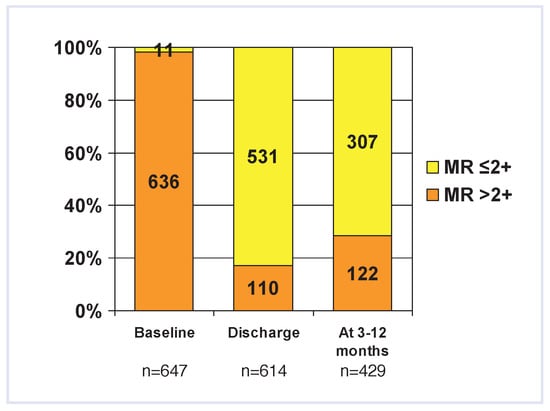
Despite these differences, acute procedural success (APS) rates (defined as an immediate reduction of MR to 2+ or less) were high throughout all reports (74– 97%). The lowest APS rates were seen in the EVER- EST I and roll-in phase of the EVEREST II study with 74%, which may be related to the per-protocol constraints with regard to the number of implanted clips (not more than 2 allowed) or to the fact that the EVER- EST trial was the only study where echocardiograms were read in a central core lab [41]. Despite these APS rates, MR tends to recur in a small group of patients with approximately three quarters (66–97%) of patients having a sustained MR reduction to 2+ or less at 3–12 months (Figure 6). In some of these patients this may be due to partial clip detachment which may occur immediately during the procedure or in-hospital (0–4%), or delayed over the ensuing months (9% at 2y follow-up in the EVEREST trial [35]). However, total clip detachment with embolisation has not yet been reported.
Figure 6.
Severity of mitral regurgitation at baseline, hospital discharge and 3–12 months after the MitraClip procedure summarised from 7 published cohorts in which data was available [28,34,35,36,37,38,39]. Note: 3–12 month data was only present in 5 of the studies [34,35,36,38,39], therefore 3–12 month follow-up data in the graph is incomplete and should be interpreted cautiously.
As a result, a number of patients (between 0 and 8% in European registries and 20–30% in the EVER- EST population) require mitral valve surgery for recurrent symptomatic MR (Table 2). Again, differences between the European and US experience are based on differences in patients’ characteristics, adherence to study protocols and length of observation period (which used to be longer for the EVEREST trials). However, in the early post-implantation period, the device can be unlocked and safely removed by the surgeon, if required, allowing unrestricted access of the mitral valve for surgical MVR. Indeed, when a repair was planned after unsuccessful MitraClip implantation, the majority (84%) were repaired with good results [35,42], however the presence of anterior or bileaflet flail or prolapse was a significant predictor of the need for mitral valve replacement [43]. Only rarely will injury of the leaflets caused by the MitraClip affect the surgical strategy towards a more complex repair technique [39]. Successful surgical MVR has been reported upto 5 years and 2 months after MitraClip implantation [44].
Impact on functional capacity
There is a clear subjective improvement of functional capacity and quality of life after MitraClip implantation in the majority of patients. At 3 to 12 months of follow-up after the procedure, 66–98% of patients are reported to be in the New York Heart Association (NYHA) functional class I–II (Table 2). Even in patients with very low ejection fractions and severe heart failure symptoms, MitraClip implantation resulted in a significant improvement of functional capacity, and the proportion of patients being in NYHA class I-II is in the range of 60–70% [31,33]. In those studies, which used more objective measurements of functional capacity, approximately a 30% increase in the six-minute walking distance, and a 30% decline in serum NT-pro- BNP levels could be observed after MitraClip implantation [31,32]. Moreover, an improvement in quality of life can be seen as evidenced by specified questionnaires (Minnesota living with heart failure questionnaire) [32]. In the EVEREST II trial, patients randomised to percutaneous MVR reported significantly higher physical quality-of-life scores at 30 days compared to patients in the surgical group [34]. However, percutaneous MVR has not yet been compared to medical therapy in a randomised trial.
Haemodynamic effects of MitraClip implantation
Two studies performed systematic invasive haemodynamic monitoring with a Swan Ganz catheter [36,45] and have documented a significant increase in cardiac index after the MitraClip procedure. One study documented a significant reduction of pulmonary artery and wedge pressures by 8 and 20%, respectively, which were predictive of an improved clinical outcome on follow-up at 7–8 months [36]. Interestingly, LV filling pressures (LVEDP) did not decrease significantly in the latter study, while Siegel and colleagues documented a significant reduction in LVEDP from 11 to 9 mm Hg [45]. These discrepancies are probably related to significant differences in patients’ baseline characteristics. The former study included predominantly patients with functional MR and impaired LV function. Therefore, it can be hypothetised that eliminating regurgitant flow into the left atrium reduced pulmonary pressures, while the acute increase in after- load (by removing the low-impedance regurgitant flow) imposed on a compromised left ventricle was responsible for the lack of change in LVEDP. Nonetheless, cardiac index increased in both studies, indicating that the positive effects of removing regurgitant flow outweighed the potentially harmful increase in afterload, and thereby improved cardiac forward output. Of note, none of the patients with successful MitraClip implantation experienced an acute low output state after the procedure [36,45]. More elaborate means of haemody- namic monitoring using simultaneous LV pressure- volume monitoring with a conductance catheter will be needed to investigate the effect of MitraClip implantation on left ventricular contractility (Figure 7).
Figure 7.
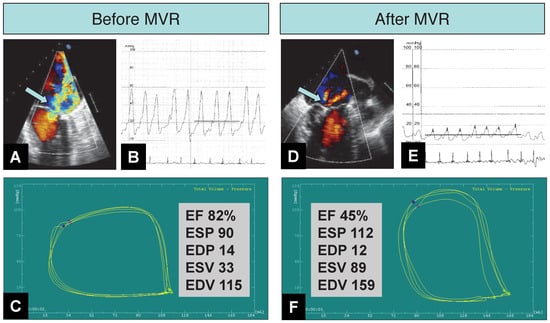
Haemodynamic and LV pump performance changes after percutaneous mitral valve repair (MVR) with three MitraClips in an 73-year-old man with prolapse of the A3 and P3 segments of the mitral valve and severe (4+) mitral regurgitation (MR). Acute procedural success is documented by a reduction of MR from (4+) (A, arrow) to (1+) (D, arrow) on transoesophageal echocardiography. Pressure tracings (B, E) show a reduction in mean pulmonary capillary wedge pressure from 22 mm Hg to 10 mm Hg. V-wave decreased from 54 mm Hg to 17 mm Hg. Consistently, the cardiac index increased from 3.6 to 4.8 l/min/m2 (measured by Fick principle). Real-time pressure volume loops (C, F) (conductance catheter, CD Leycom, Zoetermeer, Netherlands) an upward and rightward displacement of the end-systolic pressure (ESP) volume point (blue dot), and a reduction in ejection fraction (EF). Of note, end-diastolic pressures (EDP) are similar (and at low level) before and after MVR, indicating that the reduction in PCWP results from removal of the regurgitant burden on the pulmonary circulation rather than reductions in left ventricular filling pressures. EDV = end-diastolic volume; ESV = end-systolic volume.
Echocardiographic outcomes
Percutaneous MVR appears to induce reverse left ventricular remodeling as evidenced by a decrease in LV dimensions. All studies in which echocardiographic follow-up was available at 3–12 months have documented a significant reduction in LV end-diastolic and end-systolic volumes as a result of the favourable effects of chronic LV unloading [31,32,33,34,36,38,46]. LV ejection fraction remained unchanged in the majority of cohorts, although a significant increase was observed in those studies with the lowest baseline ejection fraction [31,33]. No changes were noted for left atrial dimensions. In the EVEREST II trial, LV end-diastolic and end-systolic volume decreased by 25 mL and 6 mL, respectively at 12 months in the MitraClip group, albeit to a lesser degree than in the surgical group, which is probably the result of more effective LV unloading with surgery.
Mitral valve area tends to decrease by 1.4–2.4 cm2 whereas trans-mitral pressure gradient increases by 1.3–2.4 mm Hg [28,31,32,35,38,47] after MitraClip implantation. However, transmitral pressure gradient rarely exceeds 5 mm Hg and no single case of significant mitral stenosis has been reported in any of the published cohorts so far (although one case of incorrectly diagnosed mitral stenosis leading to corrective surgery is reported in the literature [42]). Nonetheless, further long-term follow-up will be needed to show whether significant mitral stenosis may evolve after complete tissue bridging between the edges of the leaflets has taken place, particularly in patients with multi-Clip procedures.
Long-term outcomes and clinical events
Due to the recent introduction of percutaneous MVR in clinical practice, clinical follow-up is restricted and the longest observation periods of upto two years are available for the EVEREST trials which started recruiting patients in 2003. As a consequence of the large spectrum of patients included in Mitraclip cohorts across Europe and the US, clinical outcomes vary considerably. For instance, in the EVEREST I and roll-in phase of the EVEREST II trials, the survival rate at 1 and 3 years in patients achieving APS was 96 and 90%, respectively. However, “sicker” cohorts inherently had poorer outcomes with 1-year survival rates of 75–82% [31,32,33,46] and upto 50% clinical event rates (death, rehospitalisation, re-intervention/mitral valve surgery) at 1 year [32].
The EVEREST II trial was a randomised study comparing outcomes in 279 low-risk patients with moderate-to-severe or severe MR assigned to either percutaneous MVR (using the MitraClip system) or surgery in a 2:1 ratio [34]. At 12 months, the rates of the primary efficacy endpoint (composite endpoint consisting of death, mitral valve surgery/reoperative, or 3+/4+ MR) were lower for the MitraClip group compared to the surgical group (55 vs 73%), however, the MitraClip group met criteria for non-inferiority (non- inferiority p = 0.007). This difference was largely driven by a higher mitral valve surgery/re-operation rate in the MitraClip group (20 vs 2%, p <0.001), whereas there were no differences between both groups in terms of overall mortality and residual MR. Interestingly, on subgroup analysis, superior efficacy of surgery over percutaneous MVR was only present for patients with degenerative MR, but was lost for patients with functional MR. Conversely, percutaneous MVR was superior with regard to the safety endpoint consisting of major adverse events at 30 days (15 vs 48%, p <0.001). This difference was driven by a significantly higher incidence of bleeding requiring transfusion of at least two units of packed red blood cells in the surgical group. Both groups showed significant improvements in NYHA functional class and reductions in LV volumes. Thus, in summary the results of the EVEREST II trial allow the conclusion that percutaneous MVR provides increased safety while surgery provides more complete reduction of MR.
However, the EVEREST II trial included low-risk patients that were potential cadidates for mitral valve surgery. The mean age of the study population was 66 years, mean ejection fraction 60% and comorbidities were rare. Whitlow and colleagues compared outcomes of percutaneous MVR in a higher-risk population (59% functional MR) with a medically treated comparison group, albeit with a retrospective non-randomised study design [46]. Patients in the MitraClip group had a significantly better 1-year survival compared to the comparison group (76 vs 55%, p <0.047) along with an improvement in functional capacity and quality of life. While these results are encouraging, we definitely need larger randomised controlled trials to evaluate the merits of percutaneous MVR over conservative medical treatment in high-risk patients with predominantly functional MR and reduced ventricular function, who are poor candidates for surgery.
Safety of the procedure
Overall, serious life-threatening or fatal complications related to the MitraClip procedure are exceedingly rare (Table 3). Procedural mortality is very low and the majority of reports did not report any immediate procedural casualties. Similarly, the rates of major clinical complications such as stroke, myocardial infarction, acute renal failure, and septicaemia are below 5%. Urgent surgery for persistent or aggravated MR varies across different reports (0–8%). Among minor complications, the most common was access site bleeding or groin haematoma. The EVEREST II trial reported upto 13% incidence of red blood cell transfusions, however, postprocedural access site haemorrhage was only reported in 2.7% of patients.
Table 3.
Periprocedural complications from the MitraClip procedures summarised from published cohorts [28,34,35,36,37,38].
Clip-related complications are rare (<5%) but potentially deleterious. Complications from transseptal puncture may include pericardial tamponade with the need for emergency pericardiocentesis or iatrogenic atrial septal defect. Clip-related chordal rupture may result from inadvertent tangling of the device in the subvalvular apparatus and may result in acute MR worsening requiring emergency circulatory support (intraaortic balloon counterpulsation) and bail-out mitral valve surgery. Finally, displacement of a pacemaker lead in the right atrium may occur which can be grave in pacemaker-dependent patients.
Open questions and future perspectives
Despite the encouraging early results of the MitraClip procedure, open questions remain and need to be addressed in order to justify the added costs of the procedure. On one hand, although APS rates are uniformly reported to be high, MR tends to recur in a substantial proportion of patients, and approximately one quarter of patients are found to have recurrent moderate-to- severe or severe MR on mid-term follow-up. This suggests that in these patients, the edge-to-edge repair of the mitral valve may have failed to address to principal morphologic aetiology of MR. As percutaneous techniques continue to evolve [20,21], it can be envisaged that the future of percutaneous MVR may consist of a combination of devices to address the entire complexity of the mitral valve. A combination of one or more MitraClips with an anuloplasty or LV cinching device [48,49] could simultaneously correct anular dilation and tethering of the subvalvular apparatus, reduce restrictive leaflet motion and re-establish leaflet coaptation. However, whether or not emerging percutaneous anuloplasty procedures will be complementary to the MitraClip procedure remains to be seen. Furthermore, despite successful MitraClip implantation, a significant proportion of patients remain symptomatic on follow-up or develop clinical events. Further studies are eagerly needed to better characterise non-responders of MitraClip therapy and allow for identification of predictors of clinical success among candidates for Mitra- Clip therapy. Furthermore, as mentioned above, prospective randomised controlled trials are needed to explore the potential benefit of the MitraClip procedure compared to other established heart failure therapies. The results of these studies will further aid in the patient selection process and allow the MitraClip procedure to find its place among the spectrum of therapies for patients with heart failure.
Transcatheter mitral valve implantation is currently limited by the complexity of the mitral valve anatomy and the difficulty to anchor a rigid stent or frame reliably in the mitral annulus. However, several companies have devoted considerable resources to the development of transcatheter mitral valve delivery systems, some of which are expected to take the step from animal tests to first human experience shortly.
Concluding remarks
Percutaneous MVR using the MitraClip system is a novel and promising technique for the treatment of MR and has been employed in over 5,000 patients world- wide; its feasibility, high procedural success rates and safety has been established in several small single- or multi-centric registries. The randomised EVEREST II trial has shown that the MitraClip procedure provides increased safety compared to surgery. With regard to efficacy, percutaneous MVR met non-inferiority criteria compared to surgery, although less complete reduction of MR could be achieved with percutaneous MVR. There is no doubt that, as to the present moment, surgical MVR represents the gold standard for treating MR, particularly of degenerative origin. However, as the experience with this novel percutaneous technique continues to accrue, larger studies are expected that will shed more light on the appropriate patient selection and help to define the role of the MitraClip procedure among established MR and heart failure therapies.
Funding/potential competing interests
O.Gaemperli has received speaker’s honoraria and a research grant from Abbott Vascular. R.Corti has received speaker’s honoraria from Abbott Vascular and is teaching faculty at Cross- roads Institute for MitraClip. The Structural Heart Team at University Hospital Zurich (headed by R.C.) is a centre of excellence for MitraClip implantation. No other potential conflict of interest relevant to this article was reported.
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez- Sarano, M. Burden of valvular heart diseases: a population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Grigioni, F.; Enriquez-Sarano, M.; Zehr, K.J.; Bailey, K.R.; Tajik, A.J. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001, 103, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Enriquez-Sarano, M.; Nkomo, V.T.; et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005, 111, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Mitchell, G.F.; Flaker, G.C.; et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation 1997, 96, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Trichon, B.H.; Felker, G.M.; Shaw, L.K.; Cabell, C.H.; O’Connor, C.M. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003, 91, 538–543. [Google Scholar] [CrossRef]
- Ling, L.H.; Enriquez-Sarano, M.; Seward, J.B.; et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 1996, 335, 1417–1423. [Google Scholar] [CrossRef]
- Avierinos, J.F.; Gersh, B.J.; Melton, L.J., 3rd; et, al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 2002, 106, 1355–1361. [Google Scholar] [CrossRef]
- Bonow, R.O.; Carabello, B.A.; Kanu, C.; et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guide- lines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006, 114, e84–231. [Google Scholar]
- Vahanian, A.; Baumgartner, H.; Bax, J.; et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007, 28, 230–268. [Google Scholar]
- Enriquez-Sarano, M.; Schaff, H.V.; Orszulak, T.A.; Tajik, A.J.; Bailey, K.R.; Frye, R.L. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation 1995, 91, 1022–1028. [Google Scholar] [CrossRef]
- Lee, E.M.; Shapiro, L.M.; Wells, F.C. Superiority of mitral valve repair in surgery for degenerative mitral regurgitation. Eur Heart J 1997, 18, 655–663. [Google Scholar] [CrossRef]
- Tribouilloy, C.M.; Enriquez-Sarano, M.; Schaff, H.V.; et al. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation 1999, 99, 400–405. [Google Scholar] [CrossRef]
- Wu, A.H.; Aaronson, K.D.; Bolling, S.F.; Pagani, F.D.; Welch, K.; Koelling, T.M. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005, 45, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Mihaljevic, T.; Lam, B.K.; Rajeswaran, J.; et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol 2007, 49, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Fattouch, K.; Guccione, F.; Sampognaro, R.; et al. POINT: Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg 2009, 138, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Trichon, B.H.; Glower, D.D.; Shaw, L.K.; et al. Survival after coronary revascularization, with and without mitral valve surgery, in patients with ischemic mitral regurgitation. Circulation 2003, 108 (Suppl. S1), II103–II110. [Google Scholar] [CrossRef][Green Version]
- McGee, E.C.; Gillinov, A.M.; Blackstone, E.H.; et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004, 128, 916–924. [Google Scholar] [CrossRef]
- Mirabel, M.; Iung, B.; Baron, G.; et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007, 28, 1358–1365. [Google Scholar] [CrossRef]
- Goldberg, S.L.; Feldman, T. Percutaneous mitral valve interventions: over- view of new approaches. Curr Cardiol Rep 2010, 12, 404–412. [Google Scholar] [CrossRef]
- Perlowski, A.; St Goar, F.; Glower, D.G.; Feldman, T. Percutanenous therapies for mitral regurgitation. Curr Probl Cardiol 2012, 37, 42–68. [Google Scholar] [CrossRef]
- Tops, L.F.; Kapadia, S.R.; Tuzcu, E.M.; et al. Percutaneous valve procedures: an update. Curr Probl Cardiol 2008, 33, 417–457. [Google Scholar]
- Alfieri, O.; Maisano, F.; De Bonis, M.; et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001, 122, 674–681. [Google Scholar] [PubMed]
- Alfieri, O.; De Bonis, M. The role of the edge-to-edge repair in the surgical treatment of mitral regurgitation. J Card Surg 2010, 25, 536–541. [Google Scholar] [CrossRef] [PubMed]
- St Goar, F.G.; Fann, J.I.; Komtebedde, J.; et al. Endovascular edge-to-edge mitral valve repair: short-term results in a porcine model. Circulation 2003, 108, 1990–1993. [Google Scholar] [CrossRef] [PubMed]
- Fann, J.I.; St Goar, F.G.; Komtebedde, J.; et al. Beating heart catheter-based edge-to-edge mitral valve procedure in a porcine model: efficacy and healing response. Circulation 2004, 110, 988–993. [Google Scholar] [CrossRef]
- Condado, J.A.; Acquatella, H.; Rodriguez, L.; Whitlow, P.; Velez-Gimo, M.; St Goar, F.G. Percutaneous edge-to-edge mitral valve repair: 2-year follow- up in the first human case. Catheter Cardiovasc Interv 2006, 67, 323–325. [Google Scholar]
- Biaggi, P.; Gruner, C.; Jedrzkiewicz, S.; et al. Assessment of Mitral Valve Prolapse by 3D TEE Angled Views Are Key. JACC Cardiovasc Imaging 2011, 4, 94–97. [Google Scholar] [CrossRef][Green Version]
- Franzen, O.; Baldus, S.; Rudolph, V.; et al. Acute outcomes of MitraClip therapy for mitral regurgitation in high-surgical-risk patients: emphasis on adverse valve morphology and severe left ventricular dysfunction. Eur Heart J 2010, 31, 1373–1381. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Tajik, A.J.; Schaff, H.V.; et al. Echocardiographic pre- diction of left ventricular function after correction of mitral regurgitation: results and clinical implications. J Am Coll Cardiol 1994, 24, 1536–1543. [Google Scholar]
- Leung, D.Y.; Griffin, B.P.; Stewart, W.J.; Cosgrove, D.M.; 3rd Thomas, J.D.; Marwick, T.H. Left ventricular function after valve repair for chronic mitral regurgitation: predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J Am Coll Cardiol 1996, 28, 1198–1205. [Google Scholar]
- Franzen, O.; van der Heyden, J.; Baldus, S.; et al. MitraClip(R) therapy in patients with end-stage systolic heart failure. Eur J Heart Fail 2011, 13, 569–576. [Google Scholar] [CrossRef]
- Rudolph, V.; Knap, M.; Franzen, O.; et al. Echocardiographic and clinical outcomes of MitraClip therapy in patients not amenable to surgery. J Am Coll Cardiol 2011, 58, 2190–2195. [Google Scholar] [CrossRef]
- Auricchio, A.; Schillinger, W.; Meyer, S.; et al. Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol 2011, 58, 2183–2189. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.G.; et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011, 364, 1395–1406. [Google Scholar] [CrossRef]
- Feldman, T.; Kar, S.; Rinaldi, M.; et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol 2009, 54, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Gaemperli, O.; Moccetti, M.; Surder, D.; et al. Acute haemodynamic changes after percutaneous mitral valve repair: relation to mid-term outcomes. Heart 2012, 98, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzini, G.; Surder, D.; Moccetti, M.; et al. Perkutane Katheter-basierte Behandlung der schweren Mitralinsuffizienz. Erste Schweizer Erfahrungen mit dem MitraClip-System. Cardiovasc Med 2010, 13, 122–129. [Google Scholar]
- Tamburino, C.; Ussia, G.P.; Maisano, F.; et al. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur Heart J 2010, 31, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Treede, H.; Schirmer, J.; Rudolph, V.; et al. A heart team’s perspective on interventional mitral valve repair: percutaneous clip implantation as an important adjunct to a surgical mitral valve program for treatment of high-risk patients. J Thorac Cardiovasc Surg 2012, 143, 78–84. [Google Scholar] [CrossRef]
- Rogers, J.H.; Franzen, O. Percutaneous edge-to-edge MitraClip therapy in the management of mitral regurgitation. Eur Heart J. 2011, 32, 2350–2357. [Google Scholar] [CrossRef]
- Foster, E.; Wasserman, H.S.; Gray, W.; et al. Quantitative assessment of severity of mitral regurgitation by serial echocardiography in a multicenter clinical trial of percutaneous mitral valve repair. Am J Cardiol 2007, 100, 1577–1583. [Google Scholar] [CrossRef]
- Argenziano, M.; Skipper, E.; Heimansohn, D.; et al. Surgical revision after percutaneous mitral repair with the MitraClip device. Ann Thorac Surg 2010, 89, 72–80, discussion p 80. [Google Scholar] [CrossRef] [PubMed]
- Glower, D.; Young, J.N.; Alexander, J.; et al. EVEREST II Randomized Clinical Trial: A Critical Assessment of Anatomical Predictors of de novo Mitral Valve Replacement following the MitraClip Procedure. J Am Coll Cardiol 2010, 56, B25. [Google Scholar]
- Rogers, J.H.; Yeo, K.K.; Carroll, J.D.; et al. Late surgical mitral valve repair after percutaneous repair with the MitraClip system. J Card Surg 2009, 24, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.J.; Biner, S.; Rafique, A.M.; et al. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol 2011, 57, 1658–1665. [Google Scholar] [CrossRef]
- Whitlow, P.L.; Feldman, T.; Pedersen, W.R.; et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol 2012, 59, 130–139. [Google Scholar] [CrossRef]
- Herrmann, H.C.; Kar, S.; Siegel, R.; et al. Effect of percutaneous mitral repair with the MitraClip device on mitral valve area and gradient. EuroIntervention 2009, 4, 437–442. [Google Scholar] [CrossRef]
- Schofer, J.; Siminiak, T.; Haude, M.; et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 2009, 120, 326–333. [Google Scholar] [CrossRef]
- Feldman, T.; Cilingiroglu, M. Percutaneous leaflet repair and annuloplasty for mitral regurgitation. J Am Coll Cardiol 2011, 57, 529–537. [Google Scholar] [CrossRef]
- Feldman, T.; Wasserman, H.S.; Herrmann, H.C.; et al. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol 2005, 46, 2134–2140. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; et al. Recommendations for evaluation of the severity of native valvular regurgitation with two- dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003, 16, 777–802. [Google Scholar] [CrossRef]
© 2012 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.