Summary
Ever since the publication of the PARTNER trials, the spotlight has turned to neurologic events during transcatheter aortic valve implantation (TAVI). Recently published long-term follow-up data mitigated the initial concerns, however, stroke prevention during TAVI remains an important issue. This review article focuses on the currently available cerebral protection devices for TAVI and puts them in perspective of the overall “stroke issue”. Cerebral protection devices either deflect emboli (Embrella, EDD) or capture them (Claret, EmbolX). In TAVI, only first-in-human studies with those devices are currently published, with all of them showing technical feasibility. Larger trials are underway and will shed light on the role of cerebral protection devices during TAVI and their clinical impact on preventing neurologic events.
Background
The attention of transcatheter aortic valve implantation (TAVI) has been diverted away from reasonability and mortality towards reducing morbidity. This bodes well for TAVI and reflects that the procedure has become broadly accepted not only by patients for its less invasiveness, but also by the medical community due to the bulk of favourable evidence.
In the first large randomised trial in non-operable patients with severe aortic stenosis (PARTNER 1B), a significantly better 1-year survival (70% vs 50%), less re-hospitalisations and a better functional status was shown in patients treated with TAVI compared to standard medical therapy [1]. In this trial, major procedural complications during TAVI were the occurrence of cerebral strokes (5%) and vascular complications (16%).
Eight months later, the randomised PARTNER 1A trial [2] comparing TAVI with conventional open-heart surgery (SAVR) in high-risk patients showed non-inferiority of TAVI. This is remarkable, bearing in mind that most of the operators for TAVI procedures had just started their valve program and had to compete with very experienced surgeons in SAVR. Thirty-day mortality in the “as-treated analysis” was even significantly lower in the transarterial TAVI subgroup than in the SAVR group.
Neurologic events comprised of transient ischaemic attacks (TIA) and minor strokes in about one third of patients and major strokes in the remaining. Major stroke itself was not significantly more common in the TAVI group, nor was the composite endpoint of death or major stroke.
Are the two groups (TAVI and SAVR) really comparable with regard to the composite of all neurologic events? Second thoughts are at least allowed for two reasons:
Firstly, 30-day outcome was defined as the time span of 30 days after randomisation (not 30 days after the actual procedure). In the SAVR group, the time lag from randomisation to the procedure was 15 days compared to 10 days in the TAVI group. Therefore, post-procedural days included in the 30-day outcome were 15 and 20 days in SAVR and TAVI, respectively. This difference results in a reported post-procedural follow-up that is 25% shorter in the SAVR group compared to the TAVI group.
Secondly, SAVR patients are typically intubated for a longer post-procedural period than TAVI patients—a time period where TIAs or even minor strokes would go unrecognised. This might be one explanation for the very low occurrence of TIAs and minor strokes in the SAVR group (each 0.3%) and the rate of 0.0% in the subgroup of patients randomised to SAVR in the transarterial arm (while in patients in the transarterial arm undergoing TAVI these events accounted for 2.1% of all neurologic events).
Recently, longer term follow-up data of the PART-NER 1A trial were published [3], showing no difference in the rate of strokes between TAVI and SAVR up to 36 months.
Was the “stroke” issue overrated overall? Probably, however stroke prevention is undoubtedly an important issue. Intra-procedural cerebral protection is one interventional approach amongst others aiming to reduce stroke rate.
The goal of cerebral protection is reduction of embolisation to the brain during the TAVI procedure. While >80% of cerebrovascular events occur within the first two months after TAVI, only about 40–50% of those occur within the first 24–48 hours of the procedure [4,5]. Cerebral protection devices therefore at best reduce the 60-day neurologic event rate by 50% in the overall TAVI population.
The use of cerebral protection devices is of course not restricted to TAVI procedures, but might play a role in high-risk surgical and interventional procedures.
Lest we forget other interventional approaches for stroke prevention in high-risk populations: left atrial appendage occlusion [6] in patients in atrial fibrillation, patent foramen ovale closure [7] and carotid stenting [8].
Mechanism of Stroke in TAVI
When do intra-procedural strokes occur during TAVI, and is there a difference between transarterial and transapical TAVI with regard to neurologic events?
Intuitively, we would expect embolisation to occur when crossing the aortic arch with the bulky devices and during balloon inflations for valvuloplasty and valve implantation. If this was the case, then transapical TAVI would theoretically bear a smaller risk of stroke, since the valve and delivery system are introduced via an apical cut-down, thereby avoiding crossing of the aortic arch.
While the first is partly true, the latter is probably not. Most emboli occur during balloon valvuloplasty and valve positioning as assessed by transcranial doppler [9]. In MRI studies, new post-procedural perfusion defects were found in about 70–80% of patients [10,11,12], most of which were clinically silent.
No difference in new perfusion defects on MRI between patients treated with the transarterial or transapical approach was found [10], and there is no difference in clinical strokes between the two approaches [13,14]. The PARTNER 1A trial showed an even higher 1-year overall stroke and major stroke rate when using the transapical compared to the transarterial approach (14.1 vs 6.1% and 9.4 vs 3.5%) [2], but this was due to an overall sicker population in the transapical arm resulting in more strokes during follow-up [5]. According to the study of Rodes et al. [10], neither the occurrence of large aortic plaques, nor heavily calcified aortic leaflets were predictors of new perfusion defects. It is speculated, that air embolism could be one mechanism causing new perfusion defects after transapical procedures. Air can either be trapped in catheters and sheaths, or enter the body from the exposed apex during sheath retrieval in transapical procedures.
According to the above studies, patients with known cerebrovascular disease, small valve area indices, high transvalvular gradients and concomitant coronary artery disease are at highest risk for neurologic events—something worth bearing in mind when selecting higher-risk patients for the use of a cerebral protection device.
Requirements for Cerebral Protection Devices and Current Devices
The ultimate goal of cerebral protection devices is to reduce stroke rate. Since stroke is a rare complication of the procedure, cerebral embolisation measured by transcranial doppler or new perfusion defects in post-procedural MRIs are often used as surrogate primary endpoints in studies. The relevance of the measured “hits” by transcranial doppler or new perfusion defects on MRI is unknown, with the vast majority of these events occurring clinically silent (e.g., no clinical correlate or neurologic symptoms).
Placement of cerebral protection devices itself carries a certain risk of stroke, bleeding and vascular complications. The first requirement for a protection device therefore is to protect, rather than causing collateral harm—a good safety profile is most essential. In addition, in order not to significantly prolong and complicate the TAVI procedure, the device should be easy and fast deploy—and retrievable.
The device or the delivery system should not cause any endothelial damage, should not dislodge arterial plaques, should ensure proper perfusion of the protected arterial territories and should not be thrombogenic. Furthermore, they have to accommodate the frequent anatomic variations of the aortic arch anatomy [15] and are not supposed to interfere with the valve or the valve delivery system during TAVI.
Several devices with the potential to fulfil these requirements are currently developed and investigated. Some deflect emboli whereas others actually capture embolic debris (Table 1).

Table 1.
Current cerebral protection devices.
Embrella
The Embrella device (Edwards Lifesciences Ltd., Irvine, CA, USA) is introduced either through the right radial or brachial artery. The concept of the device is to deflect rather than to capture emboli. Proof-of-concept was shown in the year 2010 in a first-in-human study [16]. The study confirmed the simple handling of the device, with an additional procedural time of only 13 minutes. Whether it is an atraumatic and effective device needs further investigation in a large trial. The Embrella is an umbrella-like device that consists of 2 polyurethane membranes mounted on a nitinol frame. The device is attached to a 0.035-inch nitinol delivery cable (Figure 1A). It can be folded, sheathed and loaded into a 6F long delivery sheath, which itself is placed over the right radial or brachial artery into the ascending aorta. Then the device consisting of two petals is released from the sheath. It is pulled back and positioned at the outer curvature of the aortic arch such that the petals cover the left carotid and the innominate artery (Figure 1B). In some patients it will further (partially) cover the left subclavian artery. The polyurethane membrane has 100-µm pores to ensure proper blood circulation downstream of the device.
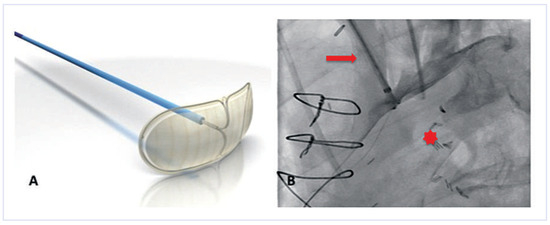
Figure 1.
The Embrella device. (A)The device consists of two petals and a delivery cable. (Courtesy of Edwards Lifesciences Ltd., Irvine, CA, USA.). (B) Once positioned at the outer curvature of the aortic arch, a contrast injection through the delivery sheath confirms proper placement. Note that the two petals of the device cover the brachiocephalic trunk and the left carotid artery. Red arrow = brachiocephalic trunk; red star = aortic lumen.
Sitting at the outer curvature of the aortic arch, the device does not interfere with the TAVI procedures, and in particular there is no interference with the large valve delivery system. Once the procedure is terminated, the device is re-sheathed using the 6F delivery sheath.
Potential pitfalls during deployment are exiting the device out of the sheath too early, thereby damaging the brachiocephalic artery, dissection of the radial artery and mobilisation of atheroma in the aortic arch or the brachiocephalic artery or insufficient withdrawal of the device such that it is not well apposed to the outer aortic curvature. None of these potential complications, however, occurred in our feasibility study.
Keystone Heart Embolic Deflection Device (EDD)
The EDD (Keystone Heart Ltd, formerly SMT Research & Development Ltd, Herzliya, Israel) is introduced via femoral access (Figure 3). It is a very similar device as the Embrella, also deflecting rather than capturing emboli (Figure 2). However there are some important differences: The device fits through a 7 French sheath, however, a 9 French sheath is often used, to facilitate recapturing of the device and allowing simultaneous placement of a pigtail through the same sheath. The device is self-positioning at the outer curvature of the aortic arch covering all three neck vessels. It is anchored in the brachiocephalic trunk and the inner curvature of the aorta by stabilizing nitinol arms. Femoral access and minimal interference with the brachiocephalic trunk are potentially less traumatic than advancing a device through the brachiocephalic trunk.
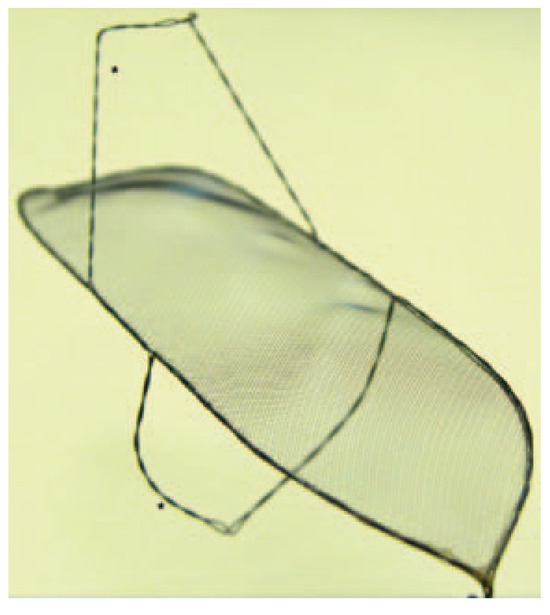
Figure 2.
Keystone Heart Embolic Deflection Device. The device consists of the deflection shield and two nitinol hoops (*). (Courtesy of Keystone Heart Ltd, Herzliya, Israel.
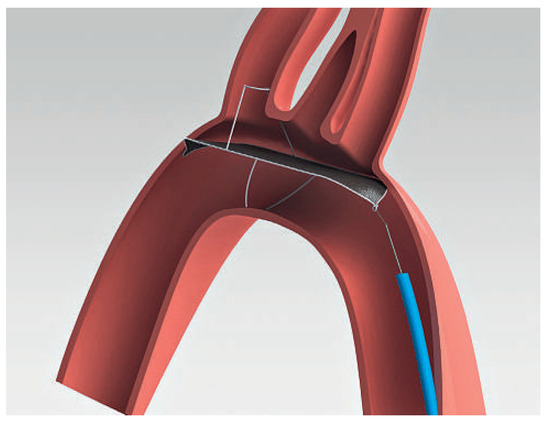
Figure 3.
SMT Embolic Deflection Device. The device is introduced by the femoral route. (Courtesy of Keystone Heart Ltd, Herzliya, Israel.).
The original design allows permanent implantation, however, so far the devices were removed immediately after the TAVI procedures.
The first-in-human experience including 15 patients was presented at TCT 2011 by Dr Pieter Stella, showing a 50% reduction in cerebral embolisation (as assessed by MRI). Given the fact that the deflection device partially protrudes to the aortic lumen, there is a potential for interference of the device with the valve delivery system. This complication, however, was not encountered in the first-in-human experience (personal communication from Dr Stella).
Whether deflected emboli either using the Embrella or the EDD can cause collateral damage down-stream (e.g., impairment of renal function) is not known and needs further investigation in larger trials.
Claret CE Pro™
The Claret CE Pro™ device (Claret Medical Inc., Santa Rosa, CA, USA) is introduced via right radial or brachial access. It contains one filter basket to be deployed in the brachiocephalic trunk, and allows introduction of a standard filter wire to the left carotid artery [17]. The system consists of a 6 French sheath, a delivery system and the brachiocephalic filter (Figure 4). The first generation device was modified in order to allow introduction over a 0.014’’ guidewire and by modifying the bend of the distal steerable tip for antegrade probing of the left carotid artery. After deployment of the proximal filter in the brachiocephalic trunk, the distal tip of the delivery system is advanced to the aortic arch and positioned in a way to allow placement of the distal filter in the left carotid artery (Figure 5 and Figure 6).
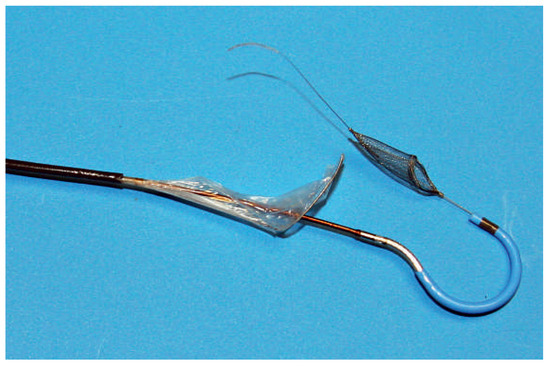
Figure 4.
Claret CE Pro. Second generation Claret CE Pro™ device with the two filters and the steerable distal tip. (Courtesy of Claret Medical Inc., Santa Rosa, CA, USA.).
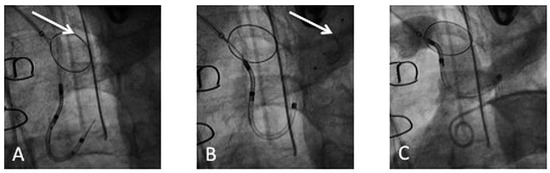
Figure 5.
Deployment of the Claret CE Pro™: (A) Deployment of the first filter in the brachiocephalic trunk (arrow). (B) Deployment of the second filter in the left common carotid (arrow). (C) Confirmation of correct device position by contrast injection in the aortic arch.
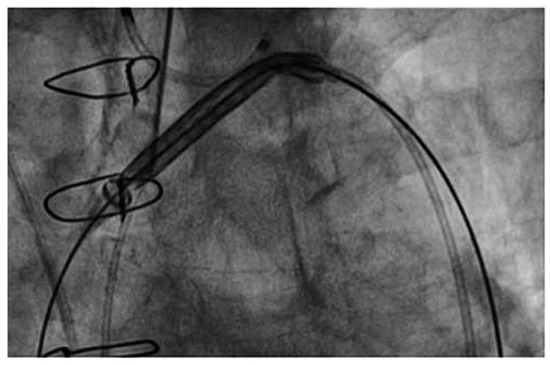
Figure 6.
Claret CE Pro. No interference of the Claret CE Pro™ device with the prosthetic valve delivery system.
The proximal filter is made of a porous polyurethane membrane and a nitinol frame. Anatomical requirements in the first-in-human study were a diameter of the brachiocephalic trunk of 9 mm and a left carotid artery diameter of at least 3 mm. While capturing of debris is appealing and the possibility to review what was captured by the device is illustrative and convincing, the device might be more demanding to deploy than the above described deflection devices: in the first-in-human study in 40 patients [17], delivery failure or imperfect deployment occurred in 25% of patients. The second generation device seemed to improve considerably on that (delivery failure reduced to 13%). Major complications occurred in 7.5% of patients, all related to radial or brachial access (1 dissection of the radial artery, 2 brachial pseudo-aneurysms; all treated surgically). After the TAVI procedure, macroscopic debris was present in >50% of patients, underlining the device’s effectiveness.
Embol-X
The Embol-X intra-aortic filtration system (Edwards Lifesciences Ltd., Irvine, CA, USA) is a filter device designed for cardiac surgery procedures and was recently used for the first time in a transaortic transcatheter valve implantation [18]. In surgical procedures, the device is deployed in the ascending aorta before release of the cross-clamp. It is introduced through a side port of the aortic cardiopulmonary bypass cannula. The device comes in five sizes accommodating various aortic diameters. The filter consists of a semi-permeable polyester mesh, allowing blood circulation through the filter, while capturing emboli with diameters >120 µm (Figure 7). In a randomised trial on >1200 surgical patients mostly atheromatous emboli were captured by the device in >95% of patients [19]. It was demonstrated that the use of the device led to a significantly lower combined endpoint (neurologic, renal, myocardial, gastrointestinal, peripheral vascular complications and death) in subgroups of patients with a low body mass index, low ejection fraction or previous history of cerebrovascular disease. This difference was mainly driven by a reduction in renal insufficiency.
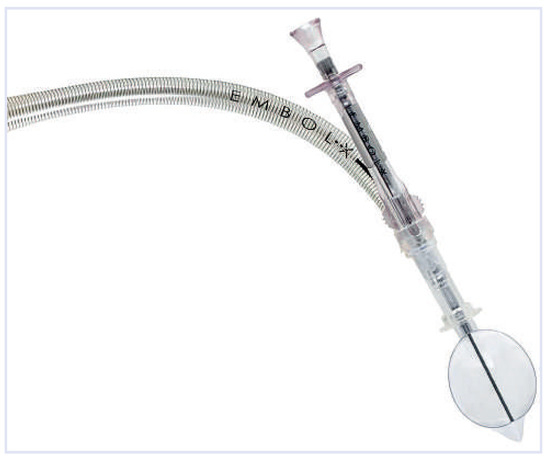
Figure 7.
Embol-X: The device consists of the delivery system and a basket to be deployed in the aorta. (Courtesy of Edwards Lifesciences Ltd., Irvine, CA, USA.).
Etienne et al. [18] successfully utilised the device in transaortic TAVI. An upper mini-sternotomy was performed and under direct visualisation of the ascending aorta, access for the transapical Ascendra delivery system (Edwards Lifesciences Ltd, Irvine, CA, USA) was gained. Distal to the first access site, the aorta was punctured again in order to introduce a 14 French sheath over which the Embol-X device was deployed in the ascending aorta. Although transaortic access is currently not broadly used, it is the only TAVI-access route allowing for concomitant use of this cerebral protection device.
Conclusions
Efforts to reduce the stroke rate are beneficial and will strengthen the position of TAVI in an environment where TAVI is already expanding from high-risk to lower risk populations. Use of cerebral protection devices is one approach to the problem, but the issues that need to be addressed are numerous. An important issue is to define the optimal anti-thrombotic regimen after TAVI. In the PARTNER trials, dual anti-platelet therapy was prescribed for six months, followed by aspirin monotherapy indefinitely. It can be speculated that an intensified anti-thrombotic regimen could reduce the early peak in cerebrovascular events post-TAVI—potentially at the price of more bleeding complications. Otherwise, newer devices and delivery systems or a true percutaneous transapical approach with apical closure [20] have the potential of being less traumatic and thereby reducing stroke rate. Left atrial appendage occlusion for stroke prevention in patients with a history of atrial fibrillation or new onset atrial fibrillation may be particularly beneficial in TAVI patients.
The use of intra-procedural cerebral protection devices is a promising approach to reduce intra-procedural stroke rate. At this stage, however, we do not have sufficient evidence to support the broad use of these devices. Several clinical studies in larger patient populations are currently underway. Whether the use of these devices is safe and whether the devices will find broad application in clinical practice or will become niche products for a few very high risk patients (e.g., those with large mobile aortic atheroma or patients with a mobile thrombus in the left atrial appendage) will largely depend on the outcome of these trials, the additional costs and the ease of use of the devices.
Funding/Potential Competing Interests
Dr Nietlispach is a Consult- ant and Proctor to Edwards Lifesciences Ltd, Irvine, California, USA.
References
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012.
- Tay, E.L.; Gurvitch, R.; Wijesinghe, N.; Nietlispach, F.; Wood, D.; Cheung, A.; et al. A high-risk period for cerebrovascular events exists after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2011, 4, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.C.; Blackstone, E.H.; Mack, M.J.; Svensson, L.G.; Kodali, S.K.; Kapadia, S.; et al. Transcatheter (TAVR) versus surgical (AVR) aortic valve replacement: Occurrence, hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Cardiovasc Surg. 2012, 143, 832–843.e13. [Google Scholar] [CrossRef] [PubMed]
- Nietlispach, F.; Gloekler, S.; Khattab, A.; Pilgrim, T.; Schmid, M.; Wenaweser, P.; et al. Percutaneous left atrial appendage closure. Eur Geriatric Med. 2012, 3, 308–311. [Google Scholar] [CrossRef]
- Wahl, A.; Juni, P.; Mono, M.L.; Kalesan, B.; Praz, F.; Geister, L.; et al. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation. 2012, 125, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Knapp, G.; Tamhane, U.; Chaturvedi, S.; Gurm, H.S. Short term and intermediate term comparison of endarterectomy versus stenting for carotid artery stenosis: systematic review and meta-analysis of randomised controlled clinical trials. BMJ. 2010, 340, c467. [Google Scholar] [CrossRef] [PubMed]
- Drews, T.; Pasic, M.; Buz, S.; Unbehaun, A.; Dreysse, S.; Kukucka, M.; et al. Transcranial Doppler sound detection of cerebral microembolism during transapical aortic valve implantation. Thorac Cardiovasc Surg. 2011, 59, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Rodes-Cabau, J.; Dumont, E.; Boone, R.H.; Larose, E.; Bagur, R.; Gurvitch, R.; et al. Cerebral embolism following transcatheter aortic valve implantation: comparison of transfemoral and transapical approaches. J Am Coll Cardiol. 2011, 57, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, P.; Knipp, S.C.; Schlamann, M.; Thielmann, M.; Al-Rashid, F.; Weber, M.; et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010, 121, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.; Muller, A.; Nahle, C.P.; Kocurek, J.; Werner, N.; Hammerstingl, C.; et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol. 2010, 55, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Schymik, G.; Walther, T.; Himbert, D.; Lefevre, T.; Treede, H.; et al. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2011, 124, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Rodes-Cabau, J.; Webb, J.G.; Cheung, A.; Ye, J.; Dumont, E.; Feindel, C.M.; et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010, 55, 1080–1090. [Google Scholar] [PubMed]
- Liechty, J.D.; Shields, T.W.; Anson, B.J. Variations pertaining to the aortic arches and their branches; with comments on surgically important types. Q Bull Northwest Univ Med Sch. 1957, 31, 136–143. [Google Scholar]
- Nietlispach, F.; Wijesinghe, N.; Gurvitch, R.; Tay, E.; Carpenter, J.P.; Burns, C.; et al. An embolic deflection device for aortic valve interventions. JACC Cardiovasc Interv. 2010, 3, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Naber, C.K.; Ghanem, A.; Abizaid, A.A.; Wolf, A.; Sinning, J.M.; Werner, N.; et al. First-in-man use of a novel embolic protection device for patients undergoing transcatheter aortic valve implantation. EuroIntervention 2012, 8, 43–50. [Google Scholar] [CrossRef]
- Etienne, P.Y.; Papadatos, S.; Pieters, D.; El Khoury, E.; Alexis, F.; Price, J.; et al. Embol-X intraaortic filter and transaortic approach for improved cerebral protection in transcatheter aortic valve implantation. Ann Thorac Surg. 2011, 92, e95–e96. [Google Scholar] [CrossRef] [PubMed]
- Banbury, M.K.; Kouchoukos, N.T.; Allen, K.B.; Slaughter, M.S.; Weissman, N.J.; Berry, G.J.; et al. Emboli capture using the Embol-X intraaortic filter in cardiac surgery: a multicentered randomized trial of 1,289 patients. Ann Thorac Surg. 2003, 76, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Nietlispach, F.; Eckstein, F.; Seeberger, M.; Osswald, S.; Kaufmann, B.A.; Reuthebuch, O. Closure of apical access site after transapical, transcatheter paravalvular leak closure. Can J Cardiol. 2012, 28, 516. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the author. Attribution-Non-Commercial-NoDerivatives 4.0.