A Narrative Review on Breast Cancer Treatment Supported by Focused and Systemic Phytotherapy
Abstract
1. Introduction
1.1. “Who Is at Risk”
1.2. Global Impact
1.3. Molecular Factors with a Significant Role in Breast Cancer Development
1.3.1. Estrogen and Progesterone
1.3.2. Aromatase and Breast Cancer
1.3.3. Cathepsin D—Lysosomal Aspartic Protease
1.3.4. Urokinase Plasminogen Activator
1.3.5. Fucosiltransferase 3 (FUT3)
1.3.6. Tyrosine Kinase Receptor
1.3.7. Deiodinase-Type 3 Enzyme—DIO3
1.4. Immune Response and Inflammation
1.5. Breast Cancer Biomarkers
1.5.1. Blood Biomarkers
1.5.2. Salivary Biomarkers
1.5.3. Urinary Tumor Markers
1.6. Breast Cancer Treatment
1.6.1. Estrogen Modulation
1.6.2. Aromatase Inhibitors
1.6.3. HER2-Positive Cancers
- -
- Trastuzumab is an antibody that targets HER2+ cancer cells. When bound to the HER2 protein, it slows or stops the growth of these cells. For advanced breast cancer, treatment is usually given with the goal of reducing the size of the tumors or slowing their development [247].
- -
- -
- Ado-trastuzumab emtansine (T-DM1) is also a targeted therapy for HER2+ advanced breast cancer. It consists of trastuzumab, along with chemotherapy. T-DM1 is approved for the treatment of HER2+ advanced breast cancer that continues to progress after treatment with trastuzumab and taxane chemotherapy. A recent study showed that T-DM1 increases overall survival more than lapatinib in addition to capecitabine in women with HER2+ advanced breast cancer [250,251].
- -
- Trastuzumab deruxtecan is also an antibody for the treatment of HER2+ advanced breast cancer. It consists of trastuzumab along with the chemotherapy drug deruxtecan. Its use is approved for the treatment of advanced HER2+ breast cancer that continues to progress after two or more treatments with drugs that target HER2+ cells [250,252].
1.6.4. Other Enzyme Inhibitors for Breast Cancer Reduction
- -
- Tyrosine kinase receptors: Since tyrosine kinases are enzymes that control cell division and tumor growth by acting at the level of HER1 and HER2 receptors, their inhibition leads to the interruption of the cell cycle, preventing tumor growth. Tyrosine kinase inhibitors approved for the treatment of advanced breast cancer are tucatinib, neratinib, and lapatinib. The addition of tucatinib to treatment with trastuzumab and chemotherapy showed increased overall survival in women with HER2+ advanced breast cancer [250,251,252].
- -
- Poly (ADP-ribose) polymerase (PARP) Enzyme Inhibitors: DNA damage and the mechanisms of its repair represent pivotal factors in the emergence of mutations that instigate and drive tumorigenesis [262]. Genetic instability, secondary to changes in the DNA molecule and the number and/or structure of chromosomes, is present in the majority of solid tumors [263]. Poly (ADP-ribose) polymerases are a group of enzymes that play a key role in signaling and repairing DNA errors. So, the inhibition of its activity is a therapeutic strategy that takes advantage of the mechanism of synthetic lethality and can be used in the treatment of tumors with specific defects in DNA repair pathways, namely in tumors with mutations in BRCA1 and BRCA2 tumor suppressor genes [264,265,266,267]. As tumor cells with a mutated BRCA gene already have trouble repairing damaged DNA, blocking PARP proteins often leads to the death of these cells [268]. Consequently, PARP deficiency impairs homologous recombination (HR), thereby promoting the dominance of non-conservative DNA repair pathways [269]. The HR process involves proteins including BRCA1, BRCA2, PALB2, ATM, CHEK1, CHEK2, and RAD51. In contrast, the enzymes PARP1 and PARP2 are foundational to the base-excision repair (BER) pathway [263]. The discovery of the PARP family of enzymes and the knowledge of their role in DNA repair pathways made it possible to develop a new class of anti-neoplastic drugs—PARP inhibitors (iPARP) [270]. iPARPs, which target the PARP enzyme, were the inaugural clinically approved agents to leverage the principle of synthetic lethality [262,264]. Synthetic lethality describes a genetic interaction in which the simultaneous functional abrogation of two genes leads to cellular demise, while the isolated functional loss of either gene maintains cell viability [263,266]. Consequently, iPARPs constitute a novel therapeutic approach for the treatment of tumors harboring BRCA1/2 mutations or those displaying a “BRCAness” phenotype, given their inherent deficiencies in HR [271]. There are several iPARPs already approved by the USA Food and Drug Administration (FDA) and the European Medicines Agency used in the treatment of breast, ovarian, pancreatic, and prostate cancer [267]. However, as with other target therapies, despite being well tolerated and widely used in the clinical practice, iPARPs resistance is common and can be developed through various molecular mechanisms [267]. iPARPs may be employed as a monotherapy or in conjunction with other agents, particularly alongside chemotherapy, immunotherapy, and targeted therapies that compromise DNA repair mechanisms [272,273].
- -
- -
- Rucaparib: The efficacy of rucaparib was evaluated in the ARIEL3 study, in which progression-free survival was estimated in patients with recurrent ovarian, fallopian tube, or primary peritoneal epithelial tumors being treated with this drug. This intervention was observed to enhance patient prognosis, even in the absence of BRCA1/2 mutations [275].
- -
- Niraparib: The NOVA study allowed the approval of niraparib in the maintenance treatment of epithelial tumors of the ovary, fallopian tubes, or primary peritoneal, recurrent and sensitive to platinum [273,276]. In this study, it was found that the benefit of niraparib is transversal to tumors of the ovary, regardless of HR status and BRCA mutations [276].
- -
- Talazoparib: It is a potent iPARP. In addition to having a high capacity to inhibit the catalytic activity of enzymes, it has greater potential to trap PARP1 in DNA errors [277]. According to the results of the EMBRACA study, talazoparib was approved for the treatment of breast tumors associated with gBRCAm, HER2-negative, locally advanced or metastatic [278].
- -
- Cyclin-dependent kinase inhibitor: Cyclin-dependent kinases (CDKs) play an essential role in regulating cell cycle progression, allowing the transition between different phases. Its activation depends on cyclins, molecules that are synthesized and degraded during the cell cycle [279]. As cell cycle regulators, their inhibition ensures that tumor cells do not enter cell division, thus preventing them from proliferating and dying, breaking the tumor growth cycle. A targeted therapy known as a CDK inhibitor stops the activity of CDK4/6 [279]. Among this type of inhibitors, three were approved by ANVISA: Palbociclib, Abemaciclib, and Ribociclib [279]. On the basis of their impressive efficacy, all three CDK4/6 inhibitors now play an important role in the treatment of patients with HR+, HER2- breast cancer; however, their optimal use still needs to be established [279].
- -
- Oncolytic viruses are gaining significant clinical value due to their effectiveness against cancer. Plant viruses, specifically, cannot infect mammalian cells, eliminating the infection-related drawbacks seen with other viral therapies. This makes them a valuable tool for manipulating tumors and inducing anti-tumor immunity [280,281]. Rather than directly replicating in or destroying cancer cells, plant virus nanoparticles (PVNPs) represent a novel class of immunostimulatory agents [281]. There are two main types of PVNPs: viral nanoparticles (VNPs), which are whole viruses with both a coat protein and internal nucleic acid, and virus-like particles (VLPs), which consist solely of the coat protein [282,283,284]. VLPs are genome-free versions of VNPs; they cannot replicate in plants and closely resemble the native structure of plant viruses. Both VLPs and VNPs can act as immune adjuvants and delivery systems for tumor-specific antigens that the human immune system can recognize.
1.7. Integrative and Complementary Medicine: Phytomedicine as the Most Expeditious Therapy
- Providing appropriate matrix components in a 3D configuration that mirrors in vivo conditions.
- Co-culturing cancer cells, endothelial cells, and other associated cells in a spatially relevant manner.
- Monitoring and controlling hypoxia levels to mimic those found in native tumors.
- Monitoring the release of angiogenic factors by cancer cells in response to hypoxia.
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Brierley, J.D.; Sullivan, R. Union for international cancer control (UICC). Cancer Syst. Control Health Prof. 2025, 126, 277. [Google Scholar]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H. NCCN Guidelines® insights: Breast cancer, version 4.2023: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2023, 21, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Shiovitz, S.; Korde, L.A. Genetics of breast cancer: A topic in evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Strategies and Plans of Action That Are Scheduled to Expire Within One Year: WHO Traditional Medicine Strategy: 2014–2023 (EB152/37). 2023. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/biblio-1567633 (accessed on 1 February 2025).
- Rossi, R.E.; Pericleous, M.; Mandair, D.; Whyand, T.; Caplin, M.E. The role of dietary factors in prevention and progression of breast cancer. Anticancer Res. 2014, 34, 6861–6875. [Google Scholar]
- Mills, G.B.; Rieger, P.T. The M. D. Anderson Cancer Care Series: Breast Cancer; Hunt, K.K., Robb, G.L., Strom, E.A., Ueno, N.T., Eds.; Springer: New York, NY, USA, 2001; pp. 55–92. [Google Scholar]
- Parkin, D.M. Global cancer statistics in the year 2000. Lancet Oncol. 2001, 2, 533–543. [Google Scholar] [CrossRef]
- Peto, J. Cancer epidemiology in the last century and the next decade. Nature 2001, 411, 390–395. [Google Scholar] [CrossRef]
- Liede, A.; Karlan, B.Y.; Narod, S.A. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: A review of the literature. J. Clin. Oncol. 2004, 22, 735–742. [Google Scholar] [CrossRef]
- Adank, M.A.; van Mil, S.E.; Gille, J.J.; Waisfisz, Q.; Meijers-Heijboer, H. PALB2 analysis in BRCA2-like families. Breast Cancer Res. Treat. 2011, 127, 357–362. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Breast Cancer Initiative Implementation Framework: Assessing, Strengthening and Scaling-Up of Services for the Early Detection and Management of Breast Cancer; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Breast cancer incidence, mortality and mortality-to-incidence ratio (MIR) are associated with human development, 1990–2016: Evidence from Global Burden of Disease Study 2016. Breast Cancer 2019, 26, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Islami, F.; Baeker Bispo, J.; Lee, H.; Wiese, D.; Yabroff, K.R.; Bandi, P.; Sloan, K.; Patel, A.V.; Daniels, E.C.; Kamal, A.H. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA A Cancer J. Clin. 2024, 74, 136–166. [Google Scholar] [CrossRef]
- Kolak, A.; Kaminska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukielka-Budny, B.; Burdan, F. Primary and secondary prevention of breast cancer. Ann. Agric. Environ. Med. 2017, 24, 549–553. [Google Scholar] [CrossRef]
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and Nutritional Supplements on Breast Cancer. Biomed. Res. Int. 2017, 2017, 7207983. [Google Scholar] [CrossRef]
- Bernstein, C.; Bernstein, H.; Payne, C.M.; Garewal, H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: Fail-safe protection against carcinogenesis. Mutat. Res. 2002, 511, 145–178. [Google Scholar] [CrossRef]
- Bhat, K.P.; Pezzuto, J.M. Natural modulators of estrogen biosynthesis and function as chemopreventive agents. Arch. Pharmacal Res. 2001, 24, 473–484. [Google Scholar] [CrossRef]
- Jordan, V.C.; Osipo, C.; Schafer, J.M.; Fox, J.E.; Cheng, D.; Liu, H. Changing role of the oestrogen receptor in the life and death of breast cancer cells. Breast 2003, 12, 432–441. [Google Scholar] [CrossRef]
- Osborne, C.K.; Schiff, R.; Fuqua, S.A.; Shou, J. Estrogen receptor: Current understanding of its activation and modulation. Clin. Cancer Res. 2001, 7, 4338s–4342s; discussion 4411s–4412s. [Google Scholar]
- Simpson, E.R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Simpson, E.R.; Zhao, Y.; Agarwal, V.R.; Michael, M.D.; Bulun, S.E.; Hinshelwood, M.M.; Graham-Lorence, S.; Sun, T.; Fisher, C.R.; Qin, K.; et al. Aromatase expression in health and disease. Recent. Prog. Horm. Res. 1997, 52, 185–213; discussion 184–213. [Google Scholar]
- Benes, P.; Vetvicka, V.; Fusek, M. Cathepsin D—Many functions of one aspartic protease. Crit. Rev. Oncol./Hematol. 2008, 68, 12–28. [Google Scholar] [CrossRef]
- Ohri, S.S.; Vashishta, A.; Proctor, M.; Fusek, M.; Vetvicka, V. The propeptide of cathepsin D increases proliferation, invasion and metastasis of breast cancer cells. Int. J. Oncol. 2008, 32, 491–498. [Google Scholar] [CrossRef]
- Vashishta, A.; Ohri, S.S.; Proctor, M.; Fusek, M.; Vetvicka, V. Role of activation peptide of procathepsin D in proliferation and invasion of lung cancer cells. Anticancer Res. 2006, 26, 4163–4170. [Google Scholar]
- Garcia, M.; Platet, N.; Liaudet, E.; Laurent, V.; Derocq, D.; Brouillet, J.P.; Rochefort, H. Biological and clinical significance of cathepsin D in breast cancer metastasis. Stem Cells 1996, 14, 642–650. [Google Scholar] [CrossRef]
- Christensen, B.; Schack, L.; Kläning, E.; Sørensen, E.S. Osteopontin is cleaved at multiple sites close to its integrin-binding motifs in milk and is a novel substrate for plasmin and cathepsin D. J. Biol. Chem. 2010, 285, 7929–7937. [Google Scholar] [CrossRef] [PubMed]
- Piwnica, D.; Touraine, P.; Struman, I.; Tabruyn, S.b.; Bolbach, G.r.; Clapp, C.; Martial, J.A.; Kelly, P.A.; Goffin, V. Cathepsin D processes human prolactin into multiple 16K-like N-terminal fragments: Study of their antiangiogenic properties and physiological relevance. Mol. Endocrinol. 2004, 18, 2522–2542. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Clark-Lewis, I.; Buri, C.; Langen, H.; Lis, M.; Mazzucchelli, L. Cathepsin D specifically cleaves the chemokines macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, and SLC that are expressed in human breast cancer. Am. J. Pathol. 2003, 162, 1183–1190. [Google Scholar] [CrossRef]
- Andreasen, P.A.; Kjoller, L.; Christensen, L.; Duffy, M.J. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int. J. Cancer 1997, 72, 1–22. [Google Scholar] [CrossRef]
- Chapman, H.A. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr. Opin. Cell Biol. 1997, 9, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Conese, M.; Blasi, F. The urokinase/urokinase-receptor system and cancer invasion. Baillieres Clin. Haematol. 1995, 8, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.; Takimoto, R.; Tamura, F.; Yoshida, M.; Ono, M.; Murase, K.; Sato, Y.; Osuga, T.; Sato, T.; Iyama, S.; et al. Fucosylated TGF-beta receptors transduces a signal for epithelial-mesenchymal transition in colorectal cancer cells. Br. J. Cancer 2014, 110, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.W.; Punnoose, E.A.; Januario, T.; Lawrence, D.A.; Pitti, R.M.; Lancaster, K.; Lee, D.; von Goetz, M.; Yee, S.F.; Totpal, K.; et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 2007, 13, 1070–1077. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Goemann, I.M.; Marczyk, V.R.; Recamonde-Mendoza, M.; Wajner, S.M.; Graudenz, M.S.; Maia, A.L. Decreased expression of the thyroid hormone-inactivating enzyme type 3 deiodinase is associated with lower survival rates in breast cancer. Sci. Rep. 2020, 10, 13914. [Google Scholar] [CrossRef]
- Visser, T.J. Mechanism of action of iodothyronine-5′-deiodinase. Biochim. Biophys. Acta 1979, 569, 302–308. [Google Scholar] [CrossRef]
- Al-Shami, K.; Awadi, S.; Khamees, A.; Alsheikh, A.M.; Al-Sharif, S.; Ala’ Bereshy, R.; Al-Eitan, S.F.; Banikhaled, S.H.; Al-Qudimat, A.R.; Al-Zoubi, R.M.; et al. Estrogens and the risk of breast cancer: A narrative review of literature. Heliyon 2023, 9, e20224. [Google Scholar] [CrossRef]
- Kim, J.; Munster, P.N. Estrogens and breast cancer. Ann. Oncol. 2025, 36, 134–148. [Google Scholar] [CrossRef]
- Wang, X.; Feng, S.; Deng, Q.; Wu, C.; Duan, R.; Yang, L. The role of estrogen in Alzheimer’s disease pathogenesis and therapeutic potential in women. Mol. Cell. Biochem. 2025, 480, 1983–1998. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; Matthews, K.A.; Meilahn, E.N. Estrogens and women’s health: Interrelation of coronary heart disease, breast cancer and osteoporosis. J. Steroid Biochem. Mol. Biol. 2000, 74, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.C. Cardiovascular effects of oestrogens. J. Steroid Biochem. Mol. Biol. 2000, 74, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, D.W.; Vasudevan, N.; Kia, H.K.; Zhu, Y.S.; Chan, J.; Garey, J.; Morgan, M.; Ogawa, S. Estrogens, brain and behavior: Studies in fundamental neurobiology and observations related to women’s health. J. Steroid Biochem. Mol. Biol. 2000, 74, 365–373. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Budish, R.A.; Kashyap, S.; Lindsey, S.H. GPER-novel membrane oestrogen receptor. Clin. Sci. 2016, 130, 1005–1016. [Google Scholar] [CrossRef]
- Zhu, B.T.; Han, G.-Z.; Shim, J.-Y.; Wen, Y.; Jiang, X.-R. Quantitative Structure-Activity Relationship of Various Endogenous Estrogen Metabolites for Human Estrogen Receptor α and β Subtypes: Insights into the Structural Determinants Favoring a Differential Subtype Binding. Endocrinology 2006, 147, 4132–4150. [Google Scholar] [CrossRef]
- Trabert, B.; Sherman, M.E.; Kannan, N.; Stanczyk, F.Z. Progesterone and Breast Cancer. Endocr. Rev. 2020, 41, 320–344. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, V.; McGowan, E.; Sherman, L.; Mancini, M.A.; Cheskis, B.J.; Edwards, D.P. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol. Endocrinol. 2007, 21, 359–375. [Google Scholar] [CrossRef]
- Bellance, C.; Khan, J.A.; Meduri, G.; Guiochon-Mantel, A.; Lombes, M.; Loosfelt, H. Progesterone receptor isoforms PRA and PRB differentially contribute to breast cancer cell migration through interaction with focal adhesion kinase complexes. Mol. Biol. Cell 2013, 24, 1363–1374. [Google Scholar] [CrossRef]
- Richer, J.K.; Jacobsen, B.M.; Manning, N.G.; Abel, M.G.; Wolf, D.M.; Horwitz, K.B. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 2002, 277, 5209–5218. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Dong, J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor alpha (mPRalpha) by progesterone receptor membrane component 1 (PGRMC1): Evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology 2014, 155, 1107–1119. [Google Scholar] [CrossRef]
- Revelli, A.; Massobrio, M.; Tesarik, J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr. Rev. 1998, 19, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen. Comp. Endocrinol. 2012, 175, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, H.; Li, S.; Wu, P.; Mao, X. The Role of Progesterone Receptors in Breast Cancer. Drug Des. Devel Ther. 2022, 16, 305–314. [Google Scholar] [CrossRef]
- Swinstead, E.; Need, E.; Grover, P.; Smith, E.; Buchanan, G. Estrogen priming is critical for effective progesterone receptor engagement with DNA in breast cancer cells: ChIP sequencing and microarray studies. In Proceedings of the Endocrine Abstracts, Florence, Italy, 5–9 May 2012. [Google Scholar]
- Tian, J.M.; Ran, B.; Zhang, C.L.; Yan, D.M.; Li, X.H. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Braz. J. Med. Biol. Res. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Smith, I.E.; Dowsett, M. Aromatase inhibitors in breast cancer. N. Engl. J. Med. 2003, 348, 2431–2442. [Google Scholar] [CrossRef]
- Simpson, E.; Rubin, G.; Clyne, C.; Robertson, K.; O’Donnell, L.; Jones, M.; Davis, S. The role of local estrogen biosynthesis in males and females. Trends Endocrinol. Metab. 2000, 11, 184–188. [Google Scholar] [CrossRef]
- Pasqualini, J.R.; Chetrite, G.; Blacker, C.; Feinstein, M.C.; Delalonde, L.; Talbi, M.; Maloche, C. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J. Clin. Endocrinol. Metab. 1996, 81, 1460–1464. [Google Scholar] [CrossRef]
- Buzdar, A.; Chlebowski, R.; Cuzick, J.; Duffy, S.; Forbes, J.; Jonat, W.; Ravdin, P. Defining the role of aromatase inhibitors in the adjuvant endocrine treatment of early breast cancer. Curr. Med. Res. Opin. 2006, 22, 1575–1585. [Google Scholar] [CrossRef]
- Brueggemeier, R.W. Update on the use of aromatase inhibitors in breast cancer. Expert. Opin. Pharmacother. 2006, 7, 1919–1930. [Google Scholar] [CrossRef]
- Kendall, A.; Dowsett, M. Novel concepts for the chemoprevention of breast cancer through aromatase inhibition. Endocr.-Relat. Cancer 2006, 13, 827–837. [Google Scholar] [CrossRef]
- Balunas, M.J.; Su, B.; Brueggemeier, R.W.; Kinghorn, A.D. Natural products as aromatase inhibitors. Anti-Cancer Agents Med. Chem. 2008, 8, 646–682. [Google Scholar] [CrossRef]
- Grube, B.J.; Eng, E.T.; Kao, Y.C.; Kwon, A.; Chen, S. White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation. J. Nutr. 2001, 131, 3288–3293. [Google Scholar] [CrossRef]
- Satoh, K.; Sakamoto, Y.; Ogata, A.; Nagai, F.; Mikuriya, H.; Numazawa, M.; Yamada, K.; Aoki, N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem. Toxicol. 2002, 40, 925–933. [Google Scholar] [CrossRef]
- Wu, A.H.; Butler, L.M. Green tea and breast cancer. Mol. Nutr. Food Res. 2011, 55, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jo, H.; Cho, J.H.; Dhanasekaran, D.N.; Song, Y.S. Resveratrol as a Tumor-Suppressive Nutraceutical Modulating Tumor Microenvironment and Malignant Behaviors of Cancer. Int. J. Mol. Sci. 2019, 20, 925. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zheng, J.; Hu, C.; He, J.; Wang, S.; Chen, Z.; Wang, Y.; Li, H.; Ge, R.-S.; Tang, Y.; et al. Cyclocurcumin potently inhibits human aromatase as a potential therapeutic agent. J. Steroid Biochem. Mol. Biol. 2025, 247, 106672. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, A.M.; Barnett, J.L.; Berman, S.A.; Li, J.; Quarless, S.; Bursztajn, S.; Lippa, C.; Nixon, R.A. Gene expression and cellular content of cathepsin D in Alzheimer’s disease brain: Evidence for early up-regulation of the endosomal-lysosomal system. Neuron 1995, 14, 671–680. [Google Scholar] [CrossRef]
- Di, Y.Q.; Han, X.L.; Kang, X.L.; Wang, D.; Chen, C.H.; Wang, J.X.; Zhao, X.F. Autophagy triggers CTSD (cathepsin D) maturation and localization inside cells to promote apoptosis. Autophagy 2021, 17, 1170–1192. [Google Scholar] [CrossRef]
- Escot, C.; Zhao, Y.; Puech, C.; Rochefort, H. Cellular localisation by in situ hybridisation of cathepsin D, stromelysin 3, and urokinase plasminogen activator RNAs in breast cancer. Breast Cancer Res. Treat. 1996, 38, 217–226. [Google Scholar] [CrossRef]
- Seo, S.U.; Woo, S.M.; Im, S.S.; Jang, Y.; Han, E.; Kim, S.H.; Lee, H.; Lee, H.S.; Nam, J.O.; Gabrielson, E.; et al. Cathepsin D as a potential therapeutic target to enhance anticancer drug-induced apoptosis via RNF183-mediated destabilization of Bcl-xL in cancer cells. Cell Death Dis. 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Handley, C.J.; Mok, M.T.; Ilic, M.Z.; Adcocks, C.; Buttle, D.J.; Robinson, H.C. Cathepsin D cleaves aggrecan at unique sites within the interglobular domain and chondroitin sulfate attachment regions that are also cleaved when cartilage is maintained at acid pH. Matrix Biol. 2001, 20, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.M.; Murphy, G. Matrix Proteinases. In Dynamics of Bone and Cartilage Metabolism; Seibel, M.J., Robins, S.P., Bilezikian, J.P., Eds.; Academic Press: Burlington, OA, Canada, 2006; pp. 181–198. [Google Scholar]
- Ji, K.; Sloane, B.F. Cancer—Proteases in the Progression and Metastasis. In Encyclopedia of Cell Biology; Bradshaw, R.A., Stahl, P.D., Eds.; Academic Press: Waltham, MA, USA, 2016; pp. 753–762. [Google Scholar]
- Guerra-Martin, M.D.; Tejedor-Bueno, M.S.; Correa-Casado, M. Effectiveness of Complementary Therapies in Cancer Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1017. [Google Scholar] [CrossRef] [PubMed]
- Glondu, M.; Coopman, P.; Laurent-Matha, V.; Garcia, M.; Rochefort, H.; Liaudet-Coopman, E. A mutated cathepsin-D devoid of its catalytic activity stimulates the growth of cancer cells. Oncogene 2001, 20, 6920–6929. [Google Scholar] [CrossRef]
- Fitzgibbons, P.L.; Page, D.L.; Weaver, D.; Thor, A.D.; Allred, D.C.; Clark, G.M.; Ruby, S.G.; O’Malley, F.; Simpson, J.F.; Connolly, J.L.; et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch. Pathol. Lab. Med. 2000, 124, 966–978. [Google Scholar] [CrossRef]
- Rochefort, H.; Garcia, M.; Glondu, M.; Laurent, V.; Liaudet, E.; Rey, J.M.; Roger, P. Cathepsin D in breast cancer: Mechanisms and clinical applications, a 1999 overview. Clin. Chim. Acta 2000, 291, 157–170. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Carew, J.S.; Espitia, C.M.; Esquivel, J.A., 2nd; Mahalingam, D.; Kelly, K.R.; Reddy, G.; Giles, F.J.; Nawrocki, S.T. Lucanthone is a novel inhibitor of autophagy that induces cathepsin D-mediated apoptosis. J. Biol. Chem. 2011, 286, 6602–6613. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, Q.; Wang, C.; Yao, D.; Zhu, L.; Pan, Y.; Zhang, J.; Bai, Y.; Shao, C. Inhibition of Cathepsin D (CTSD) enhances radiosensitivity of glioblastoma cells by attenuating autophagy. Mol. Carcinog. 2020, 59, 651–660. [Google Scholar] [CrossRef]
- Foekens, J.A.; Peters, H.A.; Look, M.P.; Portengen, H.; Schmitt, M.; Kramer, M.D.; Brunner, N.; Janicke, F.; Meijer-van Gelder, M.E.; Henzen-Logmans, S.C.; et al. The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer Res. 2000, 60, 636–643. [Google Scholar]
- De Witte, H.; Sweep, F.; Brunner, N.; Heuvel, J.; Beex, L.; Grebenschikov, N.; Benraad, T. Complexes between urokinase-type plasminogen activator and its receptor in blood as determined by enzyme-linked immunosorbent assay. Int. J. Cancer 1998, 77, 236–242. [Google Scholar] [CrossRef]
- Deng, G.; Curriden, S.A.; Wang, S.; Rosenberg, S.; Loskutoff, D.J. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J. Cell Biol. 1996, 134, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Kanse, S.M.; Kost, C.; Wilhelm, O.G.; Andreasen, P.A.; Preissner, K.T. The urokinase receptor is a major vitronectin-binding protein on endothelial cells. Exp. Cell Res. 1996, 224, 344–353. [Google Scholar] [CrossRef]
- Andreasen, P.A.; Georg, B.; Lund, L.R.; Riccio, A.; Stacey, S.N. Plasminogen activator inhibitors: Hormonally regulated serpins. Mol. Cell. Endocrinol. 1990, 68, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Brysch, W.; Schlingensiepen, K.H. Design and application of antisense oligonucleotides in cell culture, in vivo, and as therapeutic agents. Cell. Mol. Neurobiol. 1994, 14, 557–568. [Google Scholar] [CrossRef]
- Brysch, W.; Magal, E.; Louis, J.C.; Kunst, M.; Klinger, I.; Schlingensiepen, R.; Schlingensiepen, K.H. Inhibition of p185c-erbB-2 proto-oncogene expression by antisense oligodeoxynucleotides down-regulates p185-associated tyrosine-kinase activity and strongly inhibits mammary tumor-cell proliferation. Cancer Gene Ther. 1994, 1, 99–105. [Google Scholar]
- Chiang, M.Y.; Chan, H.; Zounes, M.A.; Freier, S.M.; Lima, W.F.; Bennett, C.F. Antisense oligonucleotides inhibit intercellular adhesion molecule 1 expression by two distinct mechanisms. J. Biol. Chem. 1991, 266, 18162–18171. [Google Scholar] [CrossRef]
- Wahlestedt, C.; Golanov, E.; Yamamoto, S.; Yee, F.; Ericson, H.; Yoo, H.; Inturrisi, C.E.; Reis, D.J. Antisense oligodeoxynucleotides to NMDA-R1 receptor channel protect cortical neurons from excitotoxicity and reduce focal ischaemic infarctions. Nature 1993, 363, 260–263. [Google Scholar] [CrossRef]
- Agrawal, S. Antisense oligonucleotides: Towards clinical trials. Trends Biotechnol. 1996, 14, 376–387. [Google Scholar] [CrossRef]
- Frazier, K.S. Antisense oligonucleotide therapies: The promise and the challenges from a toxicologic pathologist’s perspective. Toxicol. Pathol. 2015, 43, 78–89. [Google Scholar] [CrossRef]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Khuu, A.; Verreault, M.; Colin, P.; Tran, H.; Idbaih, A. Clinical Applications of Antisense Oligonucleotides in Cancer: A Focus on Glioblastoma. Cells 2024, 13, 1869. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Witztum, J.L.; Bennett, C.F.; Baker, B.F. RNA-Targeted Therapeutics. Cell Metab. 2018, 27, 714–739. [Google Scholar] [CrossRef] [PubMed]
- Sazani, P.; Kole, R. Therapeutic potential of antisense oligonucleotides as modulators of alternative splicing. J. Clin. Investig. 2003, 112, 481–486. [Google Scholar] [CrossRef]
- Vasconcelos, A.O.; Vieira, L.M.; Rocha, C.R.C.; Beltrao, E.I.C. The contribution of fucosyltransferases to cancer biology. Braz. J. Biol. 2024, 84, e278681. [Google Scholar] [CrossRef]
- do Nascimento, J.C.; Ferreira Sde, A.; Vasconcelos, J.L.; da Silva-Filho, J.L.; Barbosa, B.T.; Bezerra, M.F.; Rocha, C.R.; Beltrao, E.I. Fut3 role in breast invasive ductal carcinoma: Investigating its gene promoter and protein expression. Exp. Mol. Pathol. 2015, 99, 409–415. [Google Scholar] [CrossRef]
- do Nascimento, J.C.F.; Beltrao, E.I.C.; Rocha, C.R.C. High FUT3 expression is a marker of lower overall survival of breast cancer patients. Glycoconj. J. 2020, 37, 263–275. [Google Scholar] [CrossRef]
- Yuan, K.; Kucik, D.; Singh, R.K.; Listinsky, C.M.; Listinsky, J.J.; Siegal, G.P. Alterations in human breast cancer adhesion-motility in response to changes in cell surface glycoproteins displaying alpha-L-fucose moieties. Int. J. Oncol. 2008, 32, 797–807. [Google Scholar]
- Higai, K.; Ichikawa, A.; Matsumoto, K. Binding of sialyl Lewis X antigen to lectin-like receptors on NK cells induces cytotoxicity and tyrosine phosphorylation of a 17-kDa protein. Biochim. Biophys. Acta 2006, 1760, 1355–1363. [Google Scholar] [CrossRef]
- Silsirivanit, A.; Araki, N.; Wongkham, C.; Vaeteewoottacharn, K.; Pairojkul, C.; Kuwahara, K.; Narimatsu, Y.; Sawaki, H.; Narimatsu, H.; Okada, S.; et al. CA-S27: A novel Lewis a associated carbohydrate epitope is diagnostic and prognostic for cholangiocarcinoma. Cancer Sci. 2013, 104, 1278–1284. [Google Scholar] [CrossRef]
- Ohyama, C.; Kanto, S.; Kato, K.; Nakano, O.; Arai, Y.; Kato, T.; Chen, S.; Fukuda, M.N.; Fukuda, M. Natural killer cells attack tumor cells expressing high levels of sialyl Lewis x oligosaccharides. Proc. Natl. Acad. Sci. USA 2002, 99, 13789–13794. [Google Scholar] [CrossRef] [PubMed]
- Javaud, C.; Dupuy, F.; Maftah, A.; Julien, R.; Petit, J.M. The fucosyltransferase gene family: An amazing summary of the underlying mechanisms of gene evolution. Genetica 2003, 118, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Mylonas, I.; Shabani, N.; Kunert-Keil, C.; Schindlbeck, C.; Gerber, B.; Friese, K. Expression of sialyl lewis X, sialyl Lewis A, E-cadherin and cathepsin-D in human breast cancer: Immunohistochemical analysis in mammary carcinoma in situ, invasive carcinomas and their lymph node metastasis. Anticancer Res. 2005, 25, 1615–1622. [Google Scholar] [PubMed]
- Zhang, B.; van Roosmalen, I.A.M.; Reis, C.R.; Setroikromo, R.; Quax, W.J. Death receptor 5 is activated by fucosylation in colon cancer cells. FEBS J. 2019, 286, 555–571. [Google Scholar] [CrossRef]
- Kelley, S.K.; Ashkenazi, A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr. Opin. Pharmacol. 2004, 4, 333–339. [Google Scholar] [CrossRef]
- Orlinick, J.R.; Chao, M.V. TNF-related ligands and their receptors. Cell. Signal 1998, 10, 543–551. [Google Scholar] [CrossRef]
- Masum, A.A.; Yokoi, K.; Hisamatsu, Y.; Naito, K.; Shashni, B.; Aoki, S. Design and synthesis of a luminescent iridium complex-peptide hybrid (IPH) that detects cancer cells and induces their apoptosis. Bioorg Med. Chem. 2018, 26, 4804–4816. [Google Scholar] [CrossRef]
- Deng, Q.; Chen, L.; Zhang, G.; Liu, L.; Luo, S.M.; Gao, X. TRIAL-based combination therapies in cancers. Int. Immunopharmacol. 2024, 138, 112570. [Google Scholar] [CrossRef]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef]
- Huang, L.; Fu, L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm. Sin. B 2015, 5, 390–401. [Google Scholar] [CrossRef]
- Tomasello, C.; Baldessari, C.; Napolitano, M.; Orsi, G.; Grizzi, G.; Bertolini, F.; Barbieri, F.; Cascinu, S. Resistance to EGFR inhibitors in non-small cell lung cancer: Clinical management and future perspectives. Crit. Rev. Oncol. Hematol. 2018, 123, 149–161. [Google Scholar] [CrossRef]
- Campos Bastos, S. Mecanismos De Resistência Aos Inibidores Da Tirosina Cinase Do RECETOR Do Fator De Crescimento Epidérmico No Cancro De Pulmão De Não Pequenas Células. Master’s Thesis, ICBAS—School of Medicine and Biomedical Sciences of the University of Porto, Porto, Portugal, 2022. [Google Scholar]
- Hashim, G.M.; Shahgolzari, M.; Hefferon, K.; Yavari, A.; Venkataraman, S. Plant-Derived Anti-Cancer Therapeutics and Biopharmaceuticals. Bioengineering 2024, 12, 7. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jerusalem, G.; Longuespee, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, S.; Zhao, W.; Qin, S.; Chu, Q.; Wu, K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol. Cancer 2018, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Murphrey, M.B.; Quaim, L.; Rahimi, N.; Varacallo, M.A. Biochemistry, Epidermal Growth Factor Receptor. In StatPearls; StatPearls Publishing: Treasure Island, FA, USA, 2023. [Google Scholar]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Shah, M. K-ras mutations in colorectal cancer: A practice changing discovery. Clin. Adv. Hematol. Oncol. 2009, 7, 45–53, 64. [Google Scholar]
- Zaryouh, H.; Van Loenhout, J.; Peeters, M.; Vermorken, J.B.; Lardon, F.; Wouters, A. Co-Targeting the EGFR and PI3K/Akt Pathway to Overcome Therapeutic Resistance in Head and Neck Squamous Cell Carcinoma: What about Autophagy? Cancers 2022, 14, 6128. [Google Scholar] [CrossRef]
- Andl, C.D.; Mizushima, T.; Oyama, K.; Bowser, M.; Nakagawa, H.; Rustgi, A.K. EGFR-induced cell migration is mediated predominantly by the JAK-STAT pathway in primary esophageal keratinocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1227–G1237. [Google Scholar] [CrossRef]
- Benedetti, V.; Lavecchia, A.M.; Locatelli, M.; Brizi, V.; Corna, D.; Todeschini, M.; Novelli, R.; Benigni, A.; Zoja, C.; Remuzzi, G.; et al. Alteration of thyroid hormone signaling triggers the diabetes-induced pathological growth, remodeling, and dedifferentiation of podocytes. JCI Insight 2019, 4, e130249. [Google Scholar] [CrossRef]
- Kolcsar, M.; Zeces, I.A.; Kovecsi, A.; Kovacs, Z.; Gall, Z. Iodothyronine Deiodinase 3 Gene Expression in Gastrointestinal Stromal Tumors: A Pilot Study to Contribute to Risk Assessment. Cureus 2024, 16, e67426. [Google Scholar] [CrossRef] [PubMed]
- Karunakara, S.H.; Mehtani, R.; Kabekkodu, S.P.; Kumar, D.P.; Santhekadur, P.K. Genes of DLK1-DIO3 Locus and miR-379/656 Cluster is a Potential Diagnostic and Prognostic Marker in Patients with Hepatocellular Carcinoma: A Systems Biology Study. J. Clin. Exp. Hepatol. 2025, 15, 102450. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Shapiro, D.J. The immune system and inflammation in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 673–682. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef]
- Bailey, C.; Black, J.R.M.; Reading, J.L.; Litchfield, K.; Turajlic, S.; McGranahan, N.; Jamal-Hanjani, M.; Swanton, C. Tracking Cancer Evolution through the Disease Course. Cancer Discov. 2021, 11, 916–932. [Google Scholar] [CrossRef]
- Hong, J.T.; Son, D.J.; Lee, C.K.; Yoon, D.Y.; Lee, D.H.; Park, M.H. Interleukin 32, inflammation and cancer. Pharmacol. Ther. 2017, 174, 127–137. [Google Scholar] [CrossRef]
- Emens, L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 511–520. [Google Scholar] [CrossRef]
- Albini, A.; Sporn, M.B. The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer 2007, 7, 139–147. [Google Scholar] [CrossRef]
- Augimeri, G.; Gonzalez, M.E.; Paoli, A.; Eido, A.; Choi, Y.; Burman, B.; Djomehri, S.; Karthikeyan, S.K.; Varambally, S.; Buschhaus, J.M.; et al. A hybrid breast cancer/mesenchymal stem cell population enhances chemoresistance and metastasis. JCI Insight 2023, 8, e164216. [Google Scholar] [CrossRef]
- Riera Romo, M.; Perez-Martinez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Baek, S.H.; Park, T.; Kang, M.G.; Park, D. Anti-Inflammatory Activity and ROS Regulation Effect of Sinapaldehyde in LPS-Stimulated RAW 264.7 Macrophages. Molecules 2020, 25, 4089. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Wellenstein, M.D.; Coffelt, S.B.; Duits, D.E.M.; van Miltenburg, M.H.; Slagter, M.; de Rink, I.; Henneman, L.; Kas, S.M.; Prekovic, S.; Hau, C.S.; et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature 2019, 572, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Liubomirski, Y.; Lerrer, S.; Meshel, T.; Rubinstein-Achiasaf, L.; Morein, D.; Wiemann, S.; Korner, C.; Ben-Baruch, A. Tumor-Stroma-Inflammation Networks Promote Pro-metastatic Chemokines and Aggressiveness Characteristics in Triple-Negative Breast Cancer. Front. Immunol. 2019, 10, 757. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef]
- Coy-Barrera, E.D.; Cuca-Suarez, L.E. In vitro anti-inflammatory effects of naturally-occurring compounds from two Lauraceae plants. An. Acad. Bras. Cienc. 2011, 83, 1397–1402. [Google Scholar] [CrossRef]
- Jaeschke, R.; Gajewski, P.; Brozek, J. Cardiovascular thrombotic events in controlled, clinical trials of rofecoxib. Circulation 2002, 106, e18. [Google Scholar] [CrossRef][Green Version]
- Garcia-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.A.; Martinez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Forstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef]
- Duffy, M.J. Clinical uses of tumor markers: A critical review. Crit. Rev. Clin. Lab. Sci. 2001, 38, 225–262. [Google Scholar] [CrossRef]
- Duffy, M.J. Role of tumor markers in patients with solid cancers: A critical review. Eur. J. Intern. Med. 2007, 18, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Maric, P.; Ozretic, P.; Levanat, S.; Oreskovic, S.; Antunac, K.; Beketic-Oreskovic, L. Tumor markers in breast cancer--evaluation of their clinical usefulness. Coll. Antropol. 2011, 35, 241–247. [Google Scholar] [PubMed]
- Briguenti Ramalho, R.; De Luca Castro, G.; Santana Sakamoto, G.; Romana Correia Sales, A.K.; Nicole Lino do Nascimento, W.; Gomes Lourencini, Y.; Oliveira de Lima, L.; Weiler Prado, L.; Oliveira Freire do Nascimento, L.; De Almeida Andrade Pereira, L.; et al. Diagnóstico e tratamento do câncer de mama: Uma revisão de literatura. Braz. J. Implantol. Health Sci. 2024, 6, 1040–1050. [Google Scholar] [CrossRef]
- Plebani, M. Biochemical and imaging biomarkers: The search for the Holy Grail. Clin. Chem. Lab. Med. 2010, 48, 1055–1056. [Google Scholar] [CrossRef] [PubMed]
- Cunat, S.; Hoffmann, P.; Pujol, P. Estrogens and epithelial ovarian cancer. Gynecol. Oncol. 2004, 94, 25–32. [Google Scholar] [CrossRef]
- Dunn, B.M. Aspartic Proteases. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 137–140. [Google Scholar]
- Fusek, M.; Mares, M.; Vetvicka, V.; Cathepsin, D. Handbook of Proteolytic Enzymes; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 54–63. [Google Scholar]
- Pashayan, N.; Antoniou, A.C.; Ivanus, U.; Esserman, L.J.; Easton, D.F.; French, D.; Sroczynski, G.; Hall, P.; Cuzick, J.; Evans, D.G.; et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat. Rev. Clin. Oncol. 2020, 17, 687–705. [Google Scholar] [CrossRef]
- Barba, D.; Leon-Sosa, A.; Lugo, P.; Suquillo, D.; Torres, F.; Surre, F.; Trojman, L.; Caicedo, A. Breast cancer, screening and diagnostic tools: All you need to know. Crit. Rev. Oncol. Hematol. 2021, 157, 103174. [Google Scholar] [CrossRef]
- Gibson, A.F.; Broom, A.; Kirby, E.; Wyld, D.K.; Lwin, Z. The Social Reception of Women with Cancer. Qual. Health Res. 2017, 27, 983–993. [Google Scholar] [CrossRef]
- Tejeda, S.; Gallardo, R.I.; Ferrans, C.E.; Rauscher, G.H. Breast cancer delay in Latinas: The role of cultural beliefs and acculturation. J. Behav. Med. 2017, 40, 343–351. [Google Scholar] [CrossRef]
- Wan, H.H.; Zhu, H.; Chiang, C.C.; Li, J.S.; Ren, F.; Tsai, C.T.; Liao, Y.T.; Neal, D.; Esquivel-Upshaw, J.F.; Pearton, S.J. High sensitivity saliva-based biosensor in detection of breast cancer biomarkers: HER2 and CA15-3. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2024, 42, 023202. [Google Scholar] [CrossRef] [PubMed]
- Agide, F.D.; Sadeghi, R.; Garmaroudi, G.; Tigabu, B.M. A systematic review of health promotion interventions to increase breast cancer screening uptake: From the last 12 years. Eur. J. Public Health 2018, 28, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhao, W.; Zhang, J.; Chen, W.; Xie, T.; Wang, L.; Fan, W.; Xie, S.; Shen, J.; Zheng, H.; et al. Evaluation of the diagnostic value of circulating tumor cells with CytoSorter ® CTC capture system in patients with breast cancer. Cancer Med. 2020, 9, 1638–1647. [Google Scholar] [CrossRef]
- Feng, J.; Li, B.; Ying, J.; Pan, W.; Liu, C.; Luo, T.; Lin, H.; Zheng, L. Liquid Biopsy: Application in Early Diagnosis and Monitoring of Cancer. Small Struct. 2020, 1, 2000063. [Google Scholar] [CrossRef]
- Wang, X.; Kaczor-Urbanowicz, K.E.; Wong, D.T. Salivary biomarkers in cancer detection. Med. Oncol. 2017, 34, 7. [Google Scholar] [CrossRef]
- Lal, M.; Ansari, A.H.; Agrawal, A.; Mukhopadhyay, A. Diagnostic and Prognostic Potential of MiR-379/656 MicroRNA Cluster in Molecular Subtypes of Breast Cancer. J. Clin. Med. 2021, 10, 4071. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Sowa, P.M.; Downes, M.J.; Gordon, L.G. Cost-effectiveness of dual-energy X-ray absorptiometry plus antiresorptive treatment in Australian women with breast cancer who receive aromatase inhibitors. J. Bone Miner. Metab. 2017, 35, 199–208. [Google Scholar] [CrossRef]
- Smykla, A.; Walewicz, K.; Trybulski, R.; Halski, T.; Kucharzewski, M.; Kucio, C.; Mikusek, W.; Klakla, K.; Taradaj, J. Effect of Kinesiology Taping on breast cancer-related lymphedema: A randomized single-blind controlled pilot study. Biomed. Res. Int. 2013, 2013, 767106. [Google Scholar] [CrossRef]
- McCann, S.E.; Edge, S.B.; Hicks, D.G.; Thompson, L.U.; Morrison, C.D.; Fetterly, G.; Andrews, C.; Clark, K.; Wilton, J.; Kulkarni, S. A pilot study comparing the effect of flaxseed, aromatase inhibitor, and the combination on breast tumor biomarkers. Nutr. Cancer 2014, 66, 566–575. [Google Scholar] [CrossRef]
- Mao, J.J.; Farrar, J.T.; Bruner, D.; Zee, J.; Bowman, M.; Seluzicki, C.; DeMichele, A.; Xie, S.X. Electroacupuncture for fatigue, sleep, and psychological distress in breast cancer patients with aromatase inhibitor-related arthralgia: A randomized trial. Cancer 2014, 120, 3744–3751. [Google Scholar] [CrossRef] [PubMed]
- Koopaie, M.; Kolahdooz, S.; Fatahzadeh, M.; Manifar, S. Salivary biomarkers in breast cancer diagnosis: A systematic review and diagnostic meta-analysis. Cancer Med. 2022, 11, 2644–2661. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Wei, F.; Rao, S.L.; Kim, J.; Shin, H.; Cheng, J.; Tu, M.; Wong, D.T.W.; Kim, Y. Clinical validity of saliva and novel technology for cancer detection. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, L.; Paderno, A.; Piazza, C.; Pagan, E.; Bignotti, E.; Romani, C.; Bandiera, E.; Calza, S.; Del Bon, F.; Nicolai, P.; et al. Epidermal growth factor receptor detection in serum and saliva as a diagnostic and prognostic tool in oral cancer. Laryngoscope 2017, 127, E408–E414. [Google Scholar] [CrossRef]
- Koopaie, M.; Abedinejad, F.; Manifar, S.; Mousavi, R.; Kolahdooz, S.; Shamshiri, A. Salivary miRNA-21 expression as a potential non-invasive diagnostic biomarker in breast cancer. Gene Rep. 2021, 25, 101317. [Google Scholar] [CrossRef]
- Hernandez-Arteaga, A.C.; de Jesus Zermeno-Nava, J.; Martinez-Martinez, M.U.; Hernandez-Cedillo, A.; Ojeda-Galvan, H.J.; Jose-Yacaman, M.; Navarro-Contreras, H.R. Determination of Salivary Sialic Acid Through Nanotechnology: A Useful Biomarker for the Screening of Breast Cancer. Arch. Med. Res. 2019, 50, 105–110. [Google Scholar] [CrossRef]
- Farahani, H.; Amri, J.; Alaee, M.; Mohaghegh, F.; Rafiee, M. Serum and Saliva Levels of Cancer Antigen 15-3, Carcinoembryonic Antigen, Estradiol, Vaspin, and Obestatin as Biomarkers for the Diagnosis of Breast Cancer in Postmenopausal Women. Lab. Med. 2020, 51, 620–627. [Google Scholar] [CrossRef]
- Rapado-Gonzalez, O.; Majem, B.; Muinelo-Romay, L.; Lopez-Lopez, R.; Suarez-Cunqueiro, M.M. Cancer Salivary Biomarkers for Tumours Distant to the Oral Cavity. Int. J. Mol. Sci. 2016, 17, 1531. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Kaczor, T.; Tu, M.; Wei, F.; Garcia-Godoy, F.; Wong, D.T. Emerging technologies for salivaomics in cancer detection. J. Cell. Mol. Med. 2017, 21, 640–647. [Google Scholar] [CrossRef]
- Ghizoni, J.S.; Nichele, R.; de Oliveira, M.T.; Pamato, S.; Pereira, J.R. The utilization of saliva as an early diagnostic tool for oral cancer: microRNA as a biomarker. Clin. Transl. Oncol. 2020, 22, 804–812. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.I. Serum and saliva protein levels in females with breast cancer. Oncol. Lett. 2014, 8, 2752–2756. [Google Scholar] [CrossRef][Green Version]
- Myllymaki, S.M.; Mikkola, M.L. Inductive signals in branching morphogenesis—Lessons from mammary and salivary glands. Curr. Opin. Cell Biol. 2019, 61, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Laidi, F.; Bouziane, A.; Lakhdar, A.; Khabouze, S.; Rhrab, B.; Zaoui, F. Salivary expression of soluble HER2 in breast cancer patients with positive and negative HER2 status. Onco Targets Ther. 2014, 7, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Arteaga, A.; de Jesús Zermeño Nava, J.; Kolosovas-Machuca, E.S.; Velázquez-Salazar, J.J.; Vinogradova, E.; José-Yacamán, M.; Navarro-Contreras, H.R. Diagnosis of breast cancer by analysis of sialic acid concentrations in human saliva by surface-enhanced Raman spectroscopy of silver nanoparticles. Nano Res. 2017, 10, 3662–3670. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.; Qiao, Y.; Yang, J.; Shu, J.; Zhang, J.; Zhang, Z.; He, J.; Li, Z. Salivary Glycopatterns as Potential Biomarkers for Screening of Early-Stage Breast Cancer. EBioMedicine 2018, 28, 70–79. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Shu, J.; Hou, Y.; Chen, M.; Yu, H.; Ma, T.; Du, H.; Zhang, J.; Qiao, Y.; et al. Abnormal Galactosylated-Glycans recognized by Bandeiraea Simplicifolia Lectin I in saliva of patients with breast Cancer. Glycoconj. J. 2020, 37, 373–394. [Google Scholar] [CrossRef]
- Murata, T.; Yanagisawa, T.; Kurihara, T.; Kaneko, M.; Ota, S.; Enomoto, A.; Tomita, M.; Sugimoto, M.; Sunamura, M.; Hayashida, T.; et al. Salivary metabolomics with alternative decision tree-based machine learning methods for breast cancer discrimination. Breast Cancer Res. Treat. 2019, 177, 591–601. [Google Scholar] [CrossRef]
- Porto-Mascarenhas, E.C.; Assad, D.X.; Chardin, H.; Gozal, D.; De Luca Canto, G.; Acevedo, A.C.; Guerra, E.N. Salivary biomarkers in the diagnosis of breast cancer: A review. Crit. Rev. Oncol. Hematol. 2017, 110, 62–73. [Google Scholar] [CrossRef]
- Wood, N.; Streckfus, C.F. The Expression of Lung Resistance Protein in Saliva: A Novel Prognostic Indicator Protein for Carcinoma of the Breast. Cancer Investig. 2015, 33, 510–515. [Google Scholar] [CrossRef]
- Ando, W.; Kikuchi, K.; Uematsu, T.; Yokomori, H.; Takaki, T.; Sogabe, M.; Kohgo, Y.; Otori, K.; Ishikawa, S.; Okazaki, I. Novel breast cancer screening: Combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis. Sci. Rep. 2019, 9, 13595. [Google Scholar] [CrossRef]
- Kuo, H.W.; Chou, S.Y.; Hu, T.W.; Wu, F.Y.; Chen, D.J. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) and genetic polymorphisms in breast cancer patients. Mutat. Res. 2007, 631, 62–68. [Google Scholar] [CrossRef]
- Wang, T.; Nichols, H.B.; Nyante, S.J.; Bradshaw, P.T.; Moorman, P.G.; Kabat, G.C.; Parada, H., Jr.; Khankari, N.K.; Teitelbaum, S.L.; Terry, M.B.; et al. Urinary Estrogen Metabolites and Long-Term Mortality Following Breast Cancer. JNCI Cancer Spectr. 2020, 4, pkaa014. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2022, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bashar, M.A.; Hossain, M.A.; Kavey, M.R.H.; Shazib, R.; Islam, M.S.; Ansari, S.A.; Rahman, M.H. Network Pharmacology and In silico Elucidation of Phytochemicals Extracted from Ajwa Dates (Phoenix dactylifera L.) to Inhibit Akt and PI3K Causing Triple Negative Breast Cancer (TNBC). Curr. Pharm. Des. 2025, 31, 774–796. [Google Scholar] [CrossRef] [PubMed]
- Strom, E.A.; Buzdar, A.U.; Hunt, K.K. Multidisciplinary Care of the Breast Cancer Patient: Overview and Implementation. In Breast Cancer; Hunt, K.K., Robb, G.L., Strom, E.A., Ueno, N.T., Eds.; Springer: New York, NY, USA, 2001; pp. 1–19. [Google Scholar]
- Buzdar, A.U. Breast cancer in men. Oncology 2003, 17, 1361–1364; discussion 1364, 1369–1372. [Google Scholar]
- Mouridsen, H.T.; Rose, C.; Brodie, A.H.; Smith, I.E. Challenges in the endocrine management of breast cancer. Breast 2003, 12 (Suppl. S2), S2–S19. [Google Scholar] [CrossRef]
- Forbes, J.F.; Gradishar, W.J.; Ravdin, P.M. Choosing between endocrine therapy and chemotherapy—or is there a role for combination therapy? Breast Cancer Res. Treat. 2002, 75 (Suppl. S1), S37–S44; discussion S39–S57. [Google Scholar] [CrossRef]
- Gabrielson, M.; Hammarstrom, M.; Bergqvist, J.; Lang, K.; Rosendahl, A.H.; Borgquist, S.; Hellgren, R.; Czene, K.; Hall, P. Baseline breast tissue characteristics determine the effect of tamoxifen on mammographic density change. Int. J. Cancer 2024, 155, 339–351. [Google Scholar] [CrossRef]
- Jordan, V.C.; Gapstur, S.; Morrow, M. Selective estrogen receptor modulation and reduction in risk of breast cancer, osteoporosis, and coronary heart disease. J. Natl. Cancer Inst. 2001, 93, 1449–1457. [Google Scholar] [CrossRef]
- Dunn, B.K.; Ford, L.G. From adjuvant therapy to breast cancer prevention: BCPT and STAR. Breast J. 2001, 7, 144–157. [Google Scholar] [CrossRef]
- Ellmen, J.; Hakulinen, P.; Partanen, A.; Hayes, D.F. Estrogenic effects of toremifene and tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res. Treat. 2003, 82, 103–111. [Google Scholar] [CrossRef]
- Lecomte, S.; Demay, F.; Ferriere, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Fallowfield, L.; Barker, P.; Cuzick, J.; Locker, G.; Howell, A.; Group, A.T. Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res. Treat. 2006, 100, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Goss, P.E.; Qi, S.; Hu, H.; Cheung, A.M. The effects of atamestane and toremifene alone and in combination compared with letrozole on bone, serum lipids and the uterus in an ovariectomized rat model. Breast Cancer Res. Treat. 2007, 103, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Cocconi, G. First generation aromatase inhibitors—aminoglutethimide and testololactone. Breast Cancer Res. Treat. 1994, 30, 57–80. [Google Scholar] [CrossRef]
- Kijima, I.; Phung, S.; Hur, G.; Kwok, S.L.; Chen, S. Grape seed extract is an aromatase inhibitor and a suppressor of aromatase expression. Cancer Res. 2006, 66, 5960–5967. [Google Scholar] [CrossRef]
- Eng, E.T.; Ye, J.; Williams, D.; Phung, S.; Moore, R.E.; Young, M.K.; Gruntmanis, U.; Braunstein, G.; Chen, S. Suppression of estrogen biosynthesis by procyanidin dimers in red wine and grape seeds. Cancer Res. 2003, 63, 8516–8522. [Google Scholar]
- Santen, R.J.; Lobenhofer, E.K.; Afshari, C.A.; Bao, Y.; Song, R.X. Adaptation of estrogen-regulated genes in long-term estradiol deprived MCF-7 breast cancer cells. Breast Cancer Res. Treat. 2005, 94, 213–223. [Google Scholar] [CrossRef]
- Gnant, M. Management of bone loss induced by aromatase inhibitors. Cancer Investig. 2006, 24, 328–330. [Google Scholar] [CrossRef]
- Chowdhury, S.; Pickering, L.M.; Ellis, P.A. Adjuvant aromatase inhibitors and bone health. J. Br. Menopause Soc. 2006, 12, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Hannon, R.A.; Cuzick, J.; Dowsett, M.; Clack, G.; Adams, J.E. Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J. Bone Miner. Res. 2006, 21, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.; Lonning, P.E.; Krag, L.E.; Lokkevik, E.; Risberg, T.; Hagen, A.I.; Schlichting, E.; Lien, E.A.; Ofjord, E.S.; Eide, G.E.; et al. Changes in bone and lipid metabolism in postmenopausal women with early breast cancer after terminating 2-year treatment with exemestane: A randomised, placebo-controlled study. Eur. J. Cancer 2006, 42, 2968–2975. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Anderson, G.L.; Geller, M.; Col, N. Coronary heart disease and stroke with aromatase inhibitor, tamoxifen, and menopausal hormone therapy use. Clin. Breast Cancer 2006, 6 (Suppl. S2), S58–S64. [Google Scholar] [CrossRef]
- Yue, X.; Lu, M.; Lancaster, T.; Cao, P.; Honda, S.; Staufenbiel, M.; Harada, N.; Zhong, Z.; Shen, Y.; Li, R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc. Natl. Acad. Sci. USA 2005, 102, 19198–19203. [Google Scholar] [CrossRef]
- Jones, W.P.; Chin, Y.W.; Kinghorn, A.D. The role of pharmacognosy in modern medicine and pharmacy. Curr. Drug Targets 2006, 7, 247–264. [Google Scholar] [CrossRef]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef]
- Daly, J.W.; Spande, T.F.; Garraffo, H.M. Alkaloids from amphibian skin: A tabulation of over eight-hundred compounds. J. Nat. Prod. 2005, 68, 1556–1575. [Google Scholar] [CrossRef]
- Brodowska, K.M. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar]
- Szaefer, H.; Licznerska, B.; Sobierajska, H.; Baer-Dubowska, W. Breast Cancer Cytochromes P450: Chemopreventive and/or Therapeutic Targets for Naturally Occurring Phytochemicals. Molecules 2025, 30, 3079. [Google Scholar] [CrossRef]
- Balam, F.H.; Ahmadi, Z.S.; Ghorbani, A. Inhibitory effect of chrysin on estrogen biosynthesis by suppression of enzyme aromatase (CYP19): A systematic review. Heliyon 2020, 6, e03557. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; Melebari, S.A.; Khogeer, A.A.; Abdulaal, W.H.; Al-Fageeh, M.B.; Algahtani, M.; Rateb, M.E. Targeting allosteric sites of human aromatase: A comprehensive in-silico and in-vitro workflow to find potential plant-based anti-breast cancer therapeutics. J. Enzym. Inhib. Med. Chem. 2021, 36, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Hoensch, H.P.; Oertel, R. The value of flavonoids for the human nutrition: Short review and perspectives. Clin. Nutr. Exp. 2015, 3, 8–14. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic flavonoids with antimicrobial activity: A review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi Akbar Boojar, M. An Overview of the Cellular Mechanisms of Flavonoids Radioprotective Effects. Adv. Pharm. Bull. 2020, 10, 13–19. [Google Scholar] [CrossRef]
- Yahyapour, R.; Shabeeb, D.; Cheki, M.; Musa, A.E.; Farhood, B.; Rezaeyan, A.; Amini, P.; Fallah, H.; Najafi, M. Radiation Protection and Mitigation by Natural Antioxidants and Flavonoids: Implications to Radiotherapy and Radiation Disasters. Curr. Mol. Pharmacol. 2018, 11, 285–304. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, C.; Xi, S.; Qian, F.; Peng, X.; Huang, J.; Tang, F. Radioprotective Effect of Flavonoids on Ionizing Radiation-Induced Brain Damage. Molecules 2020, 25, 5719. [Google Scholar] [CrossRef]
- Moon, H.I.; Jeong, M.H.; Jo, W.S. Protective activity of C-geranylflavonoid analogs from Paulownia tomentosa against DNA damage in 137Cs irradiated AHH-1 cells. Nat. Prod. Commun. 2014, 9, 1295–1298. [Google Scholar]
- Landauer, M.R.; Harvey, A.J.; Kaytor, M.D.; Day, R.M. Mechanism and therapeutic window of a genistein nanosuspension to protect against hematopoietic-acute radiation syndrome. J. Radiat. Res. 2019, 60, 308–317. [Google Scholar] [CrossRef]
- Gvilava, I.; Ormotsadze, G.; Chkhikvishvili, I.; Giorgobiani, M.; Kipiani, N.V.; Sanikidze, T. Radioprotective Activity of Polymetoxy-Lated Flavonoids of Citrus Extract. Georgian Med. News 2018, 285, 119–124. [Google Scholar]
- Ghasemi, S.; Lorigooini, Z. A review of significant molecular mechanisms of flavonoids in prevention of prostate cancer. J. Chem. Pharm. Sci. 2016, 9, 3388–3394. [Google Scholar]
- Hamadeh, I.S.; Patel, J.N.; Rusin, S.; Tan, A.R. Personalizing aromatase inhibitor therapy in patients with breast cancer. Cancer Treat. Rev. 2018, 70, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.I.; Yu, J.S.; Zhang, Y.; Yoo, H.H. Evaluating flavonoids as potential aromatase inhibitors for breast cancer treatment: In vitro studies and in silico predictions. Chem. Biol. Interact. 2024, 392, 110927. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2020, 11, 592654. [Google Scholar] [CrossRef]
- Habli, Z.; Toumieh, G.; Fatfat, M.; Rahal, O.N.; Gali-Muhtasib, H. Emerging Cytotoxic Alkaloids in the Battle against Cancer: Overview of Molecular Mechanisms. Molecules 2017, 22, 250. [Google Scholar] [CrossRef]
- Lu, J.J.; Bao, J.L.; Chen, X.P.; Huang, M.; Wang, Y.T. Alkaloids isolated from natural herbs as the anticancer agents. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 485042. [Google Scholar] [CrossRef]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharmacother. 2018, 103, 1643–1651. [Google Scholar] [CrossRef]
- Osawa, Y.; Tochigi, B.; Tochigi, M.; Ohnishi, S.; Watanabe, Y.; Bullion, K.; Osawa, G.; Nakabayashi, Y.; Yarborough, C. Aromatase inhibitors in cigarette smoke, tobacco leaves and other plants. J. Enzym. Inhib. 1990, 4, 187–200. [Google Scholar] [CrossRef]
- Kadohama, N.; Shintani, K.; Osawa, Y. Tobacco alkaloid derivatives as inhibitors of breast cancer aromatase. Cancer Lett. 1993, 75, 175–182. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Basly, J.P.; Lavier, M.C. Dietary phytoestrogens: Potential selective estrogen enzyme modulators? Planta Med. 2005, 71, 287–294. [Google Scholar] [CrossRef]
- Seralini, G.; Moslemi, S. Aromatase inhibitors: Past, present and future. Mol. Cell. Endocrinol. 2001, 178, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, N.; Joshi, S.C.; Ahotupa, M.; Li, X.; Ammala, J.; Makela, S.; Santti, R. No evidence for the in vivo activity of aromatase-inhibiting flavonoids. J. Steroid Biochem. Mol. Biol. 2001, 78, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Goodin, M.G.; Rosengren, R.J. Epigallocatechin gallate modulates CYP450 isoforms in the female Swiss-Webster mouse. Toxicol. Sci. 2003, 76, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X. A Comprehensive Review of HER2 in Cancer Biology and Therapeutics. Genes 2024, 15, 903. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.L.; Schillaci, R. Emerging Targeted Therapies for HER2-Positive Breast Cancer. Cancers 2023, 15, 1987. [Google Scholar] [CrossRef]
- Stanowicka-Grada, M.; Senkus, E. Anti-HER2 Drugs for the Treatment of Advanced HER2 Positive Breast Cancer. Curr. Treat. Options Oncol. 2023, 24, 1633–1650. [Google Scholar] [CrossRef]
- Zimmerman, B.S.; Esteva, F.J. Next-Generation HER2-Targeted Antibody–Drug Conjugates in Breast Cancer. Cancers 2024, 16, 800. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, X.; Tai, H.; Zhong, X.; Luo, T.; Zheng, H. HER2-targeted therapies in cancer: A systematic review. Biomark. Res. 2024, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Kamta, J.; Chaar, M.; Ande, A.; Altomare, D.A.; Ait-Oudhia, S. Advancing Cancer Therapy with Present and Emerging Immuno-Oncology Approaches. Front. Oncol. 2017, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Moussavou, G.; Ko, K.; Lee, J.H.; Choo, Y.K. Production of monoclonal antibodies in plants for cancer immunotherapy. Biomed. Res. Int. 2015, 2015, 306164. [Google Scholar] [CrossRef] [PubMed]
- Hefferon, K. Reconceptualizing cancer immunotherapy based on plant production systems. Future Sci. OA 2017, 3, FSO217. [Google Scholar] [CrossRef]
- Park, C.; Kim, K.; Kim, Y.; Zhu, R.; Hain, L.; Seferovic, H.; Kim, M.H.; Woo, H.J.; Hwang, H.; Lee, S.H.; et al. Plant-Derived Anti-Human Epidermal Growth Factor Receptor 2 Antibody Suppresses Trastuzumab-Resistant Breast Cancer with Enhanced Nanoscale Binding. ACS Nano 2024, 18, 16126–16140. [Google Scholar] [CrossRef]
- Bulaon, C.J.I.; Khorattanakulchai, N.; Rattanapisit, K.; Sun, H.; Pisuttinusart, N.; Strasser, R.; Tanaka, S.; Soon-Shiong, P.; Phoolcharoen, W. Antitumor effect of plant-produced anti-CTLA-4 monoclonal antibody in a murine model of colon cancer. Front. Plant Sci. 2023, 14, 1149455. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Phakham, T.; Bulaon, C.J.I.; Khorattanakulchai, N.; Shanmugaraj, B.; Buranapraditkun, S.; Boonkrai, C.; Sooksai, S.; Hirankarn, N.; Abe, Y.; Strasser, R.; et al. Functional Characterization of Pembrolizumab Produced in Nicotiana benthamiana Using a Rapid Transient Expression System. Front. Plant Sci. 2021, 12, 736299. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Phakham, T.; Buranapraditkun, S.; Siriwattananon, K.; Boonkrai, C.; Pisitkun, T.; Hirankarn, N.; Strasser, R.; Abe, Y.; Phoolcharoen, W. Structural and In Vitro Functional Analyses of Novel Plant-Produced Anti-Human PD1 Antibody. Sci. Rep. 2019, 9, 15205. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Bulaon, C.J.I.; Strasser, R.; Sun, H.; Phoolcharoen, W. In vitro and in vivo studies of plant-produced Atezolizumab as a potential immunotherapeutic antibody. Sci. Rep. 2023, 13, 14146. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat. Med. 2013, 19, 1381–1388. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- McLornan, D.P.; List, A.; Mufti, G.J. Applying synthetic lethality for the selective targeting of cancer. N. Engl. J. Med. 2014, 371, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Branco, C.; Paredes, J. PARP Inhibitors: From the Mechanism of Action to Clinical Practice. Acta Med. Port. 2022, 35, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive, relapsed serous ovarian cancer and a BRCA mutation: Overall survival adjusted for postprogression poly(adenosine diphosphate ribose) polymerase inhibitor therapy. Cancer 2016, 122, 1844–1852. [Google Scholar] [CrossRef]
- Mirza, M.R.; Pignata, S.; Ledermann, J.A. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann. Oncol. 2018, 29, 1366–1376. [Google Scholar] [CrossRef]
- Livraghi, L.; Garber, J.E. PARP inhibitors in the management of breast cancer: Current data and future prospects. BMC Med. 2015, 13, 188. [Google Scholar] [CrossRef]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef]
- Konecny, G.E.; Kristeleit, R.S. PARP inhibitors for BRCA1/2-mutated and sporadic ovarian cancer: Current practice and future directions. Br. J. Cancer 2016, 115, 1157–1173. [Google Scholar] [CrossRef]
- Yi, M.; Dong, B.; Qin, S.; Chu, Q.; Wu, K.; Luo, S. Advances and perspectives of PARP inhibitors. Exp. Hematol. Oncol. 2019, 8, 29. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- McCann, K.E. Advances in the use of PARP inhibitors for BRCA1/2-associated breast cancer: Talazoparib. Future Oncol. 2019, 15, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.J.; Koolen, S.L.W.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Buijs, P.R.; Verhagen, J.H.; van Eijck, C.H.; van den Hoogen, B.G. Oncolytic viruses: From bench to bedside with a focus on safety. Hum. Vaccin. Immunother. 2015, 11, 1573–1584. [Google Scholar] [CrossRef]
- Shukla, S.; Hu, H.; Cai, H.; Chan, S.K.; Boone, C.E.; Beiss, V.; Chariou, P.L.; Steinmetz, N.F. Plant Viruses and Bacteriophage-Based Reagents for Diagnosis and Therapy. Annu. Rev. Virol. 2020, 7, 559–587. [Google Scholar] [CrossRef]
- Steele, J.F.C.; Peyret, H.; Saunders, K.; Castells-Graells, R.; Marsian, J.; Meshcheriakova, Y.; Lomonossoff, G.P. Synthetic plant virology for nanobiotechnology and nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1447. [Google Scholar] [CrossRef]
- Shahgolzari, M.; Pazhouhandeh, M.; Milani, M.; Yari Khosroushahi, A.; Fiering, S. Plant viral nanoparticles for packaging and in vivo delivery of bioactive cargos. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1629. [Google Scholar] [CrossRef] [PubMed]
- Masarapu, H.; Patel, B.K.; Chariou, P.L.; Hu, H.; Gulati, N.M.; Carpenter, B.L.; Ghiladi, R.A.; Shukla, S.; Steinmetz, N.F. Physalis Mottle Virus-Like Particles as Nanocarriers for Imaging Reagents and Drugs. Biomacromolecules 2017, 18, 4141–4153. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Lee, A.S.; Kim, S.M.; Heo, H.R.; Kim, C.S. Virus-like nanoparticles as a theranostic platform for cancer. Front. Bioeng. Biotechnol. 2022, 10, 1106767. [Google Scholar] [CrossRef]
- Cai, H.; Shukla, S.; Steinmetz, N.F. The Antitumor Efficacy of CpG Oligonucleotides is Improved by Encapsulation in Plant Virus-Like Particles. Adv. Funct. Mater. 2020, 30, 1908743. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Vault, viral, and virus-like nanoparticles for targeted cancer therapy. Mater. Adv. 2023, 4, 2909–2917. [Google Scholar] [CrossRef]
- Chan, S.K.; Steinmetz, N.F. microRNA-181a silencing by antisense oligonucleotides delivered by virus-like particles. J. Mater. Chem. B 2023, 11, 816–825. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Santos, C.; Ribeiro, V.; Silva, A.; Lopes, L.; Machado, J.P. Mental health benefits of traditional Chinese medicine—An umbrella review of meta-analyses. Brain Behav. Immun. Integr. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Health, U.D.o.; Services, H. Thinking About Complementary and Alternative Medicine: A Guide for People with Cancer. NIH Publication: Bethesda, MD, USA, 2005; pp. 1–20. [Google Scholar]
- Complementary Therapy. Available online: https://www.breastcancer.org/treatment/complementary-therapy (accessed on 15 December 2024).
- Che, C.-T.; George, V.; Ijinu, T.; Pushpangadan, P.; Andrae-Marobela, K. Traditional medicine. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 11–28. [Google Scholar]
- Micke, O.; Bruns, F.; Glatzel, M.; Schönekaes, K.; Micke, P.; Mücke, R.; Büntzel, J. Predictive factors for the use of complementary and alternative medicine (CAM) in radiation oncology. Eur. J. Integr. Med. 2009, 1, 19–25. [Google Scholar] [CrossRef]
- Horneber, M.; Bueschel, G.; Dennert, G.; Less, D.; Ritter, E.; Zwahlen, M. How many cancer patients use complementary and alternative medicine: A systematic review and metaanalysis. Integr. Cancer Ther. 2012, 11, 187–203. [Google Scholar] [CrossRef]
- Eschiti, V.S. Lesson from comparison of CAM use by women with female-specific cancers to others: It’s time to focus on interaction risks with CAM therapies. Integr. Cancer Ther. 2007, 6, 313–344. [Google Scholar] [CrossRef]
- Gansler, T.; Kaw, C.; Crammer, C.; Smith, T. A population-based study of prevalence of complementary methods use by cancer survivors: A report from the American Cancer Society’s studies of cancer survivors. Cancer 2008, 113, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Wanchai, A.; Armer, J.M.; Stewart, B.R. Complementary and alternative medicine use among women with breast cancer: A systematic review. Clin. J. Oncol. Nurs. 2010, 14, E45–E55. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Carvalho, C.; Bispo, R. Use of complementary and alternative medicine in a sample of women with breast cancer. Sage Open 2013, 3, 2158244013502497. [Google Scholar] [CrossRef]
- Astin, J.A.; Reilly, C.; Perkins, C.; Child, W.L.; Susan, G.K.B.C.F. Breast cancer patients’ perspectives on and use of complementary and alternative medicine: A study by the Susan, G. Komen Breast Cancer Foundation. J. Soc. Integr. Oncol. 2006, 4, 157–169. [Google Scholar] [CrossRef]
- Dobos, G.J.; Voiss, P.; Schwidde, I.; Choi, K.E.; Paul, A.; Kirschbaum, B.; Saha, F.J.; Kuemmel, S. Integrative oncology for breast cancer patients: Introduction of an expert-based model. BMC Cancer 2012, 12, 539. [Google Scholar] [CrossRef]
- Dobos, G.; Tao, I. The model of Western integrative medicine: The role of Chinese medicine. Chin. J. Integr. Med. 2011, 17, 11–20. [Google Scholar] [CrossRef]
- Tung, N. What is the optimal endocrine therapy for postmenopausal women with hormone receptor-positive early breast cancer? J. Clin. Oncol. 2013, 31, 1391–1397. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising Nanocarriers to Enhance Solubility and Bioavailability of Cannabidiol for a Plethora of Therapeutic Opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Paulus, V.; Billieux, J.; Benyamina, A.; Karila, L. Cannabidiol in the context of substance use disorder treatment: A systematic review. Addict. Behav. 2022, 132, 107360. [Google Scholar] [CrossRef] [PubMed]
- Sivesind, T.E.; Maghfour, J.; Rietcheck, H.; Kamel, K.; Malik, A.S.; Dellavalle, R.P. Cannabinoids for the Treatment of Dermatologic Conditions. JID Innov. 2022, 2, 100095. [Google Scholar] [CrossRef] [PubMed]
- Borm, K.J.; Schiller, K.; Asadpour, R.; Combs, S.E. Complementary and Alternative Medicine in Radiotherapy: A Comprehensive Review. Top. Magn. Reson. Imaging 2020, 29, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.E.; Cooper, K.L.; Relton, C.; Thomas, K.J. Prevalence of complementary and alternative medicine (CAM) use by the general population: A systematic review and update. Int. J. Clin. Pract. 2012, 66, 924–939. [Google Scholar] [CrossRef]
- Johnson, S.B.; Park, H.S.; Gross, C.P.; Yu, J.B. Complementary Medicine, Refusal of Conventional Cancer Therapy, and Survival Among Patients wsith Curable Cancers. JAMA Oncol. 2018, 4, 1375–1381. [Google Scholar] [CrossRef]
- Scaria, B.; Sood, S.; Raad, C.; Khanafer, J.; Jayachandiran, R.; Pupulin, A.; Grewal, S.; Okoko, M.; Arora, M.; Miles, L.; et al. Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends. Int. J. Mol. Sci. 2020, 21, 8480. [Google Scholar] [CrossRef]
- World Health Organization. WHO International Standard Terminologies on Traditional Chinese Medicine, Geneva. World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Kong, Y.; Zhu, X.; Zhang, X.; Li, Z.; Yin, Y.; Wang, J.; Jia, H. Traditional Chinese medicine combined with radiofrequency ablation improves primary liver cancer outcomes: A systematic review with meta-analysis. Heliyon 2023, 9, e18591. [Google Scholar] [CrossRef]
- Ma, H.; Carpenter, C.L.; Sullivan-Halley, J.; Bernstein, L. The roles of herbal remedies in survival and quality of life among long-term breast cancer survivors--results of a prospective study. BMC Cancer 2011, 11, 222. [Google Scholar] [CrossRef]
- Wargovich, M.J.; Woods, C.; Hollis, D.M.; Zander, M.E. Herbals, cancer prevention and health. J. Nutr. 2001, 131, 3034S–3036S. [Google Scholar] [CrossRef]
- He, Y.C.; He, L.; Khoshaba, R.; Lu, F.G.; Cai, C.; Zhou, F.L.; Liao, D.F.; Cao, D. Curcumin Nicotinate Selectively Induces Cancer Cell Apoptosis and Cycle Arrest through a P53-Mediated Mechanism. Molecules 2019, 24, 4179. [Google Scholar] [CrossRef]
- Pukhov, S.A.; Semakov, A.V.; Globa, A.A.; Anikina, L.V.; Afanasyeva, S.V.; Yandulova, E.Y.; Aleksandrova, Y.R.; Neganova, M.E.; Klochkov, S.G. New Conjugates of Daunorubicin with Sesquiterpene Lactones and Their Biological Activity. ChemistrySelect 2021, 6, 8446–8451. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, J.H.; Seo, D.; Gavaachimed, L.; Choi, J.; Park, S.; Min, N.Y.; Lee, D.H.; Bang, H.W.; Ham, S.W.; et al. EGCG-induced selective death of cancer cells through autophagy-dependent regulation of the p62-mediated antioxidant survival pathway. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119659. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.E.; Aleksandrova, Y.R.; Sharova, E.V.; Smirnova, E.V.; Artyushin, O.I.; Nikolaeva, N.S.; Semakov, A.V.; Schagina, I.A.; Akylbekov, N.; Kurmanbayev, R.; et al. Conjugates of 3,5-Bis(arylidene)-4-piperidone and Sesquiterpene Lactones Have an Antitumor Effect via Resetting the Metabolic Phenotype of Cancer Cells. Molecules 2024, 29, 2765. [Google Scholar] [CrossRef] [PubMed]
- Omogbadegun, Z.O. Medicinal plants-based foods for breast cancer treatment: An ethnobotanical survey and digitization. Int. J. Med. Plants Altern. Med. 2013, 1, 137–163. [Google Scholar]
- Nagykalnai, T.; Landherr, L.; Nagy, A.C. Vitamin D and breast cancer. Orv. Hetil. 2014, 155, 1091–1096. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Hashemi, M.; Mirzaei, S.; Barati, M.; Hejazi, E.S.; Kakavand, A.; Entezari, M.; Salimimoghadam, S.; Kalbasi, A.; Rashidi, M.; Taheriazam, A.; et al. Curcumin in the treatment of urological cancers: Therapeutic targets, challenges and prospects. Life Sci. 2022, 309, 120984. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef]
- Ye, Y.; Yan, J.; Cui, J.; Mao, S.; Li, M.; Liao, X.; Tong, H. Dynamic changes in amino acids, catechins, caffeine and gallic acid in green tea during withering. J. Food Compos. Anal. 2018, 66, 98–108. [Google Scholar] [CrossRef]
- Toden, S.; Tran, H.M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef]
- Palatty, P.L.; Azmidah, A.; Rao, S.; Jayachander, D.; Thilakchand, K.R.; Rai, M.P.; Haniadka, R.; Simon, P.; Ravi, R.; Jimmy, R.; et al. Topical application of a sandal wood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients undergoing external beam radiotherapy: A pilot study. Br. J. Radiol. 2014, 87, 20130490. [Google Scholar] [CrossRef]
- Han, Y.; Pei, D.; Li, W.; Luo, B.; Jiang, Q. Epigallocatechin gallate attenuates tumor necrosis factor (TNF)-alpha-induced inhibition of osteoblastic differentiation by up-regulating lncRNA TUG1 in osteoporosis. Bioengineered 2022, 13, 8950–8961. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, M.; Rahmani, S.; Zendehdel, M.; Hosseini, S.A.; Zare Javid, A. Ginseng and Cancer-Related Fatigue: A Systematic Review of Clinical Trials. Nutr. Cancer 2021, 73, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shen, T.; Kim, H.G.; Hu, W.; Cho, J.Y. 20(S)-Protopanaxadiol from Panax ginseng Induces Apoptosis and Autophagy in Gastric Cancer Cells by Inhibiting Src. Am. J. Chin. Med. 2023, 51, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Alesaeidi, S.; Miraj, S. A Systematic Review of Anti-malarial Properties, Immunosuppressive Properties, Anti-inflammatory Properties, and Anti-cancer Properties of Artemisia Annua. Electron. Physician 2016, 8, 3150–3155. [Google Scholar] [CrossRef]
- Albaqami, J.J.; Benny, T.P.; Hamdi, H.; Altemimi, A.B.; Kuttithodi, A.M.; Job, J.T.; Sasidharan, A.; Narayanankutty, A. Phytochemical Composition and In Vitro Antioxidant, Anti-Inflammatory, Anticancer, and Enzyme-Inhibitory Activities of Artemisia nilagirica (C.B. Clarke) Pamp. Molecules 2022, 27, 7119. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, K.; Wang, M.; Qiu, F. Casticin and chrysosplenol D from Artemisia annua L. induce apoptosis by inhibiting topoisomerase IIalpha in human non-small-cell lung cancer cells. Phytomedicine 2022, 100, 154095. [Google Scholar] [CrossRef]
- Terasaki, M.; Kubota, A.; Kojima, H.; Maeda, H.; Miyashita, K.; Kawagoe, C.; Mutoh, M.; Tanaka, T. Fucoxanthin and Colorectal Cancer Prevention. Cancers 2021, 13, 2379. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, W.; Huang, J.; Xie, Y.; Ma, W. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chin. Med. 2016, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Sohretoglu, D.; Huang, S. Ganoderma lucidum Polysaccharides as An Anti-cancer Agent. Anticancer. Agents Med. Chem. 2018, 18, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Emadi, S.A.; Ghasemzadeh Rahbardar, M.; Mehri, S.; Hosseinzadeh, H. A review of therapeutic potentials of milk thistle (Silybum marianum L.) and its main constituent, silymarin, on cancer, and their related patents. Iran. J. Basic. Med. Sci. 2022, 25, 1166–1176. [Google Scholar] [CrossRef]
- Bangroo, A.; Malhotra, A.; Sharma, U.; Jain, A.; Kaur, A. Biosynthesis of Zinc Oxide Nanoparticles Using Catharanthus Roseus Leaves and Their Therapeutic Response in Breast Cancer (MDA-MB-231) Cells. Nutr. Cancer 2022, 74, 1489–1496. [Google Scholar] [CrossRef]
- Hafuth, S.; Randhawa, S. Investigating the Anti-Cancer Properties of 6-Shogaol in Zingiber officinale. Crit. Rev. Oncog. 2022, 27, 15–22. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Thiruvengadam, M.; Imran, M.; Olatunde, A.; Shariati, M.A.; Bawazeer, S.; Naz, S.; Shirooie, S.; Sanches-Silva, A.; et al. Garlic (Allium sativum L.): Its Chemistry, Nutritional Composition, Toxicity, and Anticancer Properties. Curr. Top. Med. Chem. 2022, 22, 957–972. [Google Scholar] [CrossRef]
- Yosri, N.; Kamal, N.; Mediani, A.; AbouZid, S.; Swillam, A.; Swilam, M.; Ayyat, A.M.; Jantan, I. Immunomodulatory Activity and Inhibitory Effects of Viscum album on Cancer Cells, Its Safety Profiles and Recent Nanotechnology Development. Planta Med. 2024, 90, 1059–1079. [Google Scholar] [CrossRef]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.Y.; Li, B.; Zhu, W.L.; Shi, J.Y.; Jia, Q.; Li, Y.M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017, 38, 157–167. [Google Scholar] [CrossRef]
- Konkimalla, V.B.; Efferth, T. Evidence-based Chinese medicine for cancer therapy. J. Ethnopharmacol. 2008, 116, 207–210. [Google Scholar] [CrossRef]
- McLay, J.S.; Stewart, D.; George, J.; Rore, C.; Heys, S.D. Complementary and alternative medicines use by Scottish women with breast cancer. What, why and the potential for drug interactions? Eur. J. Clin. Pharmacol. 2012, 68, 811–819. [Google Scholar] [CrossRef]
- Nyga, A.; Cheema, U.; Loizidou, M. 3D tumour models: Novel in vitro approaches to cancer studies. J. Cell Commun. Signal 2011, 5, 239–248. [Google Scholar] [CrossRef]
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Hehlgans, S.; Eke, I.; Storch, K.; Haase, M.; Baretton, G.B.; Cordes, N. Caveolin-1 mediated radioresistance of 3D grown pancreatic cancer cells. Radiother. Oncol. 2009, 92, 362–370. [Google Scholar] [CrossRef]
- do Amaral, J.B. Células MCF-7 Como Modelo 3D no Estudo de Câncer de Mama Humano. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2010. [Google Scholar]
- Storch, K.; Eke, I.; Borgmann, K.; Krause, M.; Richter, C.; Becker, K.; Schrock, E.; Cordes, N. Three-dimensional cell growth confers radioresistance by chromatin density modification. Cancer Res. 2010, 70, 3925–3934. [Google Scholar] [CrossRef]
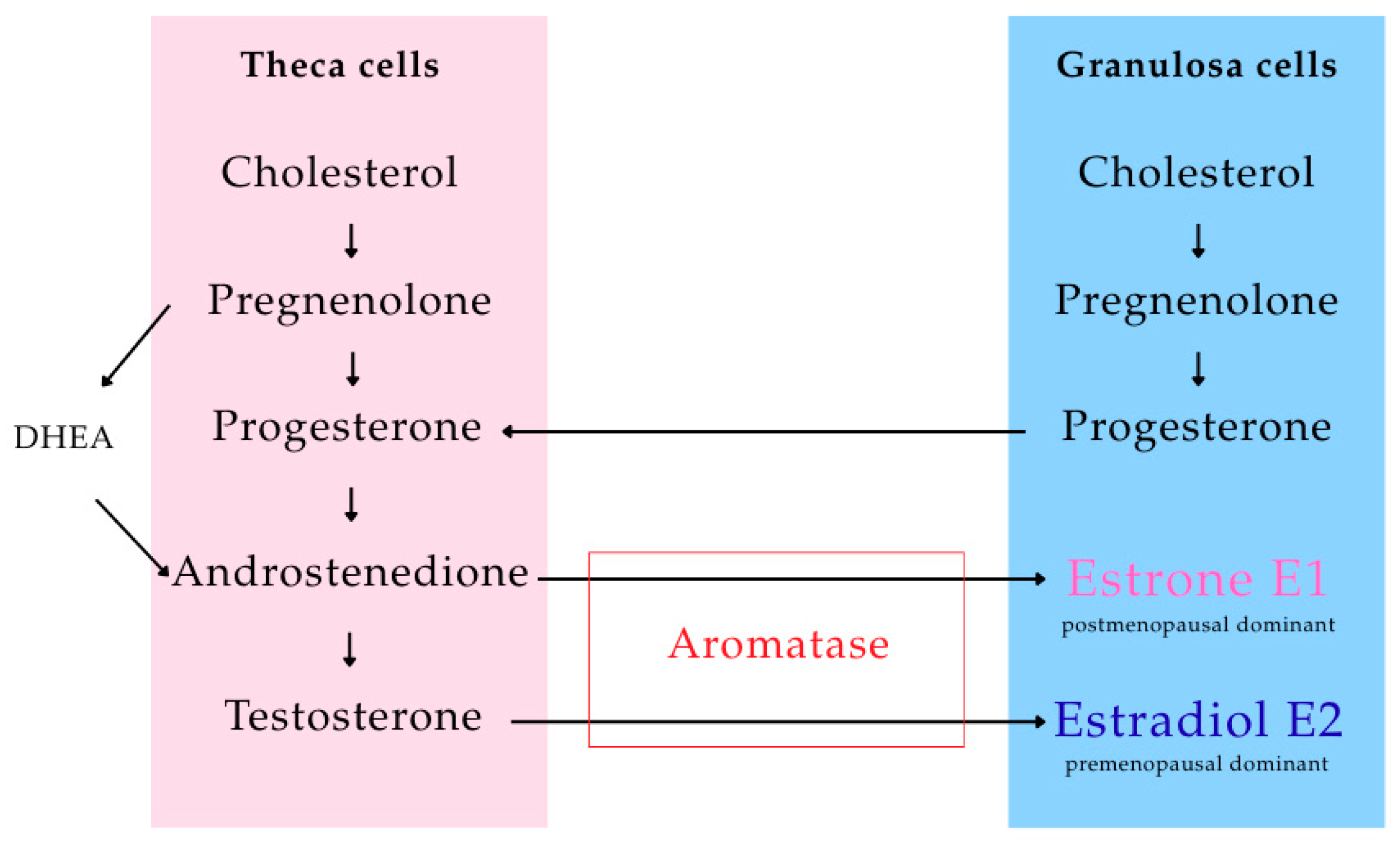

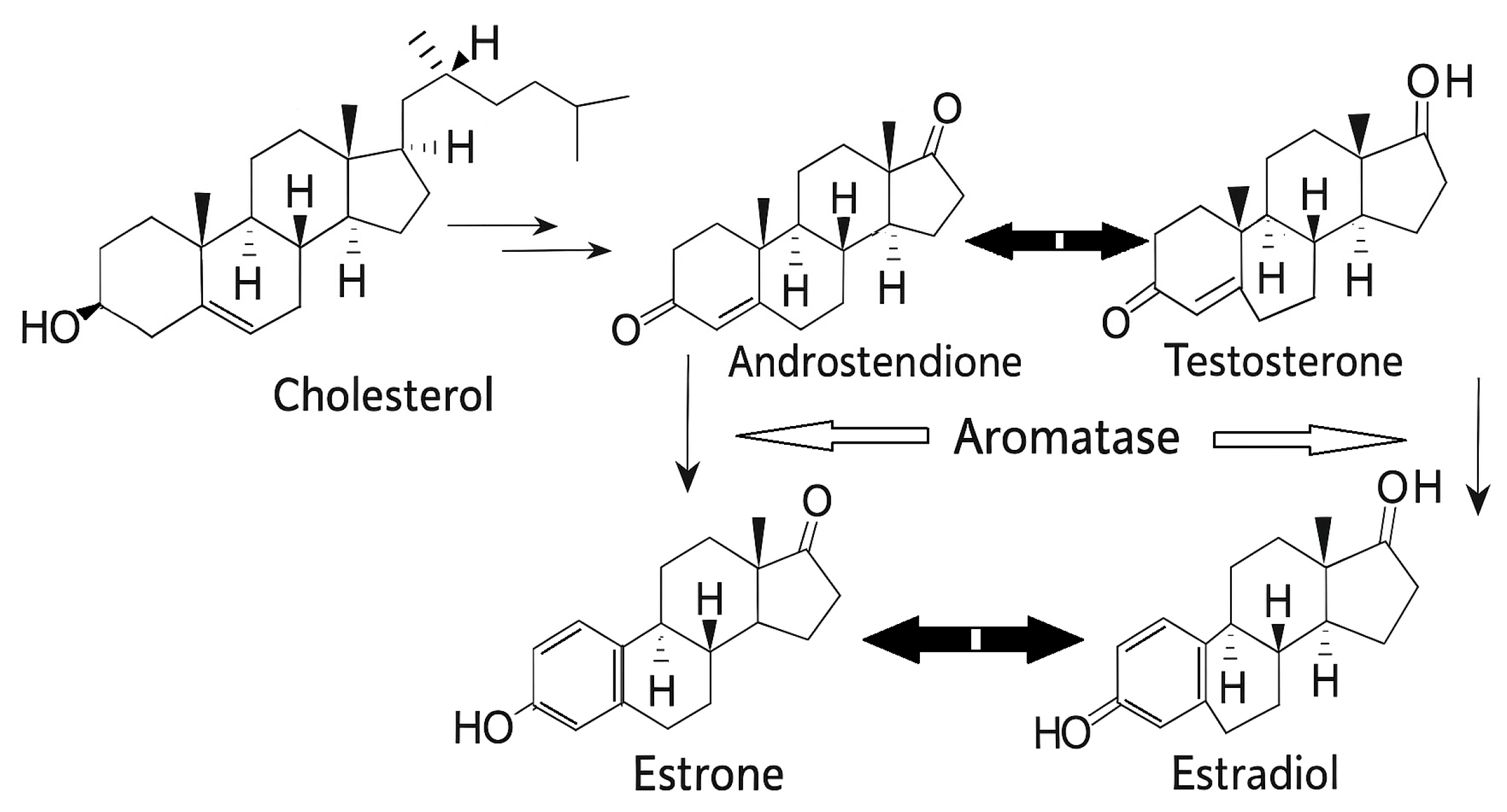
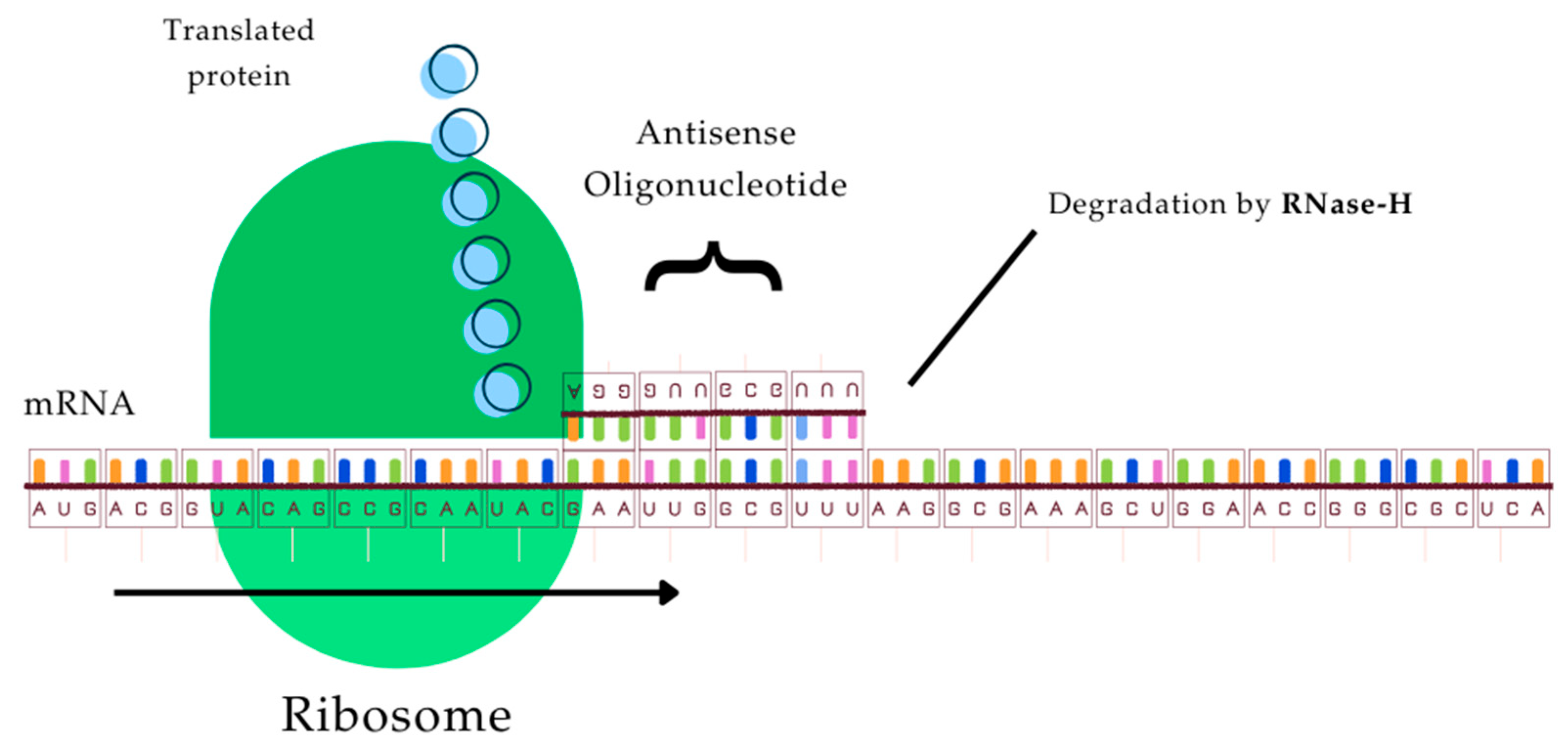
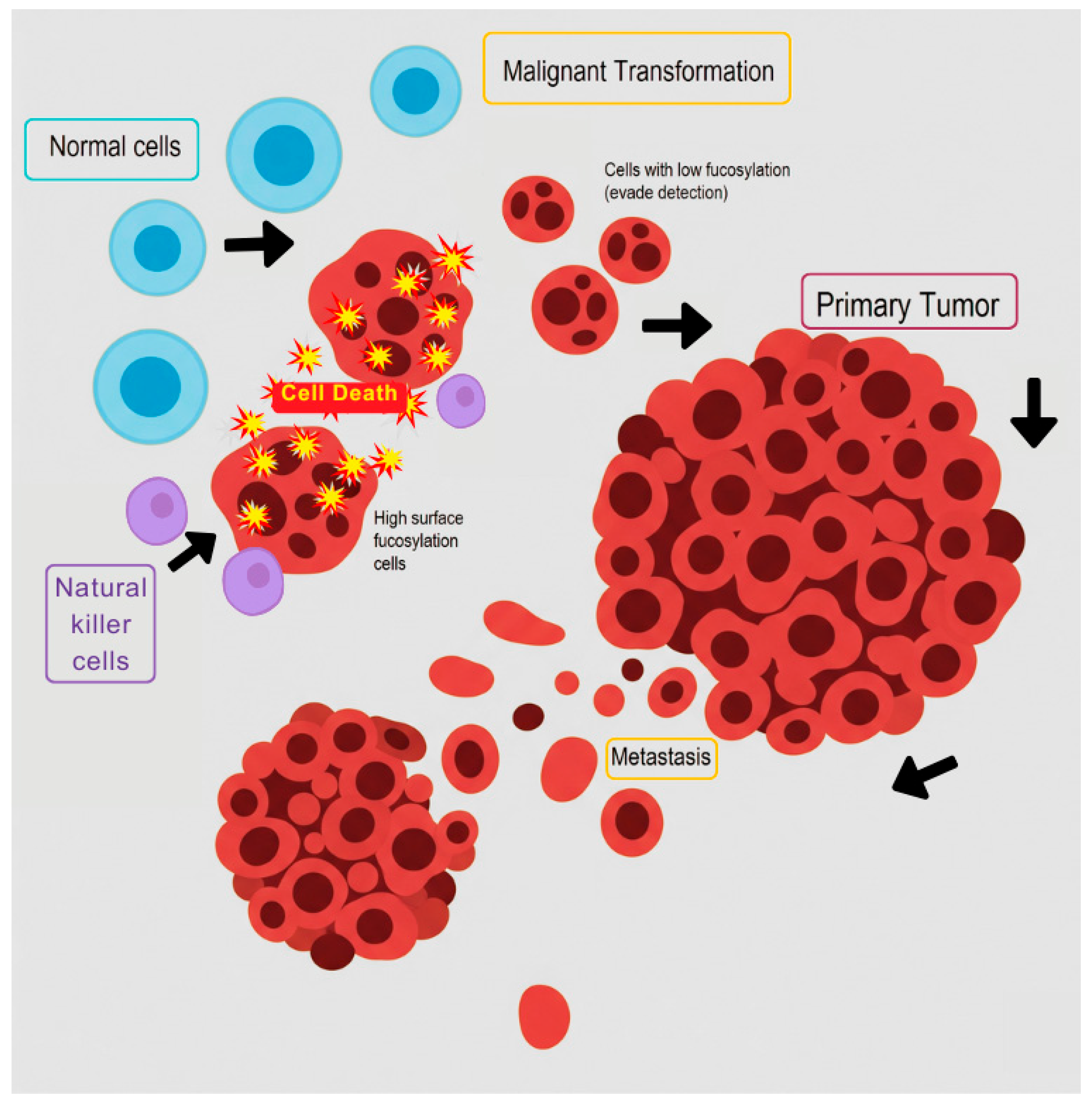
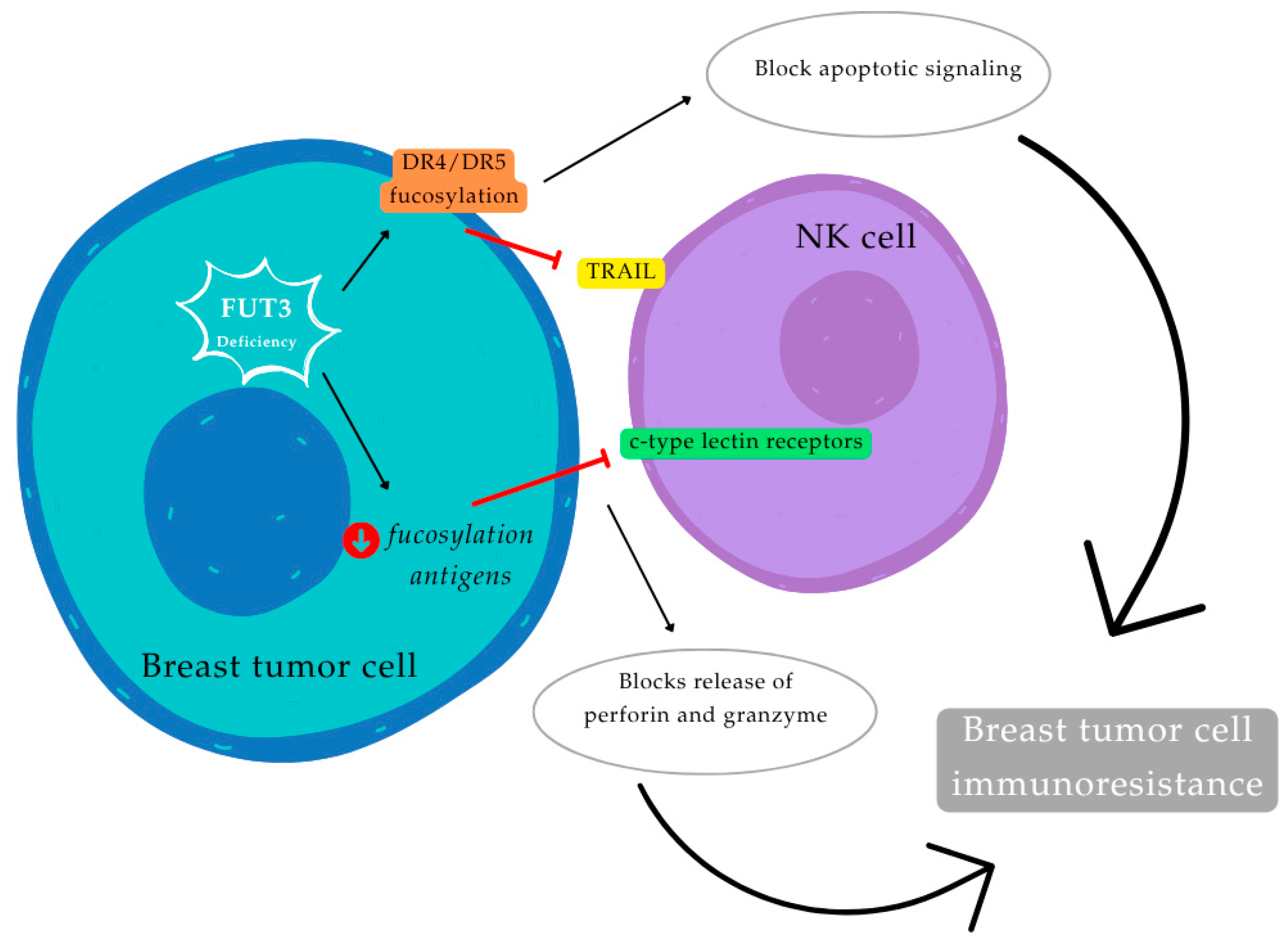
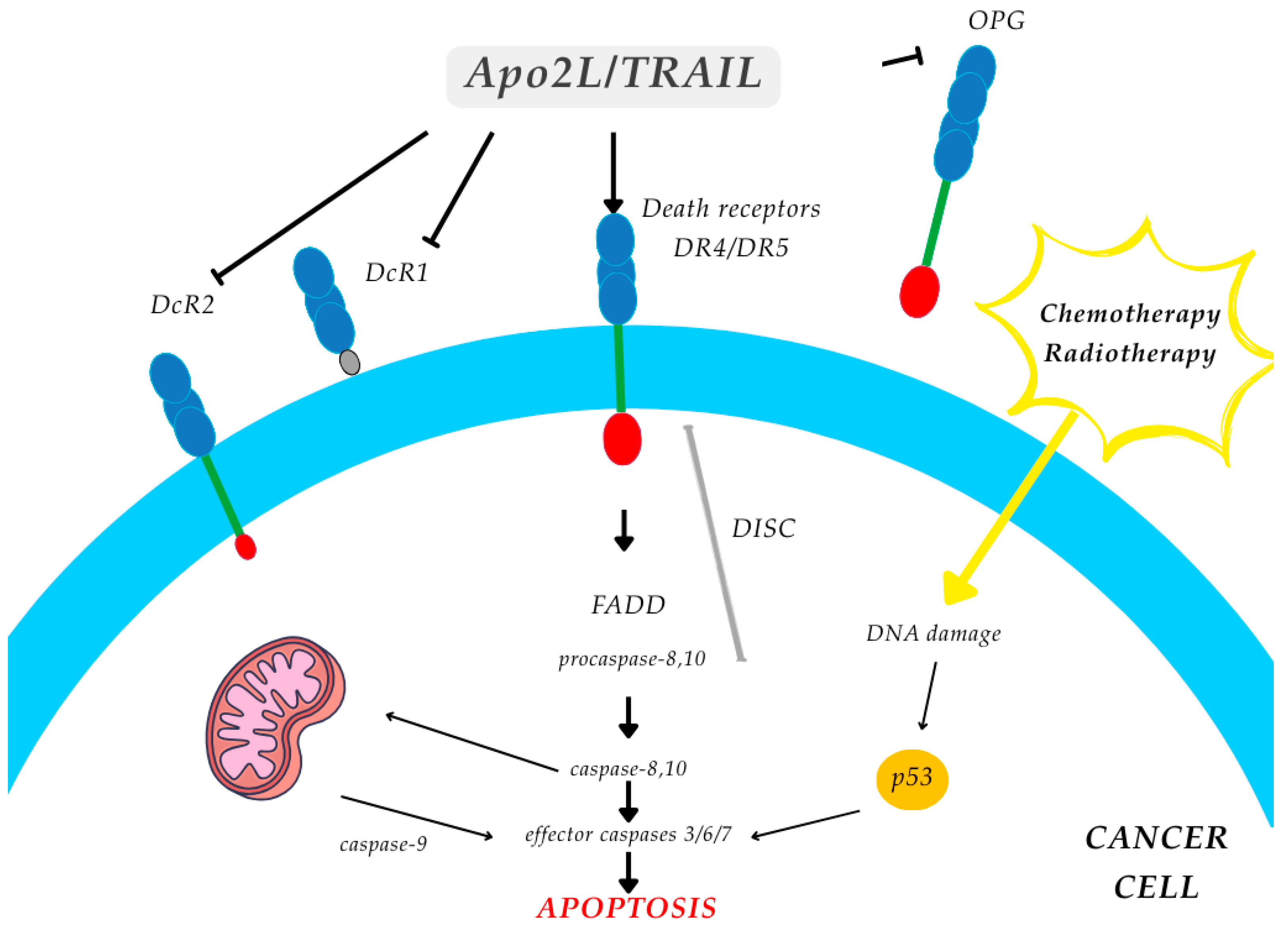
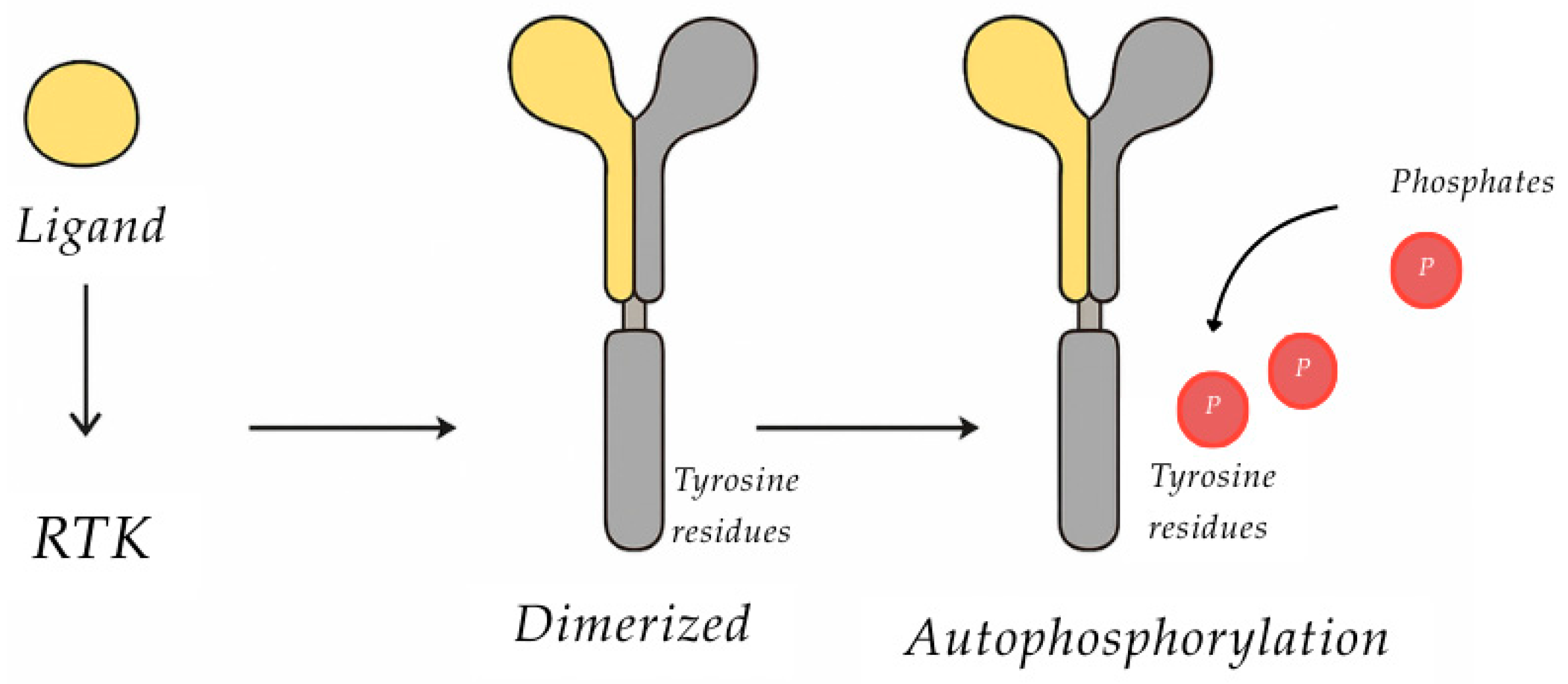
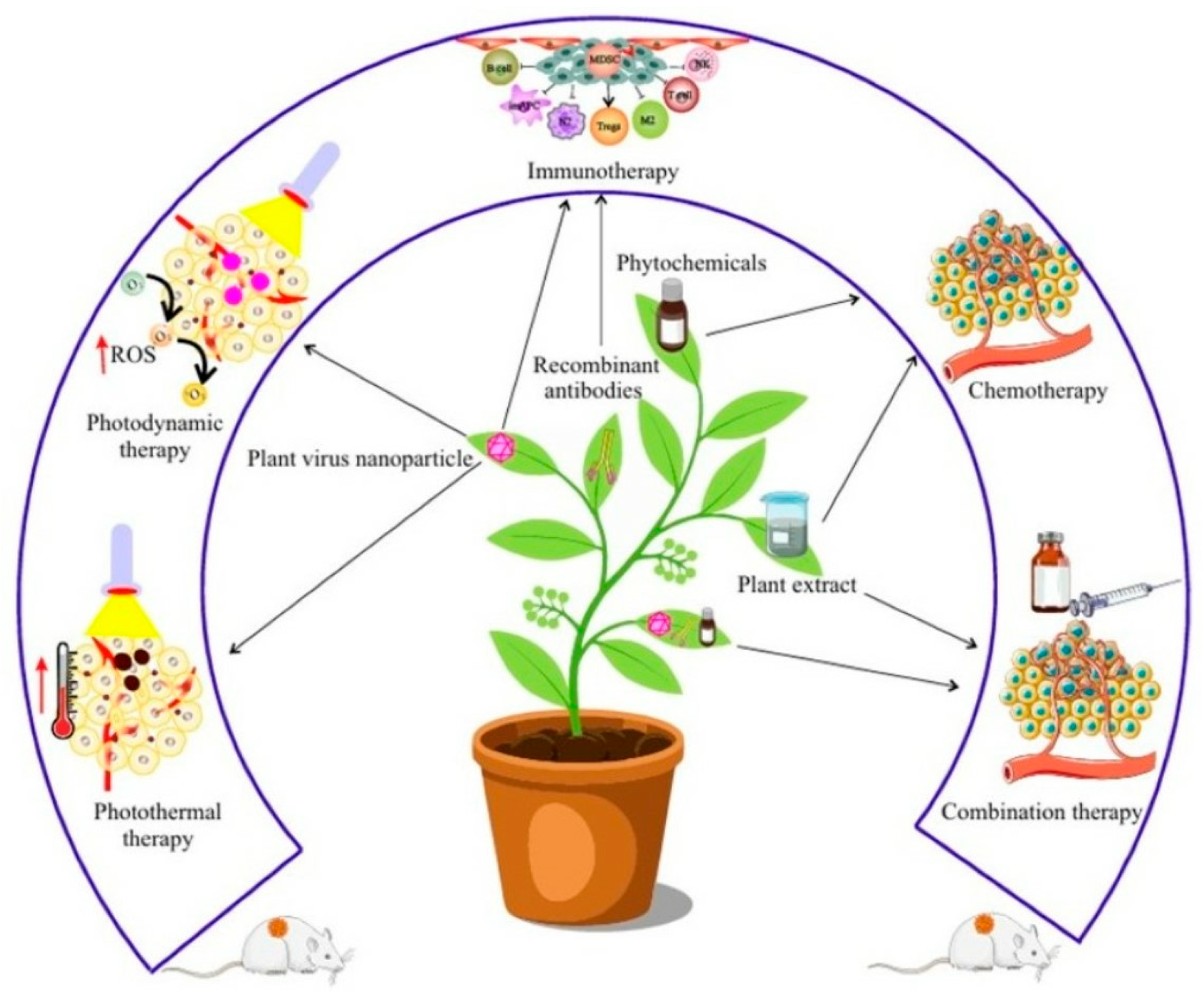
| Molecular Target | Main Mechanisms in Cancer Cells | Importance in Breast Cancer Treatment | References |
|---|---|---|---|
| Estrogens and estrogen receptors | Stimulation of cell proliferation through the ERα pathway, direct increases in rates of genetic mutations or effects on the DNA repair system. | Estrogen modulation. | [21,22,23,24] |
| Aromatase | Catalyzing the biosynthesis of estrogens (estrone and estradiol) from androgens (androstenedione and testosterone). | Aromatase inhibition. | [25,26] |
| ProCatepsin D/Catepsin D | Mitogen on cancer and stromal cells, stimulating their pro-invasive and pro-metastatic properties Facilitate cell growth at distant sites. Cleaves structural and functional proteins and peptides; inactivates chemokines such as CCL3, CCL4 and CCL21; and cleaves prolactin and osteopontin, modulating their functions. | cathepsin-D inhibition. | [27,28,29,30,31,32,33] |
| Urokinase Plasminogen Activator | Decrease cell adhesion and migration through both proteolytic and nonproteolytic mechanisms. Degrade most components of the extracellular medium directly or indirectly through activation of metalloproteinases, which subsequently degrade collagens and other matrix proteins. | Antisense oligonucleotides, antibodies, enzyme inhibitors, and recombinant and synthetic uPA and uPAR analogs. | [34,35,36] |
| Fucosiltransferase (FUT3) | Alteration of glycosylation pattern Adhesion to components of the extracellular matrix and to endothelial cells. | The expression of sLea in breast carcinoma is related to tumor stage, and higher levels were found in metastatic tumors. | [37,38] |
| Tyrosine Kinase Receptor | Control cell division and tumor growth by acting at the level of HER1 and HER2 receptors | Inhibition leads to the interruption of the cell cycle. | [39] |
| Deiodinase-type 3 enzyme | Responsible for the inactivation of thyroid hormones | Reduced expression is linked to worse prognosis. | [40,41] |
| Compound | Class | Source | Aromatase Activity |
|---|---|---|---|
| Chrysin | Flavone | Passionflower, honey | Strong (in vitro) |
| Apigenin | Flavone | Parsley, chamomile | Moderate |
| Genistein | Isoflavone | Soy | Moderate |
| Kaempferol | Flavonol | Tea, broccoli | Moderate |
| Naringenin | Flavanone | Citrus | Weak–moderate |
| Resveratrol | Stilbene | Grapes, wine | Moderate |
| Ellagic Acid | Tannin | Berries, nuts | Moderate |
| Gallic Acid | Phenolic acid | Tea, berries | Weak |
| Curcumin | Polyphenol | Turmeric | Moderate–strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, H.; Machado, J.; Alves, C.; Monteiro, M.-d.-C.; Cruz, A.; Pinho, C.; Soares, C.; Grosso, C.; Rodrigues, J.M.; Criado, M.B. A Narrative Review on Breast Cancer Treatment Supported by Focused and Systemic Phytotherapy. Nutraceuticals 2025, 5, 37. https://doi.org/10.3390/nutraceuticals5040037
Machado H, Machado J, Alves C, Monteiro M-d-C, Cruz A, Pinho C, Soares C, Grosso C, Rodrigues JM, Criado MB. A Narrative Review on Breast Cancer Treatment Supported by Focused and Systemic Phytotherapy. Nutraceuticals. 2025; 5(4):37. https://doi.org/10.3390/nutraceuticals5040037
Chicago/Turabian StyleMachado, Helena, Jorge Machado, Christian Alves, Maria-do-Céu Monteiro, Agostinho Cruz, Cláudia Pinho, Cristina Soares, Clara Grosso, Jorge Magalhães Rodrigues, and Maria Begoña Criado. 2025. "A Narrative Review on Breast Cancer Treatment Supported by Focused and Systemic Phytotherapy" Nutraceuticals 5, no. 4: 37. https://doi.org/10.3390/nutraceuticals5040037
APA StyleMachado, H., Machado, J., Alves, C., Monteiro, M.-d.-C., Cruz, A., Pinho, C., Soares, C., Grosso, C., Rodrigues, J. M., & Criado, M. B. (2025). A Narrative Review on Breast Cancer Treatment Supported by Focused and Systemic Phytotherapy. Nutraceuticals, 5(4), 37. https://doi.org/10.3390/nutraceuticals5040037










