Systematic Purification of Peptides with In Vitro Antioxidant, Antihyperglycemic, Anti-Obesity, and Antidiabetic Potential Released from Sesame Byproduct Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Extraction and Quantification
2.3. Preparation of Protein Hydrolysates of Albumins, Globulins, Prolamins, and Glutelins and Determination of the Degree of Hydrolysis
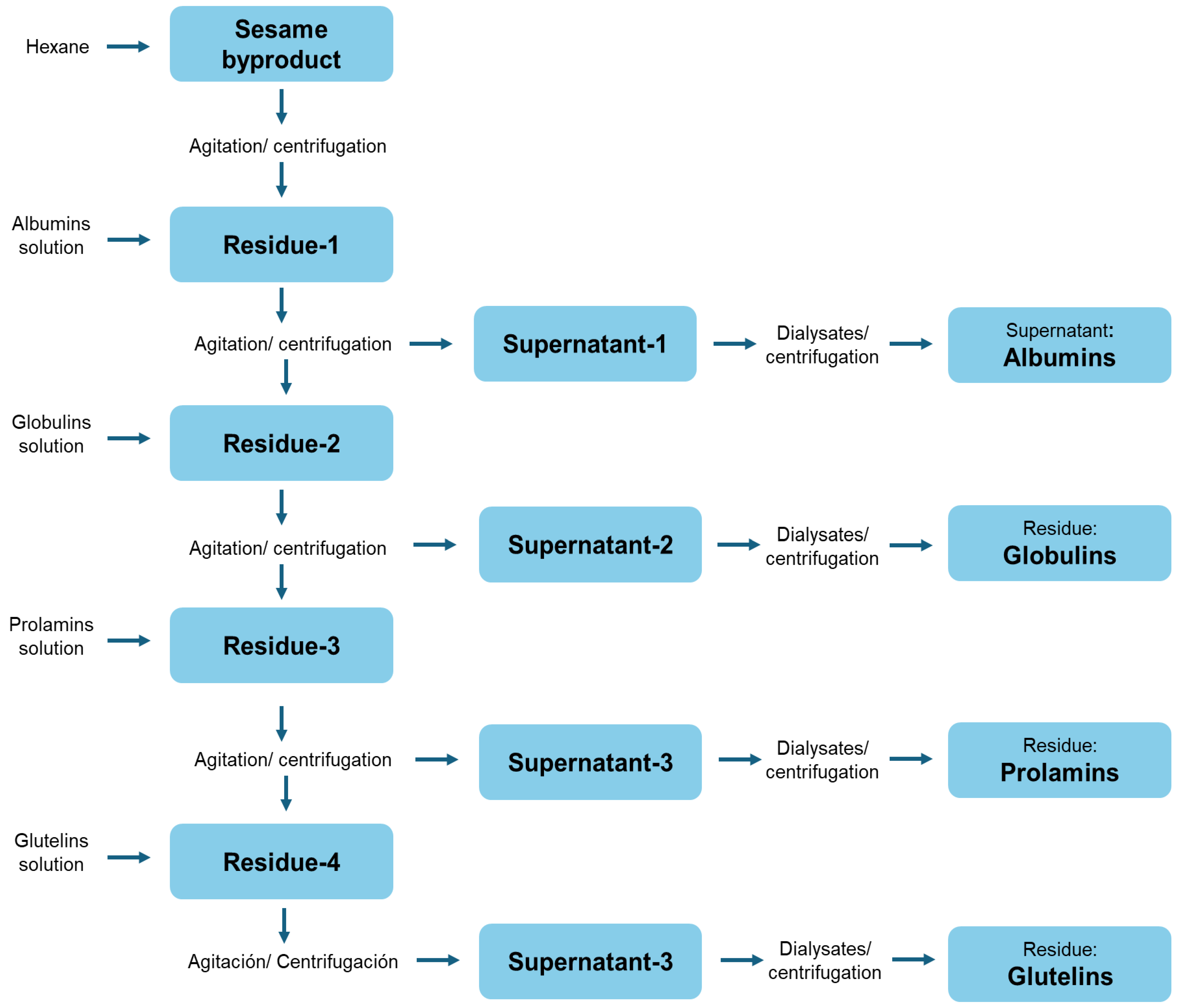
2.4. Preparation of Peptide Fractions
2.5. Determination of Total Protein Content, Protein Fractions, and Soluble Protein
2.6. Evaluation of Biological Activities
2.6.1. Determination of Antioxidant Activity
2.6.2. Determination of Antihyperglycemic Activity
2.6.3. Determination of Antidiabetic Activity
2.6.4. Determination of Anti-Obesity Activity
2.7. Identification and Purification of Peptides
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Hydrolysates and Peptide Fractions
3.1.1. Protein Content and Degree of Hydrolysis
3.1.2. Antioxidant Activity of Hydrolysates and Peptide Fractions
3.1.3. Antihyperglycemic Activity of Hydrolysates and Peptide Fractions
3.2. Characterization of Purified Peptides
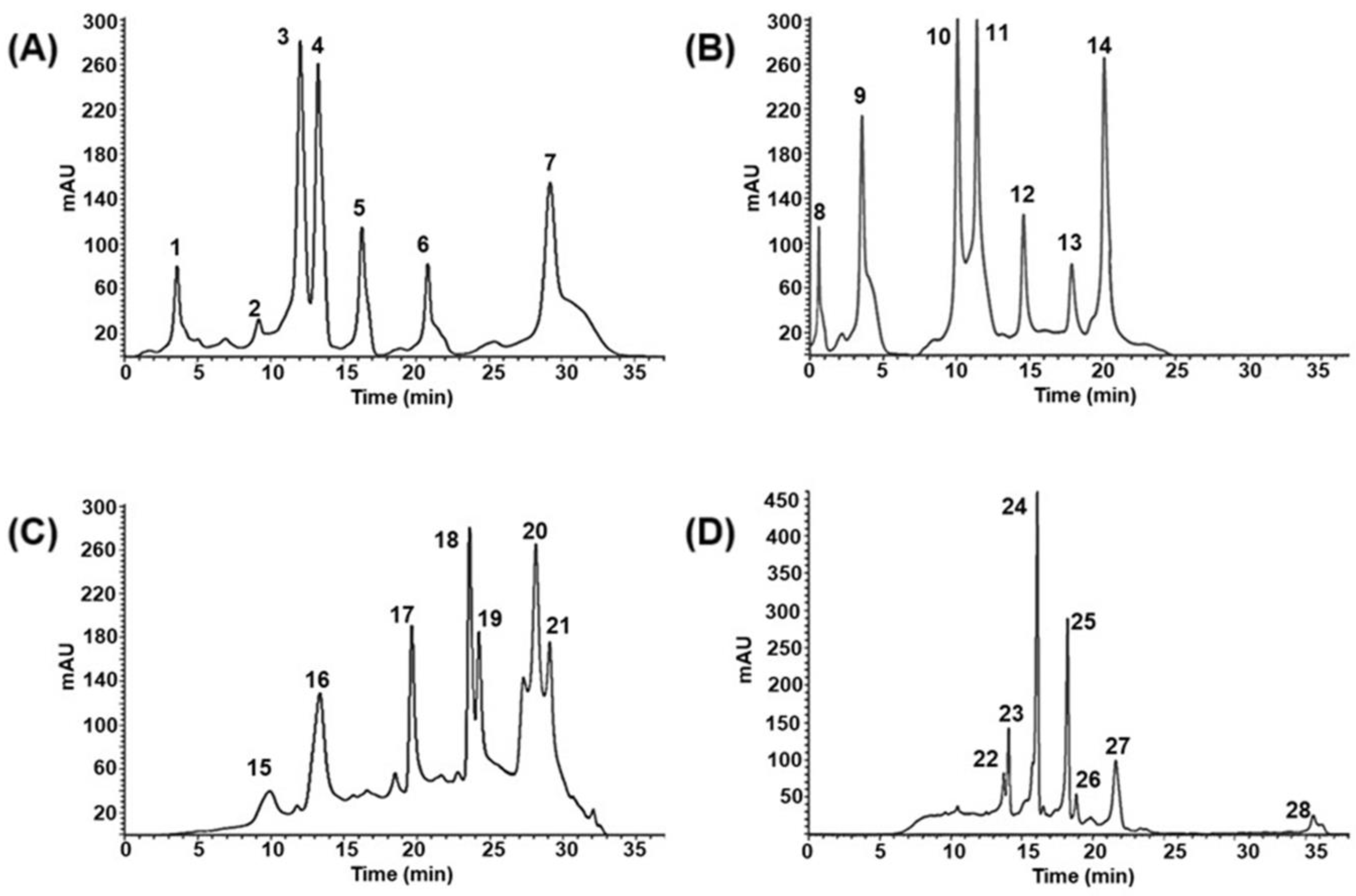
3.2.1. Peptide Profile
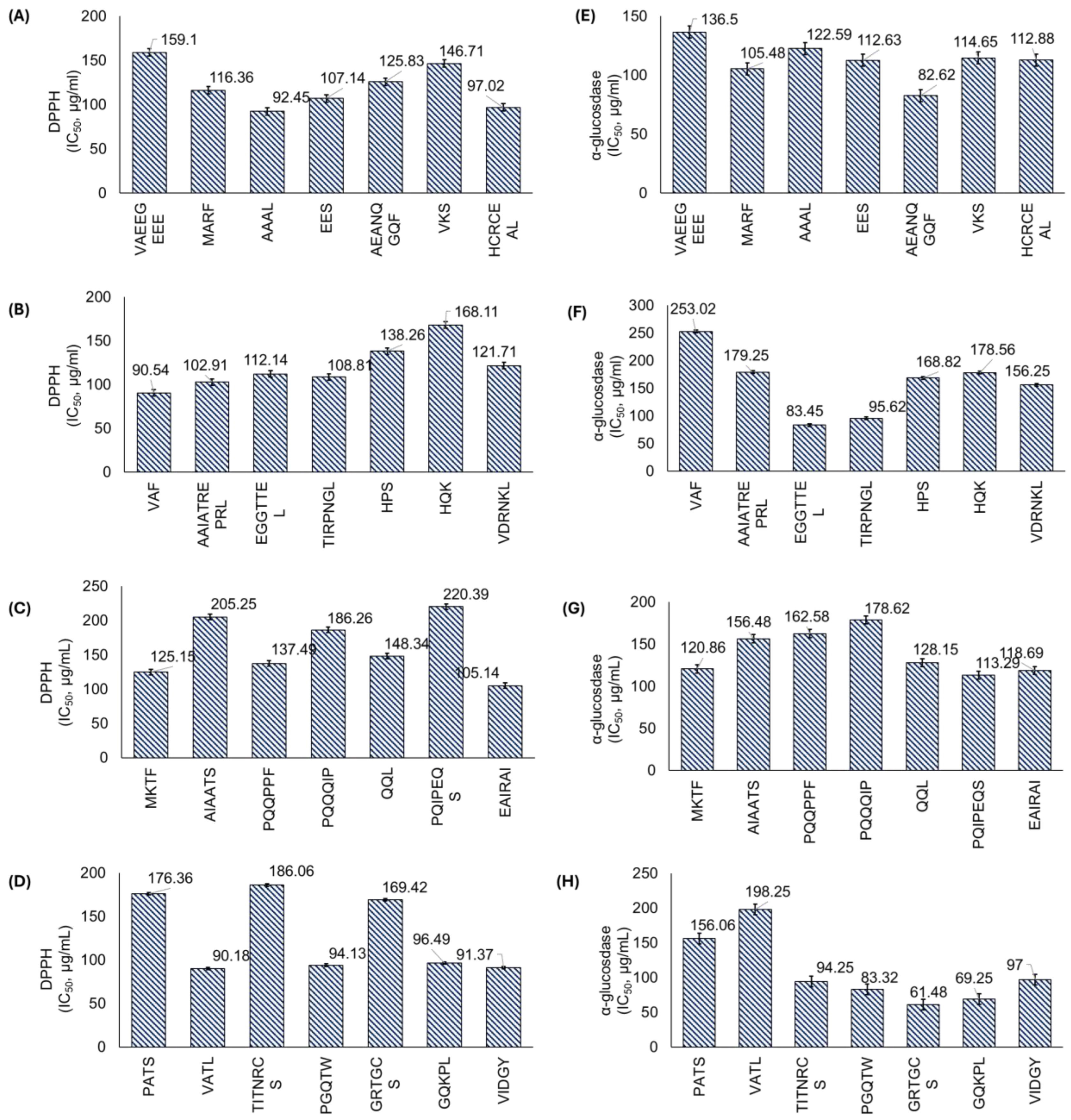
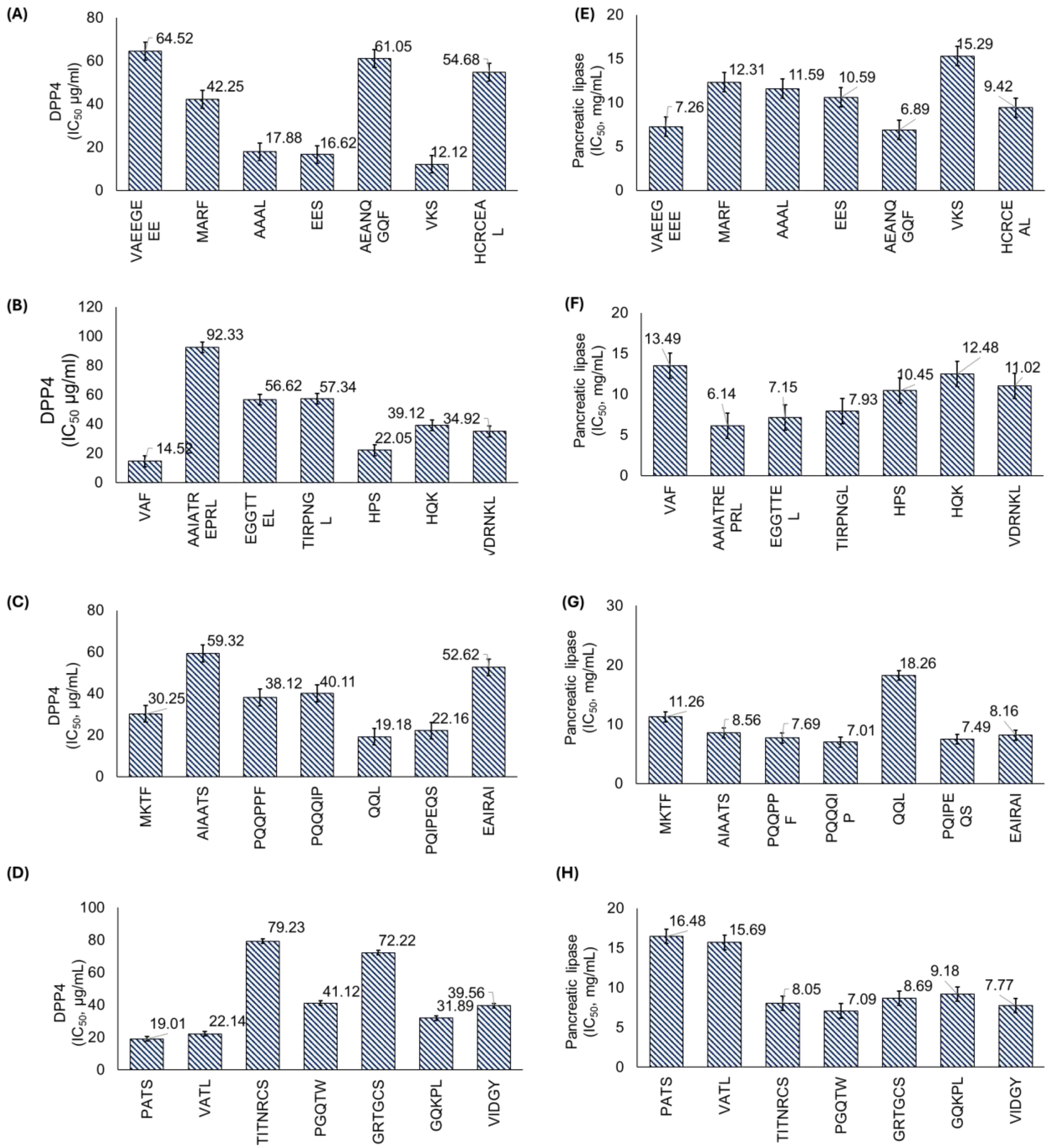
3.2.2. Biological Activities of Peptides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOEB | Sesame oil extraction byproduct |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| PMSF | Phenylmethylsulfonyl fluoride |
| EDTA | Ethylenediaminetetraacetic acid |
| ALBH | Albumin hydrolysate |
| GLOH | Globulin hydrolysate |
| GLUH | Glutenin hydrolysate |
| PROH | Prolamin hydrolysate |
| ALBF | Albumin fraction |
| GLOF | Globulin fraction |
| GLUF | Glutelin fraction |
| PROF | Prolamin fraction |
References
- Huang, L.; Cai, Y.; Fang, F.; Huang, T.; Zhao, M.; Zhao, Q.; Van der Meeren, P. Recent advance in the valorization of soy-based by-products: Extraction, modification, interaction and applications in the food industry. Food Hydrocoll. 2024, 157, 110407. [Google Scholar] [CrossRef]
- Salanță, L.C.; Fărcaş, A.C. Exploring the efficacy and feasibility of tomato by-products in advancing food industry applications. Food Biosci. 2024, 62, 105567. [Google Scholar] [CrossRef]
- Erismann, Y.; Brück, W.M.; Andlauer, W. Solid-State Fermentation of Agro-Industrial By-Products. Nutraceuticals 2025, 5, 11. [Google Scholar] [CrossRef]
- Vilas-Franquesa, A.; Montemurro, M.; Casertano, M.; Fogliano, V. The food by-products bioprocess wheel: A guidance tool for the food industry. Trends Food Sci. Technol. 2024, 152, 104652. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Hassoun, A.; Tarchi, I.; Loudiyi, M.; Boukria, O.; Cahyana, Y.; Ozogul, F.; Khwaldia, K. Transforming plant-based waste and by-products into valuable products using various “Food Industry 4.0” enabling technologies: A literature review. Sci. Total Environ. 2024, 955, 176872. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Concha, J.L.; Villada-Castillo, H.S.; Fernández-Quintero, A.; Ortega-Toro, R. Use of trout by-products (Oncorhynchus mykiss) for the production of an extruded fish feed/Uso de subproductos de trucha (Oncorhynchus mykiss) para la producción de alimento extruído para peces. Rev. Méx. Ing. Quím. 2022, 21, Alim2726. [Google Scholar] [CrossRef]
- Kaveh, S.; Darmian, Y.N.; Hashemi, S.M.B.; Abedi, E. Valorization of fishery byproducts as a sustainable development strategy: Health-beneficial activity with an emphasis on anticancer peptides and stabilization through encapsulation in liposomal systems. Appl. Food Res. 2025, 5, 100935. [Google Scholar] [CrossRef]
- Rios-Herrera, G.D.; Pedroza-Toledo, G.M.; Osuna-Ruiz, I.; Martínez-Montaño, E.; Sandoval-Gallardo, J.M.; Salazar-Leyva, J.A. Alkaline proteases from rose snapper (Lutjanus guttatus): Evaluation of their stability to chemical denaturants and potential application to hydrolyze seafood waste proteins. Appl. Biochem. Biotechnol. 2025, 197, 1946–1971. [Google Scholar] [CrossRef] [PubMed]
- Rios-Herrera, G.D.; Salazar-Leyva, J.A.; Hernández, C.; Jiménez-Gutiérrez, L.R.; Sandoval-Gallardo, J.M.; Osuna-Ruiz, I.; Martínez-Montaño, E.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; Ramirez-Perez, J.S. Production of Protein Hydrolysates Using Marine Catfish Bagre panamensis Muscle or Casein as Substrates: Effect of Enzymatic Source and Degree of Hydrolysis on Antioxidant and Biochemical Properties. Appl. Biochem. Biotechnol. 2021, 193, 3214–3231. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhao, F.; Wang, Z.; Wang, Q.; Chai, X.; Hou, G.; Meng, Q. Sesame (Sesamum indicum L.): A comprehensive review of nutritional value, phytochemical composition, health benefits, development of food, and industrial applications. Nutrients 2022, 14, 4079. [Google Scholar] [CrossRef]
- Norouzi, Z.; Nateghi, L.; Rashidi, L.; Movahhed, S. Increasing the bioactive properties of sesame seed meal as a valuable protein source through the enzymatic hydrolysis. Food Biosci. 2025, 68, 106790. [Google Scholar] [CrossRef]
- Ruiz-Armenta, X.A.; Ruiz-Armenta, J.E.; Espinoza-Moreno, R.J.; Gutiérrez-Dorado, R.; Aguilar-Palazuelos, E.; Zazueta-Morales, J.d.J.; Gómez-Favela, M.A. Aprovechamiento de subproducto de sésamo y extrusión optimizada para obtención de harina funcional con propiedades tecno-funcionales, nutricionales y antioxidante mejoradas. Acta Univ. 2022, 32, 1–20. [Google Scholar] [CrossRef]
- Noptana, R.; McClements, D.J.; McLandsborough, L.A.; Onsaard, E. Comparison of characteristics and antioxidant activities of sesame protein hydrolysates and their fractions. Heliyon 2024, 10, e27891. [Google Scholar] [CrossRef]
- Quintero-Soto, M.F.; Espinoza-Moreno, R.J.; Félix-Medina, J.V.; Salas-López, F.; López-Carrera, C.F.; Argüelles-López, O.D.; Vazquez-Ontiveros, M.E.; Gómez-Favela, M.A. Comparison of phytochemical profile and In Vitro bioactivity of beverages based on the unprocessed and extruded sesame (Sesamum indicum L.) seed byproduct. Foods 2022, 11, 3175. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Chávez, J.; Quintero-Soto, M.F.; Velarde-Barraza, R.; Rivero-Espejel, I.A.; Díaz-Peña, I.; Vázquez-Ontiveros, M.E.; Espinoza-Moreno, R.J.; Ontiveros-García, L.A.; Amillano-Cisneros, J.M.; Perales-Sánchez, J.X.K.; et al. Changes in the profile of phenolic compounds and in the antioxidant, hypoglycemic, and antidiabetic activities of a beverage based on sesame by-product caused by the simulated gastrointestinal digestion process. Beverages 2024, 10, 115. [Google Scholar] [CrossRef]
- Reis de Souza, T.C.; Aguilera-Barreyro, A.; Mariscal-Landín, G. Digestibilidad ileal estandarizada de la proteína y aminoácidos de la pasta de ajonjolí en cerdos en crecimiento. Rev. Mex. Cienc. Pecu. 2022, 12, 1292–1304. [Google Scholar] [CrossRef]
- Koysuren, B.; Oztop, M.H.; Mazi, B.G. Sesame seed as an alternative plant protein source: A comprehensive physicochemical characterisation study for alkaline, salt and enzyme-assisted extracted samples. Int. J. Food Sci. Technol. 2021, 56, 5471–5484. [Google Scholar] [CrossRef]
- Fajardo-Espinoza, F.S.; Romero-Rojas, A.; Hernández-Sánchez, H. Production of bioactive peptides from bovine colostrum whey using enzymatic hydrolysis/Producción de peptidos bioactivos a partir de suero de calostro bovino por hidrólisis enzimática. Rev. Mex. Ing. Quím. 2022, 19, 1–9. [Google Scholar] [CrossRef]
- Han, R.; Hernández Álvarez, A.J.; Maycock, J.; Murray, B.S.; Boesch, C. Comparison of alcalase- and pepsin-treated oilseed protein hydrolysates—Experimental validation of predicted antioxidant, antihypertensive and antidiabetic properties. Curr. Res. Food Sci. 2021, 4, 141–149. [Google Scholar] [CrossRef]
- Idowu, A.O.; Alashi, A.M.; Nwachukwu, I.D.; Fagbemi, T.N.; Aluko, R.E. Functional properties of sesame (Sesamum indicum Linn) seed protein fractions. Food Prod. Process. Nutr. 2021, 3, 4. [Google Scholar] [CrossRef]
- Idowu, A.O.; Famuwagun, A.A.; Fagbemi, T.N.; Aluko, R.E. Antioxidant and enzyme-inhibitory properties of sesame seed protein fractions and their isolate and hydrolyzate. Int. J. Food Prop. 2021, 24, 780–795. [Google Scholar] [CrossRef]
- Awosika, T.; Aluko, R. Inhibition of the in vitro activities of α-amylase, α-glucosidase and pancreatic lipase by yellow field pea (Pisum sativum L.) protein hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 2021–2034. [Google Scholar] [CrossRef]
- Wang, X.; Ai, X.; Zhu, Z.; Zhang, M.; Pan, F.; Yang, Z.; Wang, O.; Zhao, L.; Zhao, L. Pancreatic lipase inhibitory effects of peptides derived from sesame proteins: In silico and in vitro analyses. Int. J. Biol. Macromol. 2022, 222, 1531–1537. [Google Scholar] [CrossRef]
- Chaipoot, S.; Punfa, W.; Ounjaijean, S.; Phongphisutthinant, R.; Kulprachakarn, K.; Parklak, W.; Phaworn, L.; Rotphet, P.; Boonyapranai, K. Antioxidant, anti-diabetic, anti-obesity, and antihypertensive properties of protein hydrolysate and peptide fractions from black sesame cake. Molecules 2023, 28, 211. [Google Scholar] [CrossRef] [PubMed]
- Mensah, E.O.; Adadi, P.; Asase, R.V.; Kelvin, O.; Mozhdehi, F.J.; Amoah, I.; Agyei, D. Aloe vera and its byproducts as sources of valuable bioactive compounds: Extraction, biological activities, and applications in various food industries. PharmaNutrition 2025, 31, 100436. [Google Scholar] [CrossRef]
- Quintero-Soto, M.F.; Chávez-Ontiveros, J.; Garzón-Tiznado, J.A.; Salazar-Salas, N.Y.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; López-Valenzuela, J.A. Characterization of peptides with antioxidant activity and antidiabetic potential obtained from chickpea (Cicer arietinum L.) protein hydrolyzates. J. Food Sci 2021, 86, 2962–2977. [Google Scholar] [CrossRef]
- Félix-Medina, J.V.; Sepúlveda-Haro, A.G.; Quintero-Soto, M.F. Stability of antioxidant and hypoglycemic activities of peptide fractions of Maize (Zea mays L.) under different processes. J. Food Meas. Charact. 2023, 17, 362–370. [Google Scholar] [CrossRef]
- Xu, Y.; Galanopoulos, M.; Sismour, E.; Ren, S.; Mersha, Z.; Lynch, P.; Almutaimi, A. Effect of enzymatic hydrolysis using endo- and exo-proteases on secondary structure, functional, and antioxidant properties of chickpea protein hydrolysates. J. Food Meas. Charact. 2020, 14, 343–352. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 960.52. Microchemical determination of nitrogen. Micro-Kjeldahl Method. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MA, USA, 2012. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mojica, L.; Chen, K.; González de Mejía, E. Impact of commercial precooking of common bean (Phaseolus vulgaris) on the generation of peptides, after pepsin–pancreatin Hydrolysis, capable to inhibit dipeptidyl peptidase-IV. J. Food Sci. 2015, 80, H188–H198. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Chatterjee, R.; Dey, T.K.; Ghosh, M.; Dhar, P. Enzymatic modification of sesame seed protein, sourced from waste resource for nutraceutical application. Food Bioprod. Process. 2015, 94, 70–81. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2019, 116, 707–716. [Google Scholar] [CrossRef]
- Benjamin, M.A.Z.; Mohd Mokhtar, R.A.; Iqbal, M.; Abdullah, A.; Azizah, R.; Sulistyorini, L.; Mahfudh, N.; Zakaria, Z.A. Medicinal plants of Southeast Asia with anti-α-glucosidase activity as potential source for type-2 diabetes mellitus treatment. J. Ethnopharmacol. 2024, 330, 118239. [Google Scholar] [CrossRef]
- Qiao, H.; Bi, X.; Zhang, Y.; Liu, M.; Zu, S.; Jia, N.; Jiang, S.; Lu, Q.; Zu, Y.; Bao, Y. Enzymic polypeptide antioxidant activity and inhibitory activity on α-glucosidase and α-amylase from Paeonia ostii cake. Ind. Crops Prod. 2020, 146, 112158. [Google Scholar] [CrossRef]
- Du, T.; Xu, Y.; Xu, X.; Xiong, S.; Zhang, L.; Dong, B.; Huang, J.; Huang, T.; Xiao, M.; Xiong, T.; et al. ACE inhibitory peptides from enzymatic hydrolysate of fermented black sesame seed: Random forest-based optimization, screening, and molecular docking analysis. Food Chem. 2024, 437, 137921. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-R.; Zhang, L.; Ding, D.-G.; Chi, C.-F.; Wang, B.; Huo, J.-C. Preparation, Identification, and Activity Evaluation of Eight Antioxidant Peptides from Protein Hydrolysate of Hairtail (Trichiurus japonicas) Muscle. Mar. Drugs 2019, 17, 23. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Liu, B.-Q.; Cui, B.; Wen, L.; Xu, Z.; Chen, M.-L.; Wu, H. Alanine substitution to determine the effect of LR5 and YR6 rice peptide structure on antioxidant and anti-inflammatory activity. Nutrients 2023, 15, 2373. [Google Scholar] [CrossRef]
- Mengyuan, Z.; Chen, C.; Feng, W.; Ning, Z.; Wanyu, Y.; Tianrong, Z.; Guoyan, R.; Zhijun, Q.; Bin, Z. Identification and molecular mechanism of novel α-glucosidase inhibitory peptides from the hydrolysate of hemp seed proteins: Peptidomic analysis, molecular docking, and dynamics simulation. Int. J. Mol. Sci. 2025, 26, 2222. [Google Scholar] [CrossRef] [PubMed]
- González-Montoya, M.; Hernández-Ledesma, B.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Bioactive peptides from germinated soybean with anti-diabetic potential by inhibition of dipeptidyl peptidase-IV, α-amylase, and α-glucosidase enzymes. Int. J. Mol. Sci. 2018, 19, 2883. [Google Scholar] [CrossRef]
- Zhang, M.; Ling, Z.; Gangcheng, W.; Tongtong, L.; Xiguang, Q.; Zhang, H. Food-derived dipeptidyl peptidase IV inhibitory peptides: Production, identification, structure-activity relationship, and their potential role in glycemic regulation. Crit. Rev. Food Sci. Nutr. 2024, 64, 2053–2075. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Hirokazu, A.; Kazunori, S.; Honda, H. Enrichment and identification of pancreas lipase inhibitory peptides from food protein hydrolysate using group-selective peptide adsorption support. J. Chem. Eng. Jpn. 2025, 58, 2501969. [Google Scholar] [CrossRef]
| Feature | Sample |
|---|---|
| Protein fractions (g/100 g of flour) | |
| Albumin | 10.05 ± 0.50 b |
| Globulin | 30.53 ± 1.85 a |
| Glutelin | 7.5 ± 0.50 c |
| Prolamin | 2.08 ± 0.28 d |
| Soluble protein (g/100 g of hydrolyzed) | |
| Albumin hydrolysate | 93.54 ± 4.01 a |
| Globulin hydrolysate | 96.25 ± 0.93 a |
| Glutelin hydrolysate | 92.86 ± 1.40 a |
| Prolamin hydrolysate | 91.61 ± 2.64 a |
| Degree of hydrolysis (%) | |
| Albumin hydrolysate | 40.00 ± 2.00 b |
| Globulin hydrolysate | 45.10 ± 3.05 a |
| Glutelin hydrolysate | 36.33 ± 1.15 bc |
| Prolamin hydrolysate | 34.66 ± 3.51 c |
| Sample (1 mg/mL) | DPPH (% Inhibition) | α-Glucosidase (% Inhibition) |
|---|---|---|
| Albumin | ||
| ALBH | 43.43 ± 1.99 e | 55.63 ± 3.62 e |
| ALBF > 5 kDa | 62.48 ± 3.83 d | 61.25 ± 2.51 d |
| ALBF 3–5 kDa | 74.43 ± 2.51 c | 68.42 v 1.93 c |
| ALBF 1–3 kDa | 83.16 ± 2.52 b | 74.98 ± 1.07 b |
| ALBF < 1 kDa | 95.79 ± 2.32 a | 85.47 ± 1.67 a |
| Globulin | ||
| GLOBH | 37.10 ± 2.31 e | 47.05 ± 1.56 e |
| GLOF > 5 kDa | 51.68 ± 1.81 d | 53.10 ± 1.49 d |
| GLOF 3–5 kDa | 65.71 ± 2.65 c | 62.05 ± 0.81 c |
| AGLOF 1–3 kDa | 76.66 ± 2.52 b | 74.16 ± 0.88 b |
| GLOF < 1 kDa | 83.93 ± 2.17 a | 79.31 ± 0.95 a |
| Glutelin | ||
| GLUH | 40.17 ± 1.33 e | 42.79 ± 2.10 d |
| GLUF > 5 kDa | 58.72 ± 4.28 d | 54.14 ± 2.39 c |
| GLUF 3–5 kDa | 65.84 ± 1.45 c | 57.36 ± 1.55 c |
| GLUF 1–3 kDa | 76.87 ± 1.39 b | 64.14 ± 1.32 b |
| GLUF < 1 kDa | 86.92 ± 0.83 a | 74.93 ± 2.47 a |
| Prolamin | ||
| PROH | 25.19 ± 1.68 e | 40.14 ± 1.92 e |
| PROF > 5 kDa | 39.77 ± 2.56 d | 47.48 ± 1.45 d |
| PROF 3–5 kDa | 47.27 ± 0.46 c | 50.96 ± 0.96 c |
| PROF 1–3 kDa | 55.85 ± 3.09 b | 59.10 ± 0.09 b |
| PROF < 1 kDa | 71.40 ± 0.57 a | 65.21 ± 1.57 a |
| PM (Da) | Peptide * | PI | Hydrophobicity (kcal/mol) | Net Charge | Possible Bioactivity |
|---|---|---|---|---|---|
| ALBF < 1 kDa | |||||
| 890.36 | VAEEGEEE | 2.83 | 27.24 | −5 | ACE, DPP4 |
| 523.29 | MARF | 10.90 | 7.82 | 1 | ACE, DPP4 |
| 344.23 | AAAL | 5.60 | 8.15 | 0 | ACE, DPP4, AB |
| 363.16 | EES | 2.92 | 15.62 | −2 | DPP4 |
| 716.31 | AEANQGQ | 3.21 | 16.07 | −1 | ACE, DPP4, α-GLU |
| 332.24 | VKS | 9.88 | 10.70 | 1 | ACE, DPP4 |
| 830.37 | HCRCEAL | 7.01 | 14.88 | 0 | ACE, DPP4, α-GLU, AOX, AI |
| GLOF < 1 kDa | |||||
| 335.23 | VAF | 5.56 | 6.23 | 0 | ACE, DPP4 |
| 1096.71 | AAIATREPRL | 11.02 | 14.67 | 1 | ACE, DPP4, AB |
| 705.32 | EGGTTEL | 2.92 | 16.1 | −2 | ACE, DPP4, AOX |
| 769.45 | TIRPNGL | 11.11 | 9.73 | 1 | ACE, DPP4, AOX |
| 339.17 | HPS | 7.63 | 10.83 | 0 | ACE, DPP4 |
| 411.26 | HQK | 9.80 | 13.80 | 1 | ACE |
| 743.46 | VDRNKL | 10.14 | 15.29 | 1 | ACE, DPP4 |
| GLUF < 1 kDa | |||||
| 525.27 | MKTF | 9.93 | 8.57 | 1 | ACE, DPP4, AO |
| 532.28 | AIAATS | 5.51 | 8.99 | 0 | ACE, DPP4, AB |
| 712.37 | PQQPPF | 5.19 | 8.15 | 0 | ACE, DPP4, α-GLU |
| 709.39 | PQQQIP | 5.25 | 9.37 | 0 | ACE, DPP4, |
| 387.23 | QQL | 5.44 | 8.19 | 0 | DPP4 |
| 791.38 | PQIPEQS | 3.01 | 12.69 | −1 | ACE, DPP4, α-GLU |
| 671.41 | EAIRAI | 6.85 | 12.10 | 0 | ACE, DPP4, α-GLU, AOX |
| PROF < 1 kDa | |||||
| 374.18 | PATS | 5.18 | 9.25 | 0 | ACE, DPP4 |
| 402.27 | VATL | 5.63 | 6.94 | 0 | DPP4 |
| 793.38 | TITNRCS | 8.76 | 10.38 | 1 | ACE |
| 587.27 | PGQTW | 5.21 | 8.12 | 0 | ACE, DPP4, AOX, AT |
| 579.27 | GRTGCS | 8.76 | 12.70 | 1 | ACE, DPP4, AOX |
| 541.34 | GQKPL | 10.15 | 11.51 | 1 | ACE, DPP4, AOX |
| 565.28 | VIDGY | 3.15 | 10.40 | −1 | ACE, DPP4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Barajas, U.A.; Vázquez-Ontiveros, M.E.; Félix-Medina, J.V.; Velarde-Barraza, R.; Grimaldi-Olivas, J.C.; Badilla-Medina, C.N.; Amillano-Cisneros, J.M.; Quintero-Soto, M.F. Systematic Purification of Peptides with In Vitro Antioxidant, Antihyperglycemic, Anti-Obesity, and Antidiabetic Potential Released from Sesame Byproduct Proteins. Nutraceuticals 2025, 5, 23. https://doi.org/10.3390/nutraceuticals5030023
Mendoza-Barajas UA, Vázquez-Ontiveros ME, Félix-Medina JV, Velarde-Barraza R, Grimaldi-Olivas JC, Badilla-Medina CN, Amillano-Cisneros JM, Quintero-Soto MF. Systematic Purification of Peptides with In Vitro Antioxidant, Antihyperglycemic, Anti-Obesity, and Antidiabetic Potential Released from Sesame Byproduct Proteins. Nutraceuticals. 2025; 5(3):23. https://doi.org/10.3390/nutraceuticals5030023
Chicago/Turabian StyleMendoza-Barajas, Ulises Alan, Martha Elena Vázquez-Ontiveros, Jennifer Vianey Félix-Medina, Rosalio Velarde-Barraza, Jesús Christian Grimaldi-Olivas, Cesar Noe Badilla-Medina, Jesús Mateo Amillano-Cisneros, and María Fernanda Quintero-Soto. 2025. "Systematic Purification of Peptides with In Vitro Antioxidant, Antihyperglycemic, Anti-Obesity, and Antidiabetic Potential Released from Sesame Byproduct Proteins" Nutraceuticals 5, no. 3: 23. https://doi.org/10.3390/nutraceuticals5030023
APA StyleMendoza-Barajas, U. A., Vázquez-Ontiveros, M. E., Félix-Medina, J. V., Velarde-Barraza, R., Grimaldi-Olivas, J. C., Badilla-Medina, C. N., Amillano-Cisneros, J. M., & Quintero-Soto, M. F. (2025). Systematic Purification of Peptides with In Vitro Antioxidant, Antihyperglycemic, Anti-Obesity, and Antidiabetic Potential Released from Sesame Byproduct Proteins. Nutraceuticals, 5(3), 23. https://doi.org/10.3390/nutraceuticals5030023







